Abstract
The interneurons of the mushroom body, known as Kenyon cells, are essential for the long-term memory of olfactory associative learning in some insects. Some studies have reported that nitric oxide (NO) is strongly related to this long-term memory in Kenyon cells. However, the target molecules and upstream and downstream NO signaling cascades are not completely understood. Here we analyzed the effect of the NO signaling cascade on Na+-activated K+ (KNa) channel activity in Kenyon cells of crickets (Gryllus bimaculatus). We found that two different NO donors, S-nitrosoglutathione (GSNO) and S-nitroso-N-acetyl-dl-penicillamine (SNAP), strongly suppressed KNa channel currents. Additionally, this inhibitory effect of GSNO on KNa channel activity was diminished by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of soluble guanylate cyclase (sGC), and KT5823, an inhibitor of protein kinase G (PKG). Next, we analyzed the role of ACh in the NO signaling cascade. ACh strongly suppressed KNa channel currents, similar to NO donors. Furthermore, this inhibitory effect of ACh was blocked by pirenzepine, an M1 muscarinic ACh receptor antagonist, but not by 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) and mecamylamine, an M3 muscarinic ACh receptor antagonist and a nicotinic ACh receptor antagonist, respectively. The ACh-induced inhibition of KNa channel currents was also diminished by the PLC inhibitor U73122 and the calmodulin antagonist W-7. Finally, we found that ACh inhibition was blocked by the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME). These results suggested that the ACh signaling cascade promotes NO production by activating NOS and NO inhibits KNa channel currents via the sGC/cGMP/PKG signaling cascade in Kenyon cells.
Keywords: Na+-activated K+ channel, nitric oxide, acetylcholine, cGMP, patch clamp-electrophysiology, Kenyon cell, mushroom body
behavioral and pharmacological studies in insects such as crickets (Gryllus bimaculatus) and honeybees (Apis mellifera) have shown a high capacity for olfactory associative learning and long-term memory (Matsumoto and Mizunami 2000, 2002; Takeda et al. 1961). A recent study in the locust elucidated that the specific modulation of the synapses between the intrinsic mushroom body neurons and their postsynaptic neurons contributes to associative learning (Cassenaer and Laurent 2012). In insects, intrinsic neurons of mushroom bodies, known as Kenyon cells, may play an essential role in olfactory associative learning and memory (Matsumoto et al. 2006, 2009; Watanabe et al. 2010). Kenyon cells receive sensory information from various sites and are important for integrating multiple sensory inputs (Belle and Heisenberg 1994; Hammer and Menzel 1998; Heisenberg 2003; McGuire et al. 2001). Although the intrinsic cellular mechanisms of associative learning and memory in Kenyon cells are not completely understood, some studies have suggested that the nitric oxide (NO) signaling cascade in mushroom bodies is related to long-term memory in insects (Matsumoto et al. 2006, 2009, 2013).
NO is a membrane-permeant agent and acts as an inter- and intracellular signal in various organs including the brain. A well-known physiological effect of NO is blood vessel relaxation. NO produced from vascular endothelial cells spreads and relaxes vascular smooth muscles via the activation of soluble guanylate cyclase (sGC) and production of cGMP. Additionally, many studies in vertebrates have indicated that NO plays an important role in synaptic plasticity of neurons, which is essential for learning and long-term memory establishment. In the cerebellum, the NO-cGMP/protein kinase G (PKG) signaling cascade is required for long-term synaptic depression, which underlies the formation of motor learning (Shibuki and Okada 1991). Some reports have suggested that the NO and cGMP signaling pathways are involved in long-term synaptic potentiation in the hippocampus (Böhme et al. 1991; Boulton et al. 1995; Doyle et al. 1996; Haley et al. 1993; Lu et al. 1999; Schuman and Madison 1991; Zhuo et al. 1994). In insects, it was also proposed that NO signaling cascade within Kenyon cells participates in the formation of learning and long-term memory. Bicker et al. (1996) first showed that Kenyon cells of the locust can respond to NO via cGMP signal cascade. In crickets and locusts, the mushroom bodies show very strong nitric oxide synthase (NOS) expression, particularly in the peduncle and lobes, which is attributable to specific subpopulations of Kenyon cells (Cayre et al. 2005; Ott et al. 2007). Moreover, at least in locusts, NO-induced cGMP responses are strongest in complementary Kenyon cell subpopulations that express little or no NOS (Ott et al. 2007). The NO-cGMP/cAMP signaling cascade is required for shifts from short-term memory to long-term memory in an olfactory associative learning experiment (Matsumoto et al. 2006, 2013).
Although previous studies in some insects have pointed to the importance of the NO signaling cascade in the formation of olfactory long-term memory within mushroom bodies (that is, within Kenyon cells), the target molecules of the NO signaling cascade and the upstream mechanism that induces NO production in Kenyon cells are not fully understood. Here we analyzed the effect of the NO signaling cascade on Na+-activated K+ (KNa) channel activity. Furthermore, we hypothesized that the neuronal transmitter ACh triggers NO production in Kenyon cells, because ACh transmits olfactory information, which is a conditioning stimulus in olfactory associative learning, from the antennal lobes to the mushroom body Kenyon cells (Kreissl and Bicker 1989; Yasuyama et al. 2002, 2003) and might be related to associative learning (Watanabe et al. 2010; Williamson and Wright 2013).
METHODS
Animals.
Adult male G. bimaculatus crickets were maintained in a colony in the Department of Biology of Tokyo Gakugei University at 25–30°C with a relative humidity of 65–85% under a 12:12-h light-dark photoperiod. Crickets were fed an artificial insect diet (Oriental Yeast) and provided water. The present experiments were performed under the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences, recommended by the Physiological Society of Japan.
Kenyon cell isolation.
For the electrophysiological analyses, Kenyon cells were isolated with previously reported methods (Inoue et al. 2014; Nakamura and Yoshino 2013). The brain was carefully removed from the head capsule. The mushroom bodies were removed from the brain and placed in a 3-ml silicone chamber filled with Ca2+-free normal saline and incubated for 15 min. The mushroom bodies were then transferred to a tube containing dissociation solution (mainly papain) (Sumitomo nerve-cell culture medium, Sumitomo Bakelite, Tokyo, Japan) and incubated for 30 min at 25°C. After incubation, the pooled mushroom bodies were rinsed with normal saline and dissociated by gentle trituration with a fire-polished pipette with an inner diameter of ∼100 μm. Freshly dispersed cells were allowed to settle on the flat glass bottom of a silicone chamber mounted on the stage of an inverted microscope (Nikon 144 Diaphoto). After 20-min settling time, cells were then superfused with a high-K+ bath solution. Within a few minutes after superfusing a high-K+ solution, we started the single-channel recording and a series of experiments. Among isolated Kenyon cells, we selected large-size Kenyon cells more than ∼10 μm for the electrophysiological analysis.
Electrophysiology.
