Abstract
The existence and functional relevance of efferent optic nerve fibers in mammals have long been debated. While anatomical evidence for cortico-retinal and retino-retinal projections is substantial, physiological evidence is lacking, as efferent fibers are few in number and are severed in studies of excised retinal tissue. Here we show that interocular connections contribute to retinal bioelectrical activity in adult mammals. Full-field flash electroretinograms (ERGs) were recorded from one or both eyes of Brown-Norway rats under dark-adapted (n = 16) and light-adapted (n = 11) conditions. Flashes were confined to each eye by an opaque tube that blocked stray light. Monocular flashes evoked a small (5–15 μV) signal in the nonilluminated eye, which was named “crossed ERG” (xERG). The xERG began under dark-adapted conditions with a positive (xP1) wave that peaked at 70–90 ms and ended with slower negative (xN1) and positive (xP2) waves from 200 to 400 ms. xN1 was absent under light-adapted conditions. Injection of tetrodotoxin in either eye (n = 15) eliminated the xERG. Intraocular pressure elevation of the illuminated eye (n = 6) had the same effect. The treatments also altered the ERG b-wave in both eyes, and the alterations correlated with xERG disappearance. Optic nerve stimulation (n = 3) elicited a biphasic compound action potential in the nonstimulated nerve with 10- to 13-ms latency, implying that the xERG comes from slow-conducting (W type) fibers. Monocular dye application (n = 7) confirmed the presence of retino-retinal ganglion cells in adult rats. We conclude that mammalian eyes communicate directly with each other via a handful of optic nerve fibers. The cross talk alters retinal activity in rats, and perhaps other animals.
Keywords: centrifugal fibers, retino-retinal projection, retinopetal fibers, electroretinogram
the standard view of the visual system stipulates that the axons of retinal ganglion cells exit the eyes to form the optic nerves, with nasal fibers projecting along the contralateral optic tract and temporal fibers along the ipsilateral optic tract to the lateral geniculate nucleus and other brain areas. This view applies primarily to the adult visual system. A diversity of connections exists during embryogenesis and early development, which gets pruned to form the mature pathway (Innocenti 1981; McLoon and Lund 1982; Nakamura and O'Leary 1989). For example, it has been reported that a cluster of retinal ganglion cells (∼5–200) sends axons to the opposite eye in frogs (Bohn and Stelzner 1979, 1981; Tennant et al. 1993; Toth and Straznicky 1989), rats (Avellaneda-Chevrier et al. 2015; Bunt et al. 1983; Bunt and Lund 1981; Gastinger et al. 2006; Nadal-Nicolas et al. 2015; Sefton and Lam 1984), and rabbits (Muller and Hollander 1988). The number of retino-retinal axons peaks at birth and dwindles into adulthood, so most studies have not ascribed any particular function to the misrouted cells. Those that remain are presumed either to be in the process of degeneration (Bohn and Stelzner 1979) or to have branches that synapse with proper targets elsewhere in the visual system (Nadal-Nicolas et al. 2015; Tennant et al. 1993).
A role of retino-retinal connections in vision cannot, however, be excluded solely based on low axon counts in adulthood. Beginning with Cajal's work over a century ago, anatomical studies have shown that the optic nerves of adult mammals, including humans (Honrubia and Elliott 1968), contain sparse populations of efferent fibers that originate from multiple locations, including the brain stem and hypothalamus (Gastinger et al. 2006; Labandeira-Garcia et al. 1990; Reperant et al. 2006; Terubayashi et al. 1983), in addition to the contralateral eye. Some may even come from auditory, olfactory, and somatosensory centers (Francis et al. 2013; Nikitopoulou-Maratou et al. 1980; Spinelli and Weingarten 1966; Stell et al. 1984; Walker and Stell 1986). The efferent fibers branch widely in the eye, covering over a quarter of the retinal surface (Gastinger et al. 2006). Their branches terminate in the inner and outer plexiform layers, inner nuclear layer, and ganglion cell layer depending on fiber type (Gastinger et al. 2001; Honrubia and Elliott 1970; Schutte 1995; Usai et al. 1991). Hence, there may be few retinal efferents in adult mammals, but activation of just one could have broad influence on visual information processing.
Despite the anatomical evidence for efferent optic nerve fibers, centrifugal control of retinal function in mammals has long been a controversial subject (Brindley and Hamasaki 1966; Schnyder and Kunzle 1984). Fueling the controversy is a lack of convincing physiological evidence for efferent effects on retinal activity. Early research on the electroretinogram (ERG) noted that illumination of one eye evoked a small ERG-like signal in the other eye of cats (Auerbach and Feinsod 1973) and that the amplitude of the ERG b-wave was reduced in humans if both eyes were illuminated (Hellner 1964; Motokawa et al. 1956). However, interocular effects were subsequently attributed to methodological artifacts, including light scatter into the nonilluminated eye, consensual pupillary or blink reflexes, and volume conduction of neural signals from the illuminated eye to the contralateral eye electrode (Chou and Porciatti 2012; Horsten et al. 1961; Johnson and Massof 1982; Nagaya et al. 1962; Peachey et al. 1983; Seiple and Siegel 1983). Little to no research has since been reported on retino-retinal interactions in mammals. There has been some support for cortico-retinal interactions. Electrical stimulation of the optic tract in rabbits and dorsal raphe nucleus in rats was shown to induce antidromic spikes that invade the retina (Dodt 1956) and to alter the ERG b-wave and oscillatory potentials (OPs) (Lorincz et al. 2008). ERG changes were also observed after severing or pharmacologically blocking the optic nerve of rabbits and cats, though not always of the same form (decrease in a-wave, increase in b-wave, and no effect on b-wave but increase in OPs) (Auerbach and Feinsod 1973; Jacobson and Suzuki 1962; Molotchnikoff et al. 1989; van Hasselt 1969) and not by everyone (Brindley and Hamasaki 1962). Efferent optic nerve fibers were implicated as well by the centrifugal effect of cortical cryoinactivation (Molotchnikoff and Tremblay 1983, 1986) on retinal ganglion cell spike trains and slow-wave and REM sleep (Galambos et al. 1994, 2001) on the amplitude of light-evoked retinal and optic nerve signals in rats.
We investigated interocular communication by recording the bioelectrical activity of both eyes while visually stimulating one eye of adult rats. We describe a new light-evoked signal that can be recorded in the eye that is not illuminated. We present multiple lines of evidence that this signal is neural in origin and comes from the illuminated eye. We show that the cross talk is likely mediated by a small group of ganglion cells that send axons to the opposite eye and that the spike activity of these cells alters light responses in the recipient retina.
MATERIALS AND METHODS
Animal preparation.
Brown-Norway rats (27 males, 6–8 mo, 300–400 g; Harlan Laboratories) were housed under a 12:12-h light-dark cycle and fed a standard daily diet. On the day of experimentation the animal was anesthetized with an intraperitoneal injection of ketamine hydrochloride (75 mg/kg) and xylazine (7.5 mg/kg), supplemented as needed, and given an intramuscular injection of dexamethasone (3 mg/kg). Cannulas were surgically inserted into the trachea for mechanical ventilation, the femoral vein for intravenous drug delivery, and, in some experiments, the femoral artery for blood pressure measurement. The animal was then mounted in a stereotaxic apparatus in a lighttight booth. ECGs were recorded with needle electrodes, and body temperature was maintained with a rectal thermometer and a thermal blanket. Corneas were fitted with clear contact lenses, pupils were dilated with a drop of 1% cyclopentolate hydrochloride, and eye movements were paralyzed with an intravenous injection of gallamine triethiodide (40 mg/kg). Anesthesia and paralysis were sustained for the remainder of the experiment via an intravenous infusion of ketamine (30 mg·kg−1·h−1), xylazine (1.5 mg·kg−1·h−1), gallamine (40 mg·kg−1·h−1), dextrose (600 mg·kg−1·h−1), and 0.9% physiological saline. Data were collected while vital signs remained at acceptable levels (heart rate >225 beats/min, rectal temperature 37–38°C, blood pressure >80 mmHg). Animals were euthanized with Euthasol (50 mg/kg) given to effect at the end of experiments, which could last up to 12 h. All procedures were approved by the Institutional Animal Care and Use Committee of the University of South Florida in accordance with National Institutes of Health guidelines.
Electrophysiological recordings.
