Abstract
Primary graft dysfunction (PGD) is a possible risk factor for bronchiolitis obliterans syndrome (BOS) following lung transplantation; however, the mechanism for any such association is poorly understood. Based on TGF-β's association with acute and chronic inflammatory disorders, we hypothesized that it may play a role in the continuum between PGD and BOS. Thus, the association between PGD and BOS was assessed in a single-center cohort of lung transplant recipients. Bronchoalveolar lavage fluid concentrations of TGF-β and procollagen collected within 24 hours of transplantation were compared across the spectrum of PGD, and incorporated into Cox models of BOS. Immunohistochemistry localized expression of TGF-β and its receptor in early lung biopsies post-transplant. We found an association between PGD and BOS in both bilateral and single lung recipients with a hazard ratio of 3.07 (95% CI 1.76-5.38) for the most severe form of PGD. TGF-β and procollagen concentrations were elevated during PGD (p<0.01), and associated with increased rates of BOS. Expression of TGF-β and its receptor localized to allograft infiltrating mononuclear and stromal cells, and the airway epithelium. These findings validate the association between PGD and the subsequent development of BOS, and suggest that this association may be mediated by receptor/TGF-β biology.
INTRODUCTION
Despite advances in surgical technique and post-operative medical care, survival following lung transplantation continues to lag considerably behind other solid organ transplants, with an estimated median survival of less than 6 years.(1) The primary obstacle to long-term survival is chronic rejection in the form of bronchiolitis obliterans syndrome (BOS), which affects up to 50% of patients within 5 years, and is characterized histopathologically by obliterative bronchiolitis, a progressive fibro-obliterative process affecting the allograft small airways.(2)
A unique feature of lung transplantation appears to be an association between ostensibly non-alloimmune insults to the allograft and the subsequent development of BOS.(3-10) Primary graft dysfunction (PGD) is a form of non-alloimmune acute lung injury that arises within the first 72 hours of transplantation, and is thought to be driven primarily by ischemia-reperfusion injury.(11, 12) PGD is the leading cause of early mortality following transplantation. In addition, recent studies suggest that PGD may also be a risk factor for BOS.(13-15) Little is known about the biomolecular mechanisms that might link these processes. Transforming growth factor-beta (TGF-β) is a critical mediator of extracellular remodeling and fibroproliferation that has also been found to have pleiotropic immunoregulatory properties, and is involved in the pathogenesis of a wide variety of disease states.(16-20) The profibrotic effects of TGF-β are driven by binding and activation of the TGF-β receptor complex, which promotes synthesis of procollagens, the precursors to mature collagen. Among these, procollagen type I (PC) is the propeptide of the most abundant form of collagen in humans. Previous studies have demonstrated that TGF-β concentrations within lungs are also elevated early on in the setting of acute lung injury, the non-transplant correlate of PGD, and TGF-β plays a role in driving the fibroproliferative phase of this condition.(21-24) However the role of TGF-β with respect to PGD specifically following lung transplantation has not been described.
In this study, we characterize the relationship between PGD and BOS in a single-center cohort of bilateral and single lung recipients. Additionally, we hypothesize that the association between PGD and BOS may be mediated, in part, by TGF-β in the early post-operative period, and that bronchoalveolar lavage fluid (BALF) concentrations of TGF-β and PC measured during this critical period may serve as complementary biomarkers for the future development of BOS.
MATERIALS AND METHODS
Study design and patient population
With approval from the institutional review board of the University of California at Los Angeles (protocol number 10-001492), we conducted a retrospective study of lung transplant recipients at our institution between March 1, 2000 and August 31, 2007. To determine the risk of BOS attributable to PGD, basic clinical characteristics, pathology reports from all allograft biopsy specimens, pulmonary function tests, and chest x-rays, arterial blood gases, and inspired oxygen concentrations from within the first 72 hours of transplantation were collected. To assess the association between BALF concentrations of TGF-β and PC and the clinical outcomes of PGD and BOS, we performed a nested study of all prospectively consented subjects with BALF from 24 hours post-transplant available for research use. Subjects were managed and underwent routine follow-up according to our institutional protocols.(25) All clinical protocols remained unchanged over the study period.
Clinical variable classification
BOS, acute cellular rejection (AR), and lymphocytic bronchiolitis (LB) were defined and graded according to International Society for Heart and Lung Transplantation (ISHLT) guidelines.(26-28) A cumulative AR score was derived for each patient, representing the sum of all ‘A’ grades.(25, 29) PGD was graded immediately after transplantation (T0) and through 72 hours (T72), according to ISHLT criteria.(11) We further classified subjects as follows: “Controls” had PGD grade 0-1 at all time points; subjects with “Transient PGD” had PGD grade 2-3 at T0 with resolution to grade 0-1 at T72; subjects with “Severe PGD” had PGD grade 2-3 at T0 through T72.
In order to ensure adequate spirometric data for the diagnosis of BOS, all study subjects were required to meet the following criteria for inclusion in models analyzing the association between PGD and BOS: (1) at least 6 outpatient pulmonary function tests following hospital discharge; (2) at least 6 months of spirometric follow-up. In order to minimize bias introduced by subjects with ongoing decline in lung function immediately following the development of PGD, these criteria were specifically chosen so that all subjects had stable established peak lung function. In other words, all subjects diagnosed with BOS developed a sustained 20% reduction in FEV1 that had to occur at least 6 months after transplantation.
