Abstract
Background
Improvements in renal allograft outcomes have permitted kidney transplantation after prior kidney allograft failure as well as after nonrenal solid organ transplantation. This study compares renal allograft outcomes in the 3 groups, that is, primary, repeat, and kidney after nonrenal solid organ transplantation, where transplant group was coded as a time-dependent variable.
Methods
We retrospectively reviewed registry data for kidney transplant recipients at University of Pittsburgh Medical Center from January 2000 to December 2011. We compared overall graft survival between the 3 groups using Cox regression modeling. We calculated 1-, 3-, and 5-year graft survival and half-lives for each group where feasible.
Results
The study cohort (N = 2014) consisted of group A (primary kidney transplant, n = 1578, with 7923.2 years of follow-up time), group B (repeat kidney transplant, n = 314, with 1566.7 years of follow-up time) and group C (kidney post-nonrenal solid organ transplant, n = 176, with 844.8 years of follow-up time). Of the 1578 patients in the primary kidney transplant group, 74 later received a repeat transplant and thus also have follow-up counted in the repeat kidney transplant group. The median follow-up was 56, 53, and 55 months, respectively. The 5-year actuarial and death-censored graft survival was 68.69%, 68.79%, and 66.48% and 65.53%, 67.68%, and 62.92%, respectively (P = 0.70). There was no difference in overall graft survival in the Cox-adjusted analysis (group B: odds ratio, 1.02; 95% confidence interval, 0.84-1.26; P = 0.79; group C: odds ratio, 0.96; 95% confidence interval, 0.75-1.23; P = 0.76).
Conclusions
The adjusted kidney graft survivals in the 3 groups were similar.
Kidney transplantation is the preferred therapy for patients with end-stage renal disease (ESRD). With improvements in allograft and patient outcomes, patients with prior kidney allograft failures are increasingly becoming candidates for repeat transplantation.1 According to the annual Scientific Registry for Transplant Recipients (SRTR) report for the year 2011, approximately 12% of kidney transplant recipients had prior renal allograft failure.2 In addition, renal failure is common in recipients of other nonrenal solid organ transplants with rates of CKD of up to 16% and in those with CKD, an ESRD incidence of up to 30% over 36 months post-nonrenal organ transplantation which varies from organ to organ.3-6 With improvements in medical care, patients with nonrenal solid organ transplants are living longer to become candidates for kidney after solid organ transplantation.7 This group which constituted less than 1% of the waitlist candidates before 1995 increased to 3.3% in 2008 and were more likely to be listed preemptively (38% vs 21% in those without prior nonrenal solid organ transplantation).8 According to the SRTR annual report, 2.6% of transplants performed in year 2011 were in recipients of prior nonrenal solid organ transplants.2 Only a few previous studies have compared renal allograft outcomes in recipients who have undergone other solid organ transplants compared with those undergoing primary or repeat kidney transplantation. These previous studies were based on national databases with large center to center variations in practices and issues with nonuniform coding.9,10 The current study is a retrospective single-center study that analyzes specifically the renal allograft outcome after primary kidney, repeat kidney and kidney-after-other solid organ transplant. We evaluated various demographic, donor/recipient, and posttransplant variables across the 3 groups.
METHODOLOGY
Center and Follow-Up
We used in-center registry data for kidney transplantations at University of Pittsburgh Medical Center from January 2000 to December 2011. The follow-up data were complete up to October 2012. An institutional review board approval was obtained for retrospective usage of the collected data for the study purposes.
Study Groups
All adult recipients who underwent renal transplantation alone at our center were included. We excluded recipients of kidney combined with pancreas, liver, heart, or lung transplant. Survival time for each patient was stratified into the following groups—group A: recipients of primary kidney-alone transplants, group B: recipients of repeat kidney-alone transplants, and group C: recipients of kidney-alone transplants performed after a previous nonrenal solid organ transplant, including prior liver, heart, lung, small bowel, or pancreas transplants. Survival time was coded in a time-dependent manner, that is, follow-up for the patient's initial renal transplant was either attributed to group A or (if it occurred after a previous non-renal solid organ transplant) to group C. Follow-up time after a repeat renal transplant was then attributed to group B. Therefore, the same patients may contribute to both groups A and B.
