Abstract
Purpose of review
Development of cystic fibrosis transmembrane conductance regulator (CFTR) modulators, small molecule therapies that target the basic defect in CF, represents a new era in CF treatment. This review highlights recent progress in CF therapeutics as an example of precision medicine and personalized approaches to test CFTR modulators using preclinical model systems.
Recent findings
CFTR modulators are now clinically available for approximately 50% of the U.S. CF population. The CFTR potentiator, ivacaftor, is approved for people with CF ages two years and older with at least one gating mutation (G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, or S549R) or the R117H conductance mutation. The recent FDA approval of the corrector/potentiator combination, lumacaftor/ivacaftor, expands modulator therapy to people with CF homozygous for the F508del mutation, ages 12 years and older. Ivacaftor and lumacaftor however do not fully restore CFTR activity. Thus, next-generation correctors and potentiators are in development. Read-through agents targeting nonsense mutations and genotype agnostic treatments (gene-editing and gene therapy) are also in various phases of clinical development.
Summary
CFTR modulators promise to transform the therapeutic landscape in CF in a precision based fashion. Areas of ongoing research include developing drugs for all mutation classes so that all persons with CF can benefit from these therapies and refining preclinical assays that allow the selection of the most effective treatments on an individual basis.
Keywords: cystic fibrosis transmembrane conductance regulator, potentiators, correctors, gene therapy, model systems
INTRODUCTION
Cystic fibrosis (CF) is a genetic disorder that leads to chronic multisystem disease consisting of chronic sinopulmonary infections, malabsorption and nutritional abnormalities. It is the most common autosomal recessive life-shortening disease among Caucasians in the U.S. Though multiple organ systems are affected in this disease, lung involvement is the major cause of morbidity and mortality. From a pulmonary perspective, a cycle of chronic, persistent infections with CF-related pathogens and an excessive inflammatory response progressively damage the airways and lung parenchyma, resulting in widespread bronchiectasis and ultimately, early death from respiratory failure [1, 2].
CF is caused by mutations in a gene on chromosome 7 that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a cyclic adenosine monophosphate–regulated ion channel. CFTR functions primarily as a chloride and bicarbonate channel and controls the movement of fluid into and out of epithelial cells lining the respiratory tract, biliary tree, intestines, vas deferens, sweat ducts, and pancreatic ducts. The discovery of the gene responsible for CF 25 years ago [3] has enabled an understanding of how various mutations lead to specific alterations in the structure and function of the CFTR protein. Over 1,900 CFTR mutations and variants have been identified, although over 80% of people with CF in the U.S. possess at least one copy of the most common mutation, F508del [4]. This knowledge eventually led to the discovery and development of compounds that modulate CFTR function, thus targeting the basic defect in CF. Development of CFTR-targeted therapies represents a new era in CF treatment, one that is revolutionizing the care of CF patients.
ERA OF PRECISION MEDICINE IN CF
In 2015, the second CFTR protein modulator therapy, ivacaftor/lumacaftor (Orkambi®), was approved by the U.S. Food and Drug Administration (FDA) for individuals with CF ages 12 years and older with two copies of the F508del mutation, thus providing a potential treatment for approximately 50% of people with CF who are homozygous for F508del. The first, ivacaftor (Kalydeco®), has been available in the U.S. since 2012 as a stand-alone therapy for CF individuals with the G551D and other gating mutations. Now, more than 10,000 people living with CF can benefit from drugs that treat the underlying genetic cause of their disease.
The development and targeted use of ivacaftor and lumacaftor are great examples of how the CF community is at the forefront of the field of precision medicine. Precision medicine essentially means tailoring treatments based on an individual’s genetic makeup. President Barack Obama highlighted the pioneering advances in CF treatments in his 2015 State of the Union address and in a speech launching the Precision Medicine Initiative (https://www.nih.gov/precision-medicine-initiative-cohort-program).