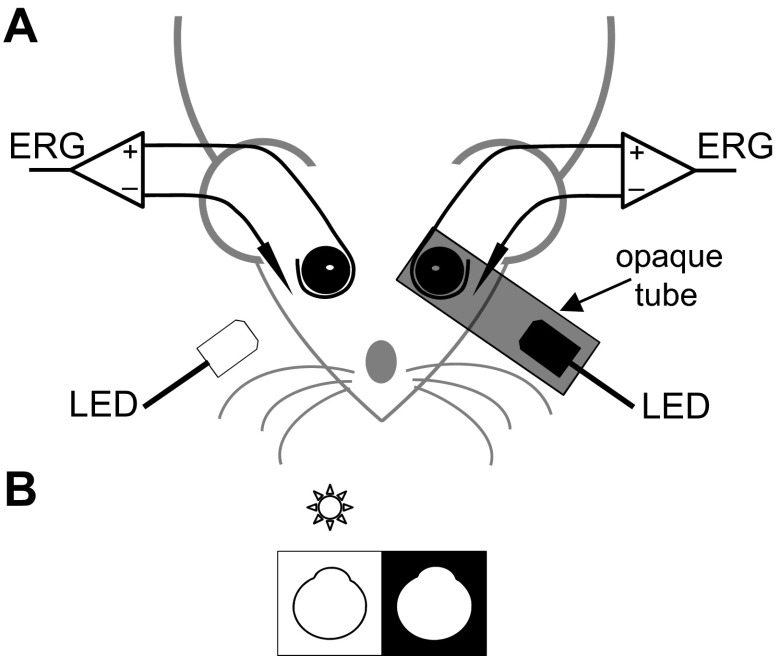
Figure 1 illustrates the experimental setup. ERGs were recorded from both eyes with a custom ring-shaped gold electrode placed on the corneal limbus. Platinum needle electrodes (Natus Neurology, Warwick, RI) inserted in the temples and tail served as references and ground, respectively. Recorded signals were differentially amplified (2,000×) and filtered (0.1–1,000 Hz) by a multichannel bioamplifier (Xcell-3x4; FHC, Bowdoin, ME) and digitized at 1,000 Hz. Light stimuli were produced by a green LED (Vishay TLCTG5800; Newark Electronics, Palatine, IL) with an 8° emittance angle positioned 1 cm in front of each cornea. One LED was encased in an opaque tube, the lumen of which covered the eye socket. The tube exit was lined externally with black tape to further block the escape of light. The LEDs had a peak wavelength of 520 nm and peak output of 2,100 cd/m2 measured with a calibrated photometer (UDT Instruments, Baltimore, MD). Animals were dark adapted for 4 h prior to data collection (Behn et al. 2003). Full-field scotopic ERGs were then recorded for a series of 200 brief (10 ms) flashes (1.32 log cd·s/m2) delivered to one or both eyes. After scotopic recording, animals were exposed for 30 min to ambient room light (15 cd/m2) from a LED strip that circumscribed the booth ceiling, and full-field photopic ERGs were recorded from the uncovered light-adapted eye while the tube-covered eye remained in darkness. All flash sequences had an interstimulus interval of 3 s, which provided sufficient time for full recovery of ERG responses under photopic conditions and represented a trade-off between data collection time and response recovery time under scotopic conditions. The animal and experiment were monitored from outside the booth with an infrared camera. Recordings were stable over the duration of anesthesia and were terminated on completion of data collection or deterioration of animal health.
Fig. 1.
Electroretinography setup. A: ring electrodes were rested on the corneas, and needle reference electrodes were inserted in the cheeks of an anesthetized paralyzed rat, while LEDs delivered a series of 10-ms flashes (interflash interval: 3 s) to either or both eyes. One of the LEDs was housed in an opaque tube that blocked stray light from entering that eye. Flashes to the other noncovered eye were presented in darkness or room light. B: diagram of the stimulus configuration in A. The star marks the flashed eye. The dark and bright borders indicate that the eye was dark or light adapted, respectively. Similar diagrams are provided in subsequent figures to illustrate the stimulus configuration.
Pharmacological injections.
Baseline ERG data were collected for a series of 200 full-field flashes delivered separately to each eye of 15 animals in darkness or room light. One eye was then injected intravitreally with a 5-μl bolus of tetrodotoxin (TTX; Abcam Biochemicals, Toronto, ON, Canada) or physiological saline as a control. TTX was dissolved in saline to produce an estimated concentration of 5 μM based on a rat vitreous volume of 40 μl (Hughes 1979). Solutions were injected 2 mm behind the limbus with a 10-μl microsyringe (Hamilton, Reno, NV) at a 45° angle to avoid contact with the lens. They were administered to dark-adapted animals under dim red illumination. After injection, conventional ERGs were recorded every 10 min for a series of 30 flashes delivered to both eyes. Once signal amplitude stabilized in the injected eye (∼30 min), full-field ERGs were recorded for a series of 200 flashes delivered to one eye and then the other. Data were collected every 30 min for up to 5 h, during which effects of TTX partially or completely washed out in some animals.
Intraocular pressure manipulations.
The anterior chamber of one eye of six animals was cannulated with a 33-gauge needle connected to a pressure transducer and a reservoir of physiological saline via a three-way stopcock and plastic tubing. The pressure transducer was calibrated before data collection with a mercury manometer. Animals were dark adapted for 4 h, after which baseline ERG data were collected for a series of 200 full-field flashes delivered separately to each eye at the resting intraocular pressure (IOP) level. The IOP of the cannulated eye was then elevated in 10-mmHg steps to the mean arterial blood pressure by varying the height of the saline reservoir. After each IOP step, conventional ERGs were recorded every 10 min for a series of 30 flashes delivered to both eyes. Once signal amplitude stabilized in the cannulated eye (∼30 min), full-field ERGs were recorded for a series of 200 flashes delivered to one eye and then the other. The process was repeated as IOP was lowered in 10-mmHg steps back to the resting level.
Compound action potential recordings.
The optic nerves of three animals were transected behind the globe, both eyes were removed, and the orbits were filled with artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, and 10 glucose (pH 7.45). ACSF-filled suction electrodes were positioned in each orbit with micromanipulators, and optic nerve stumps were gently aspirated into the glass tip of the electrodes. The tips were shaped by heat to snugly accommodate the nerves and coiled with silver chloride wire that served as a reference electrode. One pair of electrode leads was connected to the bioamplifier, and the other pair was connected to an electrical stimulator (S48; Grass Instruments, Warwick, RI). Bipolar current pulses (0.1 ms) of increasing amplitude were applied to one nerve until a compound action potential (CAP) was recorded in the opposite nerve. Once CAP threshold was identified, a series of 100 current pulses were delivered at 1 Hz for one subthreshold and several suprathreshold strengths. Evoked signals were digitized at 1,000 Hz to computer and processed off-line by averaging with respect to the stimulus onset and response onset. The latter was specified by the time of crossing of the leading edge of the CAP above a criterion level.
Retinal ganglion cell labeling.
One eye of seven animals was injected with a 10-μl bolus of fluorescent dye (mol wt 70,000, rhodamine dextran B; Life Technologies, Eugene, OR) in distilled water for the purpose of retrograde ganglion cell labeling. The dye was injected intravitreally with a 25-gauge needle and a Hamilton microsyringe in rats that were temporarily anesthetized with isoflurane. After 5–7 days the animal was euthanized, and both eyes were enucleated. Retinas were whole mounted on slides, coverslipped, and immediately viewed through a rhodamine filter with a fluorescent microscope (EVOS; Life Technologies, Grand Island, NY). The whole-mounted retinas of three animals were counterstained in Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA) prior to fluorescence imaging.
Data analysis.
Light-evoked signals were quantified in terms of amplitude, duration, and time to peak. Amplitude was measured from the peak to the trough, duration from the width at half-peak amplitude, and time to peak from the stimulus onset. Statistical analysis was performed with SigmaPlot software (San Jose, CA) using a two-sample t-test that assumed unequal variance for pairwise comparisons of raw measurements, a one-sided t-test for ratio measurements, and one-way ANOVA for comparisons of multiple data sets. Bonferroni's multiple-comparisons test was used to determine which ANOVA data sets were significantly different. Statistical significance was assessed at an α-level of 0.05.
RESULTS
Anatomical confirmation of retino-retinal connections.
Existence of retinal ganglion cells that send axons to the opposite eye of adult rats was confirmed by injecting the dye into the vitreous chamber of one eye (n = 7). The dye was uptaken by numerous retinal ganglion cells in the injected eye (Fig. 2A, top) and after several days retrogradely labeled a small number (<10) of ganglion cells in the opposite retina of every animal (Fig. 2A, bottom). The staining pattern was punctate and confined to the cell body, implying that the dye was encapsulated in vesicles carried by axonal transport from the injected eye. If intracellular diffusion were involved, the dye would be expected to label dendritic and axonal arbors as well. The soma diameter of labeled cells in the noninjected eye was between 8 and 12 μm, which falls on the low end for rat retinal ganglion cells (Danias et al. 2002). The retino-retinal ganglion cells tended to reside in the nasal portion of the eye (Fig. 2B). These findings are consistent with prior descriptions of tracer-labeled retino-retinal ganglion cells in adult rats (Avellaneda-Chevrier et al. 2015; Muller and Hollander 1988; Nadal-Nicholas et al. 2015).
Fig. 2.
Interocular connections in adult rats. A: fluorescence image of retinal ganglion cells labeled in the ipsilateral eye (top) and contralateral eye (bottom) with dye injected intravitreally in the ipsilateral eye. The dye was uptaken by thousands of cells in the ipsilateral retina and 4 cells in the contralateral retina. Cell nuclei were counterstained blue with DAPI. B: topographical map of retino-retinal ganglion cell locations across animals (1–7). Central circle is the optic disk.
