Abstract
Several studies have demonstrated low rates of local recurrence with brachytherapy-based accelerated partial breast irradiation (APBI). However, long-term outcomes on toxicity (e.g. telangiectasia) and cosmesis remain a major concern. The purpose of this study is to investigate the dosimetric feasibility of using targeted non-toxic radiosensitizing gold nanoparticles (GNPs) for localized dose enhancement to the planning target volume (PTV) during electronic brachytherapy APBI while reducing normal tissue toxicity. We propose to incorporate GNPs into a micrometer-thick polymer film on the surface of routinely used lumpectomy balloon applicators and provide subsequent treatment using a 50 kVp Xoft device. An experimentally determined diffusion coefficient was used to determine space-time customizable distribution of GNPs for feasible in-vivo concentrations of 7 mg/g and 43 mg/g. An analytical approach from previously published work was employed to estimate the dose enhancement due to GNPs as a function of distance up to 1 cm from the lumpectomy cavity surface. Clinically significant dose enhancement values of at least 1.2, due to 2 nm GNPs, were found at 1 cm away from the lumpectomy cavity wall when using electronic brachytherapy APBI. Higher customizable dose enhancement was also achieved at other distances as a function of nanoparticle size. Our preliminary results suggest that significant dose enhancement can be achieved to residual tumor cells targeted with GNPs during APBI with electronic brachytherapy.
Keywords: APBI, Dose enhancement, Gold, Nanoparticles, 50 kVp, Xoft electronic brachytherapy
Introduction
Breast cancer is the second leading cause of death among women in the World. According to the National Breast Cancer Foundation, one in eight women will be diagnosed with breast cancer in their lifetime. The treatment of breast cancer may include surgery, chemotherapy, radiotherapy (RT), etc. or combinations of these treatments. However, the primary treatment for early stage breast cancer is breast conserving surgery (BCS) [1]. Meanwhile studies revealed that the majority of breast cancer recurrences arise at or near the primary tumor site, around the lumpectomy cavity [2]; therefore radiotherapy is administered after BCS in order to kill any remaining cancerous cells around this cavity. This has led to the development and increasing use of Accelerated Partial Breast Irradiation (APBI) [3], with the prescribed dose delivered only to the lumpectomy cavity vicinity. Compared with whole breast irradiation (WBI), APBI is attractive because the number of treatment fractions is decreased by increasing (accelerating) the dose delivered per treatment fraction, which is relatively more convenient. While traditional external beam radiotherapy takes about 6–8 weeks, APBI delivers the whole course of radiation dose in only 5–7 days and the treatment may begin anywhere from 2 to 21 days post BCS [3].
APBI can be performed either as external beam RT (EBRT) or internal RT (brachytherapy). Currently, brachytherapy APBI is typically applied with the use of a balloon applicator [4,5]. To this day, there is very little information on normal tissue toxicity during APBI, and most information available is on multiple catheter interstitial brachytherapy [6]. Although APBI has the advantage of improving patient and staff convenience [2], recent studies showed higher long-term complications, such as normal tissue toxicity [7] and local recurrences [7], compared to WBI [8]. Therefore, there is a need to develop new approaches to address these limitations.
Many studies [9] have shown that introducing high atomic number (Z) contrast materials during radiotherapy could substantially increase the local dose deposition in the tumor volume due to the high photoelectric interaction cross-section of high-Z materials [10,11]. In recent years, due to its biocompatibility [12] and non-toxicity [7], several authors have focused on investigating the use of gold nanoparticles to enhance cancer treatment [9,13] effectively demonstrating significant local dose enhancement in the presence of gold nanoparticles. The dose enhancement due to interactions of low energy photons with high-Z materials is expected to be much higher than those of high energy photons. This is due to higher probability of photoelectric interaction of low energy photons with high-Z materials. As a result of this, a more significant dose enhancement is predicted to be achieved with brachytherapy sources, relative to EBRT. For the first time, in this work we investigated the dosimetric feasibility of using such gold nanoparticles as localized radiosensitizers to boost the dose to the planning target volume (PTV) with residual tumor cells during APBI, while minimizing the dose to surrounding healthy tissue. Such a new approach has potential to help reduce long-term adverse outcomes on toxicity (e.g. telangiectasia) and cosmesis.
