Abstract
Saccadic eye movements can be elicited by more than one type of sensory stimulus. This implies substantial transformations of signals originating in different sense organs as they reach a common motor output pathway. In this study, we compared the prevalence and magnitude of auditory- and visually evoked activity in a structure implicated in oculomotor processing, the primate frontal eye fields (FEF). We recorded from 324 single neurons while 2 monkeys performed delayed saccades to visual or auditory targets. We found that 64% of FEF neurons were active on presentation of auditory targets and 87% were active during auditory-guided saccades, compared with 75 and 84% for visual targets and saccades. As saccade onset approached, the average level of population activity in the FEF became indistinguishable on visual and auditory trials. FEF activity was better correlated with the movement vector than with the target location for both modalities. In summary, the large proportion of auditory-responsive neurons in the FEF, the similarity between visual and auditory activity levels at the time of the saccade, and the strong correlation between the activity and the saccade vector suggest that auditory signals undergo tailoring to match roughly the strength of visual signals present in the FEF, facilitating accessing of a common motor output pathway.
Keywords: multisensory, saccade, frontal eye field (FEF), auditory
both sounds and visual stimuli can elicit saccades, or rapid movements of the eyes that bring stimuli of interest onto the fovea. The eye movements are qualitatively similar regardless of the sensory trigger (Frens and Van Opstal 1995; Jay and Sparks 1990; Metzger et al. 2004; Yao and Peck 1997; Zambarbieri et al. 1982). This suggests that the brain must transform signals evoked by two very different forms of stimulus energy (light and sound) and beginning in separate places (the eyes and ears) into commands that converge on a common motor pathway and produce very similar patterns of muscle contractions. We seek to shed light on this signal transformation by comparing the properties of auditory- and visual-evoked activity in the primate frontal eye fields (FEF).
The FEF is important for saccade generation. Electrical stimulation of the FEF produces saccades with a short latency (Bruce et al. 1985; Robinson and Fuchs 1969). FEF lesions, whether reversible or permanent, cause deficits in saccade performance (Dias and Segraves 1999; Schiller et al. 1980, 1987; Sommer and Tehovnik 1997), as can transcranial magnetic stimulation (Priori et al. 1993; Ro et al. 1997; Wipfli et al. 2001). However, these studies have largely focused on the causal role of the FEF in visually and memory-guided saccades, leaving its functional contribution to saccades evoked by auditory stimuli unexplored.
Auditory-evoked activity has been previously reported in the primate FEF (Bruce and Goldberg 1985; Mohler et al. 1973; Schall 1991) and adjacent regions (Vaadia et al. 1986). Data from candidate homologs of the FEF in other mammalian species have generally focused exclusively on visually guided eye movements (Weyand and Gafka 1998; Weyand et al. 1999). However, many studies in the cat have revealed multisensory contributions originating from structures in the temporal lobe to signals in other oculomotor structures (e.g., Alvarado et al. 2007, 2009; Chabot et al. 2013; Meredith and Clemo 1989; Royal et al. 2009). Although these areas are probably not directly homologous to the primate FEF, such studies illustrate the likely importance of cortical contributions to multisensory behavior.
Although it is clear that the FEF contributes to saccade programming and contains auditory activity, it is not yet known how auditory activity in this structure compares with visual activity and whether the auditory activity patterns of the FEF retain significant signatures of their different sensory origin or if they have become sufficiently comparable with visual signals to account for the similarity of visual and auditory saccades. In this study, we address this question, focusing on the proportion of FEF neurons active for auditory saccades and the amount of activity evoked across the population compared with the visual-evoked activity in the same neurons.
We found a high fraction of neurons exhibiting auditory responses in FEF: from 64% at the onset of an auditory target to 87% soon before a saccade was made toward it. This proportion greatly exceeds 2–10% reported in early studies that did not use an auditory saccade task (Bruce and Goldberg 1985; Mohler et al. 1973) and approaches the 100% reported by a study that did (Russo and Bruce 1994). Auditory activity was lower than visual activity throughout most of the period from target onset until about 100–200 ms before saccade onset, illustrating that initially some signatures of sensory origin remain in the FEF. However, beginning about 100–200 ms before saccade onset, visual and auditory activity were nearly identical. This suggests that FEF activity may be evaluated by downstream targets and exert its influence on saccade programming during this specific period of time. This observation is therefore reminiscent of related findings concerning visually evoked activity and how saccades are triggered or aborted using reaction time or countermanding saccade tasks (Hanes and Schall 1995, 1996).
Our data suggest a reevaluation of the FEF as a multisensory area akin to the superior colliculus (SC) and more than just an eye field for visually guided movements. The FEF seems capable of contributing to a command to guide auditory-evoked eye movements. If the readout of the FEF occurs during the period of time when visual and auditory activity are most similar, little normalization of this command would be necessary to ensure that the eye movement is similar regardless of whether it is evoked by a visual or auditory stimulus.
MATERIALS AND METHODS
Subjects.
All procedures conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health 2011) and were approved by the Institutional Animal Care and Use Committee of Duke University. Two adult rhesus monkeys (Macaca mulatta) participated (monkey F, male, and monkey N, female). Under general anesthesia and using sterile surgical procedures, we first implanted a headpost holder to restrain the head and a scleral search coil to track eye movements (Judge et al. 1980; Robinson 1963). After recovery under veterinary observation, we trained the monkeys in the experimental task. In a second surgery, we implanted a recording cylinder (2-cm diameter) over the left or right FEF. We determined the location of the cylinder with stereotactic coordinates (24–26 mm anterior with respect to the interaural axis and 15–16 mm lateral with respect to the midline) and verified it with MRI scans at the Duke Center for Advanced Magnetic Resonance Development (Fig. 1A) and with microstimulation during the recording sessions (Bruce et al. 1985).
Fig. 1.
Experimental paradigm. A: an MRI image of the grid and electrode placement on the right FEF of monkey N. SAR, superior arcuate sulcus; IAR, inferior arcuate sulcus. B: location of visual and auditory stimuli. deg, Degrees. C: overlap saccade task. The visual or auditory target was presented while the monkey's gaze was directed toward an initial fixation light. After an overlap time of 600–900 ms, the fixation light was turned off and the monkey initiated a saccade toward the target. The row of fixation lights could be either above or below the row of saccade targets to facilitate placement of the latter in the receptive fields of FEF neurons.
Experimental setup.
The experiment took place in a dark (monkey F, male) or dimly illuminated (monkey N, female) sound-attenuated room. Dim illumination prevented normal dark-induced nystagmus in monkey N (Mulch and Lewitzki 1977) but did not provide any useful visual cues. Indeed, performance was comparable between the two subjects (see results) and with previous studies in complete darkness (Metzger et al. 2004; Mullette-Gillman et al. 2005, 2009).
The monkeys sat in a primate chair, with their heads restrained, at a distance of 150 cm from a board of stimuli. An array of nine speakers and nine light-emitting diodes (LEDs; each attached to the center of a speaker) was situated at 0° elevation on the horizontal meridian (range ±24°, increments 6°; Fig. 1B). These served as target stimuli. The board also contained LEDs that were used as fixation lights. These were presented at a variable elevation above or below the speaker array, at horizontal locations of −12, 0, and 12° (Fig. 1B).