CFTR MUTATION CLASSES
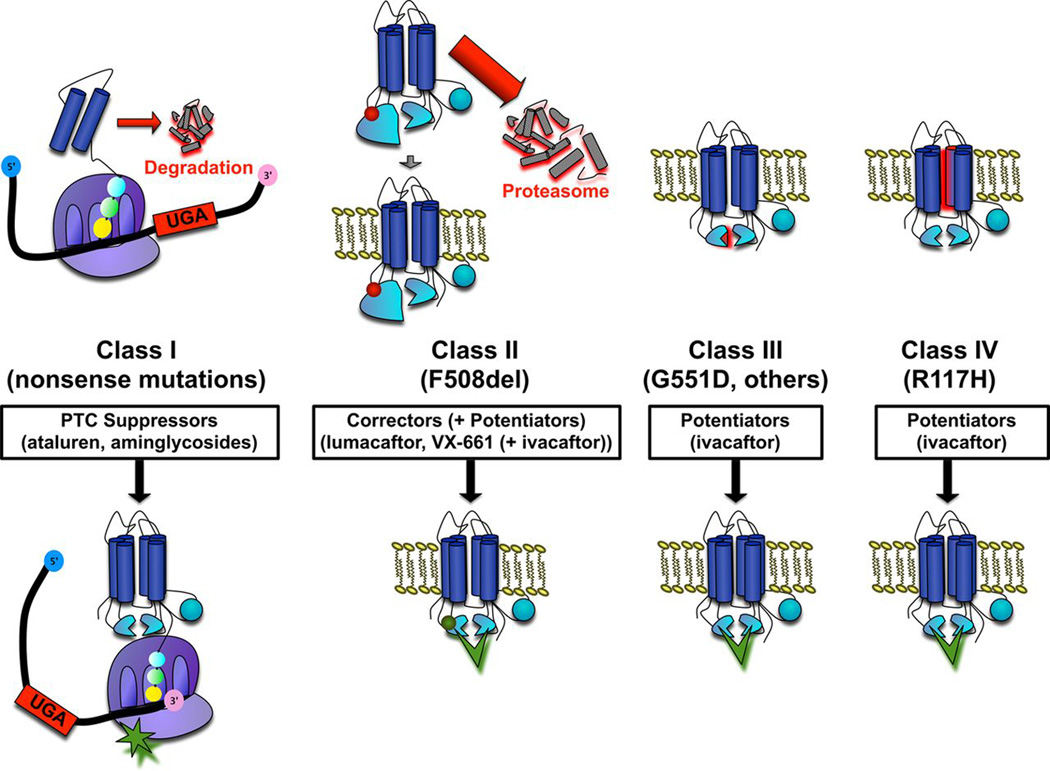
Over 1,900 mutations or variants in the CFTR gene have been identified although not all are disease-causing. CFTR mutations can be broadly categorized into five classes based on the effect of the mutation on the protein (Figure 1) [1, 5, 6]. Class I mutations result in altered RNA processing and absent or truncated CFTR protein due to nonsense, splice site or frameshift mutations. Class II mutations, which include F508del, lead to folding or maturation defects and thus, little detectable CFTR in the cell membrane. Class III mutations, typified by the G551D mutation, are gating mutations that result in CFTR protein that reaches the cell membrane but is nonfunctional secondary to gating defects that limit channel opening. Class IV and V mutations are associated with either reduced chloride conductance through the CFTR channel or reduced levels of the CFTR protein at the cell membrane, respectively. The most commonly detected class IV mutation is the R117H which has an additional layer of clinical phenotype variability based on polymorphisms in the poly-T tract in intron 8 of the CFTR gene [7]. Individuals with the R117H mutation with a 7T tract are generally expected to do well with low risk of disease progression and sweat chloride values in the intermediate to normal range, while those with a 5T tract are at higher risk of developing lung disease and may have sweat chloride values in the CF diagnostic range.
Figure 1. CFTR gene mutation classes and therapeutic approaches.
