Abstract
A series of 1,4-diphenalkylpiperidine analogs were synthesized and evaluated for their affinity and inhibitory potency at the [3H]dihydrotetrabenazine (DTBZ) binding site and [3H]dopamine (DA) uptake site on the vesicular monoamine transporter-2 (VMAT2). Results revealed that translocation of the phenethyl side chains of lobelane from C2 and C6 to C1 and C4 around the central piperidine ring slightly reduces affinity and inhibitory potency at VMAT2 with respect to lobelane. However, methoxy and flouro-substitution of either phenyl ring of these 1,4-diphenethyl analogs afforded VMAT2 inhibition comparable or higher (5-fold) affinity at the DTBZ binding and DA uptake sites relative to lobelane, whereas replacement of the 4-phenethyl moiety in these analogs with a 4-phenmethyl moiety markedly reduced affinity for the DTBZ binding and DA uptake sites by 3- and 5-fold, respectively. Among the twenty five 1,4-diphenethylpiperidine analogs evaluated, compounds containing a 4-(2-methoxyphenethyl) moiety exhibited the most potent inhibition of DTBZ binding and vesicular DA uptake. From this subgroup, analogs 8h, 8j and 8m exhibited Ki values of 9.3 nM, 13 nM and 13 nM, respectively, for inhibition of [3H]DA uptake by VMAT2, and represent some of the most potent inhibitors of VMAT2 function reported thus far.
Keywords: Vesicular monoamine transporter-2; [3H]DTBZ binding; [3H]DA uptake inhibition at VMAT2; 1,4 diphenethyl lobelane analogs; 1-phenethyl, 4-phenylmethylene derivatives of lobelane
Graphical abstract
Methamphetamine (METH) is a highly addictive psycho-stimulant and its abuse produces severe, deleterious health effects, including fatigue, dysphoric mood, anxiety, depression and symptoms of psychosis.1-3 Currently, there are no FDA-approved treatments for METH abuse. The abuse liability of METH is, at least in part, due to its interaction with the brain dopamine (DA) reward system. METH reverses the normal function of the dopamine transporter (DAT) and the vesicular monoamine transporter-2 (VMAT2) greatly increasing DA concentrations in the cytosol of dopaminergic presynaptic terminals as well as in the extracellular compartment. METH also inhibits monoamine oxidase (MAO) activity, preventing the metabolism of cytosolic DA to 3,4-dihydroxyphenylacetic acid (DOPAC), consequently increasing the availability of cytosolic DA for METH-induced reverse transport by DAT and increasing DA concentrations in the extracellular compartment, resulting in subsequent stimulation of postsynaptic DA receptors.4 Since METH inhibits DA uptake at VMAT2 and promotes DA release from vesicles to increase cytosolic DA, VMAT2 is considered an important molecular target in the search for a treatment for METH abuse.5 Therefore, molecules that can modulate function and prevent the pharmacological effects of METH may be suitable candidates for the treatment of METH addiction.
In this respect, lobeline, the principal alkaloidal constituent of Lobelia inflata, has been shown to decrease amphetamine-induced and METH-induced hyperactivity in rats and mice, and to inhibit amphetamine-evoked and METH-evoked DA release from superfused rat brain slices.6 Importantly, lobeline also decreases METH self-administration in rats without acting as a substitute reinforcer,7, 8 indicating a lack of abuse liability.
The mechanism by which lobeline reduces the reinforcing and rewarding effects of psychostimulants involves its ability to interact with VMAT2.4, 9 Lobeline inhibits VMAT2 with about 100-fold higher affinity compared to its affinity for DAT, indicating that the interaction with VMAT2 is essential for the observed decrease in the behavioral effects of METH.4, 9, 10 Unlike METH, lobeline does not inhibit MAO activity, allowing DA within the cytosol to be metabolized to DOPAC.11 Thus, lobeline diminishes the cytosolic DA pool available for METH-induced reverse transport by DAT.