The auditory stimuli were band-passed white-noise bursts (500 Hz to 18 kHz, 55-dB sound pressure level, rise time of 10 ms, variable duration) played by Cambridge SoundWorks MC50 speakers. The visual stimuli were green light spots (0.55 min of arc, luminance of 26.4 cd/m2) produced by LEDs.
Control of the behavioral paradigms and collection of eye position and neural data were accomplished using the Beethoven program (Ryklin Software). Eye position was tracked via a scleral search coil in most sessions (see Subjects). For monkey F, we also used an optical eye-tracking system (EyeLink 1000; SR Research, Ontario, Canada) in a minority of recording sessions (62 sessions with eye tracker vs. 171 sessions with scleral search coil; the total number of recording sessions in monkey F was 233).
Behavioral task.
The training procedure is described in Metzger et al. (2004). We used an overlap task to dissociate sensory-related activity from motor-related activity (Fig. 1C). A trial started with the presentation of a fixation light. The monkey was required to initiate fixation within 3,000 ms and maintain it (within a square window of ±3°) until the fixation light was extinguished. After 900-1,200 ms from fixation onset, a target (visual or auditory) was presented in one of nine possible positions (Fig. 1B). The fixation light and the target both stayed on for an overlap period of 600–900 ms, after which the fixation light was turned off and the monkey was required to make a saccade to the target. Saccades performed within 500 ms and followed by a fixation period of 200–500 ms were considered correct with a tolerance of ±4° vertical and ±3° horizontal around the target. We rewarded correct trials with a few drops of juice or water and penalized incorrect trials with a time out of 1 s.
Recordings.
We recorded single-cell extracellular activity with tungsten microelectrodes (FHC; impedance between 0.7 and 2.5 MΩ at 1 kHz). A grid system (Crist et al. 1988) and a stainless steel guide tube supported and directed the electrode. We manually inserted the guide tube through the dura and then advanced the electrode with a hydraulic pulse microdrive (Narishige MO-95) while the monkey performed visual- and auditory-guided saccade task. We isolated single neurons online using a Plexon system (Sort Client software; Plexon) and recorded the time of each action potential for offline analysis. For each isolated neuron, we qualitatively selected one fixation elevation (range −12 to +14° relative to horizontal) that allowed the best sampling of its response field (the horizontal positions of the fixation lights and the locations of the targets were the same for all recordings; Fig. 1B). Data were collected as long as the neuron was well-isolated and the monkey performed the task (average 578 ± 185 SD correct trials for each neuron).
On some sessions, we confirmed recordings from within FEF by microstimulation. After recordings, the same electrode was used to inject current in trains of bipolar pulses at 300 Hz (rectangular pulses with 0.2-ms duration, 0.1 ms between pulses, negative pulse leading). The train duration was 100 ms. Sites where a current ≤50 μA elicited a contralateral saccade were considered part of FEF (Robinson and Fuchs 1969). Adjacent sites were considered FEF if they lay between two confirmed sites or if they had similar sensory and saccade activities during the task. All neurons isolated within these sites were analyzed, including those that were not responsive during either visual or auditory trials.
Eye position was sampled at 500 Hz. We did not monitor pinna movements, as these have previously been found to be small and uncorrelated with eye movements (Groh et al. 2001; Werner-Reiss et al. 2003).
Analysis.
All analyses were conducted with custom-made routines in MATLAB (The MathWorks). This study was part of an ongoing investigation of the reference frame of target representation in FEF in comparison with the SC and parietal cortex (Lee and Groh 2012, 2014; Mullette-Gillman et al. 2005, 2009). Accordingly, we varied initial fixation position while holding the head immobile to disambiguate eye- and head-centered frames of reference. All analyses reported here, with the exception of the responsiveness and spatial selectivity analysis (Table 1 and Fig. 6), include only trials starting from the central fixation location. Thus, for the present study, reference frame is held constant.
Table 1.
Responsiveness and spatial selectivity
| Visual |
Auditory |
Both |
||||
|---|---|---|---|---|---|---|
| Total = 324 cells | n | % | n | % | n | % |
| Sensory period | ||||||
| A: Responsiveness (t-test) | 245 | 75.6 | 209 | 64.5 | 169 | 52.2 |
| B: ANOVA: head-centered target × eye position (main effect for target location or interaction between the target and fixation locations) | 142 | 43.8 | 50 | 15.4 | 30 | 9.3 |
| C: ANOVA: eye-centered target × eye position (main effect for target location or interaction between the target and fixation locations) | 148 | 45.7 | 66 | 20.4 | 34 | 10.5 |
| D: B or C | 174 | 53.7 | 87 | 26.9 | 51 | 15.7 |
| Motor period | ||||||
| E: Responsiveness (t-test) | 272 | 84.0 | 283 | 87.3 | 246 | 75.9 |
| F: ANOVA: head-centered target × eye position (main effect for target location or interaction between the target and fixation locations) | 115 | 35.5 | 96 | 29.6 | 49 | 15.1 |
| G: ANOVA: eye-centered target × eye position (main effect for target location or interaction between the target and fixation locations) | 135 | 41.7 | 100 | 30.2 | 59 | 18.2 |
| H: F or G | 160 | 49.4 | 142 | 43.8 | 89 | 27.5 |
| Sensory and motor period | ||||||
| I: D and H | 117 | 36.1 | 47 | 14.5 | 26 | 8.0 |
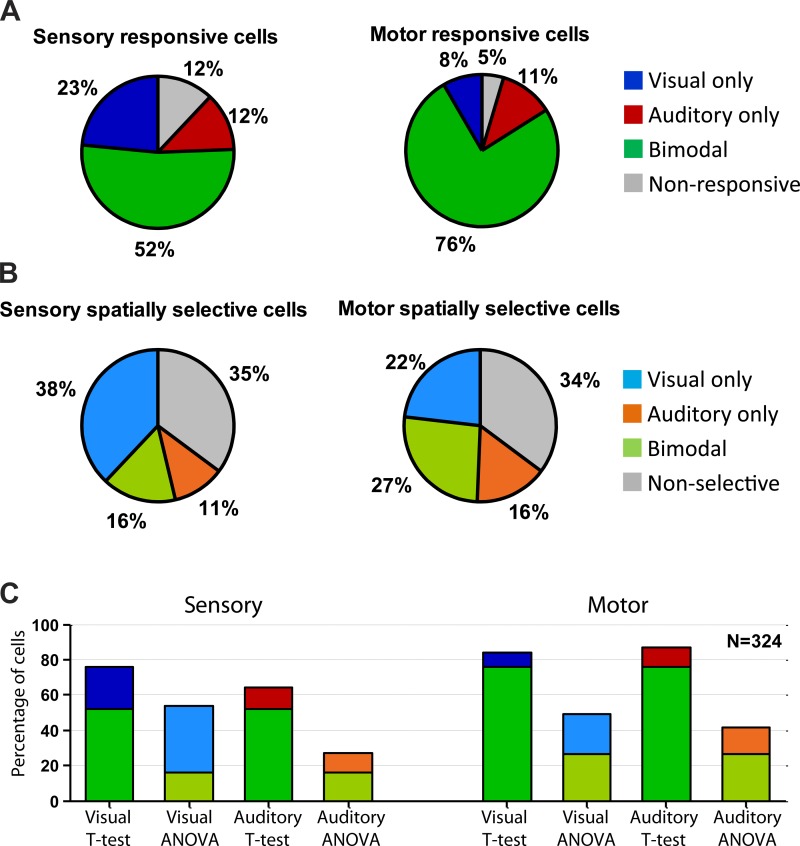
Fig. 6.
