Abstract
Intrauterine growth restriction (IUGR), a condition that occurs due to various reasons, is an important cause of fetal and neonatal morbidity and mortality. It has been defined as a rate of fetal growth that is less than normal in light of the growth potential of that specific infant. Usually, IUGR and small for gestational age (SGA) are used interchangeably in literature, even though there exist minute differences between them. SGA has been defined as having birth weight less than two standard deviations below the mean or less than the 10th percentile of a population-specific birth weight for specific gestational age. These infants have many acute neonatal problems that include perinatal asphyxia, hypothermia, hypoglycemia, and polycythemia. The likely long-term complications that are prone to develop when IUGR infants grow up includes growth retardation, major and subtle neurodevelopmental handicaps, and developmental origin of health and disease. In this review, we have covered various antenatal and postnatal aspects of IUGR.
Keywords: Intrauterine growth restriction (IUGR), small for gestational age (SGA), symmetrical IUGR, asymmetrical IUGR, placental genes, maternal genes, fetal genes, developmental origin of health and disease, thrifty phenotype (Barker hypothesis)
Introduction
Intrauterine growth restriction (IUGR) has been defined as the rate of fetal growth that is below normal in light of the growth potential of a specific infant as per the race and gender of the fetus. It has also been described as a deviation from or a reduction in an expected fetal growth pattern and is usually the result of innate reduced growth potential or because of multiple adverse effects on the fetus. The “normal” neonate is the one whose birth weight is between the 10th and 90th percentile as per the gestational age, gender and race with no feature of malnutrition and growth retardation. The terms “IUGR” and “small for gestational age (SGA)” have been used synonymously in medical literature, but there exist small differences between the two. SGA definition is based on the cross-sectional evaluation (either prenatal or postnatal), and this term has been used for those neonates whose birth weight is less than the 10th percentile for that particular gestational age or two standard deviations below the population norms on the growth charts, and the definition considers only the birth weight without any consideration of the in-utero growth and physical characteristics at birth. An IUGR is a clinical definition and applies to neonates born with clinical features of malnutrition and in-utero growth retardation, irrespective of their birth weight percentile. Henceforth, appropriate for gestational age (AGA) infants can be labeled as IUGR if they have features of in-utero growth retardation and malnutrition at the time of birth. Therefore, it is important to keep in mind that neonates with a birth weight less than the 10th percentile will be SGA, but not an IUGR if there are no features of malnutrition, and a neonate with a birth weight greater than the 10th percentile will be an IUGR in spite of being an AGA, if the infants have features of malnutrition at birth. Low birth weight (LBW) is a separate entity and should not be confused with IUGR/SGA, as the definition of LBW is based on the birth weight (less than 2,500 g) irrespective of gestational age, sex, race, and clinical features. The same definition of IUGR and SGA holds true for LBW infants too.1 In this review, IUGR and SGA have been used interchangeably, and we have covered various antenatal and postnatal features of IUGR in brief; details on these aspects can be seen in other published review articles of the author.2,3
Epidemiology of IUGR
The incidence of IUGR is six times higher in underdeveloped/developing countries when compared to that in developed countries, and this incidence can be further high in lower- and middle-income countries, as many infants are born in home with no birth records. The incidence of IUGR differs among countries, populations, and races and increases with decreasing gestational age. A large number of IUGR infants are seen in the Asian continent, which accounts for approximately 75% of all affected infants. This is followed by the African and Latin American continents. In the Asian continent countries, the highest incidences for low birth weight (LBW) and IUGR–LBW are seen in decreasing order in the following countries: Bangladesh, India, Pakistan, Sri Lanka, Cambodia, Vietnam and the Philippines, Indonesia and Malaysia, Thailand, and the People’s Republic of China (PRC).4
Definition
Small gestational age (SGA) refers to a weight below the 10th percentile for gestational age as per the population growth charts. It can be further classified as follows5:
Moderate: Birth weight from third to tenth percentile
Severe: Birth weight less than the third percentile
Classification of IUGR
There are predominately three types of IUGR: asymmetrical IUGR (malnourished babies), symmetrical IUGR (hypoplastic small for date), and mixed IUGR. This is based on various clinical and anthropometric features (Table 1). A third variety, which is usually seen in developing countries, has been named as mixed IUGR. Infants with this type have lesser number of cells and small cell size. These neonates have clinical features of both symmetrical and asymmetrical IUGR at birth. This type of IUGR results when early IUGR is affected further by placental causes in late pregnancy.6
Table 1.
Features of symmetrical and asymmetrical IUGR.
| CHARACTERISTICS | SYMMETRICAL IUGR | ASYMMETRICAL IUGR |
|---|---|---|
| Period of insult | Earlier gestation | Later gestation |
| Incidence of total IUGR cases | 20% to 30% | 70% to 80% |
| Etiology | Genetic disorder or infection intrinsic to foetus | Utero-placental insufficiency |
| Antenatal scan Head circumference, Abdominal circumference, Biparietal diameter and Femur length |
All are proportionally reduced | Abdominal circumference-decreased Biparietal diameter, Head circumference, and femur length- normal |
| Cell number | Reduced | Normal |
| Cell size | Normal | Reduced |
| Ponderal Index | Normal (more than 2) | Low (less than 2) |
| Postnatal anthropometry Weight, length and head circumference. |
Reductions in all parameters | Reduction in weight Length and Head circumference- normal (Brain sparing growth) |
| Difference between head and chest circumference in term IUGR | Less than 3 cm | More than 3 cm |
| Features of malnutrition | Less pronounced | More pronounced |
| Prognosis | Poor | Good |
Note: Adapted from Sharma D, Farahbakhsh N, Shastri S, Sharma P. Intrauterine growth restriction–part 2. J Matern Fetal Neonatal Med. 2016 Mar 15:1–12. [Epub ahead of print] PubMed PMID: 26979578 with permission.
Causes of IUGR
IUGR is the common end result of maternal, placental, fetal, or genetic factors, and IUGR can also result due to a combination of any of these factors (Fig. 1). Various maternal factors such as age of the mother, inter-pregnancy interval (less than 6 months or 120 months or more), maternal health, behavioral habits, and maternal infection affect the growth of the fetus and are responsible for causing IUGR (Table 2).2 Any mismatch between the supply of nutrient by the placenta and the demand of the fetus also leads to IUGR (Table 3). Fetal malformations, inborn error of metabolism, and chromosomal abnormalities are responsible for IUGR in a few cases (Table 4).7 With the recent advances in molecular biology and genetics, the role of various maternal, fetal, and placental genes polymorphisms has become important and has now been implicated as a cause of IUGR (Table 5).2,8–11
Figure 1.
IUGR can be the result of maternal, fetal, placental, genetic cause or can be combination of either of the combination. (Copyright images Deepak Sharma).
Table 2.
Maternal causes for intrauterine growth restriction.
| • Maternal age (less than 16 years and more than 35 years) • High altitude and maternal hypoxia • Low socioeconomic status and developing country • Ethnicity or race • Maternal substance abuse (smoking both active and passive, alcohol, illicit drugs like marijuana or cocaine) • Maternal medication (warfarin, steroids, anticonvulsants, antineoplastic, anti-metabolite, and folic acid antagonists) • Moderate to heavy physical work • Maternal pre-pregnancy height and weight (BMI less than 20, weight less than 45 kg and more than 75 kg) • Parity (none and more than 5 birth) • Inter pregnancy interval (less than 6 months or 120 months or more) • Previous delivery of a SGA newborn • Assisted reproductive technologies (ART) • Pregnancy poor medical care • Pregnancy severe maternal starvation. • Pregnancy poor weight gain • Maternal Bronchial asthma, cyanotic congenital heart diseases • Hematologic and immunologic disorders (Acquired thrombophilias, such as anti-cardiolipin antibodies and lupus anticoagulant) • Maternal medical disorders (hypertensive disorders (gestational and non-gestational), diabetes associated with vasculopathy, chronic renal disease, systemic lupus erythematosus, antiphospholipid syndrome, sickle cell disease • Pathological conditions in pregnancy like preeclampsia and diabetes associated with vasculopathy • Maternal infection and parasite infestations (TORCH, malaria, tuberculosis, urinary tract infections and bacterial vaginosis) |
Table 3.
Placental causes for intrauterine growth restriction.
| • Placental weight (weight less than 350 gram) • Abnormal uteroplacental vasculature • Placental dysfunction (PIH, pre-eclampsia) • Thrombophilia-related uteroplacental pathology • Confined placental mosaicism (CPM) • Avascular villi • Decidual or spiral artery arteritis • Multiple infarctions • Partial molar pregnancy • Syncytial knots • Chronic inflammatory lesions • Single umbilical artery • Abruptio placenta • Velamentous cord • Placental hemangioma • Placental infections (Placental malaria) • Infectious villitis • Multiple gestation • Chronic villitis of unknown etiology (CVUE) • Reduced expression of enzymes for redox regulation (thioredoxin, glutaredoxin) |
Table 4.
