Abstract
INTRODUCTION
Patient-reported outcome (PRO) measures serve to capture vital patient information not otherwise obtained by primary study endpoints. This paper examines how PROs are utilized as endpoints in industry-sponsored metastatic breast cancer clinical trials.
METHODS
A search was conducted in the clinicaltrials.gov web site for trials involving common treatments for metastatic breast cancer. Thirty-eight clinical trials were identified which included a PRO endpoint in the study, and data were extracted and summarized.
RESULTS
Overall, 17 unique PRO questionnaires and 14 concepts of measurement were identified as secondary or exploratory endpoints. The Functional Assessment of Cancer Therapy—Breast was the most frequently utilized questionnaire, commonly implemented to assess quality of life. The EORTC QLQ-C30 was also frequently used to measure quality of life or pain.
CONCLUSION
This review shares insights into the role of PROs in trials for metastatic breast cancer from which treatment developers and other stakeholders can enhance successful implementation of the patient voice into future trials.
Keywords: metastatic breast cancer, patient-reported outcomes, treatment effectiveness, endpoint development, clinical trials
Introduction
New cases of breast cancer in the United States during 2015 are projected to total 234,190, resulting in 40,730 deaths. About 61% of patients with breast cancer are diagnosed with localized (also known as Stage I) disease, while 32% are diagnosed with regional disease, in which the cancer has spread to regional lymph nodes.1 From 2005 to 2011, 98.6% of patients diagnosed with localized breast cancer achieved a five-year relative survival, as did 84.9% of patients diagnosed with regional disease. Distant disease (also known as Stage IV breast cancer) involves distant metastases that have spread to other areas of the body (eg, bones, lungs, liver, and brain). Patients diagnosed with Stage IV breast cancer exhibit a higher rate of mortality, with only 25.9% achieving a five-year relative survival between 2005 and 2011.2,3
Treatment for patients with advanced disease includes neoadjuvant chemotherapy, surgery, postsurgical radiation therapy, and systemic adjuvant therapy (including hormone therapy for those with hormone receptor-positive breast cancers).4 Breast cancers that are estrogen receptor positive (ER+) and human epidermal growth factor receptor-2 positive (HER2+) are treated with agents that target these receptors (such as tamoxifen, toremifene, and fulvestrant for ER+ breast cancers and trastuzumab, pertuzumab, and lapatinib for HER2+ breast cancers) in order to slow or stop the growth of cancer cells.5,6 ER+ breast cancers can also be treated with aromatase inhibitors, which aim to lower estrogen levels. Breast cancers that are triple negative (ie, negative for estrogen, progesterone, and human epidermal growth factor receptors) are treated with chemotherapy agents (such as anthracyclines, taxanes, and cyclophosphamide), as targeted therapies have not yet proven to be as effective; however, research is still underway.7 For patients with advanced breast cancer, current standard treatments can extend survival and improve quality of life, but rarely are curative.
Patients with breast cancer experience various symptoms, which can include swelling of all or part of the breast; breast or nipple pain; skin irritation or dimpling; and redness, scaliness, or thickening of nipple or breast skin. Both the symptoms of breast cancer and side effects associated with treatment can have a negative, sometimes debilitating, impact on the quality of life of patients, affecting such things as physical activity, psychological well-being, relationships, and family life. Patient-reported outcome (PRO) measures have been increasingly used in oncology in general, and specifically in advanced/metastatic breast cancer clinical trials, for the purpose of documenting patients’ symptoms, treatment side effects, and quality of life, since these are not observable and can only be reported by patients. A PRO is a measurement based on a report that comes directly from a patient (ie, study subject), regarding the patient’s health status and experience, without amendment or interpretation by a clinician or anyone else.8 Despite the increasing use of PROs as a means of gaging overall treatment benefit, these are usually relegated to secondary or exploratory study endpoints in favor of objective measures, such as survival or tumor size reduction. Indeed, PROs capture vital patient information not otherwise obtained by many common primary study endpoints, and as such, PROs play an important complementary role to primary endpoints.
To our knowledge, there has been no review to date of how PROs are being utilized in breast cancer clinical trials. Brim and Pearson9 documented the percentage of Phase 3 breast cancer clinical trials through 2011 that included PROs and the types of PRO measure used, but their article does not describe how these PROs were implemented and utilized to support primary endpoints. Information related to how PROs are being utilized in breast cancer trials could help drug developers understand and make decisions regarding the implementation of PRO instruments in their own trials. The purpose of this paper is to address this gap by examining how PROs are utilized in industry-sponsored metastatic breast cancer trials registered in the clinicaltrials.gov database.
