Abstract
Neurons in substantia nigra pars reticulata (SNr) are synaptically coupled by local axon collaterals, providing a potential mechanism for local signal processing. Because SNr neurons fire spontaneously, these synapses are constantly active. To investigate their properties, we recorded spontaneous inhibitory postsynaptic currents (sIPSCs) from SNr neurons in brain slices, in which afferents from upstream nuclei are severed, and the cells fire rhythmically. The sIPSC trains contained a mixture of periodic and aperiodic events. Autocorrelation analysis of sIPSC trains showed that a majority of cells had one to four active unitary inputs. The properties of the unitary IPSCs (uIPSCs) were analyzed for cells with one unitary input, using a model of periodic presynaptic firing and stochastic synaptic transmission. The inferred presynaptic firing rates and coefficient of variation of interspike intervals (ISIs) corresponded well with direct measurements of spiking in SNr neurons. Methods were developed to estimate the success probability, amplitude distributions, and kinetics of the uIPSCs, while removing the contribution from aperiodic sIPSCs. The sIPSC amplitudes were not increased upon release from halorhodopsin silencing, suggesting that most synapses were not depressed at the spontaneous firing rate. Gramicidin perforated-patch recordings indicated that the average reversal potential of spontaneous inhibitory postsynaptic potentials was −64 mV. Because of the change in driving force across the ISI, the unitary inputs are predicted to have a larger postsynaptic impact when they arrive late in the ISI. Simulations of network activity suggest that this very sparse inhibitory coupling may act to desynchronize the activity of SNr neurons while having only a small effect on firing rate.
Keywords: substantia nigra, axon collaterals, desynchronization
neurons in substantia nigra pars reticulata (SNr) are output cells of the basal ganglia that integrate afferent signals from other basal ganglia regions to control target structures in the thalamus and brain stem. SNr neurons are also synaptically interconnected by local axon collaterals, which are branches of the main axons projecting to target nuclei (Grofova et al. 1982; Juraska et al. 1977; Karabelas and Purpura 1980; Mailly et al. 2003). Their function was first demonstrated by antidromic stimulation of efferents in the thalamus and superior colliculus, which produced inhibitory postsynaptic potentials (IPSPs) in SNr neurons (Deniau et al. 1982). Later studies reported periodic, action potential-dependent spontaneous inhibitory postsynaptic currents (sIPSCs) in SNr neurons in brain-slice preparations (Chan and Yung 1999; Stanford and Lacey 1996), consistent with the rhythmic, autonomous firing of these cells in vitro, where most of their other synaptic inputs are silent (Atherton and Bevan 2005). Most recently, it was shown that direct optogenetic activation of neurons in the mouse SNr produced local inhibition that reduced the gain of optically evoked spiking (Brown et al. 2014).
In addition to controlling gain, it has been suggested that the local axon collateral synapses may function to decorrelate firing activity in the SNr, promoting steady inhibitory output to target nuclei in the thalamus and brain stem (Mailly et al. 2003; Wilson 2013). Such decorrelation could be particularly important for Parkinson's disease, in which exaggerated beta-frequency (13–30 Hz) oscillations appear in the globus pallidus external segment (GPe) and subthalamic nucleus (STN) and correlate firing of neurons in the basal ganglia output nuclei (Brown 2006; Giannicola et al. 2010; Mallet et al. 2008; Nambu and Tachibana 2014).
Despite the potential importance of local axon collateral synapses in the SNr and a good deal of anatomical characterization, much remains unknown about the functional properties of these connections. A detailed anatomical study showed that the local collaterals of each SNr neuron in rats formed an average of 84 ± 18 synaptic boutons, with a range of 3–417 (Mailly et al. 2003), and the collaterals were largely confined within subdivisions of the SNr defined by specific striatal projections. However, it is not known how the boutons are distributed among their postsynaptic targets, and the basic physiological properties of the unitary connections had not been quantified.
The goal of the present study was to characterize the strength, kinetics, reliability, and functional impact of unitary synaptic connections among SNr neurons. Because of the sparse connectivity of the system, it was not feasible to approach this task by paired whole-cell recording. However, the identification of unitary IPSCs (uIPSCs) was possible, thanks to the autonomous firing of these cells, which is highly rhythmic in brain slices. This rhythmic firing was predicted to produce regular trains of uIPSCs, possibly interspersed with synaptic failures and mixed with aperiodic sIPSCs from other sources—primarily cut axons from the striatum and GPe.
In the studies described here, we recorded sIPSCs from 81 SNr neurons in rat brain slices, finding periodic synaptic input in 56 cells. Among these cells, 31 received exactly one unitary input, and their sIPSC trains were analyzed to measure the strength, kinetics, and reliability of the uIPSCs. Because many synapses subjected to repetitive activation undergo strong depression, we also tested whether sIPSC amplitudes were increased immediately after reversible presynaptic silencing using halorhodopsin. Finally, we investigated the possible impact of sparse local inhibition on synchronized firing of SNr neurons using a network model.
MATERIALS AND METHODS
Brain-slice preparation.
All animal procedures were approved by The University of Texas at San Antonio Institutional Animal Care and Use Committee. Brain slices were obtained from 41 Sprague-Dawley rats of both sexes, aged 21–55 days. Animals were deeply anesthetized with isoflurane and were then perfused transcardially with ice-cold, low-sodium solution containing (in mM) 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 10 MgSO4, 10 d-glucose, 26 NaHCO3, and 202 sucrose (pH 7.4). Brain slices (300 μm) containing substantia nigra were prepared using a vibrating slicer and collected in artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 2 MgSO4, 10 d-glucose, 26 NaHCO3, 0.005 l-glutathione, 1 Na-pyruvate, and 1 Na-ascorbate and bubbled with 95% O2, 5% CO2. In some experiments, slices were warmed to 35°C for ∼30 min after cutting. Slices were then stored at room temperature until used. l-Glutathione, Na-pyruvate, and Na-ascorbate were not included in the ACSF used for recordings.
Voltage-clamp recording.
A slice was transferred to the microscope chamber and superfused with ACSF (34°C) containing 20 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX) or 5 μM 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) to block AMPA/kainate receptors and 5 μM (±)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid to block N-methyl-d-aspartate receptors. SNr neurons were visualized by Dodt gradient contrast microscopy (Olympus, Center Valley, PA) and recorded in whole-cell, voltage-clamp mode using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and an ITC-18 analog/digital converter (HEKA Instruments, Holliston, MA), controlled by custom software written in Igor Pro (WaveMetrics, Lake Oswego, OR). Patch pipettes were pulled from borosilicate glass (G150-4; Warner Instruments, Hamden, CT), using a P-97 puller (Sutter Instrument, Novato, CA), and usually had resistances of 3–5 MΩ. Recordings from 68 cells were obtained with pipette solution containing (in mM) 140.5 CsCl, 7.5 NaCl, 10 HEPES, 0.2 EGTA, 2 Mg-ATP, and 0.21 Na-GTP, and recordings from 13 cells were done with a KCl-based solution (CsCl replaced with KCl, otherwise as above). Neurons were held at −70 or −80 mV without correction for junction potential (approximately −3 mV, pipette relative to bath, with either pipette solution). Current data were filtered online at 10 kHz and sampled at 20 kHz.
Perforated-patch current-clamp recording.
Perforated-patch recordings were obtained as described previously (Wilson et al. 2014), using pipette solution containing (in mM) 140.5 K methylsulfate, 7.5 NaCl, 10 HEPES, 0.2 EGTA, 2 Mg-ATP, 0.21 Na-GTP, and 0.5 μg/ml gramicidin. The pipette solution was filtered before addition of gramicidin (from 0.5 mg/ml freshly prepared stock in DMSO). The final solution was mixed thoroughly but not filtered. Recording was started when the access resistance decreased enough to reveal action potentials overshooting 0 mV. Typical access resistances were ∼50 MΩ.
Halorhodopsin.
For an optogenetic test of the local origin of sIPSCs and a method to investigate possible synaptic depression, the third-generation halorhodopsin enhanced Natronomonas pharaonis halorhodopsin (eNpHR)3.0 (Gradinaru et al. 2010) was expressed in substantia nigra. Adeno-associated virus (AAV)1 CamKII eNpHR3.0 enhanced yellow fluorescent protein (EYFP; 150 nl, 3.6 × 1013 genome copies ml−1; Vector Biolabs, Malvern, PA) was injected into substantia nigra of rats, 21–25 days of age [coordinates: medial/lateral ±2.0 mm, anterior/posterior −4.5 mm, dorsal/ventral −6.8 mm from bregma]. Animals were killed, and brain slices prepared 11–18 days after virus injection. Viral protein expression in SNr was confirmed by observation of YFP in slices using ultraviolet illumination. The halorhodopsin current was activated using 590 nm light-emitting diode (LED) light (∼5 mW total power) directed at substantia nigra using an optical fiber.
IPSC detection and amplitude measurement.
Data were analyzed in Mathematica (Wolfram Research, Champaign, IL). Stable epochs of current data, 40–300 s in duration, were selected for analysis. For sIPSC detection, the current data were smoothed by convolution with a Gaussian kernel (SD = 0.4 ms) and differentiated. The SD of the noise was estimated by fitting an all-points histogram with a Gaussian curve over a range, from −5 to +5 pA/ms. The sIPSC detection level was then set at −4 times the SD of the noise. Each event time was taken at the maximum rate of fall of the current, and the event amplitude was taken as the minimum of the smoothed current within 2 ms after that time, relative to the preceding baseline current 2 ms earlier.
Autocorrelation of the sIPSC train.
To analyze the temporal pattern of sIPSCs, the set of sIPSC times was represented by a binned histogram (bin width, w = 0.001 s). The histogram was scaled by 1/w to represent event rate (events/seconds) and is referred to as the event train, E(t). The autocorrelation of E(t) was then computed as a function of the time difference
The power spectrum of E(t) was computed as the magnitude of the Fourier transform of cE(Δt) for −2 s < Δt < 2 s.
Model of synaptic inputs.
To aid in characterizing the unitary synaptic inputs, a simple model of the afferent synaptic activity was developed. It was assumed that each periodic component of the sIPSC train arises from a regularly firing presynaptic neuron with interspike interval (ISI) jitter and with a probability of failure at the unitary synaptic connection. Here, we refer to the presynaptic firing rate as f, the SD of ISIs as σ, and the rate of uIPSCs from this connection as r. Thus the unitary synaptic success probability, P(success), is r/f.
