Vasoactive intestinal polypeptide (VIP+)-expressing GABAergic interneurons are wired to suppress the firing of other interneurons in the cortex. In this study, we show that VIP+ cells are nonspecifically active across behavioral states including immobility, under anesthesia, and during visual stimulation. These cells were highly correlated with periods of increased network activity, suggesting baseline cortical activity is controlled by VIP+-mediated disinhibition. When we specifically silenced the activity of VIP+ cells, the overall activity in the network was reduced, regardless of brain state of the animal. These results suggest VIP+ interneurons are important for sustaining high activity rates in the neocortex.
Keywords: GABA, interneurons, neocortex, network, spontaneous activity
Abstract
GABAergic interneurons are positioned to powerfully influence the dynamics of neural activity, yet the interneuron-mediated circuit mechanisms that control spontaneous and evoked neocortical activity remains elusive. Vasoactive intestinal peptide (VIP+) interneurons are a specialized cell class which synapse specifically on other interneurons, potentially serving to facilitate increases in cortical activity. In this study, using in vivo Ca2+ imaging, we describe the interaction between local network activity and VIP+ cells and determine their role in modulating neocortical activity in mouse visual cortex. VIP+ cells were active across brain states including locomotion, nonlocomotion, visual stimulation, and under anesthesia. VIP+ activity correlated most clearly with the mean level of population activity of nearby excitatory neurons during all brain states, suggesting VIP+ cells enable high-excitability states in the cortex. The pharmacogenetic blockade of VIP+ cell output reduced network activity during locomotion, nonlocomotion, anesthesia, and visual stimulation, suggesting VIP+ cells exert a state-independent facilitation of neural activity in the cortex. Collectively, our findings demonstrate that VIP+ neurons have a causal role in the generation of high-activity regimes during spontaneous and stimulus evoked neocortical activity.
NEW & NOTEWORTHY
Vasoactive intestinal polypeptide (VIP+)-expressing GABAergic interneurons are wired to suppress the firing of other interneurons in the cortex. In this study, we show that VIP+ cells are nonspecifically active across behavioral states including immobility, under anesthesia, and during visual stimulation. These cells were highly correlated with periods of increased network activity, suggesting baseline cortical activity is controlled by VIP+-mediated disinhibition. When we specifically silenced the activity of VIP+ cells, the overall activity in the network was reduced, regardless of brain state of the animal. These results suggest VIP+ interneurons are important for sustaining high activity rates in the neocortex.
inhibitory interneurons play a role in shaping both spontaneous and evoked network activity as they connect densely to local excitatory principal cells (PCs) and inhibit firing (Fino and Yuste 2011; Isaacson and Scanziani 2011; Packer and Yuste 2011; Xue et al. 2014). However, one major group of cortical interneurons (Rudy et al. 2011) containing vasoactive intestinal polypeptide (VIP+) represent a specialized inhibitory class that contacts other interneurons expressing somatostatin (SOM+) (Lee et al. 2013; Pfeffer et al. 2013; Pi et al. 2013) and, to a lesser extent, interneurons expressing parvalbumin (PV+) (Hioki et al. 2013). These different inhibitory cell classes are mutually exclusive and contain almost no overlap (Fishell and Rudy 2011; Rudy et al. 2011; Xu et al. 2010). As SOM+ and PV+ cells function to inhibit PCs, VIP+ cells are situated to facilitate firing of PCs through disinhibition. Despite the clear connectivity VIP+ cells maintain in local circuits, it remains unclear how the actions of this small cell population (1–2% of all cortical neurons) could influence the excitability of large groups of pyramidal cells. Previous work has shown that VIP+ cells in the cortex are active during movement (Fu et al. 2014) and sensory processing (Pi et al. 2013) and function in gain control (Fu et al. 2014; Pi et al. 2013) and plasticity (Fu et al. 2015). Therefore, their disinhibitory role is thought to function exclusively during specific network states, suggesting a role for VIP+ cells in particular network processes. However, the neocortex is spontaneously active, even in the absence of sensory input (Kenet et al. 2003; Luczak et al. 2009; Miller et al. 2014; Mizuseki and Buzsaki 2013; Tsodyks et al. 1999). This spontaneous cortical activity is proposed to play a critical role in processes such as memory replay, sensory reverberation, and intrinsic mental states (Ji and Wilson 2007; Luczak et al. 2009; Miller et al. 2014).
A detailed characterization of the brain-state VIP+ cell correlates in the context of network activity in vivo has not been performed. VIP+ cells mainly connect to and inhibit SOM+ interneurons. Therefore, these cells should serve a generalized role in regulating network excitability by promoting excitation of pyramidal cells, although this has not been experimentally demonstrated. Furthermore, it is unclear under what behavioral and brain states the same VIP+ cells fire, and if effects on the network are brain-state dependent. Experimentally, how the suppression of VIP+ cells changes the overall activity levels in the network across both spontaneous and evoked neocortical states has not been tested. Using in vivo two-photon imaging and pharmacogenetics, we have investigated these questions.
MATERIALS AND METHODS
Animals.
All procedures were performed in accordance with and approved by the Institutional Animal Care and Use Committee at Columbia University. Mice were originally obtained from Jackson Laboratory. VIP-cre (stock no. 010908) were crossed with the Lox-stop-lox-tdTomato (td-Tomato; Ai14 strain, stock no. 007908). Animals were housed on a temperature-controlled 12:12-h light-dark cycle, and food and water were provided ad libitum. Animals of both sexes were used and were between postnatal days 60 and 100 (P60–P100) at the time of imaging. Experiments were performed at similar times of day (between 4:00 and 10:00 PM).
Surgery 1.
Mice (P30–P60) of both sex were injected stereotaxically with AAV1-syn-GCaMP6s, AAV1-syn-GCaMP6f, or AAV5-DIO-hM4Di-mCherry + AAV1-syn-GCaMP6s (Chen et al. 2013). All viruses were obtained from the vector core at the University of North Carolina-Chapel Hill (UNC Vector Core). Mice were anesthetized with isoflurane, and a small craniotomy (0.1 × 0.1 mm) was made for the insertion of a beveled injection needle (World Precision Instruments) at 2.5 mm lateral from lambda and 0.05 mm anterior to lamba and 150–200 μm below the pial surface. Virus was injected (500-1,000 nl at 80 nl/min) using a UMP3 micro syringe pump (World Precision Instruments). The needle was left in place for an additional 10 min to allow viral diffusion. Animals were given carprofen (5 mg/kg) to aid recovery. Imaging was performed between 4 and 7 wk following injection.
