The most common anatomic variation of the kidney is the horseshoe kidney, with an incidence of 1 in 400 to 800.1 According to Eurotranspant data, more then one third of horseshoe kidneys from deceased donors were discarded.2 However, successful transplantation both en bloc3 and split4 kidneys had been described. The incidence suggests that there might be potential living donors with this anatomy. Here, we report on a case of successful renal transplantation from a live donor with a horseshoe kidney, with long-term follow-up of the donor and the recipient.
CASE DESCRIPTION
A 13-year-old girl with reflux nephropathy received a paternal left half of a horseshoe kidney on her right side simultaneously with bilateral native nephrectomy on February 6, 2008. The patent had 2 potential living donors, her hypertensive 40-year-old mother (average blood pressure, 150/90 mm Hg on 2 hypotensive drugs), and her normotensive 38-year-old father with a horseshoe kidney. The patient lived in 1077 km from the nearest pediatric transplant center, so that deceased donor transplantation was not realistic.
The right half of her father's horseshoe kidney was normal, whereas the left half had pyelectasis, perhaps because of the ureter passing anterior to the isthmus, and was supplied by 5 arteries (Figures 1 and 2).
FIGURE 1.
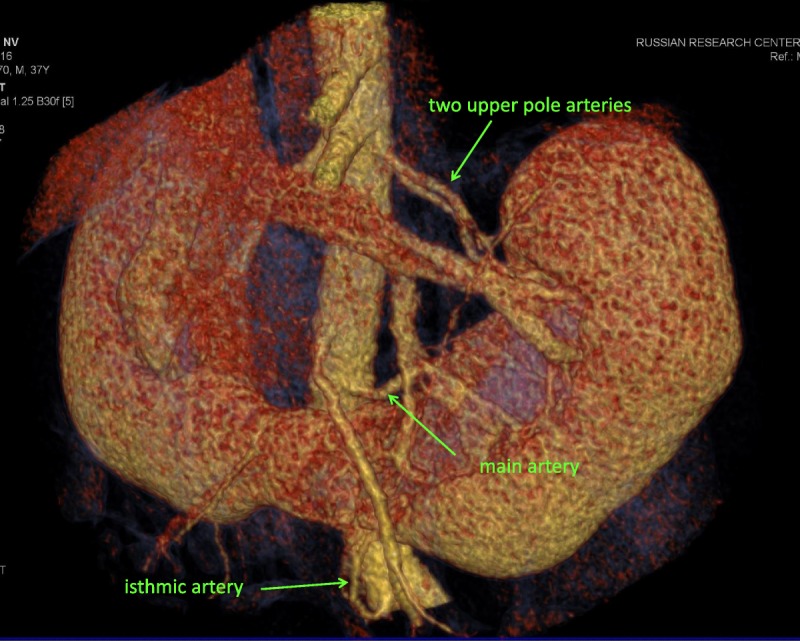
Multidetector computed tomography angiography in donor was performed using a 4-MDCT scanner (Somatom VZ, Siemens). We injected 100 mL of iohexol through a peripheral venous catheter at a rate 3.0 mL/s. Arterial phase images were acquired at 20 to 30 seconds. Image data were reconstructed with a body soft tissue algorithm in 0.5 mm and 1.5 mm thick sections. Computed tomography angiograms were interpreted in axial and coronal reformations. Was used three-dimensional imaging with volume rendering and maximum intensity projection (MIP) for evaluation of renal vessels and urinary tract.
FIGURE 2.
