In natural, everyday environments, our available action options will frequently change from moment to moment, and we must often act without knowledge of whether additional options will become available to us. Here, we provide evidence, using a target-directed reaching task, that in such situations, movements are prepared and accumulated across time for sequential action options and, while being held in working memory, can be adaptively revised based on newly acquired target information.
Keywords: action, perception, decision-making, motor planning, working memory
Abstract
Recent neural and behavioral findings provide support for the influential idea that in situations in which multiple action options are presented simultaneously, we prepare action plans for each competing option before deciding between and executing one of those plans. However, in natural, everyday environments, our available action options frequently change from one moment to the next, and there is often uncertainty as to whether additional options will become available before having to select a particular course of action. Here, with the use of a target-directed reaching task, we show that in this situation, the brain specifies a competing action for each new, sequentially presented potential target and that recently formed action plans can be revisited and updated so as to conform with separate, more newly developed, plans. These findings indicate that the brain forms labile motor plans for sequentially arising target options that can be flexibly restructured to accommodate new motor plans.
NEW & NOTEWORTHY
In natural, everyday environments, our available action options will frequently change from moment to moment, and we must often act without knowledge of whether additional options will become available to us. Here, we provide evidence, using a target-directed reaching task, that in such situations, movements are prepared and accumulated across time for sequential action options and, while being held in working memory, can be adaptively revised based on newly acquired target information.
recent neurophysiological (Baumann et al. 2009; Cisek 2007; Cisek and Kalaska 2005, 2010; Klaes et al. 2011) and behavioral (Chapman et al. 2010a, 2014; Ghez et al. 1997; Stewart et al. 2013, 2014; Tipper et al. 1998; Wood et al. 2011) studies have provided strong evidence supporting the notion that in situations in which multiple potential movement goals are presented simultaneously, the brain specifies, in parallel, multiple motor plans for these competing options before deciding on one of them. Such motor encoding of competing action goals could facilitate the incorporation of movement-related costs and constraints into decisions related to action selection and may enable more rapid responding once the target is selected (Christopoulos et al. 2015; Cisek 2006; Cisek and Pastor-Bernier 2014; Cos et al. 2011, 2012, 2014; Gallivan et al. 2015). In contrast to experiments described above, in the real world, due to continuous shifts of overt or covert attention, potential action goals often arise sequentially over time with uncertainty about the number of options that will become available before we need to act. For example, as we clamber over rough terrain, for each foot placement or handhold, we have to select, using focal attention, a single target location from among several possible alternatives that present themselves sequentially as we survey the scene. A great deal is known about how processes linked to focal attention influence visually guided movements [e.g., Song and Nakayama (2006)] and how the brain accumulates and evaluates sensory evidence over time to select an action signifying a perceptual decision (Kira et al. 2015; Yang and Shadlen 2007). However, it remains unclear how, before selecting from among multiple potential actions, we represent and integrate new motor plans with other competing motor plans already being held in working memory (WM).
It has recently been shown that in situations in which two potential reach targets are presented in parallel, the motor plans selected for the two targets interact and are “cooptimized” (Gallivan et al. 2015). Specifically, when one of the potential targets (the ambiguous target) can be reached using either wrist supination or pronation, and the other potential target (the unambiguous target) can only be reached with one of these orientations, the motor system selects and prepares a movement for the ambiguous target that has the wrist orientation required and prepared for the unambiguous target. This interaction, which can only arise if multiple, competing motor plans are specified in advance of target selection, may be advantageous in several ways. First, as we have demonstrated previously (Gallivan et al. 2015), the preparation of movements with compatible wrist orientations can reduce the time required to launch a given movement once a target is selected. Similarly, it has been shown that reaction times (RTs) are reduced when there is spatial compatibility between multiple, potential reach target locations [e.g., Bock and Arnold (1992)] and in the context of stimulus-response experiments, when the hand movement to be executed is congruent with the actions afforded by the stimulus [e.g., Tucker and Ellis (1998)]. Second, the selection of compatible trajectories, which share common movement components (e.g., wrist orientation), may reduce the WM load associated with maintaining multiple motor plans in advance of target selection.
Here, we tested several novel hypotheses related to the representation of potential targets that arise sequentially and probabilistically (i.e., with uncertainty about the number of potential targets that will be presented before a target is selected). Given the putative benefits of directly mapping potential targets into associated action plans (outlined above), we hypothesized that the brain prepares actions for sequentially arising reach options. Specifically, due to uncertainty about the number of options that will become available, we predicted that motor plans are prepared for each option as they appear and that these competing motor plans are accumulated across time. Moreover, given the putative advantages of cooptimizing motor plans, we hypothesized a bidirectional interaction between action plans for successively presented potential targets. That is, we predicted that the movement prepared for the first potential target in a sequence will influence the motor plan formed for a second, more recently presented potential target (i.e., proactive influence) and furthermore, predicted that the action prepared for the first potential target can be revisited and adaptively restructured so as to share movement components associated with the second, later potential target (i.e., retroactive influence). Our confirmation of these predictions demonstrates that the brain forms labile motor plans for sequentially arising action options that can be flexibly restructured to accommodate new motor plans.
