Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are surgical complications estimated to occur in 5% to 10% of patients. There are limited data regarding DVT/PE in the early postoperative period in liver transplant patients. The aim of this study is to determine risk factors that influence the incidence of DVT/PE and the effectiveness of prophylaxis.
Methods
We reviewed the records of 999 patients who underwent initial liver transplant between January 2000 and June 2012 at Henry Ford Hospital. In 2011, a standardized prophylactic regimen using subcutaneous (SQ) heparin was initiated. All patients that developed either upper/lower extremity DVT or PE within the first 30 days of transplant formed the cohort of this study.
Results
On multivariate analysis, only peripherally inserted central catheter (PICC) placement and SQ heparin were associated with DVT/PE. In patients receiving heparin, 3 (1.0%) had DVT/PE versus 25 (3.5%) who did not receive heparin (P = 0.03). Sixteen (6.9%) patients that had a PICC developed DVT/PE compared with 12 (1.6%) patients without a PICC (P < 0.001). In the heparin group, DVT/PE with PICC was reduced to 3 (3.0%) versus 13 (9.9%) in those with a PICC and did not receive heparin (P = 0.03). Mean time from transplant to DVT/PE diagnosis was 12.3 days. Length of hospitalization was significantly longer in patients who developed DVT/PE (18.5 vs 10.0 days, P < 0.001).
Conclusions
In this study, we demonstrated that PICC placement significantly increases the likelihood of DVT/PE in liver transplant recipients. Prophylactic SQ heparin effectively reduced DVT/PE events in this patient population.
Venous thromboembolism (VTE), with deep vein thrombosis (DVT) and pulmonary embolism (PE) as the most common manifestations, is a serious and potentially fatal complication of major abdominal surgery and is estimated to occur in 5% to 10% of patients.1 The major outcomes of VTE include significant bleeding from anticoagulation, postthrombotic syndrome, recurrence, and even death.2 Death occurs within 1 month in about 6% of those affected with DVT and 10% with PE.3 With autopsy-based PE diagnosis, the mortality rate for PE has been estimated to be as high as 30%.4 Pulmonary embolism is reported to be the most common cause of preventable death in surgical patients.5
Venous thromboembolism tends to occur due to a combination of endogenous, genetic, and environmental risk factors.6 The most common factors for VTE are hospitalization (52%), cancer (48%), and surgery (42%).3 Numerous risk factors also exist in patients who undergo orthotopic liver transplantation (OLT). Our group has previously demonstrated an increased risk of VTE in transplant recipients with diabetes, previous history of VTE, and end-stage renal disease.7 Another recent study found an increased risk of VTE associated with transplant patients discharged to rehabilitation centers, received factor VII during surgery, or had postoperative pneumonia.8 Patients with hepatitis C have been found to have an increased level of anti-phospholipid antibodies, placing them at a higher risk for thrombosis.9 In patients with end-stage liver disease, thrombotic and hemorrhagic complications can occur due to deficiencies in coagulation factors, inhibitors, and fibrinolytic mechanisms.10,11
The liver is the primary organ responsible for hemostasis because it synthesizes the majority of circulating coagulation factors and inhibitors, as well as components of the fibrinolytic system. Consequently, liver diseases are often associated with disruption in hemostasis. It was previously believed that patients with liver disease have a higher bleeding tendency, which was supported by abnormal coagulation tests including prolonged prothrombin time, activated partial thromboplastin time (aPTT), and low platelet count. These patients typically have significantly lower levels of factors responsible for coagulation, fibrinolysis, and platelet function. Interestingly, levels of von Willebrand factor (vWF) and factor VIII are elevated in these patients.12,13 It has been postulated that vWF elevation may be due to enhanced production by the endothelium or reduced clearance by the liver.14 The increased plasma levels of factor VIII may be secondary to the elevation of its carrier protein vWF. Because the platelet count does not take the elevated vWF levels into account, and because the prothrombin time and aPTT are insensitive to the plasma levels of anticoagulants, such as protein C, antithrombin, and tissue factor pathway inhibitor, they fail to reflect the true hemostatic profile and cannot predict the risk of bleeding in patients with liver disease.15-19 Studies have described the concept of rebalanced hemostasis due to reduction in both procoagulants and anticoagulants, and a marked resistance to the inhibitory action of thrombomodulin.20,21
Currently, the difficulty that physicians encounter is to determine when postreperfusion changes and coagulopathy revert in the postoperative phase to a normal or procoagulant state. Patients may be hypercoagulable early after OLT because of an imbalance between coagulation and fibrinolytic mechanisms, favoring a sustained prothrombotic state in the early postoperative phase.22 Furthermore, immunosuppressive drugs, such as cyclosporine, tacrolimus, and dexamethasone, may play a role in increasing platelet aggregation and thrombogenicity.23-25 It is well established that even as the graft regains normal function, there are transient defects in coagulation and fibrinolysis leading to altered clot formation and breakdown.22 There is currently limited data regarding VTE in the early postoperative period in liver transplant patients. The aim of this study is to identify risk factors associated with either DVT or PE and establish the effectiveness of thrombo-prophylaxis, specifically subcutaneous (SQ) heparin, in reducing the risk of DVT or PE in the OLT patient population.