The autocorrelation of the presynaptic spike train, cspike(Δt), comprises a series of peaks of equal area and increasing width, representing the first-order ISIs (T1), the second-order intervals (T2), and so on. We assume that the firing is regular enough that the probability density of T1 can be approximated by a Gaussian distribution with mean μ = 1/f and SD σ. If sequential ISIs were independent, then the variance (σ2) of the higher-order ISIs would increase linearly with the ISI order. To allow for correlations between sequential ISIs, we allow σh = hpσ, where the harmonic peak number h corresponds to the ISI order, and the power p is a free parameter. The area of each peak is equal to f. At large Δt, the peaks for high-order ISIs broaden and summate, approaching an asymptotic level equal to f2. Thus the autocorrelation of the spike train is described by an infinite sum of Gaussian components
For a finite time window, cspike(Δt) is approximated by a finite sum of Gaussian components (i.e., h = 1–100). We verified that this model provided excellent fits to spike train autocorrelations for SNr neurons in slices (see Fig. 3A).
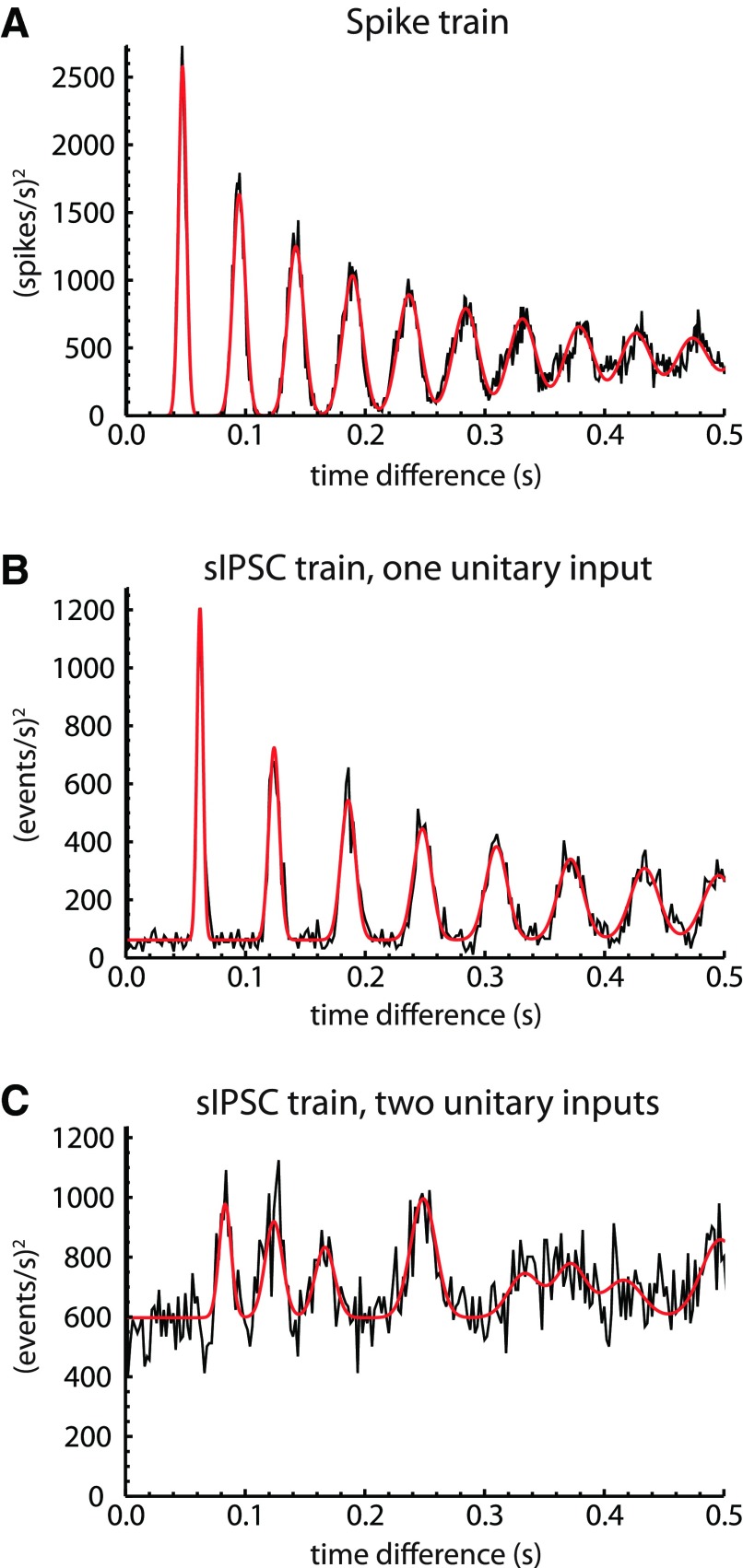
Fig. 3.
Model fit to autocorrelation of spike train and sIPSC trains. A: autocorrelation of spike train recorded from an SNr neuron using the perforated-patch method, fitted with a sum of Gaussian components (red), representing the first-order and higher-order ISIs. The fit parameters indicate a mean firing rate of 21.13 Hz and a CVISI of 0.069. B: autocorrelation of sIPSC train from a cell with 1 unitary input, fitted with a similar model, including a periodic input and an aperiodic input (see materials and methods). The fit parameters show an aperiodic event rate (r0) of 2.51 s−1, a presynaptic firing frequency (f1) of 16.15 s−1, CVISI of 0.041, and a periodic event rate (r1) of 10.89 s−1. Based on r1/f1, the unitary synaptic success probability was 0.674. C: autocorrelation of sIPSC train from a cell with 2 unitary inputs, fitted with a model, including 2 periodic inputs and an aperiodic input.
The sIPSC event train, E(t), differs from a presynaptic spike train in two respects. First, there may be multiple inputs, j = 0 to m, where input 0 is an aperiodic component [presumably miniature IPSCs (mIPSCs)], and inputs 1 to m are periodic unitary inputs, which may have different firing frequencies and are assumed to be uncorrelated with one another. Second, there are synaptic failures, so the rate of uIPSCs from input j (rj) is lower than the spike frequency of that input (fj). The autocorrelation of E(t) is the sum of the auto- and cross-correlations of the individual components
For subcomponent cj,k, the expected value at large Δt is the product of the mean rates, rj·rk. The only periodic components of cE(Δt) are the autocorrelations of the unitary event trains, cj,j(Δt), where j > 0. At large Δt, cj,j(Δt) approaches rj2. Because this asymptotic level is also equal to fj times the peak area, we see that the peak area is rj2/fj. Based on these assumptions, each subcomponent of the autocorrelation is described by the following model
For data analysis, the model was approximated by a finite sum of Gaussian components (h = 1–100) and provided good fits to sIPSC time autocorrelations with up to four periodic components (the largest number detected in the present study). However, because a sufficient number of cells showing exactly one periodic input were recorded, the data reported are restricted to these simpler cases.
Clocking method for sIPSCs.
To distinguish uIPSCs from aperiodic events, a method was developed to estimate the phase of presynaptic firing by band-pass filtering of E(t). The filter was obtained by fitting a Gaussian function to the first harmonic peak of the power spectrum PE(f), giving the center frequency (fcenter) and the SD (σP). The gain of the corresponding filter (operating on signal amplitude rather than power) is then described by a Gaussian function with the same fcenter and an SD σG = 21/2σP.
In the time domain, neglecting a scale factor, the corresponding waveform is a Gaussian-windowed cosine function with an SD σt = 1/(2πσG).
To examine the phase of event i with respect to its neighbors, we took the surrounding portion of E(t), or Ei(Δt), within a symmetrical window of ±2 s around the event time ti. To prevent the event from influencing its own phase estimate, the central event at Δt = 0 was removed. Ei(Δt) was then convolved with g(Δt) to produce the local “clock” waveform
The local clock ki(Δt) is a sinusoidal waveform with peaks that align with periodic events within the selected frequency band (i.e., fcenter ± σG; see Fig. 5). Thus the phase of event i, or θi, was determined from the positions of the two surrounding minima of ki(Δt), at time differences Δt0 and Δt1
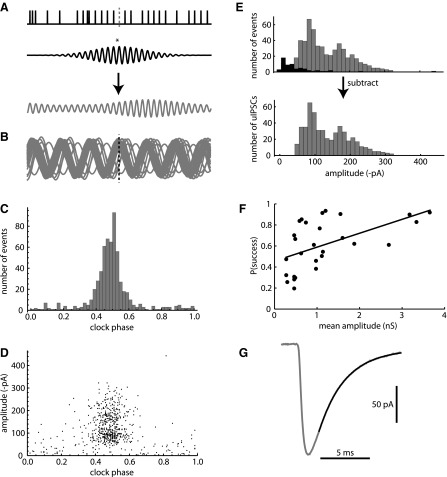
Fig. 5.
The estimation of the uIPSC amplitude distribution and kinetics. A: method for clocking sIPSCs with respect to the surrounding periodic events. Top: digitized sIPSC train surrounding a reference event, which has been removed (vertical dotted line). Middle: band-pass filter represented by a Gaussian-windowed cosine function. The filter frequency and bandwidth were determined based on the power spectrum of the entire digitized sIPSC train (see materials and methods). Bottom: local clock signal produced by convolving the digitized sIPSC train with the filter. The asterisk represents the operation of convolution. B: the local clock signals surrounding 50 reference sIPSCs. Most, but not all, of the reference events align with the peak of the clock signal. Black dotted line indicates the time of the reference event. C: phase distribution of sIPSCs with respect to the local clock signal. D: sIPSC amplitude vs. clock phase. In the example cell, most of the larger sIPSCs appeared at central phases, indicating that they came from the unitary input. E, top: amplitude distributions for “center-phase” sIPSCs at phases of 0.25–0.75 (gray bars) and “side-phase” sIPSCs at phases of 0–0.25 and 0.75–1 (black bars). Bottom: uIPSC amplitude distribution obtained by subtracting the side-phase distribution from the center-phase distribution. F: unitary synaptic success probability vs. mean uIPSC amplitude for each cell with exactly 1 unitary input (r = 0.45, P < 0.01). G: estimated average uIPSC waveform for the example cell (see materials and methods). The 20–80% rise time was 0.35 ms, and the decay time constant was 2.66 ms.
With this definition of phase, uIPSCs from the selected input are concentrated at phases near 0.5, whereas aperiodic mIPSCs are distributed uniformly across all phases.
uIPSC amplitude distribution.
