In this study, we have applied photostimulation-based approaches to local bed nucleus of the stria terminalis circuit mapping of defined neuronal types. We performed physiological and anatomical calibrations for laser scanning photostimulation via glutamate uncaging or channelrhodopsin-2-mediated optogenetic stimulation, to establish experimental conditions allowing for the accuracy of synaptic input mapping analysis. This work will help provide a template for other investigators who wish to apply these techniques to functional mapping of nuclei and other nonlayered brain structures.
Keywords: extended amygdala, synaptic connections, glutamatergic, GABAergic, glutamate uncaging, optogenetic stimulation
Abstract
The bed nucleus of the stria terminalis (BNST) is a key component of the extended amygdala and has been implicated in anxiety and addiction. As individual neurons function within neural circuits, it is important to understand local microcircuits and larger network connections of identified neuronal types and understand how maladaptive changes in the BNST neural networks are induced by stress and drug abuse. However, due to limitations of classic anatomical and physiological methods, the local circuit organization of synaptic inputs to specific BNST neuron types is not well understood. In this study, we report on the application of high-resolution and cell-type-specific photostimulation methodology developed in our laboratory to local circuit mapping in the BNST. Under calibrated experimental conditions, laser photostimulation via glutamate uncaging or channelrhodopsin-2 photoactivation evokes spiking of BNST neurons perisomatically, without activating spikes from axons of passage or distal dendrites. Whole cell recordings, combined with spatially restricted photostimulation of presynaptic neurons at many different locations over a large region, allow high-resolution mapping of presynaptic input sources to single recorded neurons in the BNST. We constructed maps of synaptic inputs impinging onto corticotrophin-releasing hormone-expressing (CRH+) BNST neurons in the dorsolateral BNST and found that the CRH+ neurons receive predominant local inhibitory synaptic connections with very weak excitatory connections. Through cell-type-specific optogenetic stimulation mapping, we generated maps of somatostatin-expressing neuron-specific inhibitory inputs to BNST neurons. Taken together, the photostimulation-based techniques offer us powerful tools for determining the functional organization of local circuits of specific BNST neuron types.
NEW & NOTEWORTHY
In this study, we have applied photostimulation-based approaches to local bed nucleus of the stria terminalis circuit mapping of defined neuronal types. We performed physiological and anatomical calibrations for laser scanning photostimulation via glutamate uncaging or channelrhodopsin-2-mediated optogenetic stimulation, to establish experimental conditions allowing for the accuracy of synaptic input mapping analysis. This work will help provide a template for other investigators who wish to apply these techniques to functional mapping of nuclei and other nonlayered brain structures.
the bed nucleus of the stria terminalis (BNST) is a relatively small structure in the basal forebrain, but it is a major component of the extended amygdala which shares embryological origin with the central nucleus of the amygdala. The BNST coordinates neuroendocrine, autonomic and somatomotor responses to anxiety and emotional aspects of stressful events, and it is identified as a key node in neural networks relevant to pathological anxiety and addiction. This area has received increased attention in light of recent physiological and functional studies (Daniel and Rainnie 2016; Deisseroth 2014; Jennings et al. 2013; Johansen 2013; Kim et al. 2013).
Based on gross anatomical and cytoarchitecture features, the BNST contains a collection of nuclei, and they can be generally divided into anterior and posterior groups by the presence of anterior commissure (Wood and Swann 2005). A number of studies have focused on the anterior dorsal BNST (adBNST) for robust identification and delineation of this region. Although specific cell types and the neurochemical makeup of the adBNST has not been thoroughly explored, it has been known that GABAergic neurons make up a predominant portion of the general makeup of adBNST, while glutamatergic neurons only account for a minority of the overall population (Nguyen et al. 2015). This is contrary to the makeup of excitatory and inhibitory neurons in the cerebral cortex in general. In addition, there are many BNST neurons expressing neuropeptides, such as corticotrophin-releasing hormone (CRH), somatostatin (SOM) and substance P (Dabrowska et al. 2011, 2013), which are critically related to emotional control of behavior (Lee and Davis 1997; Magableh and Lundy 2014; Silberman and Winder 2013).
The physiology and function of the BNST subregions or nuclei are likely determined by specific neuronal constituents and their differential circuit connections. However, most existing knowledge about intrinsic and extrinsic BNST circuit connections is derived from classic anatomical studies (Choi et al. 2007; Dong et al. 2001; Dong and Swanson 2004; McDonald 1983; Walker et al. 2003) which tend to have low spatial or cell type resolution and do not reveal functional synaptic connections. Even as in the cortex, testing connections with paired or multiple intracellular recordings would be limited to highly localized microcircuits. Due to limitations of these methods, many aspects of the circuit organization of synaptic input to specific BNST neuron types are not well understood.
In this study, we report on our application of high-resolution and cell-type specific photostimulation-based methods developed in our laboratory (Kuhlman et al. 2013; San Antonio et al. 2014; Shi et al. 2010, 2014; Sun et al. 2014; Xu et al. 2010) to local circuit mapping in the BNST. Specifically, laser scanning photostimulation (LSPS; via glutamate uncaging) combined with whole cell recording allows high-resolution mapping of intra- and inter-subregional distributions of presynaptic input sources to single neurons in the BNST. We were able to construct detailed maps of excitatory and inhibitory synaptic inputs impinging onto CRH-expressing neurons in the adBNST. Furthermore, we complemented glutamate uncaging studies with photoactivation via channelrhodopsin-2 (ChR2; light-activated cationic channel) expression in specific cell types to map presynaptic inputs from specific subset of neurons within the overall participating circuits. Under calibrated conditions, we generated maps of SOM-specific inhibitory inputs to single BNST neurons through optogenetic stimulation mapping. The application of new mapping approaches is important for understanding BNST circuit organization and function. As our results demonstrate, the new mapping experiments have allowed us to uncover local BNST circuit connections and relate local inhibitory circuit control to larger neural network operations.
METHODS and MATERIALS
All animals were handled, and experiments were conducted in accordance with procedures approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. To genetically label CRH-expressing neurons, CRH-IRES-Cre knock-in mice (Taniguchi et al. 2011) (Jackson Laboratories, stock no. 012704) were crossed with Ai9 tdTomato reporter knock-in mice (Madisen et al. 2010) (Jackson Laboratories, stock no. 007909). All experimental mice were hemizygous for both transgenes (CRH-Cre; Ai9) at the ages of postnatal 4–8 wk. Similarly, the SOM-IRES-Cre mice (Taniguchi et al. 2011) (Jackson Laboratories, stock no. 013044) were crossed to the Ai32 mouse line (Madisen et al. 2012) (Jackson Laboratories, stock no. 024109) that has a conditional allele of Rosa-CAG-LSL-ChR2(H134R)-EYFP-WPRE, to drive targeted ChR2/EYFP fusion protein expression in SOM+ BNST cells (SOM-Cre; Ai32). The SOM-Cre; Ai32 mice used for recordings were about 12 wk old, allowing for high ChR2 expression in the BNST (e.g., see Fig. 4B).
Fig. 4.
