The neuronal network of the central pattern generator (CPG) in the turtle spinal cord that produces the mixed-synergy rostral scratch has multipartite modular organization that contrasts with the classic view of bipartite half-center organization of spinal CPGs. Single-unit interneuronal recordings during both normal and deletion rostral-scratch motor patterns support the concept that the turtle rostral-scratch CPG has at least four distinct modules: knee flexor, knee extensor, hip flexor, and hip extensor.
Keywords: central pattern generator, spinal cord, module, fictive motor rhythms, scratch
Abstract
Central pattern generators (CPGs) are neuronal networks in the spinal cord that generate rhythmic patterns of motor activity in the absence of movement-related sensory feedback. For many vertebrate rhythmic behaviors, CPGs generate normal patterns of motor neuron activities as well as variations of the normal patterns, termed deletions, in which bursts in one or more motor nerves are absent from one or more cycles of the rhythm. Prior work with hip-extensor deletions during turtle rostral scratch supports hypotheses of hip-extensor interneurons in a hip-extensor module and of hip-flexor interneurons in a hip-flexor module. We present here single-unit interneuronal recording data that support hypotheses of knee-extensor interneurons in a knee-extensor module and of knee-flexor interneurons in a knee-flexor module. Members of knee-related modules are not members of hip-related modules and vice versa. These results in turtle provide experimental support at the single-unit interneuronal level for the organizational concept that the rostral-scratch CPG for the turtle hindlimb is multipartite, that is, composed of more than two modules. This work, when combined with experimental and computational work in other vertebrates, does not support the classical view that the vertebrate limb CPG is bipartite with only two modules, one controlling all the flexors of the limb and the other controlling all the extensors of the limb. Instead, these results support the general principle that spinal CPGs are multipartite.
NEW & NOTEWORTHY
The neuronal network of the central pattern generator (CPG) in the turtle spinal cord that produces the mixed-synergy rostral scratch has multipartite modular organization that contrasts with the classic view of bipartite half-center organization of spinal CPGs. Single-unit interneuronal recordings during both normal and deletion rostral-scratch motor patterns support the concept that the turtle rostral-scratch CPG has at least four distinct modules: knee flexor, knee extensor, hip flexor, and hip extensor.
patterns of motor neuron (MN) and muscle activities occur during rhythmic behaviors in vertebrates (Grillner 1981; Grillner et al. 2008a; Kiehn 2006, 2016; Stein 2005; Stein et al. 1997). Neuronal networks located in the spinal cord, termed central pattern generators (CPGs), generate excellent replicas of these motor patterns in the absence of movement-related sensory feedback (Grillner 1981; Stein 1984). Characterization of the neuronal mechanisms and the network structures responsible for generating these motor patterns is a major goal of systems neuroscience. For over a century, the spinal cord has served as an important model system to understand these network structures (Brown 1914; Kiehn 2006).
Classic spinal cord CPG studies focus on flexor/extensor rhythmic alternation and support a hypothesis of bipartite modular organization (Brown 1914; Jankowska et al. 1967; Lundberg 1981). These studies emphasize that all the flexors of a limb (hip flexors, knee flexors, etc.) are controlled by a neuronal module termed the flexor half-center and that all the extensors of a limb (hip extensors, knee extensors, etc.) are controlled by a neuronal module termed the extensor half-center (Brown 1914; Jankowska et al. 1967; Lundberg 1981). Reciprocal inhibition between the flexor and extensor half-centers is a key feature of this hypothesis. Even though this bipartite modular hypothesis has considerable explanatory value, it lacks the complexity to account for important features of many spinal motor patterns.
More recently, investigators have gathered data in support of hypotheses suggesting more complex multipartite modular organization of spinal CPGs (Ampatzis et al. 2014; Bagnall and McLean 2014; Bellardita and Kiehn 2015; Bizzi et al. 2008; Duysens et al. 2013; Giszter 2015; Giszter and Hart 2013; Grillner 1981; Grillner et al. 2008b; Grillner and El Manira 2015; Hagglund et al. 2013; Hao et al. 2014; Hinckley et al. 2015; Krouchev et al. 2006; Krouchev and Drew 2013; Lacquaniti et al. 2012; Markin et al. 2012; McCrea and Rybak 2008; McLean and Dougherty 2015; Prilutsky and Edwards 2016; Stein 1985, 2008; Zhang et al. 2014). Many of these studies conclude that the classic bipartite flexor/extensor half-center hypothesis is not sufficient to account for the specifics of spinal cord motor output and that a multipartite modular hypothesis is needed to account for important features of the experimental data.
Two prominent classes of modular multipartite hypotheses have been proposed for the spinal networks that control hindlimb motor rhythms: 1) the Grillner unit burst generator (UBG) hypothesis of organization of the cat hindlimb stepping CPG with agonist and antagonist UBGs at each degree of freedom of the cat hindlimb, e.g., hip-extensor module, hip-flexor module, knee-extensor module, knee-flexor module, etc. (see Fig. 31 of Grillner 1981) and 2) the McCrea-Rybak hypothesis that includes multiple pattern generator (PG) modules responsible for motor pattern formation (see Fig. 4 of McCrea and Rybak 2008). Each of these multipartite hypotheses asserts that several modules are needed to control hindlimb flexors and several other modules are needed to control hindlimb extensors.
The spinal network that controls rostral scratch in turtle is an attractive model system to study multipartite organization of a spinal CPG. Knee-extensor MNs are active mostly during hip-flexor motor activity and are mainly quiet during hip-extensor motor activity. This type of motor pattern is termed a mixed synergy (Stein and Smith 1997). The classic bipartite half-center hypothesis (Brown 1914; Lundberg 1981) lacks the complexity to account for mixed-synergy motor patterns.
Motor pattern deletions are variations of normal motor patterns in which bursts of MN and/or muscle activities are not expressed in one or more cycles of a motor rhythm (Stein 2008). Studies of deletions provide support for hypotheses of multipartite modular organization of spinal CPGs (Britz et al. 2015; Duysens 1977; Griener et al. 2013; Grillner and Zangger 1979; Lafreniere-Roula and McCrea 2005; Markin et al. 2012; Martinez et al. 2013; McCrea and Rybak 2008; Rybak et al. 2006; Stein 2005, 2008, 2010; Stein and Daniels-McQueen 2002, 2004; Turkin and Hamm 2004; Zhong et al. 2012). In the spinal turtle, the motor patterns of the normal rostral scratch and the hip-extensor deletion variation of the rostral scratch have been extensively studied (Robertson and Stein 1988; Stein 2005, 2008, 2010; Stein et al. 1982, 1995, 1998; Stein and Daniels-McQueen 2002, 2003, 2004; Stein and Grossman 1980). During a normal rostral scratch, there is rhythmic alternation between hip-flexor and hip-extensor MN activities and between knee-flexor and knee-extensor MN activities (Stein and Daniels-McQueen 2002, 2003, 2004). Knee-flexor motor end phases occur close to knee-extensor motor start phases; the transition from knee-flexor to knee-extensor motor activities occurs in the middle of the hip-flexor motor burst (Fig. 1 of Stein and Daniels-McQueen 2003). During a hip-extensor deletion, there is no hip-extensor motor activity and/or no quiescence between successive hip-flexor motor bursts (filled diamond in Fig. 2B of Stein and Daniels-McQueen 2002). During many hip-extensor deletions, there is rhythmic alternation between knee-flexor and knee-extensor motor activity (unfilled diamonds in Fig. 1B of Stein and Daniels-McQueen 2004).