Variables
We examined donor factors (age, sex, race), recipient factors (age, sex, race, diabetes status), transplant factors (HLA mismatch, cold ischemia time (CIT), donor source—living donor [LD] vs deceased donor [DD]), and posttransplant variables (immunosuppression, delayed graft function (DGF) and acute rejection).
Immunosuppression
All but very few recipients received lymphocyte depleting induction. To adjust for the variability of maintenance immunosuppressive regimens at our center, we also categorized recipients according to their maintenance immunosuppressive regimens in use at our center. We divided the maintenance immunosuppressive regimens into 4 groups: group 1 (recipients receiving every other day or less frequent use of immunosuppressive medications), group 2 (calcineurin inhibitor [CNI] or mammalian target of rapamycin inhibitor or mycophenolic acid or azathioprine only regimen with or without prednisone), group 3 (CNI with an antimetabolite, either mycophenolic acid or azathioprine), and group 4 (triple immunosuppressive regimen with CNI, an antimetabolite or mammalian target of rapamycin inhibitor, and prednisone).
Outcomes
We compared overall graft survival differences between the three groups using Kaplan-Meier (K-M) curves and used log rank tests to detect differences in outcome. Graft failure was also stratified according to functional allograft failure as well as those who died with a functioning kidney, for the entire follow-up period. We performed adjusted graft survival between the three groups using Cox regression modeling. We also calculated 1-, 3-, and 5-year graft survival and calculated half-lives for each group where feasible.
Subgroup Analysis
We divided the kidney transplant after nonrenal solid organ transplant group into kidney after thoracic organ (heart and lung) and kidney after abdominal organ (pancreas-alone and liver) transplant. We studied and compared the 2 groups to determine differential kidney graft survival in previous thoracic and abdominal transplant recipients using K-M curves and adjusted survival using Cox regression modeling.
Statistical Analysis
For purposes of the descriptive analyses, patients who eventually had a repeat renal transplant were excluded from the primary renal transplant group (to avoid repeat observations on a given subject). All continuous variables were compared using t test/analysis of variance and categorical variables were analyzed using χ2 test. We used K-M curves and Log rank test to compare overall allograft survival and Cox regression analysis for adjusted graft survival. For all of the survival analyses, survival time for each study group was defined as a time-varying predictor so that follow-up for patients with a primary renal transplant was attributed to group A until they received (if applicable) a repeat renal transplant. Follow-up time after the repeat renal transplant was then attributed to group B. The covariates used in the final Cox model were selected based on the unadjusted analysis and previously published literature and included recipient and donor age, donor type (LD or DD), sex, cause of ESRD, CIT, presence of DGF, induction, and maintenance immunosuppressive regimen (4 groups as described above), acute cellular rejection within 12 months and days from prior kidney or other solid organ transplant. Variables with an unadjusted P value above 0.20 were excluded from consideration in the multivariable model. All variables not significant at P less than 0.05 were then dropped to form the final multivariable model. Stata was used for all statistical analysis (StataCorp. 2013, Stata Statistical Software: Release 13; StataCorp LP, College Station, TX).
RESULTS
Study Cohort
There were a total of 2014 patients in the study cohort. Group A consisted of a total of 1578 recipients (7923.2 years of follow-up time), group B consisted of 314 recipients (1566.7 years of follow-up time), and group C consisted of 176 recipients (844.8 years of follow-up time). The details of study subjects in each group stratified by recipient and donor characteristics and transplant variables are detailed in Table 1.
TABLE 1.