To estimate the uIPSC amplitude distribution, the events were separated into two groups: a “center-phase” group, with 0.25 < θ < 0.75, and a “side-phase” group, with θ < 0.25 or θ > 0.75. An amplitude histogram was constructed for each group, using a bin width of 0.1 times the mean event amplitude for the entire data set. The uIPSC amplitude distribution, nu(A), was then estimated by subtracting the side-group histogram from the center-group histogram. In some cells, it was observed that fewer low-amplitude sIPSCs were detected at center phases compared with side phases, resulting in negative values in the subtracted distribution for the lowest amplitude bins. This artifact was attributed to a lower probability of detecting very small sIPSCs when they overlapped with larger uIPSCs. To reduce this bias, any negative counts in nu(A) were set to zero. The mean uIPSC amplitude (μA) and the variance (σA2) were then calculated from nu(A), taking each value of A as the bin center and summing across all bins
uIPSC kinetics.
To estimate the waveform of the average uIPSC, the individual event traces, Ii(Δt) = I(ti + Δt), were extracted from the raw current data (−2 ms < Δt < 10 ms), and the baseline current at Δt = −2 ms was subtracted. The events were collected in a center-phase group (collectively designated Icenter, count = ncenter) and a side-phase group (designated Iside, count = nside) as described above. The average uIPSC waveform was then estimated from the summed waveforms of the two groups
The rise time of the resulting current trace was measured from 20% to 80% of peak amplitude, and the decay phase was fitted with a single exponential function, y0 + A exp(−t/τd), over the range from 80% of peak amplitude to 10 ms after the event detection point.
sIPSP detection.
Spontaneous IPSPs (sIPSPs) were recorded during autonomous firing activity using the perforated-patch current-clamp method described above. For IPSP detection, the voltage data were smoothed by convolution with a Gaussian function (SD = 0.5 ms) and differentiated twice, computing the derivative at each time point as the slope between the two surrounding points. A detection threshold was chosen by eye based on the noise level of each cell, and the IPSP times were taken at each minimum of the second-derivative trace that exceeded the threshold in the negative direction. In some cells, the choice of threshold was verified by analyzing data obtained subsequently from the same cell in the presence of picrotoxin (100 μM) to confirm that most of the detected events were GABAA receptor-mediated IPSPs.
Estimation of sIPSP reversal potential.
To estimate sIPSP reversal potential (Erev) from the perforated-patch current-clamp data, we analyzed the detection of sIPSPs as a function of phase within the ISI. The phase of each sIPSP (ϕi) was calculated based on its detection time (ti) and the two surrounding spike times (ts− and ts+), which were each taken at the first data point positive to −20 mV
Events with phases <0.1 or >0.9 were excluded from the analysis, because it was not possible to detect sIPSPs reliably in close proximity to an action potential. A histogram of sIPSP phases was then constructed for phases of 0.1–0.9. It was generally observed that fewer sIPSPs were detected at early phases of the ISI, as expected, based on the lower driving force for the Cl− current. To estimate Erev, we found the onset voltage of each sIPSC (Vonset) and measured the amplitude of the event (Ai) from the second-derivative trace. This measure of sIPSP amplitude was chosen because it was the detection criterion and because it was relatively unaffected by the underlying interspike membrane potential (Vm) trajectory. We then determined what value of Erev could account for the difference in event detection frequency between the late portion of the ISI (0.5 < ϕ < 0.9) and the early portion (0.1 < ϕ < 0.5). For each late sIPSC, an onset voltage was selected randomly from the early portion of the same interspike trajectory, and the amplitude the late event would have had if it were moved to the early phase was calculated for an assumed Erev
The calculation was repeated for multiple values of Erev, and the number of late events giving Aearly above the detection threshold was determined. The correct value of Erev was then estimated as the value predicting the number of early events detected.
Spike timing response curve for IPSPs.
To investigate the impact of IPSPs on spike timing, spike timing response curves (STRCs) were calculated by a modification of a recently published multiple-regression method (Wilson et al. 2014). Briefly, the method assigns each input a phase based on its position in the ISI (equal to ϕi defined above). The input during each ISI is subdivided into a fixed number of bins (here, bin width = 0.1 cycle). The binned input amplitudes provide the independent variables, and the ISI length is the dependent variable for a multiple linear regression, which generates a slope coefficient (cycles/input amplitude) for each phase bin. In the present application of the method, it was necessary to account for the bias in event detection produced by the varying distance from Erev during each ISI. Thus we divided the amplitude of each event (taken as the peak negative value of the second-derivative trace) by the estimated driving force (Vonset − Erev) to obtain a value proportional to the synaptic conductance. The corrected amplitudes were then normalized by the mean value, such that normalized amplitude of unity represents an event of average conductance. The regression coefficients then indicate the shortening of the ISI (cycles) produced by one event of average conductance at each phase.
Network model.
To investigate the possible influence of sparse inhibitory coupling on a network of repetitively firing neurons driven by shared input, a simple model of 100 cells was constructed. Each cell was represented by a phase model comprising an autonomous firing rate (ω = 20 s−1) and an STRC, R(ϕ). The phase evolution of the model is perturbed by external input, Gext(t), and by the local synaptic conductance, Gsyn(t), according to the following differential equation
For simplicity, it was assumed that the external input and the local synaptic input operate through the same STRC, and the inputs were represented in the same units of inhibitory conductance. The external synaptic inhibition Gext(t) was intended to represent time-varying inhibitory input from the striatum and GPe, and the more discrete synaptic inhibition Gsyn(t) represented the local coupling of SNr cells. Synaptic conductances were scaled so that a conductance of one represented the peak of a single unitary input. Based on our experimental data (see Fig. 7F), the STRC of each model neuron was linear in shape, producing a phase delay of zero for input at phase ϕ = 0 and 0.1 cycle for an integrated inhibitory conductance equivalent to one unitary input applied at ϕ → 1. The actual phase delays differed slightly from the linear ramp profile of the model STRC because of the synaptic decay time constant τsyn = 3 ms, which results in synaptic conductance persisting for a short time after the IPSC onset. To simulate the local connections, each cell was connected to an average of four randomly chosen cells (for 100 cells, connection probability = 0.04). The synaptic interactions were simulated with a synaptic delay (1.6 ms) and an instantaneous rise. At each time when the phase of a cell reached one, the spike time was recorded and the cell's phase reset to zero, and after the synaptic delay, the synaptic conductance of each postsynaptic cell was increased by the peak unitary conductance.
Fig. 7.
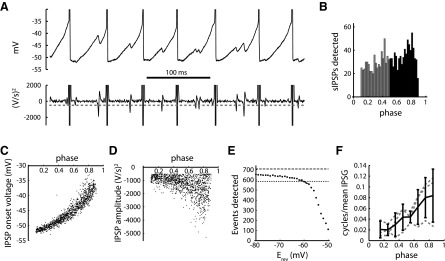
sIPSPs in SNr neurons. A, top: example of sIPSPs recorded by the gramicidin perforated-patch method during autonomous firing. The sIPSPs appear as sudden downward deflections in the interspike voltage trajectories. The pattern of sIPSPs suggests that this cell received at least 2 unitary inputs. Bottom: second derivative of voltage trace, smoothed by convolution with a Gaussian filter (0.5 ms SD). Note the large negative excursions corresponding to the onset of the sIPSPs. The sIPSPs were detected from the second-derivative trace; the dashed gray line indicates the detection threshold for this cell. B: histogram showing the number of sIPSPs detected as a function of phase within the ISI. A phase of 0 represents the previous spike time, and a phase of 1 represents the next spike time. To avoid detection artifacts associated with spikes, analysis was restricted to sIPSPs occurring at phases of 0.1–0.9. As expected for a conductance input with varying driving force (Vm − Erev), a larger number of sIPSPs were detected at phases of 0.5–0.9 (black bars) compared with phases of 0.1–0.5 (gray bars). C: Vm at the onset of each sIPSP, plotted as a function of phase. The curvature of the plot represents the interspike Vm trajectory. D: amplitude of each sIPSP, plotted as a function of phase. The amplitude was measured from the second-derivative trace used for event detection. Because of the increased driving force, larger-amplitude sIPSPs were found at late phases. E: estimation of the sIPSP reversal potential (Erev; see materials and methods). The method of estimation was to assume a range of values of Erev and based on these assumed values, calculate the number of sIPSCs that would have been detected at early phases (0.1–0.5), where the driving force is less. The graph shows the predicted number of late-phase sIPSPs (0.5–0.9) that would have been detected at randomly chosen early phases, plotted as a function of the assumed value of Erev. The dashed line indicates the total number of sIPSPs that were actually detected at late phases, and the dotted line indicates the number of sIPSPs detected at early phases. In this cell, a correct prediction was obtained with an assumed Erev of −60.4 mV, where the curve crosses the dotted line. F: spike time response curves (STRCs) for sIPSPs in 3 cells (dotted gray lines) and average STRC (black line; error bars are SD). The data indicate that sIPSPs at later phases caused greater lengthening of the ISI. IPSG, inhibitory postsynaptic conductance.
To investigate network synchronization, simulations were performed with external sine-wave input. Each neuron was initialized at a random phase chosen from a uniform distribution (0 < ϕ < 1), and the entire network was then driven by 2 s of shared sine-wave input with amplitude Aext and frequency fext
To quantify the synchrony produced by the external input, we measured the phase entropy of the population of neurons. At each time point in the simulation, a phase-probability histogram, P(ϕ), was constructed with a bin width of 0.01 cycle (n = 100 bins), and the entropy was calculated from the binned P(ϕ) values
The entropy values shown are the mean “steady-state” values for the second half of each simulation (1–2 s).
Data and statistical comparisons.
Average values are reported as means ± SD. The significance of differences between groups was evaluated using Student's t-tests, and results were considered significant when P < 0.05.
Materials.
Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). DNQX and NBQX were purchased from Tocris Bioscience (Bristol, UK).
RESULTS
Periodic sIPSCs in SNr neurons.
sIPSCs were recorded from 81 SNr neurons in brain slices. High-chloride pipette solution was used to increase the driving force for Cl− currents (calculated ECl = +3 mV; holding potential = −70 or −80 mV), and glutamatergic synaptic inputs were blocked (see materials and methods). In addition to 77 cells recorded in coronal slices, four cells were recorded in sagittal slices and showed generally similar patterns of sIPSCs. CsCl pipette solution was used for 68 cells, and an otherwise identical KCl pipette solution was used for the remaining 13 cells. We observed periodic trains of sIPSCs in 49/68 cells recorded with CsCl solution and 7/13 cells recorded with KCl solution, suggesting that most of the synapses were located sufficiently close to the soma so that the sIPSCs could be detected without blockade of dendritic K+ channels. The amplitudes and kinetics of the periodic sIPSCs were in the same range with both pipette solutions. Thus the data obtained with the two solutions were pooled.