Previously published in Rowe SM, et al. Progress in cystic fibrosis and the CF Therapeutics Development Network. Thorax. 2012 Oct;67(10):882-90.
Class I-III mutations result in minimal protein function; thus, people with two copies of these mutations typically have the classic CF phenotype including pancreatic insufficiency. Individuals with at least one Class IV or V mutation (i.e. partial function mutations) typically have residual CFTR function and sufficient pancreatic function to absorb nutrients without supplemental pancreatic enzymes (i.e. pancreatic sufficiency), and may have sweat chloride values in the CF diagnostic or intermediate ranges [8]. Although classifying mutations into distinct classes provides a useful framework, in reality many mutations including F508del result in problems with CFTR protein production and function at multiple steps adding to the complexity of achieving fully restored CFTR activity. In addition to CFTR genotype, modifier genes and environmental factors influence the variability of CF phenotype and co-morbidities and may contribute to differences in treatment response [9–11].
CFTR MODULATION
POTENTIATORS
The first CFTR modulator to gain approval for clinical use by the U.S. Food and Drug Administration (FDA) was ivacaftor (Kalydeco®, Vertex Pharmaceuticals), a CFTR “potentiator” that works by opening the dysfunctional CFTR channel present at the cell surface (Figure 1). Potentiators work on CFTR protein that has reached the cell membrane; thus, individuals with class III gating mutations and class IV conductance defects are the most likely to benefit from stand-alone potentiator therapy. Two pivotal phase III clinical trials of ivacaftor demonstrated rapid, dramatic, and sustained improvements in lung function [forced expiratory volume in 1 second (FEV1)], weight, quality of life, and biomarkers of CFTR function (sweat chloride), as well as reductions in pulmonary exacerbations in people with at least one copy of the G551D gating mutation [12, 13]. As a result, in 2012, the FDA approved ivacaftor for CF patients ages 6 years and older with the G551D mutation. A 96-week, open-label extension study of ivacaftor confirmed findings from the initial phase III studies and showed persistence of benefits in lung function, weight and quality of life over 3 years [14]. Based on further studies in individuals with other non-G551D class III gating mutations and in young children, ivacaftor has now been approved for those with eight additional gating mutations (G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, or S549R) [15] and for children as young as 2 years of age (ClinicalTrials.gov Identifier: NCT01705145). While gating mutations such as the G551D are present in only about 5% of the U.S. CF population, the dramatic success of ivacaftor proved that rescue of mutant CFTR was possible, paving the way for the development and clinical testing of additional CFTR modulators that target the majority of patients with CF.
Ivacaftor is now approved for use in individuals with the class IV conductance mutation, R117H. A 24-week, randomized, double-blind, placebo-controlled trial of ivacaftor in people ages 6 years and older with the R117H mutation (7T or 5T poly-T tracts) demonstrated decreased sweat chloride, and in patients 18 years of age and older, improved lung function and quality of life [16]. Participants ages 6–17 (n=19) did not experience statistically significant improvements in lung function or quality of life. However, the number of pediatric subjects enrolled was small, and children with the R117H mutation typically have mild disease phenotypes making detection of clinically significant changes more difficult. Phase IV post-approval studies are on-going which will provide additional information about which patients with the R117H may benefit the most from treatment with ivacaftor.
Additionally, a pilot study was recently completed testing the benefit of ivacaftor in CF subjects 12 years and older with phenotypic or molecular evidence of residual CFTR function (ClinicalTrials.gov Identifier: NCT01685801). This study was a randomized, double-blind, placebo-controlled, multiple-within-participant crossover design (N-of-1 trial), used to study subjects with rare CFTR mutations associated with residual function [17]. This study design is a unique model, in which each patient served as their own control, using precision medicine to study rare genetic mutations in a rare disease.
