Summary
The Phosphoinositide-3-kinase (PI3K) pathway is often aberrantly activated in Estrogen Receptor positive (ER+) breast cancer and therapies combining PI3K inhibitors and anti-estrogens are under clinical development. Given that many PI3K inhibitors have substantial toxicities with continuous dosing and that alternate dosing schedules are equally active, further clinical exploration is warranted.
In this issue of Clinical Cancer Research, Yang and colleagues explore alternative dosing schedules of PI3K inhibition that elicit high anti-tumor activity (1). Their observations provide a mechanistic rationale for intermittent dosing of PI3K inhibitors in the management of ER+/PIK3CA mutant breast cancer that should be considered in clinical practice.
At the height of the development of powerful anti-cancer cytotoxic therapies, the field of cancer pharmacology was characterized by the study of meticulously detailed dose schedules that maximized the benefit/toxicity ratio, particularly for multiple agent combination regimens (2). Currently, in the age of targeted therapies, this attention to classical pharmacology has been somewhat lost for a number of reasons. Targeted agents were anticipated to have a favorable safety profile since they preferentially target oncogenic drivers and even in some cases, like with monoclonal antibodies, their prolonged half-life did not allow for flexible scheduling. And yet, the promise of high target specificity is not usually the case since many of these targeted agents have substantial toxicities, highlighting the importance of dose and scheduling. Several studies have indeed compared tumor regression achieved by transient potent inhibition versus continuous inhibition of oncogenic drivers (3–5). For example, transient potent BCR-ABL inhibition by the oral ABL kinase inhibitor imatinib, which is frontline therapy for chronic myeloid leukemia (CML), is sufficient to induce tumor regression in cell culture models and in patients (3). But overall, with the majority of these agents a continued schedule had been favored over alternative dosing schedules because of a general assumption that clinical success might require prolonged target inhibition.
A particular example of a class of targeted therapies that has shown higher than anticipated toxicities with continuous administration is the case of PI3K inhibitors. This increased toxicity is particularly true for pan-PI3K inhibitors such as GDC-0941 and BKM120. In the case of BKM120 (buparlisib), we recently reported the results of the phase III study of BKM120+ fulvestrant versus placebo+ fulvestrant (6). The study met its primary endpoint of improved progression free survival but the benefit was modest. The patients who received fulvestrant alone had a progression-free survival of five months; those who received buparlisib plus fulvestrant had a progression-free survival of 6.9 months (HR 0.78; P value <0.001). Patients who had mutant PIK3CA detected in their circulating tumor DNA (ctDNA) had much better outcomes if they received buparlisib plus fulvestrant when compared with those who received fulvestrant alone: progression-free survival of 7 months in the buparlisib plus fulvestrant only group versus 3.2 months in the fulvestrant