Methods
To ensure a targeted approach when identifying relevant PRO measures, an a priori list of 24 of the most common advanced breast cancer treatments approved by the Food and Drug Administration (FDA) or in development was created. The identified treatments were selected from the major treatment classes for advanced or metastatic breast cancer: hormonal agents for the ER+ patient population, HER2-targeted agents for the HER2+ patient population, and chemotherapy for the triple-negative patient population, as well as new classes of drugs such as cyclin-dependent kinase (CDK) 4/6 inhibitors and phosphoinositide 3-kinase (PI3K) inhibitors. Treatment class determination was, in part, informed by the National Comprehensive Cancer Network Clinical Practice Guidelines.10
A search was then conducted using the clinicaltrials.gov web site on June 25, 2015, through July 29, 2015. The searches identified Phase 2 or 3 clinical trials of the 24 selected metastatic breast cancer treatments. The following search terms were used for each of three search categories: Condition = metastatic breast cancer, Intervention = [name of breast cancer therapy], and Phase = Phase 2 or Phase 3.
Clinical trials were excluded from analysis if the breast cancer treatment served only as a comparator to an investigational drug outside of the 24 selected, if the trial was withdrawn or terminated prior to completion, if no PRO was included as an endpoint in the trial, or if the trial was being sponsored by an independent Principal Investigator or nonindustry research organization.
Clinical trials identified by our search were reviewed, and the information provided was extracted and summarized, as available (see Table 1 for a listing of all information included in the clinicaltrials.gov web site). The information selected for review from the clinicaltrials.gov web site is shown in Table 2.
Table 1.
Information contained on ClinicalTrials.gov web site.
| CLINICALTRIALS.GOV LABEL | CONTENTS |
|---|---|
| Study start date | Start date for clinical trial |
| Study end date | Final data collection date for primary outcome measure |
| Current primary outcome measures | Primary endpoints* |
| Current secondary outcome measures | Secondary endpoints* |
| Current other outcome measures | Other endpoints (e.g. exploratory)* |
| Obcbcr-10-2016-093cial title | Title of clinical trial |
| Brief summary | Brief description of the clinical trial |
| Study phase | Phase 2 or Phase 3 |
| Study design | Description of clinical trial design and methodology |
| Treatment arms and interventions | Description of all treatment arms and associated treatment(s) used in clinical trial |
| Recruitment status | Whether trial is still currently recruiting |
| Eligibility criteria | Description of clinical trial study population |
| Gender | |
| Ages | |
| NCT number | Clinical trial identification code |
| Study sponsor | Clinical trial sponsor |
| Results | Description of clinical trial results, if available |
Note:
Endpoints often include the assessment measured and also may include a definition of clinical significance, and a full description of what data are collected and how they are analyzed to support a specific study objective.
Table 2.
Information from the clinicaltrials.gov web site evaluated as part of this review.
| TREATMENT NAME (GENERIC, BRAND, AND/OR INVESTIGATIONAL) |
|---|
| Clinical trial title |
| Clinical trial sponsor |
| clinicaltrials.gov identifier |
| Study phase |
| Start and end dates |
| Current recruitment status |
| PROs used in the clinical trial (name, goal, administration methods) |
| Breast cancer population included in the clinical trial |
| Clinical trial endpoints (primary, secondary, other) |
| PRO measurement concepts evaluated in the clinical trial |
| Definition of clinical significance |
| Statistical analysis methods, scoring, and interpretation |
| Additional notes (clinical trial design and methodology, etc.) |
Results
A search of clinicaltrials.gov for the selected treatments yielded a total of 1,634 clinical trials. Upon review, 74 were identified as unique trials for one of the selected treatments and also utilized a PRO endpoint. Subsequently, an additional 36 clinical trials were excluded because they were not pharmaceutical industry sponsored. The remaining 38 clinical trials were reviewed in full, and data were extracted and summarized (see Table 3).
Table 3.
Search results of clinical trials in clinicaltrials.gov for selected treatments organized by treatment class.