Proportions of responsive and spatially selective cells. A: responsiveness to visual and/or auditory targets was statistically assessed using a 2-tailed t-test comparing the activity in the sensory (left) and motor (right) periods with baseline (P < 0.05). B: spatial selectivity was assessed with an analysis of variance (ANOVA) for the sensory (left) and motor (right) periods. Details of these tests are also provided in Table 1. C: the proportions of significantly responsive neurons were generally higher when using the t-test than using the ANOVA.
To measure responsiveness and spatial selectivity (Table 1 and Fig. 6) across the population of cells, we calculated the average firing rate in three periods (Fig. 1C): 1) a baseline period, comprising the 0–500 ms of fixation before the target onset; 2) a sensory period, consisting of 0–500 ms after target onset; and 3) a motor period, starting 50 ms before the onset of the saccade to the target and ending at the offset of the saccade. The sensory period was chosen to match the ones used in our previous studies with the same task in the SC and lateral and medial intraparietal cortex (Lee and Groh 2012, 2014; Mullette-Gillman et al. 2005, 2009) and is consistent with previous studies that have noted a continuum of sensory-like and motor-like elements of FEF activity (Bruce and Goldberg 1985; Jantz et al. 2013; Lawrence et al. 2005; Mohler et al. 1973; Schall 1991; Schall and Hanes 1993; Schall et al. 1995; Schall and Thompson 1999; Segraves and Goldberg 1987; Wurtz et al. 2001). The sensory period captures both the transient and sustained visual and auditory responses to the target while the gaze is stationary on the fixation light. We have also repeated the analyses with a shorter sensory window (0–200 ms after target onset) and found similar results. The motor period, variable with saccade duration, captures the saccade related burst: we chose a 50-ms advance interval based on the average latency of stimulation-evoked saccades reported in the literature (Bruce et al. 1985). This value is within the range of average latencies of the electrically evoked saccades in 175 of our recording sites across both monkeys [70 ± 34 ms (mean ± SD)]. Saccade onset and offset were measured with a tangential velocity threshold of 25°/s. Only correct trials were included in this analysis.
Neurons were considered responsive in the visual/auditory modality and sensory/motor intervals if a 2-tailed t-test between their baseline activity and relevant response period was significant (Table 1 and Fig. 6). To accommodate the different durations of the response windows, activity was expressed in units of spikes per second. To allow comparison of the present results with our previous related studies in SC and parietal cortex (Lee and Groh 2012, 2014; Mullette-Gillman et al. 2005, 2009), we remained agnostic about reference frame and assessed the spatial selectivity of responses (firing rate in the sensory or motor period and visual or auditory modality) in both head- and eye-centered reference frames using two two-way ANOVAs. Each ANOVA involved the three levels of initial eye position (−12, 0, and +12°) as well as five levels of target location (−12 to +12° in 6° increments) defined in head-centered coordinates for the first ANOVA and in eye-centered coordinates for the second ANOVA. Cells were classified as spatially selective if either of the two ANOVAs yielded a significant main effect for target location or a significant interaction between the target and fixation locations (Table 1 and Fig. 6). These were the same inclusion criteria used for our prior studies in SC and parietal cortex (Lee and Groh 2012, 2014; Mullette-Gillman et al. 2005, 2009). In all tests, statistical significance was defined as P value <0.05; to be consistent with our previous analyses, we did not apply Bonferroni correction.
Single-cell perievent time histograms (PETH) were calculated for each target and each modality by averaging the number of action potentials within successive bins of 25 ms aligned to the target onset or the saccade onset across all correct trials involving the central fixation (Figs. 3–5). To compute the “raw” population PETH, we first obtained single PETH to all visual or auditory targets and then averaged across all neurons recorded in the two monkeys (Fig. 7, A and B). Similarly, the normalized population PETH was obtained by averaging the normalized individual PETH across all recorded cells (Fig. 7, C and D). The normalization consisted of subtracting the average baseline and dividing by the maximum activity bin observed for any target for that neuron, regardless of whether it was visual or auditory, so that the units of activity became percentage of maximum activity.
Fig. 3.
Raster plot and perievent time histogram (PETH) of an example cell. This cell responded vigorously to the onset of contralateral visual and auditory targets. The activity persisted in time until a saccade was made. A: raster plots for visual and auditory trials aligned to the target and the saccade onset. B: PETH for visual and auditory trials aligned to the target and the saccade onset. PETH were obtained by averaging the number of action potentials within running bins of 10 ms aligned to the target onset or saccade onset [smoothed using a half-triangular filter (2/3, 1/3). The points were attributed to the time of the value receiving the 2/3 weight]. Colors indicate horizontal target locations. The colored tick marks in B indicate the average saccade offset for each target location.
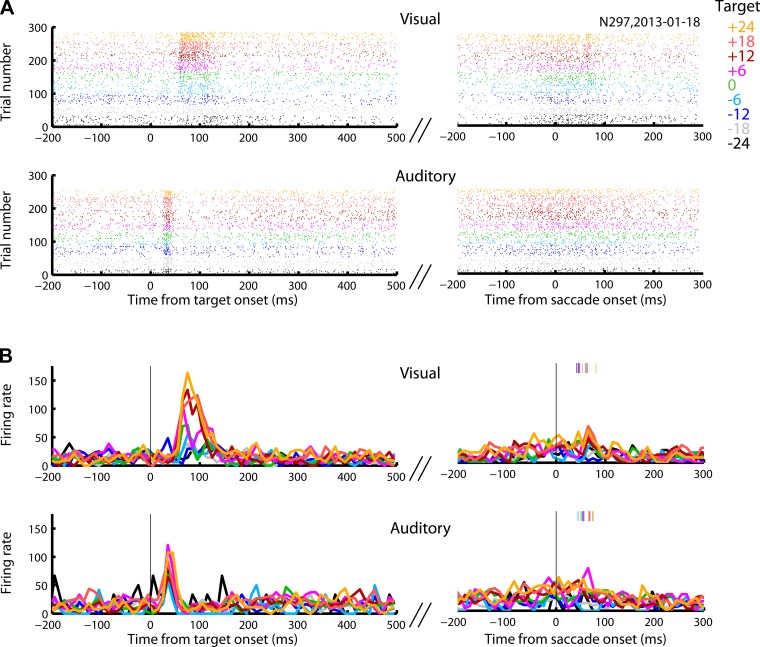
Fig. 5.