Clinical evaluation of lobeline as a pharmacotherapy for METH abuse revealed that the alkaloid is safe in METH addicted individuals.12 However, bitter taste and nausea were noted as relatively minor side-effects, likely the result of nicotinic acetylcholine receptor (nAChR) antagonism.13, 14 Another limitation was the short plasma half-life of lobeline, which would require multiple doses as a therapeutic, likely diminishing compliance. In an attempt to address these limitations, a significant drug discovery effort was initiated in which the lobeline molecule was structurally modified with the goal of improving potency and selectivity as an inhibitor of VMAT2, while eliminating affinity for nAChRs and other neurotransmitter transporters.9 Lobelane (1), a chemically defunctionalized analog of lobeline, emerged as a lead analog that exhibited a greater potency inhibiting VMAT2 function compared to lobeline, while demonstrating little affinity for nAChRs.13
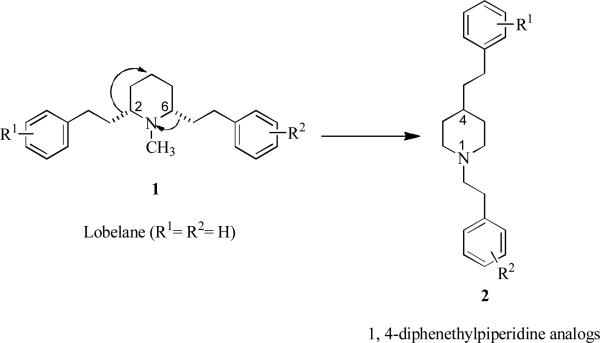
After carrying out comprehensive structure-activity relationship (SAR) studies14 with lobelane analogs, it was determined that relocation of the 2,6-phenethyl moieties in the lobelane molecule (1) to the 1,4-positions on the central piperidine heterocycle to afford 2 (R1 = R2 = H) (Fig. 1), resulted in no loss of affinity for the [3H]DBTZ binding site on VMAT2 when compared with lobelane. This structural change also maintained the low affinity for α4β2 and α7 nAChRs that was exhibited by lobelane. An added advantage was the achiral nature of 2.
Fig. 1.
Relocation of the 2,6-phenethyl moieties of lobelane (1, R1=R2=H) to the 1,4- positions on the central piperidine heterocycle to afford 1,4-diphenethylpiperidine (2, R1=R2=H).
In the current study, this new scaffold was exploited by building a small library of aromatic substituted analogs of 2, and these compounds exhibited inhibition of VMAT2 function with comparable or higher affinity relative to lobelane. Herein, we present the synthesis and evaluation of 1,4-diphenethylpiperidine and 1-phenethyl-4-benzylpiperidine analogs as inhibitors of vesicular DA uptake.
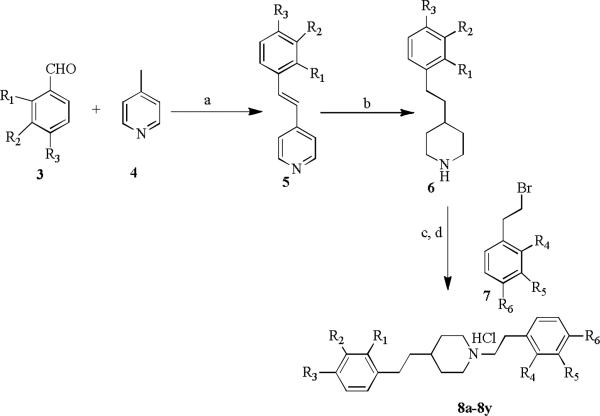
The general synthetic approach adopted for the preparation of the aromatic substituted 1,4-diphenethylpiperidine analogs is illustrated in Scheme 1. The synthesis utilizes 4-picoline (4) which is reacted with various substituted benzaldehydes (3) via Aldol condensation in acetic anhydride at reflux temperature to afford the corresponding (E)-4-styrylpyridine 5. Compound 5 is then subjected to hydrogenation with Adams catalyst (PtO2) in acetic acid under hydrogen gas (50 psi) at room temperature to afford the saturated piperidino intermediate (6). Intermediate 6 is alkylated with variously substituted phenethyl bromides (7) using K2CO3 in acetonitrile at reflux temperature to yield the corresponding 1,4-diphenethylpiperidine analogs 8a-8y, which were further converted to hydrochloride salts with 2M HCl in diethyl ether (Scheme 1).15
Scheme 1.