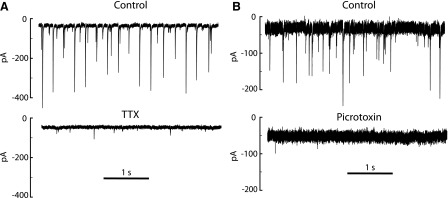
A current trace from a neuron showing clear periodic input is shown in Fig. 1A. The regular pattern of synaptic currents suggests that this cell received input from at least one autonomously firing presynaptic neuron present within the slice. Consistent with this, the periodic sIPSCs were eliminated by application of TTX (Fig. 1A). In four cells showing periodic sIPSCs that were then superfused with TTX (0.1 or 1 μM, n = 2 each), the total rate of sIPSCs was reduced from 53.2 ± 34.3 s−1 to 3.4 ± 1.3 s−1, and the remaining sIPSCs were small and aperiodic. The sIPSCs were also blocked by picrotoxin (100 μM, n = 2; Fig. 1B), consistent with the GABAergic phenotype of SNr neurons (Oertel and Mugnaini 1984; Smith et al. 1987) and previous reports that sIPSCs in these cells were sensitive to GABAA receptor antagonists (Stanford and Lacey 1996; Ye et al. 1997).
Fig. 1.
sIPSCs in SNr neurons. A: recordings in control extracellular solution (top) and in the presence of TTX (bottom; 1 μM), obtained with CsCl intracellular solution at −70 mV. B: recordings in control solution (top) and in the presence of picrotoxin (bottom; 100 μM), obtained with KCl intracellular solution at −70 mV.
Number of unitary inputs.
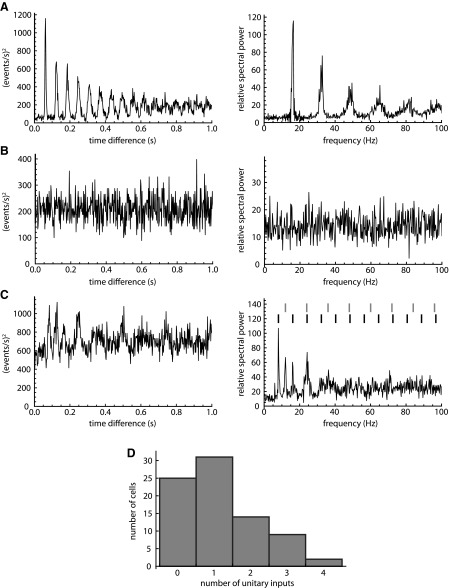
To count and characterize the unitary synaptic connections, we converted the set of sIPSC times to a digitized event train and computed its autocorrelation (see materials and methods). In a cell with one active unitary input firing regularly with some timing jitter, the autocorrelation is predicted to show a series of regularly spaced peaks that broaden with increasing Δt, eventually fusing to form a plateau. This pattern arises directly from the successive ISIs of the presynaptic neuron. Out of 81 cells recorded, 31 showed this simple pattern (e.g., Fig. 2A). In contrast, 25 cells lacked obvious periodic sIPSCs and did not show clear peaks in the autocorrelation (Fig. 2B). The remaining 25 cells showed complex patterns, suggesting the presence of more than one unitary input (Fig. 2C).
Fig. 2.
Analysis of periodicity in sIPSC trains. A–C, left: autocorrelation of the digitized sIPSC train; right: corresponding power spectrum. A: a cell with 1 active unitary input shows a single series of evenly spaced harmonic peaks in the autocorrelation and the spectrum. B: a cell with no recognizable unitary input shows no clear peaks in the autocorrelation or the spectrum. C: a cell with 2 unitary inputs shows a complex pattern of peaks in the autocorrelation and the spectrum. The vertical marks above the spectrum (right) indicate the 2 harmonic components, each with equally spaced peaks. D: histogram of number of identified unitary inputs to each SNr neuron.
The number of unitary inputs to each cell was determined by examining the spectrum obtained by Fourier transformation of the autocorrelation (Fig. 2, A–C). These spectra also comprise a series of harmonic peaks but more clearly revealed the presence of relatively low-frequency uIPSC trains that were sometimes less evident in the autocorrelation. The number of harmonic components was determined by visual inspection of each spectrum, with the aid of a custom algorithm that placed markers at integer multiples of each identified principal frequency (Fig. 2C). Based on this analysis, SNr neurons in slices received from zero to four unitary inputs (1.16 ± 1.07; Fig. 2D). As the local collaterals of SNr neurons have been shown to traverse large portions of the nucleus (Mailly et al. 2003), it is likely that more unitary connections are present in the intact SNr.
uIPSC timing is consistent with local input from SNr neurons.
With the use of a simple model of the unitary synaptic input (see materials and methods), we analyzed the sIPSC time autocorrelations of SNr neurons that were found to have only one unitary input (n = 31). The model was based on the expected autocorrelation of a regular spike train; thus we verified that spike trains of SNr neurons in slices were fitted well by the model, constrained to allow no aperiodic events or failures (Fig. 3A). The firing frequency and coefficient of variation of ISIs (CVISI) recovered from the model fit parameters closely matched the corresponding values measured directly from the spike times. For example, the illustrated cell fired at a mean rate of 21.21 spikes/s, and the CVISI was 0.070, whereas the model fit returned a harmonic frequency of 21.13 Hz and a CV of 0.069.
We then fitted the model to the sIPSC autocorrelation data for cells with a single unitary input (Fig. 3B). It was also possible to fit the model to data from cells with two unitary inputs (Fig. 3C) or even more. However, as sufficient data were available for cells with one unitary input, our characterization of uIPSC properties was restricted to this group. The model fits provided five parameters: r0, the rate of aperiodic sIPSCs; r1, the rate of uIPSCs; f1, the frequency of presynaptic action potentials; σ, the SD of presynaptic ISIs; and p, a power describing the rate of increase in SD for higher-order ISIs (i.e., sums of sequential ISIs). The values of f1 were predicted to correspond to the firing frequencies of SNr neurons recorded in the slice preparation using a noninvasive technique, whereas σ · f1 should correspond to the CVISI. To test these predictions, we compared the model fit data with direct measurements of mean firing rate and CVISI in perforated-patch recordings performed under the same conditions. From the model fits, f1 of the sIPSC trains ranged from 4.1 to 32.4 Hz (16.2 ± 7.6 Hz, mean ± SD, n = 30, excluding 1 outlier cell with f1 = 69.6 Hz), and the estimated CVISI of the presynaptic cells was 0.074 ± 0.030. These values were similar to the directly recorded spike data (firing rate = 20.6 ± 9.6 spikes/s, CVISI = 0.062 ± 0.025, n = 15), supporting the hypothesis that the periodic sIPSCs recorded in SNr neurons in brain slices arise from axon collaterals of other SNr cells.
Success probability of uIPSCs.
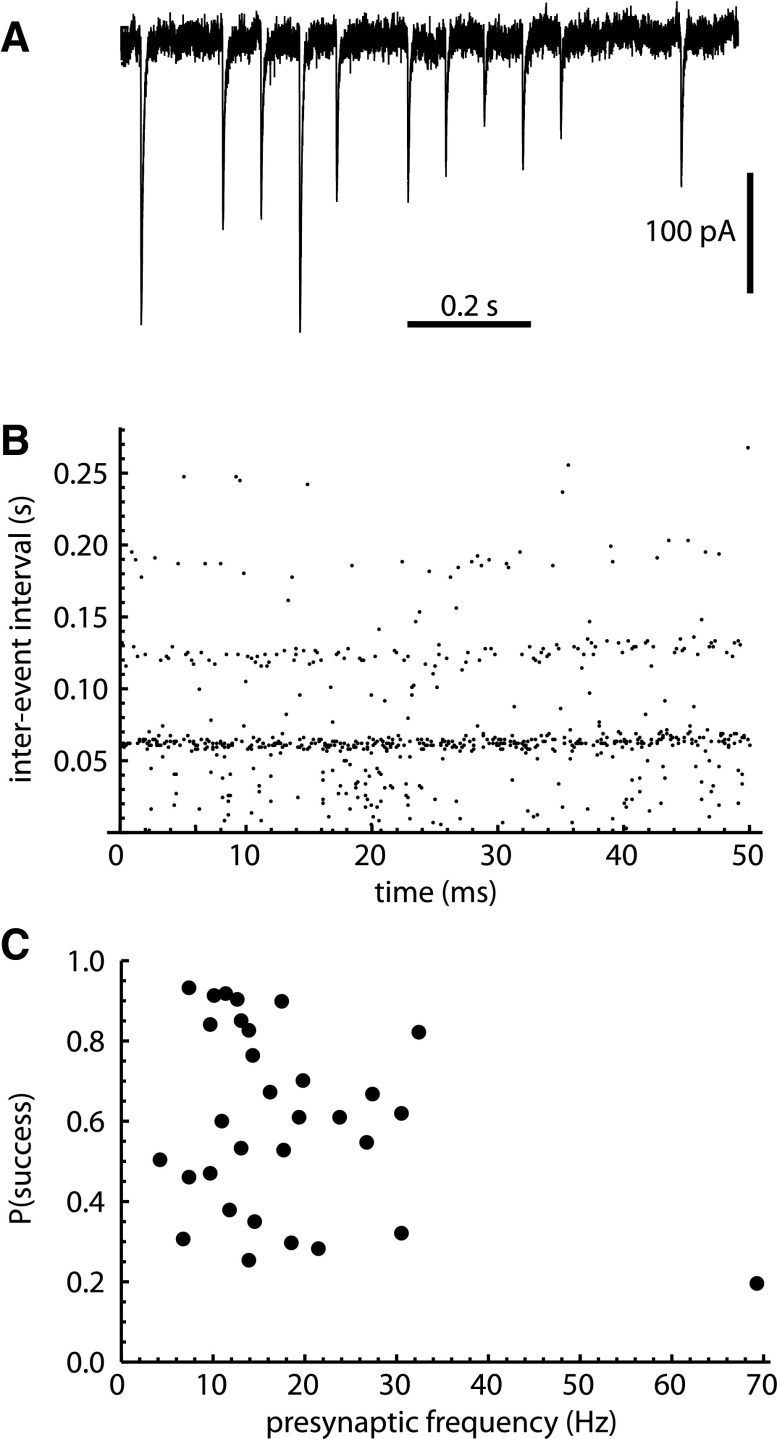
In most current traces showing a single uIPSC train, the periodic IPSCs appeared to be mixed with synaptic failures (Fig. 4A). In some cells, this was apparent from the interevent intervals, which showed multiples of the primary interval (Fig. 4B). However, the failure rate could not be determined simply by visual inspection of the data, because the times of the presynaptic action potentials were not known with certainty, and because mIPSCs were intermixed with the uIPSCs. Thus we estimated the uIPSC success probability, P(success), from the model fit parameters, dividing the uIPSC rate r1 by the frequency f1. P(success) varied widely among the 31 cells determined to have 1 unitary input, ranging from 0.20 to 0.93 (mean = 0.60, SD = 0.23). Note that P(success) represents the overall success probability for transmission at all synaptic contacts from one neuron to another, and the release probability at each individual synapse is likely to be considerably lower.