MATERIALS AND METHODS
This was a retrospective review of all 1001 patients who underwent 1107 OLTs in the period of January 2000 to June 2012 at Henry Ford Hospital. One hundred and one patients had more than 1 transplant. Only the first transplant was included in this study. Two patients with missing VTE information were excluded. All patients older than 18 years with symptomatic VTE within 30 days after OLT were included in the study. Data were gathered from patient medical records which reflects inpatient hospitalization or outpatient visits. A 30-day cutoff was established to decrease the chance of including transplant-unrelated VTE events. Techniques of OLT included the traditional caval interposition technique with or without venovenous bypass and caval sparing, or piggyback technique, without venovenous bypass. In 2011, a standardized prophylactic regimen using SQ heparin (5000 U) every 8 hours was initiated. All patients with symptomatic upper extremity DVT, lower extremity DVT, and/or PE formed the cohort of this study. Diagnosis of DVT was confirmed radiologically using Doppler ultrasound; diagnosis of PE was confirmed using computed tomography pulmonary angiography. Peripherally inserted central catheter (PICC) lines were placed posttransplant after the removal of the perioperatively placed cordis and Swan-Ganz catheter in patients requiring central venous access for induction immunosuppression and/or total parental nutrition. Rabbit antithymocyte globulin was given as an induction agent centrally per institutional protocol. Patients diagnosed with DVT or PE were treated by PICC line removal and anticoagulation.
Values for white blood cell (WBC) count, platelet count, international normalized ratio (INR), age and model for end-stage liver disease (MELD) score were collected preoperatively, 1 day postoperative and 7 days postoperative. The use of PICC line placement during the transplant hospitalization period was also included. χ2 Test of independence and Wilcoxon rank sum test were used to determine which factors were associated with VTE. Logistic regression was run to find predictors of VTE. Univariate models were initially run with the variables PICC line, SQ heparin, age, and MELD score. The final multivariate model was selected using backward selection. All variables in the final model are significant at P less than 0.05. Statistical analysis was done using SAS version 9.4.
RESULTS
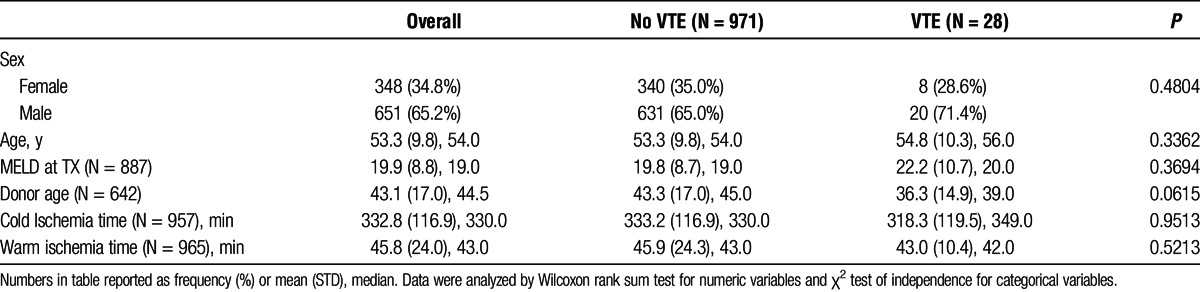
Baseline characteristics of the patient cohorts are provided in Table 1. Of the 999 patients included in the analysis, 28 (2.8%) developed posttransplant DVT or PE either during their initial hospitalization or within the first 30 days posttransplant. Eight patients with VTE events were women (28.6%) and 20 patients were men (71.4%), which were not statistically significant. The mean patient age was 54.8 years. The mean donor age in the VTE cohort was slightly lower at 36.3 years, although it was not statistically significant. The average MELD score at transplantation was 22.2. Mean cold and warm ischemia times were 318.3 and 43.0 minutes, respectively.
TABLE 1.
Patient demographics
In the VTE group, the etiology of cirrhosis are as follows: 3 patients due to alcohol, 3 patients due to hepatitis C, 1 patient due to nonalcoholic steatohepatitis, 2 patients due to hepatocellular carcinoma, and 2 patients due to autosomal-dominant polycystic kidney disease. In the VTE + PICC group: 5 patients were due to alcohol, 1 patient due to hepatitis C, 1 patient was cryptogenic, 1 patient due to hereditary hemochromatosis, 1 patient due to primary sclerosing cholangitis-ulcerative colitis, 1 patient due to nonalcoholic steatohepatitis, 2 patients due to lupus, 1 patient drug-related, 2 patients due to hepatocellular carcinoma, 1 patient was benign, and 1 patient due to autosomal-dominant polycystic kidney disease.