Fetal factors for intrauterine growth restriction.
| • Constitutional small (50–70% of SGA fetuses, with fetal growth appropriate for maternal size and ethnicity) • Chromosomal abnormalities [(trisomies 13, 18, 21), autosomal deletions, ring chromosomes and uniparental disomy] • Genetic syndromes (Bloom syndrome, Russell-Silver syndrome, Cornelia de Lange syndrome, Brachmann–de Lange syndrome, Mulibrey Nanism syndrome, Rubenstein–Taybi syndrome, Dubowitz syndrome, Seckel syndrome, Johanson–Blizzard syndrome, Fanconi syndrome, Roberts syndrome, and De Sanctis–Cacchione syndrome) • Major congenital anomalies (Tracheo-esophageal fistula, congenital heart disease, congenital diaphragmatic hernia, abdominal wall defects such as omphalocele and gastrochisis, neural tube defect like anencephaly and anorectal malformation) • Multiple gestation • Congenital infections (TORCH, Malaria, congenital HIV infection, Syphilis) • Metabolic disorders (agenesis of pancreas, congenital absence of islets of Langerhans, congenital lipodystrophy, galactosemia, generalized gangliosidosis type I, hypophosphatasia, I-cell disease, Leprechaunism, fetal phenylketonuria, transient neonatal diabetes mellitus) |
Table 5.
Genetic factors for intrauterine growth restriction.
| • Placental Genes: Placental 11B-Hydroxysteroid Dehydrogenase Type 2 mRNA reduced activity, Placental growth factor (PlGF) under-expression, SERPINA3 upregulation, Homeobox (DLX3, DLX4, MSX2 and GAX, ESX1 L, HLX1) under-expression, Cullin (CUL4B and CUL7), STOX1, NEAT1 (Nuclear Paraspeckle Assembly Transcript 1) over-expression, Trophoblastic miRNAs (micro RNA) (miRNA-424 and miRNA-141) over-expression, Anti-apoptosis Bcl-2 under-expression, Placental Insulin-like growth factor 1 (IGF1) under-expression, Placental Insulin-like growth factor 2 (IGF2) over-expression, Insulin like growth factor binding protein (IGFBP)-3 over-expression, Epidermal growth factor (EGF) under-expression |
| • Maternal Genes: Endothelin-1 (ET-1) over-expression, Leptin under-expression, Visfatin over-expression, Thrombophilia genes (factor V G1691 A or factor II A (20210)) mutation, Soluble vascular cellular adhesion molecule-1 (sVCaM-1) higher level, higher soluble e-selectin (sE-selectin) level, higher maternal serum and neonatal umbilical cord Asymmetric dimethylarginine (ADMA) levels |
| • Fetal Genes: High urinary Protein S100B, Genetic deletion of IGF1 (Insulin Like growth factor 1) and SHOX, Insulin-like growth factors 1 receptor (IGF-1R) mutation leading to decreased IGF-I-receptor function, N-terminal parathyroid hormone-related protein under-expression, Low Nitric Oxide |
Endocrine Basis of IUGR
The fetal growth depends on various hormones, namely, insulin, thyroid, adrenal hormones, and pituitary hormones. These hormones promote the growth and development of the fetus and any disruption in these hormonal levels leads to IUGR.
Insulin controls the cell number because it has direct mitogenic effects on cellular development. It leads to glucose uptake and consumption by body tissues and decreases protein breakdown. Fetal insulin acts as a signal of nutrient availability for growth and insulin deficiency will lead to IUGR. In insulin deficiency, IUGR results because of reduced uptake and utilization of nutrients.12 In preclinical trials, it has been shown that pancreatic agenesis of the fetus leads to fetal hyperglycemia and this results in secondary decrease in the maternal–fetal glucose concentration gradient; thus, there is a decrease in glucose transport to the fetus, leading to IUGR.13
Insulin-like growth factor-I (IGF-I) is positively regulated by glucose supply in the fetus. It has mitogenic properties inducing somatic cell growth and proliferation, and influences the transport of glucose and amino acids across the placenta. In the preclinical trial, it has been shown that a decreased expression of IGF-I results in markedly reduced rates of fetal growth.14 IGF-I also has positive effect on brain growth, causes an increase in oligodendrocytes and neuronal number and neuronal outgrowth, and also increases dendritic arborization and axon terminal fields.15
The role of Insulin-like growth factor-II (IGF-II), Insulin-like growth factor binding protein-2 (IGFBP-2), Insulin-like growth factor binding protein-3 (IGFBP-3), and vasoactive intestinal polypeptide (VIP) in IUGR have been proved. In the various preclinical trials, mutation in IGF-II mutation has shown to cause reduction in the fetal size, even though the effect of IGF-II mutation on human fetal growth still needs to be determined conclusively.16,17 The cellular growth is dependent on the balance between the binding protein and the IGF molecule itself. IGFBP-3 is reduced in the cord blood of infants with IUGR. VIP is the growth factor in the fetus that affects neuronal and whole body growth.18
Fetal hypothyroxinaemia leads to developmental abnormalities such as decreased oxygen consumption and oxidation of glucose, leading to decreased fetal energy supply for growth. Hypothyroidism also lowers the circulating and tissue concentrations of IGF-I.19,20
The glucocorticoid hormone does not have any significant effects on the fetal growth but has an important role in the development and maturation of fetal organs. These effects include glycogen deposition, gluconeogenesis, fatty acid oxidation, induction of surfactant production and release, structural maturation of alveoli, structural maturation of the gastrointestinal tract, increased expression of digestive enzymes, increased adrenal function, switch from fetal to adult hemoglobin synthesis, and maturation of thymus, liver, and kidney.21
Pregnancy-associated plasma protein-A (PAPP-A) is secreted by the decidua into the maternal circulation. The function of PAPP-A is to cleave IGFBP-4, a potent inhibitor of IGF action, thereby increasing the activity of local IGFs. The low circulating levels of PAPP-A in early pregnancy have been associated with an increased risk for IUGR.21
Growth hormone, which is the major hormonal regulator of postnatal growth, has no demonstrable effect on fetal growth.22
Antenatal Diagnosis of Growth Retardation
The goal of antenatal monitoring is early detection of IUGR, so that antenatal management can be optimized for better neonatal outcome. Unfortunately, in spite of these initiatives, the overall outcome of these IUGR has not changed much over time. Close monitoring will lead to changes in the time of delivery or management, but still there is controversy over the appropriate type and timing of antenatal monitoring.23
The investigation required for high-risk mothers who are susceptible of having IUGR fetus includes risk factor assessment in maternal and familial history, maternal anthropometry with maternal pre-pregnancy weight and height, maternal nutritional status, exact gestational dating, fundal height with fetal palpation, cardiotocography (CTG), ultrasound with Doppler, and accurate fetal weight measurement estimated using biometric measures (abdominal circumference [AC], head circumference [HC], biparietal diameter, and femur length [FL]). The HC/AC ratio has been used for fetuses diagnosed with asymmetric Fetal growth restriction (FGR).24 In these types of IUGR, the size of the liver is too small when compared with simultaneous head circumference and femur length, as these are not affected initially in the process of IUGR. The HC/AC ratio decreases linearly throughout pregnancy having a normal fetus and a ratio greater than two standard deviations (SD) above the mean; gestational age (GA) has been considered abnormal, as this marks a significant decrease in AC. A few studies have shown that an abnormal HC/AC ratio is more specific and have negative predictive value in detecting asymmetric IUGR when compared with symmetrical IUGR.25,26 These IUGR fetuses have a low body mass index (BMI) when compared to its normal counterpart at birth and show significant increase in BMI postnatally.27
The appropriate gestational age should be calculated using both the date of the last menstrual period and the crown-rump length of the fetus in the first trimester. A customized fetal weight growth chart can be used for specific population according to race and ethnicity for diagnosing IUGR. In order to accurately demonstrate IUGR on ultrasound, serial examinations have to be done (at least three weeks apart to minimize false-positive rates for diagnosing FGR). Abdominal circumference has a specificity and negative predictive value close to 90% for diagnosing IUGR. Traditional screening methods for fetal growth using abdominal palpation or measurement of symphysis–fundal height (SFH) have poor detection rates for IUGR, and therefore they should not be performed routinely.28
Once maternal risk factors and IUGR are identified, the mother is evaluated with fetal karyotype for chromosomal abnormalities, maternal infection including TORCH (Toxoplasma, others, rubella, cytomegalovirus, and herpes), syphilis, and malaria especially in high endemic areas. The mother should be evaluated with a detailed fetal anatomical survey, TIFFA scan (targeted imaging for fetal anomaly) and uterine artery Doppler, by a fetal medicine specialist if severe SGA is identified at the 18–20 week scan.29
Biophysical profile (BPP) reflects the fetal acid–base status and has been used to assess the risk for IUGR and to monitor the IUGR fetus. In IUGR fetuses, BPP changes follow a predetermined pattern that includes reactivity, which disappears first, followed by fetal breathing, fetal movement, and tone, and the last change is reduction in amniotic fluid.30 The Cochrane meta-analysis reported that there was insufficient evidence from various randomized controlled trials to support the use of BPP as a test of fetal well-being in high-risk pregnancies.31