MATERIALS AND METHODS
Overview of General Procedure
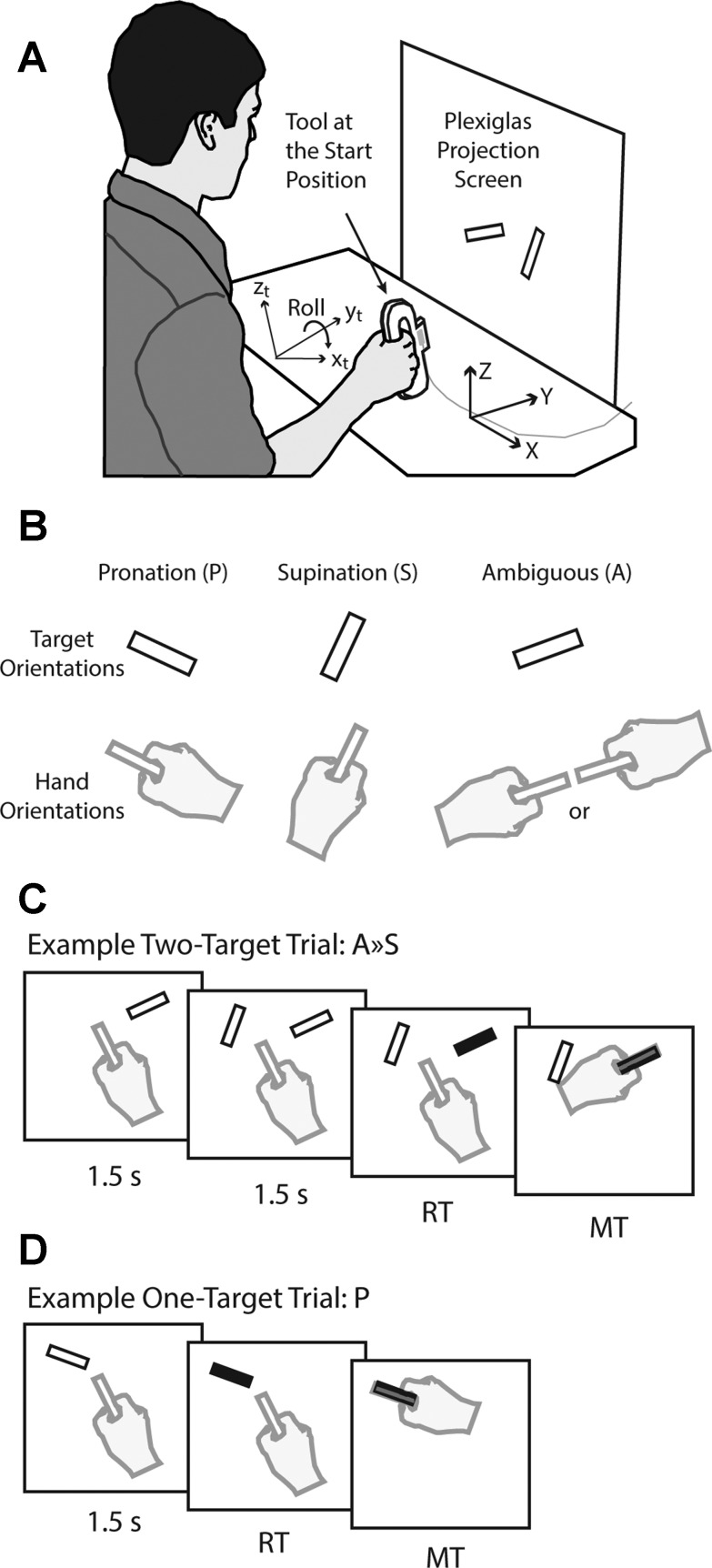
To test whether the brain specifies multiple movement plans for sequences of targets that are separated in time and to examine the extent to which such plans interact in a bidirectional fashion, we designed a reaching task in which participants were presented with a temporal sequence of potential targets, one of which would be cued eventually as the final target (Fig. 1). In brief, the majority of trials (∼75%) began with the presentation of one potential rectangular target, and then, after a 1.5-s delay, a second was presented on a vertical screen (Fig. 1C). After a further 1.5-s delay and concurrent with an auditory beep, one of the potential targets was cued (filled in), instructing the participant to move a hand-held rectangular tool-tip as quickly and accurately as possible from the start position to contact the target. Critically, on the remaining ∼25% of the trials, the first (and only) target was cued at the time the second target would have otherwise been presented (i.e., one-target trials; Fig. 1D). These one-target trials (randomly interspersed with the two-target trials) created uncertainty as to whether, on any given trial, an additional (second), potential target would become available before target selection and thus importantly encouraged participants to prepare movements for the first of two potential targets in the sequence (on two-target trials) once it appeared.
Fig. 1.
Experimental setup and trial types. A: participants moved the rectangular tip of a hand-held tool to contact targets projected onto a vertical screen. B: possible target orientations (top) and corresponding hand orientations (bottom). Note that the ambiguous target equally afforded wrist pronation and supination, as shown. C: example of a 2-target trial. Here, an ambiguous potential target is displayed, and 1.5 s later, a supination potential target is displayed while the tool is at the start position. After a further 1.5 s, the ambiguous potential target is cued (filled in) as the target, providing the go signal for the participant to move the tool-tip to the target. D: example of a 1-target trial. Here, a pronation potential target is displayed, and 1.5 s later, it is cued as the target, providing the go signal. RT, reaction time from target cuing to movement onset; MT, movement time from onset to target contact.
The orientations of the potential targets could be one of three types (Fig. 1B): a target requiring wrist pronation (P), a target requiring wrist supination (S), or an ambiguous target (A) that could be contacted equally using either wrist pronation or supination. In a variant of this task, in which the two targets were always presented simultaneously, we previously showed that when the cued target was ambiguous, participants were more likely to pronate or supinate the wrist when the noncued, unambiguous target required pronation or supination, respectively (Gallivan et al. 2015). In addition, we found that RTs were slightly faster when the chosen wrist orientation was compatible with the orientation that would have been required for the other unambiguous target. Together, these previous findings indicated that motor plans are specified for each potential target in advance of target selection and are cooptimized to improve task performance in cases of simultaneous target presentation. If in the current sequential target-presentation task, motor plans are specified sequentially for each potential target, and they interact over time, then we would expect to observe similar cooptimization in trials in which the second potential target is cued (i.e., proactive influence of the first motor plan on the second). Furthermore and perhaps more interestingly, by testing for cooptimization in trials in which the first potential target is cued, our paradigm also allowed us to examine whether the first motor plan can actually be reprogrammed so as to conform to the requirements of the second, more newly formed motor plan (i.e., retroactive influence of the second motor plan on the first).
Participants
Thirty-five right-handed participants (aged 19–26, 21 women), recruited from the student population at Queen's University, took part in this study after providing written, informed consent. Of the 18 participants who took part in the main experiment, 3 were excluded, because they always either supinated or pronated the wrist when reaching to the ambiguous target and therefore, could not contribute information that either supported or disaffirmed our experimental predictions (see below). Of the 17 participants who took part in a second follow-up experiment, 3 were excluded for the same reason. None of the participants who participated in the main experiment took part in the second experiment. The Queen's University Research Ethics Board approved the study.
Apparatus
Seated participants held a Plexiglas tool that had a 6 × 1-cm rectangular tool-tip extending from its front, which directly matched the orientation of the wrist (Fig. 1A). Rectangular targets, the same size and shape as the tool-tip, were rear projected onto a vertical screen located 15 cm from the start position of the tool-tip and ∼40 cm from the participant's eyes. The screen was covered in Plexiglas and could be contacted forcefully with the tool-tip. An electromagnetic sensor (Polhemus Liberty, Colchester, VT), embedded in the tool-tip, recorded the position and orientation of the tool-tip in three dimensions at 240 samples/s. Variants of this general type of “letter-posting” task have been successfully used by others to investigate the online control of visually guided actions (Darling and Miller 1993; Goodale et al. 1991; Gosselin-Kessiby et al. 2008; Perenin and Vighetto 1988; Torres and Zipser 2004).
The start position of the tool-tip was aligned with a sagittal plane through the participant's shoulder, as well as the midline of the screen (Fig. 1A). Targets were presented at a height of 15 cm above the table surface (z-axis), either 6.5 cm to the left or 6.5 cm to the right of screen midline or at both locations depending on the trial type. Target and tool-tip orientations were defined as 0° when the long axis of the rectangle was vertical, with the clockwise rotation from the perspective of the participant defined as positive.