Raster plot and perievent time histogram (PETH) of an example cell. This cell was activated transiently by the onset of visual targets and more strongly by the saccades to them. In the auditory modality, the response to sound onset was much smaller, but the activity during auditory saccades was comparable with that displayed on visual saccades. A and B like in Fig. 3.
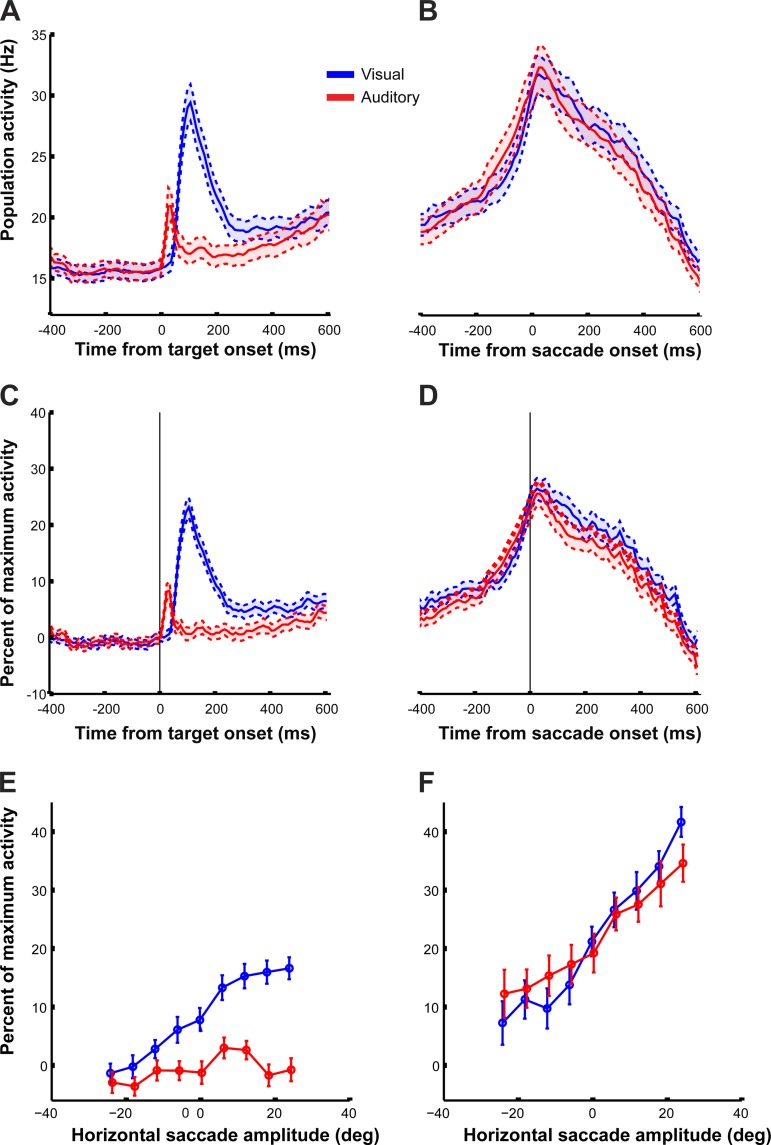
Fig. 7.
Average population activity of all neurons (324) recorded in the 2 monkeys as a function of time and horizontal target location. A: average perievent time histogram (PETH; mean ± SE) across all cells recorded for visual (blue) and auditory (red) responses aligned to the target onset. Bin size = 10 ms, no smoothing. Only trials starting from the central fixation were included. B: same as A but with traces aligned to saccade onset. C and D: same as A and B but with normalized activity (for each cell, the normalization consisted in subtracting the average baseline activity and dividing by the maximum activity level for each cell; see materials and methods). E: normalized population activity (mean ± SE) for each target during the 500-ms sensory period (see materials and methods). Only correct trials are included. Same color convention and trials inclusions as A. Auditory data are displaced slightly along the x-axis for visualization purposes. F: same as E but for the activity during the motor period.
From a similar normalized population PETH with 5-ms bins, we measured the response onset latency as the time the activity became higher than the mean ± 3 SD of the average baseline population activity before target onset.
Single-neuron spatial tuning curves were estimated by fitting a Gaussian function to the neural activity as a function of the horizontal location of the target or as a function of the horizontal amplitude of the resulting saccade. The goodness of fit was measured by comparing the coefficients of variations (r2) for each cell. For each cell, we included only correct trials starting from the central fixation position, thus eliminating reference frame as a factor. We also restricted the target locations to −18, +18° horizontal to minimize the influence of secondary saccades, as the proportion of secondary saccades increases with eccentricity for visual targets (Frost and Pöppel 1976). Note that it has previously been shown that Gaussian functions can fit response functions well even if they are monotonic, so this type of curve fit is agnostic to the question of whether auditory (or visual) response functions in the FEF are typically circumscribed or predominantly open ended (Groh et al. 2003; Porter and Groh 2006; Werner-Reiss and Groh 2008): differences between response function shape on visual vs. auditory trials have been found in the primate SC in a similar paradigm (Lee and Groh 2014).
RESULTS
Behavioral results and implications for models of saccade generation.
As has been previously reported, we observed that auditory-guided saccades are qualitatively similar to visually guided saccades. Both involve high-speed movements of the eyes, but auditory saccades are a little less accurate and slightly slower than visually guided movements to targets at the same location (Frens and Van Opstal 1995; Jay and Sparks 1990; Metzger et al. 2004; Yao and Peck 1997; Zambarbieri et al. 1982).
Figure 2, A–J, shows some examples of raw trajectories and velocity profiles of the saccades made to the same target locations in the visual and auditory conditions. Compared with visual saccades, the endpoints of auditory saccades were more variable and there were sometimes systematic displacements relative to the target (e.g., the red, auditory points in Fig. 2B are slightly above and to the left of the blue, visual points). As shown in the velocity panels of Fig. 2, F–J, the peak speeds of auditory-guided saccades were lower than those of corresponding visually guided saccades except for smaller saccades (Fig. 2, F and G). Auditory saccade trajectories could also be curved, e.g., Fig. 2, C and D, an observation previously noted (Frens and Van Opstal 1995) but will not be considered further in this study.
Fig. 2.
Saccade accuracy and velocity. A–E: examples of trajectories of visual (blue) and auditory (red) saccades to the same target locations. The black rectangles indicate the area within which a saccade was considered correct. In the low left corners is the session from which the examples were taken (N, monkey N; F, monkey F). F–J: velocity profiles of the saccades above. K: horizontal saccade accuracy (mean ± SD) for monkey F (left) and for monkey N (right). All saccades attempted within 500 ms from the go cue are included (correct and incorrect). Data pooled from all recording sessions in which eye position was sampled with a scleral search coil and the auditory data are slightly displaced along the x-axis for visualization purposes. L: vertical saccade accuracy as a function of target horizontal location (vertical location of targets did not vary). The inset shows the average vertical saccade endpoint (mean ± SD) for the 2 monkeys combined. All other details as in K. M: average auditory peak velocity as a proportion of the average visual peak velocity for the corresponding saccade amplitude range (mean ± SD, bin size = 4°). Data pooled from all recording sessions involving scleral search coil and both monkeys.