Protein measurement and immunohistochemistry
After centrifugation of BALF specimens, supernatant protein concentrations were measured using standard ELISA for TGF-β1 (R&D Systems, Minneapolis, MN) and PC (Quidel Corporation, San Diego, CA) according to manufacturers’ instructions. Five subjects with hemodynamic instability, anemia and severe PGD underwent surgical re-exploration that included allograft biopsies within the first week of transplantation, and immunohistochemical staining was performed on these biopsy specimens for TGF-β and TGF-β receptor I (TβRI).
Statistical Analysis
Baseline clinical and demographic characteristics were compared between the total and nested cohorts by the Chi-square test for categorical variables and the Mann-Whitney test for continuous variables. Cox proportional hazards (CPH) models of BOS were created for the total cohort, and stratified by transplant type. Single lung transplantation, cumulative AR score, and the presence of LB on biopsies obtained prior to the diagnosis of BOS were then included into multivariable CPH models a priori, and backward elimination was used to screen for additional relevant covariates by Wald testing at a significance level of p < 0.10. Interactions were tested in the final models by individually adding multiplicative terms. Freedom from BOS was also estimated using the Kaplan-Meier method. BALF protein concentrations were compared across PGD classes by Kruskal-Wallis testing. Subjects were then dichotomized based on levels above or below the median concentration for each protein, and CPH models of BOS were created as a function of BALF protein concentration with and without adjustment for PGD. Analyses were performed using SAS® software version 9.3 (SAS Institute Inc., Cary, NC), and figures were created using GraphPad Prism® (version 5.0, GraphPad Software, San Diego,CA).
RESULTS
Cohort characteristics
Over the study period, a total of 279 patients underwent transplantation, including 174 bilateral and 105 single lung transplants. Among the bilateral transplants, 5 heart-lung recipients were also included. 148 (53.0%) subjects had PGD grade 0-1 at all time points (T0-T72), and were included in the “Control” group. An additional 124 (44.4%) patients had PGD grade 2-3 at T0 with 76 (27.2%) improving to PGD grade ≤ 1 by T72 included in the “Transient PGD” group, and the remaining 48 (17.2%) patients were considered to have “Severe PGD.” An additional three patients were excluded from subsequent analyses for delayed onset PGD (e.g. PGD grade 1 at T0-24 progressing to grade 2-3 at T48-72). Four patients were excluded for death due to PGD prior to T72. Forty subjects were excluded from analyses of freedom from BOS due to insufficient spirometric follow-up. Of the remaining 239, all subjects had at least 6 post-transplant pulmonary functions tests over at least 6 months of follow-up. At study completion, there were a total of 684.3 person-years of BOS-free spirometric follow-up and a mean follow-up of 2.86 +/− 1.93 years among those with sufficient pulmonary function tests. A total of 98 subjects developed BOS over the study period with an estimated 50% incidence of BOS at 4.23 years following transplantation based on the Kaplan-Meier method. Seventy-five subjects had BALF collected within 24 hours post-transplant available for measurement of TGF-β and PC concentrations, and were included in the nested protein analyses. Among the nested cohort, the “Control,” “Transient PGD,” and “Severe PGD” group sample sizes were 44 (58.7%), 18 (24.0) and 13 (17.3%), respectively. Other relevant clinical characteristics for the total and nested cohort are summarized in Table 1, and the characteristics of subjects meeting spirometric criteria for inclusion in BOS analyses are summarized in Table S1. There were no statistically significant differences in any of these characteristics between the total cohort and those meeting spirometric criteria.
Table 1.
Characteristics of subjects in the total and nested cohort.
| Characteristic | Total Cohort (n=279) | Nested Cohort (n=75) | p-value* |
|---|---|---|---|
| Gender, n (%) | 0.4 | ||
| Male | 173 (62%) | 37 (49.3%) | |
| Female | 126 (38%) | 38 (50.7%) | |
| Age, yr [IQR]) | 59 [53-64] | 61 [56-65] | 0.15 |
| Age ≥ 65, n (%) | 63 (22.6) | 20 (26.7) | 0.46 |
| Transplant Type, n (%) | 0.56 | ||
| Single | 105 (37.6) | 31 (41.3) | |
| Bilateral | 174 (62.4) | 44 (58.7) | |
| Induction Agent, n (%) | 0.60 | ||
| rATG | 173 (62.0) | 44 (58.7) | |
| Basiliximab | 106 (38.0) | 31 (41.3) | |
| Cardiopulmonary bypass use, n (%) | 187 (67.0) | 47 (62.7) | 0.48 |
| Ischemia time, min [IQR] | 321 [267-372] | 321 [257-361] | 0.44 |
| Pre-transplant diagnosis, n (%) | 0.54 | ||
| Idiopathic pulmonary fibrosis | 101 (36.2) | 30 (40.0) | |
| Chronic obstructive pulmonary disease | 97 (34.8) | 21 (28.0) | |
| Other | 81 (29.0) | 24 (32.0) | |
| Pulmonary arterial hypertension | 14 (5.0) | 4 (5.3) | |
| Systemic Sclerosis | 14 (5.0) | 5 (6.7) | |
| Other interstitial lung disease | 11 (3.9) | 4 (5.3) | |
| Cystic fibrosis | 10 (3.6) | 1 (1.3) | |
| Sarcoidosis | 9 (3.2) | 3 (4.0) | |
| Bronchiolitis obliterans syndrome | 9 (3.2) | 3 (4.0) | |
| Non-CF bronchiectasis | 7 (2.5) | 1 (1.3) | |
| α1-antitrypsin deficiency | 6 (2.2) | 2 (2.7) | |
| Lymphangioleiomyomatosis | 1 (0.4) | 1 (1.3) | |
| PGD Class, n (%) | 0.77 | ||
| Controls | 148 (53.0) | 44 (58.7) | |
| Transient PGD | 76 (27.2) | 18 (24.0) | |
| Severe PGD | 48 (17.2) | 13 (17.3) |
Categorical variables were compared by the Chi-square test; continuous variables were compared by the Mann-Whitney test
IQR = Interquartile range; PGD = primary graft dysfunction.