Single-channel patch-clamp recordings from the isolated Kenyon cells were performed according to a previous report (Nakamura and Yoshino 2013). Patch pipettes were pulled from capillary tubes (G-1.5, Narishige, Tokyo, Japan) with a two-stage pipette puller (PC-10, Narishige). The tip resistance of patch pipettes was ∼5–10 MΩ. Freshly dispersed cells were allowed to settle on the flat glass bottom of a silicone chamber mounted on the stage of an inverted microscope (I×70, Olympus), and the patch electrode was positioned on the cell surface with a three-dimensional hydraulic micromanipulator (MHW-3, Narishige). The reference electrode was an Ag-AgCl wire connected to the bath solution through a 100-mM KCl-agar bridge. Single-K+ channel currents were measured in cell-attached patches by filling the patch pipettes with high-K+ solution containing (in mM) 140 KCl and 5 HEPES, buffered to pH 7.4 with Tris-Cl. We recorded the single-channel currents of the isolated Kenyon cells in the hand-made silicon chamber filled with high-K+ extracellular solution containing (in mM) 140 KCl and 44 glucose with HEPES, buffered to pH 7.4 with Tris-Cl. A previous report showed that large-conductance KNa channel currents can be recorded in Kenyon cells by addition of 10 mM NaCl to a high-K+ extracellular bath solution (Nakamura and Yoshino 2013). Additionally, it was also suggested that addition of Ca2+ to a bath solution induced Ca2+-activated K+ (KCa) currents. Therefore, for selective recordings of KNa channel currents, we added 10 mM NaCl to high-K+ extracellular solution and excluded the Ca2+ component. By using this extracellular solution, we could record single-channel currents similar to KNa channel currents of the previous study. Therefore, we judged that the currents observed in the present study are KNa channel currents. Currents were filtered at 2 kHz and digitized at 10 kHz. In all experiments, voltage clamp and voltage pulse generation were controlled with a List EPC7 patch-clamp amplifier.
Analysis of single-channel currents.
All values are shown as means ± SE, and n represents the number of cells. Single-KNa channel currents were analyzed with the methods of a previous report (Aoki et al. 2008; Nakamura and Yoshino 2013). Unitary currents were analyzed with pCLAMP 9.0 software (Axon Instruments, Union City, CA). Histograms of current amplitudes for single-channel records provided the clearest demonstration of multiple current levels. In the present study, we expressed the open probability (Po) of single channels as NPo, where N stands for the total number of functional channels in a patch. NPo was calculated with the following expression: NPo = (A1 + 2A2 + 3A3 + . . . + nAn)/(A0 + A1 + A2 + . . . + An), where A0 is the area under the curve of an all-point amplitude histogram corresponding to the current in the closed state and A1–An represents the histogram area reflecting the different open-state current levels for 1–n channels present in the patch. Histogram parameters were obtained from multiple least-squares Gaussian fits of the data with Clampfit 9.2 software (Axon Instruments).
NPo(drug application period) was calculated from the recordings during the time between 0.5 and 1.5 min after the start of drug application. NPo(control period) was calculated from the recordings during the 1.0 min prior to drug application 30 s or more.
Drugs.
The following drugs were used in this study: ACh chloride (Wako, Osaka, Japan), 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP; Tocris, Bristol, UK), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; Wako), KT5823 (Wako), NG-nitro-l-arginine methyl ester hydrochloride (l-NAME; Wako), mecamylamine hydrochloride (Sigma-Aldrich), pirenzepine dihydrochloride monohydrate (Wako), S-nitrosoglutathione (GSNO; Sigma-Aldrich), S-nitroso-N-acetyl-dl-penicillamine (SNAP; Sigma-Aldrich), U73122 (Wako), and W-7 hydrochloride (Wako). Stock solutions of 4-DAMP (100 mM in DMSO), ODQ (10 mM in DMSO), KT5823 (50 mM in DMSO), l-NAME (50 mM in DMSO), mecamylamine (10 mM in DMSO), SNAP (100 mM in DMSO), W-7 (100 mM in DMSO), and U-73122 (10 mM in DMSO) were kept in aliquots at −20°C until use, and the final concentration of DMSO in the bath was <1:1,000.
Statistical analysis.
Comparison between two treatments was performed with a paired t-test. Comparison among three treatments was performed with a repeated-measures ANOVA. If the sphericity assumption was violated in the repeated-measures ANOVA, the Greenhouse-Geisser correction was used. The difference was considered significant when P < 0.05.
RESULTS
NO released by NO donors inhibits KNa channel activity.
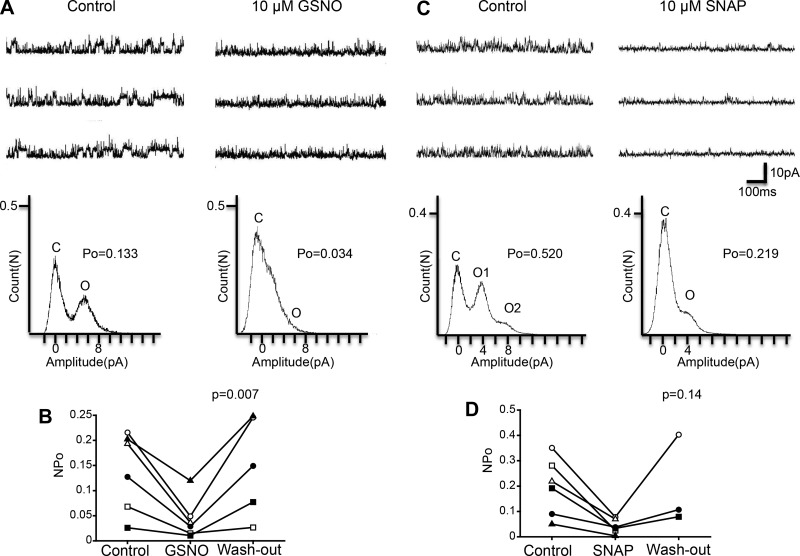
In the present study, we analyzed the effect of drug application on single-KNa channel activity at a membrane potential of +60 mV. Figure 1A shows the effect of NO on KNa channel activity with the NO donor GSNO (10 μM). As shown in a scatterplot in Fig. 1B, we compared NPo of KNa channels at three treatments (Control, GSNO, and Washout). Repeated-measures ANOVA showed that NPo was significantly different among the three treatments (Control, GSNO: n = 6, Washout: n = 5, F = 9.8, df = 2/8, P = 0.007), indicating that GSNO affects (inhibits) KNa channel activity. Next, we examined the effect of the alternate NO donor SNAP (10 μM; Fig. 1C), using an experimental protocol similar to that used with GSNO. As shown in a scatterplot in Fig. 1D, we compared NPo of KNa channels at three different treatments (Control, SNAP, and Washout). Repeated-measures ANOVA showed that the effect of SNAP on NPo was not significant (Control, SNAP: n = 6, Washout: n = 3, F = 3.3, df = 2/4, P = 0.14). We thought, however, that this result was due to the low sample number for washout: washout is only available for three of six cells. Therefore, we applied a paired t-test between control and SNAP treatment and found that NPo was significantly decreased by SNAP compared with control (n = 6, paired t-test, df = 5, P < 0.05). Taken together, these results indicate that NO released by NO donors suppresses KNa channel activity.
Fig. 1.
Effect of the NO donors GSNO and SNAP on KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in the control (left) and 10 μM GSNO (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before the application of GSNO (Control), in the presence of 10 μM GSNO (GSNO), and after washout of GSNO (Wash-out). Matching symbols connected with a line show the sequential experiment in the same cell. C, top: representative traces of KNa channel current in the control (left) and 10 μM SNAP (right) groups. Bottom: corresponding all-point amplitude histograms. D: NPo before application of SNAP (Control), in the presence of 10 μM SNAP (SNAP), and after washout of SNAP (Wash-out). B and D: repeated-measures ANOVA.
sGC/cGMP/PKG pathway is related to NO-induced inhibitory effect on KNa channel activity.