Synthesis of 1,4-diphenethylpiperidine analogs. Reagents and conditions: (a) Ac2O, reflux, 24 h, 48-60%; (b) 10% (w/v)PtO2/H2, AcOH, 50 psi, rt,12 h, 75-80%; (c) K2CO3/acetonitrile, 80 °C, 8 h, 75-80% ; (d) 2M HCl in ether.
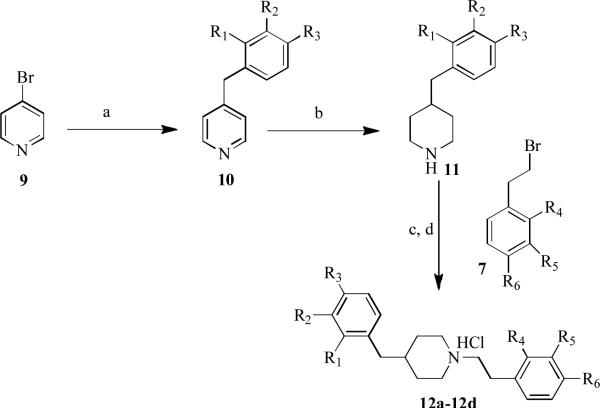
In addition, a small group of 1,4-diphenalkylpiperidine analogs were synthesized in which the 4-phenethyl moiety was replaced with a 4-benzyl moiety. The synthetic route for generating these compounds is shown in Scheme 2 and utilized 4-bromopyridine (9) as a starting point.
Scheme 2.
Synthesis of 1-phenethyl-4-benzylpiperidine analogs. Reagents and conditions: (a) Benzylmagnesium chloride, NiCl2 (dppp), THF, rt 15 h, 85-90%; (b) H2, PtO2, AcOH, 50 psi, rt, 12 h, 75-80%; (c) K2CO3/acetonitrile, 80 °C, 8 h, 75-80%; (d) 2M HCl in ether.
4-Bromopyridine was reacted with benzylmagnesium chloride using NiCl2(dppp)/THF (Kumada coupling)16 at ambient temperature to obtain the 4-benzylpyridine intermediate 10. Compound 10 was then subjected to hydrogenation with Adams catalyst (PtO2) in acetic acid under hydrogen gas (50 psi) at room temperature to obtain the saturated benzylpiperidino intermediate, 11. Compound 11 was then reacted with variously substituted phenethyl bromides (7) in the presence of K2CO3 in acetonitrile at reflux temperature to yield the corresponding 1-phenethyl-4-benzylpiperidine derivatives 12a-12d, which were further converted to hydrochloride salts with 2M HCl in diethyl ether (Scheme 2).
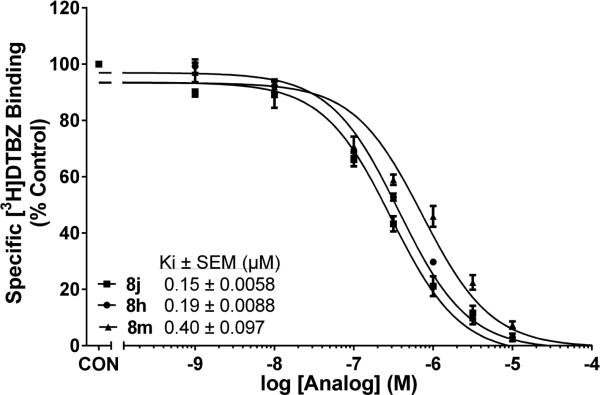
All the synthesized compounds were evaluated for affinity for the [3H]DTBZ binding site on VMAT2 and for affinity at the DA translocation site on VMAT2, using the [3H]DA uptake assay in isolated synaptic vesicles (Table 1). In the 1,4-diphenethylpiperidine series of compounds (8a-8y, Table 1), the Ki values from the [3H]DTBZ binding assay ranged from 0.15-2.8 μM. However, 80% of the analogs evaluated had Ki values < 1μM, notable exceptions being the 1-(3,4-dimethoxyphenethyl) analog 8c (Ki = 2.8 μM), and the 1,4-bis(4-methoxyphenethyl) analog 8w (Ki = 2.5 μM). The two most potent compounds in this series were analogs 8j and 8h (Fig. 2), which exhibited similar affinity in the [3H]DTBZ binding assay (Ki = 0.15 μM, and 0.19 μM, respectively), revealing a 5 to 6-fold higher affinity than lobelane (Ki = 0.97 μM)14 for the DTBZ binding site on VMAT2. It should be noted that these compounds contain a 4-(2-methoxyphenethyl) moiety.