Doppler velocities are helpful as a clinical tool specifically in the case of placental insufficiency that leads to IUGR. The various Doppler velocities that are being used for assessing fetal wellbeing and detection of IUGR are uterine artery Doppler, umbilical artery Doppler, middle cerebral artery Doppler, cerebro-placental ratio (CPR), ductus venosus Doppler, and aortic isthmus Doppler.32 Using Doppler, the maternal, placental, and fetal circulation can be simultaneously assessed. Uterine arteries provide knowledge of maternal circulation, whereas the umbilical and middle cerebral arteries give information about the fetal circulation. Uterine artery Doppler has limited sensitivity and specificity to predict adverse outcome in SGA fetuses diagnosed during the third trimester.29 Umbilical artery (UA) Doppler as a standalone standard is not valid anymore because UA Doppler is able to identify FGR because of severe placental disease, but it showed poor detection rates in cases of mild placental diseases, which are responsible for a few cases of early-onset IUGR and for a majority of cases of late-onset FGR.33 Umbilical artery Doppler provides both diagnostic and prognostic knowledge in the management of FGR. The various Doppler abnormalities seen in IUGR are increased resistance in blood vessels or absent and reverse end diastolic flow (AREDF). Increased umbilical artery Doppler perfusion index (PI) has shown good correlation of early identification of FGR, both alone or else with the cerebro-placental ratio (CPR) ratio. AREDF is usually associated with injury to various fetal organs or death. Cochrane meta-analysis showed that umbilical artery Doppler reduced the risk of perinatal deaths (risk ratio [RR] 0.71, 95% CI 0.52–0.98), lead to fewer inductions of labor (RR 0.89, 95% CI 0.80–0.99), and fewer cesarean sections (RR 0.90, 95% CI 0.84–0.97, 14 studies, 7,918 women). The authors also reported that there was no difference in operative vaginal births (RR 0.95, 95% CI 0.80–1.14), and in Apgar scores less than seven at five minutes (RR 0.92, 95% CI 0.69–1.24).34 Middle cerebral artery (MCA) tells about the compensatory vasodilation of brain blood vessels that take place in response to hypoxia (cephalization). It is considered a late manifestation, and its sensitivity and specificity increase with simultaneous use of CPR.33 Morris et al in their systematic review of 31 observational studies reported that MCA Doppler had limited predictive accuracy for adverse perinatal outcome (LR+ 2.79, 95% CI 1.10–1.67; LR – 0.56, 95% CI 0.43–0.72) and perinatal mortality (LR+ 1.36, 95% CI 1.10–1.67; LR – 0.51, 95% CI 0.29–0.89).35 The CPR is a diagnostic index and its combination improves the sensitivity of the umbilical artery and MCA for detection of FGR. This is because increased placental impedance of the uterine artery is also present with reduced cerebral resistance (MCA), leading to reduction in CPR, when other parameters are still within normal ranges.36,37 Ductus venosus (DV) Doppler is the strongest single Doppler parameter that predicts the short-term risk of fetal death in early-onset FGR and it has been shown to become abnormal only in advanced stages of fetal compromise,38,39 and shown to have good correlation with cord acidemia40 and perinatal mortality.41 There are no systematic reviews that have evaluated the effectiveness of venous Doppler as a surveillance tool in high-risk or SGA fetuses. Yagel et al in their systematic review of 18 observational studies reported that DV Doppler had moderate predictive accuracy for the prediction of perinatal mortality in high-risk fetuses with placental insufficiency with a pooled positive likelihood ratio of 4.21 (95% CI 1.98–8.96) and negative likelihood ratio of 0.43 (95% CI 0.30–0.61).42 Aortic isthmus (AoI) Doppler is associated with increased fetal mortality and neurological morbidity in cases of early-onset FGR.43 AoI Doppler shows the balance between the impedance of the brain and systemic vascular systems, and reversal of AoI flow is seen in the advanced deterioration of FGR fetus. Umbilical artery Dopplers are the most commonly used Dopplers for the detection of IUGR and its positive predictive value is increased with simultaneous measurement of other Doppler and CPR.44,45
Staging of intrauterine growth-restricted fetuses has been purposed by Mari et al and is based on fetal biometry (expected fetal weight [EFW], abdominal circumference [AC]) Doppler cardiovascular changes, amniotic fluid volume, and clinical parameters. This staging is applicable for pregnancies for any gestational age. The classification includes the following:
Stage 0: Fetuses with an EFW or an AC <10th percentile. Doppler of the UA and MCA is normal.
Stage I: Fetuses whose EFW or AC is <10th percentile plus abnormal Doppler flow of the UA or MCA.
Stage II: Fetuses whose EFW or AC is <10th percentile plus absent or reversed Doppler flow of the UA
Stage III: Fetuses whose EFW or AC is <10th percentile plus absent or reversed Doppler flow of the DV
Based on the amniotic fluid index (AFI), each IUGR fetus will be either A (AFI <5 cm) or B (AFI ≥5 cm).46
Management of the IUGR Fetus
The guidelines of the Royal college of Obstetrics and Gynaecology (RCOG) recommend the management of these IUGR fetuses including both monitoring and delivery methods. Women with an SGA fetus between 24+0 and 35+6 weeks of gestation should receive a single course of antenatal corticosteroids, when delivery is being considered. Umbilical artery Doppler should be the primary surveillance tool in the SGA fetus, as this has shown to reduce perinatal morbidity and mortality in high-risk population. Repeat surveillance of repeat Doppler will depend on the previous Doppler indices (Fig. 2).
Figure 2.
Screening for Small–for–Gestational–Age (SGA) Fetus.
Note: Reproduced from: Royal College of Obstetricians and Gynaecologists. The Investigation and Management of the Small–for–Gestational–Age Fetus. Green-top Guideline No. 31. London: RCOG; 2014, with the permission of the Royal College of Obstetricians and Gynaecologists.
CTG and ultrasound assessment of amniotic fluid should not be used, as the only form of surveillance in SGA fetuses and BPP should not be done in preterm SGA fetuses. The optimal gestation to deliver the SGA fetus will depend upon the gestational age of the fetus and Doppler study of the umbilical artery (Fig. 3). In the SGA fetus with umbilical artery, AREDF delivery by caesarean section is recommended. Early admission is recommended in women in spontaneous labor with an SGA fetus in order to instigate continuous fetal heart rate monitoring.29
Figure 3.
The Management of the Small–for–Gestational–Age (SGA) Foetus.
Notes: Reproduced from: Royal College of Obstetricians and Gynaecologists. The Investigation and Management of the Small–for–Gestational–Age Fetus. Green-top Guideline No. 31. London: RCOG; 2014, with the permission of the Royal College of Obstetricians and Gynaecologists. 1 Weekly measurement of fetal size is valuable in predicting birthweight and determining size-for-gestational age. 2 If two AC/EFW measurements are used to estimate growth, they should be at least 3 weeks apart. 3 Use cCTG when DV Doppler is unavailable or results are inconsistent – recommend delivery if STV <3 ms.
Abbreviations: AC, abdominal circumference; EFW, estimated fetal weight; Pl, pulsatility index; RI, resistance index; UA, umbilical artery; MCA, middle cerebral artery; DV, ducts venosus; FGR, fetal growth restriction; EDV, end-diastolic velocities.
Recently stage-based protocol for managing fetal growth restriction has been recommended that takes into account Doppler of different blood vessels.32,47
Prevention of IUGR
The high incidence of IUGR in developing countries is mostly because of social reasons such as gender discrimination (it leads to poor nutritional supplement to the female gender when compared to the male gender, leading to poor health and malnutrition of female that in-turn leads to IUGR fetus) and does not appear to reduce with interventions that are targeted toward the pregnant women. Adolescent and pre-pregnancy nutrition, pre-pregnancy weights, poverty, and inter-pregnancy interval are the crucial determinants of fetal growth in low- and middle-income countries. Social intervention measures such as taking care of female nutrition enrichment, delaying of age at first pregnancy, preventing female gender violence (this will lead to a decrease in gender discrimination and better female nutrition), and treating chronic disease and pregnancy-induced disorders will help have a positive effect on reducing the incidence of IUGR in developing countries. However, some evidence-based interventions have shown to reduce the incidence of IUGR.48 The evidence-based proven interventions include balanced energy protein supplemen tation,49 intermittent preventive treatment of malaria in pregnancy,48 multiple micronutrient supplementation,50 insecticide-treated nets (ITN),48 anti-platelets for preeclampsia,51,52 and smoking cessation.53
The other interventions that have been tried by various researchers in mothers who were diagnosed to have IUGR fetus, but met with variable success, are as follows:6
Bed rest to mother
Parenteral nutrition to mother
Calcium supplementation
Calcium supplementation for hypertension
Nutritional supplementation to fetus
Antihypertensive for mild to moderate hypertension
Oxygen therapy
Prophylactic antibiotic therapy to the mother
Pharmacological therapy to mother including aspirin, beta adrenergic agonist, and atrial natriuretic peptide
Nitric oxide donor
Intermittent abdominal compression
Postnatal diagnosis of IUGR
The postnatal diagnosis of IUGR infant includes clinical examination, anthropometry, Ponderal index, clinical assessment of nutrition (CAN) score, cephalization index, mid-arm circumference, and mid-arm/head circumference ratios.