PGD is a risk factor for BOS
To characterize the association between PGD and the development of BOS in our cohort, we created crude CPH models of BOS as a function of PGD severity (Table 2). Compared to the “Control” group, subjects in both the “Transient PGD” (hazard ratio [HR] = 2.03, 95% confidence interval [CI] 1.29-3.20; p = 0.002) and “Severe PGD” (HR = 3.96, 95% CI 2.33-6.73; p < 0.001) groups had significantly increased rates of BOS that incrementally increased with PGD severity. After a priori adjustment for single lung transplantation, cumulative AR score, and the presence of LB the association between BOS and both “Transient PGD” and “Severe PGD” remained significant. As expected, both cumulative AR and LB were also significant predictors of BOS independent of PGD (Table 2). We explored the utility of adjusting for additional covariates including: recipient age, gender, use of cardiopulmonary bypass, type of induction immunosuppression, ischemia time (time to second lung reperfusion in bilateral recipients), and pre-transplant diagnosis; however, backward selection excluded all of these covariates.
Table 2.
Crude and adjusted Cox proportional hazards models for freedom from BOS by PGD severity for subjects from the cohort meeting spirometric criteria for BOS analysis (n=239), further stratified by transplant type.
| Variable | Crude1 HR [95% CI] | p-value | Adjusted2 HR [95% CI] | p-value |
|---|---|---|---|---|
| Total cohort | ||||
| PGD severity | ||||
| Controls | 1.00 | 1.00 | ||
| Transient PGD | 2.03 [1.29-3.20] | 0.002 | 2.00 [1.27-3.16] | 0.003 |
| Severe PGD | 3.96 [2.33-6.73] | < 0.001 | 3.07 [1.76-5.38] | < 0.001 |
| Cumulative AR score | -- | 1.12 [1.02-1.22] | 0.02 | |
| Lymphocytic bronchiolitis | -- | 2.10 [1.20-3.68] | 0.01 | |
| Single lung transplant | -- | 1.30 [0.86-1.98] | 0.21 | |
| Bilateral lung transplants | ||||
| PGD severity | ||||
| Controls | 1.00 | 1.00 | ||
| Transient PGD | 1.91 [1.04-3.50] | 0.04 | 2.08 [1.13-3.84] | 0.02 |
| Severe PGD | 3.70 [1.94-7.05] | < 0.001 | 2.82 [1.45-5.50] | 0.002 |
| Cumulative AR score | -- | 1.13 [1.01-1.27] | 0.04 | |
| Lymphocytic bronchiolitis | -- | 1.69 [0.82-3.51] | 0.16 | |
| Single lung transplants | ||||
| PGD severity | ||||
| Controls | 1.00 | 1.00 | ||
| Transient PGD | 2.13 [1.07-4.21] | 0.03 | 1.74 [0.87-3.48] | 0.12 |
| Severe PGD | 4.25 [1.64-11.04] | 0.003 | 3.67 [1.27-10.58] | 0.02 |
| Cumulative AR score | -- | 1.13 [0.99-1.30] | 0.10 | |
| Lymphocytic bronchiolitis | -- | 2.82 [1.13-7.00] | 0.03 |
HR = hazard ratio; CI = confidence interval; BOS = bronchiolitis obliterans syndrome; PGD = primary graft dysfunction; AR = acute rejection.
Crude models included only Transient PGD and Severe PGD as independent categorical variables with Controls as reference.
All adjusted models included PGD severity, cumulative AR score, and the presence of LB on transbronchial biopsies. The adjusted model for the total cohort was also further adjusted for transplant type.