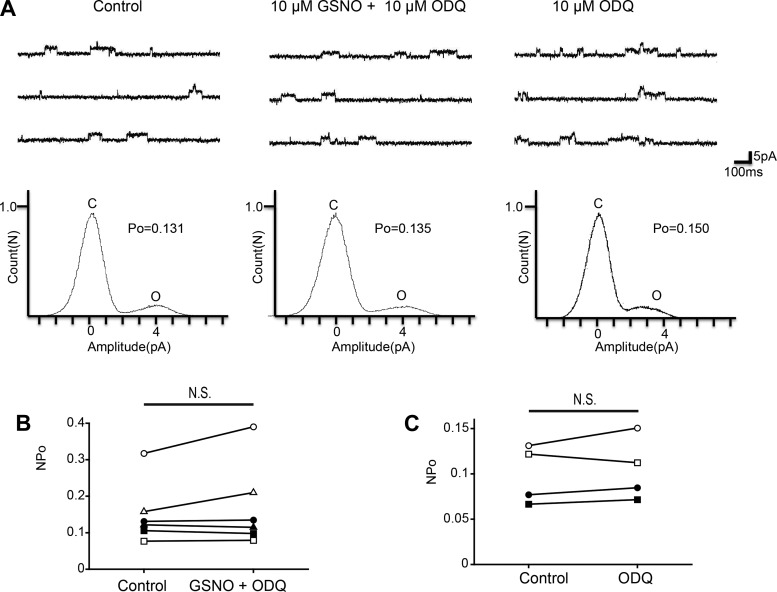
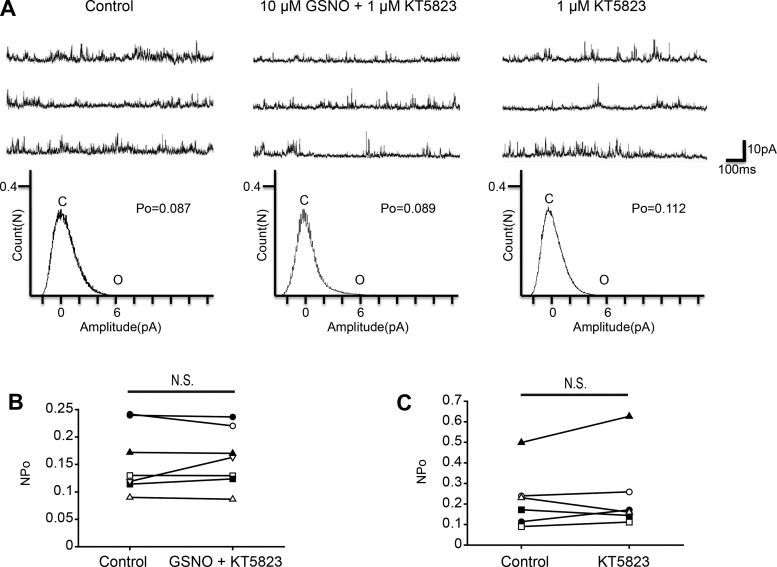
The intracellular effects of NO include stimulation of sGC and promotion of cGMP synthesis. Therefore, for the analyses of downstream pathways, we examined the sGC-cGMP pathway. To investigate the involvement of sGC, we used the membrane-permeant sGC inhibitor ODQ. Coapplication of 10 μM GSNO and 10 μM ODQ had no significant effect on the NPo of KNa channel activity (Fig. 2, A and B; n = 6, paired t-test, df = 5, P = 0.223), which suggests that ODQ blocked the inhibitory effect of NO on KNa channel currents. Additionally, the single application of 10 μM ODQ had no significant effect on NPo (Fig. 2, A and C; n = 4, paired t-test, df = 3, P = 0.408). These results indicate that NO inhibits KNa channel activity via sGC-cGMP signal cascade. The protein kinase G (PKG) is activated by cGMP and modulates various channel activities. Next, we examined whether PKG is related to the inhibitory effect of the NO-cGMP pathway. Coapplication of 10 μM GSNO and 1 μM PKG inhibitor KT5823 had no significant effect on NPo (Fig. 3, A and B; n = 7, paired t-test, df = 6, P = 0.670). Additionally, single application of 10 μM KT5823 had no significant effect on KNa channel activity (Fig. 3, A and C; n = 6, paired t-test, df = 5, P = 0.481). These results suggest that the sGC/cGMP/PKG pathway is involved in the NO-induced inhibitory effect on KNa channel activity.
Fig. 2.
Effect of the sGC inhibitor ODQ on GSNO-induced KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in the control (left), 10 μM GSNO + 10 μM ODQ (center), and 10 μM ODQ (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM GSNO + 10 μM ODQ (GSNO + ODQ). Matching symbols connected with a line show the sequential experiment in the same cell. C: NPo before drug application (Control) and in the presence of 10 μM ODQ (ODQ). B and C: paired t-test; N.S., not significant.
Fig. 3.
Effect of the PKG inhibitor KT5823 on GSNO-induced KNa channel activity in isolated Kenyon cells. A, top: representative trace of KNa channel current in the control (left), 10 μM GSNO + 1 μM KT5823 (center), and 1 μM KT5823 (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM GSNO + 1 μM KT5823 (GSNO + KT5823). Matching symbols connected with a line show the sequential experiment in the same cell. C: NPo before drug application (Control) and in the presence of 1 μM KT5823 (KT5823). B and C: paired t-test; N.S., not significant.
Acetylcholine reduces single-KNa channel currents.
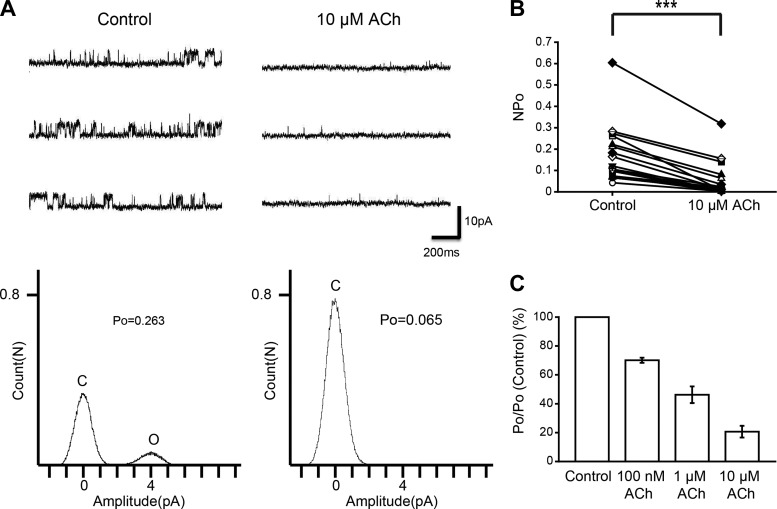
By perfusion of NO donors and cGMP signal inhibitors, we found that the NO-cGMP signaling cascade inhibits KNa channel currents. We then examined the effect of ACh on KNa channel activity to determine whether it is an upstream mechanism of the NO-cGMP signaling cascade. The NPo of KNa channel currents were drastically reduced by the application of 10 μM ACh to the bath solution, similar to the action of NO donors (Fig. 4, A and B; n = 19, paired t-test, df = 18, P < 0.001). Furthermore, we examined various concentrations of ACh. Figure 4C shows the average values of relative NPo from 3 cells; 70 ± 1.8% of control (100 nM ACh) and 46 ± 5.7% of control (1 μM ACh). These results showed that ACh inhibits single-KNa channel currents.