Methods
When the 50 kVp Xoft device is chosen for brachytherapy APBI following BCS, the balloon applicator is inserted into the lumpectomy cavity and the irradiation can be performed twice a day for 5–7 days [14]. Here, we propose to deliver GNPs via in-situ release from a GNP-loaded polymer film that is coated on the surface of the balloon applicator (Fig. 1a). Our hypothesis is that, once the Xoft balloon applicator is placed inside the lumpectomy cavity, the polymer film will begin to biodegrade, releasing GNPs in situ. As the GNPs release in tissue near lumpectomy cavity surface, the GNPs will begin to diffuse further away from the lumpectomy surface, while being irradiated by 50 kVp Xoft device subsequently. The spectra for the 50 kVp Xoft electronic brachytherapy device can be found in previous literature [15].
Figure 1.
(a) A schematic diagram of the distribution of GNPs in lumpectomy cavity surface from Xoft balloon applicator. (b) The distribution of GNPs in tissue toward residual tumor cells.
In order to calculate the dose enhancement to the target volume, the diffusion profiles of 2 and 10 nm GNPs as a function of time and position were first determined. To this end, a previous experimentally determined diffusion coefficient, D = 2.2 × 10−8 cm2 s−1 for 10 nm nanoparticles [16] was employed to calculate the value of the same coefficient but for 2 nm size nanoparticles by using the Stokes–Einstein equation (Eq. 1):
| (1) |
where, kB is Boltzmann’s constant, T is the absolute temperature, η is the dynamic viscosity of the medium and r is the radius of the nanoparticles. Stokes–Einstein equation is used to calculate the diffusion coefficient of a spherical particle moving in liquid based on the forces acting on it. We assumed that the Stokes–Einstein relation for the diffusion coefficient of nanoparticles is valid in tissue media, and that the mean viscosity is constant in the particle size and concentration ranges considered in this paper. Based on this, the concentration of GNPs at any time and at any point inside the target volume was calculated by using one dimensional solution of Fick’s second law diffusion:
| (2) |
Here, C0 is the initial concentration at anywhere in the tissue prior the burst release (considered zero), CS is the concentration on the lumpectomy cavity surface (considered Cs = 7 mg/g for case I and 43 mg/g for case II). C(x, t) is the concentration as a function of distance (x) from the surface of the lumpectomy cavity over time (t), and erf is the error function, which describes the probability of the magnitude by which the measured results deviated from the mean. The diffusion of GNPs from the lumpectomy cavity surface to the target volume is illustrated in Fig. 1b. The number of gold nanoparticles interacting with photons in the target area depends on the initial concentration as well as the diffusion rate of the nanoparticles. In this work, we considered two initial GNP concentrations (C0): 7 mg/g and 43 mg/g for a lumpectomy cavity size of 2 cm in diameter. An in-vivo animal study showed that there are no toxic side effects of GNPs when used with a 7 mg/g concentration [17]. In addition, we used 43 mg/g GNP concentration since it is the FDA approved concentration of cisplatin, which is relatively more toxic than gold [18].
We hypothesize that localized dose boost to tumor cells will result from micrometer ranged photo-/Auger electrons emitted from the high-Z GNPs due to the interactions with low energy photons during APBI. The calculated dose enhancement factor (DEF) is defined as the ratio of dose to each tumor voxel with and without GNPs. Physically, for example, if the DEF is 2, it means the delivered dose in the presence of GNPs is doubled (or 100% higher) compared to dose without GNPs. In order to calculate the DEF in the presence of GNPs, we employed an analytical calculation method, which was used in a previously published work [19,20]. Briefly, in this approach a tumor voxel is modeled as a slab of 10 μm × 10 μm × 10 μm, representing a sub-volume containing a tumor cell of diameter 10 μm. The energy deposited by an emitted electron, E, is calculated by Cole’s electron energy loss formula [21] (Eq. 3):
| (3) |
Here R = Rtot – r, where r is the distance from the photoelectron emission site and Rtot is the total range of the photoelectron (Eq. 4).
| (4) |
By integrating Eq. 4 over the range of emitted electron energies, the total energy deposited in a tumor sub-volume was calculated.
In DEF calculations, the GNPs at equal distances away from the surface of the balloon applicator are assumed to be uniformly distributed throughout the tumor cells. Therefore, the specific location of the nanoparticle in the tumor sub-volume does not alter the value of DEF. The consequence of this assumption and that of the burst release will be addressed in the discussion section. Due to the short range of photo/Auger electrons emitted by GNPs, the dose enhancement is expected to be localized almost entirely within the planning target volume (PTV) containing any residual tumor cells. This could allow for sub-volume dose boosting while sparing the dose to surrounding healthy tissue.