In the main session in both experiments, three target orientations were used: a target requiring wrist pronation, a target requiring wrist supination, or an ambiguous target that could be equally contacted using either wrist pronation or supination (Fig. 1B). The ambiguous target was rotated +65° from upright (positive corresponds to clockwise). This orientation was based on our previous work (Gallivan et al. 2015) and was slightly modified based on more recent pilot testing. The pronation target was rotated +110° (or −70°) from upright, and the supination target was rotated +20° from upright. Thus the pronation and supination targets were rotated, relative to the ambiguous target, 45° clockwise and counter-clockwise, respectively. The start position was rotated −25° from upright. Thus the magnitude of the tool-tip rotation required to contact the ambiguous target from the start position was the same regardless of whether the wrist was pronated (90° counter-clockwise) or supinated (90° clockwise). Moreover, the same magnitude of rotation was required to contact the pronation (45° counter-clockwise) and supination (45° clockwise) targets.
Experimental Procedure
Main experiment.
Participants first performed 70 one-target trials with the rectangular targets at the following orientations centered on +65° (i.e., the ambiguous target orientation) and ranging from +20° (the supination target orientation) to +110° (the pronation target orientation): 20, 30, 40, 50, 60, 65, 70, 80, 90, 100, and 110°. The numbers of trials associated with these orientations were: 4, 4, 6, 8, 8, 10, 8, 8, 6, 4, and 4, respectively. In one-half of the trials for each target orientation, the target was presented at the left target position, and in the other half, it was presented at the right target position. The order of trials was fully randomized. The aim of this initial phase was to give participants experience reaching to targets of varying orientations, including the ambiguous targets.
At the beginning of each trial in this initial phase, the start target was displayed on the screen along with a rectangle (equal in size to the tool-tip and start target), representing the projection of the tool-tip onto the screen. The color of the projected tool-tip indicated whether the tool-tip was held within 2 cm and 5° of the start position (green) or not (red). With this feedback, participants could quickly position the tool-tip at the start position. Once the tool-tip was held in the correct position for 1.5 s, the start target disappeared, and a single target was displayed. After a delay of 1.5 s, the target was cued (filled in black), and a brief auditory tone (100 ms, 1,000 Hz) sounded, together providing a “go” signal, instructing the participant to initiate a movement to contact the target quickly and accurately. After the tool-tip contacted the screen, the combined reaction and movement time—from the go signal to screen contact—and whether the trial was a “hit” or a “miss” were displayed centrally on the screen. The trial was considered a hit if the center of the tool-tip was within 2 cm of the center of the target, and the orientation of the tool-tip was within 15° of the orientation of the target. If the movement was initiated <100 ms after the go signal (i.e., before the movement could have reliably been triggered by the go signal), then the targets were removed from the screen, the message “too early” was displayed, and the trial was re-run later in the session.
The main experimental session began following these 70 one-target trials. In each trial, after the tool-tip was aligned at the start position for 1.5 s (as above), a target was presented on either the left or right for 1.5 s. In 78% of these trials, a second target was then presented (in the other location) for a further 1.5 s, while the first target remained visible. One of these two potential targets was then cued (filled in), and a tone sounded (see above), together providing the go signal to contact the target quickly and accurately. In the remaining 22% of these trials, the first target, after being presented for 1.5 s, was immediately cued (filled in), and a tone sounded, providing the go signal. After the screen was contacted, participants received the same feedback as in the 70 single target trials (described above). The sequences of events in these two- and one-target trials are illustrated in Fig. 1, C and D, respectively.
Participants completed 8 blocks of 54 trials each (432 trials in total) with rests in between. We will refer to different two-target trial types using two letters, indicating the target orientation (A, P, or S), separated by the ≫ symbol, where the first and second letters denote the first and second targets, and the cued target is boldfaced. Thus in an A ≫ P trial, an ambiguous target was presented first, a pronation target was presented second, and the first ambiguous target was cued. We will refer to one-target trials using a single bold-faced letter (A, P, or S). The following trial types, with the number of trials in parentheses, were tested: A (32), A ≫ A (16), A ≫ P (32), P ≫ A (32), A ≫ S (32), S ≫ A (32), A ≫ P (32), P ≫ A (32), P (32), P ≫ P (16), P ≫ S (8), S ≫ P (8), A ≫ S (32), S ≫ A (32), P ≫ S (8), S ≫ P (8), S (32), and S ≫ S (16). The left vs. right locations of the first and second targets were counterbalanced, and the trial order was fully randomized within blocks.
Second experiment.
The procedure for the second experiment was the same as the main experiment, with the exception that this separate testing session did not include one-target trials. That is, two potential targets were always presented before the final target was cued, and participants were informed that two targets would always appear sequentially. The same two-target trial types used in the main experiment, with the same number of trials for each type, were included, resulting in a total of 336 trials. The aim of the follow-up experiment was to assess whether participants actually prepare an action for the first target before presentation of the second target, even when they know that two targets will always be presented before target selection, and if so, whether this first prepared action can be reprogrammed to cooptimize the two motor plans.
Data Analysis
For each trial, we determined the roll angle of the tool-tip (Fig. 1A) at the time the tool-tip contacted the screen. The wrist was classified as having been supinated if the roll angle at contact was greater than −25°, indicating that the wrist rotated clockwise from the start angle, and as having been pronated if the roll angle was less than −25°, indicating that the wrist rotated counter-clockwise from its start angle. We also determined the RT for each trial, i.e., the time from target selection to movement onset, where the latter was defined as the time at which the resultant velocity of the tool-tip first exceeded 0.1 m/s. Repeated-measures ANOVA and paired t-tests with a P value of 0.05 were used to compare dependent measures across conditions.
Trial Selection
Only hit trials were included in the analyses reported in results. The percentage of successful target hits ranged from 72 to 93% across the 15 participants (mean = 84%) in the main experiment and ranged from 59 to 86% across the 14 participants (mean = 75%) in the second experiment. We also excluded trials with RTs that did not fall between 150 and 350 ms, so as ensure that movements were not initiated before the go signal and to avoid unduly long RTs. This resulted in the removal of 5 and 6% of all successful hit trials in the main and second experiments, respectively.
RESULTS
We hypothesized that if multiple action plans are specified sequentially for potential targets, as they appear, and they interact over time, then we should expect to observe bidirectional cooptimization. That is, we should find the following: 1) a proactive influence of the earlier movement plan formed for the first potential target on the plan subsequently formed for the second potential target and 2) a retroactive influence of the second, more newly developed movement plan for the second potential target on the plan previously formed for the first potential target. Critical to testing each of these two ideas (i.e., proactive and retroactive cooptimization) is that participants reliably form a motor plan for the first target in the sequence once it appears. To determine whether the inclusion of our one-target trials was successful in this regard, we compared the RTs in one-target with two-target trials. In our previous study (Gallivan et al. 2015), in which participants prepared either a single movement in one-target trials or two movements in two-target trials in which both targets were simultaneously presented, we found a 23-ms RT advantage for one-target compared with two-target trials [an advantage consistent with that reported previously, e.g., Bock and Arnold (1992); Chapman et al. (2010a); Gallivan et al. (2011); Rosenbaum et al. (1992)]. Thus if participants in the current experiment consistently prepared a movement for the first target when it was presented and before the second target was presented, then we might also expect to observe a similar RT advantage for one-target over two-target trials here. A paired t-test revealed that RT was significantly shorter (t14 = −5.30; P < 0.001), by 21 ms, in one-target (mean = 250 ms; SE = 6 ms) compared with two-target (mean = 271 ms; SE = 5 ms) trials. In the context of our previous results (and others'), this finding clearly suggests that participants consistently prepared a movement for the first potential target once it appeared and thus permitted us to investigate further whether competing action plans for sequentially presented potential targets were cooptimized proactively, retroactively, or both.