Fig. 4.
Stochastic transmission at unitary synaptic connections. A: recording from an SNr neuron showing a train of sIPSCs with clear synaptic successes and failures and a relatively low rate of aperiodic events. Note the series of regularly spaced sIPSCs interrupted by gaps of 2–3 times the shorter intervals. B: sequence of interevent intervals in the same cell, showing multiples of the most common interval length. C: unitary synaptic success probability, P(success), of each cell with exactly 1 unitary input, plotted as a function of the inferred presynaptic firing frequency, f1. Both parameters were determined by model fitting, as described in materials and methods. The correlation between f1 and P(success) was not significant.
Based on numerous reports of frequency-dependent synaptic depression in various neurons during repetitive afferent activation [e.g., Atherton et al. (2013); Connelly et al. (2010); Korn et al. (1984)], we were interested in whether the success probability would depend on the spontaneous presynaptic firing frequency. Thus we examined the relationship between f1 and P(success) (Fig. 4C). Surprisingly, there was virtually no correlation between these measures (r = −0.08), suggesting that frequency-dependent synaptic depression was not present or was compensated by other differences in synaptic properties.
Distinguishing uIPSCs and measuring their amplitudes and kinetics.
To characterize the uIPSCs, it was important to distinguish them as well as possible from other aperiodic sIPSCs with which they are intermixed. Our approach was to construct a “clock signal,” based on the surrounding event times, using band-pass filtering, based on the experimentally determined event train spectrum (see materials and methods). In the time domain, the filter is a cosine wavelet. The filter is convolved with the local event train (after removing the reference event), producing a clock signal that tracks the periodic train of neighboring events (Fig. 5, A and B). Based on the clock signal, each sIPSC was assigned a phase from zero to one, where members of the uIPSC train are expected to occur at phases near 0.5. We observe that the sIPSC phase distribution has a peak centered at a phase of 0.5, in addition to some events distributed relatively uniformly (Fig. 5C). In cells with large-amplitude uIPSCs, we generally observed that most of the larger sIPSCs were associated with the central peak of the phase distribution, whereas small-amplitude sIPSCs were observed at all phases (Fig. 5D).
To estimate the uIPSC amplitude distribution, we first subdivided the sIPSC amplitude distribution into two components: one for events with “center” phases of 0.25–0.75 and another for events with “side” phases <0.25 or >0.75. The amplitude distribution of phase-associated events (i.e., uIPSCs) was then obtained by subtracting the side distribution from the center distribution (Fig. 5E; see materials and methods). The analysis of uIPSC amplitudes was performed successfully for 28/31 cells, identified as having exactly one unitary input. In the other three cells, the subtracted amplitude distributions showed a high level of noise due to relatively high rates of aperiodic events and were not analyzed further. Based on the distributions obtained, the uIPSCs had mean conductance amplitudes of 1.17 ± 0.95 nS and amplitude CVs of 0.60 ± 0.13, not including synaptic failures. There was a significant correlation between P(success) and the mean amplitude of the uIPSCs (Fig. 5F; r = 0.45, P < 0.01). A likely explanation for this correlation is that on average, larger uIPSCs are produced by unitary connections comprising a larger number of individual release sites.
The average uIPSC waveform of each cell was estimated based on the current waveforms of the individual events in the center-phase and side-phase groups (see materials and methods). An example waveform is shown in Fig. 5G. The 20–80% rise times of the average uIPSCs were 0.44 ± 0.10 ms, and the decay time constants were 3.42 ± 1.07 ms. These data indicate that the inhibition produced by the local collateral synapses is quite fast in onset and decay. However, there was a significant correlation between the rise times and the decay time constants of the average uIPSCs from different cells (r = 0.62, P < 0.001), suggesting that the synaptic currents may be affected by electrotonic filtering. These data would be consistent with synaptic locations near but perhaps not on the postsynaptic cell bodies.
Quantal content and size.
In preliminary experiments, we did not find it feasible to perform a full quantal analysis (i.e., mean-variance analysis) on the uIPSC data, as we observed relatively little curvature in the mean-variance relationship (σA2 vs. μA) for uIPSC amplitudes when synaptic release was increased approximately two-fold using high-calcium external solution (data not shown). This observation suggested that the probability of transmitter release (p) at each individual release site is low. In support of this, unitary inputs producing small uIPSCs had overall success probabilities, P(success), as low as 0.2. Thus although we do not know the value of p, it appears reasonable to suppose that it is sufficiently low so that Poisson statistics apply. We can then estimate the quantal content (m) as −ln[1 − P(success)] and the quantal size (q) as σA2/μA. Given these assumptions, our data showed m = 1.14 ± 0.75 quanta/spike (n = 31), and q = 0.73 ± 0.54 nS (n = 28).
Optogenetic investigation of synaptic depression.
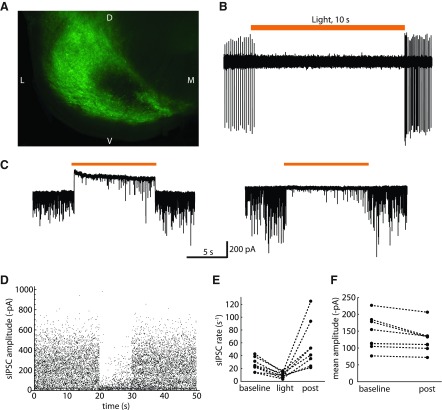
Because SNr neurons fire continuously, we expected that their axon collateral synapses might be in a continual state of short-term synaptic depression, which would recover if the firing were silenced by strong, sustained inhibition or by artificial means. To test this hypothesis, we suppressed autonomous firing using viral expression of halorhodopsin in SNr neurons (see materials and methods). Successfully targeted virus injections gave widespread viral protein expression throughout most of substantia nigra (see example in Fig. 6A, showing the ventrolateral portion of a coronal brain slice). Halorhodopsin was activated in brain slices using 590 nm LED light applied to a large area of substantia nigra via an optical fiber. In slice preparations from animals with well-targeted virus injections, most but not all neurons showed a suppression of spiking on illumination (see loose-patch recording; Fig. 6B). In voltage-clamp recordings, most cells showed a large light-evoked outward current and a suppression of sIPSCs (Fig. 6C). However, the presynaptic and postsynaptic expression of halorhodopsin did not always correspond, as a few cells that lacked a light-evoked outward current did show a strong reduction of sIPSC activity (Fig. 6C). The analyses described below were restricted to cells where periodic sIPSC activity was evident and was strongly suppressed by illumination (n = 8).
Fig. 6.
Investigation of possible synaptic depression using halorhodopsin. A: ventrolateral portion of coronal brain slice from a rat injected with AAV1 CamKII eNpHR3.0 EYFP. The green fluorescence indicates expression of virus-encoded EYFP throughout most of substantia nigra (D, dorsal; V, ventral; M, medial; L, lateral). B: loose-patch extracellular recording from a halorhodopsin-expressing SNr neuron. Orange bar indicates the timing of 590 nm illumination. Activation of halorhodopsin completely suppressed the cell's autonomous firing for 10 s. At light offset, the cell resumed firing with increased frequency. C: effect of halorhodopsin activation on sIPSCs in 2 SNr neurons. Left: cell with a large halorhodopsin current (upward shift in baseline during illumination). Right: cell with little or no halorhodopsin current. In both cells, halorhodopsin activation strongly suppressed sIPSC activity, with only a relatively low rate of large sIPSCs returning by the end of the 10-s period of illumination. D: all sIPSC amplitudes in the example cell from C, right, combining the data from 10 trials. The sIPSCs occurring immediately after light offset were not larger than the baseline events. E: mean sIPSC rates of each cell before illumination (baseline), during the light, and during the first 1 s after illumination (post). F: mean sIPSC amplitude of each cell during the baseline period and the postillumination period. The lack of increase of sIPSC amplitudes upon offset of illumination suggests that the unitary synaptic inputs were not depressed by the baseline autonomous firing.
For investigation of possible synaptic depression, halorhodopsin was activated with continuous 10 s light stimuli, as studies of other neurons suggested that this duration would be sufficient for at least partial recovery from depression [e.g., Atherton et al. (2013)]. Because of the changing presynaptic firing rates during this experiment, we did not attempt to perform analyses like those described above to distinguish the uIPSCs from other sIPSCs. In keeping with the local collateral origin of the uIPSCs, illumination suppressed the periodic components of the sIPSC trains while leaving a lower rate of small, aperiodic events. In some recordings, a lower rate of periodic sIPSCs began to appear near the end of the 10-s light stimulus, presumably because of a gradual reduction of the halorhodopsin current and/or intrinsic adaptation in the presynaptic neuron.
We measured the amplitudes of all sIPSCs (example in Fig. 6D) and obtained the mean sIPSC rates (Fig. 6E) and amplitudes (Fig. 6F) in each cell before illumination and from 0 to 1 s after illumination. During illumination, the mean sIPSC rate was reduced from 26.4 ± 10.7 s−1 to 9.6 ± 4.4 s−1 (n = 8), which was similar to the mean rate of aperiodic sIPSCs, estimated from the unperturbed sIPSC data described above (7.5 ± 4.2 s−1, n = 31). The sIPSC rates recovered almost immediately on termination of the light, in most cases, showing a transient increase above the prestimulus rate. The increase in sIPSC rate could result from an increase in presynaptic firing rate (which we often observe in SNr neurons after termination of a hyperpolarizing current step) or from an increase in the unitary synaptic success probability, P(success). If P(success) were increased by recovery from synaptic depression, then we would expect that at least those cells starting with a relatively high P(success) would also show larger sIPSC amplitudes, as multiple quanta would likely be released from the set of presynaptic terminals forming the unitary connection. In fact, even an increase in presynaptic firing rate with no change in P(success) would be predicted to increase the mean sIPSC amplitude, because in most cases, the uIPSCs are larger than the aperiodic sIPSCs. However, we did not observe an increase in sIPSC amplitude at the offset of illumination, as would be expected if collateral synapses were depressed by the continuous firing of SNr neurons. In the first second after illumination, the mean sIPSC amplitudes were 88.6 ± 9.6% of the mean baseline amplitude. The small reduction of sIPSC amplitudes after illumination was statistically significant (P < 0.05), suggesting that a small degree of synaptic facilitation may be present during baseline activity. These data suggest that the local collateral synapses are not depressed during autonomous firing.