| TREATMENT CLASS | GENERIC TREATMENT NAME | INVESTIGATIONAL/BRAND TREATMENT NAME | CLINICAL TRIALS IN WHICH TREATMENT MENTIONED*,† | CLINICAL TRIALS WITH TREATMENT MENTION INCLUDING PRO DATA† | CLINICAL TRIALS WITH PROs REVIEWED IN FULL‡ |
|---|---|---|---|---|---|
| Hormonal therapies for ER+ (n = 6) | Anastrozole | NCT01602380/Arimidex® | 51 | 9/51 | 1/9 |
| Everolimus | Afinitor®; Afinitor Disperz™ | 55 | 4/55 | 2/4 | |
| Exemestane | Aromasin®, FCE-24304 | 114 | 14/114 | 2/14 | |
| Fulvestrant | Faslodex®, ZD9238 | 74 | 4/74 | 1/4 | |
| Letrozole | DB01005/Femara® | 62 | 15/62 | 1/15 | |
| Tamoxifen | Tamoxifen citrate, Nolvadex®, Soltamox® | 193 | 19/193 | 1/19 | |
| Targeted agents for HER2+ (n = 4) | Lapatinib | Tyverb™, GW572016, GSK572016 | 86 | 10/86 | 5/10 |
| Pertuzumab | Perjeta®, 2C4 | 25 | 6/25 | 4/6 | |
| Trastuzumab emtansine | T-DM1, Kadcyla® | 18 | 2/18 | 1/2 | |
| Trastuzumab | Herceptin®, SU011248, Herclon™ | 223 | 13/223 | 1/13 | |
| Chemotherapies for ER−, PR−, and/or HER2− (n = 5) | Capecitabine | Xeloda® | 162 | 10/162 | 1/10 |
| Docetaxel | XRP6976, Taxotere®, Docefrez™ | 147 | 13/147 | 1/13 | |
| Doxorubicin | Doxorubicin hydrochloride, doxorubicin liposomal, doxorubicin hydrochloride liposomal, DB00997, Adriamycin® | 73 | 7/73 | 1/7 | |
| Gemcitabine | Gemzar®, LY188011, gemcitabine hydrochloride | 90 | 4/90 | 4/4 | |
| Paclitaxel | Taxol®, DB01229, Abraxane® | 209 | 12/209 | 0/12 | |
| CDK inhibitors for ER+, HER2− (n = 3) | Abemaciclib | LY2835219 | 5 | 5/5 | 5/5 |
| Palbociclib | PD-0332991-0054, Ibrance® | 19 | 5/19 | 4/5 | |
| Ribociclib | LEE011 | 8 | 3/8 | 2/3 | |
| Src inhibitors (n = 1) | Dasatinib | NSC-732517 | 10 | 2/10 | 1/2 |
| PI3K inhibitors (n = 4) | Alpelisib | BYL719 | 0 | 0/0 | 0/0 |
| Buparlisib | BKM120 | 1 | 1/1 | 0/1 | |
| Pictilisib | GDC-0941 | 3 | 0/3 | 0/0 | |
| Taselisib | GDC-0032 | 2 | 0/2 | 0/0 | |
| HDAC inhibitors (n = 1) | Entinostat | MS-275 | 4 | 0/4 | 0/0 |
| Total | 1,634 | 74/1,634 | 38/74 |
Notes:
Includes number of Phase 2 or 3 clinical trials with a breast cancer indication registered in clinicaltrials.gov database.
Counts not mutually inclusive.
Clinical trials were excluded from final review if the study drug was not one of the 24 treatments on the a priori list and/or if the trials were not sponsored by the pharmaceutical industry.
No pharmaceutical industry-sponsored studies among the PI3K and histone deacetylase (HDAC) inhibitor treatment classes were found to include a PRO endpoint.
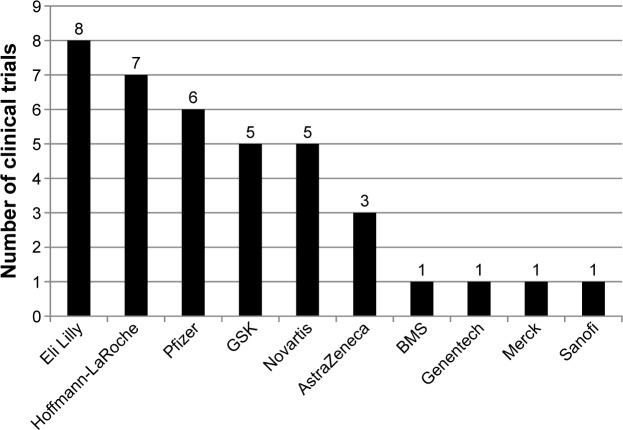
The clinical trials reviewed represented a total of 10 pharmaceutical companies sponsoring the product development. Of these, four companies each sponsored only one of the reviewed clinical trials, while the remaining six sponsored two or more. See Figure 1 for a complete list of treatment developers.
Figure 1.
Number of reviewed clinical trials including PROs by treatment developer (N = 38).
The reviewed clinical trials represented 21 open-label trials (55.3% of the total number of trials reviewed), 11 double-blind trials with placebo (28.9%), and 6 double-blind trials with active comparators (15.8%).
PRO endpoint positioning in reviewed trials
Across all clinical trials reviewed, PROs were positioned as secondary endpoints; that is, no PROs were listed as the primary study endpoint. Half of the reviewed trials (n = 19, 50.0%) evaluated change from baseline in a PRO score (one trial further specified baseline as referring to the time of randomization to the maintenance phase). The PRO endpoint designs for the remaining trials were varied; five trials assessed change over time of a PRO score (without reference to baseline or randomization), three trials evaluated time to symptom progression as assessed by a PRO (one trial further specified this as the time from randomization to the first symptom progression as measured by a PRO), and three trials described time to deterioration in PRO-assessed quality-of-life scores. In the latter case, two trials further specified time to deterioration as the time from the date of randomization to the date of at least 10% worsening (relative to baseline) of the scale score (without further improvement above the threshold), or death due to any cause. The remaining eight trials did not specify endpoint design.