Tests for Cooptimization of Selected Wrist Posture
To address these central research questions, our analysis focused on one- and two-target trials in which the ambiguous target was cued as the final target. (Note that in trials in which the pronation or supination target was cued, participants invariably pronated or supinated the wrist, respectively.) Figure 2A shows the average probability, across participants, of selecting wrist supination (pS) for all two-target trial types in which the cued target was ambiguous. To quantify whether the orientation of the noncued target influenced pS for the ambiguous target and whether presenting the ambiguous target first or second also influenced pS, our analyses first focused on the four trial types in which the noncued target was unambiguous (A ≫ P, P ≫ A, A ≫ S, and S ≫ A, where the letter before and after the ≫ symbol corresponds to the first and second presented target, respectively). Here, a two-way, repeated-measures ANOVA revealed a main effect of orientation (F1, 14 = 76.6; P < 0.001) and an interaction between orientation and presentation order (F1, 14 = 5.52; P < 0.034) but no main effect of presentation order (F1, 14 = 0.001; P = 0.98). Overall, we found that pS was greater when the noncued target required supination (mean = 0.66; SE = 0.08) compared with when the noncued target required pronation (mean = 0.39; SE = 0.08). This result is consistent with our previous study that only used simultaneous target presentation [cf. Gallivan et al. (2015)] and demonstrates that the movement prepared but not executed for the unambiguous target directly influenced the movement prepared and executed for the ambiguous target. For comparison, in Fig. 2A, the average pS from our previous study (Gallivan et al. 2015) for trials in which the cued target was ambiguous, and the noncued target was a pronation or a supination target, respectively, is represented.
Fig. 2.
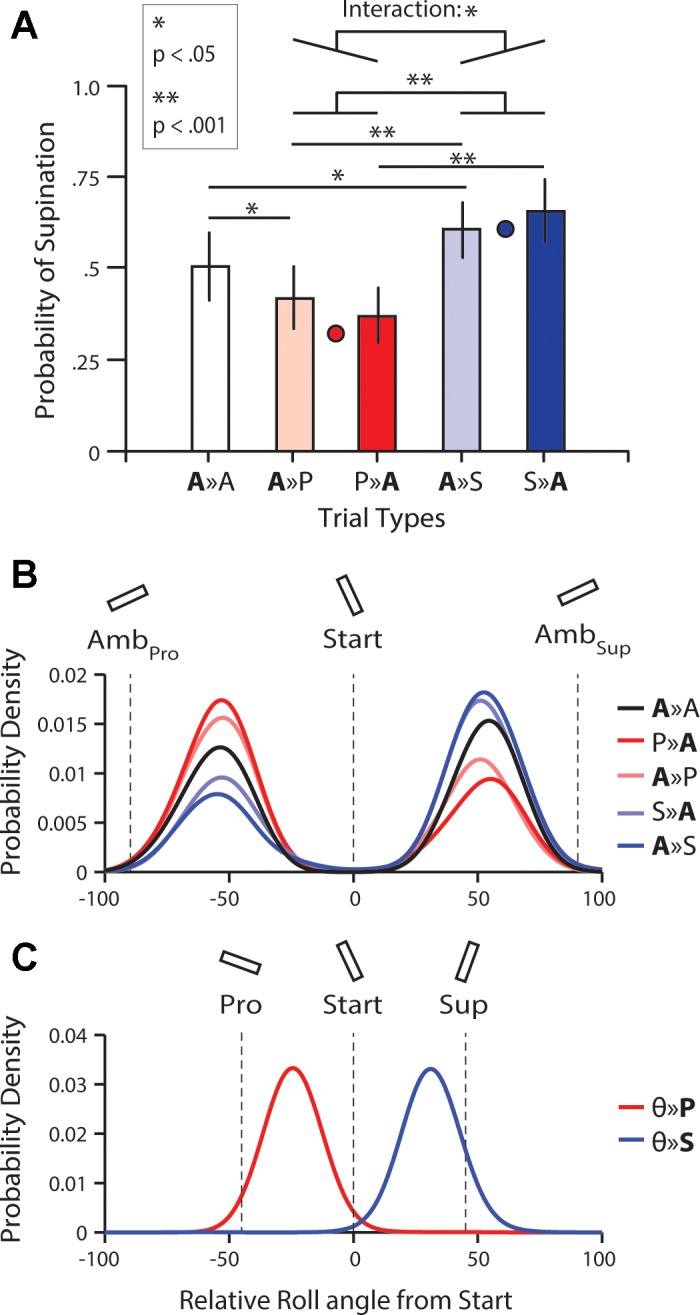
Results of the main experiment. A: average probability of selecting wrist supination (pS) for all 2-target trial types in which the cued target was ambiguous. The first and second letters represent the first and second target orientations, respectively (A = ambiguous, P = pronation, S = supination). The red and blue circles represent the average pS from a previous study (Gallivan et al. 2015) in which competing targets were presented simultaneously for trials in which the cued target was ambiguous, and the noncued target was a pronation or a supination target, respectively. B: probability density functions (fit using a Gaussian kernel with an SD of 10°) of the roll angle, relative to the start roll angle, at 30% of the Y distance to the target for different trial types. The vertical lines depict (from left to right) the relative roll angles associated with the ambiguous target when selecting pronation (AmbPro), the start position, and the ambiguous target when selecting supination (AmbSup). As clearly demonstrated by this plot, we found no evidence for the averaging of target orientation early in the movement trajectory (i.e., no values at 0° relative roll angle from start). C: corresponding functions for 2-target trials in which the pronation and supination targets were cued (θ represents all possible target orientations). Here, the vertical lines depict (from left to right) the relative roll angles associated with the pronation target (Pro), the start position, and the supination target (Sup).