Two knee-related variations of rostral scratch in the turtle have been described (Stein and Daniels-McQueen 2004). In a knee-flexor deletion, there is no knee-flexor MN activity and/or no quiescence between successive knee-extensor MN bursts. In a knee-extensor deletion, there is no knee-extensor MN activity and/or no quiescence between successive knee-flexor MN bursts. Knee-related deletions usually occur near the time of a hip-extensor deletion. The present article reports single-unit recordings obtained in the white matter of the spinal cord from the descending axons of propriospinal interneurons (INs) during knee-related deletions of turtle rostral scratch.
Single-unit recordings of propriospinal INs in the turtle during the normal rostral scratch and during hip-related variations provide support for the concept of hip-related modules in the rostral-scratch CPG (Stein and Daniels-McQueen 2002). During normal rostral scratch, hip-extensor INs (“0%-overlap INs,” active during the hip-extensor motor burst) rhythmically alternate with hip-flexor INs (“large-overlap INs,” active during all or nearly all of the hip-flexor burst). Hip-extensor INs are usually quiet during hip-extensor deletions (Fig. 4 of Stein and Daniels-McQueen 2002). This supports the concept that hip-extensor INs are members of a hip-extensor module active during the hip-extensor phase of normal rostral scratch and quiet during hip-extensor deletions. Hip-flexor INs are usually continuously active during hip-extensor deletions (Fig. 7 of Stein and Daniels-McQueen 2002). This supports the concept that hip-flexor INs are members of a hip-flexor module active during the hip-flexor burst of normal rostral scratch and continuously active during hip-extensor deletions.
The transition from knee-flexor MN activity to knee-extensor MN activity occurs midway during the hip-flexor MN burst during a normal rostral scratch cycle (Stein and Daniels-McQueen 2003, 2004). The application of Mardia's circular-circular rank correlation test (Batschelet 1981; Mardia 1975) revealed a significant positive correlation between the end phases of knee-flexor motor bursts and the start phases of knee-extensor motor bursts during normal rostral scratch (Stein and Daniels-McQueen 2003). Stein and Daniels-McQueen (2003) also used this statistical test to identify knee-related INs, termed ON-units and OFF-units, during normal rostral scratch in the turtle. ON-units are INs whose start phases are close to and positively correlated with the start phases of knee-extensor motor bursts (Fig. 4 of Stein and Daniels-McQueen 2003). OFF-units are INs whose end phases are close to and positively correlated with the start phases of knee-extensor motor activity (Fig. 5 of Stein and Daniels-McQueen 2003). Stein and Daniels-McQueen (2004) predicted that ON-units are quiet during knee-extensor deletions and that OFF-units are quiet during knee-flexor deletions. We report here single-unit interneuronal recordings that support these predictions. These data were described in a meeting abstract (Stein 2009). Our results support the concept that ON-units are knee-extensor INs and members of a knee-extensor module and that OFF-units are knee-flexor INs and members of a knee-flexor module (Fig. 1 of Stein 2008).
Fig. 1.
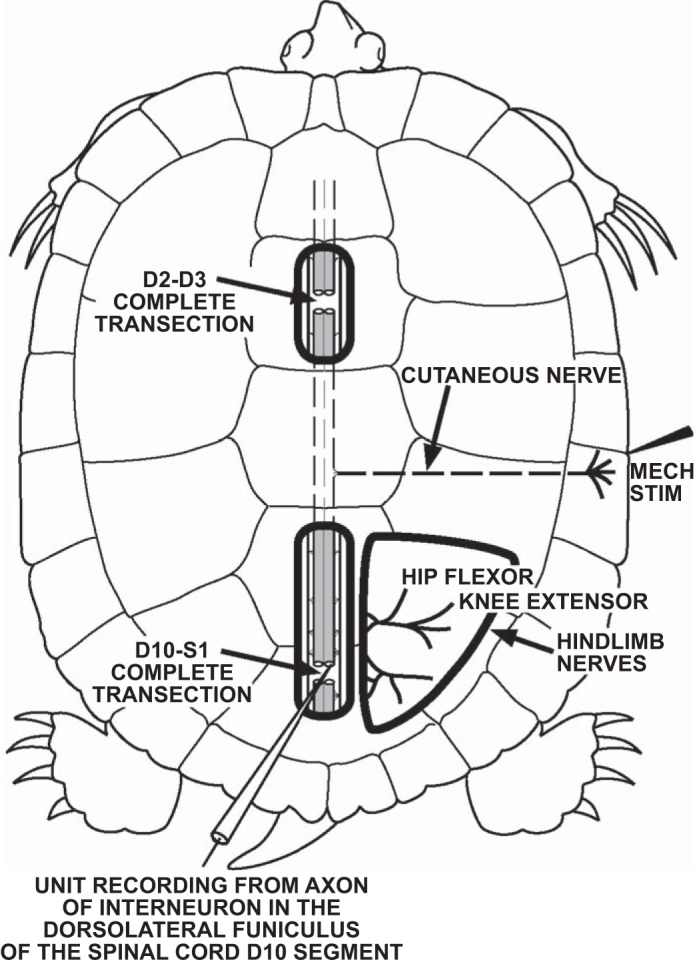
Sketch of the D3–D10 preparation. The spinal cord was completely transected in 2 locations, at the border of the D2 and D3 spinal segments just posterior to the forelimb enlargement and at the border of the D10 and S1 spinal segments within the hindlimb enlargement. ENG recordings were obtained from hip-flexor, monoarticular knee-extensor, and biarticular knee-extensor motor nerves. Single-unit recordings from descending propriospinal interneurons were obtained from the cut ends of axons in the dorsolateral funiculus at the posterior face of the D10 spinal segment. Fictive rostral scratching was evoked by mechanical stimulation of the rostral-scratch receptive field located on the shell bridge in the midbody region of the turtle. From Stein and Daniels-McQueen (2002) with permission of the Society for Neuroscience, copyright 2002.
MATERIALS AND METHODS
Red-eared turtles (n = 15 total: John K. Tucker, Illinois Natural History Survey, Brighton, IL, n = 7; Charles D. Sullivan, Nashville, TN, n = 5; Kons Scientific, Germantown, WI, n = 2; William A. Lemberger, Oshkosh, WI, n = 1), Trachemys scripta elegans (formerly Pseudemys scripta elegans), weighing 425-1,363 g, were placed on crushed ice at least 1 h before surgery to induce hypothermic analgesia (Melby and Altman 1974). Each turtle was spinalized just caudal to the forelimb enlargement by a complete spinal transection midway between the D2 and D3 dorsal roots (Mortin and Stein 1990). In addition, the spinal cord was completely transected midway between the D10 and S1 dorsal roots to produce a D3–D10 preparation; the posterior cut face of the D10 segment (Stein and Daniels-McQueen 2002) was used to record single-unit action potentials from the cut axons of descending propriospinal INs (Berkowitz and Stein 1994a, 1994b; Currie and Stein 1990) in the right dorsolateral funiculus (see Fig. 1 for a sketch of the experimental preparation). The cut ends of three peripheral nerves were prepared for electroneurographic (ENG) recordings: the right hip-flexor nerve that innervates puboischiofemoralis internus, pars anteroventralis muscle; the right monoarticular knee-extensor nerve that innervates triceps femoris, pars femorotibialis muscle; and the right biarticular knee-extensor nerve that innervates triceps femoris, pars ambiens muscle (Robertson et al. 1985; Walker 1973). These experiments were conducted during two periods, December 2000 to February 2001 and September 2005 to December 2006. All procedures were approved by the Washington University Animal Studies Committee. Some single-unit interneuronal recordings from three of these turtles were described in previous publications (Stein and Daniels-McQueen 2002, 2003). We present here additional single-unit interneuronal recordings from these 3 turtles and from 12 additional turtles.