Demographic and transplant-specific characteristics of each group
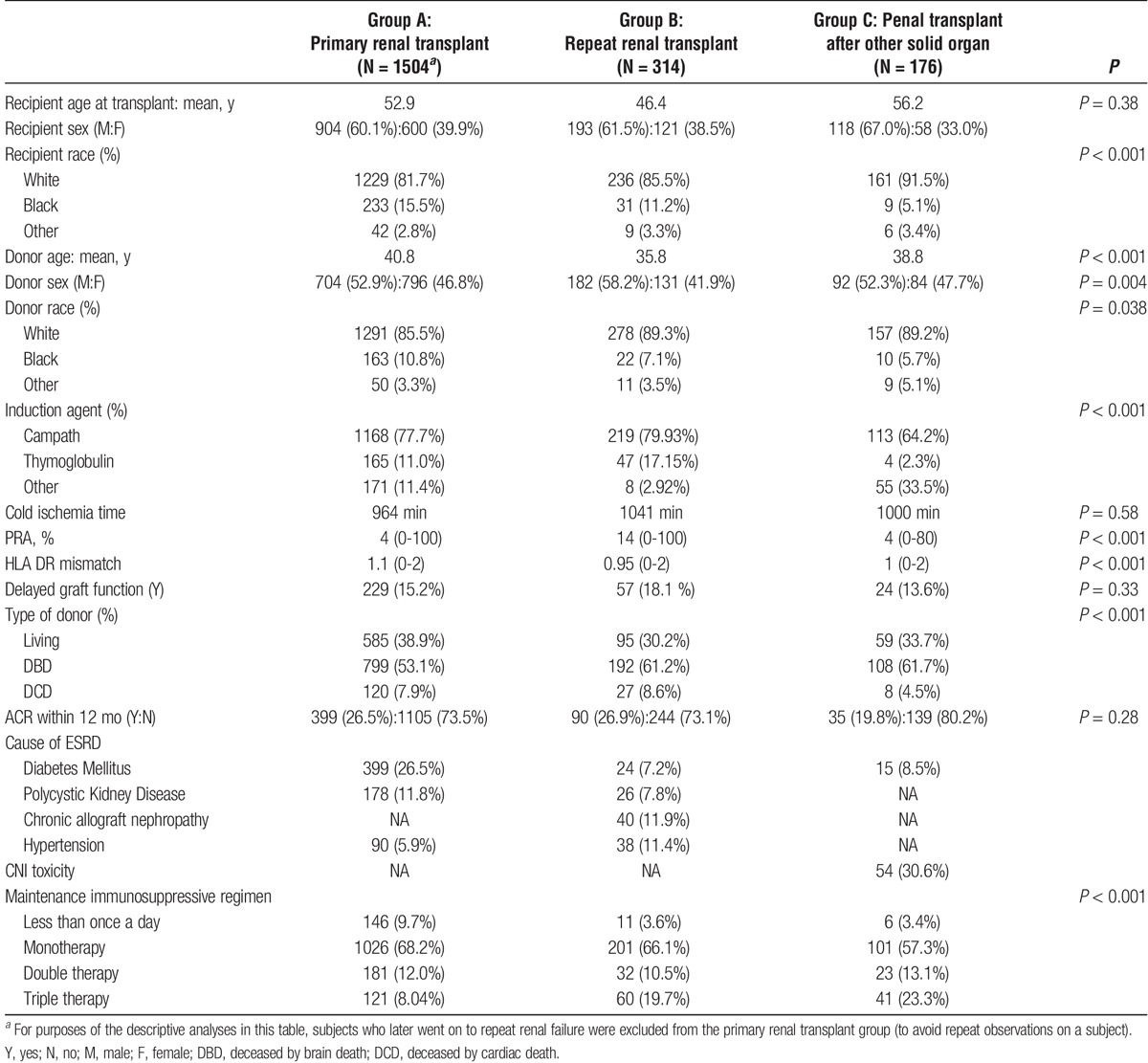
Recipient Characteristics
The mean age (in years) at transplantation for the primary, repeat renal and renal transplantation post-solid organ transplantation was 52.9, 46.4, and 56.2, respectively (P = 0.38). The proportion of male recipients in the 3 groups was 60%, 61%, and 67%, respectively. The majority of recipients in the 3 groups were white (82%, 85%, and 91%, respectively). The most common cause of ESRD in the primary renal transplant group was diabetes mellitus (26%), in the repeat renal group was chronic rejection (12%), and was presumptive CNI nephrotoxicity (31%) in the kidney after solid organ transplant group.
Donor Characteristics
The mean age of the donor (in years) for the 3 groups was 41, 36, and 39, respectively. The majority of donors in the 3 groups were white (Table 1). The proportion of LDs in the 3 groups was 39%, 30% and 34%, respectively.