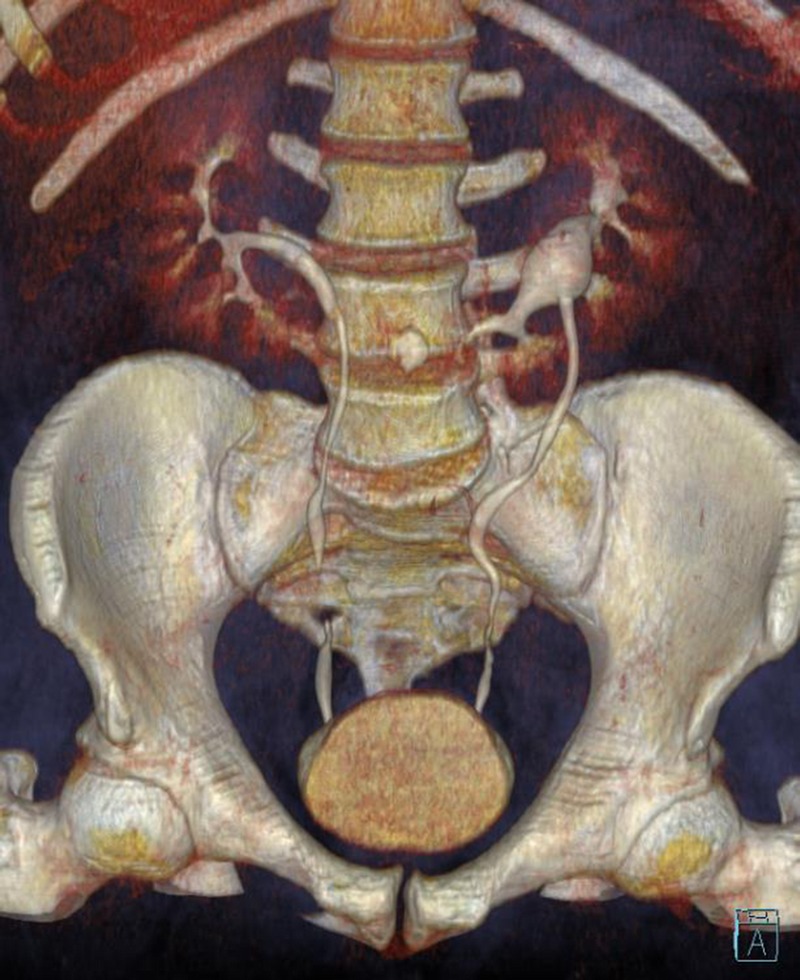
MDCT urography was performed using a 4-MDCT scanner. We injected 100 mL of iohexol through a peripheral venous catheter at a rate 3.0 mL/s. A delayed scan was obtained at 8 to 10 minutes postinjection to evaluate for urinary tract.
We preferred to use left half of the horseshoe kidney instead the hypertensive mother. The kidney was removed through a right subcostal incision. A small upper pole artery was sacrificed. The isthmus was 1 cm thick and 4 cm wide; to facilitate prompt graft removal and avoid bleeding, it was divided after bilateral clamping and oversewing with running polyglactin 2-0 suture.
With the goal of anastomosing the largest graft arteries to the largest recipient vessels, we placed the graft in an inverted position. The isthmic artery, originating in the donor from left common iliac artery, was sutured on the back table to a branch of the one of the upper pole arteries (Figure 3). Preservation of isthmic artery was essential for the ureteral blood supply. Graft revascularization was as follows: the main graft artery was anastomosed end-to-side to the common iliac artery, the first upper pole artery with reconstructed isthmic artery end-to-end to the internal iliac artery, and the second upper pole artery end-to-side to the external iliac artery (Figure 3). The vein was anastomosed to the external iliac vein. The ureter was anastomosed to the bladder in an extravesical manner with an external stent. The pyeloureteral segment was cut lengthwise and sutured transversely.
FIGURE 3.