Techniques used in the present study were described in prior publications: techniques for surgical preparation, recordings, stimulation, recognition of hip-extensor deletions, measurements of rostral-scratch motor patterns, and single-unit recognition and analyses (Stein and Daniels-McQueen 2002); techniques for studying knee-related INs in the D3–D10 preparation (Stein and Daniels-McQueen 2003); and techniques for recognizing knee-related deletions (Stein and Daniels-McQueen 2004).
Our technique of single-unit extracellular recordings from axons of descending propriospinal INs (Currie and Stein 1990) in the dorsolateral funiculus of the white matter on the posterior cut face of an eight-segment chain of turtle spinal cord has the technical advantage that excellent single-unit recordings can be obtained during the multiple cycles required to study each unit. Limitations of this technique are that we cannot determine whether the output of the IN is excitatory or inhibitory and we cannot determine whether the descending axon is ipsilateral to or contralateral to the cell body of the IN (Berkowitz and Stein 1994c; Nissen et al. 2008).
After surgery was completed, the turtle was allowed to warm up to room temperature and was immobilized with gallamine at a dosage of 6–8 mg/kg body wt. Fictive rostral-scratch motor patterns were elicited by mechanical stimulation of sites in the right rostral-scratch receptive field. In the present study, we considered those single units recorded during at least 24 cycles of normal rostral scratching while recording ENGs from the hip-flexor, monoarticular knee-extensor, and biarticular knee-extensor peripheral motor nerves. Recordings of ENGs from knee-flexor motor nerves were not attempted in the present study. Recordings of ENGs from hip-extensor motor nerves were not attempted in 12 of the turtles in this study. In the other three turtles of this study, our attempts to obtain hip-extensor ENG nerve recordings were not successful.
Each motor nerve ENG recording was digitized at 2,000 Hz (Spike2 from Cambridge Electronic Design, Cambridge, UK). The absolute value of these data was calculated (= “full-wave rectified”). The average of 20 successive full-wave rectified values was then obtained (= “integrated”). The resulting 100-Hz full-wave rectified integrated ENG (rENG) was then analyzed for bursts and for quiescence. Each point in the rENG is an average of 10 ms of ENG recordings (see Fig. 3 of Stein and Daniels-McQueen 2002).
The threshold for activity was determined for each rENG. First, each rENG was examined during an interval prior to the onset of stimulation when there were no extracellularly recorded action potentials. During this interval, the baseline amplitude of the rENG (maximum value minus minimum value) was measured. The threshold for rENG activity was set as the sum of the baseline amplitude plus the maximum value of the quiescent rENG. We defined a period of rENG activity when the rENG values were above threshold for at least five consecutive points (= 50 ms). We defined rENG quiescence when the rENG values were below threshold for at least five consecutive points (= 50 ms). A normal burst of rENG activity occurred when an interval of rENG activity with one prominent local maximum had intervals of rENG quiescence before and after the activity. We defined a normal rostral scratch cycle as a cycle that contains normal bursts of rENG activity in all three motor nerves recorded in the present study (hip-flexor, monoarticular knee-extensor, and biarticular knee-extensor motor nerves).
We used several strategies to analyze rENGs during deletions. For motor nerve deletions in which the agonist (e.g., knee extensor) nerve was recorded and the antagonist (e.g., knee flexor) nerve was not recorded, we defined a cycle with an agonist deletion when there was no burst of rENG in the agonist nerve during a cycle of hip-flexor rENG activity. In the present study, this criterion was used to define knee-extensor deletions. A knee-extensor deletion occurs when there is no burst (i.e., <5 successive suprathreshold rENG values) in monoarticular and/or biarticular knee extensor rENGs during a cycle of hip activity.
For motor nerve deletions in which the antagonist (e.g., hip flexor) motor nerve was recorded and the agonist (e.g., hip extensor) motor nerve was not recorded, we defined a cycle with an agonist deletion when there is no quiescence (i.e., <5 successive subthreshold rENG values) in the antagonist nerve between successive local maxima of rENG. The local minimum (or minima) in the antagonist nerve marked the time(s) of the deletion(s). Each time of deletion was used to mark the end of one burst and the start of the subsequent burst of the rENG. We used this strategy to analyze hip-extensor deletions and knee-flexor deletions (see also Stein and Daniels-McQueen 2002, 2004).
Our strategy to identify an agonist deletion with an analysis of the absence of quiescence in an antagonist motor nerve is based upon our prior work in which we recorded both agonist and antagonist motor nerves during agonist deletions. For example, in prior studies of turtle rostral scratch (Robertson and Stein 1988; Stein et al. 1995, 1998), we recorded both hip-flexor and hip-extensor motor activity. During hip-extensor deletions, there was an absence of quiescence between successive hip-flexor motor bursts when there was no hip-extensor motor activity. In other prior studies of turtle rostral scratch (Stein and Daniels-McQueen 2003, 2004), we recorded both knee-flexor and knee-extensor motor activity. During knee-flexor deletions, there was an absence of quiescence between successive knee-extensor motor bursts when there was no burst of knee-flexor motor activity (Stein and Daniels-McQueen 2004). In the present study, we did not record from knee-flexor and hip-extensor motor nerves in order to increase our chances of obtaining successful single-unit recordings. We still were able, however, to identify knee-flexor deletions based upon a lack of quiescence between successive knee-extensor bursts and to identify hip-extensor deletions based upon a lack of quiescence between successive hip-flexor motor bursts.
Each single-unit recording was digitized at 20 kHz. Spike2 template matching was used to identify single units in recordings with multiple units. Unit instantaneous frequency was defined as the reciprocal of interspike interval. A unit activity burst occurred when unit instantaneous frequency was >5 Hz for a portion of a cycle.
We used double-referent measurement techniques (Berkowitz and Stein 1994a; Stein and Daniels-McQueen 2002) to measure the onsets and offsets of bursts of motor activity and single-unit descending propriospinal IN activity: start phases of hip-flexor motor activity were defined as 0.00 and 1.00; end phases of hip-flexor motor activity were defined as 0.50. We used vector-averaging techniques (Batschelet 1981) to calculate mean phase ± angular deviation of start and end phases of motor bursts and single-unit bursts. We applied the Rayleigh test to determine whether the distribution of these phases was significantly different from a random distribution (Batschelet 1981).
We studied single-unit interneuronal recordings during normal rostral scratch cycles with mean start phases (ON-units) or mean end phases (OFF-units) (Stein and Daniels-McQueen 2003) between 0.20 and 0.40 (near the onsets of knee-extensor motor bursts) whose distributions of start or end phases, respectively, were positively correlated with the start phases of the monoarticular and/or the biarticular knee-extensor motor bursts (P < 0.05; Mardia's circular-circular rank-correlation test) (Batschelet 1981; Mardia 1975). A Bonferroni correction was used to account for the increase in type I error that resulted from doing multiple tests. In addition, each unit studied was recorded during at least four cycles of knee-related deletions. We report interneuronal recordings in the present paper from 22 knee-related units: 11 ON-units and 11 OFF-units that satisfied the above conditions.
RESULTS
Knee-related single-unit interneuronal recordings during normal rostral scratch.