Results
Diffusion profiles of 2 and 10 nm GNPs for 1, 3, 5 and 7 days, up to 1 cm away from the lumpectomy cavity are shown in Fig. 2a and b, respectively. The diffusion rate of GNPs significantly depends on the GNP size; 2 nm GNPs diffuse faster compared to 10 nm GNPs. Results are only shown for up to 7 days post injection, which is the typical duration of APBI treatment [22]. The ability to customize or program the position and concentration of GNPs (e.g. based on nanoparticle size) at a desired time could allow the optimization of treatment to maximize dose only to areas close to the lumpectomy cavity while minimizing dose to healthy tissue.
Figure 2.
Diffusion profiles for (a) 2 nm, (b) 10 nm GNPs, up to 1 cm at different times and positions.
Figure 3 shows DEF values 7 days post administration of GNPs for 50 kVp electronic brachytherapy. DEF values for 7 mg/g and 43 mg/g GNP concentrations of 2 and 10 nm GNPs up to 1 cm away from lumpectomy cavity surface with 1 mm increments are shown in Fig. 3. Since 2 nm GNPs diffuse faster than 10 nm GNPs, DEF values are higher for 2 nm GNPs. As expected, locations closer to lumpectomy cavity yielded greater DEF values compared to distal sites. Significant DEF values (DEF > 1.2) with 43 mg/g concentration of 2 and 10 nm GNPs were found at 10 mm and 4 mm, respectively.
Figure 3.
Semi-log plot of DEF vs. distance for 2 and 10 nm GNPs, with irradiation performed 7 days post injection using a 50 kVp Xoft electronic brachytherapy device with 7 mg/g and 43 mg/g GNP concentrations.
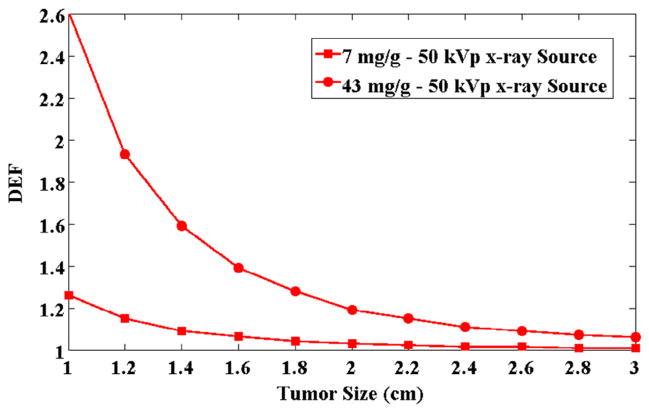
The DEF values for a range of tumor volume sizes for 7 mg/g and 43 mg/g irradiated with 50 kVp Xoft source are presented in Fig. 4. As the balloon applicators may need to be expended to varying sizes depending on each patient’s needs, which may not be known prior to GNP coating, the concentration per unit volume may also change. As the considered lumpectomy cavity volume increases, the average GNP concentration reduces, and hence the average DEF decreases.
Figure 4.
DEF values with respect to different tumor sizes for 7 mg/g and 43 mg/g concentrations of 2 nm GNPs irradiated with 50 kVp Xoft electronic brachytherapy device at 1 cm away from the lumpectomy cavity.
Discussion
In this study, the dose enhancement in the presence of GNPs to a PTV expected to contain remaining tumor cells after BCS was calculated for 50 kVp electronic brachytherapy source. The analysis of dose enhancement with respect to two initial GNP concentrations and their corresponding diffusion profiles, along with two different GNP sizes, shows that significant DEF can be achieved and customized as a function of nanoparticle size.
Previous clinical studies showed that radiation dose boosting can help prevent cancer recurrence [23]. Our preliminary results in this work indicate that, when sufficient concentrations are used, the number of GNPs diffused to tumor sub-volumes within the first 7 days could potentially result in significant DEF values. Moreover, since the range of photo- and Auger electrons is only in micrometer distances, the resulting dose boost is expected to occur in the near proximity of nanoparticles. Therefore, the biodistribution of GNPs from the lumpectomy cavity to distal areas is an important parameter in the efficacy of such nanoparticle-aided APBI.