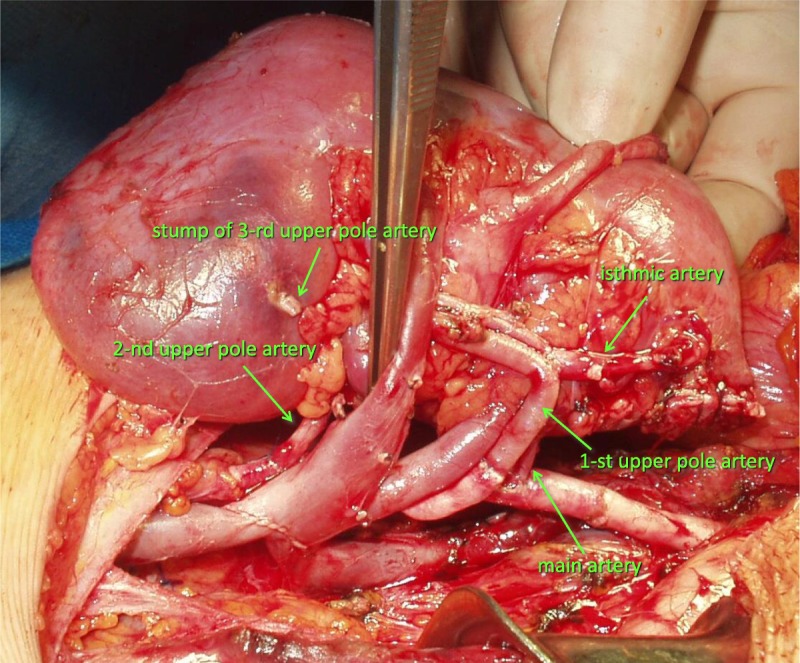
Graft revascularization. The isthmic artery, originating in the donor from left common iliac artery, was sutured on the back table to a branch of the one of the upper pole arteries. The main graft artery was anastomosed end-to-side to the common iliac artery, the first upper pole artery with reconstructed isthmic artery end-to-end to the internal iliac artery, and the second upper pole artery end-to-side to the external iliac artery. The vein was anastomosed to the external iliac vein.
The patient received alemtuzumab induction 19 days before transplantation, as it was described previously,5 with tacrolimus and mycophenolates as maintenance immunosuppression.
The immediate outcome was excellent. The recipient creatinine was 0.6 mg/dL on postoperative day 2. The donor peak creatinine level was 2 mg/dL on postoperative day 1, 2 weeks later, it was 1.3 mg/dL (his pretransplant creatinine was 1.2 mg/dL). Neither the donor nor the recipient required blood transfusions.
The recipient's graft function was good for the first four years, glomerular filtration rate (Shwartz) near 90 mL/min, she grew 27 cm, and her final height was 150 cm. After the onset of sexual activity, she developed pyelonephritis, which led to deterioration of graft function. Cystography revealed no vesicoureteral reflux. Belated vaccination against human papilloma virus was performed. Her peak creatinine level was 2.5 mg/dL 6 years posttransplant, 7.5 years posttransplant her creatinine level was 2 mg/dL. Protocol biopsies at 1 month, 1 and 3 years posttransplant were normal; biopsy at 5 years revealed severe tubulointerstitial fibrosis (Banff tubular atrophy = 3, interstitial fibrosis = 3), weak glomerulopathy (Banff chronic glomerulopathy < 1) with no evidence of rejection. To evaluate possible urine retention as a cause of pyelonephritis, we performed computed tomography in July 2015 (Figures 4 and 5), all graft arteries are clearly seen.
FIGURE 4.
MDCT urography in donor 7 years since heminephrectomy with a 320-MDCT scanner. We injected 40 mL of iodixanol through a peripheral venous catheter at a rate 3.0 mL/s. A delayed scan was obtained at 8 to 10 minutes postinjection to evaluate for urinary tract.
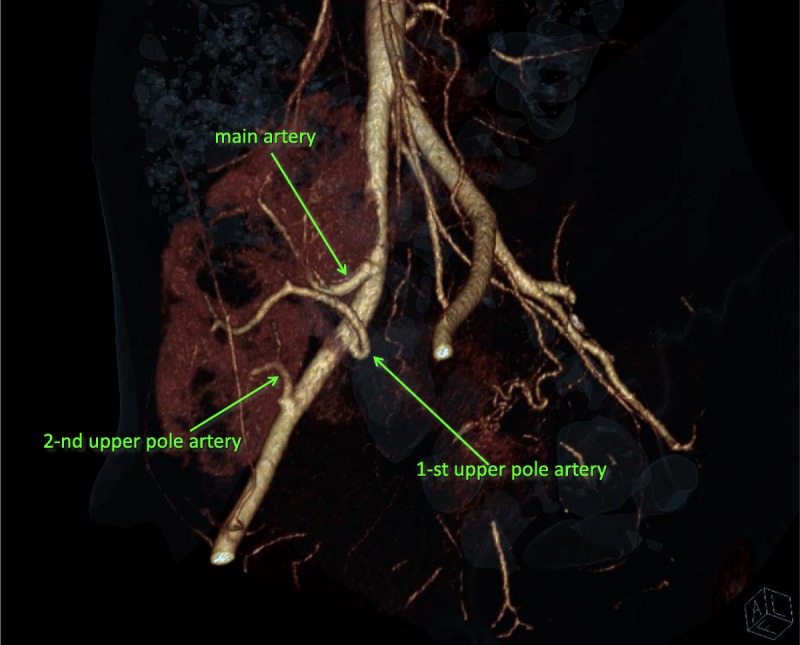
FIGURE 5.