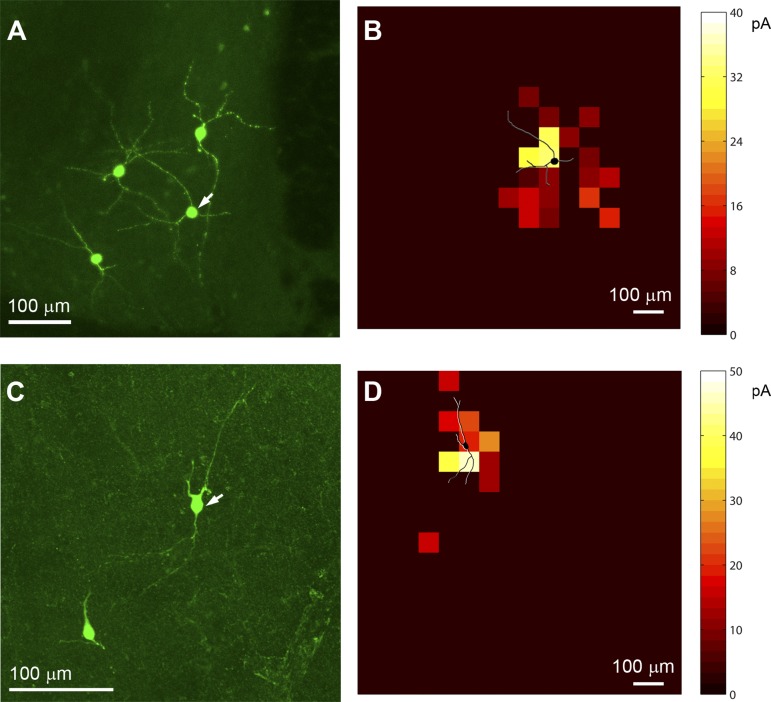
Preliminary investigation of the spatial relationship between dendritic fields and input profiles. A: confocal micrograph showing post hoc intracellular biocytin staining of the recorded CRH+ BNST neurons. Part of the image in A is shown in Fig. 1D, with an adjusted orientation. B: the overlay of the dendritic morphology (shown in black with white shading) and color-coded inhibitory input profile of the recorded CRH+ neuron (indicated by the white arrow in A). Average integrated IPSC strength per map location is color coded according to the amplitude. C and D are similarly formatted as A and B, respectively, for a different neuron.
Electrophysiology and LSPS.
For electrophysiological studies, the animals were deeply anesthetized and rapidly decapitated, and their brains were removed to make living BNST slices for whole cell recordings and circuit mapping experiments. In most animals, coronal sections of 400 μm were cut with a vibratome (VT1200S, Leica Systems) in sucrose containing artificial cerebrospinal fluid (ACSF) (in mM: 85 NaCl, 75 sucrose, 2.5 KCl, 25 glucose, 1.25 NaH2PO4, 4 MgCl2, 0.5 CaCl2, and 24 NaHCO3). Slices were incubated for at least 30 min in sucrose containing ACSF at 32°C before being transferred into slice recording chambers with normal ACSF (in mM: 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, and 10 glucose). For making BNST slices from the older SOM-Cre; Ai32 mice, we adopted an improved protocol described by Ting et al. (2014). The slices were prepared in a cutting solution (containing in mM: 92 NaCl, 2.5 KCl, 30 NaHCO3, 0.5 CaCl2·2H2O, 10 MgSO4·7H2O, 1.2 NaH2PO4, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate), and were incubated at 32°C in the N-methyl-d-glucamine (NMDG)-HEPES recovery solution (containing in mM: 93 NMDG, 93 HCl, 2.5 KCl, 30 NaHCO3, 0.5 CaCl2·2H2O, 10 MgSO4·7H2O, 1.2 NaH2PO4, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate) for 12–15 min. Throughout the cutting, incubation, and recording, the solutions were continuously supplied with 95% O2/5% CO2.
Our overall system of electrophysiological recording, photostimulation, and imaging was described previously (Xu 2011; Xu et al. 2010). Electrophysiological recordings and photostimulation were similarly performed as in references (Kuhlman et al. 2013; Shi et al. 2010; Xu et al. 2010). Whole cell recordings were performed under a differential interference contrast/fluorescent Olympus microscope (BX51WI). CRH+ neurons were targeted based on the red fluorescent protein expression in the slices of CRH-Cre; Ai9 mice. Oxygenated ACSF at room temperature was perfused into the slice recording chamber through a custom-designed flow system driven by pressurized 95% O2/5% CO2 (3 psi) at roughly 2 ml/min. Slices were examined under a ×4 objective for proper targeting of BNST neurons. To target whole cell recordings, cells were visualized at high magnification (×60 objective, 0.9 numerical aperture; LUMPlanFl/IR, Olympus). Cell bodies of recorded neurons were at least 50 μm below the surface of the slice. Patch pipettes (4–6 MΩ resistance) made of borosilicate glass were filled with an internal solution containing (in mM) 126 potassium-gluconate, 4 KCl, 10 HEPES, 4 ATP-Mg, 0.3 GTP-Na, and 10 phosphocreatine (pH 7.2, 300 mosM). Electrodes also contained 0.1% biocytin for post hoc cell labeling and further morphological identification. Once stable whole cell recordings were achieved with good access resistance (usually <30 MΩ), basic electrophysiological properties were examined through hyperpolarizing and depolarizing current injections. For the recordings in which inhibitory postsynaptic currents (IPSCs) were measured, potassium was replaced with cesium. Electrophysiological data were acquired with a Multiclamp 700B amplifier (Molecular Devices), data acquisition boards (models PCI MIO 16E-4 and 6713, National Instruments), and custom modified version of Ephus software (Suter et al. 2010). Data were digitized at 10 kHz.
LSPS was performed through a ×4 objective lens, with a laser spot diameter between 50 and 100 μm at the slice level. Stock solution of MNI-caged l-glutamate (Tocris Bioscience) was added to 20 ml of ACSF for a concentration of 0.2 mM caged glutamate. The BNST slice image, acquired through the ×4 objective, was visualized using a high-resolution digital charge-coupled device camera, and this image, in turn, was used to guide and register photostimulation sites. Laser flashes (1.5-ms duration, 15 mW) from a 355-nm UV laser (DPSS Lasers, Santa Clara, CA) were delivered to the sample with an interstimulation interval of 1.2 s, controlled via an electrooptical modulator and a mechanical shutter. Synaptic currents in patched neurons were detected under voltage clamp. By systematically surveying synaptic inputs from hundreds of different sites across a large BNST region, aggregate synaptic input maps were generated for individual neurons (see below). Because glutamate uncaging agnostically activates both excitatory and inhibitory neurons, we empirically determined the excitatory and inhibitory reversal potentials in BNST cells by glutamate uncaging at perisomatic regions while testing holding potentials to properly isolate excitatory postsynaptic currents (EPSCs) and IPSCs. Using the K+ internal solution, we voltage clamped the targeted BNST cells at −70 mV to determine LSPS-evoked EPSCs. We held the cells at +5 mV to measure IPSCs using the cesium-containing internal solution.
After all physiological assays had been completed, the brain slices were fixed in 4% paraformaldehyde in phosphate-buffered saline overnight and transferred to 30% sucrose solution in phosphate-buffered saline. The slices were stained against biocytin with 1:500 Alexa fluor 488-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA) to show the morphology of the recorded cells. Neuron reconstructions were computer-assisted and based on stacks of optical sections acquired by a Zeiss LSM780 confocal microscope (Carl Zeiss).
Local circuit input analysis of glutamate uncaging.
Photostimulation data analysis has been described in detail (Shi et al. 2010). Photostimulation induces two forms of excitatory responses: 1) those that result from direct activation of the recorded neuron's glutamate receptors; and 2) synaptically mediated responses (EPSCs) resulting from the suprathreshold activation of presynaptic excitatory neurons. Responses that occur within 10 ms of laser pulse onset were considered direct; these responses exhibited a distinct shape and occurred immediately after glutamate uncaging. Synaptic currents with such short latencies are not possible because they would have to occur before the generation of action potentials in photostimulated neurons. Therefore, direct responses need to be excluded from local synaptic input analysis. However, at some locations, synaptic responses were overriding on the relatively small direct responses and were identified and included in synaptic input analysis. LSPS-evoked EPSCs or IPSCs were quantified across the 16 × 16 mapping grid for each cell. As for individual map construction, input measurements from different stimulation sites were assigned to their corresponding anatomical locations; color-coded maps of average input amplitudes were plotted to illustrate overall input pattern to the recorded cell. The input amplitude/strength of each stimulation site was measured by the sum of individual EPSCs or IPSCs from each photostimulation site with the baseline spontaneous response subtracted and then normalized by the analysis window of 150 ms after photostimulation. This average integrated value was expressed in picoamperes (pA) for the analysis window. To quantitatively compare excitatory and inhibitory input strength/connections, we summed and measured the total ESPC and IPSC inputs across all map locations for the recorded individual cells.
Optogenetic stimulation-assisted circuit mapping.