Fig. 4.
Effect of ACh on KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in the control (left) and 10 μM ACh (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM ACh. Matching symbols connected with a line show the sequential experiment in the same cell. C: dose-response relationship between ACh concentration and the average relative Po of KNa channel currents. B: paired t-test; ***P < 0.001.
Inhibitory effects of ACh on KNa channel currents via specific receptors.
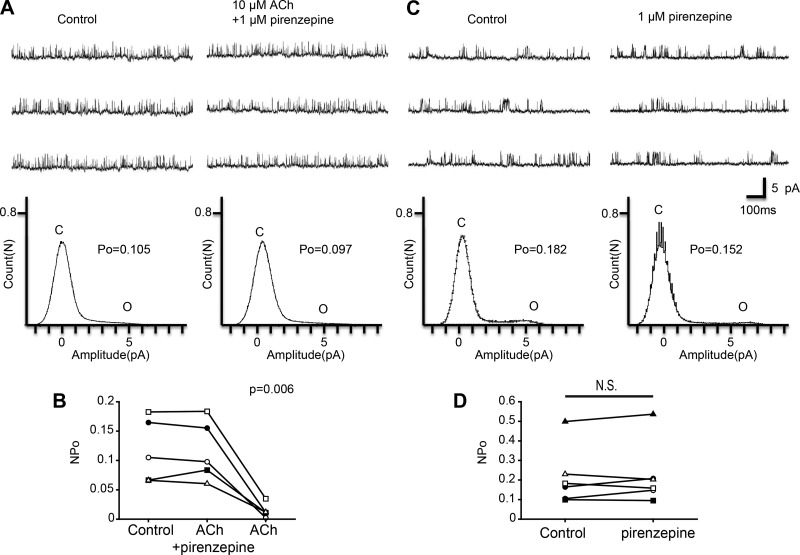
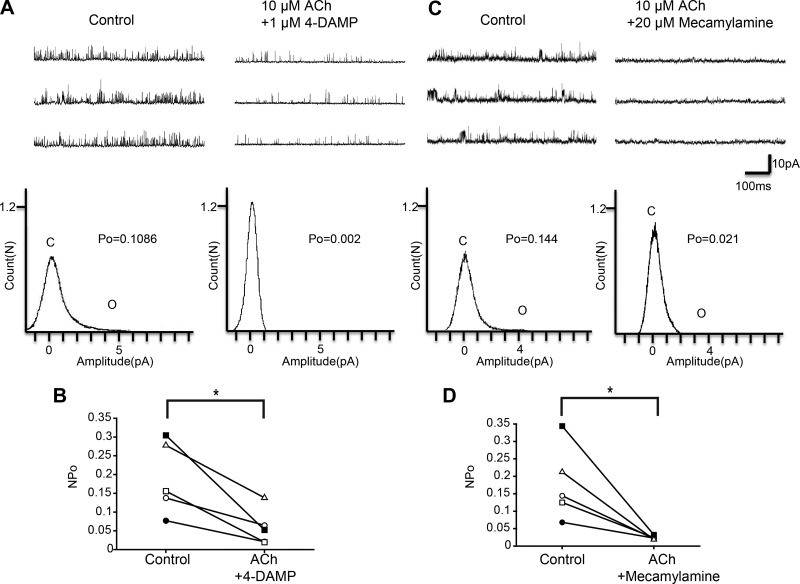
To reveal the receptor subtype involved in the observed effects of ACh, we next examined the effects of various ACh receptor antagonists. First, we examined the effect of pirenzepine, which is an M1-type muscarinic ACh receptor antagonist. As shown in Fig. 5A, we examined the effect of ACh in the presence of 1 μM pirenzepine. Figure 5B shows a scatterplot comparing the NPo at three different treatments (Control, ACh + pirenzepine, and ACh). Repeated-measures ANOVA with Greenhouse-Geisser correction showed a significant difference among the three treatments (n = 5, F = 24.1, df = 1.1/4.3, P = 0.006). Additionally, we analyzed the effect of 1 μM pirenzepine alone on KNa channel currents. Single application of pirenzepine did not significantly affect KNa channel currents (Fig. 5, C and D; n = 6, paired t-test, df = 5, P = 0.453). These results suggest that the inhibitory effect of ACh was blocked by pirenzepine and therefore M1-type muscarinic ACh receptor is involved in the inhibitory action of ACh on KNa channel activity. We also analyzed 4-DAMP and mecamylamine, an M3-type muscarinic ACh receptor antagonist and a nicotinic ACh receptor antagonist, respectively. NPo was significantly decreased by the coapplication of 10 μM ACh and 1 μM 4-DAMP (Fig. 6, A and B; n = 5, paired t-test, df = 4, P < 0.05). Furthermore, coapplication of ACh (10 μM) and mecamylamine (20 μM) also reduced NPo of KNa channel currents (Fig. 6D; n = 5, paired t-test, df = 4, P < 0.05). These results suggest that the inhibitory effect of ACh on KNa channel currents was mediated by M1-type muscarinic receptor activation but not by M3-type muscarinic receptor or nicotinic receptor activation.
Fig. 5.
Effect of the M1 muscarinic ACh receptor antagonist pirenzepine on ACh-induced KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in the control (left) and 10 μM ACh + 1 μM pirenzepine (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM ACh + 1 μM pirenzepine (ACh + pirenzepine) and the removal of pirenzepine (ACh). Matching symbols connected with a line show the sequential experiment in the same cell. C, top: representative traces of KNa channel current in the control (left) and 1 μM pirenzepine (right) groups. Bottom: corresponding all-point amplitude histograms. D: NPo before drug application (Control) and in the presence of 1 μM pirenzepine (pirenzepine). B: repeated-measures ANOVA with Greenhouse-Geisser correction. D: paired t-test; N.S., not significant.
Fig. 6.
Effect of the M3 muscarinic ACh receptor antagonist 4-DAMP and the nicotinic ACh receptor antagonist mecamylamine on ACh-induced KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in the control (left) and 10 μM ACh + 1 μM 4-DAMP (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM ACh + 1 μM 4-DAMP (ACh + 4-DAMP). Matching symbols connected with a line show the sequential experiment in the same cell. C, top: representative traces of KNa channel current in the control (left) and 10 μM ACh + 20 μM mecamylamine (right) groups. Bottom: corresponding all-point amplitude histograms. D: NPo before drug application (Control) and in the presence of 10 μM ACh + 20 μM mecamylamine (ACh + Mecamylamine). B and D: paired t-test; *P < 0.05.
PLC-Ca2+/CaM complex pathway is related to effect of ACh on KNa channel activity.