Surgery 2.
Mice were anesthetized as in surgery 1, and a titanium head plate was cemented over V1 at the location of the virus injection. For thin skull preparations, the animal was allowed to recover for an additional 2–5 days while habituation and training on the circular running disk were performed. In these cases the skull was thinned on the day of experiments and imaged between 2 and 12 h after thinning. In the case of full craniotomies, low-melting-point agar (1.5% in PBS) was placed on the brain surface with a glass coverslip and glued in place to minimize movement. For light isoflurane experiments, animals were maintained at 0.5–0.8% isoflurane plus 1.5% oxygen and were placed on a heating pad warmed to 37.5°C. Imaging was performed in the dark. After a baseline (30–60 min), animals were given clozapine-N-oxide (CNO; Sigma or Enzo Life Sciences; 2 mg/kg) dissolved in DMSO (0.05%) plus saline (0.9%). Control experiments under isoflurane were performed on animals with just AAV1-GCaMP6s (no hM4Di) that were injected with CNO or on animals with AAV1-GCaMP6s+hM4Di that were injected with DMSO + saline. We did not notice any difference between these two control groups, so data were pooled for analysis.
In vivo imaging.
Calcium imaging in vivo was performed with a two-photon Moveable Objective Microscope (MOM; Sutter Instruments) and a tunable two-photon laser (Chameleon Vision II; Coherent). GCaMP6s/f was excited at 950 nm and td-Tomato was excited at 1,040 nm. td-Tomato images were collected before each imaging session. Laser power (15–60 mW) was controlled via a pockel cell (Conoptics). Scanning and image acquisition were controlled by Sutter Instrument software (MScan; 4.07 frames/s for 512 × 512 pixels for GCaMP6s or 6.05 frames/s for 420 × 420 pixels for GCaMP6f). Emission was collected with a green (535/50 nm) and a red (610/75 nm) filter (Chroma) simultaneously on two photomultiplier tubes. Locomotion tracking was performed using an optical computer mouse placed under the running disk. Locomotion was defined as occurring when the mouse moved >1 cm/s. Small movements where the animal would make postural adjustments on the wheel could be seen but were clearly dissociable from even slow walking or brief forward motion bouts. The epochs of locomotion used for correlation analysis included the periods of time the animal was running together with 20 frames (∼4.9 s with an imaging speed of 4.07 frames/s) preceding locomotion onsets and following locomotion offsets.
For bulk loading of cortical neurons, Oregon Green BAPTA-1 AM (OGB-1 AM; Molecular Probes) was first mixed with 4 μl of pluronic acid (20% in DMSO) and further diluted in 35 μl of dye buffer (150 mM NaCl, 2.5 mM KCl, and 10 mM HEPES; pH 7.4). Sulforhodamine 101 (SR101; 50 μM; Molecular Probes) was added to the solution to label astrocytes. The dye was slowly pressure-injected into the left visual cortex at a depth of 150–200 μm at 30° angle with a micropipette (tip opening 1–2 μm) using Picospritzer II (10 psi, 8 min) under visual control of two-photon imaging (×10 water-immersion objective, 0.5 NA; Olympus). We waited 30–90 min to ensure dye uptake across a large number of cells before starting the experiment.
Visual stimulation.
Visual stimuli were generated in MATLAB (The MathWorks, Natick, MA) using the Psychophysics toolbox (Brainard 1997; Kleiner et al. 2007; Pelli 1997) and displayed on a gamma-corrected LCD monitor (Dell; 19 in., 60-Hz refresh rate) positioned 15–18 cm from the right eye, roughly at 45° to the long axis of the animal and spanning ∼114 (azimuth) × 140 (elevation) degrees of visual space. Presentation of the visual stimuli was synchronized with the image acquisition using the Sutter software (MScan) and a routine written in MATLAB such that each stimulus presentation was trigged at the beginning of frame acquisition. The actual time of stimulus presentation was detected with a silicon photodiode (Hamamatsu) attached to the right bottom corner of the screen. Mice were presented with sequences of full-field square-wave gratings against a mean luminance gray background, presented with a temporal frequency of 1.5 Hz, a spatial frequency of 0.04 cycles/deg, and 100% contrast. Gratings were presented in 12 directions of motion in 30° steps in a pseudorandom order for either 0.15, 0.5, or 1 s, followed by 3–5 s of mean luminance gray screen, and were repeated 15 times. Initial phase of the drifting gratings was kept constant across trials. During presentation of natural images, mice were presented with a set of 2,000 black-and-white images for 200 ms each, followed by 2 s of mean luminance gray screen.
Analysis.
Images were first converted to TIF format and registered to correct for x-y motion using TurboReg in ImageJ (Thevenaz et al. 1998). Regions of interest (ROIs) were drawn using the standard deviation or maximum projection image of each movie. The mean fluorescence within each cellular ROI was calculated as a function of frame and converted to the relative change in fluorescence (ΔF/F). Relative fluorescence change was calculated against the mean across the 50% of the lowest points during the previous 10-s window. During trials with visual evoked responses, relative fluorescence change was calculated against the mean response during the interstimulus interval. Visual evoked responses to drifting gratings or natural images were calculated as the average across two consecutives frames (using a frame rate of 4.07 frames/s) after stimulus onset. Sparseness was calculated according to previously published methods (Willmore and Tolhurst 2001). In brief, we calculated how few cells are active by any given image. First, we took the distribution of responses of the population to a single image and then set a threshold value for the responses to each image (1 SD of the responses). Any neural responses whose magnitudes are larger than this threshold are considered to be “on,” and responses smaller than the threshold are considered “off.” The activity sparseness is the number of cells that are off in response to a particular stimulus. Last, an “entropy” measure was calculated (Tolhurst et al. 2009). In brief, the response distribution is first normalized to have a variance of 1 and is then converted to a probability density function with a bin width of 0.2.