The above interaction between noncued target orientation and presentation order suggests an order effect; that is, when the noncued target required supination, participants were more likely to supinate when the noncued target was presented first (compare S ≫ A with A ≫ S), and when the noncued target required pronation, participants were more likely to pronate when the noncued target was presented first (compare P ≫ A with A ≫ P; i.e., stronger proactive than retroactive influence of the noncued target). However, to determine whether the effects of the noncued target on pS still held for both presentation orders, we performed follow-up t-tests. Concerning the proactive effects, we expectedly found that when the ambiguous target was presented second, pS was greater (t14 = 7.31; P < 0.001) when the noncued (first) target required supination (S ≫ A: mean = 0.66; SE = 0.08) compared with pronation (P ≫ A: mean = 0.37; SE = 0.07). Concerning the retroactive effects, we also found that when the ambiguous target was presented first, pS was greater (t14 = 6.37; P < 0.001) when the noncued second target required supination (A ≫ S: mean = 0.61; SE = 0.07) compared with pronation (A ≫ P: mean = 0.421; SE = 0.08). Together, these results importantly demonstrate that the cooptimization of motor plans occurs regardless of the order in which the ambiguous and unambiguous targets are presented. Nevertheless, they also show that the influence of the noncued target on the motor plan formed for the cued target is stronger when the noncued target is presented first rather than second (i.e., stronger proactive than retroactive influence), suggesting that there may be some resistance to revising a motor plan that has already been formed.
The effect of potential target presentation order described above (i.e., ambiguous target first vs. second), coupled with our one- vs. two-target RT effects noted at the outset, strongly suggests that action plans for the targets are specified sequentially as they appear. If this is the case, then it also suggests that the action plan developed for the second (noncued) potential target can cause the plan developed for the first (cued) potential target to be reprogrammed/updated (i.e., retroactive cooptimization). To assess this possibility further, we directly compared trials in which the first cued target was ambiguous, and the second noncued target was ambiguous (A ≫ A trials) with trials in which the first cued target was ambiguous, but the second noncued target was unambiguous (A ≫ P or A ≫ S trials). We reasoned that if plans are specified both sequentially and independently for each potential target, then pS should be the same in all of these trial types (as in all cases, the same ambiguous target, which equally affords wrist supination and pronation, is being cued). Conversely, if the action plan computed for the first target is reprogrammed following the presentation of the second target and is cooptimized based on the competing action options, pS for the cued first target (i.e., ambiguous target) should be influenced by the orientation of the second noncued target (i.e., unambiguous target). Consistent with the latter prediction, paired t-tests revealed that pS was greater (t14 = 2.53; P = 0.024) in A ≫ S trials than in A ≫ A trials (mean = 0.51; SE = 0.09) and smaller (t14 = −2.88; P = 0.012) in A ≫ P trials than in A ≫ A trials (see Fig. 2A). Based on our RT evidence above that individuals consistently prepared a movement for the first potential target in the series, once it appeared, this finding suggests that the actions associated with the second potential target can update the plan previously formed for the first target (i.e., using A ≫ A trials as a baseline for comparison).
Tests for Cooptimization during the Early Reach Trajectory
Figure 2B shows probability density functions for the same five trial types shown in Fig. 2A of the roll angle relative to the roll angle at the start position when the tool-tip reached 30% of the Y distance to the ambiguous target. Each function, or distribution, includes all trials from all participants. For comparison, Fig. 2C shows probability functions for two-target trials in which the cued target was either the pronation target (θ ≫ P, where θ represents all target orientations) or the supination target (θ ≫ S). As can be directly appreciated by visual inspection of Fig. 2B, participants, when reaching to the ambiguous target, committed early to either wrist pronation or supination, with the change in roll angle approximately one-half of the total change required to reach the target, and there is no evidence for the spatial averaging of wrist orientation [cf. Stewart et al. (2013)]. That is, bimodal distributions were observed for all trial types, and the relative roll angle was never close to 0° by the time the tool-tip reached 30% of the Y distance to the target [a point into the reach movement where spatial averaging would be expected; cf. Chapman et al. (2010a, b); Gallivan and Chapman (2014)]. Whereas this finding is certainly novel in the context of previous work that has used (or relied on) spatial averaging behavior to reveal the parallel encoding of competing movements [and/or probe the effects of distractors on reaching; see Buc Calderon et al. (2015); Chapman et al. (2015); Gallivan et al. (2011); Meegan and Tipper (1998); Stewart et al. (2014); Tipper et al. (1997, 2000)], the absence of spatial averaging effects here is perhaps to be expected, given that movements were initiated only after the target was cued.
Effects of Cooptimization on RT
In our previous study, we found that in trials in which the cued target was ambiguous, and the noncued target was unambiguous, RT was slightly improved when participants selected a wrist orientation that was compatible, as opposed to incompatible, with the orientation required for the competing noncued target. Here, we observed the same outcome; a paired t-test revealed that RT was marginally but significantly greater (t14 = 2.44; P = 0.029) in incompatible (mean = 278 ms; SE = 6 ms) compared with compatible (mean = 274; SE = 5 ms) trials. Comparison of RT in trials in which the cued target was presented first vs. trials in which it was presented second revealed no significant difference (t14 = 1.09; P = 0.294). Together, this indicates a small processing advantage when both targets are recognized as affording a common wrist orientation, an advantage that does not depend on potential target presentation order.
Results of Second Experiment
Our inclusion of one-target trials in the main experiment was guided by the fact that in our typical natural environment, there is often some uncertainty as to whether newer action options will be made available before having to select a particular plan of action. Accordingly, it is perhaps not surprising that given our inclusion of one-target trials (which were randomly interspersed with two-target trials), we report several lines of evidence showing that individuals consistently prepared movements toward the first potential target in the sequence once it appeared. We recognize, however, that there are many situations in the real world in which before making a selection, one can be fully certain about all of their available options. In such scenarios, we wondered whether individuals would still bother preparing movements for the first option as it appears or just simply wait until all options become available before forming an action plan for each (i.e., wait for simultaneous target presentation). To explore these possibilities, we performed an additional experiment in a separate group of individuals that was identical in all regards to our main experiment, with the exception that there were never any one-target trials. That is, participants in this second experiment knew and were explicitly told that a second potential target would always follow after the first.
Figure 3 shows, for this additional experiment, the average pS for all trial types in which the cued target was ambiguous. A two-way, repeated-measures ANOVA revealed a main effect of orientation (F1, 13 = 17.9; P < 0.001) but no main effect of presentation order (F1, 13 = 0.86; P = 0.37). Overall, we found that pS was greater when the noncued target required supination (mean = 0.75; SE = 0.07) compared with pronation (mean = 0.46; SE = 0.07). The analysis also revealed a significant interaction between orientation and presentation order (F1, 13 = 8.77; P = 0.011). This latter effect can be directly appreciated by visual inspection of Fig. 3; when the noncued target required pronation, pS for the ambiguous target was smaller when the noncued target was presented first (compare P ≫ A with A ≫ P), and likewise, when the noncued target required supination, pS for the ambiguous target was greater when the noncued target was presented first (compare S ≫ A with A ≫ S). However, as in the main experiment, follow-up t-tests further revealed that the noncued target orientation significantly influenced pS for both presentation orders. That is, when the ambiguous target was presented first, pS was greater (t13 = 2.94; P = 0.012) when the noncued second potential target required supination (A ≫ S: mean = 0.72; SE = 0.07) compared with pronation (A ≫ P: mean = 0.48; SE = 0.08), and when the ambiguous target was presented second, pS was greater (t13 = 5.64; P < 0.001) when the noncued first potential target required supination (S ≫ A: mean = 0.79; SE = 0.08) compared with pronation (P ≫ A: mean = 0.44; SE = 0.07).