Physiological demonstration of retino-retinal signal transmission.
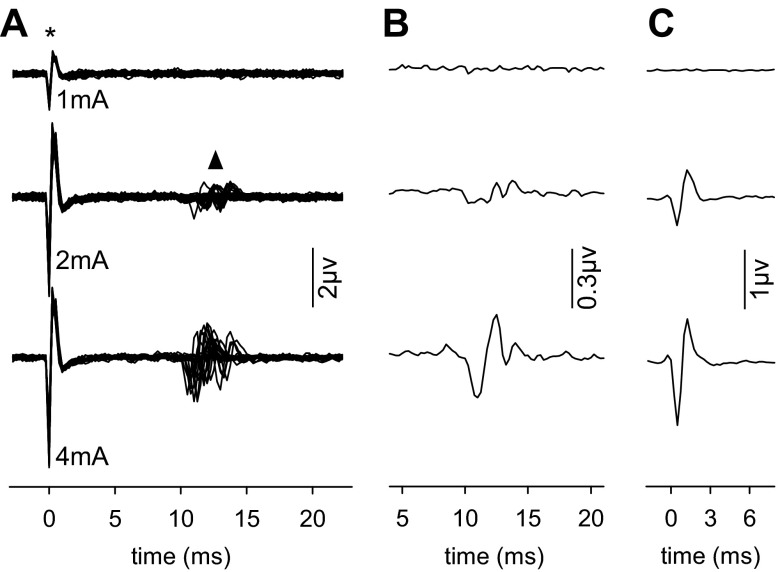
The functional status of retino-retinal efferent fibers in adult rats was assessed by excising both eyes of anesthetized animals and applying current pulses to one optic nerve stump while recording from the other nerve stump. No bioelectrical activity was observed at low current strengths, but higher strengths elicited a spikelike signal in the nonstimulated nerve (Fig. 3A), which we named “crossed CAP” (xCAP). xCAP latency ranged from 10 to 13 ms relative to pulse onset across stimulus applications and animals (n = 3). Averaging responses to a series of suprathreshold current pulses yielded a multipeaked waveform (Fig. 3B), which would normally imply the spike discharges of multiple types of ganglion cells with different conduction velocities combined to produce the signal (Sefton and Swinburn 1964). However, this is probably not the case for the xCAP because of the variability in signal latency. Averaging responses aligned by their leading edge instead of current onset reveals that the waveform is biphasic in shape and scales in amplitude with current strength (Fig. 3C), indicating that the xCAP reflects the summed activity of a single homogeneous population of efferent optic nerve fibers and that the number of active fibers and the synchrony of their discharges increased with stimulus intensity. The xCAP is not explained by volume conduction of afferent optic nerve activity to the recording electrode because the CAP has a complex multimodal waveform (Hale et al. 1979; Sefton and Swinburn 1964).
Fig. 3.
Electrical stimulation evokes signals that cross to opposite nerve. A: superposition of traces recorded in vivo from 1 optic nerve of a rat while a sequence of 50 current pulses (top: 1 mA, middle: 2 mA, bottom: 4 mA) was applied to the other optic nerve. Larger current amplitudes evoked compound action potentials (triangle) in the nonstimulated nerve. Asterisk indicates the current pulse as recorded by the bioamplification system. B: signal waveform when triggered averaged on the current pulses. C: signal waveform when triggered averaged on the leading edge of the signal itself.
Novel light-evoked interocular signal of the eye.
The full-field ERG evoked by a flash of light delivered to the dark-adapted rat eye has a stereotypic waveform, consisting of a brief negative a-wave followed by a larger positive b-wave (Fig. 4, A and B). Small OPs are often seen riding on the b-wave as well. If a single flash is presented to one eye, no bioelectrical activity is apparent in the other eye. However, when responses to a large number (>100) of monocular flashes are averaged, noise in the records decreases and a small light-evoked signal becomes detectable even in the nonflashed eye (Fig. 4, C and D). We named this signal the “crossed ERG” (xERG) since it is evoked by illumination of the opposite eye. It is not a stray-light response, as the flash was confined to the illuminated eye by an opaque tube (Fig. 1). Moreover, the xERG was recorded under both scotopic and photopic lighting conditions. Full-field flashes to the dark-adapted eye evoked a multipeaked xERG waveform when the nonflashed eye was exposed to room light (Fig. 4E), and full-field flashes to a light-adapted eye evoked a xERG of similar shape and size in the dark-adapted nonflashed eye (Fig. 4F). Any stray light that reached the nonflashed eye under photopic conditions would be orders of magnitude dimmer than the ambient illumination and much too small to evoke a light response.
Fig. 4.
A novel light-evoked signal of the eye. A: dark-adapted ERG traces of an anesthetized rat evoked by a single flash (10 ms) delivered to 1 eye while recording from the flashed (left) and nonflashed (right) eyes. B: dark-adapted ERG traces for a single flash delivered to the opposite eye. C: average dark-adapted ERG traces from the flashed (left) and nonflashed (right) eye of the same animal for 200 stimuli. A small “crossed ERG” (xERG) signal is seen in the nonflashed eye. D: average dark-adapted ERG traces for a flash sequence delivered to the opposite eye. A xERG signal can be recorded regardless of which eye is stimulated. E: average dark-adapted ERG trace of the flashed eye (left) of another rat and the xERG trace of the nonflashed eye (right) in ambient room light. F: average light-adapted ERG trace of the flashed eye (right) of the animal in E and the xERG trace of the nonflashed eye (left) in darkness.
Several control experiments were performed to further eliminate a stray-light explanation for the xERG. Figure 5A shows that no light-evoked activity is recorded in either eye if the flash is delivered through the opaque tube elsewhere on the head. Figure 5B shows that an opaque patch placed against the cornea and over the tube exit blocked not only the xERG of the nonflashed eye but also the ERG of the flashed eye, confirming that no light escaped the tube. Figure 5C shows that cutting the optic nerve of the flashed eye also eliminated the xERG in the nonflashed eye, demonstrating that the signal was not evoked by light passing through the skull to the opposite retina. Taken together, the data imply that the xERG derives from interocular signal transfer through the optic nerve, presumably via the retino-retinal projections that underlie the xCAP.
Fig. 5.
Control experiments. A: average bioelectrical activity recorded from each eye for 200 flashes delivered through an opaque tube to the forehead of a rat. B: average dark-adapted ERG of the flashed eye (left) and xERG of the nonflashed eye (right) of a rat before (top) and after (bottom) the flashed eye was covered with an opaque patch. C: average light-adapted ERG of the flashed eye (left) and xERG of the nonflashed eye (right) of another rat before (top) and after (bottom) the optic nerve was severed behind the globe of the flashed eye.
Characterization of xERG waveform.
The xERG has at least three components under dark-adapted conditions that were named xP1, xN1, and xP2 (Fig. 6). The signal begins with a positive wave (xP1) followed by a longer negative wave (xN1) and ends with a broad positive wave (xP2). Mean amplitude, time to peak, and duration of the xERG components and the dark-adapted ERG a- and b-waves were measured and compared. xP1, xN1, and xP2 amplitudes (6 ± 1, 11 ± 4, and 11 ± 5 μV, respectively; n = 16) were all measurably larger than the noise level after response averaging (Fig. 4; root mean squared = 2.1 ± 0.2 μV; n = 16) and much smaller than the a-wave [80 ± 12 μV, F(3,60) = 404, P < 0.001] and b-wave [197 ± 45 μV, F(3,60) = 261, P < 0.001]. xP1, xN1, and xP2 peak times (72 ± 9, 152 ± 25, and 288 ± 39 ms) were all much later than the a-wave [20 ± 2 ms, F(3,60) = 374, P < 0.001]. xP1 coincided in time with the b-wave (83 ± 11 ms, P > 0.999), while xN1 and xP2 peaked much later [F(3,60) = 258, P < 0.001]. xP1, xN1, and xP2 durations (44 ± 12, 99 ± 13, and 150 ± 33 ms) were all much longer than the a-wave [13 ± 2 ms, F(3,60) = 154, P < 0.001]. xP1 and xN1 were also longer in duration than the b-wave [138 ± 19 ms, F(3,60) = 79, P < 0.001], while xP2 was not (P = 0.308).
Fig. 6.
xERG properties under dark-adapted conditions. A: scotopic xERG population-average (n = 16) waveform has 3 components: xP1, xN1, and xP2. Dashed lines are ± 1 SD confidence levels. B: example dark-adapted ERG (top) and dark-adapted xERG (bottom) traces. Arrows illustrate how the amplitude, time to peak, and duration were measured for the xP1, xN1, and xP2 components of the xERG and for the a-wave and b-wave of the ERG (amplitude: peak to trough, time to peak: latency from stimulus onset, duration: width at half maximum amplitude). Arrowheads mark flash onset. C: box plots showing the mean, lower and upper quartiles, and minimum and maximum amplitude (left), time to peak (middle), and duration (right) of the ERG and xERG components.