Anthropometry
Assessment of weight at birth less than 10 centiles as per the race and sex labels a neonate SGA/IUGR. The studies have shown that the customized growth charts that have taken into account various maternal characteristics are more accurate at diagnosing fetal and neonatal IUGR.54 When antenatally diagnosed fetuses with IUGR birth weight are sought on neonatal growth curves, then these IUGR fetuses become AGA, but they have to undergo the postnatal complications of IUGR, therefore raising the suspicion for use of neonatal growth curve in these IUGR fetus.55 In symmetrical IUGR, weight, head circumference and length will be less than 10 centiles, whereas in asymmetrical IUGR, only weight will be less than 10 centiles and the rest will be as per gestation age.56
Clinical Examination (Figs. 4 and 5)
Figure 4.
This was a 36-week male neonate born to mother with severe pre-eclampsia with birth weight of 1600 grams. This baby was asymmetrical IUGR. Note loss of fat whole over the body, visible rib cage, excessive skin fold whole over the body and relatively large heads compared with rest of the body. The neonate also had excessive skin folds (more than 3) over inter-scapular and gluteal area with loss of underlying fats. There are loose folds of skin in the nape of neck and arms.
Figure 5.
Clinical features of infants at birth that are having intrauterine growth restriction. Figure Copyright Deepak Sharma.
IUGR newborns have varied typical features of malnutrition.
Large head when compared to rest of the body (brain sparing effect)
Large and wide anterior fontanelle (poor formation of membranous bones)
Absent buccal fat (old man look)
Small or scaphoid abdomen
Thin umbilical cord often stained with meconium
Decreased skeletal muscle mass and subcutaneous fat tissue
Loose, dry, and easy peelable skin
Long finger nails
Relatively large hands and feet compared to body
Skin having a loose fold of skin in the nape of neck, axilla, inter-scapular area, and gluteal region (more than three folds)
Anxious and hyper alert infant
Poor breast bud formation and immature female genitalia
If the infant is symmetrical IUGR, then these infants can have other features such as associated dysmorphic facies, congenital anomalies (suggestive of chromosomal abnormality, syndrome, or intrauterine drug exposure), and also features of congenital viral infection, especially the TORCH group (microcephaly, petechiae, blue-berry muffin [purple skin lesions result of dermal erythropoiesis], cardiac defect, hepatosplenomegaly, intracranial calcification, chorioretinitis, and cataracts).
The gestational age assessment using the Ballard scoring system is not accurate, as physical components are underscored or over scored. This is because of diminished vernix caseosa, the skin is continuously exposed to amniotic fluid, thereby leading to cracking and peeling of the skin. This in turn leads to more mature sole crease pattern, less well-formed ear cartilage, diminished breast bud (due to decreased blood flow, low estradiol level, and low subcutaneous fat), and less mature-appearing female genitalia (due to reduced fat deposit in the labia majora). There is no such problem with the neurological component of the Ballard system.57,58
Ponderal Index
Ponderal index can also be used to determine the degree of fetal malnutrition. It is calculated as ratio of body weight in grams to length in cm expressed as (PI = [weight {in gram} × 100] ÷ [length {in cm}3]). PI of less than 10 percentile reflects fetal malnutrition; PI of less than 3 percentile indicates severe fetal wasting.1,59
Mid-arm Circumference and Mid-Arm/Head Circumference Ratios (Kanawati and McLaren’s Index)
The normal value of mid-arm/head circumference ratios (MAC/HC) is 0.32–0.33 and in a term IUGR infant, a value less than 0.27 is considered features of fetal malnutrition.60
Clinical Assessment of Nutrition Score (CAN Score)
CAN score was developed by J Metcoff and is used for the assessment of nutritional status in infants at birth. It includes nine parameters, namely, hair, cheeks, neck and chin, arms, legs, back, buttocks, chest, and abdomen. The maximum score is 36 with each parameter given a maximum score of 4 and minimum score of 1, in which 4 denotes normal nutrition and 1 denotes malnutrition. A neonate with a CAN score of less than 25 is considered to be malnourished.61 Various studies that were conducted to check the efficacy of the CAN score, showed that it was better than anthropometry, Ponderal index, weight for age, MAC/HC, and BMI for the diagnosis of IUGR.62–64
Cephalization Index
This index was postulated by Harel et al and they coined the term “cephalization index”. It is the ratio of the head circumference (HC) to body weight. They showed in their study that in severe IUGR, the ratio between the brain and the body was higher, and a higher cephalization index reflected a greater degree of brain vulnerability and increased likelihood of cerebral palsy and severe psychomotor retardation.65
Short-Term Complications
The IUGR neonates are prone to acquire separate complications after birth. A few of these complications include perinatal asphyxia, meconium aspiration, persistent pulmonary hypertension, hypothermia, hypoglycemia, hyperglycemia, hypocalcemia, polycythemia, jaundice, feeding difficulties, feed intolerance, necrotizing enterocolitis, late-onset sepsis, pulmonary hemorrhage, and so on (Fig. 6, Table 6).3,58 These infants also have neuro-behavioral abnormalities66 and low serum ferritin.67
Figure 6.
Immediate neonatal complications seen in intrauterine growth restricted neonates. Figure Copyright Deepak Sharma.
Table 6.
Immediate Complications of Intrauterine Growth Restricted Newborn.
| MORBIDITY | PATHOGENESIS/PATHOPHYSIOLOGY | PREVENTION/TREATMENT |
|---|---|---|
| Intrauterine fetal death | Usually result of Placental insufficiency causing chronic hypoxia Fetal congenital malformation Maternal and fetal infection Sentinel events like Abruptio placentae, cord rupture or prolapse Placental infarcts and preeclampsia |
Needs regular antepartum and intrapartum monitoring with planned delivery. Plan delivery in case of severe/worsening fetal distress in tertiary care level center |
| Neonatal Mortality | Antepartum, intrapartum and postpartum neonatal insults Contributed by other neonatal morbidities |
Tertiary level neonatal care |
| Perinatal/Neonatal Asphyxia | Chronic fetal hypoxia superadded with acute fetal hypoxia Acute sentinel event like Abruptio placentae, cord rupture or prolapse Placental abnormalities leading to insufficiency Pre-eclampsia/eclampsia |
Needs regular Antepartum and Intrapartum surveillance Regular fetal growth monitoring by USG and plotting on customized growth chart Early detection of IUGR/SGA Delivery at appropriate time and place having appropriate neonatal facilities Delivery attended by person skilled in neonatal resuscitation |
| Hypothermia | Poor thermoregulation mechanism Increased surface area with large head Poor subcutaneous and body fat leading to less thermogenesis and lower insulation Less brown fat Deficiency of catecholamine in body Increased insensible water loss through skin Other associated neonatal morbidities like Hypoglycemia and Hypoxia |
Warm delivery room with temperature from 26 to 28 °C Use cling wrap, heated mattress and warm humidified gases in delivery room Protect heat loss by radiation, conduction, convection and evaporation. Maintain thermo-neutral temperature in nursery Early breastfeeding Rooming in with mother/Warm Transport Early skin to skin contact in delivery room |
| Hypoglycemia | Poor glycogen stores of liver and muscles Poor other alternative energy source like ketones Decreased fat (adipose tissue) Decreased ability to oxidize free fatty acids and triglycerides for gluconeogenesis Poor gluconeogenesis and glycogenesis Decreased production of glucose Low level of counter-regulatory hormones like epinephrine and glucagon Secondary to other associated comorbidities including polycythaemia, hypoxia, hypothermia Heightened insulin receptors sensitivity |