We obtained single-unit recordings from the axons of descending propriospinal INs during fictive rostral scratch generated by the D3–D10 segments of the turtle spinal cord. Each of the 22 units reported here fired in a distinct burst during normal rostral scratch (gray and black bars in Fig. 2). For each unit, the distributions of start phases and end phases were significantly different from a random distribution (P < 0.001, Rayleigh test; Batschelet 1981; Stein and Daniels-McQueen 2002). The present article is the first presentation of data from 18 of these 22 units. Data from four of these units during normal rostral scratching and during hip-extensor deletions were included in prior publications (Stein and Daniels-McQueen 2002, 2003); the present article is the first presentation of data from these four units during knee-related deletions.
Fig. 2.
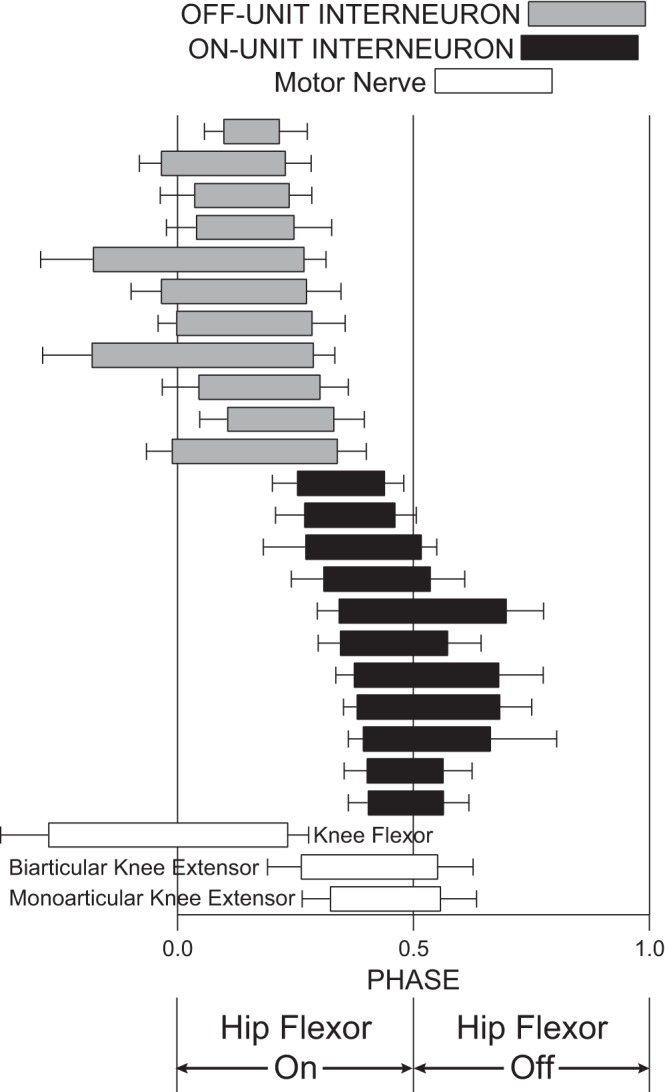
Double-referent mean ± angular deviation of start phases and end phases for 22 descending propriospinal interneurons (INs) and 3 motor nerves during normal turtle rostral scratch. Eleven INs are OFF-units; 11 INs are ON-units; 3 motor nerves (knee flexor, biarticular knee extensor, and monoarticular knee extensor) are also shown. Data for knee-flexor motor nerve from Stein and Daniels-McQueen (2003). End phases of each of the OFF-units were positively correlated with the start phases of at least 1 of the knee-extensor motor nerves. Start phases of each of the ON-units were positively correlated with the start phases of at least 1 of the knee-extensor motor nerves. OFF-units are candidate members of a knee-flexor module. ON-units are candidate members of a knee-extensor module.
The rostral-scratch motor pattern is a mixed-synergy motor pattern (Stein and Smith 1997) with knee-extensor motor activity during the latter portion of hip-flexor motor activity (open bars in Fig. 2). We define the start phases of hip-flexor motor activity as 0.0 and 1.0 phases and the end phases of hip-flexor motor activity as 0.5 phase (Berkowitz and Stein 1994a; Stein and Daniels-McQueen 2002, 2003). We studied a total of 1,298 cycles of normal rostral scratch. The mean start phase ± angular deviation of the biarticular knee-extensor motor burst was 0.26 ± 0.07, and that of the monoarticular knee-extensor motor burst was 0.32 ± 0.06. The mean end phase ± angular deviation of the biarticular knee-extensor motor burst was 0.55 ± 0.07, and that of the monoarticular knee-extensor motor burst was 0.56 ± 0.08.
The start phases of these knee-extensor motor activities are near the end phases of knee-flexor motor activities (Stein and Daniels-McQueen 2003, 2004). Knee-flexor motor activities were not recorded in the present study. The start and end phases of knee-flexor motor activity illustrated with open bars in Fig. 2 in the present article report data described by Stein and Daniels-McQueen (2003).
We studied 11 knee-related propriospinal ON-units whose start phases were close to the start phases of knee-extensor motor activity during normal rostral scratch (black bars in Fig. 2). There was a significant positive correlation of the start phases of nine of these ON-units with both the start phases of the monoarticular knee-extensor motor bursts and the start phases of the biarticular knee-extensor motor bursts (P < 0.05; Mardia's circular-circular rank correlation). The start phases of one other ON-unit were significantly correlated with the start phases of the monoarticular knee-extensor motor bursts, and the start phases of another ON-unit were significantly correlated with the start phases of the biarticular knee-extensor motor bursts. We studied a total of 11,517 action potentials in these ON-units during a total of 644 normal cycles of rostral scratch. These units fired an average of 17.9 action potentials per cycle.
We studied 11 knee-related propriospinal OFF-units whose end phases were close to the start phases of knee-extensor motor activity during normal rostral scratch (gray bars in Fig. 2). There was a significant positive correlation of the end phases of nine of these OFF-units with both the start phases of the monoarticular knee-extensor motor bursts and the start phases of the biarticular knee-extensor motor bursts (P < 0.05; Mardia's circular-circular rank correlation). The end phases of two other OFF-units were significantly correlated with the start phases of the monoarticular knee-extensor motor bursts. We studied a total of 13,866 action potentials in these OFF-units during a total of 654 normal cycles of rostral scratch. These units fired an average of 21.2 action potentials per cycle.
The 22 knee-related INs in the present study each fired during a part of the hip-flexor motor burst of a normal rostral scratch. We calculated the percentage of overlap of each unit's burst with the hip-flexor burst according to the method described by Stein and Daniels-McQueen (2002). Nineteen of the 22 units fell into the 21–60% range previously characterized as intermediate overlap (Stein and Daniels-McQueen 2002). Two units were slightly below this range, with 19.4% and 20.0% overlap. One unit was slightly above this range, with 68.0% overlap. Stein and Daniels-McQueen (2002) suggested that some of the units with intermediate percentages of overlap with the hip-flexor motor burst may be related to knee motor activity. Candidate knee-related INs with intermediate overlap with hip-flexor motor activity differ from hip-related INs with 0% overlap (candidate hip-extensor INs) and with large-percentage overlap (candidate hip-flexor INs).
Knee-related interneurons mainly fired in bursts during hip-extensor deletions.
During both normal rostral scratch and hip-extensor deletion rostral scratch, there was rhythmic alternation between knee-extensor motor activity and knee-extensor motor quiescence (Fig. 3 and Fig. 4). Knee-flexor motor activity occurs during knee-extensor motor quiescence (not shown here; see Stein and Daniels-McQueen 2003, 2004). During hip-extensor deletion rostral scratch, there was no quiescence between successive hip-flexor motor bursts (open triangles in Fig. 3 and Fig. 4). No hip-extensor motor activity occurs during a hip-extensor deletion (not shown here; see Robertson and Stein 1988; Stein and Grossman 1980; Stein and Daniels-McQueen 2002).