IPSPs and effect on spike timing.
To assess the impact of spontaneous inhibition under more realistic conditions, IPSPs were recorded in gramicidin perforated-patch current-clamp mode (Fig. 7A; see materials and methods). This method preserves the natural intracellular Cl− concentration, as well as the autonomous firing that is characteristic of SNr neurons (Atherton and Bevan 2005). IPSPs were detected as negative excursions in the second derivative of the voltage trace (Fig. 7A).
Unlike IPSCs, measured in voltage clamp with an artificially high driving force, IPSPs were small, and their amplitudes varied in size depending on the phase of the postsynaptic cell's oscillation. IPSPs were readily detected in the late part of the ISI, where Vm − Erev is large, but were much smaller and sometimes went undetected in the early part of the ISI when the cell was hyperpolarized and close to the chloride Erev (Fig. 7B). We used this effect of Vm on the IPSP amplitude to estimate Erev (Fig. 7, C–E; see materials and methods), finding the value of Erev that could account for the number of sIPSPs detected early in the ISI. In the individual example, Erev was estimated as −60.4 mV. This analysis indicated an Erev of −64 ± 3 mV for the sample of cells (n = 6). In most cells, this level was slightly below the trough of the afterhyperpolarization, indicating that IPSPs were small but not reversed at early phases, and the driving force for the IPSC was much larger at late phases of the ISI than at early phases.
In repetitively firing neurons, a brief inhibitory input is not expected to prevent an action potential but instead, delays the time of occurrence of the next spike (Wilson 2015). To quantify this effect, we determined the STRCs for sIPSPs. The STRC measures the change in ISI length (fraction of a cycle) caused by a synaptic input as a function of the relative time in the ISI (phase), at which the input arrives (Netoff et al. 2005; Wang et al. 2012). It reflects the combination of the cell's intrinsic phase resetting curve, the amplitude and time course of the synaptic conductance change, and the phase dependence of the synaptic current driving force. We calculated the STRCs using a variation of a previously published multiple-regression method, taking the inputs at each phase of the ISI as the independent variables and the ISI length as the dependent variable (Wilson et al. 2014) (see materials and methods). With the present data, STRC estimation was complicated by the phase-dependent differences in IPSP detection, which result in only large-amplitude events being detected at early phases. This bias was corrected by normalizing each input (represented by a pulse) by the estimated Vm − Erev at event onset. The success of the multiple-regression analysis was evaluated by the r2 value of the fit, indicating the fraction of the variance in ISI length that was accounted for by the linear-regression equation. Of the six cells in which sIPSPs were detected and Erev estimated, three gave r2 values >0.1 and were accepted. Figure 7F shows the STRCs for these individual cells and the average of the three STRCs. Each STRC slopes upward to the right, and the average is almost linear. Although it was not possible to analyze IPSPs at the earliest and latest phases, the portion of the average STRC that was determined is reasonably well described by a line with a zero y-intercept. These data indicate that spontaneous inhibitory inputs cause a progressively greater delay in spike timing when they arrive later in the ISI. This effect can be attributed, in large part, to the increase in Cl− driving force during the rising part of the interspike Vm trajectory.
Synaptic delay.
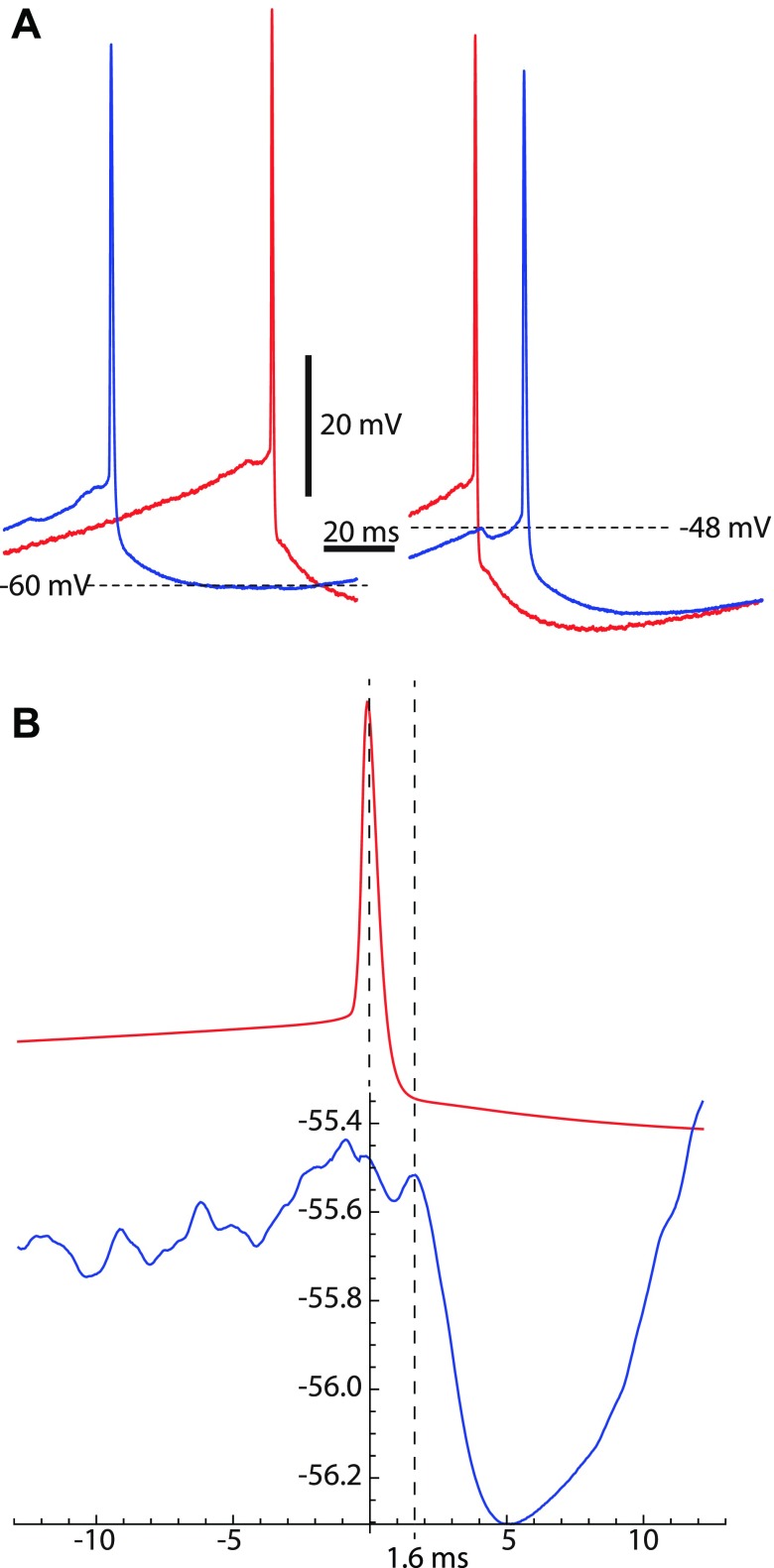
Attempts to detect synaptic interconnections in paired recordings of SNr neurons were mostly unsuccessful, presumably because of the sparse nature of the connection. We recorded spontaneous firing from 25 pairs of neurons in cell-attached mode or perforated-patch current clamp. In 24 pairs, spike time cross-correlograms were flat, with no suggestion of direct or indirect connectivity. In a single recorded pair, shown in Fig. 8A, there was a one-way direct connection, visible as a unitary IPSP (uIPSP) in one cell whenever there was an action potential in the other. This connection showed the same properties seen in sIPSPs described above, including its amplitude, duration, and variation of IPSP amplitude with postsynaptic Vm. Although this was the only instance of its kind, we used it to obtain an estimate of the conduction time between the cells, as there was no other way to make that measurement. The averaging of the postsynaptic neuron's Vm around the peak of the action potential in the presynaptic neuron yielded an estimate of 1.6 ms for the synaptic conduction time (Fig. 8B).
Fig. 8.
Synaptic latency of inhibitory coupling. A: IPSPs in a connected pair of SNr cells. The synaptic connection was 1-way, with the cell shown in red being presynaptic to the one shown in blue. The amplitude, time course, and phase dependence of the IPSP were similar to those seen for periodic sIPSPs. B: Vm of the presynaptic and postsynaptic neurons averaged on the peak of the presynaptic cell's action potential. The onset of the IPSP in the postsynaptic neuron occurred 1.6 ms after the peak of the action potential.
Effect of sparse inhibitory connections on synchronization in a model network.
The properties of the unitary synaptic connections among SNr neurons—in particular, their sparse distribution, large amplitude, fast kinetics, and strong impact at the end of the ISI—suggested to us that they might contribute to decorrelation of the activity of the cell population (Wilson 2013). It is generally thought that correlations in SNr arise from oscillations in the spiking activity of upstream nuclei, particularly GPe and STN, which become more prominent in Parkinson's disease (Alavi et al. 2013; Kühn et al. 2005; Mallet et al. 2008; Nambu and Tachibana 2014; Steigerwald et al. 2008). To investigate the generation of correlations in SNr, we constructed a model network of 100 neurons (schematic in Fig. 9A; see materials and methods). Each cell was a simple phase model with an autonomous firing frequency of 20 Hz and intrinsic noise sufficient to produce a typical CVISI of ∼0.05 in the absence of external input. In one set of simulations, the cells were uncoupled (control network). In the other simulations (coupled network), each cell was connected to an average of four other cells. The postsynaptic effect of each connection was represented by an increment in inhibitory conductance that began at a latency of 1.6 ms, decayed with a time constant of 3 ms, and produced a phase-dependent delay of the next action potential in accordance with the measured STRC. This choice of response amplitudes probably overestimates the strength of collateral synapses on average, because the STRC measurements required large IPSPs, but it is certainly within the range of naturally occurring uIPSPs.