Fig. 3.
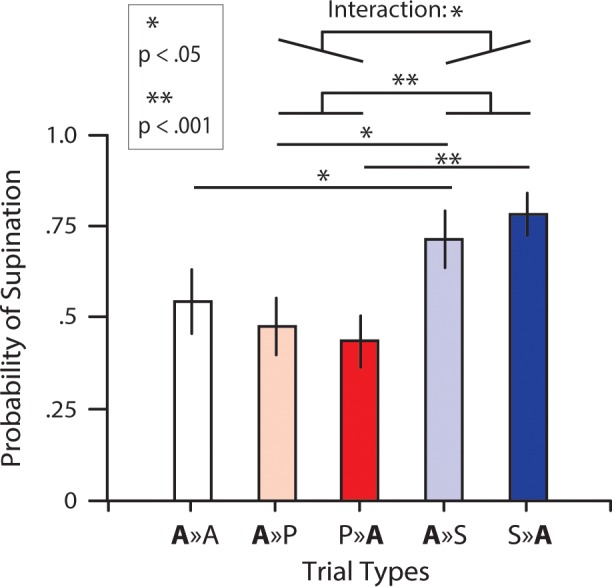
Results of the second experiment. Average probability of selecting wrist supination (pS) for all 2-target trial types in which the cued target was ambiguous. The first and second letters represent the first and second target orientations, respectively (A = ambiguous, P = pronation, S = supination).
As in the main experiment, to assess further the reprogramming effect (i.e., whether the motor plan for the first potential target is recomputed following the presentation of the second potential target), we compared trials in which the first cued target was ambiguous, and the second noncued target was ambiguous (A ≫ A trials) with trials in which the first cued target was ambiguous, but the second noncued target was unambiguous (A ≫ P or A ≫ S trials). Consistent with that observed in the main experiment, we found that pS was greater (t13 = 2.448; P = 0.029) in A ≫ S (mean = 0.72; SE = 0.07) trials than in A ≫ A trials (mean = 0.55, SE = 0.08) and smaller, although not significantly so (t13 = 1.1; P = 0.291), in A ≫ P trials (mean = 0.48, SE = 0.08) than in A ≫ A trials.
The results of this second experiment indicate that the target presentation order effects and reprogramming effects described in our main experiment are not related to the fact that in that main experiment, participants had uncertainty as to whether a second potential target would appear before target selection (recall in the main experiment that in ∼25% of the trials, the first presented target was cued without the second target ever being shown). This indicates that individuals, even when not encouraged to do so, naturally prepare actions for potential targets as they appear and flexibly update pre-existing plans to be compatible with newer plans. Together, this suggests that the formation of an action plan for each sequential action option reflects a largely automatic process that occurs in spite of participants' explicit knowledge of whether or not newer options will become available.
DISCUSSION
Recent studies have provided evidence that in situations in which multiple potential reach targets are presented simultaneously, the brain prepares motor plans for each competing option before selecting one of them to be executed (Chapman et al. 2010a; Cisek 2007; Cisek and Kalaska 2005, 2010; Gallivan et al. 2011; Klaes et al. 2011; Stewart et al. 2014). Moreover, by sharing common movement components across these motor plans, they can often be launched more quickly and thereby, cooptimized so as to improve task performance (Gallivan et al. 2015). Here, we investigated the encoding of competing action options that appear sequentially over time, as often occurs in natural, dynamic environments. With the use of a task in which potential reach targets were presented in series before one was cued as the target, we found evidence that individuals successively prepared actions for both the first and second potential targets, each as they appeared, and that these plans interacted in a bidirectional fashion. That is, not only did we find that the movement planned (and executed) for the second target reliably shared kinematic components (i.e., wrist orientation) with the movement prepared for the first target (i.e., proactive influence), but we also found that the movement plan formed for the first target could be reformed so that it borrowed kinematic components from the plan formed for the second, newer target (i.e., retroactive influence). Together, our findings suggest that movements previously considered and presumably being held in WM (as they can influence the wrist posture selected on the second target) can be adaptively revised based on the actions associated with more newly presented targets.
Previous work has shown that when two potential reach targets are simultaneously presented, activity in sensorimotor areas of the brain reflects these competing options in parallel before a target is selected (Cisek and Kalaska 2005; Klaes et al. 2011). One interpretation of this activity, consistent with Gibson's (1979) highly influential notion of action “affordances,” is that it represents the encoding of a movement plan toward each of the potential targets (Cisek 2007). However, it is entirely plausible that this activity instead represents visual properties of the potential targets (e.g., their locations/directions) and/or some general purpose WM buffer that maintains the locations of the targets so as to make a decision about response choice (Cisek and Kalaska 2005). Most of the studies that have examined the representation of competing reach options at the behavioral level have used variants of the “go-before-you-know” task in which participants are presented with multiple potential targets and required to launch a movement toward them before knowing which potential target will be cued as the final target after movement onset (Chapman et al. 2010a, 2014; Ghez et al. 1997; Hudson et al. 2007; Stewart et al. 2013, 2014; Tipper et al. 1998). These studies have shown that the direction of the initial reach vector corresponds to a spatial average of the reach directions to the potential targets. Recently, with the use of go-before-you-know tasks, in which movement and target directions were dissociated, we showed that this spatial averaging behavior does not likely arise from the planning of a single movement toward a visually averaged target location and instead, is more consistent with the specification of competing motor plans for these potential targets (Stewart et al. 2014). Moreover, we have provided evidence that control policies for competing motor plans may also be averaged (Gallivan et al. 2016). It has recently been suggested that the initial movement direction in the go-before-you-know tasks minimizes the cost of movement corrections made once the target is cued (Haith et al. 2015). Given this, one possibility is that the averaging of motor plans occurs precisely because it approximates an optimal solution for reducing the cost of such corrections (Hudson et al. 2007). However, it is also possible that participants deliberatively plan and execute a single movement that minimizes the costs of correction and that only resembles a motor average. Ultimately, it can be difficult to draw clear conclusions about the nature of the mechanisms that underlie motor planning in cases of target uncertainty from spatial averaging behavior alone.
Recently, with the use of a variant of the task used in the current study, in which the potential targets were always presented simultaneously, we provided direct evidence, via the cooptimization of action plans (i.e., the sharing of movement components across potential targets), that competing motor plans are considered for multiple potential targets in advance of target selection (Gallivan et al. 2015). This finding is noteworthy, given that the structure of the task in which target selection provided the cue for movement initiation (as here) did not actually require that participants consider the movements afforded by each target beforehand. That is, because we did not enforce any reaction or movement time requirements, individuals could have just as easily performed the task by simply waiting until the final target is cued before forming and then executing a single reach movement to that particular target.