The analytical approach used to calculate the DEF values in this work was validated in comparison with the Monte Carlo results of previous publications, which had shown significant dose enhancement. In previously published work by Berbeco et al. [24],, results of both Monte Carlo and analytical approach were compared and found remarkably similar (~>90%). A recent study by Sinha et al. [20] considered the release of GNPs from the surface of a brachytherapy spacer using the same analytical approach, and also showed significant DEF values similar to the ones presented here. In each of these studies, the GNPs were assumed to start diffusing immediately upon administration. However, for case 1, in practice, GNPs will be incorporated into a polymer film, and their release rate from such films must be accounted for. The release can be programmed or customized by modifying the polymer film parameters, such as polymer weight or type.
The release of GNPs within tumor sub-volumes may not be homogenous, resulting in spatial variations of the DEF values. Nevertheless, previous studies show that the biodistribution and diffusion of nanoparticles are not homogeneous [16]. Some nanoparticles tend to accumulate around blood vessels whereas others tend to penetrate inside cells and stop diffusing. As a first step, this study investigates the feasibility of this approach in the presence of nanoparticles homogeneously distributed as opposed to an unknown inhomogeneous distribution. Moreover, specific locations of GNPs were not considered in the tumor sub-volume or the cell. Therefore, compared to the values presented here, the actual dose enhancement to each cell may be different on a small scale. The dose enhancement to high-value targets, e.g., the nucleus, is expected to be higher for GNPs closer to the target and lower for GNPs farther away from the target. A recent study [25] shows that nuclear targeting with nanoparticles is achievable and could thus be employed here to maximize damage to the tumor cells DNA.
In general, a number of possible methods exist that can be employed to improve targeting of tumor cells. Functionalization of GNPs to specifically target tumor cells could provide significant amount of dose boost to tumor cells while sparing the healthy tissue. Increased radiation dose to tumor cells would permit the decrease of primary radiation, resulting in lower exposure to normal tissue. This would potentially help overcome long-term side effects currently associated with APBI.
Furthermore, as mentioned previously, APBI may begin anywhere from 2 to 21 days after BCS [3]. In this work, DEF values were calculated assuming APBI was performed immediately after the BCS. If GNPs were administrated inside the lumpectomy cavity at the end of the surgery, and APBI was chosen to be performed several days later, GNPs could diffuse farther in the tumor sub-volume. This mechanism could potentially lead to the presence of higher number of GNPs released from the initial location (e.g. lumpectomy cavity surface), resulting in higher DEF values. Hence this would provide more flexibility for customizing the DEF. However, such an approach may require significant changes in current treatment protocols and likely would need approval/consent from patients.
Although two justifiable concentrations are considered in this work, the maximum amount of GNPs that can be incorporated with polymer films is yet to be determined experimentally. These initial concentrations may change depending on the GNP loading capacity of polymer films. Consequently, clinically achievable GNP concentrations in humans may yield DEF values different from those presented here. However, our results demonstrate feasibility providing valuable insight for planning experimental investigations.
Conclusion
Our preliminary results suggest that significant dose enhancement can be achieved to residual tumor cells targeted with GNPs during APBI with electronic brachytherapy. The findings motivate further studies, including experimental work toward development of nanoparticle-aided APBI as one approach that can potentially address current normal tissue/skin toxicity and cosmesis limitations to APBI.