The xERG has a slightly different shape under light-adapted conditions (Fig. 7). The signal begins and ends with positive components, but the negative component is not apparent in the population-average waveform. The mean amplitude, time to peak, and duration of the xERG components and light-adapted ERG b-wave were measured and compared. xP1 and xP2 amplitudes (7 ± 3 and 8 ± 6 μV, respectively; n = 11) were both larger than the 2-μV noise level after response averaging and much smaller than the b-wave [128 ± 26 μV, F(3,36) = 242, P < 0.001]. xP1 and xP2 peak times (87 ± 7 and 325 ± 28 ms) were both later than the b-wave [63 ± 4 ms, F(3,20) = 546, P < 0.001]. xP1 duration (52 ± 10 ms) was not significantly different from b-wave duration (66 ± 11 ms, P = 0.308), while xP2 duration was much longer [169 ± 21 ms, F(3,20) = 110, P < 0.001].
Fig. 7.
xERG properties under light-adapted conditions. A: photopic xERG population-average (n = 11) waveform has 2 components: xP1 and xP2. Dashed lines are ± 1 SD confidence levels. B: example light-adapted ERG (top) and light-adapted xERG (bottom) traces. Arrows illustrate how the amplitude, time to peak, and duration were measured for the xP1 and xP2 components of the xERG and the b-wave of the ERG (amplitude: peak to trough, time to peak: latency from stimulus onset, duration: width at half maximum amplitude). Arrowheads mark flash onset. C: box plots showing the mean, lower and upper quartiles, and minimum and maximum amplitude (left), time to peak (middle), and duration (right) of the ERG and xERG components.
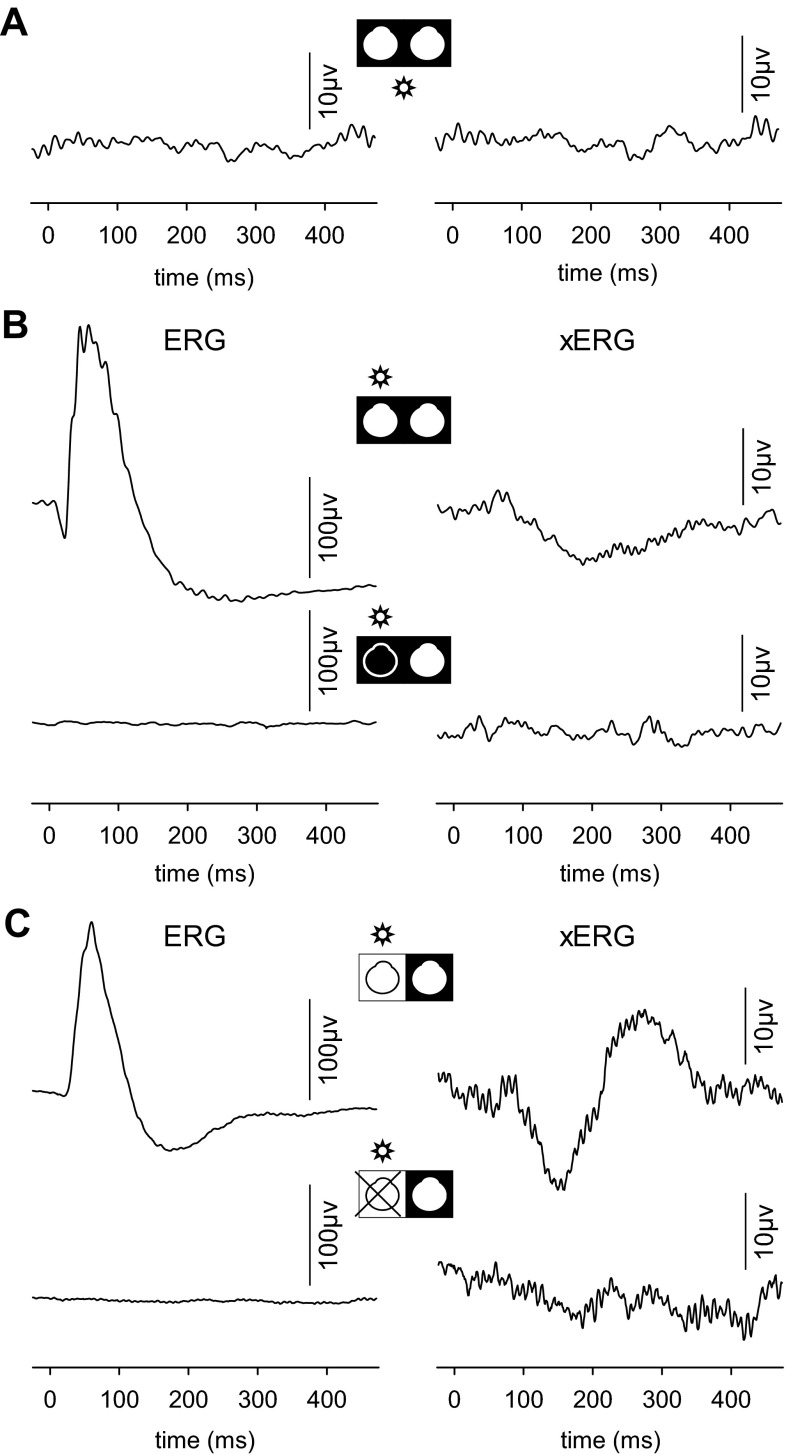
Since monocular illumination evokes bioelectrical activity in both eyes, binocular stimulation should induce both an ERG and a xERG in each eye. The signal interaction was examined by delivering full-field flashes to one or both eyes simultaneously. Monocular and binocular ERGs looked virtually identical, but subtraction of the two records yielded a difference signal (Fig. 8). The signal resembled the xERG of the nonilluminated eye for monocular flashes to a first approximation. The goodness of fit between the two records (R2) averaged 0.52 ± 0.16 across animals (n = 6). This is significantly greater than the level expected by chance (R2 = 0.03 ± 0.02) given by the goodness of fit between the difference signal and average noise records (Fig. 5). The similarity of waveforms suggests that the xERG contributes a very small but measurable component to the flash ERG of rats.
Fig. 8.
xERG contribution to the full-field flash ERG. A: ERG recorded from 1 eye for full-field flashes to both eyes of a rat. The recorded eye was exposed to room light and the other eye to darkness. B: ERG recorded from the same eye as in A for flashes delivered only to that eye. C: comparison of the xERG (thin trace) recorded from the same eye as in A for flashes delivered to the other eye and the signal produced by subtracting the monocular and binocular ERGs in A and B (thick trace). The records were normalized in amplitude to facilitate comparison of waveforms.
Effect of TTX on flash ERG and xERG.
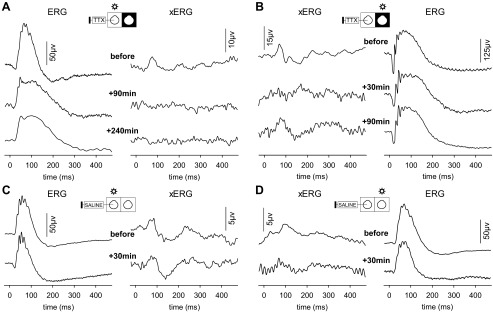
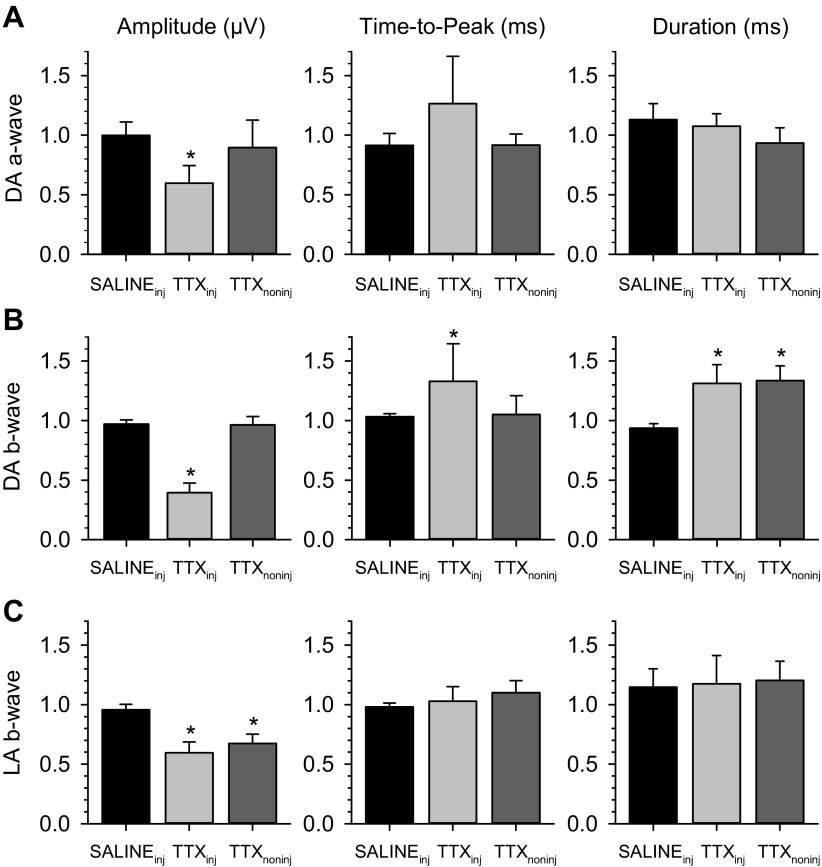
To confirm that the xERG is neural in origin, a bolus of TTX was injected in one eye of anesthetized rats. TTX is a potent blocker of voltage-gated sodium channels and thereby arrests action potential transmission in and out of the retina. In every animal (n = 15) TTX completely eliminated the xERG in the nonflashed eye regardless of whether the flash was delivered to the injected eye (Fig. 9A) or the noninjected eye (Fig. 9B, +30 min). The xERG returned after a few hours to the injected eye (Fig. 9B, +90 min) of some animals (n = 3) as TTX was slowly cleared from the eye but not to the noninjected eye at applied concentrations. Intravitreal injection of an equivalent bolus of physiological saline had little to no effect on bioelectrical activity in either eye (Fig. 9, C and D). Effects of TTX were not limited to the xERG. The flash ERG of the injected eye was altered under dark-adapted and light-adapted conditions (Fig. 10, A–C, left). The b-wave was particularly affected. On average, the scotopic amplitude was reduced by 60 ± 8% (P < 0.001; n = 6), delayed by 20 ± 17 ms (P = 0.047), and prolonged by 45 ± 23 ms (P = 0.020), while the photopic amplitude was reduced by 40 ± 15% (P < 0.001; n = 5) with time to peak and duration remaining unchanged (Fig. 11). Similar effects of TTX on the flash ERG have been reported previously (Bui and Fortune 2004; Mojumder et al. 2008). More interestingly, the flash ERG of the noninjected eye was also altered (Fig. 10, A–C, right). The changes were specific to the contralateral b-wave, which was prolonged in duration by 40 ± 8 ms (P < 0.001; n = 6) under dark-adapted conditions and reduced in amplitude by 33 ± 8% (P = 0.011; n = 5) under light-adapted conditions (Fig. 11). The ERG returned to normal after several hours in the noninjected eye of some animals (n = 2; Fig. 10B) but not the injected eye. Effects of TTX on the contralateral ERG suggest that interocular signals communicated by efferent optic nerve fibers modify the response dynamics of retinal neurons.