PROs in reviewed trials
PRO questionnaires across all trials
A total of 17 unique PROs were identified as secondary endpoints across the 38 reviewed clinical trials. There were 66 total instances in which a PRO questionnaire was utilized; thus, more than one questionnaire was administered in most of the reviewed trials. The most commonly identified PRO questionnaire across all clinical trials reviewed was the Functional Assessment of Cancer Therapy—Breast (FACT-B), which was used in 18 trials (47.4%). Other PRO questionnaires commonly used in the reviewed clinical trials were the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire—Core 30 (EORTC QLQ-C30, n = 14, 36.8%), the EuroQol 5-Dimensions (EQ-5D, n = 8, 21.1%), and the EORTC QLQ—Breast Cancer Module (EORTC QLQ-BR23, n = 5, 13.2%). See Table 4 for a complete listing of PRO questionnaires.
Table 4.
PRO questionnaires identified in reviewed clinical trials.
| PRO QUESTIONNAIRE NAME | ABBREVIATION | NUMBER OF REVIEWED CLINICAL TRIALS USING PRO n (%)* |
|---|---|---|
| Functional Assessment of Cancer Therapy—Breast | FACT-B | 18 (47.4%) |
| European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire—Core 30 | EORTC QLQ-C30 | 14 (36.8%) |
| EuroQol 5-Dimensions | EQ-5D | 8 (21.1%) |
| EORTC QLQ—Breast Cancer Module | EORTC QLQ-BR23 | 5 (13.2%) |
| Brief Pain Inventory | BPI | 3 (7.9%) |
| Functional Assessment of Cancer Therapy—General | FACT-G | 3 (7.9%) |
| MD Anderson Symptom Inventory | MDASI | 2 (5.3%) |
| Hospital Anxiety and Depression Scale | HADS | 2 (5.3%) |
| Rotterdam Symptom Checklist | RSCL | 2 (5.3%) |
| EuroQol 5-Dimension 5-Level | EQ-5D-5L | 2 (5.3%) |
| Functional Assessment of Chronic Illness Therapy—Fatigue | FACIT-F | 1 (2.6%) |
| Functional Assessment of Cancer Therapy—Endocrine Symptoms | FACT-ES | 1 (2.6%) |
| Multidimensional Assessment of Fatigue | MAF | 1 (2.6%) |
| Pittsburgh Sleep Quality Index | PSQI | 1 (2.6%) |
| Short Form Health Survey-36 | SF-36 | 1 (2.6%) |
| Somatic and Psychological Health Report questionnaire (somatic subscale) | SPHERE | 1 (2.6%) |
| Subject Significance Questionnaire | SSQ | 1 (2.6%) |
Note:
Counts not mutually exclusive.
PRO questionnaires by treatment class
The most broadly used PRO questionnaires across treatment classes were the FACT-B and the EORTC QLQ-C30 (each used in trials involving four of the five treatment classes identified), followed by the EQ-5D, used in trials involving three of the five treatment classes. Trials involving HER2+ treatments used the FACT-B more frequently (n = 9, 81.8% of trials) than trials involving other treatment classes, while the CDK inhibitor clinical trials tended to favor the EORTC QLQ-C30 (n = 7, 63.6% of trials). However, comparisons of the use of PRO questionnaires among treatment classes are complicated by the disparity between the larger numbers of trials involving ER+ and HER2+ treatments versus other treatments, and more precise conclusions are not possible. See Table 5 for a complete listing of PRO questionnaires used across treatment classes.
Table 5.
Use of PRO questionnaires across treatment classes.
| PRO | CDK INHIBITOR (n = 11) n (%) | HER2+ (n = 11) n (%) | ER+ (n = 8) n (%) | TRIPLE-NEGATIVE (n = 7) n (%) | Src INHIBITOR (n = 1) n (%) |
|---|---|---|---|---|---|
| FACT-B | 3 (27.3%) | 9 (81.8%) | 5 (62.5%) | 1 (14.3%) | – |
| EORTC QLQ-C30 | 7 (63.6%) | 1 (9.1%) | 3 (37.5%) | 3 (42.9%) | – |
| EQ-5D | 3 (27.3%) | 3 (27.3%) | 2 (25.0%) | – | – |
| EORTC QLQ-BR23 | 3 (27.3%) | – | 2 (25.0%) | – | – |
| BPI | 2 (18.2%) | – | – | – | 1 (100.0%) |
| FACT-G | – | 2 (18.2%) | 1 (12.5%) | – | – |
| MDASI | 1 (9.1%) | – | – | 1 (14.3%) | – |
| HADS | – | – | 1 (12.5%) | 1 (14.3%) | – |
| RSCL | – | – | – | 2 (28.6%) | – |
| EQ-5D-5L | 2 (18.2%) | – | – | – | – |
| FACIT-F | – | – | – | 1 (14.3%) | – |
| FACT-ES | – | – | 1 (12.5%) | – | – |
| MAF | – | 1 (9.1%) | – | – | – |
| PSQI | – | – | – | 1 (14.3%) | – |
| SF-36 | – | – | – | 1 (14.3%) | – |
| SPHERE | – | – | – | 1 (14.3%) | – |
| SSQ | – | – | – | 1 (14.3%) | – |
PRO questionnaires by clinical trial phase
Among the clinical trials reviewed, there were a total of 14 Phase 2 trials and a total of 24 Phase 3 trials. In the Phase 2 trials, seven PRO questionnaires were identified, while 16 were identified in the Phase 3 trials.