Fig. 3.
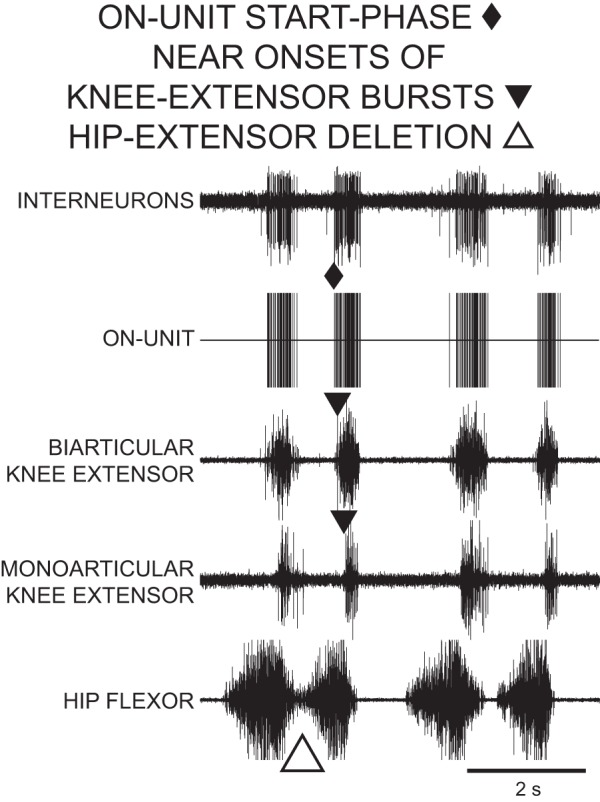
Recordings of an ON-unit descending propriospinal interneuron during 4 cycles of rostral scratch in the D3–D10 preparation. Top trace, descending propriospinal interneurons; vertical lines in 2nd trace represent the timing of action potentials in the ON-unit, the largest unit in top trace. The start phase of the ON-unit was near the start phases of knee-extensor motor activities. The first cycle ends with a hip-extensor deletion variation of rostral scratch with no quiescence between 2 successive hip-flexor bursts. The other 3 cycles are normal rostral scratch cycles with hip-flexor bursts alternating with hip-flexor quiescence. The ON-unit is active during the latter portion of each hip-flexor burst in all 4 cycles.
Fig. 4.
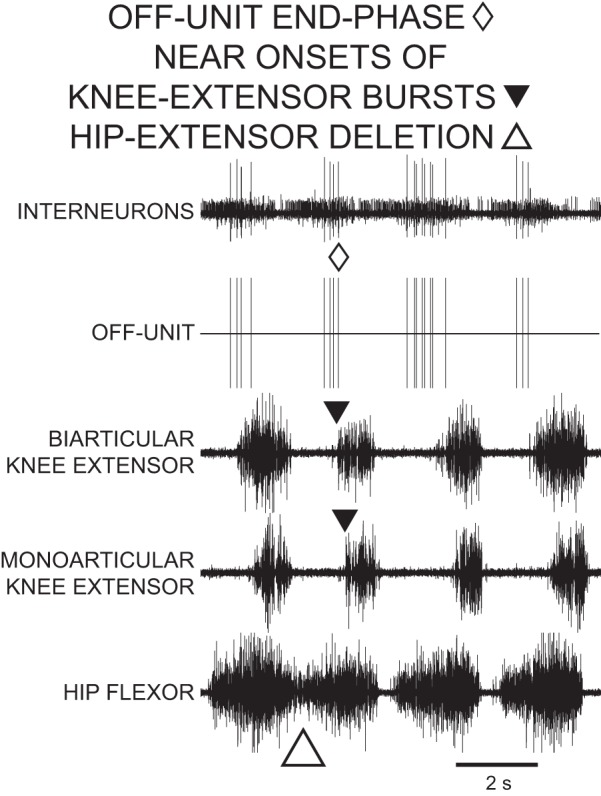
Recordings of an OFF-unit descending propriospinal interneuron during 4 cycles of rostral scratch in the D3–D10 preparation. Top trace, descending propriospinal interneurons; vertical lines in 2nd trace represent the timing of action potentials in the OFF-unit, the largest unit in top trace. The end phase of the OFF-unit was near the start phases of knee-extensor motor activities. The first cycle ends with a hip-extensor deletion variation of rostral scratch with no quiescence between 2 successive hip-flexor bursts. The other 3 cycles are normal rostral scratch cycles with hip-flexor bursts alternating with hip-flexor quiescence. The OFF-unit is active during the initial portion of each hip-flexor burst in all 4 cycles.
During normal rostral scratch and during hip-extensor deletion rostral scratch, 1) knee-related INs mainly fired in bursts, 2) ON-unit start phases were near the onsets of knee-extensor MN bursts and the ON-unit fired in a burst during the latter portion of the hip-flexor MN burst (Fig. 3), and 3) OFF-unit end phases were near the onsets of knee-extensor bursts and the OFF-unit fired in a burst during the initial portion of each hip-flexor MN burst (Fig. 4). During 91 cycles of hip-extensor deletions, the knee-related units in the present study fired in bursts during 70 of these cycles. In the remaining 21 cycles of hip-extensor deletions, the units were quiet in 8 of the cycles, fired a single action potential in 1 cycle, and fired continuously in 12 cycles. These observations that knee-related INs mainly fired in bursts during hip-extensor deletions are consistent with earlier observations (Stein and Daniels-McQueen 2003). These observations are also similar to those previously obtained for intermediate-overlap INs during hip-extensor deletions (Stein and Daniels-McQueen 2002).
Knee-extensor INs (ON-units) were mainly quiet during knee-extensor deletions.
During a rostral scratch with a knee-extensor deletion, there were no monoarticular knee-extensor and no biarticular knee-extensor MN activities (Fig. 5). The ON-unit shown in Fig. 5 fired in a burst during the knee-extensor MN bursts of normal rostral scratch and was quiet (q in Fig. 5) during knee-extensor deletion rostral scratch (open ellipses in Fig. 5).
Fig. 5.
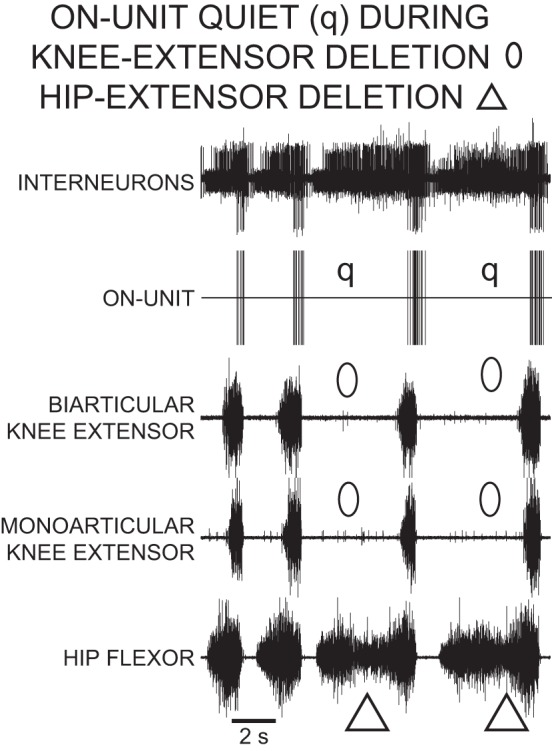
Recordings of an ON-unit descending propriospinal interneuron during 6 cycles of rostral scratch in the D3–D10 preparation. Top trace, descending propriospinal interneurons; vertical lines in 2nd trace represent the timing of action potentials in the ON-unit, the largest unit in top trace. The first, second, fourth, and sixth cycles of this episode are normal rostral scratch cycles. In each of the normal rostral scratch cycles, the start phase of the ON-unit was near the start phases of knee-extensor motor activities. The third and fifth cycles end with a hip-extensor deletion variation of rostral scratch with no hip-flexor quiescence between 2 successive hip-flexor motor bursts. In each of the cycles with a hip-extensor deletion, there is also a knee-extensor deletion in which there is no activity in each of the knee-extensor nerves. The ON-unit is quiet (q) during each of the knee-extensor deletions. These recordings support the concept that the ON-unit is a knee-extensor interneuron and a member of the knee-extensor module.