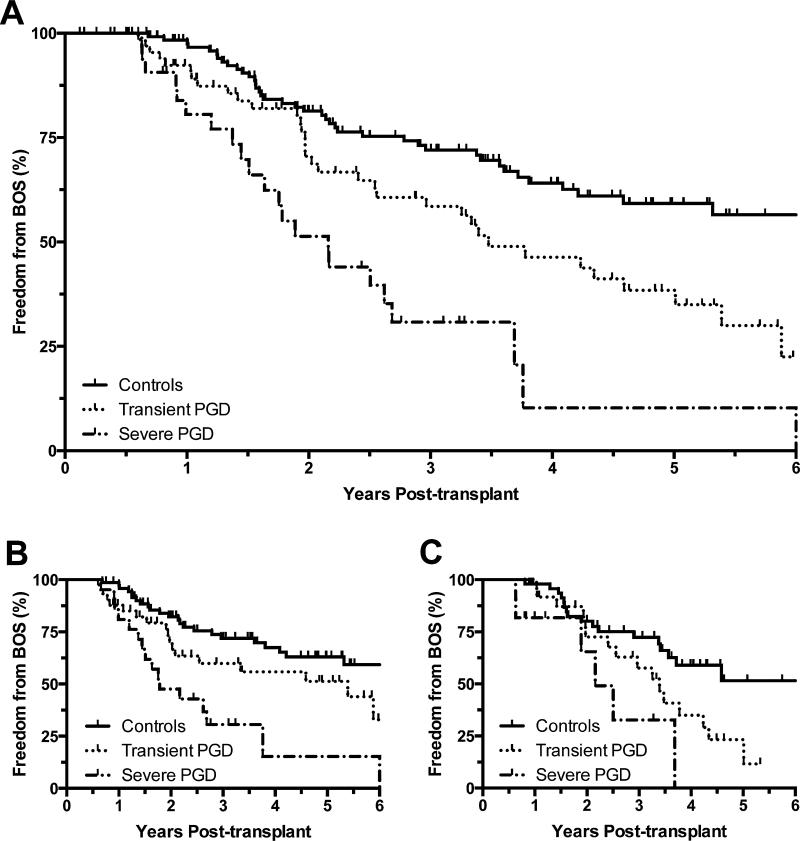
Given conflicting literature regarding the association between PGD and BOS following single lung transplantation,(13-15, 30) we also created similar CPH models of BOS after stratification of our cohort by transplant type (Table 2). In crude models of BOS as well as models adjusted for cumulative AR score and the presence of LB, there continued to be an association between increasingly severe PGD and BOS in both single and bilateral lung recipients. Relative to controls, single lung recipients with either “Transient PGD” or “Severe PGD” had adjusted hazard ratios for BOS of 1.74 (95% CI 0.87-3.48; p = 0.12) and 3.67 (95% CI 1.27-10.58; p = 0.02), respectively. Notably, point estimates of the relative hazards for BOS attributable to PGD were comparable between single and bilateral lung recipients (Table 2). In parallel with these CPH models for BOS, Kaplan-Meier plots of freedom from BOS were constructed for the total cohort and stratified by transplant type (Figure 1).
Figure 1.
Kaplan-Meier plots of freedom from BOS by PGD severity for: (A) the total cohort of transplant recipients, demonstrating log-rank hazard ratios of 1.98 (95% CI 1.32-3.52; p = 0.002) and 3.57 (95% CI 3.45-14.95; p < 0.001) for transient and severe PGD, respectively; (B) bilateral lung transplant recipients, demonstrating log-rank hazard ratios of 1.87 (95% CI 1.05-3.89; p = 0.04) and 3.53 (95% CI 2.73-14.87; p < 0.001) for transient and severe PGD, respectively; and (C) single lung transplant recipients, demonstrating log-rank hazard ratios of 2.36 (95% CI 1.31-4.85; p = 0.04) and 3.44 (95% CI 1.88-31.34; p = 0.01) for transient and severe PGD, respectively.
Augmented TGF-β concentrations in BALF from within 24 hours of transplantation are associated with the development of PGD
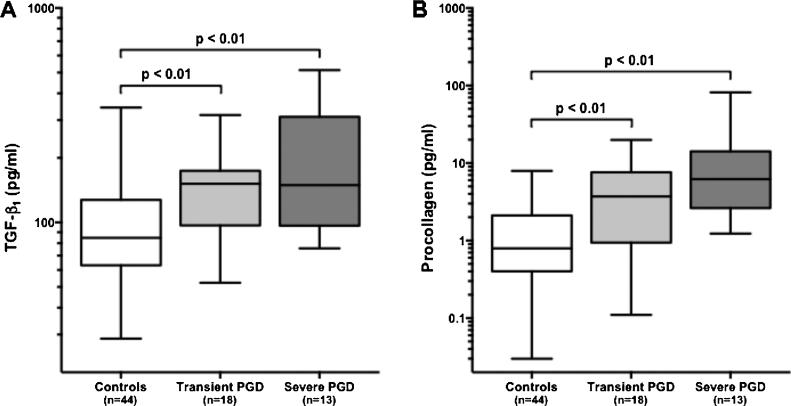
Having established an association between PGD and BOS in our cohort, we then explored the role of TGF-β during PGD within a nested cohort of lung transplant recipients (n=75) from whom BALF collected within 24 hours of transplantation was available. We found that compared to controls, subjects with either transient or severe PGD had significantly higher BALF concentrations of TGF-β (p = 0.007 and p = 0.002, respectively) (Figure 2A). Between subjects with transient and severe PGD, the difference in BALF concentrations of TGF-β was not significant.
Figure 2.
Concentrations of (A) TGF-β and (B) procollagen in BALF collected within 24 hours of transplantation. Compared to controls, concentrations of TGFb and procollagen were significantly elevated in subjects with either transient and severe PGD. Between the latter groups, differences in BALF concentration for either protein did not meet statistical significance.
There is a strong correlation between BALF concentrations of TGF-β and PC
Previous studies have demonstrated that biologically active TGF-β upregulates the production of PC.(31-33) Based on these data we evaluated the BALF samples from the nested cohort of transplant recipients for concentrations of PC. We found a strong correlation between the log-transformed protein concentrations of TGF-β and PC (Pearson's r = 0.64, p < 0.001). Furthermore, we found that compared to controls, subjects with either transient or severe PGD had significantly higher BALF concentrations of PC (p= 0.003 and p < 0.001, respectively) (Figure 2B). Between subjects with transient PGD and severe PGD, the difference in BALF PC concentration was not significant.