Using the Cre-directed ChR2 expression in SOM-expressing BNST neurons, we were able to map circuits between presynaptic neurons, defined by ChR2 expression, and postsynaptic neurons, defined by targeted patching. The photostimulation hardware and software system was identical, except for the blue laser (Crystalaser, Reno, NV) to be delivered to the sample. Spatial maps of SOM connectivity strength to each patched BNST neuron were derived by systematically stimulating ChR2-expressing SOM cells at a mapping grid of 256 different sites arranged in a 16 × 16 matrix spanning the adBNST (see Fig. 5). Spatially restricted optogenetic activation of ChR2-expressing SOM cells was accomplished under calibrated conditions (see below) using a 473-nm blue laser (30 mW, 0.01–2.5 ms, laser spot diameter, ∼50 μm). Each stimulation consisted of three repeated laser flashes with an intertrial interval of 1.2 s. During optogenetic stimulation experiments, the ionotropic glutamate receptor antagonists (10 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 5 μM CCP) was added to the bath solution to block excitatory synaptic input (GABAergic transmission is unaffected) and avoid any potential disinhibition effects. Because interneurons can also be connected by electrical synapses, we blocked gap junctions using 100 μM carbenoxolone. Whole cell voltage-clamp recordings were made from the recorded BNST neurons to measure photoactivation-evoked IPSCs at +5 mV in voltage clamp mode with the cesium-containing internal solution.
Fig. 5.
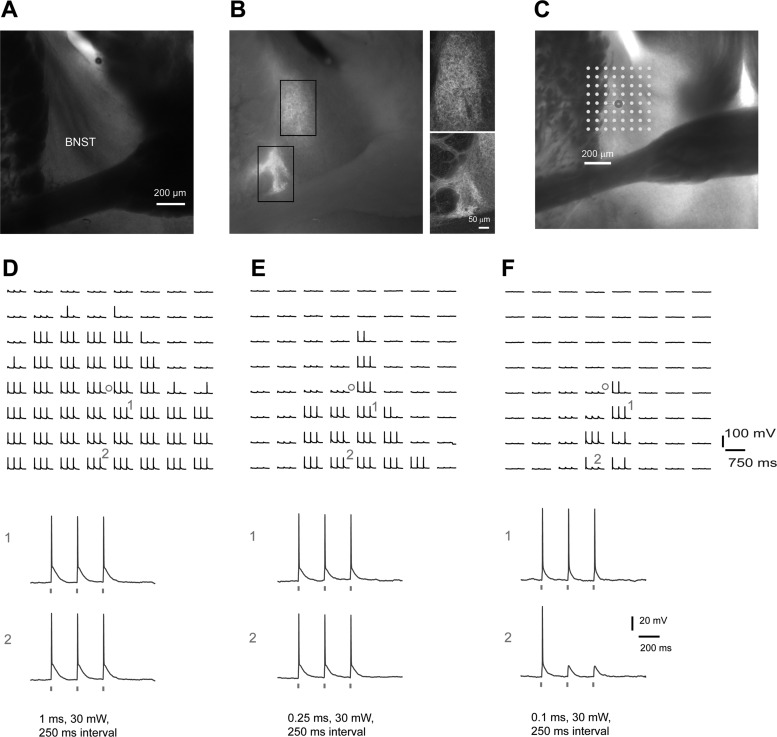
Robustness of spike generation by optogenetic stimulation depends on laser power and perisomatic proximity. A and B: bright-field and epifluorescent images of a SOM-Cre; Ai32 mouse brain slice show that ChR2 expression is concentrated in the dorsolateral BNST, including the oval BNST. The smaller images by the main panel in B are confocal images to show more details in the rectangular marked regions in the epifluorescent image. C: the SOM-Cre; Ai32 BNST slice image is superimposed with an array of laser photostimulation sites (8 × 8 dots spaced at 65 μm × 65 μm); the BNST neuron (cell body location indicated by a small circle) was recorded in whole cell current clamp mode. D–F show blue laser (473 nm) evoked spiking profiles of the same recorded neurons in response to different laser illumination durations (1, 0.25, and 0.1 ms at 30 mW, respectively). The ChR2-mediated photoactivation responses at the labeled sites (1 and 2) are shown separately below. Note that evoked spikes by longer stimulation durations do not restrict to perisomatic regions. In addition, more distal regions from the cell body tend to respond less robustly to repeated laser flashes. The results shown in D suggest a lack of spatially specific activation due to high laser power (total energy). Thus calibration is critical for interpreting optogenetic stimulation mapping of local circuit inputs.
Under calibrated conditions (see below), we found that photoactivation-evoked spikes from ChR2-expressing cells can be restricted to perisomatic regions. For the accuracy of synaptic input analysis, we used a repeated laser flash stimulation protocol, and “hits” were only scored when the recorded neuron responded to all three laser pulses in any given mapping location. IPSC peaks were detected within the window of between 3 and 50 ms after each laser flash, with an empirically determined threshold based on background spontaneous IPSCs (see Fig. 6E). The average IPSC peak amplitude/strength (in pA) of each stimulation site was measured in the analysis window.
Fig. 6.
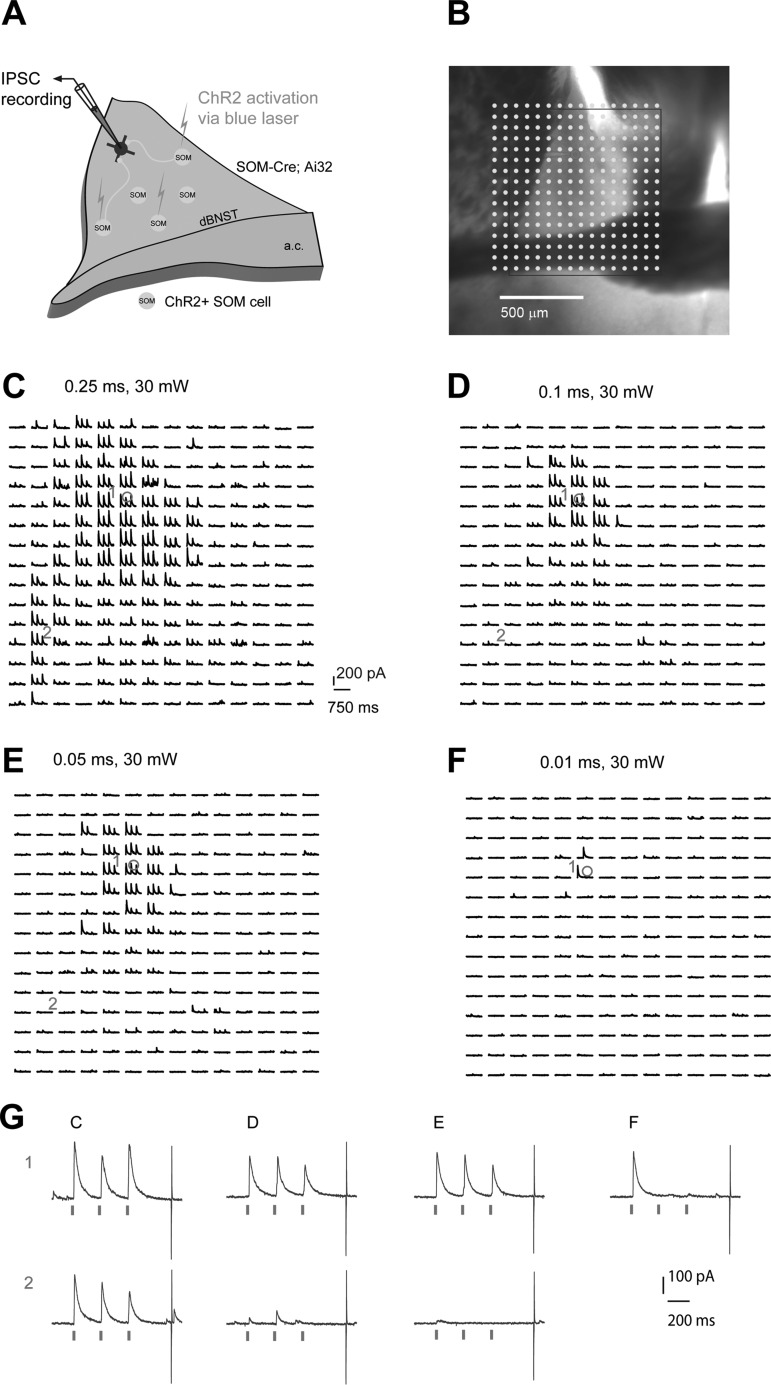
Cell-type-specific optogenetic stimulation mapping at different laser powers. A: schematic of mapping SOM+ inhibitory IPSCs to individually recorded BNST neurons in local circuits in SOM-Cre; Ai32 mouse slices. B: a representative mapping grid with ChR2 photoactivation sites (dots) is superimposed to the slice image. C–F: ChR2-evoked IPSC responses from the rectangular region in B from the recorded BNST neuron are shown with different laser illumination durations (0.25, 0.1, 0.05 and 0.01 ms at 30 mW, respectively). G: example IPSC responses recorded from the neuron from C–F are shown. The repeated blue laser flashes (represented by three ticks beneath the traces in G) were applied to each map location.