These trends were confirmed over all saccades recorded in this study in Fig. 2, K–M. In the horizontal dimension, the average endpoints of auditory-guided saccades were nearly as closely correlated with target location as were visually guided saccades: the red and blue lines are both close to the line of slope one (Fig. 2K; average error 1.2° for visual and 3.9° for auditory trials; t-test, P < 0.001) with the auditory saccades also showing greater scatter (SD 3.6° for visual and 6.8° for auditory; F test, P < 0.001). The vertical error for visual and auditory saccades to the same target location were smaller and showed less scatter (Fig. 2L; mean vertical error ± standard deviation on visual vs. auditory trials: −0.1 ± 1.6 vs. 0.4 ± 2.5°; t-test, P value <0.001; F test, P value <0.001). The auditory vertical accuracy is better than previously reported (Jay and Sparks 1990), probably due to the predictable nature of our targets: the vertical location did not vary. Peak speed covaried with amplitude, a signature characteristic distinguishing saccades from other types of eye movements (Bahill et al. 1975). However, for a given saccade amplitude, peak speed was on average lower if the target was auditory than if it was visual (Fig. 2M). The average peak velocity on auditory trials was 76% of the velocity on visual trials. There was no noticeable difference in saccade reaction time on visual and auditory trials, but it should be noted that this was not a reaction-time task: the overlap paradigm imposed a delay between the stimulus onset and the cue to make the eye movement.
Together, these observations confirm the results of previous studies that compared visually vs. auditory-guided saccades (Corneil et al. 2002; Frens and Van Opstal 1995; Hughes et al. 1998; Jay and Sparks 1990; Metzger et al. 2004; Yao and Peck 1997; Zambarbieri et al. 1982) and provide context for considering how neuronal signals in the brain generate such movements in response to sound vs. sight. Specifically, the behavioral results indicate that the command signal reaching the eye muscles may be largely similar on visual and auditory trials (i.e., the movements are clearly saccades regardless of target modality). However, there must be some differences in the command signal (perhaps inherited from the sensory inputs or perhaps originating within the motor stage) to account for the lower accuracy and slower peak velocity. We evaluated FEF activity in light of these factors to attempt to determine what aspects of FEF activity might account for both the similarities and differences between visual and auditory saccades.
Neural activity patterns: similarities and differences by target modality.
We recorded 324 neurons (233 from the left FEF of monkey F and 91 from the right FEF of monkey N). Most neurons displayed bursts of activity during saccades to either visual or auditory targets (Table 1). However, they did not always respond identically on visual and auditory trials despite the similarity of eye-movement responses.
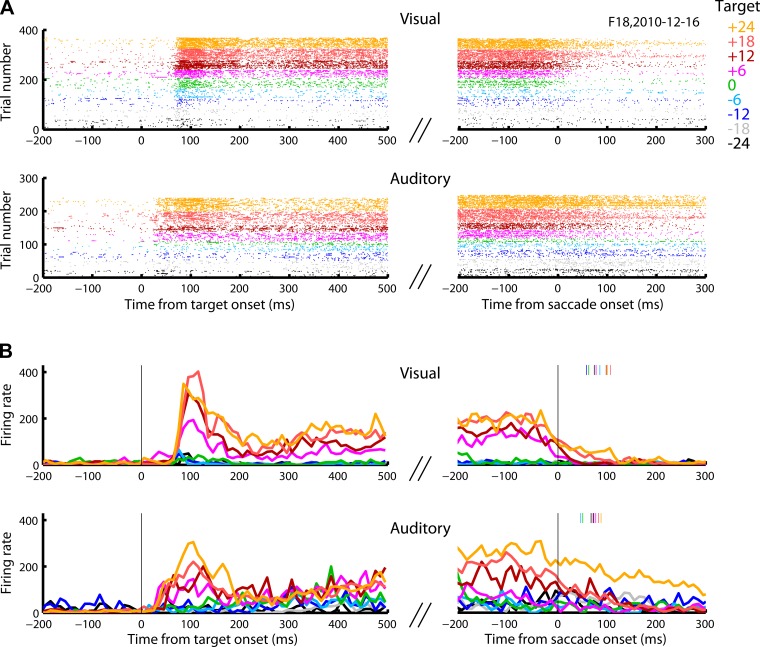
Three example neurons showing activity in response to the onset of the target and/or in conjunction with the saccade are illustrated in Figs. 3–5. These neurons illustrate the range of visual vs. auditory and sensory vs. motor activity in our data set. The neuron in Fig. 3 responded robustly to either visual or auditory targets, and the activity remained elevated as long as the stimulus persisted, decaying at the time a saccade was made toward the target. The neuron in Fig. 4 responded to either visual or auditory stimuli shortly after target onset and exhibited a second slight increase in activity in conjunction with the visually guided eye movement, whereas the neuron in Fig. 5 had stronger motor-related activity. Despite exhibiting a motor-related burst on auditory trials, this latter neuron responded very weakly (Fig. 5) to the onset of sound targets, although it responded more vigorously on visual trials.
Fig. 4.
Raster plot and perievent time histogram (PETH) of an example cell. This cell responded to the onset of visual and auditory targets and weakly during the saccade toward the visual targets. The response to auditory target onset was briefer, faster, and less spatially selective than the response to a visual target onset. The motor burst in the visual modality was weaker than the sensory response. A and B like in Fig. 3.
In total, about half of the neurons in our sample exhibited sensory responses to both visual and auditory targets (Fig. 6A, left; 2-tailed t-test comparing the activity to baseline, P < 0.05; see also Table 1 and materials and methods). About three-quarters exhibited activity for both target modalities during the motor period (Fig. 6A, right). After bimodal responsiveness, visual-only responsiveness was the next most common category for the sensory period (23% visual-only and 12% auditory-only; Fig. 6A, left). In contrast, in the motor period (perisaccadic period; see materials and methods), the percentages of unimodal visual or auditory cells were comparable (11% auditory-only and 8% visual-only; Fig. 6A, right). Nonresponsiveness to any modality was the least common category (Fig. 6A). Results were largely similar when an ANOVA was used to test for spatial selectivity but with overall lower proportions (roughly 1/3 of cells failed to show a significant dependence on target location by this categorical measure; Fig. 6B). This may indicate that some receptive fields were suboptimally sampled in this study. Figure 6C shows a direct comparison between the proportions of responsive (t-test) and spatial-selective (ANOVA) cells, indicating the total proportions of visually and auditory-responsive neurons for each measure and time period.
We next considered the aggregate activity of FEF neurons on auditory compared with visual trials. It is thought that the population of neurons in the FEF (or SC) provide the command signal specifying the goal of saccades (Hanes and Wurtz 2001; Lee et al. 1988; Quaia et al. 1998). Given that individual neurons exhibited varying strengths and types of responses, what is the net level of activity in the FEF on auditory vs. visual trials, and how does this vary in time?