To estimate the frames where VIP+ cells were most active, we used a threshold (mean + 2 SD) of the first derivative of the ΔF/F and identified frames where the instantaneous change in ΔF/F was above this threshold. This technique mainly captured the frames when the cell underwent the largest positive-going shift in florescence. To avoid false positives, we used an additional step, where active frames that had ΔF/F < 0.15 were not included. Neuropil (NP) subtraction was performed on all cell ROIs. The correlation between variables was assessed by using the cross-correlation function in MATLAB with a window of 10 s. The maximum value of this cross-correlation function was used to assess the relationship between two variables. Partial correlation was also calculated, which assesses the linear relation between two variables while accounting for and removing the variance associated with a third variable. The NP for each cell was defined as the mean ΔF/F in the region of the extracellular space between the 300th and 500th closest pixels that did not include any other active neuron or ROI. Each pixel was 0.62 μm for the 512 × 512-pixel field of view (FOV). The final ΔF/F used for analysis was calculated as the raw ΔF/Fcell − (0.7 × ΔF/FNP). We assessed the effect of changing the NP correction factor from 0.7 to 0.8 and 0.95. A higher correction factor decreased the amplitude of responses, as expected; however, using a factor of 0.95 did not change the relative difference between correlation measures. To calculate the speed modulation of the neurons and the network, we separated the speed into 10 quantiles and removed the periods where there was no locomotion. The mean network ΔF/F was then averaged for each speed bin. The same speed bin boundaries were used for baseline and CNO conditions. For the analysis of spontaneous activity, we used the standard deviation of the ΔF/F trace in each state to assess the relative activity of the cell across a given state (locomotion, nonlocomotion, or anesthesia).
To analyze the response to visual stimulation, we used only reliably responding cells. A responsive cell was any cell that responded to a particular stimulus with a mean of at least 6%. Reliability (δ) was determined according to δ = (μmax − μblank)/(σmax − σblank), where μmax and σmax are the mean and standard deviation of the response to the preferred stimulus, respectively, and μblank and σblank are the mean and standard deviation of the response to the blank stimulus, respectively. Neurons were considered reliable if δ > 1. Only visually responsive and reliable cells were chosen for further analysis. To calculate tuning curves, we averaged the evoked responses (ΔF/F) over two frames following stimulus presentation. We then averaged the response over the number of repetitions (5–15) per stimulus direction (12 directions). Direction tuning curves generated from OGB-1 fluorescence are comparable to those recorded with electrophysiological techniques (Kerlin et al. 2010; Marshel et al. 2011). Orientation selectivity index (OSI) was computed as follows OSI = (μmax − μorth)/(μmax+ μorth), where μmax is the mean response to the preferred orientation and μorth is the mean response to the orthogonal orientation (average of both directions).
In vitro experiments.
In vitro slice experiments were performed using coronal slices from double transgenic VIP-cre::SOM-GFP(GIN) mice. Mice were injected with the DIO-hM4Di-mCherry targeting VIP+ cells. This method allowed identification of VIP+ cells (with mCherry) and postsynaptic SOM+ cells (with green fluorescent protein, GFP) in the same slice. Virus specificity was verified with immunohistochemistry, where 199/212 hM4Di-mCherry cells (94%) expressed VIP (Fig. 3A). Acute brain slices (300 μm) from P60–P100 mice were cut in chilled and continuously gassed (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.1 NaH2PO4, 26 NaHCO3, 0.1 pyruvic acid, 0.5 l-glutamine, 0.4 ascorbic acid, and 25 d-glucose. After a 1-h recovery period in 37°C ACSF, slices were maintained in ACSF at room temperature. Patch pipettes (4–8 MΩ) were filled with K-gluconate-based intracellular solution, and recordings were performed at room temperature. Connectivity between VIP+ cells and SOM+ cells was tested using 2-ms, 2-nA step pulses inducing the presynaptic cell to fire at 50 Hz. After the establishment of connections between VIP+ and SOM+ cells, CNO (5 μM) was bath-applied to the slice to activate the hM4Di receptor on VIP+ cells and prevent synaptic release (Stachniak et al. 2014).
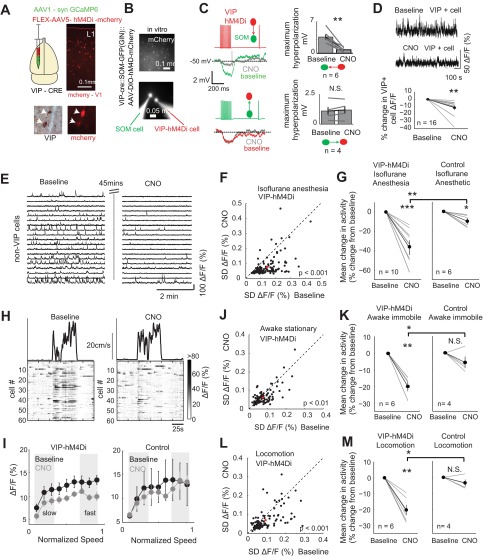
Fig. 3.