group (HR 0.56; P value <0.001. However, up to 25 percent of the patients who received buparlisib experienced serious adverse effects, including hyperglycemia and an increase in markers of liver damage leading to treatment discontinuation or dose reductions. Overall, the median exposure to BKM120 was limited to two months of therapy due to the observed toxicities. Similar toxicities have also been reported from the FERGI study on GDC-0941 (7). In retrospect, this high toxicity observed is not surprising as the PI3K pathway is also critical for normal cell survival where it regulates essential cellular processes including cell growth, proliferation, metabolism, and homeostasis (8).
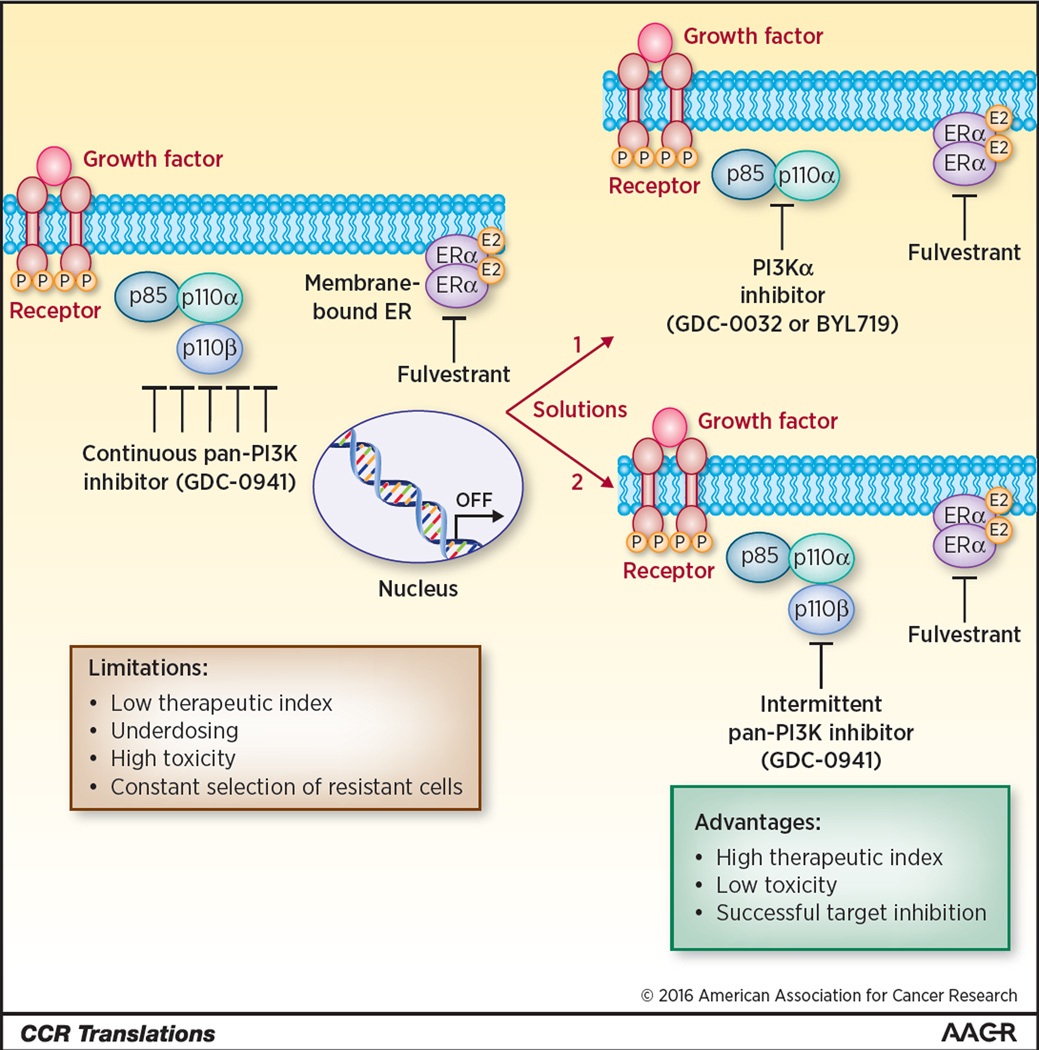
In order to deliver the full potential of PI3K inhibitors, two possible non-mutually exclusive solutions to the high toxicity observed with pan-PI3K inhibitors come to mind (Fig. 1). The first is to explore new scheduling alternatives that may be less toxic. A second ongoing approach is to develop isotype specific PI3Ka inhibitors that may have a better safety profile. Yang and colleagues have explored in their manuscript the first approach: studying new scheduling alternatives with Pictilisb (GDC-0941), a class I pan-PI3K with a recommended dose of 330 mg once-daily (9). They explored different doses and schedules: 1) weekly treatment with a high dose of GDC-0941 (800mg/kg), 2) daily treatment with a low dose of GDC-0941 (100mg/kg), or 3) bi-daily treatment with a low dose of GDC-0941 for 3 consecutive days per week (100mg/kg). Transient, complete PI3K inhibition stopped cell growth in vitro more efficiently that longer-term, continuous PI3K inhibition. Pharmacokinetic analyses revealed that high dose of GDC-0941 increased the drug’s half-life, exposure and mean residence time. Pharmacodynamics studies demonstrated that high dose GDC-0941 inhibited the PI3K signaling for 72 hours in comparison with 6–9 hours for the low dose. While these different treatment schedules elicited different patterns of pathway activation/inhibition, proliferation or apoptosis, they all evoked robust anti-cancer effects in combination with fulvestrant. Hence, both metronomic (daily) and intermittent (weekly) inhibition of PI3K in combination with fulvestrant induces tumor regression and combinatory treatment was more successful than single agents alone.
Figure 1.
Scheduling PI3K inhibition for ER+ breast cancer: limitations and possible solutions.
Receptor protein tyrosine kinase (RPTK) activation by growth factor stimulation results in the activation of phosphatidylinositol-3 kinase (PI3K) pathway, which can be inhibited by the pan PI3K inhibitor (GDC-0941). However, continuous administration of GDC-0941 leads to a low therapeutic index, significant toxicity and potential clonal selection of tumor cells resistant to therapy (left panel). Presented on the right panel, solutions include two possible options: 1) new scheduling alternatives of pan-PI3K inhibitors which can induce less toxicity while preserving efficacy or 2) the administration of PI3K alpha inhibitors. PI3K inhibition will be combined with the administration of fulvestrant to elicit robust anti-cancer effects in ER+/PIK3CA-mutant breast cancer.