Statistical analyses.
All data are reported as means ± SE of the mean. When comparing two independent groups, normally distributed data were analyzed using a Student's t-test. In the case data were not normally distributed, a Mann-Whitney U-test was used. A P value (≤0.05) was considered statistically significant.
RESULTS
LSPS mapping of excitatory and inhibitory synaptic circuits to defined BNST neurons.
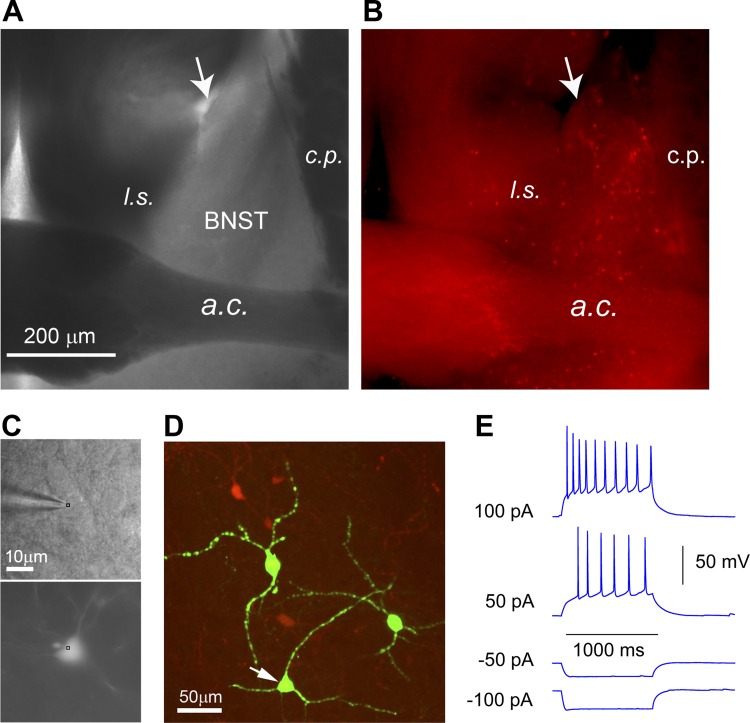
LSPS-based mapping techniques have been widely applied for analyzing cortical circuits (Dantzker and Callaway 2000; Kuhlman et al. 2013; Oviedo et al. 2010; Shepherd et al. 2003; Sun et al. 2014; Weiler et al. 2008; Xu and Callaway 2009). Here we applied this approach for local BNST circuit mapping. CRH expressing neurons are known to be important for BNST function, but their local functional circuit connections have not been investigated. Targeted recordings of CRH+ cell types were facilitated by use of CRH-Cre; Ai9 mice, which express red fluorescent proteins (tdTomato) in CRH-expressing neurons (Fig. 1, A and B), thus enabling consistent sampling of the targeted cell types. CRH+ neurons in the BNST have been studied and were reported to have heterogeneous morphological and electrophysiological phenotypes (Larriva-Sahd 2004, 2006; Silberman et al. 2013). Our post hoc intracellular biocytin staining of the recorded CRH+ BNST neurons showed diverse cell morphology (Fig. 1, C and D). We found most of the CRH+ cells recorded in the adBNST showed an electrophysiology phenotype exemplified by regular action potential spiking evoked by depolarizing current injection (Fig. 1E).
Fig. 1.
Genetic label guided recordings of CRH+ neurons in the BNST slices. To genetically label CRH-expressing neurons, CRH-Cre mice are crossed to the Ai9 tdTomato reporter mice to produce CRH-Cre; Ai9 mice, which express red fluorescent proteins (tdTomato) in CRH-expressing neurons. A and B: bright-field (A) and epi-fluorescent (B) images of a CRH-Cre; Ai9 mouse brain slice show the distribution of CRH-expressing neurons in the BNST. The anterior dorsal BNST (adBNST) is anatomically enclosed by the lateral ventricle (see the arrow), lateral septum (l.s.), caudate putamen (c.p.), and anterior commissure (a.c.). C: CRH+ neurons expressing tdTomato fluorescent proteins (bottom) are targeted for recordings (top) under a high-power objective. D: post hoc visualization of recorded neuronal morphology via intracellular biocytin staining (green). The arrowhead points to the recorded CRH neuron shown in C. E: most CRH+ neurons recorded from anterior dorsolateral BNST show a regular action potential firing phenotype.
Our laser scanning system included an X-Y pair of scan mirrors, the scan lens, the tube lens, and the objective lens (Xu et al. 2010). The mirrors delivered the laser beam through a scan lens; the beam entered the microscope and was focused by a custom-made UV-transmitting tube lens. The beam under-filled the back aperture of the microscope objective to provide a more columnar (as opposed to conical) illuminating beam, keeping the mapping as two-dimensional as possible by reducing the axial resolution. Various laser stimulation positions could be achieved through galvanometer-driven X-Y scanning mirrors, as the mirrors and the back aperture of the objective were in conjugate planes, translating mirror positions into different scanning locations at the objective lens focal plane. Given that the UV laser spot is about 50–100 μm at each location, it covers the surface area of between 7,850 μm2 and 31,400 μm2. The average neuron densities across dorsal BNST subregions are estimated to be 165.9 cells/mm2 (Nguyen et al. 2015). Thus each laser stimulation is estimated to stimulate, on average, 3.25 BNST neurons at one surface plane. Based on empirical measurements using caged fluorescein gels, the laser penetrates at least 100 μm into the slice. This leads to an estimate of photostimulating 13 BNST neurons per laser stimulation. This estimate may be on the low side, because average neuron densities vary across the BNST.
For our mapping experiments, a standard stimulus grid (16 × 16 stimulation sites, 65 μm2 spacing) was used to cover the adBNST. The LSPS site spacing was empirically determined to capture the smallest predicted distance in which photostimulation differentially activates adjacent neurons. With an interstimulation interval of 1.2 s, laser stimulation via glutamate uncaging was delivered sequentially in a nonraster, nonrandom sequence, following a “shifting-X” pattern designed to avoid revisiting the vicinity of recently stimulated sites (Shepherd et al. 2003). The scanning approach was very efficient, as it took about 5 min to map the entire grid of 256 sites. For mapping local excitatory circuit connections to CRH+ neurons, whole cell voltage-clamp recordings were made from the recorded neurons to measure photostimulation-evoked EPSC responses at the holding potential at −70 mV (using the potassium-containing internal solution). Inhibitory functional connections to single CRH+ BNST neurons were assessed by recording IPSCs at the holding potential of +5 mV (using the cesium-containing internal solution) while laser scanning across the mapping grid.
Before proceeding to map local BNST circuit inputs, we performed control experiments to determine the spatial extent of neuronal responses at the stimulation site. Using our experimental conditions (0.2 mM MNI-caged glutamate, Tocris Bioscience; 355-nm UV laser 15 mW, 1.5 ms), caged glutamate was activated in a spatially restricted region of the brain slice by UV photolysis; only neurons located within <100 μm of the site of photostimulation fired action potentials, occurring within about 10–150 ms post-photostimulation (Fig. 2). The spatial precision/resolution of photostimulation was assessed by the photostimulation-evoked spiking distance between the recorded neuron and the stimulation site. Overall, the spatial resolution was 80 ± 9 μm (n = 10 cells). It also can be inferred that evoked synaptic currents reflect direct connections onto the recorded cell, from the cells at or near the photostimulation sites in different BNST subregions. The excitability profiles further indicate that spiking occurs only upon direct stimulation, and not through excitation of synaptically coupled neurons. Taken together, these data validate the use of LSPS for mapping direct local BNST circuit connections.