VIP+ cell suppression reduces spontaneous network activity across behavioral states. A, top: setup showing the injection of AAV5-DIO-hM4Di in V1 of VIP-cre mice. Bottom, mCherry and VIP diaminobenzidine immunohistochemistry showing the localization of mCherry to VIP+ cells. B: in vitro paired recording between a VIP+ and SOM+ cell. VIP mice were crossed with SOM-GFP mice and injected with hM4Di-mCherry to label VIP+ cells. In these mice, paired recordings were then performed identifying the VIP+ cells with mCherry and the SOM+ cells with GFP. C: the electrophysiological identification of a reciprocally connected VIP+ and SOM+ cell. Note that CNO blocks the VIP→SOM inhibitory synapse without influencing the SOM→VIP synapse. Right, group data for all experiments. VIP→SOM connections were reduced by CNO (3.6 ± 0.6 vs. 0.8 ± 0.2 mV, z = 2.8, P = 0.005, n = 6), whereas SOM→VIP synapses did not change (1.1 ± 0.4 vs. 1.2 ± 0.5 mV, n = 4). D: example in vivo experiment showing raw data of a VIP+ cell ΔF/F trace from movies before and after CNO (top). Group data (bottom) show the mean ΔF/F for 14 VIP+ cells during baseline and CNO conditions (P = 9.8 × 10−4). E: example ΔF/F GCaMP6s traces from non-VIP cells pre- (baseline) and post-CNO in an anesthetized animal expressing the hM4Di receptor targeted in VIP+ cells. Baseline and CNO recordings were separated by 45 min. F: example experiment showing the activity of single neurons pre- and post-CNO under light isoflurane anesthesia. Activity was assessed as the SD of the ΔF/F trace. Each point represents 1 cell. Cells shown in E are from this experiment. G: group data showing the mean change in population activity in each experiment. CNO reduced neural activity in VIP-hM4Di animals and slightly in controls (the small reduction in controls is likely due to run down of activity during the experiment), and the change was significantly greater in the VIP-cre-hM4Di animals (n = 10) relative to controls (n = 6, z = 2.9, P = 0.004). H: example epochs showing locomotion-associated activity in the population of non-VIP+ neurons pre- and post-CNO. I: mean network ΔF/F as a function of normalized running speed during baseline and after CNO in experimental (left) and control (right) mice. ΔF/F during both fast and slow speed was reduced in experimental animals (fast, P = 0.012; slow, P = 0.023) and in controls (fast, P = 0.59; slow, P = 0.55). J: example experiment showing activity of single neurons pre- and post-CNO in the awake immobile state. K: group data showing the mean change in population activity in each experiment. Activity was reduced in VIP-hM4Di animals (n = 6, z = 2.99, P = 0.003) but not in control animals (n = 4, z = 1.08, P = 0.28). Neural activity was reduced significantly more in VIP-hM4Di animals relative to controls (z = 2.5, P = 0.01). L and M: same conventions as J and K but for locomotion analysis. Locomotor-associated activity was reduced in VIP-hM4Di animals (n = 6, z = 3.0, P = 0.003) but not in control animals (n = 4, z = 1.1, P = 0.28). Neural activity was reduced significantly more in VIP-hM4Di animals relative to controls (z = 2.5, P = 0.01). For individual experiments, n = 94 ± 13 cells for VIP-hM4Di mice and n = 74 ± 14 cells for controls. *P < 0.05; **P < 0.01; ***P < 0.001; N.S., not significant.
Statistics.
Two-sided Wilcoxon rank sum tests were performed between conditions or groups unless otherwise stated. In cases where multiple comparisons were performed (e.g., Fig. 2E), the significance value threshold was adjusted using the Bonferroni-Holm correction, and the threshold for significance was set at P < 0.05. Data are means ± SE.
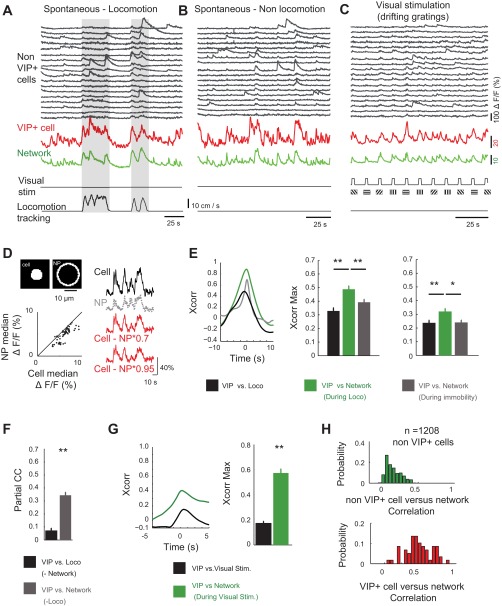
Fig. 2.
VIP+ cells correlate with the excitatory network across brain and behavioral state. A and B: segment of an imaging session showing florescence (ΔF/F, %) from a subsample of 18 non-VIP+ cells as the mouse runs on a disk (A) and while the mouse rests quietly in the dark (B). Activity of a representative VIP+ cell (red) and the overall average non-VIP+ network activity (Network; green) are shown (bottom). C: an epoch of the same cells shown in A and B, but during visual stimulation using drifting gratings (see materials and methods). D, top: an example cell contour and neuropil (NP) mask. For all cells, ΔF/F of the NP (ΔF/FNP) was subtracted from ΔF/F of the cell (ΔF/Fcell) to correct for large NP activity that could contaminate cellular measurements. Bottom, scatter plot showing the median ΔF/F for each VIP+ cell and NP. Cells had consistently greater activity than the NP (54/59 cells). At right are recordings from an example VIP+ cell showing the raw ΔF/F (black), NP ΔF/F (gray), and the cell ΔF/F after NP subtraction using 2 different NP correction factors [ΔF/Fcell − (ΔF/FNP × 0.7) or ΔF/Fcell − (ΔF/FNP × 0.9)]. E, left: example cross-correlations (Xcorr). Group data (n = 59 cells) show the correlation between VIP+ cells and locomotion (black), between VIP+ cells and the network (green), and between VIP+ cells and the network excluding locomotion bouts (gray). Middle, cross-correlation peak data (Xcorr Max). The correlation of VIP+ cell vs. network (during locomotion) was greater than that of the VIP+ cell vs. locomotion and the VIP+ cell vs. network (immobile) correlations (z = 4.0, P = 5.3 × 10−5 and z = 2.7, P = 0.007, respectively). Right, the same correlations as in middle panel but with a more conservative NP subtraction factor of 0.95 (z = 2.8, P = 0.005 and z = 2.28, P = 0.02). F: partial correlation (partial CC) between VIP+ cells and locomotion, after effects of the network (black) are removed, and the correlation between VIP+ cells and the network after the influence of locomotion (gray) is removed (z = 7.7, P = 1.4 × 10−14). G, left: example cross-correlations between VIP+ cell activity and the network (green) and between VIP+ cells and the dynamics of visual stimulation (Visual Stim.; black). Right, maximum values of the cross-correlations. Cells in the awake state (n = 11) and under anesthesia (n = 48) were pooled for analysis (z = 7.8, P = 3.3 × 10−15). H: histogram of correlations between non-VIP+ cells and the network (green) and between VIP+ cells and the network (red). For this analysis, all non-VIP cells were pooled and compared with VIP+ cell correlations (n = 59 VIP+ cells and 1,208 non-VIP cells, z = 11.5, P = 9 × 10−31). Nine mice were used in the analyses in D, E, F, and H, and 6 mice were used in the analysis in G. *P < 0.05; **P < 0.01.