Monitoring Blood sugar for initial 48–72 hours of post-natal life as per the protocol Early breast feeding within one hour of birth and if required formula supplementation Intravenous glucose when sugar is less than 25 mg/dl or symptomatic neonate |
| Hyperglycaemia | Low insulin production secondary to immature pancreas Insulin resistance Too much exogenous glucose infusion Increased epinephrine and glucagon level |
Sugar monitoring as per protocol Avoid high glucose concentration administration Treatment of symptomatic hyperglycaemia with infusion titration and insulin |
| Hypocalcemia | Decreased transfer of calcium in-utero Secondary to hypophosphatemia induced by chronic hypoxia. Immaturity of parathyroid glands |
Calcium supplementation Monitoring of calcium levels |
| Polycythaemia/Hyperviscosity/Leukoneutropenia | Placental insufficiency causes chronic intra-uterine hypoxia that leads to high fetal erythropoietin Transfusion of blood from mother to foetus |
Monitor haematocrit at 2, 12 and 24 hours after birth Regular feeding Prevent excessive postnatal weight loss Fluid supplementation and partial exchange transfusion if symptomatic |
| Persistent pulmonary hypertension (PPHN) | Abnormal pulmonary vasculature with thickened tunica media up-to intra-acinar arteries as result of chronic in-utero hypoxia Secondary to other associated morbidities like birth asphyxia, hypoglycemia, hypothermia, hypocalcemia, polycythaemia, hypoglycemia, and sepsis |
Avoid hypoxia and hyperoxia Normalization of metabolic milieu Cardiovascular support Selective and non-selective pulmonary vasodilator Mechanical ventilation if required |
| Pulmonary Haemorrhage |
Abnormal pulmonary vasculature Secondary to other associated co-morbidities like hypothermia, polycythaemia, asphyxia and neonatal sepsis |
Gentle ventilation Management of co-morbidities Supportive care for pulmonary hemorrhage |
| Meconium aspiration | Chronic in-utero hypoxia Intrapartum hypoxia secondary to any sentinel event |
Regular monitoring during intrapartum for meconium passage No role of amnio-infusion for prevention of meconium aspiration syndrome (MAS) Resuscitation as per the NRP 2015 guidelines Establish regular respiration. No role of tracheal suctioning for both vigorous/depressed newborns born with meconium stained liquor |
| Bronchopulmonary dysplasia (BPD) | Antenatal hits to fetal lung like chorioamnionitis, fetal infection and preeclampsia Abnormal pulmonary vasculature Post-natal insults to neonatal lungs like ventilation, hypoxia, hyperoxia, neonatal sepsis and Patent ductus arteriosus |
Antibiotics to mother in case of chorioamnionitis Gentle ventilation Preventing hypoxia, hyperoxia, and neonatal sepsis |
| Feed intolerance/Necrotizing enterocolitis (NEC) | Decreased intestinal perfusion secondary to redistribution of blood to vital organ in response to chronic hypoxia Focal intestinal ischemia Poor motility |
Minimal enteral nutrition to be given Protocolised increase in daily feeds Cautious start of enteral feeding Use of probiotics and lactoferrin Use only breast milk (either owns mothers milk or donor milk) Supportive treatment in case of development of NEC |
| Renal Problems | Chronic in-utero hypoxia and perinatal asphyxia leads to renal tubular injury | Cardiovascular support Maintain adequate renal perfusion |
| Immunodeficiency | Chronic in-utero and post-natal malnutrition Congenital infection Reduced number of T and B lymphocytes Poor immunological maturity |
Early, aggressive and optimal nutrition Promoting breast feeding Prevention of neonatal sepsis |
| Retinopathy of prematurity (ROP) | Intrauterine hypoxia Altered levels of growth factors Diminished antioxidant capacity Post-natal insults like hyperoxia, hypoxia, and sepsis |
Targeted saturation (90–95%) ROP screening of susceptible Treatment if required |
| Ferritin | Low levels Defective transport through placenta Increased premature delivery |
Long-Term Complications
These infants are prone to have poor growth and neurodevelopment outcome when they reach the school-going age and adulthood. They are also more susceptible to develop adult-onset diseases in their infancy and adolescence (Fig. 7).
Figure 7.
Increased risk for various physical and neurodevelopmental problems in intrauterine growth restricted neonates when they reach their childhood and adulthood. Figure Copyright Deepak Sharma.
Physical Developmental Outcome
The postnatal factor that affects the growth in these infants includes the cause of growth retardation (most important), postnatal nutrition, parents’ economic status, and the social environment in which these infants grow. Infants with symmetrical IUGR because of less cell numbers at birth are underdeveloped postnatally and usually remain small throughout their lives. On the other hand, those with asymmetrical IUGR have good prognosis and have good postnatal growth because of normal cell numbers.68–70 In the study conducted by Leger et al, the investigator concluded that in these IUGR infants, the final height and individual height gain is impacted by the mother’s height, father’s height, and birth length.71
Neurodevelopmental Outcome
The IUGR infants are more prone to develop subtle to major cognitive and neurodevelopmental abnormalities. Many interventions (antenatal and postnatal) have been done to reduce the brain damage that occurs because of IUGR and leads to poor neurodevelopmental outcome. These include taurine and melatonin supplementation, Newborn Individuali zed Developmental and Assessment Program System (NIDCAP) and targeted medical rehabilitation.72 The common neurological problems observed in these children include the following:73–76
Lower scores on cognitive testing77
Difficulties in schools or require special education
Gross motor and minor neurologic dysfunction
Behavioral problems (attention deficit hyperactivity syndrome)
Growth failure
Lower strength and work capacity
Cerebral palsy
Low social competence
Poor academic performance
Lower levels of intelligence
Hyperactive behavior
Poor perceptual performance
Poor visuo-motor perception, motor incompetence, reading, and mathematics learning
Developmental Origin of Health and Disease
Barker, in his observational studies, showed that infants who were born in the 1920s and 1930s with low weight, when they grew up to adulthood had high incidence of coronary heart disease, diabetes mellitus, hyperinsulinemia, and hypercholesterolemia.78–80 This observation was confirmed in other studies and it was postulated that fetal life insult gave pathway to these adult diseases.81–83 This was known initially as fetal origin of adult disease (FOAD), that has been replaced now with the term “developmental origin of health and disease (DoHaD).”84 Three different hypotheses have been purposed for this causal relationship, namely, fetal insulin hypothesis and mature onset diabetes of the young (MODY) genes, thrifty genotype, and thrifty phenotype (Barker hypothesis). The Barker hypothesis is the most accepted theory for DoHaD.85 These IUGR infants are susceptible to a number of adult diseases in their lifetime (Table 7 and Fig. 8).86
Table 7.
Various “developmental origin of health and diseases (DoHaD)” seen in IUGR neonates in adulthood.
| • Hypertension • Ischemic Heart disease/Stroke • Type 2 diabetes mellitus • Kidney disease • Liver disease • Hypercholesterolemia • Metabolic syndrome X • Obesity • Lung abnormalities- reactive airways disease • Cancer- breast, ovarian, colon, lung, blood • Schizophrenia/Parkinsonism • Alzheimer disease • Polycystic ovarian syndrome, premature pubarche • Shortened life span • Depression, anxiety, bipolar disorder • Immune dysfunction • Osteoporosis • Social problems • Poor cognitive performance |
Note: Adapted from Sharma D, Farahbakhsh N, Shastri S, Sharma P. Intrauterine growth restriction–part 2. J Matern Fetal Neonatal Med. 2016 Mar 15:1–12. [Epub ahead of print] PubMed PMID: 26979578 with permission
Figure 8.
Figure showing various adult disease the IUGR infant is prone to develop in his adulthood as per “Developmental origin of health and diseases (DoHaD)”. IUGR infants undergoes epigenetic modification in-utero and postnatally have abnormal nutrition and growth leading to various disease of adulthood in these infants. Figure Copyright Deepak Sharma.