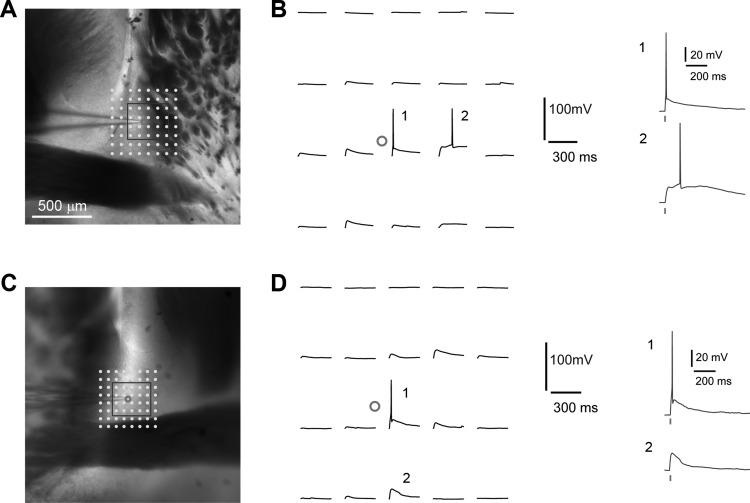
Fig. 2.
Photostimulation-evoked excitation profiling of BNST neurons demonstrates spatial precision of LSPS. A and B, and C and D, show two example excitation profiles of BNST neurons. The spatial resolution of LSPS-evoked excitability of BNST neurons was determined by measuring the LSPS-evoked spike distance relative to soma location. The BNST neurons (cell bodies indicated by small circles) were recorded in whole cell current clamp mode, and LSPS was delivered in a mapping grid (8 × 8, 75 μm2 and 65 μm2 for A and C, respectively) centered around the cell soma. LSPS-evoked action potentials or subthreshold depolarization at the labeled sites (1 and 2) are separately shown by B and D. Note the relative delay in B-2 vs. B-1, which suggests dendritic stimulation. The tick indicates laser photostimulation (1.5 ms, 15 mW).
As illustrated in Fig. 3, the LSPS approach involves first recording from a single neuron, then sequentially stimulating at other sites via uncaging of caged glutamate to generate action potentials from neurons in those sites; recording from the potential postsynaptic neuron allows one to determine whether there is actual synaptic input from that particular site. Maps of either excitatory or inhibitory synaptic input to the targeted neuron are generated by scanning the laser beam to stimulate hundreds of potential presynaptic sites in a stimulation matrix. Thus LSPS enables the construction of detailed maps of synaptic inputs impinging onto specific types of BNST neurons.
Fig. 3.
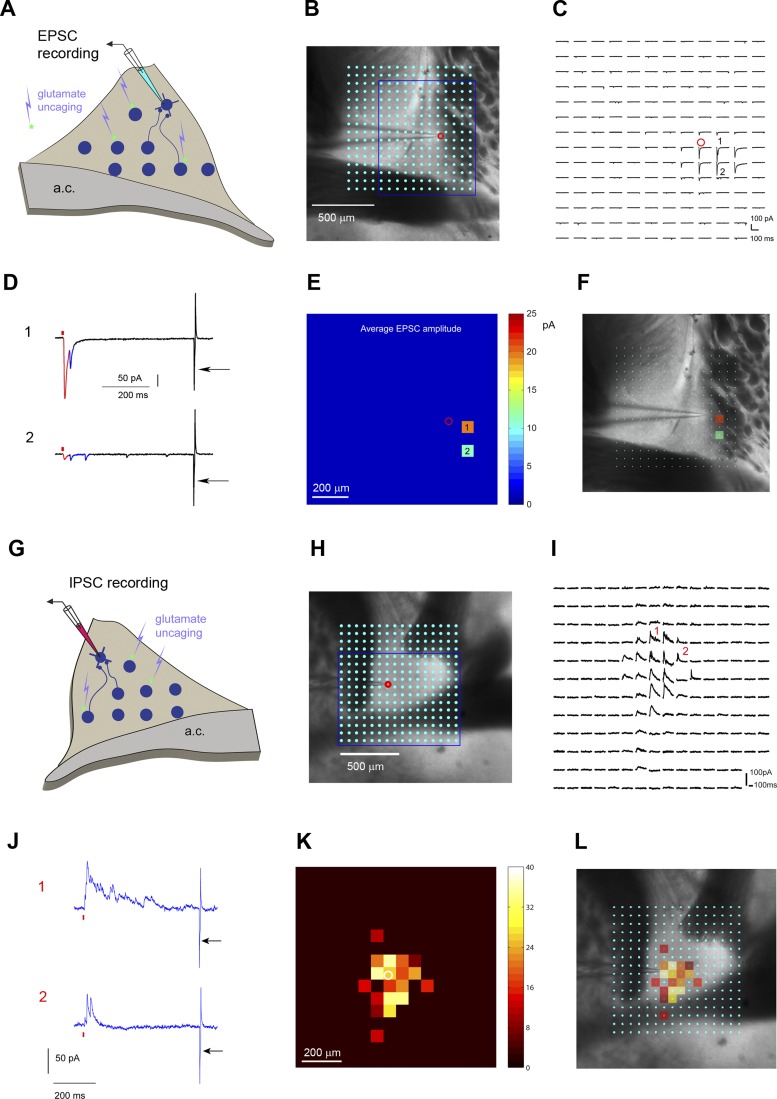
Weak excitatory vs. strong inhibitory local circuit connections to CRH-expressing neurons in the BNST. A–F: mapping excitatory synaptic inputs to an example CRH+ neuron. A: schematic of laser scanning photostimulation (LSPS) mapping of local excitatory synaptic connections to individually recorded CRH+ neurons in the BNST slice. B: the BNST slice image is superimposed with an array of laser photostimulation sites (16 × 16 cyan dots spaced at 65 μm × 65 μm). LSPS maps the broad spatial pattern of synaptic inputs for the neuron of interest and distinguishes direct uncaging responses (e.g., red highlights in example responses 1 and 2 in D) and synaptically mediated responses (blue highlights in example responses 1 and 2) to assess circuit inputs from presynaptic neuronal spiking. The amplitudes and latencies of direct and synaptic input responses systematically differ, thus allowing clear categorization. Empirically, responses within the 10-ms window from laser onset are considered direct and exhibit a distinct shape (shorter rise time) and occur immediately after glutamate uncaging (shorter latency). Synaptic events (i.e., EPSCs, downward deflecting) are measured with the analysis window of >10–160 ms post-photostimulation. See methods and materials for details. C: the raw photostimulation response traces corresponding to the stimulation sites in the encircled map grid (blue rectangle) are plotted for 250 ms, beginning at the photostimulation onset. D: the responses at labeled sites (1 and 2) are expanded and separately shown. The red tick indicates laser photostimulation (1.5 ms, 15 mW). The recoding access resistance was monitored by a current injection response (5 mV, 5 ms) shown by the arrow. E: the quantitative color-coded EPSC input heat map showing the overall sparse spatial distribution and weak strength of excitatory inputs to the recorded cell in the BNST. The map is constructed from the responses as shown in C; input responses per location are quantified in terms of average integrated EPSC strength within the analysis window and color coded according to the amplitude. F: the input response sites are overlaid on the bright-field image to show anatomical position. G–L: mapping inhibitory synaptic inputs to an example CRH+ neuron. They are similarly formatted as in A–F. G: schematic of LSPS mapping of inhibitory synaptic connections to individually recorded CRH+ neurons in the BNST slice. H: the BNST slice image is superimposed with an array of laser photostimulation sites (16 × 16 cyan dots spaced at 65 μm × 65 μm). I: in contrast to sparse and weak excitatory local input, local inhibitory input to the BNST is extensive and robust, as shown by the raw photostimulation response traces corresponding to the stimulation sites shown in H (blue rectangular region). The response traces are plotted for 250 ms, beginning at the photostimulation onset. J: the responses at labeled sites (1 and 2) are expanded and separately shown. The red tick indicates laser photostimulation (1.5 ms, 15 mW). Synaptic events (IPSCs, upward deflecting) are measured with the analysis window of >10–160 ms post-photostimulation. The recoding access resistance was monitored by a current injection response (5 mV, 5 ms) shown by the arrow. K: the quantitative color-coded inhibitory input heat map based on measuring raw photostimulation responses. The scale codes the integrated IPSC strength. L: the input response sites are overlaid on the bright-field image.