We recorded 237 action potentials from a total of 11 ON-units during 130 cycles of knee-extensor deletions. In 95 of these cycles, the ON-unit was quiet. In the remaining 35 cycles, the ON-unit fired a single spike in 8 cycles and fired in a burst in 27 cycles. The number of ON-unit action potentials during each of these 130 cycles of knee-extensor deletions was significantly less than the number of ON-unit action potentials during each of the 644 cycles of normal rostral scratch (P < 0.001, Mann-Whitney U-test) (Siegel 1956). The 1.8 action potentials per cycle produced by ON-units during knee-extensor deletions was considerably less than the 17.9 action potentials per cycle produced by ON-units during normal rostral scratch.
There were two ON-units that were responsible for 143 action potentials during 12 of the cycles with knee-extensor deletions. During 11 of these cycles, the ON-unit fired in a burst; during 1 cycle, the ON-unit fired a single spike. For the other nine ON-units, there were 94 action potentials during 118 cycles of knee-extensor deletions. In 95 of these cycles, the ON-unit was quiet. In the remaining 23 cycles, the ON-unit fired a single spike in 7 cycles and fired in a burst in 16 cycles. For this population of nine ON-units, there was an average of 0.8 action potentials per burst. Thus most of the ON-units studied here were mainly quiet during knee-extensor deletions. The quiescence of ON-units during knee-extensor deletions was similar to the quiescence of knee-extensor MNs during knee-extensor deletions. This result supports the concept that ON-units are knee-extensor INs that are members of a knee-extensor module (Stein 2008). Note that the members of the knee-extensor module (ON-units, knee extensor MNs) have properties that are different from those of the members of the hip-extensor module (0%-overlap INs, hip-extensor MNs; Stein and Daniels-McQueen 2002). The present recordings support the concept that the members of the knee-extensor module are not members of the hip-extensor module and vice versa.
Knee-flexor INs (OFF-units) were mainly quiet during knee-flexor deletions.
During a rostral scratch with a knee-flexor deletion, there was no quiet period between successive knee-extensor MN bursts (open circle in Fig. 6). The OFF-unit shown in Fig. 6 fired in a burst during knee-extensor MN quiescence of normal rostral scratch and was quiet during knee-flexor deletion rostral scratch (q in Fig. 6).
Fig. 6.
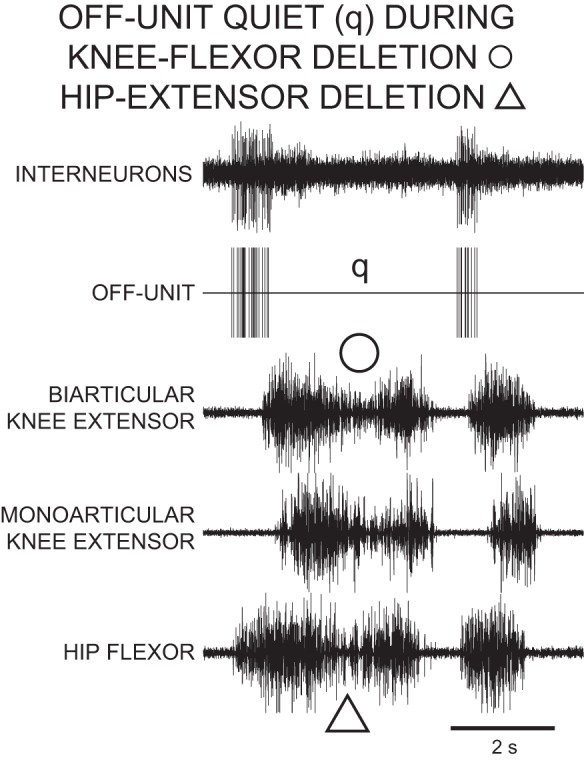
Recordings of an OFF-unit descending propriospinal interneuron during 3 cycles of rostral scratch in the D3–D10 preparation. Top trace, descending propriospinal interneurons; vertical lines in 2nd trace represent the timing of action potentials in the OFF-unit, the largest unit in top trace. The third cycle of this episode is a normal rostral scratch cycle. The first cycle of this episode ends with a hip-extensor deletion. There is a knee-flexor deletion near the beginning of the second cycle. There is no quiescence between successive knee-extensor bursts of the first and second cycles. In each of the cycles that begin with knee-extensor quiescence (first and third), there is a burst of activity in the OFF-unit with an end phase near the start phases of knee-extensor motor activities. In the second cycle that begins with a knee-flexor deletion, the OFF-unit is quiet (q). These recordings support the concept that the OFF-unit is a knee-flexor interneuron and a member of the knee-flexor module.
We recorded from eight OFF-units during 42 cycles of knee-flexor deletions. For the other three OFF-units in this study, we recorded from those units only during knee-extensor deletions and not during knee-flexor deletions. These eight OFF-units were quiet for 41 of these knee-flexor deletion cycles. In the other cycle, an OFF-unit fired four action potentials. The number of action potentials during each of these 42 cycles of knee-flexor deletions was significantly less than the number of action potentials in these eight OFF-units during each of 514 cycles of normal rostral scratch (P < 0.001, Mann-Whitney U-test; Siegel 1956). The 0.1 action potentials per cycle produced by these OFF-units during knee-flexor deletions was considerably less than the 24.4 action potentials per cycle produced by these units during normal rostral scratch. The quiescence of OFF-units during knee-flexor deletions was similar to the quiescence of knee-flexor MNs during knee-flexor deletions. This result supports the concept that OFF-units are knee-flexor INs that are members of a knee-flexor module (Stein 2008). Note that the members of the knee-flexor module (OFF-units, knee-flexor MNs) have properties that are different from those of the members of the hip-flexor module (large-overlap INs, hip-flexor MNs; Stein and Daniels-McQueen 2002). The present recordings support the concept that the members of the knee-flexor module are not members of the hip-flexor module and vice versa.
Knee-extensor INs (ON-units) were active during knee-flexor deletions.
We recorded ON-units during 23 cycles of knee-flexor deletions. During 19 of these cycles, the ON-unit fired continuously. This continuous activity of ON-units during knee-flexor deletions was similar to the activity of knee-extensor MNs during knee-flexor deletions. During the other four cycles, the ON-unit fired in a burst.
Knee-flexor INs (OFF-units) were active during knee-extensor deletions.
We recorded OFF-units during 137 cycles of knee-extensor deletions. During 17 of these cycles, the OFF-unit fired continuously. The continuous activities of these OFF-units during knee-extensor deletions were similar to the continuous activities of knee-flexor MNs during knee-extensor deletions (Stein and Daniels-McQueen 2004). During five of these cycles, the OFF-unit fired a single action potential. During the remaining 115 of these knee-extensor deletion cycles, the OFF-unit fired in a burst.
DISCUSSION
Spinal CPGs are multipartite.