Table 1.
1,4-Diphenalkylpiperidine analogs inhibit [3H]DTBZ binding and [3H]DA uptake at VMAT2
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | [3H]DTBZ Binding Ki (μM) | [3H]DA Uptake Ki (nM) |
|---|---|---|---|---|---|---|---|---|
| Lobelane (1) | 0.97 ± 0.191 | 45 ± 8.02 | ||||||
| 8a | H | H | H | H | H | H | 1.87 ± 0.251 | 120 ± 15 |
| 8b | H | H | H | OCH3 | H | H | 0.26 ± 0.026 | 30 ± 2.0 |
| 8c | H | H | H | H | OCH3 | OCH3 | 2.80 ± 0.11 | 56 ± 9.0 |
| 8d | H | H | H | H | OCH3 | H | 0.27 ± 0.038 | 22 ± 0.87 |
| 8e | H | H | H | H | H | OCH3 | 1.62 ± 0.19 | 46 ± 3.7 |
| 8f | H | H | H | Cl | H | H | 0.38 ± 0.0058 | 47 ± 6.3 |
| 8g | H | H | H | H | H | F | 1.82 ± 0.079 | 32 ± 3.8 |
| 8h | OCH3 | H | H | OCH3 | H | H | 0.19 ± 0.0088 | 9.3 ± 0.6 |
| 8i | OCH3 | H | H | H | H | H | 0.69 ± 0.050 | 22 ± 2.8 |
| 8j | OCH3 | H | H | H | OCH3 | H | 0.15 ± 0.0058 | 13 ± 1.5 |
| 8k | OCH3 | H | H | H | H | OCH3 | 0.50 ± 0.10 | 43 ± 2.8 |
| 8l | OCH3 | H | H | Cl | H | H | 0.19 ± 0.020 | 20 ± 3.0 |
| 8m | OCH3 | H | H | H | H | F | 0.40 ± 0.097 | 13 ± 2.1 |
| 8n | H | OCH3 | H | H | H | H | 0.32 ± 0.015 | 56 ± 8.6 |
| 8o | H | OCH3 | H | OCH3 | H | H | 0.32 ± 0.055 | 40 ± 7.5 |
| 8p | H | OCH3 | H | H | OCH3 | H | 0.23 ± 0.019 | 85 ± 10 |
| 8q | H | OCH3 | H | H | H | OCH3 | 0.45 ± 0.13 | 130 ± 23 |
| 8r | H | OCH3 | H | Cl | H | H | 0.46 ± 0.098 | 420 ± 49 |
| 8s | H | OCH3 | H | H | H | F | 0.96 ± 0.047 | 69 ± 4.0 |
| 8t | H | H | OCH3 | H | H | H | 0.51 ± 0.087 | 110 ± 7.0 |
| 8u | H | H | OCH3 | OCH3 | H | H | 0.42 ± 0.019 | 83 ± 9.0 |
| 8v | H | H | OCH3 | H | OCH3 | H | 0.23 ± 0.047 | 75 ± 4.0 |
| 8w | H | H | OCH3 | H | H | OCH3 | 2.5 ± 0.27 | 160 ± 12 |
| 8x | H | H | OCH3 | Cl | H | H | 0.23 ± 0.020 | 40 ± 7.7 |
| 8y | H | H | OCH3 | H | H | F | 0.47 ± 0.067 | 60 ± 5.5 |
| 12a | H | H | H | OCH3 | H | H | 2.8 ± 0.31 | 130 ± 15 |
| 12b | H | H | H | H | OCH3 | H | 1.5 ± 0.10 | 77 ± 9.8 |
| 12c | H | H | H | H | OCH3 | OCH3 | 3.0 ± 0.17 | 240 ± 34 |
| 12d | H | H | H | H | H | OCH3 | 1.9 ± 0.43 | 180 ± 11 |
Fig. 2.