Transplant Characteristics
The depleting antibody Alemtuzumab was the major induction agent in the 3 groups (78%, 80%, and 64%, respectively). The duration of CIT and incidence of DGF was not statistically different in the 3 groups. The use of deceased by brain death donors was 53%, 61%, and 62%, respectively. The use of deceased by cardiac death donors was 7.9%, 8.6%, and 4.6%, respectively. The cumulative incidence of acute T-cell rejection within 12 months posttransplant was not statistically different between the 3 groups (26%, 26%, and 20%, respectively; P = 0.283). The 2-year cumulative incidence of acute T-cell rejection in the 3 groups was 32.3%, 34.7%, and 27.8%, respectively (P = 0.29), and the 5-year cumulative incidence of acute T cell rejection was 40.8%, 42.3%, and 33.5%, respectively (P = 0.13). The details of maintenance immunosuppressive regimen stratified by 3 groups are detailed in Table 1. Among group B (repeat transplants), the majority of patients, that is, 73% (n = 243) were second transplants, 20% (n = 68) were third transplants, and the rest had greater than 3 prior kidney transplants.
Follow-Up and Graft Outcomes
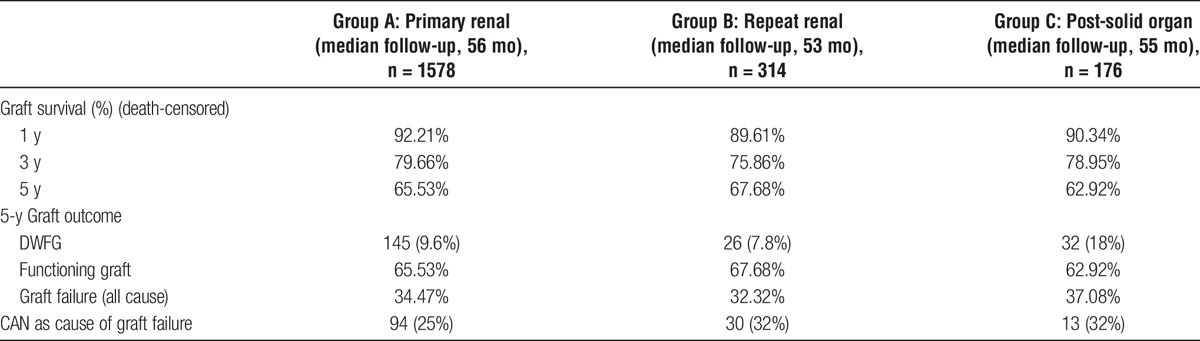
All recipients were followed up at our center, and median follow-up for groups A, B, and C were 56, 53, and 55 months, respectively (range, 1-175 months for the whole group). The 5-year death-censored graft survival was 65.53%, 67.68%, and 62.92%, respectively, in the 3 groups (P = 0.70). The 1-, 3-, and 5-year death-censored survival for each group is summarized in Table 2. The estimated cumulative incidence of graft failure (including graft loss from death with a functioning graft) for groups A, B, and C was 34.8% 33.6%, and 37.1%, respectively. Chronic allograft nephropathy was the most common cause of graft loss for group A (25%, n = 94) and group B (32%, n = 30). Death with a functioning graft was the commonest cause of graft loss in group C (18%, n = 32). During the study period, 9.6% (n = 145) patients in group A, 7.8% (n = 26) in group B, and 18% (n = 32) in group C died with a functioning graft.
TABLE 2.
Detailing the kidney graft outcomes in the 3 groups
Cox Regression Model for Graft Outcomes
A Cox regression model was constructed using kidney graft failure and death with functioning graft as the outcome of interest, incorporating variables detailed in the Methods section. In our study cohort, using primary kidney transplant (group A) as the reference, there were no differences in overall graft survival between the 3 groups (group B [repeat kidney]: odds ratio, 1.02; 95% confidence interval, 0.84-1.26; P = 0.793; group C [kidney post solid organ transplant]:odds ratio, 0.96; 95% CI, 0.75-1.23; P = 0.764). The adjusted survival curves for the 3 groups intercepted each other (Figure 1). The significant determinants of graft outcome in this study were recipient age (P < 0.001), acute cellular rejection within 12 months (P < 0.001), immunosuppressive regimen (P = 0.002), donor type (P < 0.001), and diabetes as cause of ESRD (P < 0.001).
FIGURE 1.