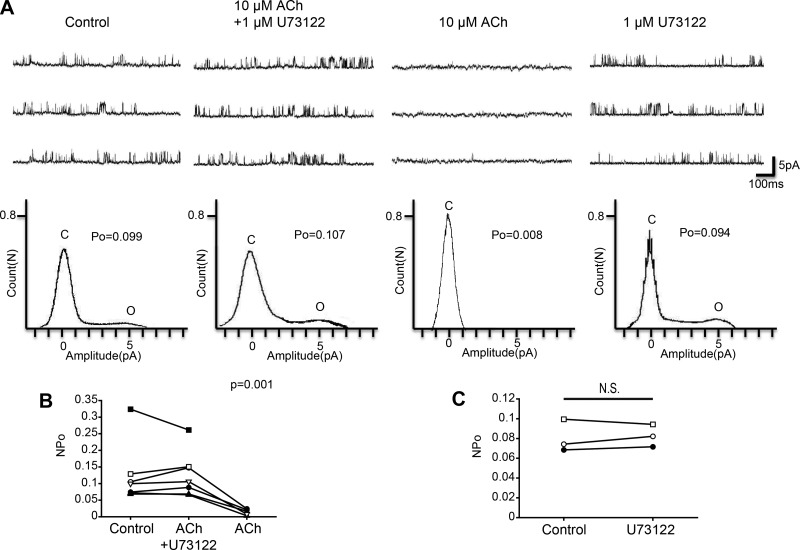
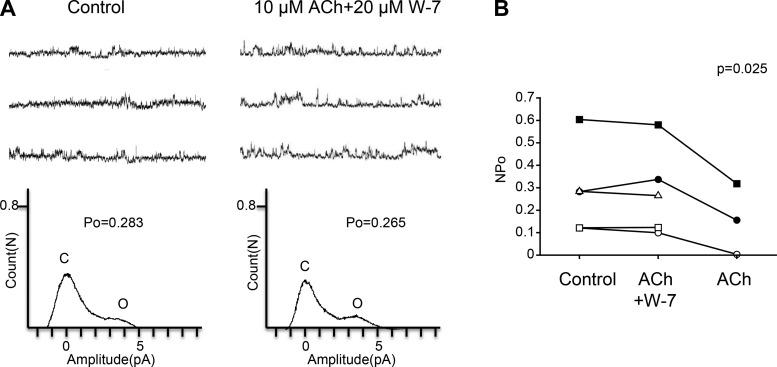
The M1 muscarinic receptor is a Gq/11-coupled receptor, and Gq/11 stimulates PLC. Therefore, to analyze the downstream pathway of the M1 muscarinic receptor we examined the role of PLC using U73122, a PLC inhibitor. As shown in Fig. 7A, we first examined the effect of ACh in the presence of U73122 and then examined the effect of removal of U73122 from a bath solution. Figure 7B shows a scatterplot comparing the NPo at three different treatments (Control, ACh + U73122, and ACh). Repeated-measures ANOVA showed a significant difference among the three treatments (Control, ACh + U73122: n = 7, ACh: n = 5, F = 37.7, df = 2/8, P = 0.001). Additionally, we analyzed the effect of 1 μM U73122 alone on KNa channel currents. Single application of U73122 did not significantly affect KNa channel currents (Fig. 7C; n = 3, paired t-test, df = 2, P = 0.655). These results indicate that ACh acting on the M1-type muscarinic receptor is coupled with PLC in Kenyon cells. PLC functions to convert phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (IP3), and IP3 increases the intracellular Ca2+ concentration by inducing the release of Ca2+ from the endoplasmic reticulum. We examined the involvement of CaM, a common Ca2+-binding messenger protein, by using W-7, a CaM inhibitor, in this ACh-induced pathway. As shown in Fig. 8A, we examined the effect of ACh in the presence of W-7. Figure 8B shows a scatterplot comparing the NPo at three different treatments (Control, ACh + W-7, ACh). Repeated-measures ANOVA analysis showed a significant difference among three treatments (Control, ACh + W-7: n = 5, ACh: n = 3, F = 10.6, df = 2/4, P = 0.025). These results suggest that CaM is involved in the inhibitory action of ACh on KNa channel activity.
Fig. 7.
Effect of PLC inhibitor U73122 on ACh-induced KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in (from left to right) control, 10 μM ACh + 1 μM U73122, 10 μM ACh, and 1 μM U73122 groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before the drug application (Control) and in the presence of 10 μM ACh + 1 μM U73122 (ACh + U73122) and the removal of U73122 (ACh). Matching symbols connected with a line show the sequential experiment in the same cell. C: NPo before drug application (Control) and in the presence of 1 μM U73122 (U73122). B: repeated-measures ANOVA. C: paired t-test; N.S., not significant.
Fig. 8.
Effect of the CaM inhibitor W-7 on ACh-induced KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in the control (left) and 10 μM ACh + 20 μM W-7 (right) groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM ACh + 20 μM W-7 (ACh + W-7) and the removal of W-7 (ACh). Matching symbols connected with a line show the sequential experiment in the same cell. B: repeated-measures ANOVA.
Inhibitory effect of ACh is related to NOS activation.
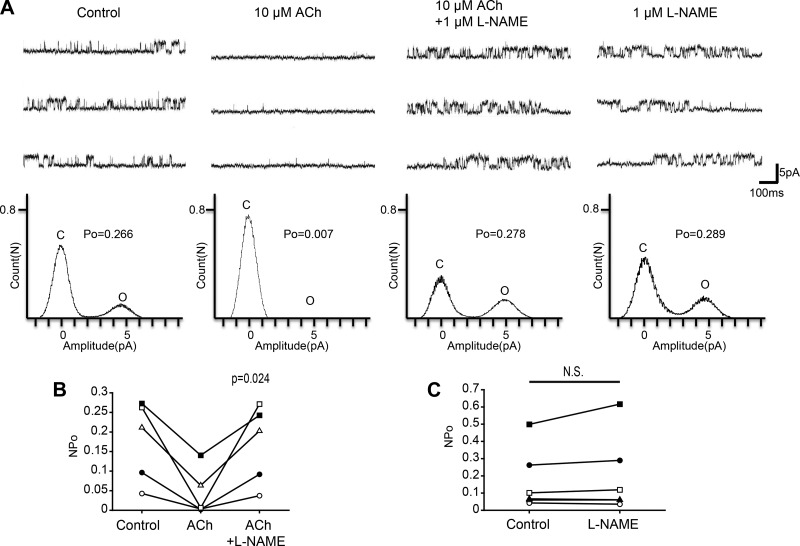
In vascular endothelial cells of vascular smooth muscle, ACh also activates CaM by increasing the intracellular Ca2+ concentration and the activated Ca2+/CaM complex stimulates NOS. Therefore, we hypothesized that Ca2+/CaM complex activation via the ACh pathway in Kenyon cells stimulates NOS and induces NO synthesis. We examined this hypothesis using 1 μM l-NAME, which is a NOS inhibitor. As shown in Fig. 9A, we first examined the effect of ACh and then examined the effect of adding l-NAME to the bath solution. Figure 9B shows a scatterplot comparing the NPo at three different treatments (Control, ACh, and ACh + l-NAME). Repeated-measures ANOVA with Greenhouse-Geisser correction showed a significant difference among the three treatments (n = 5, F = 12, df = 1.0/4.1, P = 0.024). Additionally, we analyzed the effect of 1 μM l-NAME alone on KNa channel currents. Single application of l-NAME did not significantly affect KNa channel currents (Fig. 9C; n = 6, paired t-test, df = 5, P = 0.249). These results suggest that ACh inhibits KNa channel activity via NOS activation in Kenyon cells.