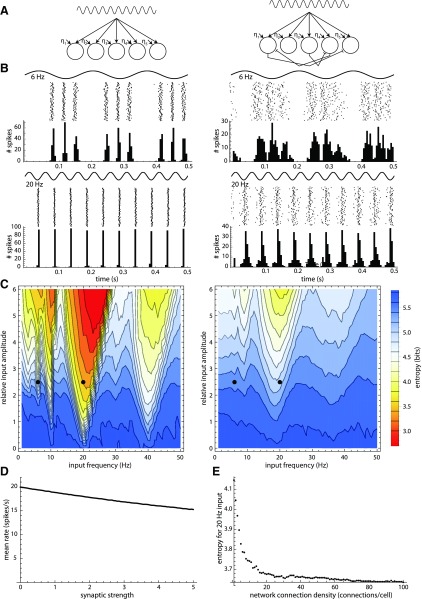
Fig. 9.
Effects of sparse inhibitory coupling in a model network. A: model configuration. Left: uncoupled control network. The network had 100 cells that received a common sine-wave input and independent white noise (η). Each cell was represented by a phase model with an autonomous firing frequency of 20 Hz and a linear STRC. Right: sparsely coupled network. Each of the 100 cells made and received an average of 4 inhibitory connections distributed randomly among the cell population. Each unitary connection produced a phase shift in its postsynaptic neurons calculated from the STRC. B: spike rasters and peristimulus-time histogram (last 0.5 s of 2 s simulation) for shared 6 Hz inputs (top) and 20 Hz inputs (bottom), with amplitudes of 2.5 times the peak conductance of the uIPSC. Left: uncoupled control network. Right: coupled network. With the 6-Hz input, the cells usually fired 3 spikes on each input cycle; with the 20-Hz input, the cells usually fired once per cycle. In each case, the addition of sparse coupling reduced the degree of synchrony produced by the shared input. C: steady-state entropy of cell phases for simulations with shared sine-wave inputs of varying frequency (1–50 Hz) and amplitude (0–6 nS) delivered to the control network (left) and the coupled network (right). Warm colors (red, orange, yellow) indicate low entropy, showing synchronization of the cells by the shared input. Sparse coupling reduced the ability of shared input to synchronize the network at all effectively synchronizing input amplitudes and frequencies. Dots indicate the input frequencies and amplitude used for the examples in B. D: effect of sparse coupling on average network firing rate in the absence of the sinusoidal input. Synaptic strength was varied from 0 to 5 times the amplitude used in the simulations above. Note the relatively weak effect of coupling on mean firing rate. E: effect of the synaptic density of inhibitory coupling on desynchronization. The shared sinusoidal input frequency was 20 Hz, and its amplitude was 3 nS. The maximal desynchronizing effect (highest entropy) was obtained with the sparsest connectivity.
To simulate network synchronization by common input, the cells were initialized at random phases and then driven by shared sine-wave inputs. Each simulation was 2 s in duration. The sine-wave input was treated as a continuously varying inhibitory conductance with amplitude scaled to the local uIPSC so that the same STRC could be used to calculate phase changes caused by common input. This choice also reflects the fact that most synaptic inputs to the SNr neuron are inhibitory, arising from the striatum and GPe. Examples in Fig. 9B show the synchronization of firing produced by shared sinusoidal inputs at the cells' intrinsic firing rate (20 Hz) and at a lower rate (6 Hz). Shared sinusoidal input not only modulated the firing rates of the neurons in time with the input oscillation but also entrained their phases so that all of the neurons fired at almost the same time (Fig. 9B). Small differences in firing time after entrainment were caused by independent sources of noise in each cell. Sparse inhibitory coupling very effectively disrupted the synchronizing effect of the shared input without disturbing the slower modulation of firing by the input oscillation (Fig. 9B). Because synchrony was disrupted, population firing more closely represented the waveform of the input sinusoid in the presence of coupling than in its absence.
To examine a wider range of inputs, a matrix of stimulus frequencies and amplitudes was delivered to the uncoupled and coupled networks. The resulting degree of network synchrony was measured by the entropy of phase distributions across all cells. In this measure, phase is binned, the probability distribution of phases is calculated for the entire network, and the entropy of this distribution is calculated at each time point. The contour plots in Fig. 9C show the phase entropy averaged over 1 s at steady state. Lower entropy indicates more synchrony. The maximum entropy possible in the network depends on the total number of cells in the network, the firing rate, and the resolution at which phase is measured (the bin width for the phase-probability histogram), so the entropy of the coupled network was compared with that of the uncoupled network for all conditions. As expected, the highest levels of synchrony were obtained when the stimulus frequency was commensurable with the cells' intrinsic firing frequency (i.e., 1/3, 1/2, 1, or 2 times the firing frequency). As the stimulus amplitude was increased, the synchronization became stronger, and the ranges of synchronizing frequencies became wider. With the comparison of the left and right panels, we see that the addition of sparse inhibitory coupling reduced the degree of synchrony obtained for all input frequencies and amplitudes capable of synchronizing the network.
As expected, inhibitory coupling did reduce the firing rate of the population overall, but this effect was small when coupling was sparse. The effect on rate was calculated by measuring the average firing rate across all cells in the absence of shared input, with and without sparse (4 inputs/cell) coupling. The effect on rate increased with increased synaptic strength but was relatively small even when the local synapses were made unrealistically strong (Fig. 9D).
The desynchronizing effect of local inhibitory coupling depended strongly on the connectivity of the network. As shown in Fig. 9E for 20 Hz input, desynchronization was greatest when the connectivity was most sparse. For a given total amount of inhibition, increasing connectivity among SNr cells rapidly reduced the desynchronizing effect. This effect depended specifically on the average number of connections received by each SNr neuron and did not depend on the total size of the network.
DISCUSSION
The results of this study show that most SNr neurons receive inhibitory synaptic input from other SNr cells. Because these cells fire rhythmically in brain slices, the unitary synaptic inputs could be characterized based on their timing, even though the synaptic connections are sparse and not amenable to paired recording. By taking advantage of the periodic nature of SNr cell firing, we could determine the number of unitary inputs received by each cell and estimate the distribution of uIPSC amplitudes and the time course of the synaptic currents. The results indicate that each SNr cell received approximately one active unitary input in the slice preparation, with a range of zero to four unitary inputs detected. The amplitudes of the unitary synaptic conductances varied from ∼0.3 to >3 nS, with typical values on the order of 1 nS. The uIPSC kinetics were fast, with 20–80% rise times of ∼0.4 ms and decay time constants of ∼3 ms. Perforated-patch recordings showed that the average sIPSC Erev was −64 mV. Consistent with the small difference between Vm and Erev early in the ISI, spontaneous inhibitory inputs produced the largest sIPSPs and had the largest effects on spike timing when they arrived near the end of the interval.
Correspondence with anatomical data.
Our average of slightly more than one unitary input per cell probably underestimates the number of connections present in the intact SNr, because both the local axon collaterals and the dendrites of SNr neurons extend for hundreds of microns in three dimensions (Cebrián et al. 2007; Mailly et al. 2001, 2003). Mailly et al. (2003) reported that SNr neurons formed an average of 84 local collateral boutons, which were often distributed in one to four small clusters. Our data are consistent with the possibility that each cluster forms a unitary connection on a single postsynaptic cell. On average, our analysis suggests that the quantal content of the uIPSCs is small, ranging from 0.2 to 3 quanta/spike, with an average of slightly >1 quantum/spike. If an axon collateral with 80 boutons formed 4 unitary connections, each comprising 20 individual synapses, then this quantal content could be achieved with a low release probability of ∼0.05.
Quite different conclusions on the number of unitary connections among SNr neurons were drawn from a recent study using mouse brain slices (Brown et al. 2014). The authors reported that channelrhodopsin excitation of SNr neurons in slices produced IPSCs, 50–100 times the average amplitude of sIPSCs. (The study did not distinguish periodic from aperiodic sIPSCs.) They also observed a total rate of 75–300 sIPSCs s−1, which was argued to be consistent with 50 or more active unitary inputs in the slice preparation. Each unitary input was reported to be much weaker than the uIPSCs characterized here, similar to a typical mIPSC, with an average conductance of ∼150 pS. In contrast, our average data in rat brain slices showed 1.16 unitary inputs/cell, 16.2 spikes s−1 input−1, and 0.6 uIPSCs spike−1, together accounting for ∼11 uIPSCs s−1. The uIPSCs we recorded were eightfold larger, with a mean conductance of ∼1.2 nS, excluding synaptic failures. It might be expected that a larger fraction of the unitary synaptic connections would be preserved in the mouse slice preparations, due to the smaller overall size of SNr in the mouse. However, the scale difference clearly cannot account for a 50-fold difference in the number of connected cells, suggesting that the distribution of local collateral synapses might differ between the two species. Unfortunately, there are no quantitative anatomical data available on the SNr collateral system in mice. If the number of active unitary inputs in rat slices had been as large as suggested for the mouse, then our analytical approach for characterizing individual unitary inputs would have been impossible.
Absence of synaptic depression.
In many neurons, long trains of presynaptic action potentials cause profound synaptic depression. For example, synapses formed by external GPe afferents on STN neurons depressed severely when stimulated at frequencies similar to the autonomous firing rates of GPe neurons (Atherton et al. 2013), and GPe afferents to SNr neurons also showed substantial depression during stimulus trains (Connelly et al. 2010). Thus it might be expected that IPSCs produced by active unitary inputs to SNr neurons would be in a constant state of depression, which could be relieved by a long pause in presynaptic firing.
Contrary to this expectation, our experiments using halorhodopsin showed that suppressing presynaptic activity for 10 s did not lead to increased sIPSC amplitudes when the activity was allowed to resume. This result suggests that the local collateral synapses do not undergo short-term depression during autonomous firing activity, although the possibility of depression that recovers extremely slowly cannot be excluded. Similarly, in the mouse, optogenetic stimulation at 10–20 s−1 produced only moderate depression of SNr IPSCs (Brown et al. 2014). In addition, a previous study using antidromic stimulation of rat SNr efferents (Deniau et al. 1982) showed nearly constant IPSPs produced by the local axon collaterals in response to trains of four stimuli at intervals of 6–7 ms. This result suggests that short bursts of fast firing are also insufficient to cause substantial depression. The apparent lack of synaptic depression during autonomous firing and short bursts is consistent with a low release probability. However, a rapid replenishment process is also required to maintain the supply of readily releasable vesicles during continuous firing activity. For example, with a typical autonomous firing rate of 20 spikes/s and a release probability of 0.05, substantial depression would be predicted if recovery did not occur with a time constant of <1 s.