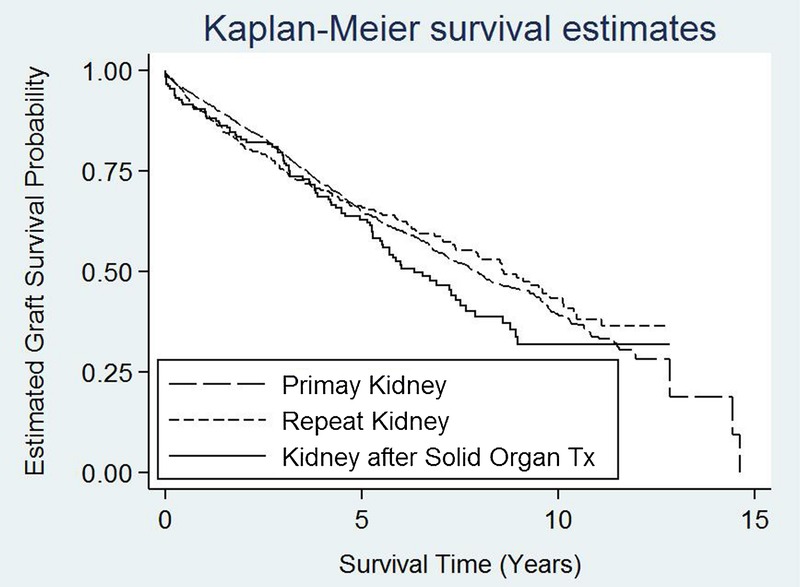
Adjusted kidney allograft survival curves for the primary renal transplant, repeat renal and kidney after solid organ transplants. Primary kidney: reference. Repeat kidney: 1.02 (95% CI 0.84-1.26, P = 0.79). Kidney post-solid organ: 0.96 (95% CI, 0.75-1.23; P = 0.76). 95% CI, 95% confidence interval.
Subgroup Analysis
We further compared renal allograft survival in previous recipients of thoracic (heart and lung, n = 64) and abdominal organ (pancreas-alone and liver, n = 100) transplants. The details of the demographic and transplant variables in the 2 groups are shown in Table 3. The mean number of days between the thoracic organ and subsequent kidney transplant was 3056 days (range, 671-6482 days) and for the abdominal transplant group was 2853 days (range, 1-8842 days). The 2 groups differed in terms of acute cellular rejection within 12 months posttransplant (9.4% in the thoracic group vs 27% in the abdominal group, P = 0.006). The actuarial 5-year kidney survival after thoracic transplant was 78% and after abdominal organ transplant was 55% (P = 0.03). The median time to renal allograft failure was 72 months for the abdominal transplant group, whereas the thoracic transplant group had not yet reached this end point by the end of study period. In our study cohort, based on K-M estimates, we found significantly better renal allograft survival after thoracic transplant as compared with post-abdominal transplant (P = 0.037) (Figure 2). On further analysis using Cox regression model after adjusting for all mentioned variables, kidney recipients after thoracic organ transplant had better overall graft survival as opposed to those after abdominal organ transplant (P = 0.046). In this analysis, recipient age and days between previous organ transplant and kidney transplant were significant predictors of posttransplant graft survival (P = 0.003 and 0.04, respectively).
TABLE 3.
Detailing demographic variables and transplant characteristics of the 2 kidney transplant after solid organ transplant groups
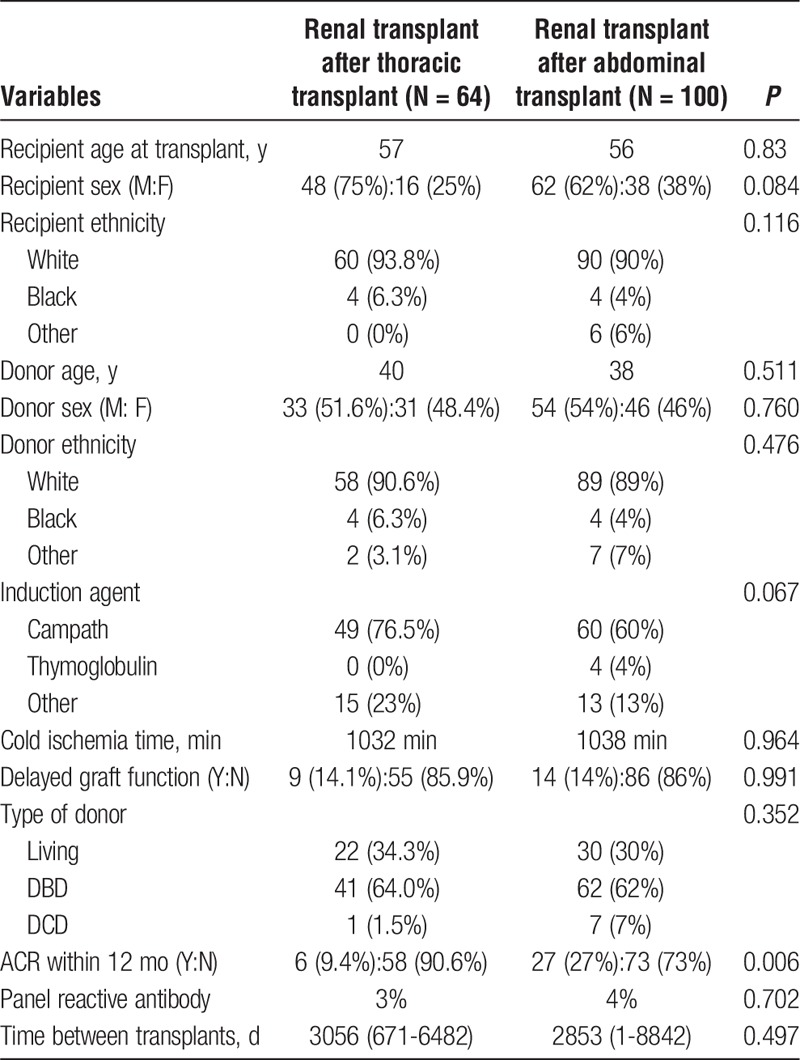
FIGURE 2.
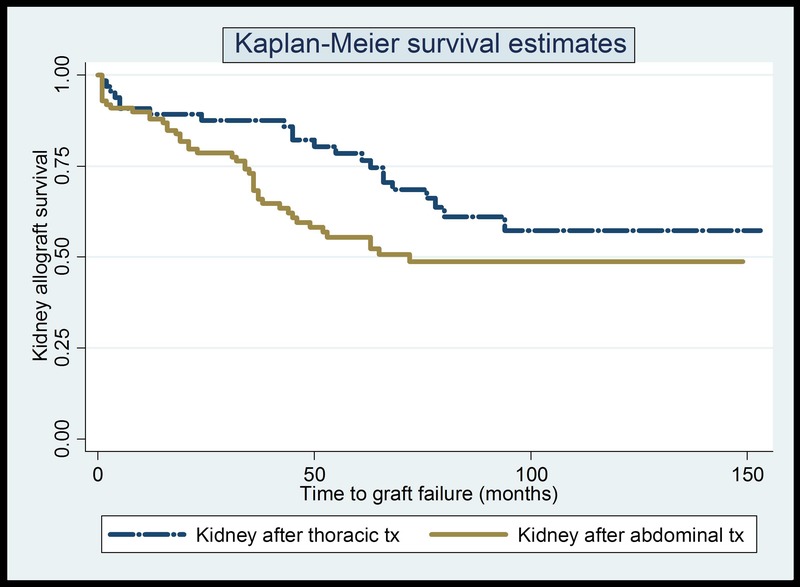
Kidney allograft survival curves for the thoracic and abdominal transplant group.
DISCUSSION
The increasing number of patients being placed on the kidney transplant waiting list with either late kidney allograft failure or ESRD following nonrenal solid organ transplantation prompted us to perform this analysis. In an SRTR registry analysis, Srinivas et al8 had reported increased mortality on the kidney waiting list for recipients of prior solid organ transplants and that a kidney transplant offered them mortality benefit. However, to