Augmented TGF-β concentrations in BALF from within 24 hours of transplantation are associated with the development of BOS
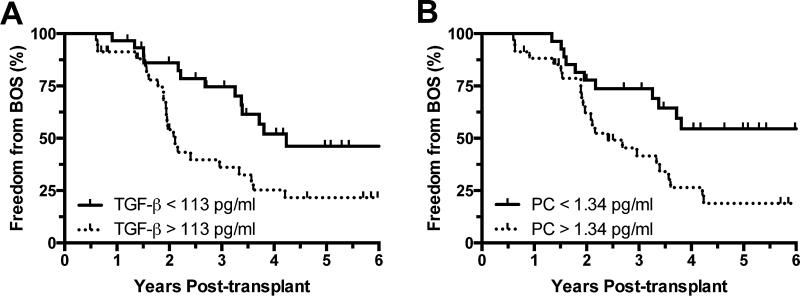
With previous studies demonstrating that increased expression of TGF-β can be associated with both acute and chronic lung injury,(24) we explored the association between increased TGF-β as well as PC concentrations in the BALF, and the long-term outcome of BOS. Thus, subjects meeting spirometric criteria for BOS analyses in the nested cohort (n=70) were then dichotomized based on median BALF concentrations of either TGF-β or PC (113 pg/ml and 1.34 pg/ml, respectively), and we constructed CPH models of BOS comparing subjects with high BALF concentrations of the proteins to those with concentrations below the medians. In crude CPH models, high levels of either protein were associated with increased rates of BOS (Table 3). These findings were consistent with Kaplan-Meier analyses demonstrating significantly reduced freedom from BOS in recipients with high concentrations of BALF TGF-β or PC (Figure 3A and B). Interestingly, after adjusting for PGD severity in parallel multivariable CPH models, high BALF TGF-β concentration remained a statistically significant independent predictor of BOS with HR = 2.61 (95% CI 1.26-5.39; p = 0.01). Conversely, the association between BALF PC and BOS was considerably dampened and no longer significant in multivariable models after adjustment for PGD severity. Among subjects in the nested cohort with a minimum of 3 years of spirometric follow-up (n=56), receiver operating characteristic analyses for the outcome of BOS at 3 years yielded areas under the curve for BALF TGF-β and PC of 0.77 (95% CI 0.64-0.90; p = 0.002) and 0.72 (95% CI 0.58-0.86; p = 0.008), respectively. There were no statistically significant associations between BALF concentrations of either protein and the subsequent development of AR or LB (data not shown).
Table 3.
Crude and adjusted Cox proportional hazards models for freedom from BOS by BALF protein concentration within the nested cohort of subjects meeting spirometric criteria for BOS analysis (n=70).
| Variable | Crude1 HR [95% CI] | p-value | Adjusted2 HR [95% CI] | p-value |
|---|---|---|---|---|
| BALF TGF-β concentration | ||||
| [TGF-β] < 113 pg/ml | 1.00 | 1.00 | ||
| [TGF-β] > 113 pg/ml | 2.54 [1.28-5.01] | 0.007 | 2.61 [1.26-5.39] | 0.01 |
| Transient PGD | -- | 1.54 [0.71-3.32] | 0.27 | |
| Severe PGD | -- | 4.47 [1.87-10.69] | 0.001 | |
| BALF PC concentration | ||||
| [PC] < 1.34 pg/ml | 1.00 | 1.00 | ||
| [PC] > 1.34 pg/ml | 2.32 [1.16-4.65] | 0.02 | 1.45 [0.63-3.37] | 0.39 |
| Transient PGD | -- | 1.86 [0.79-4.34] | 0.15 | |
| Severe PGD | -- | 3.39 [1.24-9.29] | 0.02 |
HR = hazard ratio; CI = confidence interval; BOS = bronchiolitis obliterans syndrome; PGD = primary graft dysfunction; BALF = bronchoalveolar lavage fluid; TGFβ = transforming growth factor-beta; PC = procollagen.
Crude models included only the respective protein BALF concentration dichotomized by values above or below median concentration.
All adjusted models included the respective protein concentration as in crude analyses, along with further adjustment for PGD severity as covariates.
Figure 3.
Kaplan-Meier plots of freedom from BOS dichotomized by BALF protein concentrations above or below median levels for (A) TGF-β, demonstrating a log-rank hazard ratio of 2.43 (95% CI 1.33-4.84; p = 0.01) for [TGF-β] > 113 pg/ml and (B) procollagen, demonstrating a log-rank hazard ratio of 2.26 (95% CI 1.19-4.32; p = 0.01) for [PC] > 1.34 pg/ml.