Fig. 9.
TTX abolishes the xERG. A: full-field flash ERG (left) and xERG (right) recorded before (top), 1.5 h after (middle), and 4 h after (bottom) intravitreal injection of TTX in the flashed eye of a rat. B: full-field flash ERG (right) and xERG (left) recorded before (top), 0.5 h after (middle), and 1.5 h after (bottom) injection of TTX in the nonflashed eye of another rat. C: flash ERG (left) and xERG (right) recorded before (top) and 0.5 h after (bottom) equivalent intravitreal injection of saline in the flashed eye. D: flash ERG (right) and xERG (left) recorded before (top) and 0.5 h after (bottom) equivalent intravitreal injection of saline in the nonflashed eye.
Fig. 10.
TTX alters the ERG in both eyes. A: dark-adapted ERGs recorded for full-field flashes to both eyes of a rat before and 2 h after injection of TTX in 1 eye. B: flash ERGs recorded from both eyes of another rat before, 1 h after, and 2 h after injection of TTX in 1 eye. The injected eye was light adapted, while the noninjected eye was dark adapted. C: flash ERGs recorded from both eyes of another rat before and 1 h after injection of TTX in 1 eye. The injected eye was dark adapted, while the noninjected eye was light adapted.
Fig. 11.
Summary of TTX effects on the ipsilateral and contralateral ERG. A: normalized amplitude, time to peak, and duration of the dark-adapted (DA) a-wave of saline-injected, TTX-injected, and TTX noninjected eyes. Maximum postinjection values were normalized by preinjection values. B: normalized amplitude, time to peak, and duration of the dark-adapted b-wave of injected and noninjected eyes. C: normalized amplitude, time to peak, and duration of the light-adapted (LA) b-wave of injected and noninjected eyes. Bars give group mean ± standard deviation. Asterisks indicate data sets for which pre- and postinjection measurements were statistically different.
Effect of elevated IOP on ERG and xERG.
It is known that ganglion cells cease firing at high IOP levels because of onset of retinal ischemia (Grehn et al. 1984). To further confirm that the xERG derives from efferent optic nerve activity, the anterior chamber of one eye of anesthetized rats was cannulated with a needle connected to a variable-height reservoir of saline. The dark-adapted ERG b-wave progressively decreased in amplitude and eventually disappeared (Fig. 12A, left) as IOP was increased from baseline to near the arterial blood pressure (22 and 80 mmHg, respectively, for this animal). The a-wave was relatively insensitive to IOP elevation, in agreement with prior findings in rat (Bui et al. 2013). Light flashes evoked a xERG in the opposite eye (Fig. 12A, right) that also disappeared at high IOP in every animal tested (n = 3). The disappearance of the xERG in the noncannulated eye coincided with the loss of the ERG b-wave in the cannulated eye. Both signals reappeared when IOP was lowered back to baseline. The pressure effect was unidirectional in that a xERG was still recorded in the cannulated eye at IOP levels that eliminated the ERG b-wave in that eye (Fig. 12B). This is more evidence against a stray-light explanation of the xERG. The data imply that acute ocular hypertension eliminated the xERG in the noncannulated eye because it blocked inner retinal signaling and afferent optic nerve spiking in the cannulated eye, while the noncannulated eye was not subjected to the pressure block so efferent optic nerve signals from that eye still produced a xERG in the cannulated eye. Moreover, IOP elevation altered the contralateral flash ERG (Fig. 12C). Similar to the effect of TTX, the dark-adapted b-wave of the noncannulated eye lengthened in duration by 45 ± 15 ms (P = 0.035) at high IOP and returned to normal when baseline IOP was restored (n = 3 of 4).
Fig. 12.
Acute IOP elevation alters the ERG and xERG. A: full-field flash ERGs (left) and xERGs (right) recorded simultaneously from a rat in darkness as the IOP of the flashed eye was raised from the resting level of 22 mmHg (top) to near mean arterial pressure of 80 mmHg and lowered back to the resting level (bottom). IOP level is indicated next to each trace. B: full-field dark-adapted ERG (right) and xERG (left) recorded from the same rat for flashes delivered to the noncannulated eye with the cannulated eye at an IOP of 72 mmHg. A xERG was recorded at all IOP levels, including 1 shown for which the flash ERG of that eye lacked a b-wave in A. C: dark-adapted flash ERGs recorded from both eyes of another rat with IOP at the resting level of 15 mmHg, increased to 70 mmHg, and returned back to the resting level.
DISCUSSION
The existence of direct retino-retinal projections in mammals has long been known, but evidence that the projections are retained into adulthood has been scant. Using modern immunohistochemical methods, two recent studies demonstrated that a small group (5–25) of retinal ganglion cells send axon collaterals to the contralateral retina in adult mice and rats (Avellaneda-Chevrier et al. 2015; Nadal-Nicolas et al. 2015). Since the number of retino-retinal ganglion cells was reported to vary between animals and rat strains (Nadal-Nicolas et al. 2015), we investigated our cohort of animals and confirmed that retrograde tracers label ganglion cell bodies (<10) in the contralateral retina of Brown-Norway rats at age P180 and above, which is late adulthood and beyond the age span of previous studies. Our results give a lower bound on retino-retinal ganglion cell counts in old-age rats because intravitreal tracer injection does not guarantee that all efferent fiber terminals sequester and transport tracer at a sufficient level for visual detection of efferent cell bodies in the opposite eye. The existence of these cells means that not all retinopetal fibers come from the brain, as commonly implied (Gastinger et al. 2006; Reperant et al. 2006). Information about axonal and dendritic tree morphology of retino-retinal ganglion cells is needed to move beyond mere soma sightings and begin dissecting the connectivity pattern of interocular circuits.
The functional integrity of retino-retinal projections was demonstrated here for the first time in mammals. We electrically stimulated one optic nerve of adult rats and observed in the other nonstimulated optic nerve a biphasic compound action potential (xCAP). The xCAP must come from efferent optic nerve fibers because the CAP generated by afferent optic nerve fibers is known to have a different waveform. We investigated whether retino-retinal fibers can communicate visual information by recording the bioelectrical activity evoked in one eye by flashes delivered to the other eye. Since retinal efferents are few in number, many records were averaged in order to detect a putative intraocular signal buried in the noise. Our results show that monocular light flashes elicit a small multipeaked signal in the nonflashed eye of adult rats (xERG). The novel signal has presumably escaped notice because it is severalfold smaller than the conventional flash ERG. Our results also show that experimental treatments that eliminated the xERG in one eye altered the ERG of the nontreated eye. The binocular effects suggest that retino-retinal projections serve a presently unknown purpose and are not just an anomaly of visual system development.