Through LSPS mapping, we were able to describe the broad spatial pattern of synaptic inputs to CRH+ neurons. We found that CRH+ BNST neurons had weak and sparse excitatory circuit connections (Fig. 3, A–F). Despite good access resistance, most CRH+ neurons had little or no local EPSC connections. In comparison, local inhibitory connections to CRH+ neurons were strong and extensive, ranging about 500 μm in a vertical dimension (Fig. 3, G–L). The average total EPSC and IPSC inputs to the recorded CRH+ neurons were 92.9 ± 32.1 pA (mean ± SE, n = 7 cells) and 1,182.7 ± 339.7 pA (n = 8 cells), respectively (P < 0.0005, Mann-Whitney U-test). Although not reporting in this study, we also found similar results with non-CRH+ BNST neurons. The extent of strong inhibitory connections is consistent with earlier findings showing that GABAergic neurons make up a predominant portion of the general makeup of adBNST and glutamatergic neurons only account for a minority of the overall population (Daniel and Rainnie 2016; Larriva-Sahd 2006; Nguyen et al. 2015). Given that BSNT receives strong extrinsic excitatory inputs from the amygdala and other brain regions (Daniel and Rainnie 2016; Kim et al. 2013), strong local inhibitory connections can play an important role in modulation of these extrinsic inputs.
Because both the detailed neuronal morphology revealed by biocytin staining and input mapped profiles were available, we explored the spatial relationship between dendritic fields and input profiles by examining their overlays, as illustrated in Fig. 4. Our preliminary investigation indicated a correlation between the input profiles of CRH neurons (n = 5 cells) and their dendritic morphology. The CRH neurons received strong inhibitory inputs around their perisomatic regions, and most of their inhibitory inputs appeared to be within the range of dendritic fields.
Cell-type-specific optogenetic stimulation mapping of BNST synaptic inputs.
The LSPS approach via glutamate uncaging can effectively map the overall inhibitory connections from diverse BNST cell types to targeted cell types, but it is not possible to identify specific contributions of precise inhibitory cell types to the total inhibitory synaptic input to the recorded neurons, because glutamate uncaging activates different types of inhibitory neurons agnostically. Thus, while it is important to examine overall inhibitory inputs to defined BNST cell types using LSPS, we mapped cell-type-specific inhibitory connections using optogenetic methods that genetically target and selectively photoactivate specific subsets of BNST neurons.
We first achieved Cre-directed ChR2 expression and photoactivation of SOM+ BNST cells and established calibration for our experimental interpretations. We focused on SOM+ cells, because SOM+ cells appear abundant in the lateral region of the adBNST (Fig. 5, A and B) and these neurons are associated with emotional behavioral control of the BNST (Magableh and Lundy 2014). Following high ChR2 expression in adult brains of the cross-bred pups (SOM-Cre; Ai32), living brain slices were prepared, and whole cell recordings were performed from ChR2/YFP-expressing SOM+ cells, which exhibited robust photoactivation-evoked spikes to repeated laser flashes (473 nm, 30 mW, ≤1 ms) (Fig. 5, C–F). Although the ability to optically stimulate ChR2-expressing neurons is a revolutionizing technical advancement (Boyden 2011; Boyden et al. 2005), its application in local circuit mapping has not been formally validated (Katzel et al. 2011). There are reasonable concerns that ChR2 photoactivation may not have a sufficient mapping resolution, as action potentials have been seen by the direct activation of axons and distal dendrites in EMX1-Cre; Ai32 excitatory neurons (Madisen et al. 2012) or virally ChR2-transduced neurons, which may express ChR2 at very high levels (Petreanu et al. 2007, 2009).
During our calibration experiments, we found that photoactivation-evoked spikes from ChR2-expressing cells can be restricted to perisomatic regions using an appropriate photostimulation protocol. We recorded from SOM+ cells in whole cell current clamp mode while examining their spiking profiles in response to spatially restricted blue laser stimulation (laser spot diameter, ∼50 μm) in a map grid of 64 locations (individually spaced at 65 × 65 μm) centered around recoded cell body locations. Although spikes were robustly generated across many locations, we found that evoked spikes by high laser power (with 1 ms or longer stimulation durations) lost spatial specificity of spike generation (Fig. 5, C and D). Photoactivated spikes occurred even when stimulation was applied to outside the BNST, including the caudate putamen. The light-evoked spike activation was likely due to high-power laser diffusion and reflection at slice stimulation sites. In comparison, using sufficient but lower power laser (e.g., 0.1-ms duration), photoactivated spikes were more restricted to BNST domains (Fig. 5, E and F). Furthermore, we found that, with the same laser power, more distal regions from the cell body tend to respond less robustly to repeated laser flashes (Fig. 5F). Thus we improved the effective resolution of our ChR2-based mapping approach by taking advantage of our observations and using a restrictive criterion for detecting photoactivated responses. We used this repeated stimulation protocol, and “hits” were only scored when the recorded neuron responded with spikes to all three laser flashes in any given mapping location (Fig. 5F). Under the appropriate laser power (i.e., 0.1 ms), reliable measurements of perisomatically triggered action potentials were obtained from SOM+, ChR2-expressing BNST neurons within a range of 65–130 μm (86.7 ± 21.7 μm; n = 3 cells; Fig. 5F). The spikes occurred within about 5–10 ms post ChR2 photostimulation. This spatial specificity of ChR2-mediated spiking photoactivation was comparable to the resolution of glutamate uncaging (Fig. 2). With this calibrated approach, it is possible that direct inhibitory connections to target cell types can be mapped by ChR2 photoactivation of somatic spiking of presynaptic inhibitory neurons at the stimulation sites.
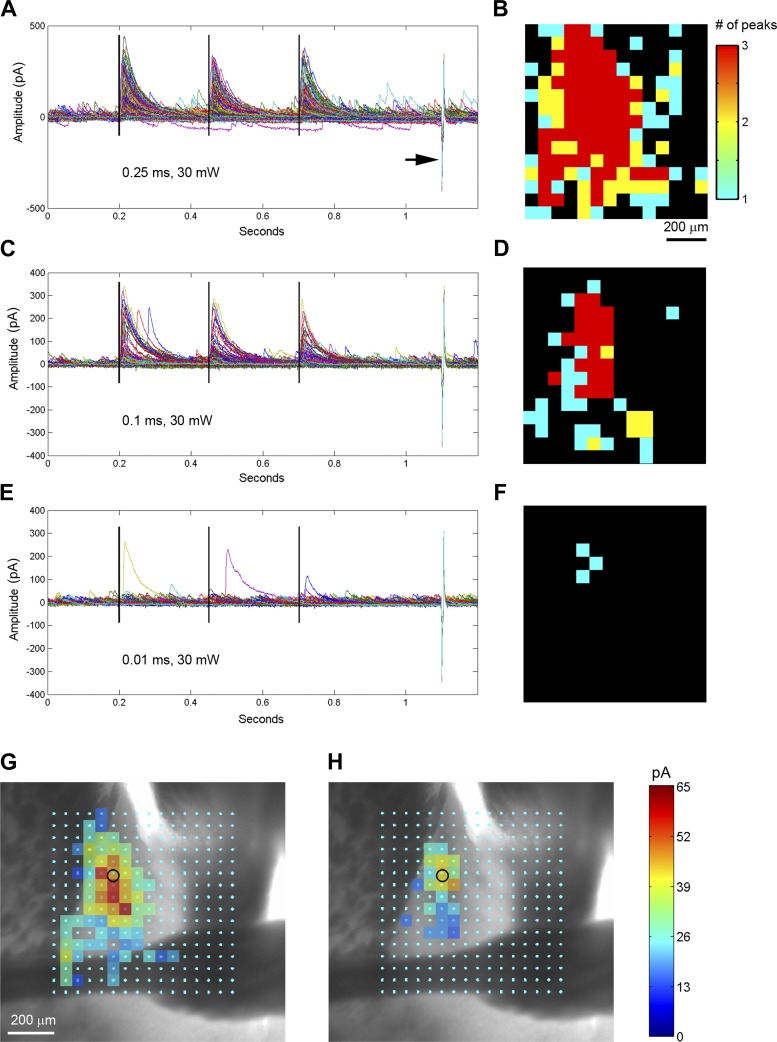
Then as shown in Fig. 6, A and B, spatially restricted 473-nm blue laser was sequentially applied at many different stimulation sites across the BNST brain slice during optogenetic stimulation mapping; SOM+ inhibitory functional connections to single BNST neurons were assessed by recording IPSCs at the holding potential of +5 mV. Guided by ChR2-mediated spiking profiles, we performed optogenetic stimulation mapping at different laser powers per different durations (0.25 ms, 0.1 ms, 0.05 ms, and 0.01 ms). Except for the duration of 0.01 ms, focal photoactivation of ChR2-expressing SOM+ neurons induced stimulus-locked IPSCs in the recorded BNST cells (Fig. 6, C–G). Consistent with the larger spiking radius of SOM+ cells by 0.25-ms photoactivation, wider input locations at this condition were seen compared with the photoactivation conditions of 0.1 and 0.05 ms. This observation was verified in five BNST neurons with photoactivation using multiple durations. For synaptic input analysis, we used the three “hit” criterion, and only map locations with three detected IPSCs were selected for further map construction (Fig. 7, A–F). The IPSC peaks were detected within the window of between 3 and 50 ms to each laser flash, with a threshold determined by background spontaneous IPSCs. Using this strategy, the IPSC inputs appeared to be robust and specific.