Fetal Insulin Hypothesis and MODY Genes
This hypothesis was proposed by Hattersley et al and it pointed to the association that existed between the genes causing both LBW and increased risk of type 2 diabetes mellitus. They postulated that genetically determined insulin resistance will lead to decreased insulin-mediated growth in the fetus and that it will also lead to insulin resistance in adulthood leading to type 2 diabetes. As a result of insulin resistance, these IUGR infants have abnormal vascular development in fetal life and early childhood, that lead to increased risk of hypertension and vascular disease. They concluded that predisposition to type 2 diabetes and vascular disease is common output of both genetic and fetal environmental factors. This fetal insulin hypothesis is supported by individuals who suffer from MODY type 2. These individuals have mutations in the glucokinase gene that result in decreased insulin secretion, reduced fetal growth, and MODY2.87
Thrifty Genotype
This theory was purposed by Neel in 1962 and he purposed that the genes that are responsible for causing diabetes in any individual have been retained in the genome of all individuals because of natural selection, as they are beneficial to the infants. These genes have greater capacity to store fat during starvation and undernourishment, and in recent times, these genes have started contributing toward detrimental body conditions because of overeating and lack of exercise, thereby leading to early onset of obesity.88,89
Thrifty Phenotype (Barker Hypothesis)
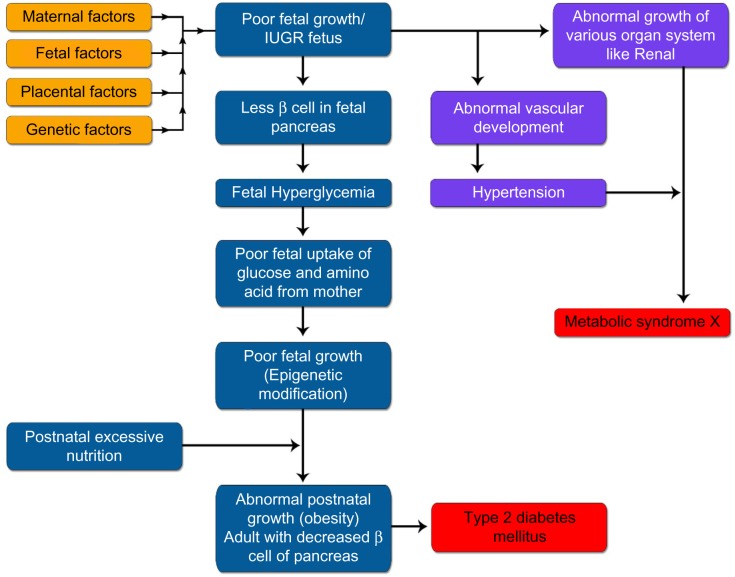
This is the most accepted hypothesis for explaining DoHaD. This hypothesis proposes that early-life environment has long-term effects on the latter life.90 A majority of present days’ work done to confirm the relationship between IUGR and DoHaD is being done to establish this theory. It states that when the antenatal environmental conditions were adverse for the growing fetus because of any reasons concerning the maternal (maternal environment, maternal genome, and microbiome), placental, or fetal aspects, the fetus adopted itself to this hostile environment to survive in-utero. These fetal adaptations include the brain-sparing effect at the expense of other organ systems, and reduced production and sensitivity to the fetal insulin and ILGF-I and also by the upregulation of the hypothalamo-pitutary adrenal (HPA) axis. This metabolic programming (epigenetic modification ie, those molecular mechanisms affecting gene expression patterns without causing alterations in DNA base sequence) occurs at the critical time window of fetal development; hence, these epigenetic changes become permanent or “programmed” in the genes of the fetus.91 These antenatal epigenetic changes are superadded with postnatal mismatched (normal or excessive nutrition) and suboptimal environment (sedentary life, less physical activity, and sedentary life habits) and are also influenced by child’s genetics and epigenetics, and this postnatal mismatching leads to abnormal growth and make them susceptible to DoHaD (Fig. 9).92 These epigenetic changes are responsible for the prevalence of obesity in childhood and adulthood and also results in other DoHaD (Table 7). These epigenetic modifications can be a result of environmental chemicals, nutritional perturbations during development, and prenatal stress. These modifications lead to altered gene expression further leading to altered cell proteins, or altered numbers and/or location of cells.84 Recently, DOHaD working groups have been established in Australia and New Zealand to work on this area and they have predefined goals and motives. ActEarly: this birth cohorts working group was established in 201493 There are many cohort studies going on around the world to verify this hypothesis of DoHaD and many completed studies have shown that both antenatal and postnatal programing are responsible for DoHaD. The Southampton Women’s Survey (SWS) enrolled between 1998 and 2002, 12,579 female patients aged 20 to 34 years and pre-pregnancy characteristics were obtained. They had around 3,000 live births, and these newborns were studied for birth phenotype and their outcome in infancy and childhood.94 The results of this study showed that continued exposure to diets of low quality across early childhood was linked to adiposity at the age of six years95; the timing of the eruption of primary dentition (one to two years of age) is affected by maternal smoking during pregnancy, socioeconomic status, size at birth, maternal ethnicity, and physical activity (assessed by reported walking speed)96; higher maternal dietary glycemic index (GI) and glycemic load (GL) in early pregnancy were associated with greater adiposity in childhood97; and consumption of higher oily fish during late pregnancy was associated with reduced aortic stiffness in the child at the age of nine years, with potential long-term consequences for later cardiovascular risk.98 Taveras et al studied 559 children in Project Viva and measured the length and weight at birth, six months, and three years. They showed that more rapid increases in weight for length in the first six months of life were associated with sharply increased risk of obesity at three years of age. They postulated that changes in weight status in infancy can be a greater risk for later obesity more than weight status at birth. This could be because of new programming effects seen postnatally.99 Perng et al studied these children of Project Viva and reported that the BMI Z score gain after one year was associated with greater mid-childhood adiposity and this was not affected by birth size, and the long-term influence of weight gain during the first postnatal year could depend on size at birth.100 The same cohort was followed till 10 years of age and the results showed that more rapid gain in BMI during the first six postnatal months and in the preschool years (till three years) may lead to higher systolic blood pressure in mid-childhood (6–10 years) regardless of the size at birth.101
Figure 9.
Barker Hypothesis (Thrifty phenotype) explaining the Fetal Origin of Adult Disease (FOAD) or “Developmental origin of health and diseases (DoHaD)” in IUGR infants. Figure Copyright Deepak Sharma.
Conclusions
IUGR is an important health problem of developing countries around the world. There are multiple causes for IUGR including maternal, fetal, placental, and genetic factors. Mothers with high risk factors for IUGR fetus should be followed up closely for any complications. The IUGR fetus needs an early diagnosis and management so that neonatal and perinatal mortality can be minimized. SGA is defined a neonate who is born with weight less than 10 centile for gestational age. There are mainly two types of IUGR, symmetrical and asymmetrical depending on the gestation of onset and etiology of IUGR. These infants with IUGR have both short-term and long-term complications, which make them high-risk neonates. The short-term problems include perinatal asphyxia, meconium aspiration, persistent pulmonary hypertension, hypothermia, hypoglycemia, hyperglycemia, hypocalcemia, polycythemia, jaundice, feeding difficulties, feed intolerance, necrotizing enterocolitis, late-onset sepsis, and pulmonary hemorrhage. The long-term problems include abnormal physical growth and neurodevelopmental outcome. These infants are more likely to develop adult onset disease because of fetal epigenetic changes. These infants need to be monitored for both short-term and long-term complications (Fig. 10).
Figure 10.

Follow up programme of infants who are born with intrauterine growth restriction. Figure copyright Deepak Sharma.
Acknowledgments
My thanks are due to Shri Keshave Dev Sharma and Smt Rajkumari Sharma, my parents, who have been a continuous source of inspiration and hard work. I also thank Dr. Sweta Sharma, my wife and Dr. Pradeep Sharma, my younger brother, who have always been supportive and helped me in all issues related to manuscript preparation.
Footnotes
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 739 words, excluding any confidential comments to the academic editor.