The most frequently identified questionnaire in both Phase 2 and Phase 3 trials was the FACT-B (Phase 2: n = 7, 50.0%; Phase 3: n = 11, 45.8%), followed by the EORTC QLQ-C30 (Phase 2: n = 4, 28.6%; Phase 3: n = 10, 41.7%). All of the questionnaires identified in Phase 2 clinical trials were also identified in the Phase 3 trials, with the exception of the Multidimensional Assessment of Fatigue (MAF). See Table 6 for a list of PRO questionnaires by trial phase.
Table 6.
PRO questionnaires use in Phase 2 and Phase 3 trials.
| PRO QUESTIONNAIRE | PHASE 2 TRIALS USING PRO (n = 14) n (%)* | PHASE 3 TRIALS USING PRO (n = 24) n (%)* |
|---|---|---|
| FACT-B | 7 (50.0%) | 11 (45.8%) |
| EORTC QLQ-C30 | 4 (28.6%) | 10 (41.7%) |
| EQ-5D | 3 (21.4%) | 5 (20.8%) |
| EORTC QLQ-BR23 | 1 (7.1%) | 4 (16.7%) |
| BPI | 2 (14.3%) | 1 (4.2%) |
| FACT-G | – | 3 (12.5%) |
| MDASI | 1 (7.1%) | 1 (4.2%) |
| HADS | – | 2 (8.3%) |
| RSCL | – | 2 (8.3%) |
| EQ-5D-5L | – | 2 (8.3%) |
| FACIT-F | – | 1 (4.2%) |
| FACT-ES | – | 1 (4.2%) |
| MAF | 1 (7.1%) | – |
| PSQI | – | 1 (4.2%) |
| SF-36 | – | 1 (4.2%) |
| SPHERE | – | 1 (4.2%) |
| SSQ | – | 1 (4.2%) |
Note:
Counts not mutually exclusive.
PRO questionnaires by industry sponsor
When evaluating the frequency of PRO questionnaires by treatment developer, Pfizer, GSK, and Hoffmann-LaRoche were using the FACT-B most frequently (n = 5, 83.3%; n = 4, 80.0%; and n = 4, 57.1%, respectively), and this was the only PRO questionnaire used in the trials sponsored by AstraZeneca (n = 3, 100%). The EORTC QLQ-C30 was the questionnaire most commonly used in trials sponsored by Novartis (n = 5, 100.0%) and Eli Lilly (n = 4, 50.0%). In the single reviewed clinical trial sponsored by Sanofi, a total of six different PRO questionnaires were being used; only Eli Lilly, across its eight sponsored trials, used more.
PRO measurement concepts identified in the reviewed clinical trials
PRO measurement concepts across clinical trials
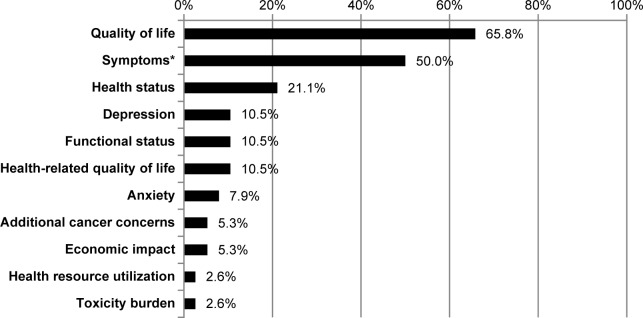
By far, quality of life was the most commonly reported PRO concept of measurement; it was identified in endpoints among 65.8% (n = 25) of the reviewed clinical trials. In 13 studies, quality of life in general was identified as the concept of measurement, while in others, it was further specified to include assessments of well-being: physical (n = 12), functional (n = 10), emotional (n = 7), and/or social/family (n = 7).
Symptoms were also commonly measured as PRO endpoints, being mentioned in 19 (50.0%) of the clinical trials reviewed. The most frequently assessed symptom concept was pain, assessed in six (15.8%) of the reviewed trials. Some trials specified symptoms by etiology [cancer related (n = 1, 2.6%) or breast cancer-related (n = 6, 15.8%)] or by dimension [symptom burden (n = 2, 5.3%) or symptom progression (n = 1, 2.6%)]. Other trials indicated specific symptom concepts that were measured, including hot flushes, cold sweats, night sweats, lack of energy, headaches, mood swings, nausea, weight gain, vomiting, diarrhea, feeling bloated, vaginal discharge, vaginal irritation, vaginal bleeding, vaginal dryness, breast tenderness, fatigue, dyspnea, insomnia, appetite loss, and constipation. One clinical trial (2.6%) specified symptom concepts by body system: gastrointestinal (nausea, weight gain, vomiting, diarrhea, and feeling bloated), gynecological (vaginal discharge, vaginal irritation, vaginal bleeding, vaginal dryness, discomfort with intercourse, lost interest in sex, and breast tenderness), neuropsychological (lack of energy, nervous feeling, lightheaded/dizzy, headaches, mood swings, and feeling irritable), or vasomotor (hot flushes, cold sweats, night sweats, and sleeping difficulties).