the best of our knowledge, this is the first study analyzing and side by side comparing the renal allograft outcomes of primary kidney, repeat kidney, and kidney after nonrenal solid organ transplants. In our current study cohort, after accounting for various clinical variables, overall kidney allograft survival between the 3 groups was similar and not statistically significant. Previously, Gondos et al11 had compared kidney graft survival in the United States and Europe and had reported respective 5- and 10-year graft survival in US populations to be 71%/46% (whites), 73%/48% (Hispanics), and 62%/34% (African-Americans). A registry study by Magee et al10 reported unadjusted 1-, 3-, and 5-year graft survival rates for repeat LD and DD kidney transplants to be significantly lower than that observed for primary LD and DD transplants. The 5-year graft survival for repeat LD and DD transplants was 76% and 63% compared with 81% and 68%, respectively, for LD and DD primary renal transplants. The corresponding 5-year kidney graft survival for primary and repeat transplants at our center were 66% and 68%, respectively, and thus were similar to the average of LD and DD transplants reported in the above Organ Procurement and Transplant Network (OPTN) analysis by Magee et al. Cecka1 reported 3-year graft survival rates for repeat and multiple DD transplants to be 77% and 73%, respectively based on an UNOS analysis in 2001. Our 3-year graft survival for repeat transplants was 76%. As reported by Magee et al, the majority of patients in our repeat kidney transplantation group were second time recipients.10 The average wait time before the second repeat transplant in our cohort was 4000 days which is similar to the 10 years reported in the OPTN analysis by Magee et al. The average wait before the third and fourth repeat transplants were 4069 days and 3722 days in our cohort, respectively. Gjertson12 reported determinants of repeat renal transplant survival to be first graft survival duration, young recipient age, white race, female sex, body mass index less than 30, lower degree of sensitization, better functional status, LD, short CIT, fewer HLA mismatches and lesser re-exposure to mismatches. We also found recipient age and sex and donor age and race to be significant determinants of graft outcome in our study. In addition, presence of DGF, acute cellular rejection at 12 months and maintenance immunosuppression were also found to be significant predictors of graft outcome in our study. Information on reexposure to mismatches, functional status, and duration of survival of the previous allograft were not available in our database and is thus a limitation of our study.