ACADEMIC EDITOR: Praveen Kumar, Editor in Chief
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: DS, SS. Analyzed the data: SS, PS. Wrote the first draft of the manuscript: DS. Contributed to the writing of the manuscript: SS, PS. Agree with manuscript results and conclusions: DS, PS, SS. Jointly developed the structure and arguments for the paper: DS, PS, SS. Made critical revisions and approved final version: DS, PS, SS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71(2):159–63. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction–part 1. J Matern Fetal Neonatal Med. 2016;7:1–11. [Google Scholar]
- 3.Sharma D, Farahbakhsh N, Shastri S, Sharma P. Intrauterine growth restriction – part 2. J Matern Fetal Neonatal Med. 2016;0(0):1–12. [Google Scholar]
- 4.de Onis M, Blössner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52(Suppl 1):S5–15. [PubMed] [Google Scholar]
- 5.Lee PA, Chernausek SD, Hokken-Koelega ACS, Czernichow P, International Small for Gestational Age Advisory Board International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics. 2003;111(6 pt 1):1253–61. doi: 10.1542/peds.111.6.1253. [DOI] [PubMed] [Google Scholar]
- 6.Singh M. Disorders of weight and gestation. In: Singh M, editor. In Care of the Newborn. 5th ed. New Delhi: Sagar Publications; 1999. pp. 224–45. [Google Scholar]
- 7.Hendrix N, Berghella V. Non-placental causes of intrauterine growth restriction. Semin Perinatol. 2008;32(3):161–5. doi: 10.1053/j.semperi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Tranquilli AL, Bezzeccheri V, Giannubilo SR, Scagnoli C, Mazzanti L, Garzetti GG. Amniotic levels of nitric oxide in women with fetal intrauterine growth restriction. J Matern Fetal Neonatal Med. 2003;13(2):115–8. doi: 10.1080/jmf.13.2.115.118. [DOI] [PubMed] [Google Scholar]
- 9.Serin S, Bakacak M, Ercan Ö, et al. The evaluation of Nesfatin-1 levels in patients with and without intrauterine growth restriction. J Matern Fetal Neonatal Med. 2016;29(9):1409–13. doi: 10.3109/14767058.2015.1049524. [DOI] [PubMed] [Google Scholar]
- 10.Coata G, Pennacchi L, Bini V, Liotta L, Renzo GCD. Soluble adhesion molecules: marker of pre-eclampsia and intrauterine growth restriction. J Matern Fetal Neonatal Med. 2002;12(1):28–34. doi: 10.1080/jmf.12.1.28.34. [DOI] [PubMed] [Google Scholar]
- 11.Laskowska M, Laskowska K, Leszczyńska-Gorzelak B, Oleszczuk J. Asymmetric dimethylarginine in normotensive pregnant women with isolated fetal intrauterine growth restriction: a comparison with preeclamptic women with and without intrauterine growth restriction. J Matern Fetal Neonatal Med. 2011;24(7):936–42. doi: 10.3109/14767058.2010.535873. [DOI] [PubMed] [Google Scholar]
- 12.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127(5):515–26. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 13.Carver TD, Anderson SM, Aldoretta PA, Esler AL, Hay WW. Glucose suppression of insulin secretion in chronically hyperglycemic fetal sheep. Pediatr Res. 1995;38(5):754–62. doi: 10.1203/00006450-199511000-00020. [DOI] [PubMed] [Google Scholar]
- 14.D’Ercole AJ, Dai Z, Xing Y, et al. Brain growth retardation due to the expression of human insulin like growth factor binding protein-1 in transgenic mice: an in vivo model for the analysis of IGF function in the brain. Brain Res Dev Brain Res. 1994;82(1–2):213–22. doi: 10.1016/0165-3806(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 15.Ye P, Carson J, D’Ercole AJ. In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. J Neurosci. 1995;15(11):7344–56. doi: 10.1523/JNEUROSCI.15-11-07344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delhanty PJ, Han VK. The expression of insulin-like growth factor (IGF)-binding protein-2 and IGF-II genes in the tissues of the developing ovine fetus. Endocrinology. 1993;132(1):41–52. doi: 10.1210/endo.132.1.7678219. [DOI] [PubMed] [Google Scholar]
- 17.Wood TL, Rogler L, Streck RD, et al. Targeted disruption of IGFBP-2 gene. Growth Regul. 1993;3(1):5–8. [PubMed] [Google Scholar]
- 18.Hill JM, Agoston DV, Gressens P, McCune SK. Distribution of VIP mRNA and two distinct VIP binding sites in the developing rat brain: relation to ontogenic events. J Comp Neurol. 1994;342(2):186–205. doi: 10.1002/cne.903420204. [DOI] [PubMed] [Google Scholar]
- 19.Saki F, Dabbaghmanesh MH, Ghaemi SZ, Forouhari S, Ranjbar Omrani G, Bakhshayeshkaram M. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int J Endocrinol Metab. 2014;12(4):e19378. doi: 10.5812/ijem.19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. 2010;281(2):215–20. doi: 10.1007/s00404-009-1105-1. [DOI] [PubMed] [Google Scholar]
- 21.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–69. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 22.Fowden AL. Endocrine regulation of fetal growth. Reprod Fertil Dev. 1995;7(3):351–63. doi: 10.1071/rd9950351. [DOI] [PubMed] [Google Scholar]
- 23.GRIT Study Group A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG. 2003;110(1):27–32. doi: 10.1046/j.1471-0528.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 24.Campbell S, Thoms A. Ultrasound measurement of the fetal head to abdomen circumference ratio in the assessment of growth retardation. Br J Obstet Gynaecol. 1977;84(3):165–74. doi: 10.1111/j.1471-0528.1977.tb12550.x. [DOI] [PubMed] [Google Scholar]
- 25.Crane JP, Kopta MM. Prediction of intrauterine growth retardation via ultrasonically measured head/abdominal circumference ratios. Obstet Gynecol. 1979;54(5):597–601. [PubMed] [Google Scholar]
- 26.Divon MY, Guidetti DA, Braverman JJ, Oberlander E, Langer O, Merkatz IR. Intrauterine growth retardation – a prospective study of the diagnostic value of real-time sonography combined with umbilical artery flow velocimetry. Obstet Gynecol. 1988;72(4):611–4. [PubMed] [Google Scholar]
- 27.da Costa IT, Leone CR. Intrauterine growth restriction influence on the nutritional evolution and growth of preterm newborns from birth until discharge. Rev Paul Pediatr. 2009;27(1):15–20. [Google Scholar]
- 28.Illanes S, Soothill P. Management of fetal growth restriction. Semin Fetal Neonatal Med. 2004;9(5):395–401. doi: 10.1016/j.siny.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Royal College of Obstetricians & Gynaecologists Small-for-Gestational-Age Fetus, Investigation and Management (Green-top Guideline No. 31) [Internet] 2015. [cited 2015 Dec 17]. Available at: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg31/
- 30.Miller J, Turan S, Baschat AA. Fetal growth restriction. Semin Perinatol. 2008;32(4):274–80. doi: 10.1053/j.semperi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Lalor JG, Fawole B, Alfirevic Z, Devane D. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database Syst Rev. 2008;(1):CD000038. doi: 10.1002/14651858.CD000038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueras F, Gratacos E. Stage-based approach to the management of fetal growth restriction. Prenat Diagn. 2014;34(7):655–9. doi: 10.1002/pd.4412. [DOI] [PubMed] [Google Scholar]
- 33.Oros D, Figueras F, Cruz-Martinez R, Meler E, Munmany M, Gratacos E. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet Gynecol. 2011;37(2):191–5. doi: 10.1002/uog.7738. [DOI] [PubMed] [Google Scholar]
- 34.Alfirevic Z, Stampalija T, Gyte GML. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2013;11:CD007529. doi: 10.1002/14651858.CD007529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris RK, Say R, Robson SC, Kleijnen J, Khan KS. Systematic review and meta-analysis of middle cerebral artery Doppler to predict perinatal wellbeing. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):141–55. doi: 10.1016/j.ejogrb.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Arbeille P, Maulik D, Fignon A, et al. Assessment of the fetal PO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol. 1995;21(7):861–70. doi: 10.1016/0301-5629(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 37.Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992;79(3):416–20. doi: 10.1097/00006250-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Ferrazzi E, Bozzo M, Rigano S, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol. 2002;19(2):140–6. doi: 10.1046/j.0960-7692.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 39.Cosmi E, Ambrosini G, D’Antona D, Saccardi C, Mari G. Doppler, cardiotocography, and biophysical profile changes in growth-restricted fetuses. Obstet Gynecol. 2005;106(6):1240–5. doi: 10.1097/01.AOG.0000187540.37795.3a. [DOI] [PubMed] [Google Scholar]
- 40.Hecher K, Snijders R, Campbell S, Nicolaides K. Fetal venous, intracardiac, and arterial blood flow measurements in intrauterine growth retardation: relationship with fetal blood gases. Am J Obstet Gynecol. 1995;173(1):10–5. doi: 10.1016/0002-9378(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 41.Baschat AA, Gembruch U, Weiner CP, Harman CR. Qualitative venous Doppler waveform analysis improves prediction of critical perinatal outcomes in premature growth-restricted fetuses. Ultrasound Obstet Gynecol. 2003;22(3):240–5. doi: 10.1002/uog.149. [DOI] [PubMed] [Google Scholar]
- 42.Yagel S, Kivilevitch Z, Cohen SM, et al. The fetal venous system, Part II: ultrasound evaluation of the fetus with congenital venous system malformation or developing circulatory compromise. Ultrasound Obstet Gynecol. 2010;36(1):93–111. doi: 10.1002/uog.7622. [DOI] [PubMed] [Google Scholar]
- 43.Fouron JC, Gosselin J, Raboisson MJ, et al. The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency. Am J Obstet Gynecol. 2005;192(2):497–503. doi: 10.1016/j.ajog.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Mäkikallio K, Jouppila P, Räsänen J. Retrograde aortic isthmus net blood flow and human fetal cardiac function in placental insufficiency. Ultrasound Obstet Gynecol. 2003;22(4):351–7. doi: 10.1002/uog.232. [DOI] [PubMed] [Google Scholar]
- 45.Fouron JC, Skoll A, Sonesson SE, Pfizenmaier M, Jaeggi E, Lessard M. Relationship between flow through the fetal aortic isthmus and cerebral oxygenation during acute placental circulatory insufficiency in ovine fetuses. Am J Obstet Gynecol. 1999;181(5 pt 1):1102–7. doi: 10.1016/s0002-9378(99)70089-x. [DOI] [PubMed] [Google Scholar]
- 46.Mari G, Hanif F, Drennan K, Kruger M. Staging of intrauterine growth-restricted fetuses. J Ultrasound Med. 2007;26(11):1469–77. doi: 10.7863/jum.2007.26.11.1469. quiz 1479. [DOI] [PubMed] [Google Scholar]
- 47.Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86–98. doi: 10.1159/000357592. [DOI] [PubMed] [Google Scholar]
- 48.Bhutta ZA, Das JK, Bahl R, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384(9940):347–70. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 49.Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015;6:CD000032. doi: 10.1002/14651858.CD000032.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2015;11:CD004905. doi: 10.1002/14651858.CD004905.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jabeen M, Yakoob MY, Imdad A, Bhutta ZA. Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths. BMC Public Health. 2011;11(suppl 3):S6. doi: 10.1186/1471-2458-11-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009;(3):CD001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001;108(8):830–4. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 55.Marconi AM, Ronzoni S, Bozzetti P, Vailati S, Morabito A, Battaglia FC. Comparison of fetal and neonatal growth curves in detecting growth restriction. Obstet Gynecol. 2008;112(6):1227–34. doi: 10.1097/AOG.0b013e31818bdc7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wollmann HA. Intrauterine growth restriction: definition and etiology. Horm Res. 1998;49(Suppl 2):1–6. [PubMed] [Google Scholar]
- 57.Rosenberg A. The IUGR newborn. Semin Perinatol. 2008;32(3):219–24. doi: 10.1053/j.semperi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Murki S, Sharma D. Intrauterine growth retardation – a review article. J Neonatal Biol. 2014. Available at: http://www.omicsgroup.org/journals/neonatal-biology-abstract.php?abstract_id=25766.