The other ongoing approach is to study agents that are specific for the PI3KCA isoform such as BYL719 and GDC-0032. In extended phase I-II clinical trials these agents have been shown to have a more favorable safety profile than pan-PI3K inhibitors (10, 11). In fact, both are currently in phase III testing in combination with fulvestrant in patients with advanced ER+/PIK3CA-mutant breast cancer (NCT02437318, 12). Even with their improved toxicity profile, intermittent schedule of PI3K inhibitors should be considered especially if these agents are to be administered in the adjuvant setting.
In summary, the work by Yang and colleagues support the testing of intermittent pathway inhibition in the treatment of ER+ and PIK3CA-mutant breast cancer. Their critical finding is that potent target inhibition by the PI3K inhibitor remains effective even if it may be achieved transiently. From the clinical point of view, a different range of schedules and doses should be considered and optimized in early stage clinical trials. This is especially important for those compounds where toxicity has precluded them from continuous therapy. Moreover, further pre-clinical studies such as those performed by Yang et al can address the temporal response and pharmacodynamic effects of other combinatory treatments. This would provide rationale for treatment scheduling that might increase the therapeutic window of new agents and combinations. In conclusion, classical pharmacological principles of dose and schedule continue to be critically important in the era of precision medicine and carefully preclinical exploration of different schedules may enable improved clinical trials design and delivery of efficacious combination regimens.
Acknowledgments
The authors thank the members of the Baselga laboratory for critical reading of the manuscript.
Grant Support
E. Toska holds a fellowship from the Terri Brodeur Breast Cancer Foundation. J. Baselga was supported by the Breast Cancer Research Foundation and the NIH under award number R01CA190642.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Yang W, Hosford SR, Dillon LM, Shee K, Liu SC, Bean JR, et al. Strategically timing inhibition of phosphatidylinositol 3-kinase to maximize therapeutic index in estrogen receptor alpha-positive, PIK3CA-mutant breast cancer. Clin Cancer Res. 2016 Jan 5; doi: 10.1158/1078-0432.CCR-15-2276. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep. 1977;61:1307–1317. [PubMed] [Google Scholar]
- 3.Shah NP, Kasap C, Weier C, Balbas M, Nicoll JM, Bleickardt E, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Chew HK, Somlo G, Mack PC, Gitlitz B, Gandour-Edwards R, Christensen S, et al. Phase I study of continuous and intermittent schedules of lapatinib in combination with vinorelbine in solid tumors. Ann Oncol. 2012;23:1023–1029. doi: 10.1093/annonc/mdr328. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Wahab O, Klimek VM, Gaskell AA, Viale A, Cheng D, Kim E, et al. Efficacy of intermittent combined RAF and MEK inhibition in a patient with concurrent BRAF- and NRAS-mutant malignancies. Cancer Discov. 2014;4:538–545. doi: 10.1158/2159-8290.CD-13-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselga J, Im S-A, Iwata H, Clemons M, Ito Y, Awada A, et al. PIK3CA status in circulating tumor DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resisatnt HR+/HER2-advanced breast cancer (BC): first results from the randomized, phase III BELLE-2 trial [abstract]. Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposum; 2015 Dec 8–12; San Antonio, TX. Philadelphia (PA): AACR; 2015. Abstract nr S6-01. [Google Scholar]
- 7.Krop I, Johnston S, Mayer IA, Dickler M, Ganju V, Forero-Torres A, et al. The FERGI phase II study of the PI3K inhibitor pictilisib (GDC-0941) plus fulvestrant vs fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)-resistant advanced or metastatic breast cancer–Part I results [abstract]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2014 Dec 9–13; San Antonio, TX. Philadelphia (PA): AACR. Cancer Res. 2015;75(9 Suppl) Abstract nr S2-02. [Google Scholar]
- 8.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 9.Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juric D, Rodon J, Gonzalez-Angulo A, Burris H, Bendell J, Berlin J, et al. BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety. PK, efficacy results from the first-in-human study [abstract]. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, IL. Philadelphia (PA): AACR. Cancer Res. 2012;72(8 Suppl) Abstract nr CT-01. [Google Scholar]
- 11.Juric D, Krop I, Ramanathan RK, Xiao J, Sanabria S, Wilson TR, et al. GDC-0032, a beta isoform-sparing PI3K inhibitor: Results of a first-in-human phase Ia dose escalation study [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR. Cancer Res. 2013;73(8 Suppl) Abstract nr LB-64. [Google Scholar]
- 12.Baselga J, Cortes J, De Laurentiis M, Dieras V, Harbeck N, Im Y-H, et al. SANDPIPER: phase III study of the PI3-kinase (PI3K) inhibitor taselisib (GDC-0032) plus fulvestrant in patients (pts) with estrogen receptor (ER)-positive, HER2-negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA mutant tumors. J Clin Oncol. 2015;33 (suppl; abstr TPS629) [Google Scholar]