Acknowledgments
The authors acknowledge funding support from NIH/NCI 1 K01 CA172478-01
References
- 1.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Jagsi R, Haffty BG. External-beam accelerated partial-breast irradiation: exploring the limits of tolerability. J Clin Oncol. 2013;31:4029–31. doi: 10.1200/JCO.2013.51.1717. [DOI] [PubMed] [Google Scholar]
- 3.Lori L, Vanyo MD. Accelerated Partial Breast Irradiation (APBI) The Robert and Beverly Lewis Family Cancer Care Center; 2009. [Google Scholar]
- 4.Ivanov O, Dickler A, Lum BY, Pellicane JV, Francescatti DS. Twelve-month follow-up results of a trial utilizing Axxent electronic brachytherapy to deliver intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol. 2011;18:453–8. doi: 10.1245/s10434-010-1283-x. [DOI] [PubMed] [Google Scholar]
- 5.Dickler A, Ivanov O, Francescatti D. Intraoperative radiation therapy in the treatment of early-stage breast cancer utilizing xoft axxent electronic brachytherapy. World J Surg Oncol. 2009;7:24. doi: 10.1186/1477-7819-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wazer DE, Kaufman S, Cuttino L, DiPetrillo T, Arthur DW. Accelerated partial breast irradiation: an analysis of variables associated with late toxicity and long-term cosmetic outcome after high-dose-rate interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2006;64:489–95. doi: 10.1016/j.ijrobp.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12:2313–33. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith GL, Xu Y, Buchholz TA, Giordano SH, Jiang J, Shih YC, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827–37. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngwa W, Kumar R, Sridhar S, Korideck H, Zygmanski P, Cormack RA, et al. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine (Lond) 2014;9:1063–82. doi: 10.2217/nnm.14.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Liu X, Jin X, He P, Zheng X, Dai Z, et al. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low-and high-LET radiations. Phys Med. 2015;31:210–18. doi: 10.1016/j.ejmp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Gehrke C, Oates R, Ramachandran P, Deloar HM, Gill S, Kron T. Automatic tracking of gold seed markers from CBCT image projections in lung and prostate radiotherapy. Phys Med. 2015;31:185–91. doi: 10.1016/j.ejmp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Grant SA, Spradling CS, Grant DN, Fox DB, Jimenez L, Grant DA, et al. Assessment of the biocompatibility and stability of a gold nanoparticle collagen bioscaffold. J Biomed Mater Res A. 2014;102:332–9. doi: 10.1002/jbm.a.34698. [DOI] [PubMed] [Google Scholar]
- 13.Elbialy NS, Fathy MM, Khalil WM. Preparation and characterization of magnetic gold nanoparticles to be used as doxorubicin nanocarriers. Phys Med. 2014;30:843–8. doi: 10.1016/j.ejmp.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Park CC, Yom SS, Podgorsak MB, Harris E, Price RA, Jr, Bevan A, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) Emerging Technology Committee report on electronic brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:963–72. doi: 10.1016/j.ijrobp.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 15.Poludniowski G, Landry G, DeBlois F, Evans PM, Verhaegen F. SpekCalc: a program to calculate photon spectra from tungsten anode x-ray tubes. Phys Med Biol. 2009;54:N433–8. doi: 10.1088/0031-9155/54/19/N01. [DOI] [PubMed] [Google Scholar]
- 16.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–31. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/ x-ray sources. Phys Med Biol. 2009;54:4889–905. doi: 10.1088/0031-9155/54/16/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [accessed 03.14];FDA-Database. 2010 < http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018057s080lbl.pdf>.
- 19.Ngwa W, Makrigiorgos GM, Berbeco RI. Gold nanoparticle-aided brachytherapy with vascular dose painting: estimation of dose enhancement to the tumor endothelial cell nucleus. Med Phys. 2012;39:392–8. doi: 10.1118/1.3671905. [DOI] [PubMed] [Google Scholar]
- 20.Sinha N, Cifter G, Sajo E, Kumar R, Sridhar S, Nguyen PL, et al. Brachytherapy application with in situ dose painting administered by gold nanoparticle eluters. Int J Radiat Oncol Biol Phys. 2015;91(2):385–92. doi: 10.1016/j.ijrobp.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole A. Absorption of 20-eV to 50,000-eV electron beams in air and plastic. Radiat Res. 1969;38:7–33. [PubMed] [Google Scholar]
- 22.Sanders ME, Scroggins T, Ampil FL, Li BD. Accelerated partial breast irradiation in early-stage breast cancer. J Clin Oncol. 2007;25:996–1002. doi: 10.1200/JCO.2006.09.7436. [DOI] [PubMed] [Google Scholar]
- 23.Kuban DA, Levy LB, Cheung MR, Lee AK, Choi S, Frank S, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79:1310–17. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Berbeco RI, Ngwa W, Makrigiorgos GM. Localized dose enhancement to tumor blood vessel endothelial cells via megavoltage X-rays and targeted gold nanoparticles: new potential for external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:270–6. doi: 10.1016/j.ijrobp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Uertz J, Yohan D, Chithrani BD. Peptide modified gold nanoparticles for improved cellular uptake, nuclear transport, and intracellular retention. Nanoscale. 2014;6:12026–33. doi: 10.1039/c4nr02535k. [DOI] [PubMed] [Google Scholar]