Possible role in decorrelation of SNr activity.
The function of local collateral inhibition in the SNr has long been enigmatic. Like other sparse local connections, it is omitted from most functional schemes for the basal ganglia, because it is overshadowed by the powerful afferent fiber systems. SNr neurons fire autonomously, and most of their afferents are GABAergic, so firing rate changes are largely driven by afferent inhibition and disinhibition [e.g., Wilson (2015)]. Of course, inhibitory feedback is a mechanism of gain control in this situation [e.g., Brown et al. (2014)], as in others. Increases in firing rate in SNr neurons will produce an increase in local inhibition that may partly counteract the effect of disinhibition from the striatum and elsewhere. However, in this antagonistic interaction between afferent disinhibition and feedback inhibition, sparse local feedback in the SNr seems far outmatched by the overpowering effect of striatal and pallidal afferents.
In repetitively firing neurons, an alternative function for sparse recurrent inhibition is offered by the phase-dependent responses of cells to their synaptic input. Even a weak synaptic input may have a substantial effect on spike timing if it systematically arrives at the right time relative to the firing of a postsynaptic cell. Our results show that for SNr cells, an inhibitory input is most effective if it occurs just before the postsynaptic neuron would have fired anyway, and it acts to delay the next action potential. In contrast, synaptic inhibition occurring early in the ISI of a postsynaptic cell has little effect. By taking advantage of this specific timing relationship, an extremely sparse input can have a powerful effect, even in the presence of other stronger but less systematically timed synaptic inputs.
Synaptic interactions of this kind between coupled oscillators have been widely studied using mathematical models and computer simulations. These studies often stress the fact that mutual inhibition in networks of coupled oscillators can have a synchronizing function [e.g., van Vreeswijk et al. (1994); Wang et al. (2012); Wang and Buzsáki (1996); Whittington et al. (1995)], but they show that it can also be desynchronizing, especially when synaptic currents are brief. Antisynchronous firing of pairs of neurons, coupled by artificial inhibitory synapses with fast kinetics, has been shown experimentally in GPe cells, whose firing rates and IPSC time courses are similar to those in SNr (Wilson 2013). Antisynchrony in cell pairs is prognostic of a desynchronizing effect of mutual inhibition in a larger network [e.g., Wilson (2013)]. Thus the shape of the STRC and the brief time course of the uIPSCs are primarily responsible for the desynchronization observed in our model. In addition, desynchronization was promoted by extremely sparse connectivity, as indicated by the inverse relationship between network entropy and connectivity in our modeling results. For desynchronization, sparse but fast and strong collateral inhibition is preferred over a more connected network arrangement with slower, weaker uIPSCs. In this role, extremely sparse connectivity may, in fact, be optimal.
The decorrelating effect of sparse, recurrent inhibition in the SNr may be a factor in maintaining the independence of firing of cells in this structure, which is believed to be essential for its normal function. Normally, basal ganglia output neurons, such as those in the SNr, do not show correlated firing, despite the input correlations that must be present because millions of striatal neurons converge on a much smaller number of cells in the substantia nigra [see, e.g., Wilson (2013)]. However, in Parkinson's disease and in experimental models of the disease, firing becomes correlated in the beta (13–30 Hz) range (Alavi et al. 2013; Nambu and Tachibana 2014; Weinberger et al. 2012). Correlations in the basal ganglia output nuclei probably arise from powerfully correlated beta-frequency oscillations of their inputs. Each cycle of the beta oscillation generally includes several spikes in parkinsonian humans or monkeys, where SNr neurons fire at relatively high rates (Elias et al. 2008; Maltête et al. 2007), whereas in rats, the beta oscillation can induce 1:1 phase locking of SNr spiking (Brazhnik et al. 2012). Our simulations suggest that largely irrespective of the relative frequencies of oscillating input and spike output, sparse local connectivity could reduce the ability of shared input to drive synchronized spiking. Thus the degree to which parkinsonian beta oscillation can synchronize activity in the output nuclei may be determined, at least in part, by the effectiveness of the recurrent inhibitory network in which the cells are embedded.
GRANTS
Support for this work was provided by the National Institute of Neurological Disorders and Stroke (Grant NS047085) and a grant from The Stevens Foundation, as well as computational support from Computational System Biology Core, funded by the National Center on Minority Health and Health Disparities (G12MD007591).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H.H. and C.J.W. conception and design of research; M.H.H. and C.J.W. performed experiments; M.H.H. and C.J.W. analyzed data; M.H.H. and C.J.W. interpreted results of experiments; M.H.H. and C.J.W. prepared figures; M.H.H. drafted manuscript; M.H.H. and C.J.W. edited and revised manuscript; M.H.H. and C.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Sharmon Lebby for excellent technical assistance.
REFERENCES
- Alavi M, Dostrovsky JO, Hodaie M, Lozano AM, Hutchison WD. Spatial extent of beta oscillatory activity in and between the subthalamic nucleus and substantia nigra pars reticulata of Parkinson's disease patients. Exp Neurol 245: 60–71, 2013. [DOI] [PubMed] [Google Scholar]
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci 25: 8272–8281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JF, Menard A, Urbain N, Bevan MD. Short-term depression of external globus pallidus-subthalamic nucleus synaptic transmission and implications for patterning subthalamic activity. J Neurosci 33: 7130–7144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci 32: 7869–7880, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Pan WX, Dudman JT. The inhibitory microcircuit of the substantia nigra provides feedback gain control of the basal ganglia output. Elife 3: e02397, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Bad oscillations in Parkinson's disease. J Neural Transm Suppl 70: 27–30, 2006. [DOI] [PubMed] [Google Scholar]
- Cebrián C, Parent A, Prensa L. The somatodendritic domain of substantia nigra pars reticulata projection neurons in the rat. Neurosci Res 57: 50–60, 2007. [DOI] [PubMed] [Google Scholar]
- Chan PK, Yung WH. Inhibitory postsynaptic currents of rat substantia nigra pars reticulata neurons: role of GABA receptors and GABA uptake. Brain Res 838: 18–26, 1999. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Schulz JM, Lees G, Reynolds JN. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J Neurosci 30: 14854–14861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Kitai ST, Donoghue JP, Grofova I. Neuronal interactions in the substantia nigra pars reticulata through axon collaterals of the projection neurons. Exp Brain Res 47: 105–113, 1982. [DOI] [PubMed] [Google Scholar]
- Elias S, Ritov Y, Bergman H. Balance of increases and decreases in firing rate of the spontaneous activity of basal ganglia high-frequency discharge neurons. J Neurophysiol 100: 3086–3104, 2008. [DOI] [PubMed] [Google Scholar]
- Giannicola G, Marceglia S, Rossi L, Mrakic-Sposta S, Rampini P, Tamma F, Cogiamanian F, Barbieri S, Priori A. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson's disease. Exp Neurol 226: 120–127, 2010. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141: 154–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grofova I, Deniau JM, Kitai S. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J Comp Neurol 208: 352–368, 1982. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Wilson CJ, Groves PM. The substantia nigra of the rat: a Golgi study. J Comp Neurol 172: 585–600, 1977. [DOI] [PubMed] [Google Scholar]
- Karabelas AG, Purpura DP. Evidence for autapses in the substantia nigra. Brain Res 200: 467–473, 1980. [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS, Burnod Y, Triller A. Regulation of efficacy at central synapses. J Neurosci 4: 125–130, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp Neurol 194: 212–220, 2005. [DOI] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Mahon S, Menetrey A, Thierry AM, Glowinski J, Deniau JM. Dendritic arborizations of the rat substantia nigra pars reticulata neurons: spatial organization and relation to the lamellar compartmentation of striato-nigral projections. J Neurosci 21: 6874–6888, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Deniau JM. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J Neurosci 23: 5247–5257, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Márton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci 28: 14245–14258, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltête D, Jodoin N, Karachi C, Houeto JL, Navarro S, Cornu P, Agid Y, Welter ML. Subthalamic stimulation and neuronal activity in the substantia nigra in Parkinson's disease. J Neurophysiol 97: 4017–4022, 2007. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tachibana Y. Mechanism of parkinsonian neuronal oscillations in the primate basal ganglia: some considerations based on our recent work. Front Syst Neurosci 8: 74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, Banks MI, Dorval AD, Acker CD, Haas JS, Kopell N, White JA. Synchronization in hybrid neuronal networks of the hippocampal formation. J Neurophysiol 93: 1197–1208, 2005. [DOI] [PubMed] [Google Scholar]
- Oertel WH, Mugnaini E. Immunocytochemical studies of GABAergic neurons in rat basal ganglia and their relations to other neuronal systems. Neurosci Lett 47: 233–238, 1984. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A, Seguela P, Descarries L. Distribution of GABA-immunoreactive neurons in the basal ganglia of the squirrel monkey (Samiri sciureus). J Comp Neurol 259: 50–64, 1987. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci 16: 7566–7573, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald F, Pötter M, Herzog J, Pinsker M, Kopper F, Mehdom H, Deuschl G, Volkmann J. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J Neurophysiol 100: 2515–2524, 2008. [DOI] [PubMed] [Google Scholar]
- van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci 1: 313–321, 1994. [DOI] [PubMed] [Google Scholar]
- Wang S, Chandrasekaran L, Fernandez FR, White JA, Canavier CC. Short conduction delays cause inhibition rather than excitation to favor synchrony in hybrid neuronal networks of the entorhinal cortex. PLoS Comput Biol 8: e1002306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16: 6402–6413, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Alavi M, Hodaie M, Lozano AM, Moro E, Dostrovsky JO. Oscillatory activity in the globus pallidus internus: comparison between Parkinson's disease and dystonia. Clin Neurophysiol 123: 358–368, 2012. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373: 612–615, 1995. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Active decorrelation in the basal ganglia. Neuroscience 250: 467–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Oscillators and oscillations in the basal ganglia. Neuroscientist 21, 530–539, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Barraza D, Troyer T, Farries MA. Predicting the responses of repetitively firing neurons to current noise. PLoS Comput Biol 10: e1003612, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Tse AC, Yung W. Taurine inhibits rat substantia nigra pars reticulata neurons by activation of GABA- and glycine-linked chloride conductance. Brain Res 749: 175–179, 1997. [DOI] [PubMed] [Google Scholar]