Other measurement concepts described in the PRO endpoints were global or overall health status (n = 8, 21.1%), health-related quality of life (n = 4, 10.5%), and depression (n = 4, 10.5%). Two clinical trials (5.3%) did not specify the PRO measurement concepts assessed. See Figure 2 for the PRO measurement concepts being assessed in the reviewed clinical trials.
Figure 2.
Percentage of PRO measurement concepts identified in the reviewed clinical trials (N = 38).
Note: *Pain, hot flushes, cold sweats, night sweats, lack of energy, headaches, nausea, weight gain, vomiting, diarrhea, feeling bloated, vaginal discharge, vaginal irritation, vaginal bleeding, vaginal dryness, breast tenderness, fatigue, dyspnea, insomnia, appetite loss, constipation, lightheadedness, dizziness, sleeping dibcbcr-10-2016-093culties, and/or discomfort.
PRO measurement concepts by treatment class
The most broadly assessed PRO concept of measurement across treatment classes was symptoms (assessed in clinical trials involving all treatment classes), followed closely by quality of life, which was assessed in clinical trials involving five out of the six treatment classes. Quality of life was the most frequently assessed PRO measurement concept in trials involving HER2+ treatments (n = 11, 100.0% of trials) and triple-negative treatments (n = 6, 85.7% of trials). Only trials involving CDK inhibitors reported toxicity burden as an assessed PRO concept of measurement. See Table 7 for a breakdown of PRO measurement concepts assessed according to treatment class.
Table 7.
Concepts of measurement assessed according to treatment class.
| PRO MEASUREMENT CONCEPT | CDK INHIBITORS (n = 11) n (%) | HER2+ (n = 11) n (%) | ER+ (n = 8) n (%) | TRIPLE-NEGATIVE (n = 7) n (%) | Src INHIBITORS (n = 1) n (%) |
|---|---|---|---|---|---|
| Quality of life | 3 (27.2%) | 11 (100.0%) | 5 (62.5%) | 6 (85.7%) | – |
| Symptoms | 6 (54.5%) | 5 (45.5%) | 3 (37.5%) | 4 (57.1%) | 1 (100.0%) |
| Health status | 6 (54.5%) | – | 1 (12.5%) | 1 (14.3%) | – |
| Depression | 1 (9.1%) | – | 2 (25.0%) | 1 (14.3%) | – |
| Functional status | 1 (9.1%) | – | 1 (12.5%) | 2 (28.5%) | – |
| Health-related quality of life | 2 (18.1%) | – | 2 (25.0%) | – | – |
| Anxiety | 1 (9.1%) | – | 2 (25.0%) | – | – |
| Additional cancer concerns | – | 1 (9.1%) | – | 1 (14.3%) | – |
| Economic impact | – | 1 (9.1%) | – | 1 (14.3%) | – |
| Fatigue | – | 1 (9.1%) | – | 1 (14.3%) | – |
| Discomfort | – | – | 1 (12.5%) | – | – |
| Health resource utilization | – | 1 (9.1%) | – | – | – |
| Sleep quality | – | – | – | 1 (14.3%) | – |
| Toxicity burden | 1 (9.1%) | – | – | – | – |
Frequency of PRO questionnaire administration in the reviewed trials
The reviewed clinical trials provided information about the frequency of administration for 15 of the 17 identified PRO questionnaires. Overall, most were administered with a frequency ranging from every 3 to 12 weeks. PRO questionnaires that were administered more frequently (daily, weekly, or every two weeks) tended to be those that assessed symptoms, including fatigue, anxiety, or depression. Such PRO questionnaires included the MDASI, HADS, FACIT-F, PSQI, and SPHERE; the one exception was the SF-36, a quality-of-life measure that was administered weekly in one trial. Conversely, PRO questionnaires that were administered less frequently (after 24 weeks or every 56, 124, or 136 weeks) tended to be those that assessed quality of life (EORTC QLQ-C30) or overall health status (EQ-5D and EQ-5D-5L). These results are presented in Table 8.
Table 8.