Fig. 7.
Physiological and anatomical calibrations are required for generation of accurate functional maps. A: the plot of all IPSC responses (0.25 ms, 30 mW, Fig. 6C) with baseline subtraction. The three vertical black lines denote the laser stimulation at 200 ms, 450 ms and 700 ms. The recoding access resistance was monitored by a current injection response (5 mV, 5 ms) shown by the arrow. B: the map coding locations with different numbers of detected IPSCs in response to 3 repeated optogenetic stimulation per location. IPSC peaks are detected within the window of between 3 and 50 ms to each laser flash, with an empirically determined threshold of 50 pA. The threshold matched the spontaneous IPSC amplitude shown in E. Only sites with 3 detected IPSCs are selected for further map construction. C and D: the plot of all IPSC responses (0.1 ms, 30 mW, Fig. 6D) are similarly formatted as in A and B, respectively. E and F: the plot of all IPSC responses (0.01 ms, 30 mW, Fig. 6F) are similarly formatted as in A and B, respectively. Under this stimulation condition (0.01 ms), no single location showed repeated IPSC responses, indicating insufficient laser power for robust circuit mapping. G and H: the SOM+ inhibitory input response sites (B, D) are overlaid on the bright-field image to show anatomical position. The small black circle indicates the cell body location. The scale codes the average peak IPSC amplitude. Presence of stimulation response in the anterior commissure in G indicates nonspecific input mapping under the high-power laser condition.
To further calibrate stimulation parameters and ensure the accuracy of synaptic input maps, we performed anatomical assessments of SOM+ input responses. In Fig. 7, G and H, where photoactivation conditions of 0.25 ms and 0.1 ms were applied, the detected input sites are overlaid on the slice image to show anatomical position. Because there is no ChR2 expression in the anterior commissure (fiber of passage, Fig. 5B), the presence of stimulation responses in Fig. 6G is critical, indicating nonspecific input mapping under the high-power laser condition (0.25 ms). Similarly, there were nonspecific input sites in the caudate putamen at 0.25-ms photoactivation. In comparison, 0.1-ms photoactivation did not produce those nonspecific input sites. We obtained this calibration from a total of five BNST neurons. For these cells, the average peak input strength across all map sites per cell was 39.5 ± 9.9 pA and 13.3 ± 3.6 pA for the photoactivation durations of 0.25 ms and 0.1 ms, respectively (n = 5 cells; P = 0.02, t-test). The input map locations were correlated to strong ChR2 expression and SOM+ neuronal distribution in the dorsolateral BNST (Figs. 5B and 7H). The spatial pattern of SOM+ neuron-specific inputs to the recorded BNST neuron resembles that revealed by the LSPS via glutamate uncaging. Taken together, this calibrated approach (0.1 ms, 30 mW, repeated photoactivation) was determined to be appropriate for subsequent mapping of local SOM+ inhibitory inputs.
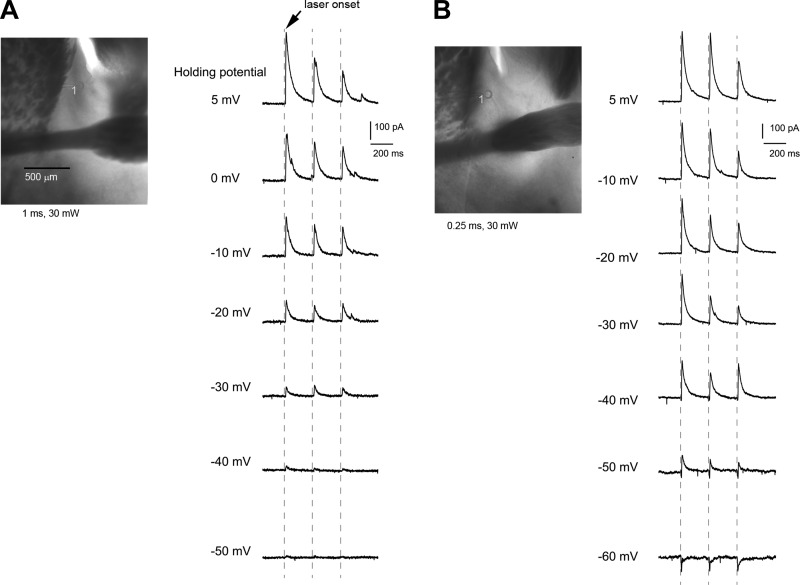
We mapped both SOM+ and non-SOM+ cells that were distinguished by their light-evoked responses at holding membrane potentials toward −50 mV to −60 mV when using the internal cesium recording solution (Fig. 8). Our preliminary recordings indicated that SOM+ neurons innervated both SOM+ and non-SOM+ BNST neurons (Fig. 8). Their SOM-specific input strength across all map sites per cell appeared to be similar. As for SOM+ cells, their average peak input strength was 32.4 ± 14.8 pA and 11.2 ± 5.5 pA for the photoactivation durations of 0.25 ms and 0.1 ms, respectively (n = 3 cells). As for non-SOM+ cells, their average peak input strength was 44.8 ± 14.5 pA and 14.8 ± 5.5 pA for the photoactivation durations of 0.25 ms and 0.1 ms, respectively (n = 3 cells). These data confirm strong inhibitory connections in the BNST revealed by the LSPS approach via glutamate uncaging.
Fig. 8.
SOM-Cre; Ai32 cells are distinguished from non-SOM+ cells by their light-evoked responses at holding membrane potentials toward −50 mV to −60 mV, using the cesium-containing internal solution. A: non-SOM+ cell. Example of ChR2-photoactivated current responses measured by whole cell voltage clamp at different holding membrane potentials is shown. The recoded cell (indicated by the circle) is located in the oval BNST and photoactivated at a perisomatic site (label, 1). Note the absence of downward deflecting currents at −40 and −50 mV. B: SOM+ cell. Example of ChR2-photoactivated current responses measured by whole cell voltage clamp at different holding membrane potentials is shown. The recoded cell is located in the oval BNST, and photoactivated at a perisomatic site (label, 1). Note the presence of downward deflecting currents at −40, −50 and −60 mV, which were caused by direct activation of ChR2-gated cation currents. In comparison, both cells showed strong upward deflecting currents (IPSCs) beyond −20 mV, which resulted from presynaptic SOM-Cre; Ai32 neuronal spiking.