Cellular sources of TGF-β and TβRI during severe PGD
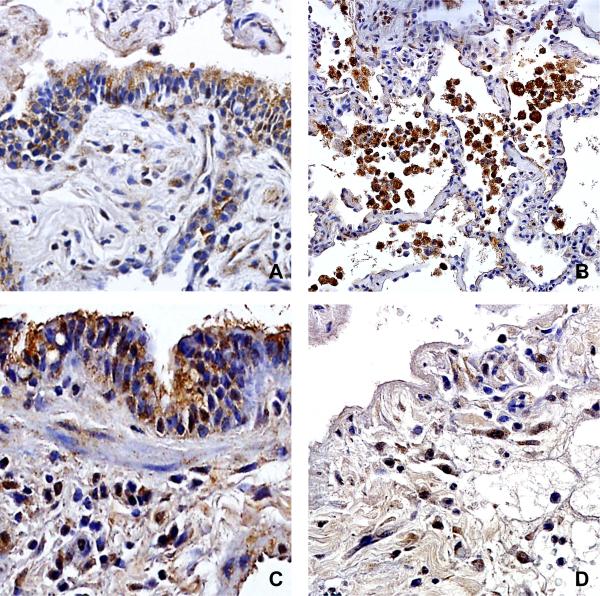
With our results demonstrating an association between early BALF TGF-β concentrations and the subsequent development of BOS, we investigated the cellular sources of TGF-β within the allograft during PGD. Immunohistochemical staining was performed on representative surgical lung biopsies from subjects with severe PGD (n=5). We found that TGF-β protein was expressed by allograft bronchial epithelial cells, subepithelial infiltrating mononuclear cells, as well as alveolar macrophages (Figure 4A and B). Importantly, TβRI was predominantly expressed by allograft airway epithelium, peri-airway and interstitial infiltrating mononuclear cells and stromal cells, as well as alveolar macrophages (Figure 4C and D).
Figure 4.
Immunohistochemical staining for TGF-β localizing expression to (A) allograft small airway epithelial cells and subepithelial infiltrating mononuclear cells, as well as (B) alveolar macrophages. TβRI expression was noted in (C) small airway epithelial cells, subepithelial stromal cells and infiltrating mononuclear cells, as well as (D) infiltrating stromal cells and mononuclear cells within the interstitium.
DISCUSSION
In this study we examine the association between PGD and BOS in a mixed cohort of single and bilateral lung transplant recipients. We demonstrate that compared to patients with minimal PGD (grade 0-1 at T0-72), patients with PGD grade 2-3 at T0 are at increased risk of subsequently developing BOS, and patients with the most severe form of PGD, persisting through T72 are at the greatest risk. These findings are consistent with prior studies in bilateral transplant recipients, and corroborate the trend toward increased risk of BOS in subjects with PGD who fail to improve within 72 hours of transplantation suggested by Huang, et al.(13) Indeed, one advantage of our classification of PGD is that it accounts for both the physiological and temporal severity of PGD throughout the early post-transplant period rather than at isolated time points, and delineates the added incremental risk associated with early PGD that fails to improve over time.
In contrast to two prior studies that failed to demonstrate such an association among single lung recipients, the association between PGD and BOS in our cohort persisted after adjustment for transplant type, and was present in stratified analyses of either single or bilateral transplant recipients.(15, 30) The application of the ISHLT PGD grading system to single lung recipients must be approached cautiously. PGD grading presumes that the severity of graft injury correlates with impairment in overall gas exchange. In single lung recipients, the degree of hypoxemia is further modulated by the severity of disease within the native lung and the extent of physiological redistribution of perfusion to functional regions of either lung. Moreover, in subjects transplanted for underlying obstructive lung disease, the presence of native lung hyperinflation and consequent allograft volume loss may create a particular challenge. This anatomical interaction may not only affect gas exchange but could also confound the interpretation of radiographic abnormalities otherwise seen in PGD, potentially biasing studies toward the null due to misclassification. Misclassification bias may also extend to the diagnosis of chronic rejection as progressive native lung hyperinflation may mimic the declining spirometry seen in BOS. Of note, obstructive lung disease was an indication for transplantation in a minority of our subjects, while it accounted for the vast majority of recipients in the prior studies investigating PGD and BOS following single lung transplantation.(15, 30) We acknowledge that the magnitude of any association between PGD and BOS may differ in single lung recipients due to these and other factors. However, if PGD is in fact a risk factor for progressive allograft dysfunction culminating in BOS, we can find no biologically plausible explanation for this association to be entirely unique to bilateral lung recipients.
Elucidating the mechanisms that promote BOS following non-alloimmune insults such as PGD is critical to explaining and potentially improving the relatively poor long-term outcomes compared to other solid organs. As with non-transplant forms of lung injury, PGD may transition to a fibroproliferative phase leading to reduced post-operative lung function and long-term morbidity and mortality. However, our spirometric inclusion criteria for the analyses of BOS included only subjects with stable to improving lung function over the first 6 months post-transplant. Furthermore, the increased rate of spirometric decline among recipients with PGD extends for multiple years following transplantation as suggested by Kaplan-Meier analyses in this and other studies.(13-15) Therefore, we hypothesized that TGF-β would be a potential link between early allograft injury and the later development of fibroplasia of the allograft airways. We found increased concentrations of BALF TGF-β are present during increasingly severe PGD. One of TGF-β's main attributes during fibroproliferative diseases is its interaction with stromal cells, which induces the expression of PC, thereby leading to the deposition of new extracellular matrix.(31-33) To explore the in vivo biological significance of TGF-β during PGD, we evaluated the concentrations of PC in the same BALF. Similar to TGF-β, we found that concentrations of PC in BALF paralleled the severity of PGD. Moreover, the concentrations of these proteins were strongly correlated. These data imply that TGF-β's augmentation during ischemia reperfusion injury promotes the expression of PC, and may contribute to fibroproliferation in the setting of PGD. This is supported by both human and animal studies involving acute lung injury, which have shown that TGF-β expression correlates with decreases in PaO2/FiO2 ratio as well as fibroplasia of the lung.(22, 24, 34-36) While these studies support the role of TGF-β in the pathogenesis of non-transplant acute lung injury as well as PGD, they do not explain the connection between acute post-transplant injury and the later onset of BOS.