Fig. 9.
Effect of the NOS inhibitor l-NAME on ACh-induced KNa channel activity in isolated Kenyon cells. A, top: representative traces of KNa channel current in (from left to right) control, 10 μM ACh, 10 μM ACh + 1 μM l-NAME, and 1 μM l-NAME groups. Bottom: corresponding all-point amplitude histograms. C and O, closed and complete opening levels, respectively. B: NPo before drug application (Control) and in the presence of 10 μM ACh (ACh) and the coapplication of 10 μM ACh + 1 μM l-NAME (ACh + l-NAME). Matching symbols connected with a line show the sequential experiment in the same cell. C: NPo before drug application (Control) and in the presence of 1 μM l-NAME (l-NAME). B: repeated-measures ANOVA with Greenhouse-Geisser correction. C: paired t-test; N.S., not significant.
DISCUSSION
In the present study, we first showed that the NO-sGC/cGMP/PKG signaling cascade inhibits KNa channel currents in mushroom body Kenyon cells of the cricket G. bimaculatus. Additionally, we found that ACh suppresses KNa channel activity via the M1 muscarinic receptor/PLC/CaM/NOS signaling pathway. Therefore, our studies suggest that ACh, which conveys olfactory information to Kenyon cells, is the trigger of NO production and modulation of KNa channel activity via the NO-sGC/cGMP/PKG signaling pathway. This modulation might be related to the formation of long-term memory in olfactory associative learning.
NO strongly inhibits KNa channel activity via sGC/cGMP/PKG signaling pathway.
In the present study, we showed that two different NO donors, GSNO and SNAP, strongly inhibit KNa channel activity in Kenyon cells. GSNO and SNAP are both sources of NO but have different chemical/physical properties, including their molecular structures and water solubility. These distinct NO donors had a similar inhibitory effect on KNa channel currents, suggesting that NO suppresses KNa channel activity in Kenyon cells. NO is known to directly modulate ion channels via nitrosylation and has indirect effects via a second messenger cascade (Ahern et al. 2002). The present study showed that the inhibitory effect of GSNO on KNa channel activity is largely blocked by inhibitors of sGC and PKG. Additionally, our previous report showed that a cell-permeant cGMP analog, 8-BrcGMP, inhibited KNa channel activity similar to NO donors (Aoki et al. 2008). These results and the previous reports indicate that NO indirectly modulates KNa channel currents via the sGC/cGMP/PKG signaling pathway in Kenyon cells. Previous reports have suggested that the NO-cGMP signaling cascade modulates ion channels and/or receptor activity and is involved in various physiological functions, such as the formation of memory (Abi-Gerges et al. 2001; Hölscher and Rose 1992; Oh 1995; Schröder et al. 2003). Similarly, in Kenyon cells, the NO-cGMP signaling cascade is reported to play an important role in the formation of long-term memory in olfactory associative learning (Matsumoto et al. 2006, 2009). Therefore, NO-cGMP modulation of KNa channel currents might be related to the formation of long-term memory in Kenyon cells.
ACh suppresses KNa channel currents via M1 muscarinic receptor signaling pathway.
Our present study showed that ACh inhibits KNa channel activity similar to NO donors. Therefore, we analyzed the downstream cascade of ACh and its relation to the NO signaling pathway. First, we examined what kind of ACh receptors are associated with ACh's inhibitory effect on KNa channel activity. ACh receptors are widely divided into nicotinic and muscarinic receptors. While many analyses of physiological and molecular properties of nicotinic ACh receptors have been reported, the pharmacological and molecular properties of muscarinic ACh receptors in insects have been poorly understood. Actually, six distinct muscarinic ACh receptor subtypes (m1, m2, m3, m4, m5, m6) have been identified in the vertebrates, whereas until now only one gene encoding muscarinic ACh receptor has been cloned in insects (Onai et al. 1989; Shapiro et al. 1989). However, although the properties of insect muscarinic receptors are not completely understood, some reports have suggested that in insects there are also multiple muscarinic ACh receptor subtypes, which have M1/M3-like and/or M2-like properties (Hannan and Hall 1993; Trimmer 1995). In the present study, we found that this inhibitory effect of ACh was blocked by an antagonist of M1 muscarinic receptors, pirenzepine, but was not blocked by the M3 muscarinic receptor antagonist 4-DAMP or the nicotinic acetylcholine receptor antagonist mecamylamine. Additionally, ACh inhibition was eliminated by an inhibitor of PLC, which is known to be coupled with M1 muscarinic receptors. These results strongly suggest that ACh inhibits KNa channel currents via the M1 muscarinic receptor signaling pathway. In the present study, the nicotinic ACh receptor inhibitor mecamylamine did not block the inhibitory effect of ACh on KNa channel activity. However, mecamylamine-sensitive nicotinic ACh receptors are thought to be related to the formation of olfactory memory and learning in mushroom bodies (Watanabe et al. 2010). In the present study, we used a Ca2+-free bath solution to record only KNa channel currents, based on previous methods (Aoki et al. 2008; Nakamura and Yoshino 2013). Additionally, mushroom body Kenyon cells express functional α-bungarotoxin-sensitive nicotinic receptors in addition to mecamylamine-sensitive nicotinic receptors (Cayre et al. 1999). Therefore, it is possible that Ca2+ influx caused by nicotinic ACh receptor activation might affect ion channel activity in Kenyon cells. Interestingly, a previous study of insect neurons indicated that nicotinic receptors are functionally coupled to the NO-cGMP signaling pathway (Zayas et al. 2002). Future studies analyzing the effect of both muscarinic and nicotinic receptors on ion channel activities in Kenyon cells are required.
ACh signaling pathway promotes NO production via Ca2+/CaM-NOS activation.
Muscarinic ACh receptor signaling pathways are suggested to stimulate the NO-cGMP signaling cascade via Ca2+/CaM-dependent activation of NOS (Hu and el-Fakahany 1993; McKinney et al. 1990; Thompson et al. 1995). We found that the inhibitory effect of ACh is blocked by the CaM inhibitor W-7 and the NOS inhibitor l-NAME, suggesting that the ACh inhibition of KNa channel activity in Kenyon cells is related to Ca2+/CaM and NOS activation. The M1 receptor-PLC signaling pathway is known to induce the production of IP3 and the release of Ca2+ from IP3-sensitive Ca2+ stores, which successively may promote Ca2+/CaM-NOS activation. In Kenyon cells, the release of Ca2+ from IP3-sensitive Ca2+ stores has not been detected. In a future study, we hope to analyze the function of IP3-sensitive Ca2+ stores in Kenyon cells.
Possible site of NO production.