RESULTS
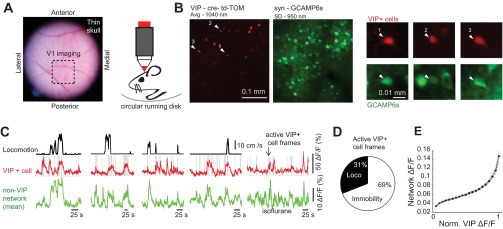
To investigate the interaction between VIP+ cells and the network in which they are embedded, we used two-photon Ca2+ imagining (with syn-GCaMP6) in awake and anesthetized VIP-cre::td-Tomato mice (materials and methods) while monitoring the spontaneous neural activity patterns in layers 2/3 of mouse visual cortex (Fig. 1, A and B). These mice allow for GCaMP Ca2+ signals to be measured both from VIP+ cells (td-Tomato+) and non-VIP (td-Tomato−; putative pyramidal cells) cell populations. VIP+ cells were previously shown to be specifically active during locomotion (Fu et al. 2014; Reimer et al. 2014). We also observed this locomotion–VIP+ cell coupling (Fig. 1C). However, we found that of the frames where VIP+ cells were active, only 31 ± 3% occurred during locomotion, whereas the remainder occurred during nonlocomotion (%locomotion vs. %nonlocomotion, P = 2 × 10−15, n = 59 cells, 5 ± 1 VIP+ cells/FOV; Fig. 1D). We averaged the activity across all non-VIP+ cells and referred to this as the network (80 ± 8 cells/FOV; green traces in Figs. 1C and 2, A–C). In general, VIP+ cell activity appeared to increase during periods of increased activity in the network (Fig. 1E), even in states of immobility and anesthesia (Fig. 1C). To quantify this relationship, pairwise correlations were calculated between 1) locomotion and VIP+ cell activity, 2) locomotion and the network, 3) VIP+ cell activity and the network during locomotion, and 4) VIP+ cell activity and the network during immobility (Fig. 2). Because the Ca2+ activity transients in interneurons are typically lower than in excitatory cells, they are more susceptible to influence from the background neuropil, which could artificially increase the correlation with the network. However, the VIP+ florescence was greater than the neuropil (ΔF/F = 30 ± 3%; Fig. 2D). The correlation between VIP+ cell activity and the network during locomotion was significantly greater than between VIP+ cell activity and locomotion itself (0.49 ± 0.02 vs. 0.33 ± 0.02, P < 0.001; Fig. 2E). Moreover, the correlation between VIP+ cell activity and the network, ignoring locomotion periods, was less than that occurring during locomotion (0.39 ± 0.02, P < 0.01; Fig. 2E), demonstrating that the interaction between VIP+ cells and the network is strengthened by locomotion. The statistical difference between correlations did not depend on the manner in which the neuropil was subtracted (Fig. 2E). VIP+ cells therefore resemble the definition of network “choristers” described recently (Okun et al. 2015). Because there also was a strong correlation between the network and locomotion (0.51 ± 0.02; not shown), we performed pairwise partial correlations to remove effects of the network on the correlation between VIP+ activity and locomotion. Partial correlation between VIP+ cells and locomotion was reduced to 0.07 ± 0.02 after the dynamics of network activity were accounted for (Fig. 2F), whereas the partial correlation between VIP+ cells and the network was 0.34 ± 0.02 after locomotion was accounted for. These data suggest that the interaction between locomotion and VIP+ cells was mediated by correlated changes in the network. In addition to locomotion and nonlocomotion spontaneous activity, VIP+ cells were recruited by visual stimulation (drifting gratings and natural images) during both the awake state (n = 11 cells; Fig. 2, C and G) and under light anesthesia (n = 48), as reported previously (Kerlin et al. 2010; Mesik et al. 2015). The correlation between VIP+ cells and the network during visual stimulation was consistently higher than for the time series of visual stimulation (0.57 ± 0.03 vs. 0.17 ± 0.02, P = 3 × 10−15; Fig. 2G). In total, VIP+ cells were more correlated with network than were members of the non-VIP population itself (0.14 ± 0.02 vs. 0.48 ± 0.02, P = 9 × 10−31; Fig. 2H), likely due to the higher heterogeneity of the non-VIP population. An increase in VIP+ cell activity together with increased network activity during periods of locomotion, awake immobility, anesthesia, and visual stimulation reflects a more general interaction between increases in VIP+ cell activity and increases in local network activity. Whether VIP+ cells are facilitating these periods of high activity in each state through disinhibition or following it through excitation by nearby pyramidal cells (Porter et al. 1998) is not clear.
Fig. 1.
VIP+ cells are active across brain and behavioral states. A: a photomicrograph showing the thin skull preparation used for 2-photon Ca2+ imaging in awake head-fixed mice. An area of left primary visual cortex (V1) is shown with a dashed box. B, left: an example field of view in V1 showing the 2-photon imaging of td-Tomato (td-TOM; 1,040-nm excitation; red) and GCaMP6s (950-nm excitation; green). Right, examples of active VIP+ cells as seen in both channels. The GCaMP image is a standard deviation (SD) projection across the entire movie. The td-TOM image is an average of a short movie taken at 1,040 nm before GCaMP imaging. C: 4 examples, taken from 4 different mice, showing locomotion speed, the activity from a VIP+ cell, and the mean activity from non-VIP+ cells (putative excitatory network). At far right is an example taken from a mouse under light isoflurane anesthetic. Note that although VIP+ cells are active during locomotion, they are also highly active outside locomotion, when the animal is immobile on the disk, and in the dark. Gray lines indicate frames where VIP+ cells were active. D: the overall mean percentage of active VIP+ cell frames that occurred during locomotion and immobility. E: the mean relationship between VIP+ cell activity and the overall excitatory network activity, showing increased activity in the network as a function of increased VIP+ cell activity. Norm., normalized; n = 59 VIP+ cells in 9 mice were used for this analysis.