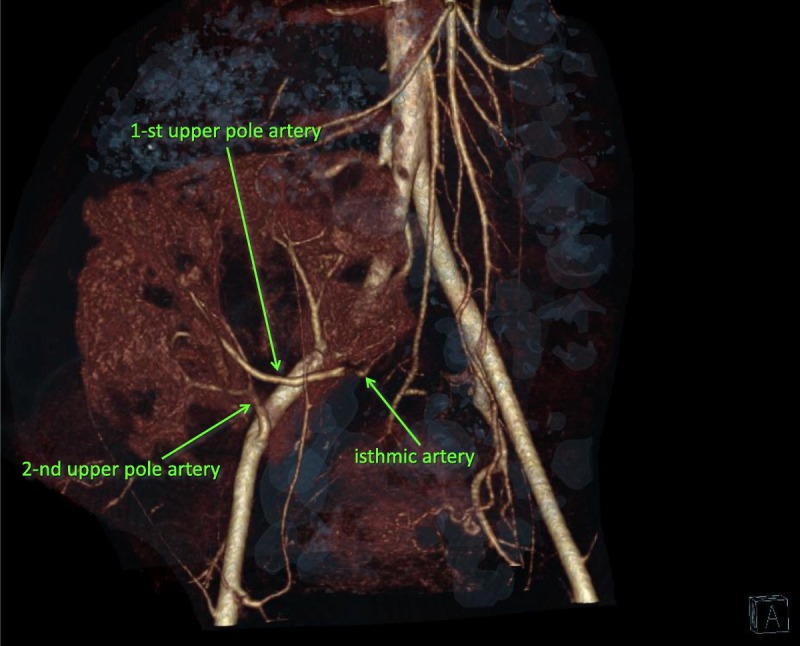
MDCT angiography 7 years after transplantation was performed using a 320-MDCT scanner. We injected 40 mL of iodixanol through a peripheral venous catheter at a rate 3.0 mL/s. Arterial phase images were acquired at 20 to 30 seconds. Three main graft arteries are clearly seen.
Seven years since heminephrectomy, the donor's serum creatinine was 1.6 mg/dL, his blood pressure was 130/80 mm Hg, and he had 221 mg/day of proteinuria. Because of suggestion that horseshoe kidneys are associated with an increased occurrence of renal pelvic tumors,1 we performed computed tomography in July 2015 and found no pathology (Figure 6).
FIGURE 6.
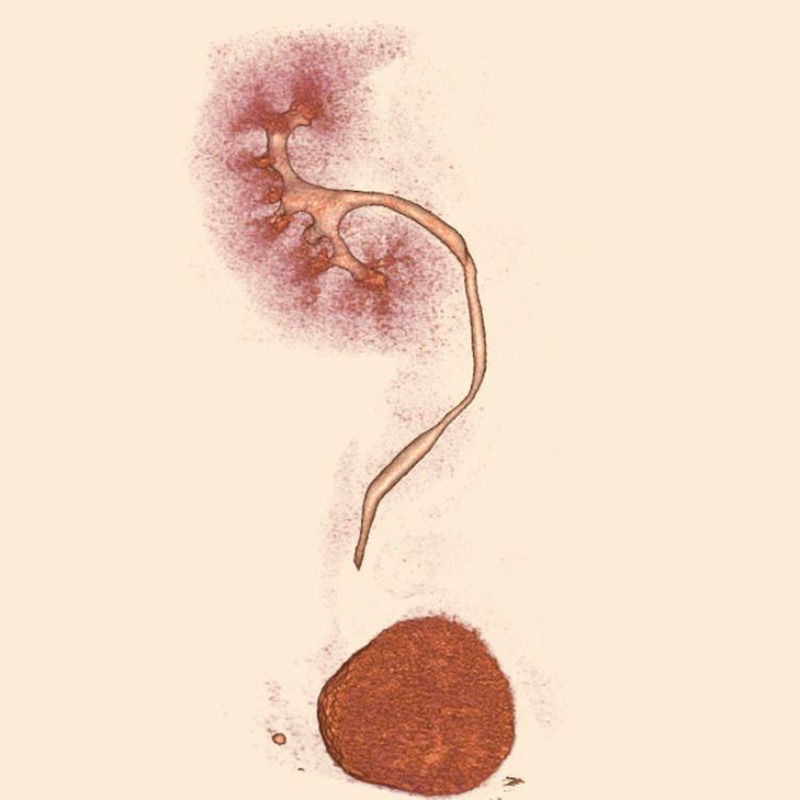
MDCT angiography 7 years after transplantation with a 320-MDCT scanner. Isthmic artery originates from first upper pole artery.