CORRECTORS
In 2015, the first combination corrector/potentiator treatment, lumacaftor/ ivacaftor (Orkambi®, Vertex Pharmaceuticals), was approved for use in patients homozygous for the F508del mutation, ages 12 years and older. CFTR “correctors” work by increasing trafficking of the CFTR protein to the cell membrane (Figure 1). The F508del mutation (a class II mutation with defective trafficking) has been the target of intense research due to its high prevalence within the CF community. Restoring CFTR function in those with the F508del mutation appears to require a combination approach with a corrector to increase the amount of protein at the cell surface plus a potentiator to increase gating of the abnormal CFTR channel. The potentiator, ivacaftor, had been previously studied as monotherapy in patients with the F508del mutation and found to have no substantial effect on CFTR function or clinical outcomes [18]. FDA approval was based on one phase II trial [19] and two phase III randomized, double-blind, placebo-controlled studies of lumacaftor/ivacaftor (n=1,108 subjects) which demonstrated modest but sustained improvements in lung function and decreased rates of pulmonary exacerbations over 24-weeks, along with a good safety profile [20]. Although the approval of a CFTR modulator for the almost 50% of individuals with CF who are homozygous for the F508del mutation was an enormous step forward in the treatment of CF, the clinical gains are modest, and second generation correctors are already in clinical trials with the hope of restoring more CFTR function [21, 22].
READ-THROUGH AGENTS
The CFTR modulator program also includes treatment strategies directed towards suppression of premature termination codons (class I mutations) present in approximately 10% of U.S. CF patients (Figure 1). The discovery that aminoglycoside antibiotics can allow the ribosome to “read-through” premature termination codons resulting in full-length functional protein [23] has led to investigations of the small molecule ataluren (PTC124, PTC Therapeutics). Following a series of phase II trials which demonstrated modest improvements in primary outcomes [24, 25] a large phase III failed to demonstrate a significant improvement in FEV1, the primary endpoint, but did demonstrate a 5.7% increase in lung function compared to placebo in a subset of individuals who were not treated with inhaled tobramycin, which is now believed to alter the efficacy of ataluren [26]. Ataluren is currently being studied in another phase III trial in subjects not taking inhaled tobramycin. Additionally, studies involving synthetic aminoglycoside derivatives and other compounds which suppress premature termination codons are proceeding [27].
GENE THERAPY AND GENE EDITING
Gene therapy has been under investigation since the CF gene was discovered in 1989. Various vectors have been tested for DNA-based gene delivery in CF, including recombinant adenoviral and adeno-associated viral vectors as well as non-viral, synthetic (e.g. plasmid) vectors [28]. The goal of gene therapy is to use a vector to effectively deliver a functional CFTR DNA to the nucleus of the lung epithelial cells, such that a functional CFTR protein can be transcribed in place of the mutated CFTR gene. The largest Phase I/IIa safety and Phase IIb multi-dose gene therapy clinical trials were published in 2015, testing a plasmid-based, non-viral gene therapy vector in CF subjects in the United Kingdom [29, 30]. In this randomized, double-blind, placebo-controlled study, subjects received monthly doses of plasmid-based gene therapy via nebulization for 12 months. At the end of treatment, subjects receiving gene therapy showed stabilization of lung function compared to a decline seen in the placebo group. Overall, treated subjects had a 3.7% improvement in FEV1 compared to placebo. There were no significant toxicities with treatment.
The development of RNA-based recombinant lentivirus vectors and an emerging gene editing technology (e.g. CRISPR/Cas9) have the promise to make the repair of the mutated CFTR gene a future possibility [31–33]. While these systems have not yet been studied clinically in CF, they offer the ultimate opportunity for precision medicine; correcting the specific CFTR mutation in the patient.