DISCUSSION
In this study, we have applied the photostimulation-based approaches to local BNST circuit mapping of defined neuronal types. We have performed physiological and anatomical calibrations for LSPS via glutamate uncaging or ChR2-mediated optogenetic stimulation, to establish experimental conditions allowing for the accuracy of synaptic input mapping analysis. The mapping resolution is defined by the spatial specificity of laser photostimulation, with which evoked action potentials are restricted to a small population of BNST neurons at or close to the location of each photostimulation site. In calibrating our approaches, we found that photostimulation-evoked spikes from recorded BNST cells can be restricted to perisomatic regions (around 100 μm), without activating spikes from their axons of passage or distal dendrites. Thus whole cell recordings from targeted cell types (such as CRH+ cells), combined with spatially restricted photostimulation of presynaptic neurons at many different locations over a large region, allow high-resolution mapping of presynaptic input sources in the BNST to the cell types of interest.
On the basis of the functional circuit mapping, our study has showed sparse excitatory vs. dense inhibitory local inputs to CRH neurons in the mouse BNST. Although CRH+ BNST neurons have robust and extensive inhibitory inputs, there is considerable variability among individual neurons in terms of input pattern and strength (see Figs. 3 and 4, and related quantitative results). We will perform further rigorous experiments to examine this and correlate input strength and pattern to cell morphology. Our findings appear to be consistent with a recent study (Turesson et al. 2013) that examined the intrinsic connections of rat BNST using glutamate uncaging in a relatively coarse manner. They reported that glutamate uncaging via UV LED light (150 μm in diameter and 50 ms in duration) elicited GABAergic inhibitory postsynaptic potentials more frequently than excitatory postsynaptic potentials (Turesson et al. 2013). These and further studies will provide important information for understanding the general principles of BNST local circuit organization. In addition, to understand BNST-associated circuit mechanisms underlying anxiety disorders and drug addiction, it is critical to understand how stress and drug use causes maladaptive changes localized to local circuit connections to impact synaptic inputs of identified neuronal types in the BNST. It is known that, in rodents, chronic stress exposure elicits structural changes in the BNST, including increased dendritic arborizations of BNST neurons (Pego et al. 2008; Vyas et al. 2003). But the functional impact of these neuronal morphological changes is unclear. Thus it would be important to use the photostimulation-based mapping approaches to further understand the functional impacts and maladaptive BNST circuit responses of specific neuron types and examine the network level of effects.
As demonstrated in the present work, as an alternative method of photostimulation via glutamate uncaging, optogenetic stimulation mapping is a powerful approach for local BNST circuit mapping. This new development makes it possible to target not only specific subregions, but also specific subset of neurons within their participating circuits. Genetically encoded ChR2 can also enhance the ability of photostimulation in targeting defined cell types, as glutamate uncaging indiscriminately stimulates all neurons expressing glutamate receptors. However, a caveat is that ChR2 photoactivation has an issue of spatial precision of photostimulation as action potentials can be elicited by photostimulating the soma and dendrites as well as the axon. Therefore, we devoted great efforts to work out a robust and specific photoactivation strategy for local circuit mapping. Our physiological and anatomical calibration experiments helped to determine proper parameters to generate accurate SOM+ functional input maps. Using the calibrated conditions, ChR2 photoactivation-evoked action potentials from perisomatic regions of SOM+, ChR2-expressing BNST neurons; there was no optically evoked responses in the anterior commissure (fiber of passage) or the caudate putamen bordering the BNST. The map locations of SOM-specific input corresponded to strong ChR2 expression and SOM+ neuronal distribution in the dorsolateral BNST. Taken together, we foresee that the high-resolution and cell-type specific photostimulation approaches will be useful in determining the functional organization of local circuits of specific BNST neuron types.
GRANTS
This work was partially funded by US National Institutes of Health Grants NS-078434 and MH-105427. This work was also made possible, in part, through access to the confocal facility of the Optical Biology Shared Resource of the Cancer Center Support Grant (CA-62203) at the University of California, Irvine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.X. conception and design of research; X.X., T.I., R.S., W.F., and F.B. performed experiments; X.X. analyzed data; X.X. interpreted results of experiments; X.X. and Y.S. prepared figures; X.X. drafted manuscript; X.X. and T.C.H. edited and revised manuscript; X.X., T.I., Y.S., R.S., T.C.H., W.F., and F.B. approved final version of manuscript.
REFERENCES
- Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol Rep 3: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27: 2025–2034, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology 36: 1312–1326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 7: 156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG. Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology 41: 103–125, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci 3: 701–707, 2000. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Circuit dynamics of adaptive and maladaptive behaviour. Nature 505: 309–317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev 38: 192–246, 2001. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol 468: 277–298, 2004. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP. Neuroscience: anxiety is the sum of its parts. Nature 496: 174–175, 2013. [DOI] [PubMed] [Google Scholar]
- Katzel D, Zemelman BV, Buetfering C, Wolfel M, Miesenbock G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat Neurosci 14: 100–107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496: 219–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501: 543–546, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriva-Sahd J. Juxtacapsular nucleus of the stria terminalis of the adult rat: extrinsic inputs, cell types, and neuronal modules: a combined Golgi and electron microscopic study. J Comp Neurol 475: 220–237, 2004. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol 497: 772–807, 2006. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 17: 6434–6446, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15: 793–802, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman AT, Sunkin SM, Oh SW, Zariwala AH, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magableh A, Lundy R. Somatostatin and corticotrophin releasing hormone cell types are a major source of descending input from the forebrain to the parabrachial nucleus in mice. Chem Senses 39: 673–682, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the bed nucleus of the stria terminalis: a Golgi study in the rat. Brain Res Bull 10: 111–120, 1983. [DOI] [PubMed] [Google Scholar]
- Nguyen AQ, Dela Cruz JAD, Sun Y, Holmes TC, Xu X. Genetic cell targeting uncovers specific neuronal types and distinct subregions in the bed nucleus of the stria terminalis. J Comp Neurol. 2015. December 30. doi: 10.1002/cne.23954 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo HV, Bureau I, Svoboda K, Zador AM. The functional asymmetry of auditory cortex is reflected in the organization of local cortical circuits. Nat Neurosci 13: 1413–1420, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci 27: 1503–1516, 2008. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 457: 1142–1145, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Antonio A, Liban K, Ikrar T, Tsyganovskiy E, Xu X. Distinct physiological and developmental properties of hippocampal CA2 subfield revealed by using anti-Purkinje cell protein 4 (PCP4) immunostaining. J Comp Neurol 522: 1333–1354, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38: 277–289, 2003. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ikrar T, Olivas ND, Xu X. Bidirectional global spontaneous network activity precedes the canonical unidirectional circuit organization in the developing hippocampus. J Comp Neurol 522: 2191–2208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Nenadic Z, Xu X. Novel use of matched filtering for synaptic event detection and extraction. PLoS One 5: e15517, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci 33: 950–960, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry 4: 42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Rep 7: 269–280, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter BA, O'Connor T, Iyer V, Petreanu LT, Hooks BM, Kiritani T, Svoboda K, Shepherd GM. Ephus: multipurpose data acquisition software for neuroscience experiments. Front Neural Circuits 4: 100, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71: 995–1013, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 1183: 221–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesson HK, Rodriguez-Sierra OE, Pare D. Intrinsic connections in the anterior part of the bed nucleus of the stria terminalis. J Neurophysiol 109: 2438–2450, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res 965: 290–294, 2003. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216, 2003. [DOI] [PubMed] [Google Scholar]
- Weiler N, Wood L, Yu J, Solla SA, Shepherd GM. Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci 11: 360–366, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience 135: 155–179, 2005. [DOI] [PubMed] [Google Scholar]
- Xu X. High precision and fast functional mapping of brain circuitry through laser scanning photostimulation and fast dye imaging. In: Laser Scanning, Theory and Applications, edited by Wang CC. Rijeka, Croatia: InTech, 2011, p. 113–132. [Google Scholar]
- Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci 29: 70–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Olivas ND, Levi R, Ikrar T, Nenadic Z. High precision and fast functional mapping of cortical circuitry through a combination of voltage sensitive dye imaging and laser scanning photostimulation. J Neurophysiol 103: 2301–2312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]