1,4-Diphenethylpiperidines inhibit [3H]DTBZ binding to VMAT2 in rat brain vesicles. Concentration-response curves for inhibition of [3H]DTBZ binding for compounds 8j, 8h and 8m. Control (CON) represents [3H]DTBZ binding in the absence of compound. Data are mean (± S.E.M.) specific [3H]DTBZ binding expressed as a percentage of control (1250 ± 50 fmol/mg). n = 11 rats for control condition; n = 3-5 rats/compound.
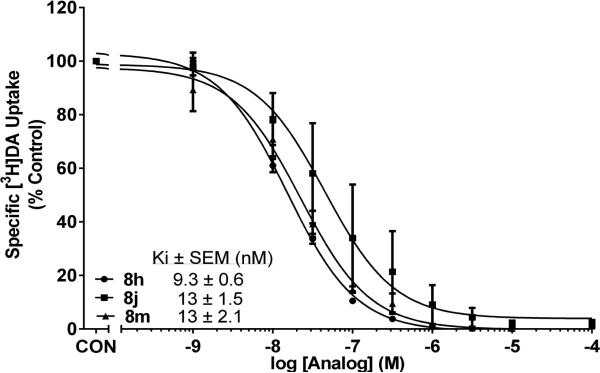
Evaluation of compounds 8a-8y in the [3H]DA uptake assay revealed Ki values in the range 9.3-420 nM. The most potent compounds in the series were analogs containing a 4-(2-methoxyphenethyl) moiety (8h-8m). Three compounds in this group exhibited high affinity (Fig. 3), i.e., Ki values of 9.3 nM (8h), 13 nM (8j) and 13 nM (8m), and were 4 to 5-fold more potent than lobelane (Ki = 45 nM)15 inhibiting [3H]DA uptake at VMAT2. Analogs incorporating a 4-(3-methoxyphenethyl) or a 4-(4-methoxyphenethyl) moiety (i.e., compounds 8n-8s and 8t-8y, respectively) generally were less potent inhibitors of [3H]DA uptake, with Ki values in the range 40-420 nM and 40-160 nM, respectively. Interestingly, further analysis of the data for compounds 8a-8y revealed a positive correlation between affinity for the DTBZ binding and DA uptake sites on VMAT2 (Spearman r value = 0.443, p<0.05), suggesting that these compounds may inhibit VMAT2 function via an allosteric interaction with the DTBZ binding site; or alternatively, they may interact with both the binding and substrate translocation sites on VMAT2.
Fig. 3.
1,4-Diphenethylpiperidines inhibit [3H]DA uptake at VMAT2 in rat striatal vesicles. Concentration-response curves for inhibition of [3H]DA uptake for compounds 8j, 8h and 8m, which exhibited highest affinity for the DA translocation site on VMAT2. Control (CON) represents [3H]DA uptake in the absence of compound. Data are mean (± S.E.M.) specific [3H]DA uptake expressed as a percentage of control (16.1 ± 1.94 pmol/min/mg), n = 11 rats for control condition; n = 3-4 rats/compound.
A subset of compounds (12a-12d) in which the 4-phenethyl moiety was replaced with a 4-benzyl moiety, also was evaluated in the [3H]DTBZ binding and [3H]DA uptake assays. While these compounds exhibited affinities (Ki = 1.5-3.0 μM) for the DTBZ binding site comparable to lobelane (Ki = 0.97 μM), they exhibited lower potency than their corresponding 4-phenethyl analogs in the [3H]DA uptake assay, with Ki values in the range 77-240 nM. In contrast to the positive correlation between affinity for the DTBZ binding and DA uptake sites for compounds 8a-8y, Spearman analysis revealed no correlation for compounds 12a-12d bearing a 4-benzyl moiety; however, this may be due to the limited number of compounds in the 4-benzyl series.