Orthotopic liver transplantation patients that developed VTE stayed in the hospital for an average of 24.8 days compared with 14.2 days by patients without VTE (P < 0.0001, Wilcoxon rank sum test). Peripherally inserted central catheter line placement was not associated with an increased length of stay in VTE patients (P > 0.05, Wilcoxon rank sum test). The minimum time to develop VTE after transplant was 2 days. The mean time to VTE within 30 days of transplant was 12.3 days.
Peripherally inserted central catheter line placement in OLT patients was assessed to determine whether it affects the risk of VTE. From the 766 patients who did not have a PICC line placed during their initial hospitalization, 12 (1.6%) patients developed VTE within the first 30 days posttransplant (Figure 1). Seven (58.3%) of the DVTs were located in the upper extremity, 3 (25.0%) in the lower extremity, and 2 (16.7%) were PEs. From the 233 patients who had a PICC line placed, 16 (6.9%) patients developed VTE (Figure 1). Seven (41.2%) of the DVTs were located in the upper extremity, 6 (35.3%) in the lower extremity, and 4 (23.5%) were PEs (P > 0.05, χ2 test of independence). One patient developed simultaneous lower-extremity DVT and PE.
FIGURE 1.
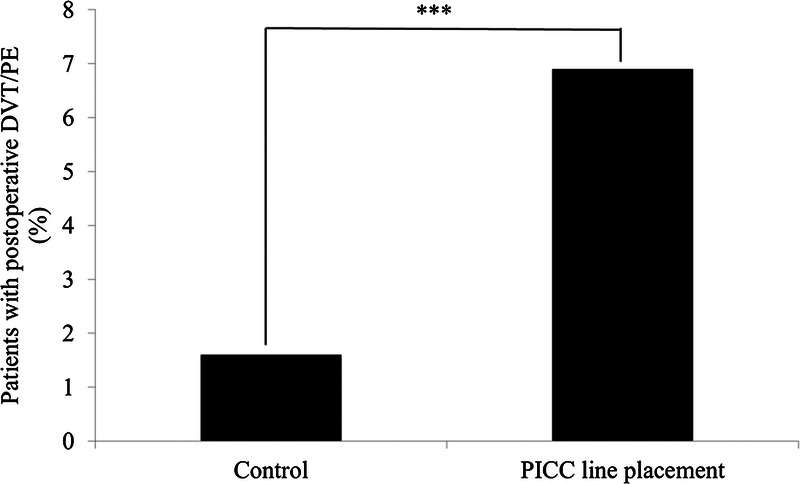
PICC line placement and risk of VTE. The use of a PICC line in OLT patients during hospitalization was identified to determine whether it had an impact on posttransplant VTE. PICC line placement was found to significantly increase the incidences of VTE in OLT patients. Data were analyzed by χ2 test of independence. ***P < 0.0001.
Prophylactic SQ heparin was given to patients as a preventative strategy for VTE. From the 288 patients who took SQ heparin, only 3 (1.0%) patients developed VTE within 30 days posttransplant. Twenty-five of 711 (3.5%) OLT patients that did not take SQ heparin developed posttransplant VTE within the 30-day period (Figure 2).
FIGURE 2.
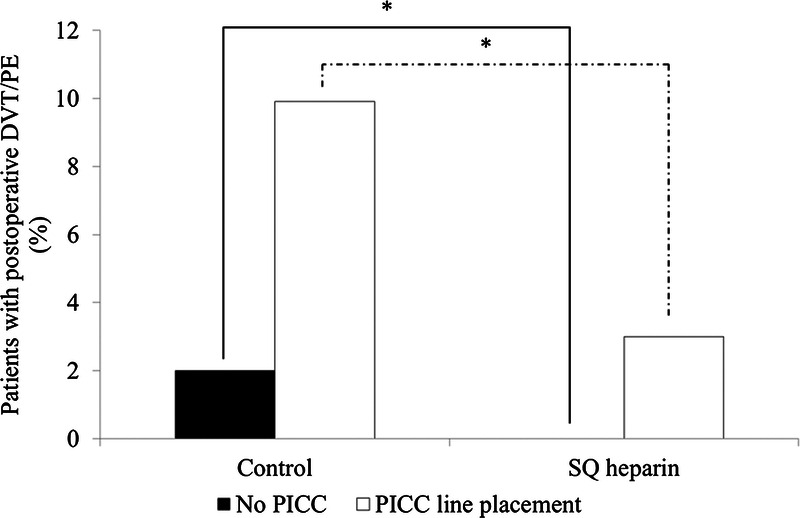
Effectiveness of SQ heparin on reducing the risk of VTE. SQ heparin was given prophylactically to OLT patients to prevent posttransplant VTE. SQ heparin significantly decreased the incidences of VTE in OLT patients, in both groups with or without PICC line placement. Data were analyzed by χ2 test of independence. *P < 0.05.