- 59.Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966;37(3):403–8. [PubMed] [Google Scholar]
- 60.Kanawati AA, McLaren DS, Abu-Jawdeh I. Failure to thrive in Lebanon. I. Experience with some simple somatic measurements. Acta Paediatr Scand. 1971;60(3):309–16. doi: 10.1111/j.1651-2227.1971.tb06662.x. [DOI] [PubMed] [Google Scholar]
- 61.Metcoff J. Clinical assessment of nutritional status at birth. Fetal malnutrition and SGA are not synonymous. Pediatr Clin North Am. 1994;41(5):875–91. doi: 10.1016/s0031-3955(16)38836-8. [DOI] [PubMed] [Google Scholar]
- 62.Mehta S, Tandon A, Dua T, Kumari S, Singh SK. Clinical assessment of nutritional status at birth. Indian Pediatr. 1998;35(5):423–8. [PubMed] [Google Scholar]
- 63.Soundarya M, Basavaprabhu A, Raghuveera K, Baliga B, Shivanagaraja B. Comparative assessment of fetal malnutrition by anthropometry and CAN Score. Iran J Pediatr. 2012;22(1):70–6. [PMC free article] [PubMed] [Google Scholar]
- 64.Adebami OJ, Owa JA. Comparison between CANSCORE and other anthropometric indicators in fetal malnutrition. Indian J Pediatr. 2008;75(5):439–42. doi: 10.1007/s12098-008-0069-7. [DOI] [PubMed] [Google Scholar]
- 65.Harel S, Tomer A, Barak Y, Binderman I, Yavin E. The cephalization index: a screening device for brain maturity and vulnerability in normal and intrauterine growth retarded newborns. Brain Dev. 1985;7(6):580–4. doi: 10.1016/s0387-7604(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 66.Padidela RNR, Bhat V. Neurobehavioral assessment of appropriate for gestational and small for gestational age babies. Indian Pediatr. 2003;40(11):1063–8. [PubMed] [Google Scholar]
- 67.Mukhopadhyay K, Yadav RK, Kishore SS, Garewal G, Jain V, Narang A. Iron status at birth and at 4 weeks in preterm-SGA infants in comparison with preterm and term-AGA infants. J Matern Fetal Neonatal Med. 2012;25(8):1474–8. doi: 10.3109/14767058.2011.643328. [DOI] [PubMed] [Google Scholar]
- 68.Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW. Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics. 1999;104(3):e33. doi: 10.1542/peds.104.3.e33. [DOI] [PubMed] [Google Scholar]
- 69.Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW. Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 1998;152(12):1225–31. doi: 10.1001/archpedi.152.12.1225. [DOI] [PubMed] [Google Scholar]
- 70.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38(5):733–9. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 71.Leger J, Limoni C, Collin D, Czernichow P. Prediction factors in the determination of final height in subjects born small for gestational age. Pediatr Res. 1998;43(6):808–12. doi: 10.1203/00006450-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Fu W, Liu J. Neurodevelopment in children with intrauterine growth restriction: adverse effects and interventions. J Matern Fetal Neonatal Med. 2016;29(4):660–8. doi: 10.3109/14767058.2015.1015417. [DOI] [PubMed] [Google Scholar]
- 73.Allen MC. Developmental outcome and followup of the small for gestational age infant. Semin Perinatol. 1984;8(2):123–56. [PubMed] [Google Scholar]
- 74.Guellec I, Lapillonne A, Renolleau S, et al. Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127(4):e883–91. doi: 10.1542/peds.2010-2442. [DOI] [PubMed] [Google Scholar]
- 75.Morsing E, Asard M, Ley D, Stjernqvist K, Marsál K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127(4):e874–82. doi: 10.1542/peds.2010-1821. [DOI] [PubMed] [Google Scholar]
- 76.Kutschera J, Urlesberger B, Maurer U, Müller W. Small for gestational age – Somatic, neurological and cognitive development until adulthood. Z Geburtshilfe Neonatol. 2002;206(2):65–71. doi: 10.1055/s-2002-30139. [DOI] [PubMed] [Google Scholar]
- 77.Løhaugen GCC, Oslash;stgård HF, Andreassen S, et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163(2):447–53. doi: 10.1016/j.jpeds.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 78.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301(6746):259–62. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 80.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bo S, Cavallo-Perin P, Scaglione L, Ciccone G, Pagano G. Low birthweight and metabolic abnormalities in twins with increased susceptibility to Type 2 diabetes mellitus. Diabet Med. 2000;17(5):365–70. doi: 10.1046/j.1464-5491.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- 82.Barnett AH, Eff C, Leslie RD, Pyke DA. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981;20(2):87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- 83.Baird J, Osmond C, MacGregor A, Snieder H, Hales CN, Phillips DI. Testing the fetal origins hypothesis in twins: the Birmingham twin study. Diabetologia. 2001;44(1):33–9. doi: 10.1007/s001250051577. [DOI] [PubMed] [Google Scholar]
- 84.Heindel JJ, Balbus J, Birnbaum L, et al. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156(10):3416–21. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matharu K, Ozanne SE. The fetal origins of disease and associations with low birthweight. NeoReviews. 2004;5(12):e522–6. [Google Scholar]
- 86.Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care. 2011;41(6):158–76. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hattersley AT, Tooke JE. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet. 1999;353(9166):1789–92. doi: 10.1016/S0140-6736(98)07546-1. [DOI] [PubMed] [Google Scholar]
- 88.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 89.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? 1962. Bull World Health Organ. 1999;77(8):694–703. [PMC free article] [PubMed] [Google Scholar]
- 90.Fukuoka H. DOHaD (developmental origins of health and disease) and birth cohort research. J Nutr Sci Vitaminol (Tokyo) 2015;61(Suppl):S2–4. doi: 10.3177/jnsv.61.S2. [DOI] [PubMed] [Google Scholar]
- 91.Huang RC, Prescott SL, Godfrey KM, Davis EA. Assessment of cardiometabolic risk in children in population studies: underpinning developmental origins of health and disease mother–offspring cohort studies. J Nutr Sci. 2015;4:e12. doi: 10.1017/jns.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 93.Prescott SL, Allen K, Armstrong K, et al. The establishment of DOHaD working groups in Australia and New Zealand. J Dev Orig Health Dis. 2016;7:1–7. doi: 10.1017/S2040174416000167. [DOI] [PubMed] [Google Scholar]
- 94.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: the Southampton Women’s Survey. Int J Epidemiol. 2006;35(1):42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okubo H, Crozier SR, Harvey NC, et al. Diet quality across early childhood and adiposity at 6 years: the Southampton Women’s Survey. Int J Obes (Lond) 2015;39(10):1456–62. doi: 10.1038/ijo.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ntani G, Day PF, Baird J, et al. Maternal and early life factors of tooth emergence patterns and number of teeth at 1 and 2 years of age. J Dev Orig Health Dis. 2015;6(4):299–307. doi: 10.1017/S2040174415001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okubo H, Crozier SR, Harvey NC, et al. Maternal dietary glycemic index and glycemic load in early pregnancy are associated with offspring adiposity in childhood: the Southampton Women’s Survey. Am J Clin Nutr. 2014;100(2):676–83. doi: 10.3945/ajcn.114.084905. [DOI] [PubMed] [Google Scholar]
- 98.Bryant J, Hanson M, Peebles C, et al. Higher oily fish consumption in late pregnancy is associated with reduced aortic stiffness in the child at age 9 years. Circ Res. 2015;116(7):1202–5. doi: 10.1161/CIRCRESAHA.116.305158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perng W, Hajj H, Belfort MB, et al. Birth size, early life weight gain, and mid-childhood cardiometabolic health. J Pediatr. 2016;173:122.e–130.e. doi: 10.1016/j.jpeds.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perng W, Rifas-Shiman SL, Kramer MS, et al. Early weight gain, linear growth, and mid-childhood blood pressure: a prospective study in project viva. Hypertension. 2016;67(2):301–8. doi: 10.1161/HYPERTENSIONAHA.115.06635. [DOI] [PMC free article] [PubMed] [Google Scholar]