There are two neural routes by which light-evoked signals in one eye could be communicated to the other eye of mammals: directly via retinal-retinal efferent fibers and indirectly via efferent fibers from cortical or subcortical neurons that receive visual input. There could be a nonneural route if the eyes are connected by an interorbital communicating artery (Ruskell 1962), although hormonal transmission would seem much too slow to explain xERG response times. Based on rat optic nerve length and optic chiasm width (∼23 mm eye to eye), the xCAP latency translates to a conduction velocity of 2–3 m/s, which is below the range of X- and Y-type ganglion cells but within the range of W-type cells (Hale et al. 1979). The anatomical and physiological properties of W cells are diverse and not known for every subtype, so it is plausible that a subpopulation could project directly to the opposite eye. Alternatively, the faster-conducting X- and Y-type fibers could perhaps relay afferent signals to cortical or subcortical circuits, which then send back efferent signals in time to produce a xCAP in the opposite eye. The extrasynaptic processing might explain the observed dispersion in xCAP timing. However, measurements of cortico-retinal transmission time do not appear to support such an explanation. Travel from the raphe nucleus to the optic chiasm of rats was clocked around 9 ms (Lorincz et al. 2008), which is comparable to xCAP latency without accounting for signal transmission from the eye to the brain stem, synaptic processing by target neurons, and transmission down the contralateral optic nerve. Hence, the simplest interpretation of our results is that retino-retinal (W type) optic nerve fibers produce the xCAP and, by inference, the early components of the xERG.
xERG—interocular signal or methodological artifact?
The xERG was investigated from multiple independent directions to guard against possible sources of methodological error. The most often cited and suspected source of possible error is stray light. Our control experiments demonstrate that this is not the case in our experimental setup, as 1) an opaque tube confined flashes to the illuminated eye, 2) the signal disappears when the eyes are covered or when the optic nerve is transected, and 3) the signal disappears after injection of TTX or increase of IOP in the nonflashed eye. Similarly, a visuomotor reflex can be excluded as a potential source, as 1) the animal was continuously infused with paralytics, 2) pupil movements were pharmacologically blocked, 3) the xP1 time to peak is much shorter than the latency of pupil contractions and dilations (290–490 ms) in rats (Clarke 2007), and 4) the signal is affected by IOP manipulations. Finally, volume conduction of bioelectrical activity from the flashed eye to the contralateral eye electrode is unlikely, as the waveform 1) is different from the measured ERG, 2) is largely unaffected by light adaptation, and 3) disappears after cutting the optic nerve or injecting TTX in the nonflashed eye. The latter observation also implies that it is not a visual signal passively conducted from cortical or subcortical circuits, although the waveform looks strikingly similar to field potentials that were recorded in the rat optic chiasm after light flashes (Galambos et al. 2000, 2005) and brain stem stimulation (Lorincz et al. 2008). Together, our results collectively point to the conclusion that the xERG is an interocular signal communicated from one retina to the other via the optic nerve.
One methodological limitation of the present work should be noted. The opaque tube used to prevent light leakage restricted LED illumination to the central retina of the stimulated eye, which would lower ERG amplitudes relative to studies that employ true Ganzfeld illumination. This preferential stimulation of the central retina might explain the stronger than usual effect of TTX on a- and b-wave amplitudes for dark-adapted conditions. Under such a hypothesis some LED light is scattered into peripheral retina and acts as background stimulation similar to ambient illumination, for which TTX application is reported to decrease ERG amplitude (Mojumder et al. 2008). Ganzfeld stimulation of the ipsilateral eye may also alter the amplitude, timing, and waveform of the xERG, a possibility that will be investigated in future studies.
Origins of ERG and xERG waveforms.
Our results do not directly address the origin of the different peaks of the xERG waveform. However, it is presumed that the xP1 component, and perhaps xN1 and xP2 components too, derives from efferent spike trains coming from retino-retinal ganglion cells in the illuminated eye. The time to peak of optic tract responses to a bright spot flashed over ganglion cell receptive field centers ranges from 50 to 150 ms depending on cell type and stimulus condition (Heine and Passaglia 2011), which fits with xP1 time to peak. The xP1 and xN1 amplitude and waveform are also comparable to the scotopic threshold response, which is a small positive-then-negative ERG signal evoked by very dim flashes in darkness that is attributed to ganglion cell spike activity in rats (Bui and Fortune 2004; Naarendorp et al. 2001). Since efferent fibers are few in number and branch widely across the retina, the xERG probably reflects spike-evoked currents in retinal neurons of the nonilluminated eye more than efferent spikes themselves. The contralateral effects of TTX and IOP on the ERG b-wave suggest that bipolar cells may be one efferent target. Alternatively, the latter components of the xERG could reflect, in whole or part, efferent signals from cortical and subcortical circuits. Determining the precise origin of the various xERG peaks requires surgical dissection of visual pathways and pharmacological blockade of specific retinal circuits, which were beyond the scope of this study.
The origin of the conventional full-field flash ERG waveforms is well defined and characterized (Frishman and Wang 2011; Weymouth and Vingrys 2008). The leading edge of the a-wave reflects photoreceptor activity, the ascending shoulder of the b-wave derives mainly from on-type bipolar cells, and the OPs are thought to reflect amacrine-ganglion cell interactions in the inner retina. We observed that TTX significantly reduced scotopic a- and b-wave amplitude and increased b-wave time to peak and duration. TTX also reduced photopic b-wave amplitude. The amplitude reductions could be due to the injected volume or a shift in adaptation state caused by the injection procedure, but control injections of saline argue against this. Prior studies found that TTX had negligible effect on the dark-adapted a-wave in rats under fully dark-adapted conditions (Bui and Fortune 2004; Mojumder et al. 2008). This study employed different stimulation parameters (e.g., non-Ganzfeld, nonbroadband), which may also have contributed to observed a-wave changes. Reported effects of TTX on the b-wave have been mixed, but similar reduction, delay, and prolongation were seen in rats (Bui and Fortune 2004; Mojumder et al. 2008). The b-wave changes can be attributed to the presence of voltage-gated sodium channels in cone bipolar cells (Mojumder et al. 2008; Pan and Hu 2000) since the flash intensity used in this study likely stimulated the cone system. In addition, we observed that TTX significantly increased the scotopic b-wave duration and decreased the photopic b-wave amplitude of the noninjected eye. The contralateral ERG changes could perhaps signify that rats have an interorbital artery. However, the scotopic b-wave amplitude and time to peak were not altered and b-wave duration was similarly prolonged even though any TTX transported to the opposite eye would be much lower in concentration. Furthermore, IOP elevation not only eliminated the ERG b-wave in the cannulated eye, as described previously (Bui et al. 2005, 2013; Kong et al. 2009), but also eliminated the xERG and altered the ERG in the noncannulated eye. The contralateral ERG changes resembled those of TTX, in that the b-wave was longer in duration under scotopic conditions and smaller in amplitude under photopic conditions. Interestingly, a decrease in photopic b-wave amplitude was noted at high IOP levels in the noncannulated eye of rats in a prior study (Tsai et al. 2014). We conclude that TTX injection and IOP elevation blocked optic nerve output from the illuminated eye and the resultant loss of interocular communication altered retinal processing in the other eye.
Possible function of retinal efferents and interocular communication.
The function of efferent optic nerve fibers is poorly understood in mammals. What is known about their physiological properties comes primarily from research on the isthmo-optic nucleus of birds, which sends a sizable projection to the eyes (∼10,000 nerve fibers) that is well studied as a result. It has been suggested that efferent feedback mediates a dynamic adaptation process that adjusts retinal sensitivity in conjunction with neural command signals for eye and head movements (Miles 1972). Other suggestions are that efferent feedback serves to stabilize the retinal image (Woodson et al. 1995), highlight retinal activity of visual interest in one or both eyes (Uchiyama 1999), or initiate tracking circuits that follow shadows cast by objects in the scene (Wilson and Lindstrom 2011). These functions all require a fairly coordinated feedback system that appears unique to birds. On the basis of immunohistochemical staining and tract tracing, it has been suggested that efferent feedback in mammals may be part of a circadian arousal system (Gastinger et al. 2006). This is because neurons that project to the retina reside in the areas of the hypothalamus and brain stem that vary in activity level according to the sleep/wake cycle and efferent nerve terminals in the retina stain for histamine and serotonin, which are neurotransmitters that modulate the firing rates of retinal ganglion cells. Perhaps retino-retinal efferent fibers act in concert with cortico-retinal efferent fibers while the animal is awake to tune the visual system for ambient lighting conditions, providing information about environmental illumination that allows retinal neurons to anticipate changes in adaptation state as the eyes and head move.
Interocular communication might play a role in human vision as well. There are some physiological processes and phenomena still awaiting detailed understanding for which retino-retinal fibers can offer a viable mechanism. These phenomena include binocular interactions in dark adaptation (Makous et al. 1976), interocular transfer of dark and light adaptation (Auerbach et al. 1992; Auerbach and Peachey 1984), interocular transfer of motion aftereffects (Wade et al. 1993), interocular suppression and contrast gain control (Baker et al. 2007a, 2007b; Baker and Meese 2007; Denny et al. 1991; Eysteinsson et al. 1993), binocular capture (Raghunandan 2011), and bilateral ERG changes following monocular IOP elevation (Lovasik et al. 2005) and eye manipulation (Francis et al. 2013). More research on retinal efferents in a variety of mammalian species is clearly needed, and the discovery of the xERG provides a much-needed tool for investigating this sparse subpopulation of optic nerve fibers that is otherwise difficult to access electrophysiologically.