To address this question, we selectively inhibited VIP+ cell activity using pharmacogenetics (Armbruster et al. 2007; Dong et al. 2010). VIP-cre or VIP-cre::tdTomato mice were injected in V1 with AAV5-DIO-hM4Di-mCherry, which expresses the exogenous hM4Di receptor in VIP+ cells (Fig. 3A). When the hM4Di receptor is activated by the specific agonist clozapine-N-oxide (CNO), synaptic release is inhibited, effectively removing the cell population from the local circuit (Dong et al. 2010; Stachniak et al. 2014). In vitro patch-clamp data from slices of VIP-cre::SOM-GFP mice confirmed that the activation of the hM4Di in VIP+ cells selectively blocks their output to SOM+ cells without impacting SOM+ cell → VIP+ cell connections (Fig. 3, B and C), and in vivo, CNO application reduced the overall activity of VIP+ cells (Fig. 3D). We were principally interested in how VIP+ cell suppression influences the activity in non-VIP+ cells (presumably excitatory cells) in V1. In lightly anesthetized mice, the suppression of VIP+ cells led to a pronounced overall decrease in spontaneous neural activity (n = 102 ± 9 cells/experiment; Fig. 3, E–G) and produced periods of prolonged cortical silence (Fig. 3E). Across experiments, the effects of CNO in VIP-cre-hM4Di mice (−37 ± 6%, n = 10 mice) were significantly greater than in control mice (−9 ± 3%, n = 7 mice) injected with CNO without the hM4Di receptor (z = 2.9; P = 0.004). In 9/10 individual experiments, VIP+ cell suppression significantly decreased the activity (pre- vs. post-CNO, P < 0.05), whereas in control mice only 1/6 mice showed a decrease pre- vs. post-CNO. In awake head-fixed mice (Fig. 3, H–M), VIP+ cell suppression reduced overall activity rates during awake immobility (−20.1 ± 3.1%; Fig. 3, J and K) and locomotion (−20.0 ± 2.7%; Fig. 3, L and M) significantly more than CNO injection in control animals (−5.7 ± 3.4% immobility, −3.5 ± 2.0% locomotion). In individual animals, 5/6 mice showed a significant decrease pre- vs. post-CNO during immobility (P < 0.05; and 1/6, P = 0.06), and during locomotion, 6/6 mice showed a significant activity reduction (P < 0.05), whereas in control experiments, 1/4 mice exhibited a significant decrease, specifically during immobility. Because cells in V1 are modulated by running speed (Erisken et al. 2014; Keller et al. 2012; Polack et al. 2013; Saleem et al. 2013; Vinck et al. 2015), we also assessed the speed modulation of the network pre- and post-VIP+ cell suppression. The speed-associated increase in network activity was also attenuated by VIP+ cell suppression at both “slow” and “fast” speeds (see materials and methods; Fig. 3I). Collectively, these data show that VIP+ cell-mediated disinhibition facilitates spontaneous activity under conditions of anesthesia, nonlocomotion, and locomotion.
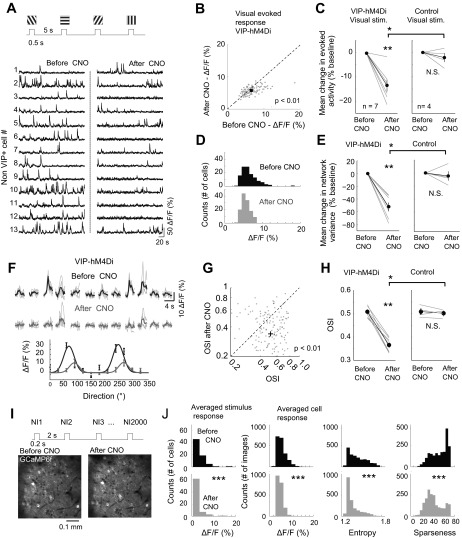
Finally, we assessed how VIP+ cell suppression impacted aspects of visual evoked activity. We presented drifting gratings and natural images to anesthetized or awake mice and determined how removal of VIP+ cells impacted the evoked response of layer 2/3 cells (Fig. 4A). We used either OGB or GCaMP6f for imaging in experiments with drifting gratings (materials and methods). During presentation of drifting gratings in both the anesthetized and awake conditions (in the absence of locomotion), VIP+ suppression reduced the mean and network variance of the visual evoked activity (mean = −13.7 ± 2.4%, variance = −52.5 ± 6.8% relative to before CNO administration, n = 7 mice, 180 trials each, 115 ± 27 cells/mouse; Fig. 4, B–E) and the overall population variance significantly more than control experiments (mean = −2.1 ± 1.8%, variance −4.6 ± 9.8% relative to before CNO, n = 4 mice, 180 trials, 121 ± 21 cells/mouse; Fig. 4, C and E). Essentially, VIP+ cell suppression decreased the averaged evoked response and narrowed the distribution of evoked response amplitudes. In individual mice, 6/7 animals showed statistically significant changes pre- vs. post-CNO (P < 0.05), whereas only one mouse (1/4) showed statistically different responses following control experiments. These results are consistent with the recent intracellular data showing that optogenetically suppressing VIP+ cells increased inhibitory synaptic drive in excitatory neurons (presumably from local SOM cells) in response to visual inputs (Zhang et al. 2014). In addition to the reduction in response magnitude, the OSI (see materials and methods) was significantly reduced by VIP+ cell suppression (0.507 ± 0.008 vs. 0.369 ± 0.011, n = 124 ± 11 cells/experiment; Fig. 3, F–H), whereas the OSI in control experiments did not change pre- and post-CNO (0.509 ± 0.012 vs. 0.502 ± 0.008, respectively, 105 ± 25 cells/experiment). In total, all animals in VIP-hM4Di experiments had significantly reduced mean OSI (P < 0.05), whereas none of the control experiments exhibited a significant change in OSI pre- vs. post-CNO. Despite this pronounced reduction in OSI following VIP+ cell suppression, the network as a whole was changed as evidenced by changes in spontaneous activity. Therefore, future studies should test how VIP+ cells control the OSI by using more temporally specific techniques, such as optogenetics, to avoid changes in network state that may affect evoked responses. We also presented natural images (Fig. 4I), and again evoked responses were altered (Fig. 4J) such that the mean cell response across stimuli significantly decreased (4.380 ± 0.416 vs. 3.276 ± 0.284 pre- and post-CNO, respectively; all cells were pooled from n = 2 mice). The statistics of the population per stimulus were also altered such that the mean response decreased (4.43 ± 0.16 vs. 3.32 ± 0.19 pre- and post-CNO, respectively), entropy decreased (1.47 ± 0.01 vs. 1.36 ± 0.02 pre- and post-CNO, respectively), and sparseness decreased (53.82 ± 2.22 vs. 41.24 ± 1.29 pre- and post-CNO, respectively). This decrease in evoked responses, together with reduced entropy and sparseness, indicates that without VIP+ cells, the visual response changes from a highly selective strong response to particular features to a smaller, more uniform response across features of visual space.
Fig. 4.