Of the 233 OLT patients with PICC line placement, 132 patients did not take SQ heparin. Thirteen (9.9%) patients that had a PICC line and did not take SQ heparin developed VTE within 30 days posttransplant. Of the 101 patients who had a PICC line and took SQ heparin, only 3 (3.0%) patients developed posttransplant VTE (Figure 2).
On multivariate analysis, PICC line placement in OLT patients resulted in a 6.3 times greater odds (95% CI, 2.9-13.8) of developing posttransplant VTE within the 30-day posttransplant period (P < 0.0001). Prophylactic SQ heparin was associated with a decrease in transplant-associated VTE, as patients without SQ heparin prophylaxis had a 5 times greater odds (95% CI, 0.1-0.7) of developing VTE (P < 0.01).
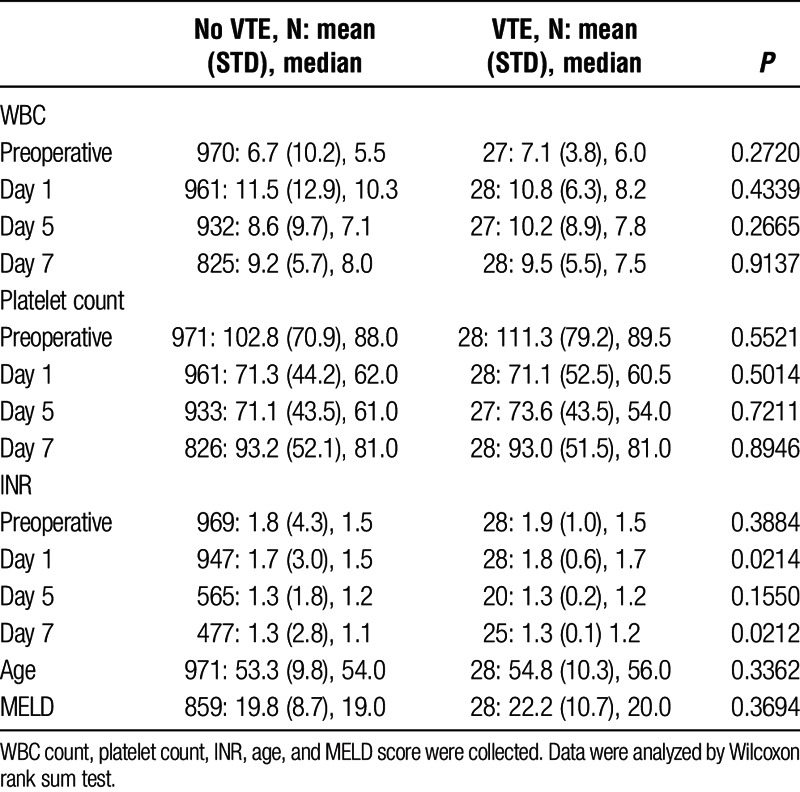
White blood cell count, platelet count, INR, age, and MELD score were collected as possible factors associated with posttransplant VTE. Values for WBC count, platelet count, and INR were collected preoperative, day 1, day 5, and day 7 posttransplant. Using the Wilcoxon rank sum test, however, there was no difference between these factors and VTE in this study (Table 2).
TABLE 2.
Possible predictive and risk factors for posttransplant VTE
DISCUSSION
A general population study that monitored 21 680 persons for VTE events over 7.6 years demonstrated that about half of all thrombotic events were considered secondary to triggering factors, with multiple risk factors present in 65% of cases.3 We previously found that diabetes, previous history of VTE, and the diagnosis of end-stage renal disease all increase the risk of VTE in OLT patients.7 Another retrospective study associated an increased risk of DVT after transplantation with patients who were discharged to rehabilitation centers, received factor VII during surgery, or had postoperative pneumonia.8 In our present study, we discovered that PICC line placement also significantly increases the risk of VTE. A meta-analysis review of 29 503 hospital patients demonstrated a risk of DVT at 2.7% with PICC line placement,26 compared with 6.9% in our transplant-specific population. In the meta-analysis review, PICC line was associated with a higher risk of DVT than central venous catheters, with the highest proportion in patients who were critically ill (13.91%) or with cancer (6.67%). As we demonstrate a baseline VTE incidence of 1.6% in OLT patients without PICC in our study, it is possible that both OLT and PICC line placement act synergistically as risk factors for VTE. We initially began using PICC lines to limit the risk of infection associated with central lines based on earlier data.27 However, based on our study, it is clear that the decision to insert PICC lines in liver transplant recipients should be guided by evaluating its benefits versus the risk for VTE.