This led us to explore the possibility that TGF-β activity in the early post-operative period may have long-term sequelae such as the subsequent development of BOS. We found that high concentrations of TGF-β in BALF collected within 24 hours of transplantation were associated with increased rates of BOS, and a similar association was seen for BALF PC in crude analyses. Together, this indicates at least two potential mechanisms that could link PGD to BOS. The first involves the self-perpetuation of receptor/TGF-β biology that eventually leads to fibroproliferative changes in the lung.(37-39) The second involves the early influence of this biological axis on stromal cells that can alter their phenotype, allowing for a relentless fibroproliferative process that persists after the resolution of acute injury and inflammation.(40, 41)
Notably, after adjusting for PGD severity, high BALF TGF-β concentrations remained a significant independent predictor of BOS, while the association between PC and BOS was no longer significant. This suggests that receptor/TGF-β biology may promote the development of BOS through mechanisms independent of or in addition to its role in promoting PC during PGD. Thus, we performed immunohistochemical analyses of both TGF-β and TβRI on lung tissue samples obtained during PGD. Both TGF-β and its receptor were expressed by interstitial and peri-airway infiltrating mononuclear cells, as well as allograft airway epithelial cells. Additionally, TβRI was also expressed by interstitial and peri-airway stromal cells. Collectively this suggests that early on, TGF-β is important for the expression of procollagen, in keeping with its known involvement in acute lung injury. (22, 24, 34-36) Prior studies have found increased concentrations of TGF-β in BALF from patients just prior to the development of BOS as well as after its diagnosis.(42-45) Mechanistically, inhibition of TGF-β signaling has also been shown to decrease fibro-obliteration in animal models of obliterative bronchiolitis.(46-48) In light of these findings, the expression of TGF-β and TβRI within and surrounding the allograft airways (the main anatomical targets in BOS) during PGD further supports the contention that receptor/TGF-β biology perpetuates fibroplasia of the allograft bronchioles, thereby implicating this axis in the continuum from PGD to BOS. Expression of both TGF-β and TβRI by the same cell types during PGD also suggests that this may be in part through autocrine induction of TGF-β expression.
In addition to the shortcomings inherent to any retrospective study design, we acknowledge that this study has several limitations. Though we made every effort to adjust for potential confounding in our analyses of the total cohort, data was unavailable to allow further adjustment for several established risk factors of BOS. Sample size limited any further stratification of the cohort to further assess for example the impact of transplant indication on the association between PGD and BOS in single lung recipients. Furthermore, while the association between PGD and BOS persisted in models stratified by transplant type, small sample size limited the statistical power to adjust for multiple covariates in stratified analyses. In light of the prior publications failing to demonstrate this association in single lung recipients and the relatively small number (n=105) of these subjects in our study, we admit that the association between PGD and BOS specifically among single lung transplant recipients requires further validation. Biopsy specimens for immunohistochemistry were available only from transplant recipients with severe PGD. We acknowledge that comparison of these immunohistochemistry findings with specimens from controls would have complemented the results of BALF protein analyses. Finally, any role for BALF protein concentrations as biomarkers for recipients at greatest risk of early BOS requires validation, and we cannot exclude the possibility that TGF-β signaling may play an active role in the biology underlying PGD, but still be an epiphenomenon of the association between PGD and BOS. Further studies are necessary to clarify and confirm the mechanistic role TGF-β biology may play in the link between early injury and chronic dysfunction of the allograft.
In summary, our results further support the presence of an association between PGD and BOS in a mixed cohort including bilateral as well as single lung recipients. Nested analyses of BALF indicate that receptor/TGF-β biology is upregulated in the setting of PGD. Furthermore, tissue immunohistochemical staining of TGF-β and its receptor localized the activity of this axis to sites of recent injury as well as sites involved in the later development of BOS, suggesting this biology's involvement in the mechanisms linking PGD and BOS.
Supplementary Material
ACKNOWLEDGMENTS
We thank the study participants, as well as the research staff at the University of California at Los Angeles. This study was supported by NIH grants R01HL112990, UL1TR000124, and U01AI113315.