VIP+ cell suppression reduces visual evoked activity and network variability. A: the experimental paradigm where square-wave drifting gratings (12 directions of motion, 15 repetitions) were presented for 0.5 s, with a 5-s interstimulus interval pre- and post-CNO administration. Bottom, representative traces from 13 non-VIP cells pre- and post-CNO in a VIP-cre mouse selectively expressing the hM4Di receptor in VIP+ cells during drifting gratings presentation. Similar to spontaneous data, the overall activity level was reduced. B: scatter plot from a representative experiment in an animal expressing the hM4Di receptor in VIP+ cells, showing the distribution of the mean evoked response (averaged across stimuli) pre- and post-CNO. Each dot represents the mean from a single cell. C: group data showing the mean evoked response of non-VIP cells pre- and post-CNO. At right is the same experiment performed in 3 animals not expressing the hM4Di receptor but given CNO and 1 animal expressing the hM4Di receptor but given saline (n = 6, z = 3.3, P = 0.001 for the VIP-hM4Di group, and z = 1.07, P = 0.28 for the control group; z = 2.17, P = 0.03 for VIP-hM4Di vs. control manipulation). In C, E, and H, OGB was used in 5/7 mice and GCaMP6f in 2/7 mice; for controls, OGB was used in 2/4 mice and GCaMP6f in 2/4 mice. D: histogram of evoked responses (averaged across stimuli) for the experiment shown in B. E: group data showing the population variance of evoked-response of non-VIP cells pre- and post-CNO. The population variance was reduced by VIP+ suppression (n = 7, z = 3.3, P = 0.001) but not in control animals (z = 1.07, P = 0.28). Therefore, evoked responses show a significantly narrower distribution of evoked response amplitudes across cells and collectively show that neurons are less responsive and more homogeneous in their response. The response variance was reduced significantly more in VIP-hM4Di animals relative to controls (z = 2.55, P = 0.01). F: example single cell showing (with OGB labeling) single trials (light gray) and mean evoked responses as a function of stimulus direction before (black) and after VIP+ suppression (dark gray). Tuning curves used to calculate OSI are shown at bottom for these data. G: example experiment showing the change in OSI pre- and post-CNO following VIP+ cell suppression. H: group data across experiments for VIP+ suppression (left) vs. control (right). The OSI was reduced in VIP-hM4Di animals (n = 7, z = 3.01, P = 0.002) but not controls (z = 0.43, P = 0.67, n = 4). The OSI was reduced significantly more in VIP-hM4Di animals relative to controls (z = 2.55, P = 0.01). I: a maximum projection image of a field of view from 1 GCaMP6f experiment in which we presented 2,000 natural images (NI1: NI2000) pre- and post-CNO while imaging the same cells. Images were obtained online from http://www.naturephoto-cz.com/. J: example data from natural image experiments, showing the changes in stimulus response statistics, including average activity across cells (same as D), as well as statistics across stimuli, including single-cell evoked responses, entropy, and sparseness.*P < 0.05; **P < 0.01; ***P < 0.001; N.S., not significant.
DISCUSSION
The present data demonstrate that VIP+ cells are active during an array of brain and behavioral states and that their ongoing activity dynamics correlate with and facilitate activity in the neocortex. Previous studies have shown that VIP+ cells in visual cortex correlate specifically with locomotion. However, we show that in addition to this locomotion-associated activity, VIP+ cells fire outside locomotion periods, during awake immobility, during light anesthesia, and, of course, during visual stimulation. Therefore, these cells are not strictly locomotion sensitive. The activity of VIP+ cells correlates strongly with the mean overall population of non-VIP+ cells (presumably excitatory neurons), suggesting VIP+ cells may increase network firing by disinhibition. Finally, we show that VIP+ cells serve a disinhibitory role in all states, because VIP+ suppression reduced spontaneous activity under anesthesia, during awake immobility, during locomotion, and during visual stimulation. Therefore, there appears to be a state-independent relationship between the amount of activity in layer 2/3 and the activity in VIP+ cells, and VIP+ cells function to maintain high neural firing rates across different brain and behavioral states.
Previously, VIP+ cell loss of function experiments have been performed with either photolytic lesions of a small subset of VIP+ cells (Fu et al. 2014) or tetanus toxin-mediated lesions (Fu et al. 2015). It was shown that the gain in the visual response during locomotion (Niell and Stryker 2010) was abolished by lesion of VIP+ cells. However, the effect on locomotion-associated spontaneous network activity, spontaneous activity in general, or visual evoked responses following VIP+ cell suppression has not been reported. Therefore, our results extend the previous finding that VIP+ cells in V1 provide disinhibition of visual evoked activity during locomotion, by showing that disinhibition extends throughout all behavioral and brain states, including visual stimulation without locomotion. Given that most VIP+ activity occurred outside of locomotion, studying these cells in all behavioral contexts will be an important next step in determining the role of these neurons in cortical computations.
The activity of VIP+ neurons and the mechanisms of local interactions with the network are likely driven by distinct mechanisms in different states. During visual stimulation, the thalamocortical circuit likely engages VIP+ cells through a monosynaptic (Fu et al. 2015) or polysynaptic excitatory circuit from the thalamus. However, during spontaneous activity or locomotion, top-down (Zhang et al. 2014) or cholinergic inputs (Alitto and Dan 2012; Eckenstein et al. 1988; Letzkus et al. 2011) may be responsible for depolarizing these cells. Although the circuit mechanisms responsible for VIP+ cell firing may vary across states, their strong correlation with the network and their disinhibitory influence appear to be generalizable. Recently, it was shown that VIP+ cells undergo subthreshold depolarization during arousal, increasing in a positive manner with pupil diameter (Reimer et al. 2015). These data suggest activity of VIP+ cells outside of locomotion may be due to arousal associated increases in pupil diameter. However, arousal was also shown to decrease the overall activity of excitatory cells in V1 (Vinck et al. 2015). Therefore, future study is required to rigorously parse out the effects of general arousal, pupil fluctuation, and locomotion on VIP+ cell firing and network excitability. The mechanism by which VIP+ cell suppression leads to a decrease in overall activity in the network is likely mediated by increased SOM+ cell firing, because VIP+ cells connect most densely to SOM+ cells, especially in V1 (Lee et al. 2013; Pfeffer et al. 2013; Pi et al. 2013). Because SOM+ cells are inhibitory, and VIP+ cells provide inhibitory inputs to SOM+ cells, VIP+ cell suppression likely leads to an increase in SOM+ firing and, consequently, an increase in dendritic inhibition and reduced activity levels in the network. However, future experiments with simultaneous imaging of multiple interneuron cell types will be necessary to determine the precise mechanism by which VIP+ cells interact with other interneurons populations to shape the network activity dynamics.