Multiple studies have demonstrated that many procoagulants are altered in liver transplant recipients. One study showed that factors V and VII did not return to normal activity until 3 days after transplant,22 whereas another study found elevated levels of factor VIII.20 von Willebrand factor was demonstrated to be substantially elevated in patients for up to 10 days post-OLT.28 ADAMTS13, a vWF-cleaving protease, has been found to decrease from transplantation, and is associated with thrombosis in different diseases including sepsis and myocardial infarction.29,30 Furthermore, an elevation in plasminogen activator inhibitor 1 was observed up to day 7 posttransplant,31 and antiphospholipid antibodies have been found in patients with cirrhosis.32
Several anticoagulant proteins also show delayed recovery. A study reported antithrombin deficiency in 81% of patients up to 3 days posttransplant, and 57% were still deficient on day 5.22 Similarly, Proteins C and S levels were deficient in 24% and 21% of OLT patients at day 3, and 20% and 10% at day 5, respectively. Although the LETS study demonstrated that patients with hereditary protein C deficiency were associated with a relative risk of 6.5 to develop thrombosis, low protein S levels were not correlated to thrombotic risk.33 Thrombomodulin was also shown to be less effective in plasma samples taken from OLT patients.20
Prothrombin time and aPTT, which are sensitive only to procoagulant proteins, are substantially prolonged despite an equal or higher thrombin generation potential in OLT patients, and are not clinically reliable to evaluate hemodynamic alterations in this population.34 Patients with cirrhosis may have features of accelerated fibrinolysis,35,36 whereas patients with acute liver failure present with inhibited fibrinolysis.35 Thrombocytopenia is less common in acute liver failure, although the procoagulant and anticoagulant deficiencies are generally more pronounced than in chronic liver failure.37,38 In cholestatic liver diseases, the hemostatic deficiencies are more mild than that in patients with parenchymal hepatic disease.39,40 Patients with nonalcoholic fatty liver disease are relatively prothrombotic, as reflected by the high incidence of VTE in this population.37,41
White blood cells are also thought to play a role in the development of VTE in a situation where transplant patients receive corticosteroids as well as other immunosuppressive drugs. However, our study did not find any significant differences in WBC count, platelet count, INR, age, or MELD score in liver transplant recipients who developed VTE. Similarly, another retrospective case-control study in Virginia also found that platelet count, INR, and MELD score were ineffective predictors of VTE.42 However, the authors in that study found low serum albumin to be an independent predictor for VTE. Multiple studies have also linked low serum albumin as an independent risk factor for VTE in cirrhotic patients, specifically with albumin lower than 1.9 g/dL.42-45 Another study found an increased risk of VTE in cirrhotic patients younger than 45 years, beyond which the risk was modestly lower in compensated cirrhotic patient.46 In our study, we did not find an association between age and VTE events in the OLT patient population. An understanding of the risk factors for VTE is necessary to minimize the incidence of this disease in the transplant patient population, and further studies are needed to discover which markers can effectively predict a patient's risk for VTE.
Vascular diseases account for 21% to 50% of deaths in transplant patients after 5 years.47-50 In a large population-based study, patients with liver disease were shown to have an increased risk for VTE events compared to the general population.51 A hypercoagulative state has been demonstrated to worsen cirrhosis, possibly through Ito cell activation or as a consequence of hepatic microthrombi.51-54 A recent study has shown that prophylactic enoxaparin therapy decreases the risk of decompensation in cirrhosis.55 The overall DVT incidence after liver transplantation has ranged between 2.7% and 8.6% in studies.8,56 Despite these findings, there is currently limited data regarding VTE prophylaxis in liver transplant recipients in the immediate postoperative phase. There is tremendous potential for the use of thromboprophylaxis in patients at risk for VTE, and it is estimated that up to 25% of all VTE events may be avoided.57 In our study, we demonstrated that prophylactic SQ heparin significantly reduces VTE events. Although antithrombin deficiency in the early