Abbreviations
- PGD
Primary graft dysfunction
- BOS
Bronchiolitis obliterans syndrome
- TGF-β
Transforming growth factor-beta
- PC
Procollagen type I
- BALF
Bronchoalveolar lavage fluid
- AR
Acute (cellular) rejection
- LB
Lymphocytic bronchiolitis
- ISHLT
International Society for Heart and Lung Transplantation
- T0
Time immediately after lung transplantation
- T72
72 hours following lung transplantation
- TβRI
TGF-β receptor I
- CPH
Cox proportional hazards
- HR
Hazard ratio
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31(10):1073–86. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, Derhovanessian A, Wallace WD, Lynch JP, 3rd, Belperio JA. Bronchiolitis obliterans syndrome: the achilles' heel of lung transplantation. Semin Respir Crit Care Med. 2013;34(3):336–51. doi: 10.1055/s-0033-1348467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11(10):2190–6. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 4.Weigt SS, Copeland CA, Derhovanessian A, Shino MY, Davis WA, Snyder LD, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant. 2013;13(4):919–27. doi: 10.1111/ajt.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–7. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5(8):2031–6. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vos R, Vanaudenaerde BM, De Vleeschauwer SI, Van Raemdonck DE, Dupont LJ, Verleden GM. De novo or persistent pseudomonal airway colonization after lung transplantation: importance for bronchiolitis obliterans syndrome? Transplantation. 2008;86(4):624–5. doi: 10.1097/TP.0b013e318182295d. author reply 35-6. [DOI] [PubMed] [Google Scholar]
- 8.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85(5):771–4. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 9.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31(4):707–13. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 10.D'Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Christie JD. Primary graft dysfunction. Clinics in chest medicine. 2011;32(2):279–93. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8(11):2454–62. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 15.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26(10):1004–11. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134(3):392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhurst RJ. TGF beta signaling in health and disease. Nature genetics. 2004;36(8):790–2. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 18.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proceedings of the American Thoracic Society. 2012;9(3):111–6. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 20.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. The New England journal of medicine. 2000;342(18):1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 21.Wesselkamper SC, Case LM, Henning LN, Borchers MT, Tichelaar JW, Mason JM, et al. Gene expression changes during the development of acute lung injury: role of transforming growth factor beta. Am J Respir Crit Care Med. 2005;172(11):1399–411. doi: 10.1164/rccm.200502-286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, et al. TGF-beta is a critical mediator of acute lung injury. The Journal of clinical investigation. 2001;107(12):1537–44. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahy RJ, Lichtenberger F, McKeegan CB, Nuovo GJ, Marsh CB, Wewers MD. The acute respiratory distress syndrome: a role for transforming growth factor-beta 1. American journal of respiratory cell and molecular biology. 2003;28(4):499–503. doi: 10.1165/rcmb.2002-0092OC. [DOI] [PubMed] [Google Scholar]
- 24.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Critical care medicine. 2003;31(4 Suppl):S258–64. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 25.Weigt SS, Elashoff RM, Huang C, Ardehali A, Gregson AL, Kubak B, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9(8):1903–11. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12(5):713–6. [PubMed] [Google Scholar]
- 27.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 28.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 29.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999;159(3):829–33. doi: 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 30.Burton CM, Iversen M, Milman N, Zemtsovski M, Carlsen J, Steinbruchel D, et al. Outcome of lung transplanted patients with primary graft dysfunction. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2007;31(1):75–82. doi: 10.1016/j.ejcts.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, et al. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. The American journal of pathology. 1997;150(3):981–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Massague J, Cheifetz S, Endo T, Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(21):8206–10. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. The Journal of clinical investigation. 1990;85(6):1833–43. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A, et al. Tumor necrosis factor-alpha and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166(5):651–6. doi: 10.1164/rccm.2109004. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107(1):196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 36.Hill JD, Ratliff JL, Parrott JC, Lamy M, Fallat RJ, Koeniger E, et al. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg. 1976;71(1):64–71. [PubMed] [Google Scholar]
- 37.Kim SJ, Jeang KT, Glick AB, Sporn MB, Roberts AB. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. The Journal of biological chemistry. 1989;264(12):7041–5. [PubMed] [Google Scholar]
- 38.Lafyatis R, Denhez F, Williams T, Sporn M, Roberts A. Sequence specific protein binding to and activation of the TGF-beta 3 promoter through a repeated TCCC motif. Nucleic acids research. 1991;19(23):6419–25. doi: 10.1093/nar/19.23.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. The Journal of biological chemistry. 1988;263(16):7741–6. [PubMed] [Google Scholar]
- 40.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. The Journal of clinical investigation. 1997;100(4):768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen B, Polunovsky V, White J, Blazar B, Nakhleh R, Jessurun J, et al. Mesenchymal cells isolated after acute lung injury manifest an enhanced proliferative phenotype. The Journal of clinical investigation. 1992;90(5):1778–85. doi: 10.1172/JCI116052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Gamel A, Sim E, Hasleton P, Hutchinson J, Yonan N, Egan J, et al. Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. J Heart Lung Transplant. 1999;18(9):828–37. doi: 10.1016/s1053-2498(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 43.Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, et al. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation. 2000;70(2):362–7. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 44.Magnan A, Mege JL, Escallier JC, Brisse J, Capo C, Reynaud M, et al. Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1431–6. doi: 10.1164/ajrccm.153.4.8616577. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez AM, Nunley DR, Rojas M, Roman J. Activation of Tissue Remodeling Precedes Obliterative Bronchiolitis in Lung Transplant Recipients. Biomarker insights. 2008;3:351–9. doi: 10.4137/bmi.s686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alho HS, Maasilta PK, Vainikka T, Salminen US. Platelet-derived growth factor, transforming growth factor-beta, and connective tissue growth factor in a porcine bronchial model of obliterative bronchiolitis. Experimental lung research. 2007;33(6):303–20. doi: 10.1080/01902140701539745. [DOI] [PubMed] [Google Scholar]
- 47.Jonigk D, Merk M, Hussein K, Maegel L, Theophile K, Muth M, et al. Obliterative airway remodeling: molecular evidence for shared pathways in transplanted and native lungs. The American journal of pathology. 2011;178(2):599–608. doi: 10.1016/j.ajpath.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6(9):2080–8. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.