We found that suppressing VIP+ cells decreased the OSI of V1 neurons. Suppression of VIP+ cells by photoablation was previously shown to abolish the locomotor modulation of visual inputs in V1 cells (Fu et al. 2014). However, the change in OSI was not reported. Our data show that pharmacogenetic suppression of VIP+ cells does decrease OSI, even in the absence of locomotion. Therefore, VIP+ cells likely play a role in modulating thalamocortical visual signals across different behavioral states. A caveat to these experiments is that the basal level of network activity is changed following VIP+ cell suppression (Fig. 3); therefore, the state of the cortex prior to visual stimulation will inevitably be changed. This change in the baseline state of cortex likely impacts the fidelity and magnitude of thalamocortical processing. Therefore, these results cannot completely separate the impact of network state vs. genuine suppression of VIP+ cells because these cells appear to be rather important for regulating baseline ongoing firing in the cortex. Use of precisely timed bidirectional optogenetic manipulation may provide a way forward for future experiments.
Collectively, our data provide novel evidence that VIP+ interneurons play a causal role in governing spontaneous and evoked neuronal activity in superficial cortical layers and that this influence is generalizable across behavioral and brain state. VIP+ cells are active not only during locomotion (Fu et al. 2014; Reimer et al. 2014) but also during awake immobility, under light anesthesia, or in response to visual stimulation. Moreover, VIP+ cell activity dynamics closely couple with the activity dynamics of the network, and suppression of VIP+ cells shifts the network to a low-activity regime. Thus VIP+ cells may form a positive feedback loop with the nearby excitatory cells, which is also suggested by the exponential shape of the curve describing the interaction between VIP+ cell and non-VIP cell activity (Fig. 1E). Future study should be devoted to parsing out the degree to which the interaction between top-down, retina-thalamo-cortical, and local circuits control the firing of VIP+ cells in different behavioral states and how VIP+ cells mediate different state-dependent computations.
GRANTS
This project was supported by the Canadian Institute for Health Research (J. Jackson), Marie Curie International Outgoing Fellowship (I. Ayzenshtat), Human Frontier Science Program Long-Term Fellowship (M. M. Karnani), and National Eye Institute Grants DP1EY024503 and R01EY011787. This material is based upon work supported by, or in part by, the U.S. Army Research Laboratory and the U.S. Army Research Office under contract W911NF-12-1-0594 (Multidisciplinary University Research Initiatives).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J., I.A., M.M.K., and R.Y. conception and design of research; J.J., I.A., and M.M.K. performed experiments; J.J., I.A., and M.M.K. analyzed data; J.J., I.A., M.M.K., and R.Y. interpreted results of experiments; J.J. and I.A. prepared figures; J.J. and I.A. drafted manuscript; J.J., I.A., M.M.K., and R.Y. edited and revised manuscript; J.J., I.A., M.M.K., and R.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Darcy Peterka, Yeonsook Shin, and Alexa Semonche for technical support. We thank Kira Poskanzer and Romain Goutagny for comments on previous versions of the manuscript.
REFERENCES
- Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci 6: 79, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Allen JA, Farrell M, Roth BL. A chemical-genetic approach for precise spatio-temporal control of cellular signaling. Mol Biosyst 6: 1376–1380, 2010. [DOI] [PubMed] [Google Scholar]
- Eckenstein FP, Baughman RW, Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience 25: 457–474, 1988. [DOI] [PubMed] [Google Scholar]
- Erisken S, Vaiceliunaite A, Jurjut O, Fiorini M, Katzner S, Busse L. Effects of locomotion extend throughout the mouse early visual system. Curr Biol 24: 2899–2907, 2014. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron 69: 1188–1203, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci 34: 535–567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Kaneko M, Tang Y, Alvarezbuylla A, Stryker MP. A cortical disinhibitory circuit for enhancing adult plasticity. Elife 4: e05558, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell 156: 1139–1152, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Okamoto S, Konno M, Kameda H, Sohn J, Kuramoto E, Fujiyama F, Kaneko T. Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J Neurosci 33: 544–555, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 72: 231–243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–107, 2007. [DOI] [PubMed] [Google Scholar]
- Keller GB, Bonhoeffer T, Hubener M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 74: 809–815, 2012. [DOI] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003. [DOI] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67: 858–871, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D. “What's new in Psychtoolbox-3?” Perception 36 (Suppl): 1, 2007. [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16: 1662–1670, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480: 331–335, 2011. [DOI] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62: 413–425, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, Callaway EM. Functional specialization of seven mouse visual cortical areas. Neuron 72: 1040–1054, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesik L, Ma WP, Li LY, Ibrahim LA, Huang ZJ, Zhang LI, Tao HW. Functional response properties of VIP-expressing inhibitory neurons in mouse visual and auditory cortex. Front Neural Circuits 9: 22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Ayzenshtat I, Carrillo-Reid L, Yuste R. Visual stimuli recruit intrinsically generated cortical ensembles. Proc Natl Acad Sci USA, 111, E4053–E4061, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Buzsáki G. Preconfigured, skewed distribution of firing rates in the hippocampus and entorhinal cortex. Cell Rep 4: 1010–1021, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65: 472–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Steinmetz NA, Cossell L, Iacaruso MF, Ko H, Bartho P, Moore T, Hofer SB, Mrsic-Flogel TD, Carandini M, Harris KD. Diverse coupling of neurons to populations in sensory cortex. Nature 521: 511–515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31: 13260–13271, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16: 1068–1076, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature 503: 521–524, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16: 1331–1339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci 10: 3617–3628, 1998. [DOI] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84: 355–362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerlingleffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71: 45–61, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M. Integration of visual motion and locomotion in mouse visual cortex. Nat Neurosci 16: 1864–1869, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82: 797–808, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7: 27–41, 1998. [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Smyth D, Thompson ID. The sparseness of neuronal responses in ferret primary visual cortex. J Neurosci 29: 2355–2370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286: 1943–1946, 1999. [DOI] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86: 740–754, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore B, Tolhurst DJ. Characterizing the sparseness of neural codes. Network 12: 255–270, 2001. [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518: 389–404, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511: 596–600, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Hoangdo JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345: 660–665, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]