postoperative phase is of concern for the effectiveness of heparin as a prophylaxis, our study identifies SQ heparin as a viable prophylactic tool to prevent VTE in OLT patients. In support of thromboprophylaxis, studies have found that heparin did not increase the risk of bleeding in patients with cirrhosis.58,59 Other therapies include enoxaparin, which has been shown to prevent portal vein thrombosis and liver decompensation in patients with advanced cirrhosis.55,59 Aspirin use has been demonstrated to have an 80% relative risk reduction in hepatic artery thrombosis.60 In breast cancer patients, prophylactic treatment with warfarin reduced the risk of chemotherapy-induced thrombosis.61
As a retrospective clinical study, we were limited to symptomatic patients captured in medical records and did not evaluate patients with asymptomatic VTE. We also did not adjust for other risk factors that are both associated with PICC line requirement and VTE, thus it is possible that PICC lines are merely a marker of greater underlying severity of illness or an unmeasured confounder. Similarly, SQ heparin prophylaxis may have been avoided in patients with greater bleeding risk, such as difficult operations or multiple comorbidities, which could potentially skew the data. Despite these limitations, however, we believe that SQ heparin should be used as a prophylaxis in OLT patients suspected to be at high risk for VTE. We did not pursue details specific to PICC device or site, because our focus was simply on the presence of the PICC rather than a study focusing on PICC-related factors. We decided to use unfractionated heparin as opposed to enoxaparin due to its shorter half-life, lack of contraindication with renal insufficiency, and low cost. Although newer anticoagulants, such as dabigatran, rivaroxaban, and apixaban, have the advantages of oral intake and lack of requirement for laboratory monitoring, there is currently no reversible agent for these drugs in case of active bleeding, and the limited data regarding their use in cirrhotic patients restrict their clinical applicability. Because of the lack of a clinically applicable coagulation test that reliably predicts the risk of bleeding or thrombosis, further studies are needed to identify patients who are most susceptible for VTE and would benefit most from SQ heparin prophylaxis. In liver transplant recipients who require a PICC line for central venous access, we recommend SQ heparin prophylaxis to minimize the risk of postoperative VTE and its associated complications.
Footnotes
1 March 2016.
The authors declare no funding or conflicts of interest.
J.Y. participated in the study design, acquisition of data, interpretation of data, drafting and revising version to be published. D.A.B. participated in the study design, acquisition of data, interpretation of data, drafting and revising version to be published. C.B. participated in the data analysis, interpretation of data, critical revision and final approval of the version to be published. M.K. participated in the study design, interpretation of data, and final approval. A.Y. participated in the study design, interpretation of data, and final approval. M.S.A. participated in the study design, interpretation of data, and final approval. G.T.S. participated in the concept and study design, analysis and interpretation of data, critical revision and final approval of version to be published.
REFERENCES
- 1. Anderson FA, Wheeler HB. Venous thromboembolism. Risk factors and prophylaxis. Clin Chest Med. 1995; 16: 235– 251. [PubMed] [Google Scholar]
- 2. Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007; 44: 62– 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004; 117: 19– 25. [DOI] [PubMed] [Google Scholar]
- 4. Heit JA, Silverstein MD, Mohr DN, et al. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med. 1999; 159: 445– 453. [DOI] [PubMed] [Google Scholar]
- 5. Agnelli G. Prevention of venous thromboembolism in surgical patients. Circulation. 2004; 110(24 Suppl 1): IV4– 12. [DOI] [PubMed] [Google Scholar]
- 6. Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999; 353: 1167– 1173. [DOI] [PubMed] [Google Scholar]
- 7. Salami A, Qureshi W, Kuriakose P, et al. Frequency and predictors of venous thromboembolism in orthotopic liver transplant recipients: a single-center retrospective review. Transplant Proc. 2013; 45: 315– 319. [DOI] [PubMed] [Google Scholar]
- 8. Annamalai A, Kim I, Sundaram V, et al. Incidence and risk factors of deep vein thrombosis after liver transplantation. Transplant Proc. 2014; 46: 3564– 3569. [DOI] [PubMed] [Google Scholar]