Frequency of PRO questionnaire administration.
| PRO QUESTIONNAIRE | DAILY | WEEKLY | EVERY 2 WEEKS | EVERY 3 WEEKS | EVERY 4 WEEKS | EVERY 6 WEEKS | EVERY 8 WEEKS | EVERY 9 WEEKS | EVERY 12 WEEKS | AFTER 24 WEEKS | EVERY 36 WEEKS | AFTER 56 WEEKS | AFTER 124 WEEKS | AFTER 136 WEEKS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FACT-B | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓✓✓ | ✓ | ||||||||
| EORTC QLQ-C30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| EQ-5D health status | ✓ | |||||||||||||
| EORTC QLQ-BR23 | ✓ | ✓ | ✓ | |||||||||||
| BPI | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| FACT-G | ✓ | ✓ | ✓✓✓ | |||||||||||
| MDASI | ✓ | ✓ | ||||||||||||
| HADS | ✓ | |||||||||||||
| RSCL* | ||||||||||||||
| EQ-5D-5L | ✓ | ✓ | ✓ | |||||||||||
| FACIT-F | ✓ | |||||||||||||
| FACT-ES | ✓ | ✓ | ✓ | |||||||||||
| MAF | ✓ | ✓ | ||||||||||||
| PSQI | ✓ | |||||||||||||
| SF-36 | ✓ | |||||||||||||
| SPHERE | ✓ | |||||||||||||
| SSQ* |
Notes:
Frequency of administration data not reported for these PROs. Pindicates one clinical trial with this frequency of administration.
Discussion
In demonstrating the value of a cancer treatment to regulators, providers, payers, and ultimately to patients themselves, it is often desirable that the treatment not only prolongs survival and inhibits tumor growth but also does so in ways that are not detrimental to patients’ quality of life, symptom experience, and overall perceived health status. A PRO can measure such described treatment effects and provide additional support and understanding of treatment value, including the impact of the treatment’s efficacy and safety on a patient. Advancements in the treatment of metastatic breast cancer, along with an increased awareness by stakeholders of the value of the patient perspective, have led to an increase in the use of PROs in clinical trials and efforts to understand how PRO questionnaires can best be developed, selected, and implemented to evaluate treatment efficacy and safety. This review across common metastatic breast cancer treatments aimed to describe PRO endpoints in these clinical trials. In addition, it sought to determine whether factors such as treatment class, treatment developer, or clinical trial phase influence how PROs might be utilized.
PRO questionnaires and their associated concepts of measurement, when utilized in the trials reviewed, were positioned as secondary or exploratory endpoints, playing supporting roles to more objective measures such as survival and tumor-related outcomes. Most frequently, the PRO endpoint was measured as the change over time from baseline in a PRO total or subscale score. While there was some variation, the impact concept most frequently measured as an endpoint across treatment classes, developers, and trial phases was quality of life, assessed with the FACT-B (either its total score or the scores of one or more subscales) or the EORTC QLQ-C30; in some trials, quality of life was further defined by specific impacts such as physical functioning. Symptoms (most often pain) assessed by the EORTC QLQ-C30 or the EORTC QLQ-BR23, among others, were also frequently identified in the PRO endpoints.
While not all of the reviewed trials described the frequency with which PRO questionnaires were being administered, it was garnered from the information available that this could be relative to the concepts being assessed. For instance, PRO questionnaires assessing concepts of measurement that tend to change slowly over time (eg, quality of life and overall health status) tended to be less frequently administered than those assessing concepts that may be more variable (eg, symptoms and fatigue).
Whether they aim to assess a patient’s global or specific health experiences, PRO questionnaires serve to incorporate the patient perspective into clinical research and practice. PRO questionnaires can be used to measure patients’ perceptions of disease-related signs, symptoms, and/or impacts, as well as treatment-related side effects, and the data collected can serve as evidence for the safety and efficacy of new and existing therapies from the patient perspective. In the development of cancer treatments specifically, treatment efficacy may often be similar between products; and therefore, PRO questionnaires may be particularly valuable for measuring and demonstrating differences in perceived treatment-related side effects between products.11
Among the 38 industry-sponsored trials in advanced breast cancer reviewed as part of this research, the FACT-B and EORTC QLQ-C30 were found to be the most frequently utilized PRO questionnaires. The FACT-B may have been the most frequently utilized PRO questionnaire due to the fact that in addition to including questions about the cancer symptom and impact experience more broadly, it also incorporates items specific to breast cancer. Additionally, there is documentation that the FACT-B was developed in line with best measurement practices,8 in that its content was generated based on expert and patient input, and specifically through qualitative research that included patients with breast cancer.12
In contrast, the second most frequently used PRO questionnaire in this review, the EORTC QLQ-C30, was not developed through qualitative research with patients. However, as part of its field testing, the lung cancer patients who completed the questionnaire were asked to comment on the clarity and ease of completing the questions,13 indicating that there is evidence that it was evaluated in terms of whether patients were able to respond meaningfully to the EORTC QLQ-C30. The EORTC QLQ-C30 is a generic cancer measure but has the advantage of being shorter than the FACT-B and likely less burdensome for patients to complete. Both questionnaires assess similar domains, such as physical and emotional functioning as well as disease- and treatment-related symptoms, and use the same recall period (seven days or the past week). In this review, the FACT-B was found to be commonly implemented to assess quality of life, while the EORTC QLQ-C30 was frequently used to measure quality of life or pain.