Spinal cord CPGs serve as model systems to study mechanisms of production of rhythmic motor behaviors by neuronal networks (Brown 1914; Grillner 1981; Stein 2013). A variety of methods reveal multipartite organization of CPGs: deletions (Stein 2008); clusters in motor-pattern phase space (Krouchev et al. 2006; Krouchev and Drew 2013); motor primitives (Giszter and Hart 2013); factorization of muscle activity patterns (Dominici et al. 2011); neurogenetics (Hinckley et al. 2015; Talpalar et al. 2013); and optogenetics (Hagglund et al. 2013; Hinckley et al. 2015). These observations do not support the classical view that spinal cord CPGs are bipartite with a flexor half-center module that controls all the flexors of a limb and an extensor half-center module that controls all the extensors of a limb (Brown 1911, 1914; Jankowska et al. 1967; Lundberg 1981). The present report provides additional support for the existence of multipartite spinal cord CPGs with descriptions of single-unit recordings from knee-related INs during turtle rostral scratch.
Deletions are variations of normal motor patterns that reveal multiple modules in the turtle rostral-scratch CPG.
At the motor pattern level, we have analyzed turtle rostral scratch hip-extensor deletions (Robertson and Stein 1988; Stein et al. 1982, 1995, 1998; Stein and Daniels-McQueen 2002, 2003, 2004; Stein and Grossman 1980) and both knee-extensor deletions and knee-flexor deletions (Stein and Daniels-McQueen 2003, 2004). These motor pattern deletions support the existence of at least four specific modules in the turtle rostral-scratch CPG: hip-flexor module, hip-extensor module, knee-flexor module, and knee-extensor module.
Figure 1 of Stein (2008) sketches our working hypothesis for the synaptic interactions between these modules. The components of this working hypothesis are 1) reciprocal inhibitory connections between the hip-flexor module and the hip-extensor module; 2) reciprocal inhibitory connections between the knee-flexor module and the knee-extensor module; and 3) synaptic outputs from hip modules that provide synaptic inputs to knee modules. The present report supports the concept that specific module(s) are quiet during specific deletions. Future work is required to understand the specifics of all the synaptic interactions among these modules during normal cycles and during deletion cycles of rostral scratch.
The modular structure of our working hypothesis has features similar to the Grillner (1981) UBG hypothesis. In addition, the modular structure of our working hypothesis has features consistent with the modules in the PG layer in a two-level hypothesis similar to that proposed by McCrea and Rybak (2008). To determine whether the UBG hypothesis or a two-level hypothesis is a better descriptor of the turtle CPG network, it is necessary to determine whether the occurrence of a deletion causes a reset or does not cause a reset in the motor rhythm. We are not able to make that determination with the data available in the present study. Our data therefore cannot be used to discriminate between the UBG hypothesis and the two-level hypothesis.
Additional experimental evidence for this modular structure of the turtle rostral-scratch CPG was obtained with intracellular recordings from MNs during normal and hip-extensor deletion rostral scratch (Robertson and Stein 1988; Stein et al. 1982). In particular, during the hip-extensor phase of a normal rostral scratch, excitatory postsynaptic potentials drive action potentials in hip-extensor MNs and inhibitory postsynaptic potentials occur in hip-flexor MNs. During a hip-extensor deletion rostral scratch, there are no action potentials in hip-extensor MNs as well as an absence of the prominent excitatory postsynaptic potentials in hip-extensor MNs and the prominent inhibitory postsynaptic potentials in hip-flexor MNs (Fig. 2 of Robertson and Stein 1988). These synaptic recordings support the following predictions: 1) there is a hip-extensor module that includes a population of hip-extensor INs that fire during the hip-extensor MN phase of a normal rostral scratch cycle, and 2) the INs in the hip-extensor module are quiet during a hip-extensor deletion cycle. Stein and Daniels-McQueen (2002) obtained single-unit recordings from 0%-overlap INs that fired during the hip-extensor phase of normal rostral scratch cycles; these hip-extensor INs were mainly quiet during hip-extensor deletion rostral scratch cycles.
Single-unit recordings from knee-related interneurons during deletions support a multipartite CPG for turtle rostral scratch.
The focus of the present report is on turtle knee-related INs first characterized by Stein and Daniels-McQueen (2003) during normal rostral scratch: knee-extensor INs active during the latter portion of the hip-flexor MN burst (ON-units) and knee-flexor INs active during the initial portion of the hip-flexor MN burst (OFF-units). During hip-extensor deletions, knee-extensor INs continue to fire during the latter portion of the hip-flexor burst (Fig. 3) and knee-flexor INs continue to fire during the initial portion of the hip-flexor MN burst (Fig. 4). The bursting activities of these knee-related INs during hip-extensor deletions contrast with the quiescence of hip-extensor INs during hip-extensor deletions (Fig. 4 of Stein and Daniels-McQueen 2002) and with the continuous activity of hip-flexor INs during hip-extensor deletions (Fig. 7 of Stein and Daniels-McQueen 2002). This is strong evidence that knee-extensor INs are not members of the hip-extensor module and that knee-flexor INs are not members of the hip-flexor module.
The major results of the present report are observations that ON-units are mainly quiet during knee-extensor deletions (Fig. 5) and that OFF-units are mainly quiet during knee-flexor deletions (Fig. 6). These findings support the concepts that ON-units are knee-extensor INs and along with knee-extensor MNs are members of a knee-extensor module and that OFF-units are knee-flexor INs and along with knee-flexor MNs are members of a knee-flexor module (Fig. 1 of Stein 2008). Future work with paired recordings from knee-related INs and MNs during turtle rostral scratch can provide additional tests of these concepts.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-30786 to P. S. G. Stein.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.S.G.S. and S.D.-M. conception and design of research; P.S.G.S., S.D.-M., J.L., and Z.L. performed experiments; P.S.G.S., S.D.-M., J.L., Z.L., and T.S.C. analyzed data; P.S.G.S., S.D.-M., J.L., Z.L., and T.S.C. interpreted results of experiments; P.S.G.S. prepared figures; P.S.G.S. drafted manuscript; P.S.G.S., S.D.-M., J.L., Z.L., and T.S.C. edited and revised manuscript; P.S.G.S., S.D.-M., J.L., Z.L., and T.S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gavin Perry for software development and Dr. Ari Berkowitz for editorial comments. We thank John K. Tucker of the Illinois Natural History Survey, Brighton, IL for his kind donation of seven of the turtles used in this study.
Present addresses: Z. Liu, Dept. of Diagnostic Radiology, Boston University Medical Center, 820 Harrison Ave., FGH Building (3rd floor), Boston, MA 02118; T. S. Corman, Dept. of Genetics, Perelman School of Medicine, University of Pennsylvania, 415 Curie Blvd., Philadelphia, PA 19104.