- 9. Violi F, Ferro D, Basili S, et al. Increased rate of thrombin generation in hepatitis C virus cirrhotic patients. Relationship to venous thrombosis. J Investig Med. 1995; 43: 550– 554. [PubMed] [Google Scholar]
- 10. Carmassi F, Morale M, De Negri F, et al. Modulation of hemostatic balance with antithrombin III replacement therapy in a case of liver cirrhosis associated with recurrent venous thrombosis. J Mol Med (Berl). 1995; 73: 89– 93. [DOI] [PubMed] [Google Scholar]
- 11. Mammen EF. Coagulation abnormalities in liver disease. Hematol Oncol Clin North Am. 1992; 6: 1247– 1257. [PubMed] [Google Scholar]
- 12. Hollestelle MJ, Poyck PP, Hollestelle JM, et al. Extra-hepatic factor VIII expression in porcine fulminant hepatic failure. J Thromb Haemost. 2005; 3: 2274– 2280. [DOI] [PubMed] [Google Scholar]
- 13. Hollestelle MJ, Geertzen HG, Straatsburg IH, et al. Factor VIII expression in liver disease. Thromb Haemost. 2004; 91: 267– 275. [DOI] [PubMed] [Google Scholar]
- 14. Weeder PD, Porte RJ, Lisman T. Hemostasis in liver disease: implications of new concepts for perioperative management. Transfus Med Rev. 2014; 28: 107– 113. [DOI] [PubMed] [Google Scholar]
- 15. Massicotte L, Beaulieu D, Roy JD, et al. MELD score and blood product requirements during liver transplantation: no link. Transplantation. 2009; 87: 1689– 1694. [DOI] [PubMed] [Google Scholar]
- 16. Caldwell S, Shah N. The prothrombin time-derived international normalized ratio: great for Warfarin, fair for prognosis and bad for liver-bleeding risk. Liver Int. 2008; 28: 1325– 1327. [DOI] [PubMed] [Google Scholar]
- 17. Tripodi A, Mannucci PM. Abnormalities of hemostasis in chronic liver disease: reappraisal of their clinical significance and need for clinical and laboratory research. J Hepatol. 2007; 46: 727– 733. [DOI] [PubMed] [Google Scholar]
- 18. Segal JB, Dzik WH, TMHCT Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005; 45: 1413– 1425. [DOI] [PubMed] [Google Scholar]
- 19. Massicotte L, Beaulieu D, Thibeault L, et al. Coagulation defects do not predict blood product requirements during liver transplantation. Transplantation. 2008; 85: 956. [DOI] [PubMed] [Google Scholar]
- 20. Lisman T, Bakhtiari K, Pereboom IT, et al. Normal to increased thrombin generation in patients undergoing liver transplantation despite prolonged conventional coagulation tests. J Hepatol. 2010; 52: 355– 361. [DOI] [PubMed] [Google Scholar]
- 21. Tripodi A, Primignani M, Chantarangkul V, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009; 137: 2105– 2111. [DOI] [PubMed] [Google Scholar]
- 22. Stahl RL, Duncan A, Hooks MA, et al. A hypercoagulable state follows orthotopic liver transplantation. Hepatology. 1990; 12(3 Pt 1): 553– 558. [DOI] [PubMed] [Google Scholar]
- 23. Remuzzi G, Bertani T. Renal vascular and thrombotic effects of cyclosporine. Am J Kidney Dis. 1989; 13: 261– 272. [DOI] [PubMed] [Google Scholar]
- 24. Huang LQ, Whitworth JA, Chesterman CN. Effects of cyclosporin A and dexamethasone on haemostatic and vasoactive functions of vascular endothelial cells. Blood Coagul Fibrinolysis. 1995; 6: 438– 445. [DOI] [PubMed] [Google Scholar]
- 25. Bombeli T, Müller M, Straub PW, et al. Cyclosporine-induced detachment of vascular endothelial cells initiates the intrinsic coagulation system in plasma and whole blood. J Lab Clin Med. 1996; 127: 621– 634. [DOI] [PubMed] [Google Scholar]
- 26. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013; 382(9889): 311– 325. [DOI] [PubMed] [Google Scholar]
- 27. Safdar N, Maki DG. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005; 128: 489– 495. [DOI] [PubMed] [Google Scholar]
- 28. Pereboom IT, Adelmeijer J, van Leeuwen Y, et al. Development of a severe von Willebrand factor/ADAMTS13 dysbalance during orthotopic liver transplantation. Am J Transplant. 2009; 9: 1189– 1196. [DOI] [PubMed] [Google Scholar]
- 29. Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009; 101: 239– 247. [PubMed] [Google Scholar]
- 30. Andersson HM, Siegerink B, Luken BM, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012; 119: 1555– 1560. [DOI] [PubMed] [Google Scholar]
- 31. Velasco F, Villalba R, Fernandez M, et al. Diminished anticoagulant and fibrinolytic activity following liver transplantation. Transplantation. 1992; 53: 1256– 1261. [DOI] [PubMed] [Google Scholar]
- 32. Amitrano L, Brancaccio V, Guardascione MA, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology. 2000; 31: 345– 348. [DOI] [PubMed] [Google Scholar]
- 33. van der Meer FJ, Koster T, Vandenbroucke JP, et al. The Leiden Thrombophilia Study (LETS). Thromb Haemost. 1997; 78: 631– 635. [PubMed] [Google Scholar]