Thus, although the FACT-B was found to be the most frequently used questionnaire in this review, either PRO questionnaire (the FACT-B or EORTC QQL-C30) may be deemed most suitable by a sponsor depending on the intended context of use. The FDA has recommended to sponsors that they carefully select PRO questionnaires that can be shown as fit for the purpose of their research aims.8 Specifically, PRO questionnaires selected for use in a trial to support labeling claims should have appropriate documentation of content validity, that is, evidence that the questionnaire captures concepts that are important and relevant to the target patient population and are measured in ways that these patients can understand and meaningfully respond to. If no such documentation exists, it may need to be established by sponsors; alternatively, sponsors are not limited to using existing PRO questionnaires, and a de novo PRO questionnaire can be developed to meet a sponsor’s study objectives. Furthermore, this research identified that in most of the reviewed trials, more than one questionnaire was administered, and sponsors may choose to utilize multiple PRO questionnaires to meet different research objectives.
Although some conclusions have been drawn from the study findings, comparisons between treatment classes or treatment developers were limited by the disparities between subcategories. For example, the low frequency of trials reviewed in some treatment classes relative to others makes it difficult to draw meaningful conclusions about differences and similarities. This review was also limited by the information made available in the clinical trial registry; while some treatment developers provided detailed endpoint statements, including the target measurement concepts, PRO measure, and the way in which scores would be evaluated, often the PRO endpoints were lacking one or more of these details.
Finally, this review focused only on industry-sponsored trials, excluding those sponsored by an independent Principal Investigator or nonindustry research organization. It has previously been found that industry-sponsored breast cancer studies typically consist of smaller, single-arm studies that are more likely to focus on advanced disease and also are more likely to publish positive results compared to nonpharmaceutical research.14 Thus, the lack of comparison between industry-sponsored and nonpharmaceutical research is one gap of this review and an important direction for future research to consider. Moreover, these findings relied on a sample of published trials that dated back to 2003 and may or may not be upheld if reevaluated over a decade later.
Conclusion
As industry sponsors, drug trialists, regulators, payers, and other stakeholders turn more attention to the patient perspective, one would expect PROs to increase as complementary measures to traditional endpoints and become an even more critical part of treatment evaluation. This review shares insights into the role of PROs in trials for metastatic breast cancer from which treatment developers and other stakeholders can learn to improve their successful implementation in future trials.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 691 words, excluding any confidential comments to the academic editor.
FUNDING: This publication and its associated research were funded by Novartis. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the research: YH, MK, NG, AS, RL, CF, and IM. Analyzed the data: YH, MK, NG, and RL. Wrote the first draft of the manuscript: YH, MK, NG, RL, and JS. Contributed to the writing of the manuscript: YH, MK, NG, RL, and JS. Agree with manuscript results and conclusions: YH, DG, MK, NG, JS, AS, RL, CF, and IM. Jointly developed the structure and arguments for the paper: YH, MK, NG, AS, and RL. Made critical revisions and approved final version: YH, DG, MK, NG, AS, and RL. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER Stat Fact Sheets: Female Breast Cancer. 2015. [Accessed October 13, 2015]. Available at: http://seer.cancer.gov/statfacts/html/breast.html.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Stages of Breast Cancer. 2014. [Accessed February 2, 2015]. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/breast/Patient/page2.
- 4.American Cancer Society Treatment of Invasive Breast Cancer, By Stage. 2014. [Accessed February 2, 2015]. Available at: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-treating-by-stage.
- 5.American Cancer Society Hormone Therapy for Breast Cancer. 2014. [Accessed February 2, 2015]. Available at: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-treating-hormone-therapy.
- 6.Oostra DR, Macrae ER. Role of trastuzumab emtansine in the treatment of HER2-positive breast cancer. Breast Cancer (Dove Med Press) 2014;6:103–113. doi: 10.2147/BCTT.S67297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav BS, Sharma SC, Chanana P, Jhamb S. Systemic treatment strategies for triple-negative breast cancer. World J Clin Oncol. 2014;5:125–133. doi: 10.5306/wjco.v5.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health . Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. FDA Office of Communications, Division of Drug Information; Silver Spring, MD: 2009. [Google Scholar]
- 9.Brim RL, Pearson SD. The use and reporting of patient-reported outcomes in phase III breast cancer trials. Clin Trials. 2013;10:243–249. doi: 10.1177/1740774513475529. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 1.2016. 2015. [Accessed November 23, 2015]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 11.Zagadailov E, Fine M, Shields A. Patient-reported outcomes are changing the landscape in oncology care: challenges and opportunities for payers. Am Health Drug Benefits. 2013;6:264–274. [PMC free article] [PubMed] [Google Scholar]
- 12.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Peppercorn J, Blood E, Winer E, Partridge A. Association between pharmaceutical involvement and outcomes in breast cancer clinical trials. Cancer. 2007;109:1239–1246. doi: 10.1002/cncr.22528. [DOI] [PubMed] [Google Scholar]