REFERENCES
- Ampatzis K, Song J, Ausborn J, El Manira A. Separate microcircuit modules of distinct V2a interneurons and motoneurons control the speed of locomotion. Neuron 83: 934–943, 2014. [DOI] [PubMed] [Google Scholar]
- Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science 343: 197–200, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. New York: Academic, 1981. [Google Scholar]
- Bellardita C, Kiehn O. Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 25: 1426–1436, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: phase analyses. J Neurosci 14: 5105–5119, 1994a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: broad tuning to regions of the body surface. J Neurosci 14: 5089–5104, 1994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A, Stein PS. Descending propriospinal axons in the hindlimb enlargement of the red-eared turtle: cells of origin and funicular courses. J Comp Neurol 346: 321–336, 1994c. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Cheung VC, d'Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev 57: 125–133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz O, Zhang J, Grossmann KS, Dyck J, Kim JC, Dymecki S, Gosgnach S, Goulding M. A genetically defined asymmetry underlies the inhibitory control of flexor-extensor locomotor movements. eLife 4: e04718, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond 84: 308–319, 1911. [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of evolution of function in the nervous system. J Physiol 48: 18–46, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SN, Stein PS. Cutaneous stimulation evokes long-lasting excitation of spinal interneurons in the turtle. J Neurophysiol 64: 1134–1148, 1990. [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 334: 997–999, 2011. [DOI] [PubMed] [Google Scholar]
- Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol 40: 737–751, 1977. [DOI] [PubMed] [Google Scholar]
- Duysens J, De Groote F, Jonkers I. The flexion synergy, mother of all synergies and father of new models of gait. Front Comput Neurosci 7: 14, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF. Spinal primitives and intra-spinal micro-stimulation (ISMS) based prostheses: a neurobiological perspective on the “known unknowns” in ISMS and future prospects. Front Neurosci 9: 72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Hart CB. Motor primitives and synergies in spinal cord and after injury—the current state of play. Ann NY Acad Sci 1279: 114–126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griener A, Dyck J, Gosgnach S. Regional distribution of putative rhythm generating and pattern forming components of the mammalian locomotor CPG. Neuroscience 250: 644–650, 2013. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., sect. 1, vol. II, p. 1179–1236, 1981. [Google Scholar]
- Grillner S, El Manira A. The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol 31: 244–249, 2015. [DOI] [PubMed] [Google Scholar]
- Grillner S, El Manira A, Kiehn O, Rossignol S, Stein PS. Networks in motion. Brain Res Rev 57: 1, 2008a. [Google Scholar]
- Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res Rev 57: 2–12, 2008b. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 34: 241–261, 1979. [DOI] [PubMed] [Google Scholar]
- Hagglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci USA 110: 11589–11594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao ZZ, Meier ML, Berkowitz A. Rostral spinal cord segments are sufficient to generate a rhythm for both locomotion and scratching but affect their hip extensor phases differently. J Neurophysiol 112: 147–155, 2014. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Alaynick WA, Gallarda BW, Hayashi M, Hilde KL, Driscoll SP, Dekker JD, Tucker HO, Sharpee TO, Pfaff SL. Spinal locomotor circuits develop using hierarchical rules based on motorneuron position and identity. Neuron 87: 1008–1021, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand 70: 369–388, 1967. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–236, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev N, Drew T. Motor cortical regulation of sparse synergies provides a framework for the flexible control of precision walking. Front Comput Neurosci 7: 83, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96: 1991–2010, 2006. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol 590: 2189–2199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 94: 1120–1132, 2005. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Half-centres revisited. Adv Physiol Sci 1: 155–167, 1981. [Google Scholar]
- Mardia KV. Statistics of directional data. J R Stat Soc B 37: 349–393, 1975. [Google Scholar]
- Markin SN, Lemay MA, Prilutsky BI, Rybak IA. Motoneuronal and muscle synergies involved in cat hindlimb control during fictive and real locomotion: a comparison study. J Neurophysiol 107: 2057–2071, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Tuznik M, Delivet-Mongrain H, Rossignol S. Emergence of deletions during treadmill locomotion as a function of supraspinal and sensory inputs. J Neurosci 33: 11599–11605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Dougherty KJ. Peeling back the layers of locomotor control in the spinal cord. Curr Opin Neurobiol 33: 63–70, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby EC, Altman NH. Handbook of Laboratory Animal Science, Vol. 1. Cleveland, OH: CRC, 1974. [Google Scholar]
- Mortin LI, Stein PS. Cutaneous dermatomes for the initiation of three forms of the scratch reflex in the spinal turtle. J Comp Neurol 295: 515–529, 1990. [DOI] [PubMed] [Google Scholar]
- Nissen UV, Moldovan M, Hounsgaard J, Glover JC. Organization of projection-specific interneurons in the spinal cord of the red-eared turtle. Brain Behav Evol 72: 179–191, 2008. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Edwards DH (Editors). Neuromechanical Modeling of Posture and Locomotion. New York: Springer, 2016. [Google Scholar]
- Robertson GA, Mortin LI, Keifer J, Stein PS. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. J Neurophysiol 53: 1517–1534, 1985. [DOI] [PubMed] [Google Scholar]
- Robertson GA, Stein PS. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol 404: 101–128, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577: 617–639, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill, 1956. [Google Scholar]
- Stein PS. Central pattern generators in the spinal cord. In: Handbook of the Spinal Cord, Vols. 2 and 3: Anatomy and Physiology, edited by Davidoff RA. New York: Dekker, p. 647–672, 1984. [Google Scholar]
- Stein PS. Neural control of the vertebrate limb: multipartite pattern generators in the spinal cord. In: Comparative Neurobiology: Modes of Communication in the Nervous System, edited by Cohen MJ, Strumwasser F. New York: Wiley, p. 245–253, 1985. [Google Scholar]
- Stein PS. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 213–229, 2005. [DOI] [PubMed] [Google Scholar]
- Stein PS. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Rev 57: 118–124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS. Modules for rostral scratch pattern generation in the turtle spinal cord (Abstract). In: Proceedings of the Conference on Cellular and Network Functions in the Spinal Cord, Madison, WI, p. 4, 2009. [Google Scholar]
- Stein PS. Alternation of agonists and antagonists during turtle hindlimb motor rhythms. Ann NY Acad Sci 1198: 105–118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS. Molecular, genetic, cellular, and network functions in the spinal cord and brainstem. Ann NY Acad Sci 1279: 1–12, 2013. [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci 22: 6800–6809, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Timing of knee-related spinal neurons during fictive rostral scratching in the turtle. J Neurophysiol 90: 3585–3593, 2003. [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J Neurophysiol 91: 2380–2384, 2004. [DOI] [PubMed] [Google Scholar]
- Stein PS, Grillner S, Selverston AI, Stuart DG. (Editors). Neurons, Networks, and Motor Behavior. Cambridge, MA: MIT Press, 1997. [Google Scholar]
- Stein PS, Grossman ML. Central program for scratch reflex in turtle. J Comp Physiol A 140: 287–294, 1980. [Google Scholar]
- Stein PS, McCullough ML, Currie SN. Reconstruction of flexor/extensor alternation during fictive rostral scratching by two-site stimulation in the spinal turtle with a transverse spinal hemisection. J Neurosci 18: 467–479, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS, Robertson GA, Keifer J, Grossman ML, Berenbeim JA, Lennard PR. Motor neuron synaptic potentials during fictive scratch reflex in turtle. J Comp Physiol A 146: 401–409, 1982. [Google Scholar]
- Stein PS, Smith JL. Neural and biomechanical control strategies for different forms of vertebrate hindlimb motor tasks. In: Neurons, Networks, and Motor Behavior, edited by Stein PS, Grillner S, Selverston AI, Stuart DG. Cambridge, MA: MIT Press, p. 61–73, 1997. [Google Scholar]
- Stein PS, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci 15: 4343–4355, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500: 85–88, 2013. [DOI] [PubMed] [Google Scholar]
- Turkin VV, Hamm TM. Changes in locomotor drive potentials and cycle characteristics associated with deletions during fictive locomotion. In: Abstract Viewer/Itinerary Planner. Washington DC: Soc. Neurosci., Program Number 883.14, 2004. [Google Scholar]
- Walker WF. The locomotor apparatus of testudines. In: Biology of the Reptilia, Vol. 4, edited by Gans C, Parsons TS. New York: Academic, p. 1–100, 1973. [Google Scholar]
- Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M. V1 and V2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 82: 138–150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol 590: 4735–4759, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