Malaterre et al. (2002) showed that the large Kenyon cells project their dendrites into the posterior calyx whereas the small Kenyon cells project into the anterior calyx in the cricket Acheta domesticus. Cayre et al. (2005) first showed that NOS mRNA is expressed in the cell bodies of the large Kenyon cells in A. domesticus. Later, Takahashi et al. (2009) showed that silencing of NOS expression by systemic RNAi impairs long-term memory formation in the cricket G. bimaculatus. Using NADPH diaphorase staining and anti-citrulline immunoreactivity, Cayre et al. (2005) demonstrated that NOS was highly expressed in the calyx zona externa as well as in the large cells over the posterior calyx. In the present study, we selectively used large Kenyon cells (more than ∼10 μm) to analyze the effects of ACh and NO on KNa channel activity. We found that almost all large Kenyon cells responded to the application of NO donors and ACh. Whether the small Kenyon cells respond to NO donor or ACh should be determined in future studies. Although our study used cell bodies, we think that NOS protein is also expressed in the dendrites of calyx neuropile and axons in the peduncle and lobes of Kenyon cells as reported by Cayre et al. (2005). NO is probably produced within a calyx region in large Kenyon cells, modifies the ion channels within its own plasma membrane, and diffuses into neighboring cells at the level of the calyx region including smaller Kenyon cells (NO-receptive Kenyon cells).
Physiological importance of ACh-NO modulation of KNa channel activity.
In the present study, we showed that the ACh-NO signaling cascade modulates KNa channel activity in Kenyon cells. The KNa channels are encoded by two genes, Slack and Slick (Bhattacharjee et al. 2003; Joiner et al. 1998; Yuan et al. 2003). Because KNa channels are activated by intracellular Na+, KNa channel activities are coupled with the Na+ influx through Na+ channels. Notably, studies of central cultured neurons dissociated from olfactory bulbs have reported a strong relationship between KNa channels and persistent Na+ channels (Budelli et al. 2009; Hage and Salkoff 2012). Recently, similar functional coupling was demonstrated in Kenyon cells of the cricket G. bimaculatus (Takahashi and Yoshino 2015). This coupling mechanism has been shown to play an important role in producing membrane oscillation because this oscillation is eliminated by applying the persistent Na+ channel blocker TTX or the KNa channel blocker quinidine (Inoue et al. 2014). During this membrane oscillation, persistent Na+ channel currents contribute to the depolarization phase and KNa channel currents contribute to the subsequent hyperpolarization phase (Inoue et al. 2014). Therefore, the ACh-NO cascade's inhibition of KNa channels may induce the long-lasting depolarization and increase the excitability of Kenyon cells.
Recently, Kosakai et al. (2015) provided evidence that NO augments single-L-type Ca2+ channel currents via cGMP/PKG signaling pathway. The authors suggested that an increase in Ca2+ influx mediated by NO/cGMP/PKG signaling pathway may be responsible for spike afterhyperpolarization by activating large-conductance Ca2+-activated K+ (BK) channels and this increase in afterhyperpolarization contributes to accelerate the recovery of Na+ channels from voltage-dependent inactivation following an action potential. Additionally, our preliminary study indicates that NO increases Na+ currents (both INaf and INap). As a result of the coordinated action of NO on ionic channels (inhibition of IKNa, excitation of INaf, INap, ICa, and IKCa), membrane excitability of Kenyon cells seems to increase. Kosakai et al. (2015) also showed that NO increases the frequency and the number of action potentials elicited by depolarizing current injection similar to the action of ACh (Terazima and Yoshino 2010) in cricket Kenyon cells. These reports seem to further support our hypothesis that ACh-NO acts as an excitatory modulator on Kenyon cells by interacting with many ion channels. Although the role of this ACh/NO excitatory modulation in the formation of learning and memory within mushroom bodies is not completely understood, Matsumoto et al. (2006, 2013) suggested the importance of the NO-cGMP/cAMP signal cascades in long-term olfactory learning. One possibility is that the increased number of spikes within the Kenyon cells induced by ACh-NO signaling may modify the synaptic plasticity between presynaptic Kenyon cells and postsynaptic output neurons via spike timing-dependent plasticity as Cassenaer and Laurent (2012) showed in the locust. This modulation of synaptic plasticity might contribute to the formation of associative learning memory in mushroom bodies. In olfactory associative learning memory, octopamine and dopamine, which convey the information of an unconditioning stimulus, play an essential role. These monoamines also modulate KNa channel activities in cricket Kenyon cells (Aoki et al. 2008). Further physiological analyses of the interactive manner between ACh-NO and octopamine/dopamine signaling cascades in Kenyon cells will reveal the mechanisms underlying long-term learning memory in insects.
GRANTS
This work was supported by Grants-in-Aid for Scientific Research (C) 24570083 and 15K07145.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.H. and M.Y. conception and design of research; M.H. and M.Y. performed experiments; M.H. and M.Y. analyzed data; M.H. and M.Y. interpreted results of experiments; M.H. and M.Y. prepared figures; M.H. and M.Y. drafted manuscript; M.H. and M.Y. edited and revised manuscript; M.H. and M.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Professor Dr. Kenji Karino (Tokyo Gakugei University) for his help with statistical analysis.
Present address of M. Hasebe: Dept. of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo, Japan.
REFERENCES
- Abi-Gerges N, Fischmeister R, Méry PF. G protein-mediated inhibitory effect of a nitric oxide donor on the L-type Ca2+ current in rat ventricular myocytes. J Physiol 531: 117–130, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25: 510–517, 2002. [DOI] [PubMed] [Google Scholar]
- Aoki K, Kosakai K, Yoshino M. Monoaminergic modulation of the Na+-activated K+ channel in Kenyon cells isolated from the mushroom body of the cricket (Gryllus bimaculatus) brain. J Neurophysiol 100: 1211–1222, 2008. [DOI] [PubMed] [Google Scholar]
- Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263: 692–695, 1994. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci 23: 11681–11691, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G, Schmachtenberg O, De Vente J. The nitric oxide/cyclic GMP messenger system in olfactory pathways of the locust brain. Eur J Neurosci 8: 2635–2643, 1996. [DOI] [PubMed] [Google Scholar]
- Böhme GA, Bon C, Stutzmann JM, Doble A, Blanchard JC. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol 199: 379–381, 1991. [DOI] [PubMed] [Google Scholar]
- Boulton CL, Southam E, Garthwaite J. Nitric oxide-dependent long-term potentiation is blocked by a specific inhibitor of soluble guanylyl cyclase. Neuroscience 69: 699–703, 1995. [DOI] [PubMed] [Google Scholar]
- Budelli G, Hage TA, Wei A, Rojas P, Jong YJ, O'Malley K, Salkoff L. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci 12: 745–750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature 482: 47–52, 2012. [DOI] [PubMed] [Google Scholar]
- Cayre M, Buckingham D, Yagodin S, Sattelle DB. Cultured insect mushroom body neurons express functional receptors for acetylcholine, GABA, glutamate, octopamine, and dopamine. J Neurophysiol 81: 1–14, 1999. [DOI] [PubMed] [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Holstein GR, Forni C, Nicolas S, Aouane A, Strambi C, Strambi A. A role of nitric oxide in sensory-induced neurogenesis in an adult insect brain. Eur J Neurosci 21: 2893–2902, 2005. [DOI] [PubMed] [Google Scholar]