TOWARD PERSONALIZED THERAPIES IN CF
While ivacaftor and lumacaftor have proven efficacious in large clinical trials, variable clinical responses were observed in these trials, underscoring the need to screen drugs on an individual level. Researchers are developing model systems, derived from biopsies of airway and intestinal epithelial cells and blood samples from individuals with CF, and recent breakthroughs in stem cell biology now allow for expansion and storage of these cells [34]. In vitro preclinical testing of CFTR modulator drugs can then be performed to select the most effective drugs or combinations for that particular individual (i.e. personalized medicine). The use of primary human airway epithelial cells grown in culture has been exploited successfully in the development of CFTR modulators. For example, the analysis of primary human airway epithelial monolayers derived from a G551D/F508del donor provided the preclinical data to justify the evaluation of ivacaftor in human subjects, despite the absence of efficacy data in an animal model [35]. Because access to bronchial epithelial cells is limited, nasal airway epithelial cells are also being examined as a model system for CFTR therapeutics. Primary nasal epithelial cells share many phenotypic features with human bronchial epithelial cells [36, 37]. Intestinal organoids or ‘enteroids’ grown from stem cells obtained from intestinal biopsy tissue serve as another model system to test CFTR modulators, as activation of CFTR leads to organoid swelling [38]. In addition, circulating monocytes and macrophages are being studied to determine whether these readily available circulating immune cells are a valuable source of cells to investigate CFTR modulators. Being able to screen drug efficacy at the individual level using airway or intestinal cells or blood samples acquired from patients with CF will facilitate personalized medicine in CF.
CONCLUSION
The recent development of CFTR modulators promises to transform the therapeutic landscape in CF in a precision based fashion. FDA approvals of the CFTR modulators ivacaftor and lumacaftor/ivacaftor combination therapy demonstrate the effectiveness of potentiators and correctors in people with specific CF genotypes. Ongoing improvements in modulator therapy as well as advancements in gene therapy and gene editing systems are paving the way for therapies targeting the basic defect in all people with CF. Model systems of airway and intestinal epithelial cells from individuals with CF to test CFTR modulators represent just how customized care could become once a broader range of CFTR drugs becomes available.
KEY POINTS.
CFTR mutations result in different functional protein consequences, ranging from complete protein absence to defective protein activity at the cell surface.
CFTR modulators have been successfully developed and are now approved for use in CF.
Potentiators increase the gating or opening of the mutant CFTR channel on the cell surface while correctors stabilize and chaperone the misfolded CFTR protein to the cell surface.
In vitro preclinical testing of CFTR modulator drugs in airway and intestinal epithelial cells from individuals with CF is being investigated to determine whether this can further personalize the approach to treatment.
Acknowledgments
Acknowledgements
None
Financial support and sponsorship
This review was supported by funding from the Cystic Fibrosis Foundation (MARTIN14A0, SAGEL11CS0) and the National Institutes of Health (K23HL114883).
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
REFERENCES
- 1.Welsh MJR BW, Accurso FJ, Cutting G. Cystic fibrosis. In: Scriver CRB AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th. McGraw-Hill; 2001. pp. 5121–5188. [Google Scholar]
- 2.Anselmo MAL LC. Cystic fibrosis: Overview. In: Taussig LML LI, editor. Pediatric Respiratory Medicine. 2nd. Elsevier Health Sciences; 2008. pp. 845–857. [Google Scholar]
- 3.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 4.Sosnay PR, Siklosi KR, Van Goor F, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cutting GR. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. doi: 10.1038/nrg3849. * This comprehensive review outlines current understanding of CF genetics and variations in phenotype, and highlights how elucidating the underlying disease mechanism is leading to new treatments.