Here, we report the same cooptimization behavior in the current experiment but in a task in which potential targets are presented sequentially over time. The previous study is important, because it provided compelling evidence that competing motor plans are prepared in advance of selecting one to execute. In contrast, the significance of the current study, which also shows that multiple competing plans are considered, lies in elucidating the fundamental nature of these plans. Specifically, the present findings indicate that the brain forms labile motor plans for sequentially arising action options and that these plans can be flexibly updated to either share parameters with old (proactive influence) or newly formed (retroactive influence) motor plans. In this regard, the current task affords us some particularly unique insights into the mechanisms that support action reprogramming. Previous studies investigating this topic have tended to focus on cases in which individuals must discard or cancel a prepared action plan in favor of a new, alternative action (Buch et al. 2010; Hartwigsen et al. 2012; Nashed et al. 2014; Neubert et al. 2010; Verbruggen et al. 2010). Typically, in these paradigms, immediately before the execution of an action plan, the target or action required is switched on the participants, and they must quickly reprogram a new, corresponding movement. Here, however, we focused on a much different question: can a recently formed action plan for the first target in a series be revisited and reprogrammed based on the parameters of an action plan generated for a newer, second target? Even though such reprogramming is not actually required by our task (as the originally prepared movement would equally satisfy the demands of the task), we provide evidence that the blueprints of newer plans can revise those that had been developed previously for an earlier potential target.
Why should the motor system cooptimize action plans for competing targets presented sequentially over time? One possible reason, suggested by both the current results and those from our previous work (Gallivan et al. 2015), is that the selection of a wrist orientation for the ambiguous target that is compatible with the competing unambiguous target may lead to an RT advantage. That is, by exploiting redundancies in the actions afforded by competing targets and preparing competing movements that share common kinematic components, each action plan might be launched more quickly. According to a recently proposed framework of motor planning (Wong et al. 2015), RT benefits may either stem from a facilitation of processes related to deciding on the goal of the action (e.g., perceiving the target objects and choosing between them) or alternatively, a facilitation of decision processes related to specifying the features of movement (e.g., preparing the kinematics of how the motor goal will be achieved). In our task, the RT benefit observed when individuals select the compatible wrist posture presumably reflects an advantage in specifying the final kinematics of the movement, as the perceptual requirements associated with both compatible and incompatible trials (e.g., target identification and cuing, application of task rules, etc.) are identical. Nevertheless, we recognize that this RT advantage, documented both here and in our previous work (group means of 6 and 8 ms, respectively), is very small and thus unlikely to be much of a factor in driving cooptimization behavior.
Another, perhaps more plausible reason for the observed cooptimization behavior is that selection of the compatible wrist orientation might optimize the deployment of WM resources. WM operations, which support the active maintenance and manipulation of behaviorally relevant information over short temporal intervals, such as the time between each of the target presentations and movement execution in the current study, are expected to play an important role in our task. First, our RT results indicate that a movement plan (i.e., information about how to move) is formed for a given target immediately after it is presented. Second, the proactive and retroactive effects that we observe indicate that the existing movement plan, which in the case of ambiguous targets, is not uniquely specified by the viewed target, can be revised (i.e., the information about how to move can be manipulated). Together, these findings suggest that in our task, movement plans are being maintained briefly in WM, a view that is consistent with neurophysiological findings [e.g., Cisek and Kalaska (2005)]. Although the role of WM in supporting sensory and/or perceptual processing has been examined thoroughly (Sreenivasan et al. 2014), its role in supporting the processes of motor control, although equally well appreciated (D'Esposito and Postle 2015), remains far less studied. Nevertheless, given the limited capacity of WM (Luck and Vogel 2013), just as it has been shown that sensory representations should prioritize task-relevant over task-irrelevant information (Vogel et al. 2005), so too should the sensorimotor system prioritize certain types of motor information over others. With regard to the present study, this might mean prioritizing the representation of certain potential movements over others and allocating more WM resources to encoding movements that might be more optimal for achieving the goals of the task. For example, the reprogramming of initially prepared movements for the first target in a series, so as to be compatible with some of the kinematics (wrist orientation) required for the second, newer target in a series, would necessarily constrain the range of hand postures that must be concurrently held in WM in advance of either the first or second target being selected.
Notably, our findings demonstrate that participants did not always cooptimize for each trial. At the level of sensorimotor processing, this may indicate some level of noise, either in initially encoding the visual orientation of the target (i.e., noise in perception) or in mapping that orientation onto the movement(s) afforded by that particular target (i.e., noise in sensorimotor transformation). In addition to these factors, the inherent noise in competitive interactions between competing plans in most models (Cisek 2006; Wang 2008) would make consistent cooptimization from trial to trial that much more unlikely. Nevertheless, our pattern of effects clearly demonstrates that participants did not use an explicit cognitive strategy during the task. Indeed, had they done so, we would have expected that participants always select the compatible wrist posture (i.e., always select pronation for the cued ambiguous target when the noncued target required pronation and always select supination for the cued ambiguous target when the noncued target required supination). Together, this suggests that the cooptimization of action plans, when observed, arises from automatic sensorimotor processes occurring outside of individuals' conscious awareness.
To summarize, here, we show, using a sequential target presentation reaching task, that participants appear to specify a movement plan for each potential target once it appears and that previously formed plans (for the first potential target) can be reprogrammed based on the encoding of more newly formed plans (for the second competing potential target). In certain respects, this latter finding resonates with the phenomenon of retroactive interference, whereby newly learned information impedes the recall of previously learned information; the exception here is we putatively describe a case of retroactive facilitation, whereby newly formed plans adaptively modify previously formed independent plans, thereby cooptimizing movements across multiple potential targets.
GRANTS
Support for this research is provided by the Natural Sciences and Engineering Research Council of Canada, The Wellcome Trust, the Human Frontiers Science Program, the Royal Society, and the Canadian Institutes of Health Research Postdoctoral Fellowships Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P.G., C.S.C., D.M.W., and J.R.F. conception and design of research; N.A.R.B. performed experiments; J.P.G., D.M.W., and J.R.F. analyzed data; J.P.G., D.M.W., and J.R.F. interpreted results of experiments; J.P.G., D.M.W., and J.R.F. prepared figures; J.P.G., D.M.W., and J.R.F. drafted manuscript; J.P.G., C.S.C., D.M.W., and J.R.F. edited and revised manuscript; J.P.G., C.S.C., D.M.W., and J.R.F. approved final version of manuscript.