Figure 7 summarizes the differences in time course and magnitude of visual- and auditory-evoked activity in the population perievent time histogram (PETH) including every neuron in our sample. Consistent with the trends from the example cells, the FEF as a whole responded more rapidly to the onset of auditory targets than visual targets. The average response onset latency (measured as the time the activity becomes higher than the mean ± 3 SD of the average baseline population activity before target onset, using 5-ms bins; see materials and methods) was 55 ms for visual trials and 20 ms for auditory trials, comparable to values estimated in an EEG study in humans (Kirchner et al. 2009). The response to auditory targets was also much weaker than that to visual targets (Fig. 7, A and C). However, beginning about 100–200 ms before the saccade, the aggregate activity was indistinguishable on visual vs. auditory trials. This was true both when considering the raw activity (Fig. 7, A and B) and when the activity of each contributing cell was normalized relative to its peak response (Fig. 7, C and D; see materials and methods). The timing was consistent with the latency of movements triggered by electrical stimulation of the FEF, which was on the order of 70 ± 34 (mean ± SD) for low current stimulation in our data set (n = 175 sites after recordings; see materials and methods). The aggregate similarity of visual and auditory activity at the time of the saccade held true across the range of target locations tested (Fig. 7, E and F). The slight compression of auditory activity relative to visual activity (higher for ipsilateral targets, lower for contralateral ones) might contribute to similar compression of auditory saccade accuracy relative to visual saccade accuracy (slight tendency for auditory saccades to undershoot; see Fig. 2K).
Response and saccade variability.
To gain insight into the causes of the greater variability of auditory saccades compared with visually guided ones, we compared how well the FEF activity correlated with horizontal target location vs. the horizontal saccade amplitude. If the greater variability in auditory saccade endpoints arises at least partially from sources within auditory sensory regions antecedent to the FEF, then FEF activity should be less well-correlated with target location on auditory vs. visual trials. However, if this is the only source of variability, then FEF activity should be equally well-correlated with the resulting movement vector on both visual and auditory trials.
To assess this, we evaluated the trial-by-trial variability of the visual and auditory responses grouped by target location or saccade endpoint by comparing the goodness of fit of Gaussian spatial tuning curves to the horizontal target locations vs. the horizontal saccade amplitude (see materials and methods).
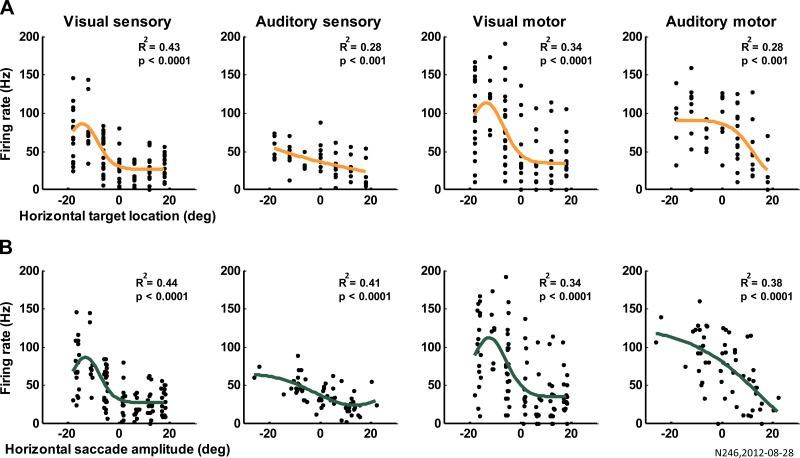
Figure 8 shows the Gaussian fits of the activity of one cell as a function of horizontal target locations (Fig. 8A) and horizontal saccade amplitude (Fig. 8B) for each modality and epoch of activity analyzed. The fit improves when considering the saccade amplitude vs. the horizontal target locations. This is confirmed at the population level (324 cells; Fig. 9) with a 3-way ANOVA examining the effect of fit type (horizontal target locations vs. horizontal saccade amplitude), modality (visual vs. auditory), and time window (sensory vs. motor). The average neural activity in FEF better correlated with the horizontal saccade amplitude than with the horizontal target locations for both sensory and motor epochs of the trials and in either visual or auditory modality (significant main effect of fit type, P value = 6 × 10−7; Fig. 9, saccade bars higher than target bars in all conditions). The activity of FEF represented both the movement and target better on visual than on auditory trials (significant main effect of modality, P value = 2 × 10−27; blue bars higher than red/orange bars), suggesting that sources of greater variability in activity on auditory trials occur both before and after (or in parallel with) the FEF. However, the fit improved from the sensory to the motor times for auditory trials but not for visual trials (significant interaction between time and modality, P value = 7 × 10−4). Furthermore, the improvement of the goodness of fit for saccade amplitude vs. target (r2 saccade − r2 target) was larger for auditory than visual trials (t-test, P value = 0.0144), suggesting that variability in the signals reaching the FEF is slightly greater relative to post-FEF sources on auditory trials than for visual trials.
Fig. 8.
Example of Gaussian fits. The activity of 1 example cell is modeled as a Gaussian function of horizontal target locations (A) and horizontal saccade amplitude (B). From left to right, the activity was computed as firing rate in the sensory and motor windows for visual and auditory trials as indicated above the panels. R2 and the P value for the individual goodness of fit are reported.
Fig. 9.
Response variability and saccade variability. Mean ± standard error coefficients of determination of the Gaussian fits of sensory and motor neural activity vs. horizontal target location (target) and horizontal saccade amplitude (saccade). *Significant comparisons; P values are in the main text.
In summary, the greater variability in saccade endpoint on auditory trials may derive from a combination of sources before the FEF (such as imperfection in the auditory localization mechanisms) as well as after the FEF, with the pre-FEF sources making the larger contribution.
DISCUSSION
Overall, our results demonstrate that the FEF is amply responsive to auditory stimuli in the context of an auditory saccade task and resolve previous questions about the auditory responsiveness of this structure (Bruce and Goldberg 1985; Mohler et al. 1973; Russo and Bruce 1994; Schall 1991). It is likely that, together with the SC, the FEF plays a causal role in controlling such saccades (Bruce and Goldberg 1985; Jay and Sparks 1984, 1987a,b; Kadunce et al. 2001; Lee and Groh 2012, 2014; Meredith and Stein 1986; Meredith et al. 1992; Mohler et al. 1973; Schall 1991; Schiller et al. 1980). However, there remain important signal transformations that must occur within the FEF or between the FEF and the eye muscles.
Specifically, the auditory signals in the FEF are initially weaker than visual signals. After the auditory target onset, significant responses occur in fewer neurons, and the population average of the activity is lower either because of the smaller proportion of active neurons or because the neurons that do exhibit auditory-evoked activity nevertheless respond less strongly to a sound than to a visual stimulus. These differences are reduced or eliminated as the saccade approaches: the level of activity, and the proportion of active neurons, becomes substantially more similar across the delay intervening between target onset and saccade. Previous studies that did not involve an auditory guided-saccade would likely have only observed these lower levels of auditory activity seen during the sensory period (Bruce and Goldberg 1985; Mohler et al. 1973; Schall 1991); during the motor period, our results more closely match those of Russo and Bruce (1994), who also employed a task involving auditory-guided saccades (although they used a reaction-time paradigm that did not expressly separate sensory- and motor-related activity).