- 6.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 7.Thauvin-Robinet C, Munck A, Huet F, et al. The very low penetrance of cystic fibrosis for the R117H mutation: A reappraisal for genetic counselling and newborn screening. J Med Genet. 2009;46:752–758. doi: 10.1136/jmg.2009.067215. [DOI] [PubMed] [Google Scholar]
- 8.Paranjape SM, Zeitlin PL. Atypical cystic fibrosis and CFTR-related diseases. Clin Rev Allergy Immunol. 2008;35:116–123. doi: 10.1007/s12016-008-8083-0. [DOI] [PubMed] [Google Scholar]
- 9.Cutting GR. Modifier genes in mendelian disorders: The example of cystic fibrosis. Ann N Y Acad Sci. 2010;1214:57–69. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borowitz D, Durie PR, Clarke LL, et al. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 11.Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration. 2000;67:117–133. doi: 10.1159/000029497. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKone EF, Borowitz D, Drevinek P, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the G551D-CFTR mutation: A phase 3, open-label extension study (persist) Lancet Respir Med. 2014;2:902–910. doi: 10.1016/S2213-2600(14)70218-8. ** This open-label extension study of adults and children with the G551D mutation treated with ivacaftor for 96 weeks demonstrated durable improvements in lung function and weight in those who had previously received ivacaftor, and improvements similar to those seen in the placebo-controlled trials in participants initiating ivacaftor. This study supported findings from the earlier placebo-controlled phase 3 trial of ivacaftor and provided reassurance about safety and continued efficacy.
- 15. De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. ** This paper reports results from the Konnection study , a two-part, double-blind, placebo-controlled crossover study of ivacaftor in participants ages 6 and older with a non-G551D gating mutation followed by a 16-week open-label extension. Over 8 weeks of treatment, participants on ivacaftor had significant improvements in lung function (FEV1), body-mass index and quality of life (CFQ-R) scores. These improvements were maintained over 24 weeks. This study lead to FDA approval of ivacaftor for people with the non-G551D gating mutations G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, and S549R.
- 16. Moss RB, Flume PA, Elborn JS, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117his-CFTR mutation: A double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–533. doi: 10.1016/S2213-2600(15)00201-5. ** This paper reports results from a 24-week, placebo-controlled, double-blind, randomized clinical trial of ivacaftor in participants with CF ages 6 and older with the residual function mutation, R117H. Treatment with ivacaftor resulted in significant decreases in sweat chloride, improvements in quality of life (CFQ-R) scores and improvement in lung function (FEV1) in a subgroup analysis of participants 18 years and older. This study lead to the FDA approval for ivacaftor in people with the R117H mutation ages 6 and older.
- 17. Nick JA RD, St Clair C, Jones MC, Li H, Higgins M Study team T. Effect of ivacaftor in patients with cystic fibrosis, residual CFTR function, and FEV1 ≥ 40% of predicted, n-of-1 study. Pediatr Pulmonol. 2014;(Supplement 38):A196. * Abstract describing N-of-1 trial design testing ivacaftor in subjects with rare, partial function mutations, in which subjects were used as their own controls.
- 18.Flume PA, Liou TG, Borowitz DS, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142:718–724. doi: 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyle MP, Bell SC, Konstan MW, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: A phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–538. doi: 10.1016/S2213-2600(14)70132-8. * This phase 2 study provided the safety and efficacy data needed to support phase 3 randomized clinical trials of the combination CFTR modulator, lumacaftor/ivacaftor for people with CF homozygous for the F508del mutation.
- 20. Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMc1510466. ** This paper reports results from the pivotal phase 3 trials of ivacaftor/lumacaftor in people homozygous for the F508del muation, TRAFFIC and TRANSPORT. Both studies met their primary input of lung function (FEV1) improvement over 24 weeks and showed significantly decreased risk of pulmonary exacerbations in those on lumacaftor/ivacaftor. These studies led to FDA approval of lumacaftor/ivacaftor for people ages 12 and older homozygous for the F508del mutation.
- 21. Davies JC. The future of CFTR modulating therapies for cystic fibrosis. Curr Opin Pulm Med. 2015;21:579–584. doi: 10.1097/MCP.0000000000000211. * This review summarizes current CFTR modulator therapies and highlights areas of unmet need including expanding treatment to all patients regardless of genetic mutations, continued long-term surveillence studies of modulators to confirm safety and disease modification, improving equity of access to treatment and a focus on early prevention.