REFERENCES
- Baumann MA, Fluet MC, Scherberger H. Context-specific grasp movement representation in the macaque anterior intraparietal area. J Neurosci 29: 6436–6448, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Arnold K. Motor control prior to movement onset: preparatory mechanisms for pointing at visual targets. Exp Brain Res 90: 209–216, 1992. [DOI] [PubMed] [Google Scholar]
- Buc Calderon C, Verguts T, Gevers W. Losing the boundary: cognition biases action well after action selection. J Exp Psychol Gen 144: 737–743, 2015. [DOI] [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci 30: 1395–1401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CS, Gallivan JP, Wong JD, Wispinski NJ, Enns JT. The snooze of lose: rapid reaching reveals that losses are processed more slowly than gains. J Exp Psychol Gen 144: 844–863, 2015. [DOI] [PubMed] [Google Scholar]
- Chapman CS, Gallivan JP, Wood DK, Milne JL, Ansari D, Culham J, Goodale MA. Counting on the motor system: rapid action planning reveals the format- and magnitude-dependent extraction of numerical quantity. J Vis 14: 1–19, 2014. [DOI] [PubMed] [Google Scholar]
- Chapman CS, Gallivan JP, Wood DK, Milne JL, Culham JC, Goodale MA. Reaching for the unknown: multiple target encoding and real-time decision-making in a rapid reach task. Cognition 116: 168–176, 2010a. [DOI] [PubMed] [Google Scholar]
- Chapman CS, Gallivan JP, Wood DK, Milne JL, Culham JC, Goodale MA. Short-term motor plasticity revealed in a visuomotor decision-making task. Behav Brain Res 214: 130–134, 2010b. [DOI] [PubMed] [Google Scholar]
- Christopoulos V, Bonaiuto J, Andersen RA. A biologically plausible computational theory for value integration and action selection in decisions with competing alternatives. PLoS Comput Biol 11: e1004104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362: 1585–1599, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26: 9761–9770, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 3: 801–814, 2005. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010. [DOI] [PubMed] [Google Scholar]
- Cisek P, Pastor-Bernier A. On the challenges and mechanisms of embodied decisions. Philos Trans R Soc Lond B Biol Sci 369: pii: 20130479, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos I, Belanger N, Cisek P. The influence of predicted arm biomechanics on decision making. J Neurophysiol 105: 3022–3033, 2011. [DOI] [PubMed] [Google Scholar]
- Cos I, Duque J, Cisek P. Rapid prediction of biomechanical costs during action decisions. J Neurophysiol 112: 1256–1266, 2014. [DOI] [PubMed] [Google Scholar]
- Cos I, Medleg F, Cisek P. The modulatory influence of end-point controllability on decisions between actions. J Neurophysiol 108: 1764–1780, 2012. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol 66: 115–142, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Miller GF. Transformations between visual and kinesthetic coordinate systems in reaches to remembered object locations and orientations. Exp Brain Res 93: 534–547, 1993. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Barton KS, Chapman CS, Wolpert DM, Flanagan JR. Action plan co-optimization reveals the parallel encoding of competing reach movements. Nat Commun 6: 7428, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS. Three-dimensional reach trajectories as a probe of real-time decision-making between multiple competing targets. Front Neurosci 8: 215, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS, Wood DK, Milne JL, Ansari D, Culham JC, Goodale MA. One to four, and nothing more: nonconscious parallel individuation of objects during action planning. Psychol Sci 22: 803–811, 2011. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Logan L, Wolpert DM, Flanagan JR. Parallel specification of competing sensorimotor control policies for alternative action options. Nat Neurosci 19: 320–326, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Favilla M, Ghilardi MF, Gordon J, Bermejo R, Pullman S. Discrete and continuous planning of hand movements and isometric force trajectories. Exp Brain Res 115: 217–233, 1997. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The Ecological Approach to Visual Perception. Boston: Houghton Mifflin, 1979. [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature 349: 154–156, 1991. [DOI] [PubMed] [Google Scholar]
- Gosselin-Kessiby N, Messier J, Kalaska JF. Evidence for automatic on-line adjustments of hand orientation during natural reaching movements to stationary targets. J Neurophysiol 99: 1653–1671, 2008. [DOI] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. Hedging your bets: intermediate movements as optimal behavior in the context of an incomplete decision. PLoS Comput Biol 11: e1004171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Bestmann S, Ward NS, Woerbel S, Mastroeni C, Granert O, Siebner HR. Left dorsal premotor cortex and supramarginal gyrus complement each other during rapid action reprogramming. J Neurosci 32: 16162–16171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson TE, Maloney LT, Landy MS. Movement planning with probabilistic target information. J Neurophysiol 98: 3034–3046, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira S, Yang T, Shadlen MN. A neural implementation of Wald's sequential probability ratio test. Neuron 85: 861–873, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes C, Westendorff S, Chakrabarti S, Gail A. Choosing goals, not rules: deciding among rule-based action plans. Neuron 70: 536–548, 2011. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn Sci 17: 391–400, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meegan DV, Tipper SP. Reaching into cluttered visual environments: spatial and temporal influences of distracting objects. Q J Exp Psychol A 51: 225–249, 1998. [DOI] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Rapid online selection between multiple motor plans. J Neurosci 34: 1769–1780, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci USA 107: 13240–13245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain 111: 643–674, 1988. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Vaughan J, Barnes HJ, Jorgensen MJ. Time course of movement planning: selection of handgrips for object manipulation. J Exp Psychol Learn Mem Cogn 18: 1058–1073, 1992. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. J Vis 6: 982–995, 2006. [DOI] [PubMed] [Google Scholar]
- Sreenivasan KK, Curtis CE, D'Esposito M. Revisiting the role of persistent neural activity during working memory. Trends Cogn Sci 18: 82–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BM, Baugh LA, Gallivan JP, Flanagan JR. Simultaneous encoding of the direction and orientation of potential targets during reach planning: evidence of multiple competing reach plans. J Neurophysiol 110: 807–816, 2013. [DOI] [PubMed] [Google Scholar]
- Stewart BM, Gallivan JP, Baugh LA, Flanagan JR. Motor, not visual, encoding of potential reach targets. Curr Biol 24: R953–R954, 2014. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Howard LA, Houghton G. Action-based mechanisms of attention. Philos Trans R Soc Lond B Biol Sci 353: 1385–1393, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP, Howard LA, Houghton G. Behavioral consequences of selection from population codes. In: Attention and Performance, edited by Monsell S and Driver J. Cambridge, MA: MIT Press, 2000, p. 223–245. [Google Scholar]
- Tipper SP, Howard LA, Jackson SR. Selective reaching to grasp: evidence for distractor interference effects. Vis Cogn 4: 1–38, 1997. [Google Scholar]
- Torres EB, Zipser D. Simultaneous control of hand displacements and rotations in orientation-matching experiments. J Appl Physiol 96: 1978–1987, 2004. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R. On the relations between seen objects and components of potential actions. J Exp Psychol 24: 830–846, 1998. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci USA 107: 13966–13971, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature 438: 500–503, 2005. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Decision making in recurrent neuronal circuits. Neuron 60: 215–234, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AL, Haith AM, Krakauer JW. Motor planning. Neuroscientist 21: 385–398, 2015. [DOI] [PubMed] [Google Scholar]
- Wood DK, Gallivan JP, Chapman CS, Milne JL, Culham JC, Goodale MA. Visual salience dominates early visuomotor competition in reaching behavior. J Vis 11: pii: 16, 2011. [DOI] [PubMed] [Google Scholar]
- Yang T, Shadlen MN. Probabilistic reasoning by neurons. Nature 447: 1075–1080, 2007. [DOI] [PubMed] [Google Scholar]