There is a paucity of literature examining renal allograft outcome after nonrenal solid organ transplants. The 1-, 3-, and 5-year actuarial graft survival in LD and DD kidney transplant after liver transplant was lower than that in kidney transplant-alone recipients (N = 678, 1997-2008, OPTN database). However, death-censored graft survival was similar.9 The death-censored graft survival in our cohort was also similar across the 3 groups (Table 2).
In regard to subgroup analysis, there is little published data comparing kidney transplant outcomes comparing recipients of prior thoracic versus abdominal transplants. In their analysis of OPTN database from 1997 to 2008, Gonwa et al9 reported a 5-year death-censored renal allograft survival of 77% after orthotopic liver transplant compared to 79% for kidney-alone DD transplant. In a separate OPTN analysis from 2009, the 5-year renal allograft survival after previous pancreas-alone transplant was 59%. In comparison, in our study, the 5-year actuarial renal graft survival for kidney transplant after orthotopic liver or pancreas transplantation was 55%. We did not have an adequate number of kidney-after-liver and kidney-after-pancreas transplants for further subanalysis. The outcomes of renal allograft after thoracic transplant have not been reported before. In our cohort, the 1-, 3-, and 5-year actuarial kidney graft survival after previous thoracic organ transplantation was 89%, 87%, and 78%, respectively. In our unadjusted analysis, there was a significantly better kidney graft survival in the thoracic versus abdominal transplant group, and this remained borderline significant in the adjusted Cox regression analysis. One of the potential factors explaining this difference could be the practice of continued maintenance of higher overall immunosuppression after thoracic transplants (lung and heart) when compared with abdominal transplants (especially liver) leading to higher rates of immunological graft injury and loss. Also, a lower mortality benefit of kidney transplant after liver transplant as compared with other solid organ transplants has been reported before.8 Also, in fact in our study, the difference in kidney graft survival after thoracic or abdominal transplants diminished when death-censored graft outcomes were compared (data not shown).
Previous studies have also reported recipient body mass index, functional status, length of ESRD treatment before transplant, relationship between donor and recipient, donor history of diabetes, hypertension, terminal creatinine, sharing outside of recovering donation service area, and donor-to-recipient weight ratio as factors influencing renal allograft outcomes.13-16 Our analysis could not address these points due to the limited data on the abovementioned variables. However, the impact of some of these variables is likely adequately captured by variables already used in our model, such as incidence of DGF, duration of CIT, and incidence of acute cellular rejection within the first year.
The strengths of the study include a single-center experience over 10 years with good follow-up and uniformity in clinical practices, adequate numbers in each group, and complete data collection on most variables of interest. This report differs from large multicentric registries in that the subjects in all 3 groups come from the same center with likely similar organ and patient selection criteria and other center-specific practices which could differentially influence the outcomes in the 3 groups had they been from different centers. The limitations of this study include the following: single-center, retrospective observational design, and limited follow-up time. In addition, the majority of patients in three groups were whites. Because race is known to affect renal graft outcomes, the study results may not be generalizable to non-white races. Another potential limiting factor of our study includes the unconventional immunosuppressive regimen practiced at our institute over the study period. We have stratified patients according to 4 different immunosuppressive regimens including triple therapy, dual therapy (without steroids), monotherapy, and less frequent immunosuppressive medications for our analysis in an effort to minimize the impact of this variable, but there is a possibility of residual confounding from this variable. The variable maintenance immunosuppression was significant in the Cox regression analysis (P = 0.002). However, the numbers in each group were not enough in the 3 groups to carry out a meaningful subgroup analysis. As such, the use of unconventional immunosuppressive regimens limits the generalizability of the results of this report.