- 22.Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: Cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol. 2015;50(Suppl 40):S3–S13. doi: 10.1002/ppul.23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedwell DM, Kaenjak A, Benos DJ, et al. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat Med. 1997;3:1280–1284. doi: 10.1038/nm1197-1280. [DOI] [PubMed] [Google Scholar]
- 24.Kerem E, Hirawat S, Armoni S, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: A prospective phase II trial. Lancet. 2008;372:719–727. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- 25.Sermet-Gaudelus I, Boeck KD, Casimir GJ, et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am J Respir Crit Care Med. 2010;182:1262–1272. doi: 10.1164/rccm.201001-0137OC. [DOI] [PubMed] [Google Scholar]
- 26. Kerem E, Konstan MW, De Boeck K, et al. Ataluren for the treatment of nonsense-mutation cystic fibrosis: A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2:539–547. doi: 10.1016/S2213-2600(14)70100-6. * Randomized, double-blind, placebo-controlled clinical trial testing a "read-through" agent, ataluren, in patients ages 6 and older with nonsense CFTR mutations for 48 weeks. Results revealed no signficant improvement in lung function in the overall treated population, though there was evidence it may be beneficial in patients not taking chronic inhaled tobramycin.
- 27. Xue X, Mutyam V, Tang L, et al. Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor. Am J Respir Cell Mol Biol. 2014;50:805–816. doi: 10.1165/rcmb.2013-0282OC. * Study evaluating a novel synthetic aminoglycoside derivative developed for premature termination codon suppression showing evidence of improved CFTR activity in a series of complementary CFTR nonsense mutation CF models.
- 28. Gill DR, Hyde SC. Delivery of genes into the CF airway. Thorax. 2014;69:962–964. doi: 10.1136/thoraxjnl-2014-205835. * Review article describing various potential gene delivery vectors available and under study for gene transfer into the CF lung.
- 29. Alton EW, Armstrong DK, Ashby D, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:684–691. doi: 10.1016/S2213-2600(15)00245-3. ** Largest randomized, double blind, placebo-controlled clinical trial of gene therapy delivered via inhalation in CF in ages 12 and older. Results showed a modest difference in lung function compared to controls due to FEV1 stabilization in the treatment arm compared to a decline in lung function in the placebo arm over 1 year.
- 30. Alton EW, Boyd AC, Porteous DJ, et al. A phase I/IIa safety and efficacy study of nebulized liposome-mediated gene therapy for cystic fibrosis supports a multidose trial. Am J Respir Crit Care Med. 2015;192:1389–1392. doi: 10.1164/rccm.201506-1193LE. * This letter to the editor provided data that supported and informed the design of the phase 2b trial detailed in reference 29.
- 31.Griesenbach U, Inoue M, Meng C, et al. Assessment of f/hn-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am J Respir Crit Care Med. 2012;186:846–856. doi: 10.1164/rccm.201206-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by crispr/cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Bellec J, Bacchetta M, Losa D, et al. CFTR inactivation by lentiviral vector-mediated RNA interference and crispr-cas9 genome editing in human airway epithelial cells. Curr Gene Ther. 2015;15:447–459. doi: 10.2174/1566523215666150812115939. [DOI] [PubMed] [Google Scholar]
- 34. Ikpa PT, Bijvelds MJ, de Jonge HR. Cystic fibrosis: Toward personalized therapies. Int J Biochem Cell Biol. 2014;52:192–200. doi: 10.1016/j.biocel.2014.02.008. * This review summarizes the development of mutation-specific and personalized therapies for CF and describes the use of in vitro preclinical assays to test CFTR modulators on an individual basis.
- 35.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDougall CM, Blaylock MG, Douglas JG, et al. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Courcey F, Zholos AV, Atherton-Watson H, et al. Development of primary human nasal epithelial cell cultures for the study of cystic fibrosis pathophysiology. Am J Physiol Cell Physiol. 2012;303:C1173–C1179. doi: 10.1152/ajpcell.00384.2011. [DOI] [PubMed] [Google Scholar]
- 38.Dekkers JF, Wiegerinck CL, de Jonge HR, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]