GRANTS
This work was partially supported by National Eye Institute Grant R21 EY-023376 and by a Thomas R. Lee Award from the Bright Focus Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.T. and C.L.P. performed experiments; X.T. and C.L.P. analyzed data; X.T., R.T., and C.L.P. interpreted results of experiments; X.T. and C.L.P. prepared figures; X.T. drafted manuscript; X.T., R.T., and C.L.P. approved final version of manuscript; R.T. and C.L.P. edited and revised manuscript; C.L.P. conception and design of research.
ACKNOWLEDGMENTS
We thank Geoffrey Arden, John Dowling, and Neal Peachey for helpful comments. Kayla Ficarrotta and Sarah Davis provided assistance with data processing.
REFERENCES
- Auerbach E, Beller AJ, Henkes HE, Goldhaber G. Electric potential of retina and cortex of cats evoked by monocular and binocular photic stimulation. Vision Res 1: 166–182, 1961. [Google Scholar]
- Auerbach E, Feinsod M. Centrifugal effects on the cat electroretinogram after section of one optic nerve. Doc Ophthalmol 34: 47–55, 1973. [DOI] [PubMed] [Google Scholar]
- Auerbach E, Peachey N. Interocular transfer and dark adaptation to long-wave test lights. Vision Res 24: 1043–1048, 1984. [DOI] [PubMed] [Google Scholar]
- Auerbach E, Dörrenhaus A, Cavonius CR. Changes in sensitivity of the dark-adapted eye during concurrent light adaptation of the other eye. Vis Neurosci 8: 359–363, 1992. [DOI] [PubMed] [Google Scholar]
- Avellaneda-Chevrier VK, Wang X, Hooper ML, Chauhan BC. The retino-retinal projection: tracing retinal ganglion cells projecting to the contralateral retina. Neurosci Lett 591: 105–109, 2015. [DOI] [PubMed] [Google Scholar]
- Baker DH, Meese TS. Binocular contrast interactions: dichoptic masking is not a single process. Vision Res 47: 3096–3107, 2007. [DOI] [PubMed] [Google Scholar]
- Baker DH, Meese TS, Georgeson MA. Binocular interaction: contrast matching and contrast discrimination are predicted by the same model. Spat Vis 20: 397–413, 2007a. [DOI] [PubMed] [Google Scholar]
- Baker DH, Meese TS, Summers RJ. Psychophysical evidence for two routes to suppression before binocular summation of signals in human vision. Neuroscience 146: 435–448, 2007b. [DOI] [PubMed] [Google Scholar]
- Behn D, Doke A, Racine J, Casanova C, Chemtob S, Lachapelle P. Dark adaptation is faster in pigmented than albino rats. Doc Ophthalmol 106: 153–159, 2003. [DOI] [PubMed] [Google Scholar]
- Bohn RC, Stelzner DJ. Aberrant retino-retinal pathway during early stages of regeneration in adult Rana pipiens. Brain Res 160: 139–144, 1979. [DOI] [PubMed] [Google Scholar]
- Bohn RC, Stelzner DJ. The aberrant retino-retinal projection during optic nerve regeneration in the frog. II. Anterograde labeling with horseradish peroxidase. J Comp Neurol 196: 621–632, 1981. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Hamasaki DI. Evidence that the cat's electroretinogram is not influenced by impulses passing to the eye along the optic nerve. J Physiol 163: 558–565, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley GS, Hamasaki DI. Histological evidence against the view that the cat's optic nerve contains centrifugal fibres. J Physiol 184: 444–449, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci 46: 202–213, 2005. [DOI] [PubMed] [Google Scholar]
- Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. J Physiol 555: 153–173, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui BV, He Z, Vingrys AJ, Nguyen CTO, Wong VH, Fortune B. Using the electroretinogram to understand how intraocular pressure elevation affects the rat retina. J Ophthalmol 2013: 262467, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SM, Lund RD. Development of a transient retino-retinal pathway in hooded and albino rats. Brain Res 211: 399–404, 1981. [DOI] [PubMed] [Google Scholar]
- Bunt SM, Lund RD, Land PW. Prenatal development of the optic projection in albino and hooded rats. Brain Res 282: 149–168, 1983. [DOI] [PubMed] [Google Scholar]
- Chou TH, Porciatti V. The bioelectric field of the pattern electroretinogram in the mouse. Vis Neurosci 53: 8086–8092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RJ. Shaping the pupil's response to light in the hooded rat. Exp Brain Res 176: 641–651, 2007. [DOI] [PubMed] [Google Scholar]
- Danias J, Shen F, Goldblum D, Chen B, Ramos-Esteban J, Podos SM, Mittag T. Cytoarchitecture of the retinal ganglion cells in the rat. Invest Ophthalmol Vis Sci 43: 587–594, 2002. [PubMed] [Google Scholar]
- Denny N, Frumkes TE, Barris MC, Eysteinsson T. Tonic interocular suppression and binocular summation in human vision. J Physiol 437: 449–460, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt E. Centrifugal impulses in rabbit's retina. J Neurophysiol 19: 301–307, 1956. [DOI] [PubMed] [Google Scholar]
- Dunlop SA, Tee LB, Rodger J, Harvey AR, Roberts JD, Beazley LD. Development of visual projections follows an avian/mammalian-like sequence in the lizard Ctenophorus ornatus. J Comp Neurol 453: 71–84, 2002. [DOI] [PubMed] [Google Scholar]
- Eysteinsson T, Barris MC, Denny N, Frumkes TE. Tonic interocular suppression, binocular summation, and the visual evoked potential. Invest Ophthalmol Vis Sci 34: 2443–2448, 1993. [PubMed] [Google Scholar]
- Francis JH, Abramson DH, Marr BP, Brodie SE. Ocular manipulation reduces both ipsilateral and contralateral electroretinograms. Doc Ophthalmol 127: 113–122, 2013. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Wang M. Electroretinogram of human, monkey and mouse. In: Adler's Physiology of the Eye, edited by Levin LA, Nilsson SF, Ver H, oeve J, Wu SM, Kaufman PL, Alm AJ. New York: Saunders Elsevier, 2011. [Google Scholar]
- Galambos R, Juhasz G, Kekesi AK, Nyitrai G, Szilagyi N. Natural sleep modifies the rat electroretinogram. Proc Natl Acad Sci USA 91: 5153–5157, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R, Juhasz G, Lorincz M, Szilagyi N. The human retinal functional unit. Int J Psychophysiol 57: 187–194, 2005. [DOI] [PubMed] [Google Scholar]
- Galambos R, Szabo-Salfay O, Barabas P, Palhalmi J, Szilagyi N, Juhasz G. Temporal distribution of the ganglion cell volleys in the normal rat optic nerve. Proc Natl Acad Sci USA 97: 13454–13459, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R, Szabo-Salfay O, Szatmari E, Szilagyi N, Juhasz G. Sleep modifies retinal ganglion cell responses in the normal rat. Proc Natl Acad Sci USA 98: 2083–2088, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Barber AJ, Khin SA, McRill CS, Gardner TW, Marshak DW. Abnormal centrifugal axons in streptozotocin-diabetic rat retinas. Invest Ophthalmol Vis Sci 42: 2679–2685, 2001. [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Tian N, Horvath T, Marshak DW. Retinopetal axons in mammals: emphasis on histamine and serotonin. Curr Eye Res 31: 655–667, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grehn F, Grusser OJ, Stange D. Effect of short-term intraocular pressure increase on cat retinal ganglion cell activity. Behav Brain Res 14: 109–121, 1984. [DOI] [PubMed] [Google Scholar]
- Hale PT, Sefton AJ, Dreher B. A correlation of receptive field properties with conduction velocity of cells in the rat's retino-geniculo-cortical pathway. Exp Brain Res 35: 425–442, 1979. [DOI] [PubMed] [Google Scholar]
- Heine WF, Passaglia CL. Spatial receptive field properties of rat retinal ganglion cells. Vis Neurosci 28: 403–417, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellner KA. Efferent inhibition of single ERG-responses by flicker-stimulation of the contralateral eye in man. Doc Ophthalmol 18: 431–439, 1964. [DOI] [PubMed] [Google Scholar]
- Honrubia FM, Elliott JH. Efferent innervation of the retina. I. Morphologic study of the human retina. Arch Ophthalmol 80: 98–103, 1968. [DOI] [PubMed] [Google Scholar]
- Honrubia FM, Elliott JH. Efferent innervation of the retina. II. Morphologic study of the monkey retina. Invest Ophthalmol 9: 971–976, 1970. [PubMed] [Google Scholar]
- Hornsten GP, Wildeboer-Venema FN, Winkelman JE. Studies on centrifugal effects on the retina: a corneo-negative potential in a non-illuminated eye. Arch Int Physiol Biochim 69: 431–442, 1961. [DOI] [PubMed] [Google Scholar]