- Doyle C, Hölscher C, Rowan MJ, Anwyl R. The selective neuronal NO synthase inhibitor 7-nitro-indazole blocks both long-term potentiation and depotentiation of field EPSPs in rat hippocampal CA1 in vivo. J Neurosci 16: 418–424, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage TA, Salkoff L. Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J Neurosci 32: 2714–2721, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley JE, Malen PL, Chapman PF. Nitric oxide synthase inhibitors block long-term potentiation induced by weak but not strong tetanic stimulation at physiological brain temperatures in rat hippocampal slices. Neurosci Lett 160: 85–88, 1993. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjection of octopamine in honeybees. Learn Mem 5: 146–156, 1998. [PMC free article] [PubMed] [Google Scholar]
- Hannan F, Hall LM. Muscarinic acetylcholine receptors in invertebrates: comparisons with homologous receptors from vertebrates. EXS 63: 98–145, 1993. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci 4: 266–275, 2003. [DOI] [PubMed] [Google Scholar]
- Hölscher C, Rose SP. An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci Lett 145: 165–167, 1992. [DOI] [PubMed] [Google Scholar]
- Hu J, el-Fakahany EE. Role of intercellular and intracellular communication by nitric oxide in coupling of muscarinic receptors to activation of guanylate cyclase in neuronal cells. J Neurochem 61: 578–585, 1993. [DOI] [PubMed] [Google Scholar]
- Inoue S, Murata K, Tanaka A, Kakuta E, Tanemura S, Hatakeyama S, Nakamura A, Yamamoto C, Hasebe M, Kosakai K, Yoshino M. Ionic channel mechanisms mediating the intrinsic excitability of Kenyon cells in the mushroom body of the cricket brain. J Insect Physiol 68: 44–57, 2014. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, Gribkoff VK, Kaczmarek LK. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat Neurosci 1: 462–469, 1998. [DOI] [PubMed] [Google Scholar]
- Kosakai K, Tsujiuchi Y, Yoshino M. Nitric oxide augments single Ca2+ channel currents via cGMP-dependent protein kinase in Kenyon cells isolated from the mushroom body of the cricket brain. J Insect Physiol 78: 26–32, 2015. [DOI] [PubMed] [Google Scholar]
- Kreissl S, Bicker G. Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. J Comp Neurol 286: 71–84, 1989. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11: 3218–3226, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano VC, Armengaud C, Gauthier M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. J Comp Physiol A 187: 249–254, 2001. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci 19: 10250–10261, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaterre J, Strambi C, Chiang AS, Aouane A, Strambi A, Cayre M. Development of cricket mushroom bodies. J Comp Neurol 452: 215–227, 2002. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Hatano A, Unoki S, Mizunami M. Stimulation of the cAMP system by the nitric oxide-cGMP system underlying the formation of long-term memory in an insect. Neurosci Lett 467: 81–85, 2009. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Hirashima D, Terao K, Mizunami M. Roles of NO signaling in long-term memory formation in visual learning in an insect. PLoS One 8: e68538, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Mizunami M. Olfactory learning in the cricket Gryllus bimaculatus. J Exp Biol 203: 2581–2588, 2000. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Mizunami M. Lifetime olfactory memory in the cricket Gryllus bimaculatus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 295–299, 2002. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Unoki S, Aonuma H, Mizunami M. Critical role of nitric oxide-cGMP cascade in the formation of cAMP-dependent long-term memory. Learn Mem 13: 35–44, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science 293: 1330–1333, 2001. [DOI] [PubMed] [Google Scholar]
- McKinney M, Bolden C, Smith C, Johnson A, Richelson E. Selective blockade of receptor-mediated cyclic GMP formation in N1E-115 neuroblastoma cells by an inhibitor of nitric oxide synthesis. Eur J Pharmacol 178: 139–140, 1990. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Yoshino M. A novel GABAergic action mediated by functional coupling between GABAB-like receptor and two different high-conductance K+ channels in cricket Kenyon cells. J Neurophysiol 109: 1735–1745, 2013. [DOI] [PubMed] [Google Scholar]
- Oh S. The generation of nitric oxide and its roles in neurotransmission and neurotoxicity. Keio J Med 44: 53–61, 1995. [DOI] [PubMed] [Google Scholar]
- Onai T, FitzGerald MG, Arakawa S, Gocayne JD, Urquhart DA, Hall LM, Fraser CM, McCombie WR, Venter JC. Cloning, sequence analysis and chromosome localization of a Drosophila muscarinic acetylcholine receptor. FEBS Lett 255: 219–225, 1989. [DOI] [PubMed] [Google Scholar]
- Ott SR, Philippides A, Elphick MR, O'Shea M. Enhanced fidelity of diffusive nitric oxide signalling by the spatial segregation of source and target neurones in the memory centre of an insect brain. Eur J Neurosci 25: 181–190, 2007. [DOI] [PubMed] [Google Scholar]
- Schröder F, Klein G, Fiedler B, Bastein M, Schnasse N, Hillmer A, Ames S, Gambaryan S, Drexler H, Walter U, Lohmann SM, Wollert KC. Single L-type Ca2+ channel regulation by cGMP-dependent protein kinase type I in adult cardiomyocytes from PKG I transgenic mice. Cardiovasc Res 60: 268–277, 2003. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science 254: 1503–1506, 1991. [DOI] [PubMed] [Google Scholar]
- Shapiro RA, Wakimoto BT, Subers EM, Nathanson NM. Characterization and functional expression in mammalian cells of genomic and cDNA clones encoding a Drosophila muscarinic acetylcholine receptor. Proc Natl Acad Sci USA 86: 9039–9043, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature 349: 326–328, 1991. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Hamada A, Miyawaki K, Matsumoto Y, Mito T, Noji S, Mizunami M. Systemic RNA interference for the study of learning and memory in an insect. J Neurosci Methods 179: 9–15, 2009. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Yoshino M. Functional coupling between sodium-activated potassium channels and voltage-dependent persistent sodium currents in cricket Kenyon cells. J Neurophysiol 114: 2450–2459, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K. Classical conditioned response in the honey bee. J Insect Physiol 6: 168–179, 1961. [Google Scholar]
- Terazima E, Yoshino M. Modulatory action of acetylcholine on the Na+-dependent action potentials in Kenyon cells isolated from the mushroom body of the cricket brain. J Insect Physiol 56: 1746–1754, 2010. [DOI] [PubMed] [Google Scholar]
- Thompson SH, Mathes C, Alousi AA. Calcium requirement for cGMP production during muscarinic activation of N1E-115 neuroblastoma cells. Am J Physiol Cell Physiol 269: C979–C985, 1995. [DOI] [PubMed] [Google Scholar]
- Trimmer BA. Current excitement from insect muscarinic receptors. Trends Neurosci 18: 104–111, 1995. [PubMed] [Google Scholar]
- Watanabe H, Matsumoto CS, Nishino H, Mizunami M. Critical roles of mecamylamine-sensitive mushroom body neurons in insect olfactory learning. Neurobiol Learn Mem 95: 1–13, 2010. [DOI] [PubMed] [Google Scholar]
- Williamson SM, Wright GA. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216: 1799–1807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schümann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol 445: 211–226, 2002. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schürmann FW. Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster. J Comp Neurol 466: 299–315, 2003. [DOI] [PubMed] [Google Scholar]
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37: 765–773, 2003. [DOI] [PubMed] [Google Scholar]
- Zayas RM, Qazi S, Morton DB, Trimmer BA. Nicotinic-acetylcholine receptors are functionally coupled to the nitric oxide/cGMP-pathway in insect neurons. J Neurochem 83: 421–431, 2002. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature 368: 635–639, 1994. [DOI] [PubMed] [Google Scholar]