DISCUSSION
Since 1998, there have been 9 cases of horseshoe kidney transplantation from live donors published6 with maximal follow-up of 54 months. Regardless of the absence of donor morbidity and acceptable recipient outcomes, transplant centers in the developed world still would decline live donors with horseshoe kidney. Partially, it can be explained by the fact that majority of published cases (8 of 9) came from countries where deceased donor transplantation is not available to all patients (Japan, India, Turkey, Mexico). In Russia, deceased donor programs cover less than half of the population. However, organ shortage is recognized and a growing universal problem. Use of live donors with horseshoe kidney cannot impact on the organ shortage, but some patients can benefit.
The major concern is donor safety. Horseshoe kidney anatomy is obtained in details during routine kidney donor workup. If a kidney remnant will not differ from a normal kidney, donor safety is not compromised, and in these settings, heminephrectomy is not less safe than hemihepatectomy in a live liver donor. Our case has the longest follow-up, both in the donor and the recipient. The transplantation was the result of a combination of circumstances—no other good donors available except for a hypertensive mother, no options for deceased donor transplantation, one half of the horseshoe was completely normal, the other half was deemed transplantable. With his kidney remnant (compare Figures 2 and 6), the donor seems do not differ from usual live kidney donors.
CONCLUSIONS
Renal transplantation from a live donor with a horseshoe kidney is feasible.
Footnotes
Published online 22 December 2015.
The authors declare no funding or conflicts of interest.
M.M.K. participated in research design, in the performance of the research, and in the writing of the article. N.N.B. participated in the performance of the research, in data analysis, and in the writing of the article. A.K.Z. participated in the performance of the research and in data analysis. V.V.K. participated in data analysis. T.N.G. participated in data analysis.
This work was carried out at the Petrovsky National Research Center of Surgery, Moscow, Russian Federation.
REFERENCES
- 1. Bauer SB. Anomalies of the kidney and ureteropelvic junction. In: Walsh PC, Retik AB, Vaughan ED, Jr, Wein AJ, editors. Campbell's Urology, 7th ed, Vol 2 Philadelphia: W. B. Saunders; 1998: 1725. [Google Scholar]
- 2. Stroosma OB, Smits JM, Schurink GW, et al. Horseshoe kidney transplantation within the Eurotransplant region: a case control study. Transplantation. 2001; 72: 1930– 1933. [DOI] [PubMed] [Google Scholar]
- 3. Corcoran AT, Shapiro R, Kayler LK. Transplantation of a horseshoe kidney. Transplantation. 2007; 83: 828– 829. [DOI] [PubMed] [Google Scholar]
- 4. Tan HP, Samaniego MD, Montgomery RA, et al. Donor horseshoe kidneys for transplantation. Transplantation. 2001; 72: 869– 873. [DOI] [PubMed] [Google Scholar]
- 5. Kaabak MM, Babenko NN, Samsonov DV, et al. Alemtuzumab induction in pediatric kidney transplantation. Pediatr Transplant. 2013; 17: 168– 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Justo-Janeiro JM, Orozco EP, Reyes FJ, et al. Transplantation of a horseshoe kidney from a living donor: case report, long term outcome and donor safety. Int J Surg Case Rep. 2015; 15: 21– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]