In summary, translocation of the phenethyl side chains of lobelane from the 2,6- to the 1,4- position on the central piperidine ring has afforded a new scaffold that has been utilized to discover novel inhibitors of VMAT2 function with inhibitory potency comparable to, or greater than that of lobelane. Among the twenty five 1,4-diphenthylpiperidine analogs tested, compounds containing a 1-(2-methoxyphenethyl) moiety exhibited the most potent inhibition of vesicular DA uptake. From this subgroup, analogs 8h, 8j and 8m exhibited the highest affinity with Ki values of 9.3 nM, 13 nM and 13 nM, respectively, for inhibition of [3H]DA uptake by VMAT2. These compounds represent some of the most potent inhibitors of VMAT2 function reported thus far, and were considered worthy of further investigation as potential clinical candidates for treatment of METH abuse.
Supplementary Material
Acknowledgements
This research was supported by NIH U01 DA13519, TR000117 and NIH/COBRA P20 GM109005 grants, and an Arkansas Research Alliance (ARA) Scholar award. Methods for the [3H]DTBZ binding assay and [3H]DA uptake assay, and full characterization data for all synthetic products can be found in the Supporting Information.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Iyo M, Namba H, Yanagisawa M, Hirai S, Yui N, Fukui S. Prog Neuropsychopharmacol. Biol. Psychiatry. 1997;21:789. doi: 10.1016/s0278-5846(97)00079-1. [DOI] [PubMed] [Google Scholar]
- 2.Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. J. Psychiatry Neurosci. 2006;31:301. [PMC free article] [PubMed] [Google Scholar]
- 3.Shoptaw SJ, Kao U, Heinzerling K, Ling W. Cochrane Database Syst. Rev. 2009:CD003021. doi: 10.1002/14651858.CD003021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwoskin LP, Crooks PA. Biochem. Pharmacol. 2002;63:89. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 5.Sulzer D, Sonders MS, Poulsen NW, Galli A. Prog. Neurobiol. 2005;75:406. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. J. Pharmacol. Exp. Ther. 2001;296:1023. [PubMed] [Google Scholar]
- 7.Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. J. Pharmacol. Exp. Ther. 2001;298:172. [PubMed] [Google Scholar]
- 8.Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Psychopharmacology (Berl) 2003;165:397. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- 9.Crooks PA, Zheng G, Vartak AP, Culver JP, Zheng F, Horton DB, Dwoskin LP. Curr. Top. Med. Chem. 2011;11:1103. doi: 10.2174/156802611795371332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. J. Pharmacol. Exp. Ther. 1997;280:1432. [PubMed] [Google Scholar]
- 11.Alachkar A, Brotchie JM, Jones OT. Neurosci. Res. 2010;68:44. doi: 10.1016/j.neures.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Jones R. Double-blind, placebo-controlled, cross- over assessment of intravenous methamphetamine and sublingual lobeline interactions. 2007 https://clinicaltrials.gov/ct2/archive/NCT00519259.
- 13.Court JA, Perry EK, Spurden D, Lloyd S, Gillespie JI, Whiting P, Barlow R. Brain Res. 1994;667:118. doi: 10.1016/0006-8993(94)91721-3. [DOI] [PubMed] [Google Scholar]
- 14.Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm SD, Dwoskin LP. J. Pharmacol. Exp. Ther. 2004;310:1035. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- 15.Crooks PA, Dwoskin LP, John PC, Nickell JR, Zheng G. PCT Int. Appl. 2014 WO 2014144064.
- 16.Kumada M. Pure Appl. Chem. 1980;52:669. [Google Scholar]
- 17.Zheng G, Dwoskin LP, Deaciuc AG, Zhu J, Jones MD, Crooks PA. Bioorg. Med. Chem. 2005;13:3899. doi: 10.1016/j.bmc.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding D, Nickell JR, Dwoskin LP, Crooks PA. Bioorg. Med. Chem. Lett. 2015;25:2613. doi: 10.1016/j.bmcl.2015.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.