The aggregate amount of auditory-evoked activity is quantitatively similar to visually evoked activity for the last 100–200 ms before the saccade (Fig. 7, B and D). This may correspond to the activity in the FEF reaching a threshold actually to trigger the movement (Brown et al. 2008; Hanes and Schall 1996; Jantz et al. 2013; Schall et al. 2011). The latency of movements triggered by low-current electrical stimulation of the FEF in our study was about 70 ± 34 ms, whereas shorter latencies have been observed in other studies with stronger stimulation parameters (Tu and Keating 2000), but the point is the same: if the FEF is evaluated by downstream structures during a window beginning about 35–105 ms before the saccade, visual and auditory activity are largely similar during that period of time.
The similarity in the overall amount of activity on visual vs. auditory trials in the period of time leading up to saccade onset argues against one theoretical explanation for lower auditory saccade velocity. Models of the saccade pulse-step generator often call two input signals: a trigger signal to initiate a movement and a goal signal to specify what movement to make. If a saccade is triggered before the activity level specifying the goal has reached its asymptote, the saccade may start more slowly and reach a lower peak velocity. The similarity (Sparks and Hartwich-Young 1989) of the visual and auditory signals from 100 to 200 ms before saccade onset tends to argue against this interpretation as both types of signals have reached comparable levels well before the saccade begins.
The lower accuracy on auditory compared with visual trials is well-known, but the neural contributions to this effect are less clear. Here, we focused on response variability and its potential relationship to behavioral variability, following a rationale developed to evaluate decision-related signals in discrimination tasks (e.g., Britten et al. 1996). Our results suggest that only a portion of the greater variability in saccade endpoint on auditory trials stems from sources before the FEF. Sound location must be computed based on interaural timing differences, interaural level differences, and spectral cues, each of which has error associated with it. However, these errors are likely reflected in the auditory information reaching the FEF, whether directly from areas such as the auditory cortex or indirectly from areas such as the medial geniculate nucleus, the inferior colliculus, or the superior olivary complex. It is somewhat surprising that additional sources of error appear to be introduced at the level of the FEF or its successors in the oculomotor pathway such as the SC and the cerebellum. Auditory signals can reach such structures by parallel routes (Huang et al. 1982; Sparks and Hartwich-Young 1989).
There remain numerous other aspects of the visual and auditory codes in the FEF to be explored. For example, the auditory representation of space in the SC reflects a hybrid of head- and eye-centered coordinates during the sensory period but evolves to be fully eye-centered by the time of the movement (Jay and Sparks 1984, 1987a,b; Lee and Groh 2012; Populin et al. 2004). Does the frame of reference of the FEF show a similar evolution? Our preliminary results suggest that the representation of the FEF is more consistently hybrid than that of the SC (Lee and Groh 2012), a subject we are currently investigating.
Likewise, the nature of the receptive fields on auditory trials, and whether they are circumscribed like visual receptive fields or monotonically open ended as in other auditory areas (Groh et al. 2003; Porter and Groh 2006; Werner-Reiss and Groh 2008; for review, see Groh 2014), is an important aspect of the computations that unfold between sensory input and motor output. In the primate SC, auditory saccades evoke activity patterns that form a meter or rate code for sound location and saccade vector, whereas visual saccades evoked activity patterns that form a map of visual stimulus location and saccade vector (Lee and Groh 2014). This aspect of the code for auditory space in FEF has yet to be explored.
In total, the FEF appears to act as an important partner with the SC in generating motor commands to stimuli regardless of their original modality. Differences between the activity patterns evoked on visual vs. auditory trials, or in the FEF vs. the SC, will be informative regarding how the brain generates movements guided by multiple types of inputs.
GRANTS
Financial support for the research was provided by National Institute of Neurological Disorders and Stroke Grant R01-NS-50942-05 to J. M. Groh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.C.C. and J.M.G. conception and design of research; V.C.C. and D.S.P. performed experiments; V.C.C. analyzed data; V.C.C. and J.M.G. interpreted results of experiments; V.C.C. prepared figures; V.C.C. and J.M.G. drafted manuscript; V.C.C., M.A.S., and J.M.G. edited and revised manuscript; V.C.C., D.S.P., M.A.S., and J.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for expert technical assistance from Jessi Cruger, Karen Waterstradt, Christie Holmes, and Tom Heil. We have benefitted from thoughtful discussions with Jungah Lee, Dave Bulkin, Debbie Ross, Shawn Willett, Jeffrey Mohl, Kurtis Gruters, and Bryce Gessell.
REFERENCES
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci 29: 6580–6592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci 27: 12775–12786, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204, 1975. [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. [DOI] [PubMed] [Google Scholar]
- Brown JW, Hanes DP, Schall JD, Stuphorn V. Relation of frontal eye field activity to saccade initiation during a countermanding task. Exp Brain Res 190: 135–151, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce C, Goldberg M. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635, 1985. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734, 1985. [DOI] [PubMed] [Google Scholar]
- Chabot N, Mellott JG, Hall AJ, Tichenoff EL, Lomber SG. Cerebral origins of the auditory projection to the superior colliculus of the cat. Hear Res 300: 33–45, 2013. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Van Wanrooij M, Munoz DP, Van Opstal AJ. Auditory-visual interactions subserving goal-directed saccades in a complex scene. J Neurophysiol 88: 438–454, 2002. [DOI] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988. [DOI] [PubMed] [Google Scholar]
- Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol 81: 2191–2214, 1999. [DOI] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ. A quantitative study of auditory-evoked saccadic eye movements in two dimensions. Exp Brain Res 107: 103–117, 1995. [DOI] [PubMed] [Google Scholar]
- Frost D, Pöppel E. Different programming modes of human saccadic eye movements as a function of stimulus eccentricity: indications of a functional subdivision of the visual field. Biol Cybern 23: 39–48, 1976. [DOI] [PubMed] [Google Scholar]
- Groh JM. Making Space: How the Brain Knows Where Things Are. Cambridge, MA: Harvard Univ. Press, 2014. [Google Scholar]
- Groh JM, Kelly KA, Underhill AM. A monotonic code for sound azimuth in primate inferior colliculus. J Cogn Neurosci 15: 1217–1231, 2003. [DOI] [PubMed] [Google Scholar]
- Groh JM, Trause AS, Underhill AM, Clark KR, Inati S. Eye position influences auditory responses in primate inferior colliculus. Neuron 29: 509–518, 2001. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Countermanding saccades in macaque. Vis Neurosci 12: 929–937, 1995. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol 85: 804–815, 2001. [DOI] [PubMed] [Google Scholar]
- Huang CM, Liu G, Huang R. Projections from the cochlear nucleus to the cerebellum. Brain Res 244: 1–8, 1982. [DOI] [PubMed] [Google Scholar]
- Hughes HC, Nelson MD, Aronchick DM. Spatial characteristics of visual-auditory summation in human saccades. Vision Res 38: 3955–3963, 1998. [DOI] [PubMed] [Google Scholar]