In conclusion, this current single-center study demonstrates similar kidney allograft survival in patients undergoing primary kidney transplantation, repeat kidney transplantation, and kidney transplantation after other nonrenal solid organ transplants, after adjusting for important potential confounders.
Footnotes
Published online 4 May 2016.
The authors declare no funding or conflicts of interest.
P.S. was involved in conception, design, analysis, and writing of the article. X.G. was involved in data analysis and interpretation. R.M. was involved in design, interpretation of data, and editing of the article. D.L. was involved in design of the study, data analysis, and interpretation and editing. C.W. was involved in design and writing of the article. R.N. was involved in design and data analysis. C.P. was involved in revision and editing of the article. A.T. was involved in the design and editing of the article. S.H. was involved in conception, design, analysis, writing of the article.
REFERENCES
- 1.Cecka JM. The UNOS Renal Transplant Registry. Clin Transpl. 2002:1–20. [PubMed] [Google Scholar]
- 2.Kidney, 2011 SRTR & OPTN Annual Data Report 2011 [cited 2014 4/27/2014]. Available from: http://srtr.transplant.hrsa.gov/annual_reports/2011/flash/01_kidney/index.html#/2/zoomed.
- 3.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003. ;349:931–940. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg A, Thompson ME, Griffith BJ, et al. Cyclosporine nephrotoxicity in cardiac allograft patients—a seven-year follow-up. Transplantation. 1990. ;50:589–593. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt CM, Arons RR. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation. 2004;78:1351–1355. [DOI] [PubMed] [Google Scholar]
- 6.Ishani A, Erturk S, Hertz MI, et al. Predictors of renal function following lung or heart-lung transplantation. Kidney Int. 2002;61:2228–2234. [DOI] [PubMed] [Google Scholar]
- 7.Chandrakantan A, de Mattos AM, Naftel D, et al. Increasing referral for renal transplant evaluation in recipients of nonrenal solid-organ transplants: a single-center experience. Clin J Am Soc Nephrol. 2006;1:832–836. [DOI] [PubMed] [Google Scholar]
- 8.Srinivas TR, Stephany BR, Budev M, et al. An emerging population: kidney transplant candidates who are placed on the waiting list after liver, heart, and lung transplantation. Clin J Am Soc Nephrol. 2010;5:1881–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonwa TA, McBride MA, Mai ML, et al. Kidney transplantation after previous liver transplantation: analysis of the organ procurement transplant network database. Transplantation. 2011;92:31–35. [DOI] [PubMed] [Google Scholar]
- 10.Magee JC, Barr ML, Basadonna GP, et al. Repeat organ transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1424–1433. [DOI] [PubMed] [Google Scholar]
- 11.Gondos A, Döhler B, Brenner H, et al. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation. 2013;95:267–274. [DOI] [PubMed] [Google Scholar]
- 12.Gjertson DW. A multi-factor analysis of kidney regraft outcomes. Clin Transpl. 2002:335–349. [PubMed] [Google Scholar]
- 13.Arnol M, Prather JC, Mittalhenkle A, et al. Long-term kidney regraft survival from deceased donors: risk factors and outcomes in a single center. Transplantation. 2008. ;86:1084–1089. [DOI] [PubMed] [Google Scholar]
- 14.Coupel S, Giral-Classe M, Karam G, et al. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int. 2003;64:674–680. [DOI] [PubMed] [Google Scholar]
- 15.Guedes AM, Malheiro J, Fonseca I, et al. Over ten-year kidney graft survival determinants. Int J Nephrol. 2012;2012:302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Xu TZ, Chen JH, et al. Factors influencing second renal allograft survival: a single center experience in China. Transpl Immunol. 2009;20:150–154. [DOI] [PubMed] [Google Scholar]