- 34. Tripodi A, Caldwell SH, Hoffman M, et al. Review article: the prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007; 26: 141– 148. [DOI] [PubMed] [Google Scholar]
- 35. Lisman T, Bakhtiari K, Adelmeijer J, et al. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012; 10: 1312– 1319. [DOI] [PubMed] [Google Scholar]
- 36. Rijken DC, Kock EL, Guimarães AH, et al. Evidence for an enhanced fibrinolytic capacity in cirrhosis as measured with two different global fibrinolysis tests. J Thromb Haemost. 2012; 10: 2116– 2122. [DOI] [PubMed] [Google Scholar]
- 37. Lisman T, Caldwell SH, Burroughs AK, et al. Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol. 2010; 53: 362– 371. [DOI] [PubMed] [Google Scholar]
- 38. Munoz SJ, Stravitz RT, Gabriel DA. Coagulopathy of acute liver failure. Clin Liver Dis. 2009; 13: 95– 107. [DOI] [PubMed] [Google Scholar]
- 39. Pihusch R, Rank A, Göhring P, et al. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002; 37: 548– 555. [DOI] [PubMed] [Google Scholar]
- 40. Ben-Ari Z, Panagou M, Patch D, et al. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol. 1997; 26: 554– 559. [DOI] [PubMed] [Google Scholar]
- 41. Kargili A, Cipil H, Karakurt F, et al. Hemostatic alterations in fatty liver disease. Blood Coagul Fibrinolysis. 2010; 21: 325– 327. [DOI] [PubMed] [Google Scholar]
- 42. Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006; 101: 1524– 1528. [DOI] [PubMed] [Google Scholar]
- 43. Gulley D, Teal E, Suvannasankha A, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008; 53: 3012– 3017. [DOI] [PubMed] [Google Scholar]
- 44. Walsh KA, Lewis DA, Clifford TM, et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013; 47: 333– 339. [DOI] [PubMed] [Google Scholar]
- 45. Anthony Lizarraga W, Dalia S, Reinert SE, et al. Venous thrombosis in patients with chronic liver disease. Blood Coagul Fibrinolysis. 2010; 21: 431– 435. [DOI] [PubMed] [Google Scholar]
- 46. Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010; 8: 800– 5. [DOI] [PubMed] [Google Scholar]
- 47. Borg MA, van der Wouden EJ, Sluiter WJ, et al. Vascular events after liver transplantation: a long-term follow-up study. Transpl Int. 2008; 21: 74– 80. [DOI] [PubMed] [Google Scholar]
- 48. Abbasoglu O, Levy MF, Brkic BB, et al. Ten years of liver transplantation: an evolving understanding of late graft loss. Transplantation. 1997; 64: 1801– 1807. [DOI] [PubMed] [Google Scholar]
- 49. Rabkin JM, de La Melena V, Orloff SL, et al. Late mortality after orthotopic liver transplantation. Am J Surg. 2001; 181: 475– 479. [DOI] [PubMed] [Google Scholar]
- 50. Albeldawi M, Aggarwal A, Madhwal S, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012; 18: 370– 375. [DOI] [PubMed] [Google Scholar]
- 51. Søgaard KK, Horváth-Puhó E, Grønbaek H, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol 2009; 104: 96– 101. [DOI] [PubMed] [Google Scholar]
- 52. Jairath V, Burroughs AK. Anticoagulation in patients with liver cirrhosis: complication or therapeutic opportunity? Gut. 2013; 62: 479– 482. [DOI] [PubMed] [Google Scholar]
- 53. Calvaruso V, Maimone S, Gatt A, et al. Coagulation and fibrosis in chronic liver disease. Gut. 2008; 57: 1722– 1727. [DOI] [PubMed] [Google Scholar]
- 54. Wanless IR, Wong F, Blendis LM, et al. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995; 21: 1238– 1247. [PubMed] [Google Scholar]
- 55. Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012; 143: 1253– 1260. [DOI] [PubMed] [Google Scholar]
- 56. Ishitani M, Angle J, Bickston S, et al. Liver transplantation: incidence and management of deep venous thrombosis and pulmonary emboli. Transplant Proc. 1997; 29: 2861– 2863. [DOI] [PubMed] [Google Scholar]
- 57. Pini M, Spyropoulos AC. Prevention of venous thromboembolism. Semin Thromb Hemost. 2006; 32: 755– 766. [DOI] [PubMed] [Google Scholar]
- 58. Gómez Cuervo C, Bisbal Pardo O, Pérez-Jacoiste Asín MA. Efficacy and safety of the use of heparin as thromboprophylaxis in patients with liver cirrhosis: a systematic review and meta-analysis. Thromb Res. 2013; 132: 414– 419. [DOI] [PubMed] [Google Scholar]
- 59. Intagliata NM, Henry ZH, Shah N, et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014; 34: 26– 32. [DOI] [PubMed] [Google Scholar]
- 60. Vivarelli M, La Barba G, Cucchetti A, et al. Can antiplatelet prophylaxis reduce the incidence of hepatic artery thrombosis after liver transplantation? Liver Transpl. 2007; 13: 651– 654. [DOI] [PubMed] [Google Scholar]
- 61. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994; 343: 886– 889. [DOI] [PubMed] [Google Scholar]