- Jantz JJ, Watanabe M, Everling S, Munoz DP. Threshold mechanism for saccade initiation in frontal eye field and superior colliculus. J Neurophysiol 109: 2767–2780, 2013. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks D. Localization of auditory and visual targets for the initiation of saccadic eye movements. In: Comparative Perception, Vol. 1: Basic Mechanisms, edited by Berkley MA and Stebbins WC. New York: John Wiley & Sons, 1990, p. 527. [Google Scholar]
- Jay MF, Sparks DL. Auditory receptive fields in primate superior colliculus shift with changes in eye position. Nature 309: 345–347, 1984. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. J Neurophysiol 57: 22–34, 1987a. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. II. Coordinates of auditory signals. J Neurophysiol 57: 35–55, 1987b. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Stein BE. The influence of visual and auditory receptive field organization on multisensory integration in the superior colliculus. Exp Brain Res 139: 303–310, 2001. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Barbeau EJ, Thorpe SJ, Regis J, Liegeois-Chauvel C. Ultra-rapid sensory responses in the human frontal eye field region. J Neurosci 29: 7599–7606, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BM, White RL 3rd, Snyder LH. Delay-period activity in visual, visuomovement, and movement neurons in the frontal eye field. J Neurophysiol 94: 1498–1508, 2005. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332: 357–360, 1988. [DOI] [PubMed] [Google Scholar]
- Lee J, Groh JM. Auditory signals evolve from hybrid- to eye-centered coordinates in the primate superior colliculus. J Neurophysiol 108: 227–242, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Groh JM. Different stimuli, different spatial codes: a visual map and an auditory rate code for oculomotor space in the primate superior colliculus. PLoS One 9: e85017, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR. Auditory cortical projection from the anterior ectosylvian sulcus (Field AES) to the superior colliculus in the cat: an anatomical and electrophysiological study. J Comp Neurol 289: 687–707, 1989. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol 56: 640–662, 1986. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Wallace MT, Stein BE. Visual, auditory and somatosensory convergence in output neurons of the cat superior colliculus: multisensory properties of the tecto-reticulo-spinal projection. Exp Brain Res 88: 181–186, 1992. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Mullette-Gillman OA, Underhill AM, Cohen YE, Groh JM. Auditory saccades from different eye positions in the monkey: implications for coordinate transformations. J Neurophysiol 92: 2622–2627, 2004. [DOI] [PubMed] [Google Scholar]
- Mohler CW, Goldberg ME, Wurtz RH. Visual receptive fields of frontal eye field neurons. Brain Res 61: 385–389, 1973. [DOI] [PubMed] [Google Scholar]
- Mulch G, Lewitzki W. Spontaneous and positional nystagmus in healthy persons demonstrated only by electronystagmography: physiological spontaneous nystagmus or “functional scar”? Arch Otorhinolaryngol 215: 135–145, 1977. [DOI] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Cohen YE, Groh JM. Eye-centered, head-centered, and complex coding of visual and auditory targets in the intraparietal sulcus. J Neurophysiol 94: 2331–2352, 2005. [DOI] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Cohen YE, Groh JM. Motor-related signals in the intraparietal cortex encode locations in a hybrid, rather than eye-centered, reference frame. Cereb Cortex 19: 1761–1775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 2011. [Google Scholar]
- Populin LC, Tollin DJ, Yin TC. Effect of eye position on saccades and neuronal responses to acoustic stimuli in the superior colliculus of the behaving cat. J Neurophysiol 92: 2151–2167, 2004. [DOI] [PubMed] [Google Scholar]
- Porter KK, Groh JM. The “other” transformation required for visual-auditory integration: representational format. Prog Brain Res 155: 313–323, 2006. [DOI] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Rothwell JC, Day BL, Marsden CD. Some saccadic eye movements can be delayed by transcranial magnetic stimulation of the cerebral cortex in man. Brain 116: 355–367, 1993. [DOI] [PubMed] [Google Scholar]
- Quaia C, Aizawa H, Optican LM, Wurtz RH. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J Neurophysiol 79: 2097–2110, 1998. [DOI] [PubMed] [Google Scholar]
- Ro T, Henik A, Machado L, Rafal RD. Transcranial magnetic stimulation of the prefrontal cortex delays contralateral endogenous saccades. J Cogn Neurosci 9: 433–440, 1997. [DOI] [PubMed] [Google Scholar]
- Robinson D. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Fuchs AF. Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol 32: 637–648, 1969. [DOI] [PubMed] [Google Scholar]
- Royal DW, Carriere BN, Wallace MT. Spatiotemporal architecture of cortical receptive fields and its impact on multisensory interactions. Exp Brain Res 198: 127–136, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Frontal eye field activity preceding aurally guided saccades. J Neurophysiol 71: 1250–1253, 1994. [DOI] [PubMed] [Google Scholar]
- Schall J. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol 66: 559–579, 1991. [DOI] [PubMed] [Google Scholar]
- Schall J, Hanes D. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366: 467–469, 1993. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci 15: 6905–6918, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Purcell BA, Heitz RP, Logan GD, Palmeri TJ. Neural mechanisms of saccade target selection: gated accumulator model of the visual-motor cascade. Eur J Neurosci 33: 1991–2002, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci 22: 241–259, 1999. [DOI] [PubMed] [Google Scholar]
- Schiller P, True S, Conway J. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol 44: 1175–1189, 1980. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57: 1033–1049, 1987. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey's frontal eye field. J Neurophysiol 58: 1387–1419, 1987. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res 116: 229–249, 1997. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev Oculomot Res 3: 213–255, 1989. [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol 84: 1103–1106, 2000. [DOI] [PubMed] [Google Scholar]
- Vaadia E, Benson DA, Hienz RD, Goldstein MH. Unit study of monkey frontal cortex: active localization of auditory and of visual stimuli. J Neurophysiol 56: 934–952, 1986. [DOI] [PubMed] [Google Scholar]
- Werner-Reiss U, Groh JM. A rate code for sound azimuth in monkey auditory cortex: implications for human neuroimaging studies. J Neurosci 28: 3747–3758, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Reiss U, Kelly KA, Trause AS, Underhill AM, Groh JM. Eye position affects activity in primary auditory cortex of primates. Curr Biol 13: 554–562, 2003. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Gafka AC. Activity of neurons in area 6 of the cat during fixation and eye movements. Vis Neurosci 15: 123–140, 1998. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Updyke BV, Gafka AC. Widespread distribution of visual responsiveness in frontal, prefrontal, and prelimbic cortical areas of the cat: an electrophysiologic investigation. J Comp Neurol 405: 99–127, 1999. [PubMed] [Google Scholar]
- Wipfli M, Felblinger J, Mosimann UP, Hess CW, Schlaepfer TE, Muri RM. Double-pulse transcranial magnetic stimulation over the frontal eye field facilitates triggering of memory-guided saccades. Eur J Neurosci 14: 571–575, 2001. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA, Pare M, Ferraina S. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res 41: 3399–3412, 2001. [DOI] [PubMed] [Google Scholar]
- Yao L, Peck CK. Saccadic eye movements to visual and auditory targets. Exp Brain Res 115: 25–34, 1997. [DOI] [PubMed] [Google Scholar]
- Zambarbieri D, Schmid R, Magenes G, Prablanc C. Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res 47: 417–427, 1982. [DOI] [PubMed] [Google Scholar]