- Hughes A. A schematic eye for the rat. Vision Res 19: 569–588, 1979. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Growth and reshaping of axons in the establishment of visual callosal connections. Science 212: 824–827, 1981. [DOI] [PubMed] [Google Scholar]
- Jacobson JH, Suzuki TA. Effect of optic nerve section on the ERG. Arch Ophthalmol 67: 791–801, 1962. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Massof RW. The photomyoclonic reflex: an artefact in the clinical electroretinogram. Br J Ophthalmol 66: 368–378, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YX, Crowston JG, Vingrys AJ, Trounce IA, Bui VB. Functional changes in the retina during and after acute intraocular pressure elevation in mice. Invest Ophthalmol Vis Sci 50: 5732–5740, 2009. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia JL, Guerra-Seijas MJ, Gonzalez F, Perez R, Acuna C. Location of neurons projecting to the retina in mammals. Neurosci Res 8: 291–302, 1990. [DOI] [PubMed] [Google Scholar]
- Lorincz ML, Olah M, Juhasz G. Functional consequences of retinopetal fibers originating in the dorsal raphe nucleus. Int J Neurosci 118: 1374–1383, 2008. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Gagnon M. Experimentally reduced perfusion of one eye impairs retinal function in both eyes. Optom Vis Sci 82: 850–857, 2005. [DOI] [PubMed] [Google Scholar]
- Makous W, Teller D, Boothe R. Binocular interaction in the dark. Vision Res 16: 473–476, 1976. [DOI] [PubMed] [Google Scholar]
- McLoon S, Lund RD. Transient retinofugal pathways in the developing chick. Exp Brain Res 45: 277–284, 1982. [DOI] [PubMed] [Google Scholar]
- Miles FA. Centrifugal control of the avian retina. I. Receptive field properties of retinal ganglion cells. Brain Res 48: 65–92, 1972. [DOI] [PubMed] [Google Scholar]
- Mojumder DK, Sherry DM, Frishman LJ. Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol 586: 2551–2580, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotchnikoff S, Lachapelle P, Casanova C. Optic nerve blockade influences the retinal responses to flash in rabbits. Vision Res 29: 957–963, 1989. [DOI] [PubMed] [Google Scholar]
- Molotchnikoff S, Tremblay F. Influence of the visual cortex on responses of retinal ganglion cells in the rat. J Neurosci Res 10: 397–409, 1983. [DOI] [PubMed] [Google Scholar]
- Molotchnikoff S, Tremblay F. Visual cortex controls retinal output in the rat. Brain Res Bull 17: 21–32, 1986. [DOI] [PubMed] [Google Scholar]
- Motokawa K, Nakagawa D, Kohata T. Electrophysiological studies of binocular stereoscopic vision. J Comp Physiol Psychol 49: 398–403, 1956. [DOI] [PubMed] [Google Scholar]
- Muller M, Hollander H. A small population of retinal ganglion cells projecting to the retina of the other eye. An experimental study in the rat and the rabbit. Exp Brain Res 71: 611–617, 1988. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Sato Y, Cajdric A, Hubbard NP. Absolute and relative sensitivity of the scotopic system of rat: electroretinography and behavior. Vis Neurosci 18: 641–656, 2001. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Valiente-Soriano FJ, Salinas-Navarro M, Jimenez-Lopez M, Vidal-Sanz M, Agudo-Barriuso M. Retino-retinal projection in juvenile and young adult rats and mice. Exp Eye Res 134: 47–52, 2015. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Oishi S, Kuno M. The central influence upon the electroretinogram evoked by double flashes. Arch Ophthalmol 68: 532–537, 1962. [Google Scholar]
- Nakamura H, O'Leary DD. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci 9: 3776–3795, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitopoulou-Maratou G, Vassiliou GA, Kepetzis M, Molyvdas PA. ERG alterations induced by sound. Neurochem Int 1C: 355–365, 1980. [DOI] [PubMed] [Google Scholar]
- Pan ZH, Hu HJ. Voltage-dependent Na+ currents in mammalian retinal cone bipolar cells. J Neurophysiol 84: 2564–2571, 2000. [DOI] [PubMed] [Google Scholar]
- Peachey NS, Sokol S, Moskowitz A. Recording the contralateral PERG: effect of different electrodes. Invest Ophthalmol Vis Sci 24: 1514–1516, 1983. [PubMed] [Google Scholar]
- Raghunandan A. Binocular capture: the effects of spatial frequency and contrast polarity of the monocular target. Vision Res 51: 2369–2377, 2011. [DOI] [PubMed] [Google Scholar]
- Reperant J, Ward R, Miceli D, Rio JP, Medina M, Kenigfest NB, Vesselkin NP. The centrifugal visual system of vertebrates: a comparative analysis of its functional anatomical organization. Brain Res Rev 52: 1–57, 2006. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The orbital arteries in the rabbit. Am J Ophthalmol 53: 96–107, 1962. [PubMed] [Google Scholar]
- Schnyder H, Kunzle H. Is there a retinopetal system in the rat? Exp Brain Res 56: 502–508, 1984. [DOI] [PubMed] [Google Scholar]
- Schutte M. Centrifugal innervation of the rat retina. Vis Neurosci 12: 1083–1092, 1995. [DOI] [PubMed] [Google Scholar]
- Sefton AJ, Lam K. Quantitative and morphological studies on developing optic axons in normal and enucleated albino rats. Exp Brain Res 57: 107–117, 1984. [DOI] [PubMed] [Google Scholar]
- Sefton AJ, Swinburn M. Electrical activity of lateral geniculate nucleus and optic tract of the rat. Vision Res 4: 315–328, 1964. [DOI] [PubMed] [Google Scholar]
- Seiple WH, Siegel IM. Recording the pattern electroretinogram: a cautionary note. Invest Ophthalmol Vis Sci 24: 796–798, 1983. [PubMed] [Google Scholar]
- Spinelli DS, Weingarten M. Afferent and efferent activity in single units of the cat's optic nerve. Exp Neurol 15: 347–362, 1966. [DOI] [PubMed] [Google Scholar]
- Stell WK, Walker SE, Chohan KS, Ball AK. The goldfish nervus terminalis: a luteinizing hormone-releasing hormone and molluscan cardioexcitatory peptide immunoreactive olfactoretinal pathway. Proc Natl Acad Sci USA 81: 940–944, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant M, Bruce SR, Beazley LD. Survival of ganglion cells which form the retino-retinal projection during optic nerve regeneration in the frog. Vis Neurosci 10: 681–686, 1993. [DOI] [PubMed] [Google Scholar]
- Terubayashi H, Fujisawa H, Itoi M, Ibata Y. Hypothalamo-retinal centrifugal projection in the dog. Neurosci Lett 40: 1–6, 1983. [DOI] [PubMed] [Google Scholar]
- Thanos S. Genesis, neurotrophin responsiveness, and apoptosis of a pronounced direct connection between the two eyes of the chick embryo: a natural error or a meaningful developmental event? J Neurosci 19: 3900–3917, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Straznicky C. Retino-retinal projections in three anuran species. Neurosci Lett 104: 43–47, 1989. [DOI] [PubMed] [Google Scholar]
- Tsai TI, Bui BV, Vingrys AJ. Effect of acute intraocular pressure challenge on rat retinal and cortical function. Invest Ophthalmol Vis Sci 55: 1067–1077, 2014. [DOI] [PubMed] [Google Scholar]
- Uchiyama H. The isthmo-optic nucleus: a possible neural substrate for visual competition. Neurocomputing 26–27: 565–571, 1999. [Google Scholar]
- Usai C, Ratto GM, Bisti S. Two systems of branching axons in monkey's retina. J Comp Neurol 308: 149–161, 1991. [DOI] [PubMed] [Google Scholar]
- van Hasselt P. Effects of optic nerve section on the double-flash ERG in unanaesthetised rabbits. A study of centrifugal influences on the ERG. Ophthalmologica 159: 65–70, 1969. [DOI] [PubMed] [Google Scholar]
- Wade NJ, Swanston MT, de Weert CM. On interocular transfer of motion aftereffects. Perception 22: 1365–1380, 1993. [DOI] [PubMed] [Google Scholar]
- Walker SE, Stell WK. Gonadotropin-releasing hormone (GnRF), molluscan cardioexcitatory peptide (FMRFamide), enkephalin and related neuropeptides affect goldfish retinal ganglion cell activity. Brain Res 384: 262–273, 1986. [DOI] [PubMed] [Google Scholar]
- Weymouth AE, Vingrys AJ. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog Retin Eye Res 27: 1–44, 2008. [DOI] [PubMed] [Google Scholar]
- Woodson W, Shimizu T, Wild JM, Schimke J, Cox K, Karten HJ. Centrifugal projections upon the retina: an anterograde tracing study in the pigeon (Columbia livia). J Comp Neurol 362: 489–509, 1995. [DOI] [PubMed] [Google Scholar]
- Wilson M, Lindstrom SH. What the bird's brain tells the bird's eye: the function of descending input to the avian retina. Vis Neurosci 28: 337–350, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]