Abstract
Adaptation of neural responses to repeated muscle stretching likely represents implicit learning to minimize muscle resistance to perturbations. To test this hypothesis, the forearm was placed on a horizontal manipulandum. Elbow flexors or extensors compensated an external load and were stretched by 20° or 70° rotations. Participants were instructed not to intervene by intentionally modifying the muscle resistance elicited by stretching. In addition to phasic stretch reflexes (SRs), muscle stretching was associated with inhibitory periods (IPs) in the ongoing muscle activity starting at minimal latencies of ∼35 ms. The SR amplitude decreased dramatically across 5–12 trials and was not restored after a resting period of 3–5 min, despite the increase in stretch amplitude from 20° to 70°, but IPs remained present. When SRs were suppressed, stretching of originally nonstretched, antagonist muscles initiated after the rest period showed immediate SR suppression while IPs remained present in the first and subsequent trials. Adaptation to muscle stretching thus includes features characteristic of implicit learning such as memory consolidation and generalization. Adaptation may be achieved by central shifts in the threshold positions at which muscles begin to be activated. Shifts are thought to be prepared in advance and triggered with stretch onset. Threshold position resetting provides a comprehensive explanation of the results in the broader context of the control of posture, movement, and motor learning in the healthy and damaged nervous system.
Keywords: motor control, stretch reflex, habituation, EMG, inhibitory reaction, threshold position resetting, spasticity, clasp-knife phenomenon
the stretch reflex (SR) contributes to active resistance of muscles to lengthening elicited by external forces. Since Sherrington (1906), it has been recognized that, together with other, proprioceptive and cutaneous reflexes, the SR plays a fundamental role in the control and stabilization of posture and movement (Shemmell et al. 2010; Stein and Capaday 1988; Zehr and Stein 1999).
Reflexes cannot be considered in isolation from central influences on α- and γ-motoneurons (MNs) since these influences predetermine how reflexes function (Feldman and Orlovsky 1972; Wei et al. 2014). Such influences can be mediated by spinal and supraspinal systems via γ-MNs and pre-, post-, mono-, and/or polysynaptic projections to α-MNs (Capaday et al. 1991; Feldman and Orlovsky 1972; Hultborn 2006; Jankowska et al. 1981; Matthews 1959; Nichols and Steeves 1986).
Central control of SRs is usually interpreted as a modulation of gain (change in EMG response per unit of muscle stretch) or as reflex gating—phase-dependent inhibition and facilitation of reflex pathways to α-MNs, as is often observed during locomotion (Evarts and Tanji 1974; Sillar 1991; Wei et al. 2014). Starting from Matthews (1959), it has been shown that practically all spinal and supraspinal systems primarily influence spatial thresholds for motoneuronal recruitment—the threshold muscle lengths (λ) or respective joint angle(s) at which muscles begin to be activated (Asatryan and Feldman 1965; Capaday et al. 1991; Feldman and Orlovsky 1972; Nichols and Steeves 1986; Raptis et al. 2010). Therefore, changes in SR gain and reflex gating can be considered as secondary, emergent effects of changes in the spatial thresholds for muscle activation (Feldman 2015).
SRs contribute to the stability of body posture during standing, but during repeated tilting of the platform on which subjects stood, phasic SR responses of calf muscles were attenuated, since such responses posed a threat to balance (Horak et al. 1989; Nashner 1976; Schieppati and Nardone 1995). SR responses can also be observed in arm muscles (e.g., Rothwell et al. 1986). Preactivated, arm muscles have intrinsic elasticity and damping (length- and velocity-dependent properties, respectively) that might be sufficient for stability of arm position, particularly if the SR is tested when the arm is placed on a horizontal manipulandum. In this case, SR responses mediated by afferent feedback can be attenuated during repetitive stretching, as observed by Rothwell et al. (1986). Functionally, this could be done to minimize unnecessary and tedious stretch responses and/or prevent possible damage of active sarcomeres from overstretching. As in the standing postural task, arm SR adaptation to repetitive stretches was gradual, spanning several trials, and could involve anticipatory reactions prepared at central spinal and supraspinal levels in advance of and triggered in response to muscle stretching (Crago et al. 1976; Lewis et al. 2006; Manning et al. 2012). Indeed, SR attenuation is just one of many ways of controlling SRs that, in many motor tasks, play an essential role in the stability of posture and movement (Feldman 2015).
In the present study, we tested the hypothesis that adaptation of neural responses to muscle stretching represents implicit learning to minimize muscle resistance to repeated perturbations when such responses are not critical for stability of posture and movement. If so, one can predict that reactions to muscle stretching are prepared in advance and triggered in response to muscle stretching. These reactions may suppress not only phasic SRs but also the ongoing muscle activity. Previous studies of responses to repeated muscle stretching focused on adaptation or habituation of phasic SRs (Rothwell et al. 1986; Wolf and Segal 1996). The possibility that such responses can be associated with transient or permanent suppression of the background EMG activity was not considered in those studies. We also tested the hypothesis that adaptation to muscle stretching includes such features characteristic of motor learning as memory consolidation and generalization. Finally, we addressed the question of whether or not the learning process is accomplished through a gradual increase in the spatial thresholds for muscle activation. Functionally, threshold shifts can be used to control not only reflexes and the ongoing muscle activation but also posture and movement in task-specific ways (Feldman 2015). Therefore, our findings can be considered in a broader context of the control of motor action (see discussion). Preliminary results have been reported in abstract form (Turpin et al. 2015).
METHODS
Subjects.
A total of 19 healthy right-hand-dominant subjects (10 women and 9 men; age: 22–38 yr) signed an informed consent form to participate in the study, which was reviewed and approved by the Institutional Ethics Committee (CRIR) in accordance with the Declaration of Helsinki.
Setup and protocol.
Subjects sat in a comfortable dental chair with a back support. The semisupinated right forearm was placed in a rigid custom-made cast attached to a horizontal manipulandum that could be rotated about a vertical axis aligned with that of the elbow joint (Fig. 1A). The shoulder was in an abducted and extended position (∼30° from the neutral position). A servo-controlled motor (Mavilor Motors, MT 2000) applied torques to the manipulandum to passively stretch elbow flexors or extensors through an arc of 20° or 70°. Flexors were stretched from an initial position of ∼50° (180° = full elbow extension), and extensors were stretched from an initial position of ∼120°. Before each stretch, subjects compensated a constant torque of 2–3 Nm (∼10–15% maximum voluntary contraction) at the specified initial position. The torque was applied to the manipulandum opposing, depending on the chosen torque direction, either flexion or extension (Fig. 1A). Feedback of the elbow position was presented on a screen, and subjects were required to maintain a stable forearm position within a 3° window. Vision of the position became unavailable when stretches were initiated. Stretch onsets were randomized within the range of 0.5–1 s after the initial position was stabilized. Participants were instructed not to intervene by consciously changing the muscle resistance elicited by stretching.
Fig. 1.
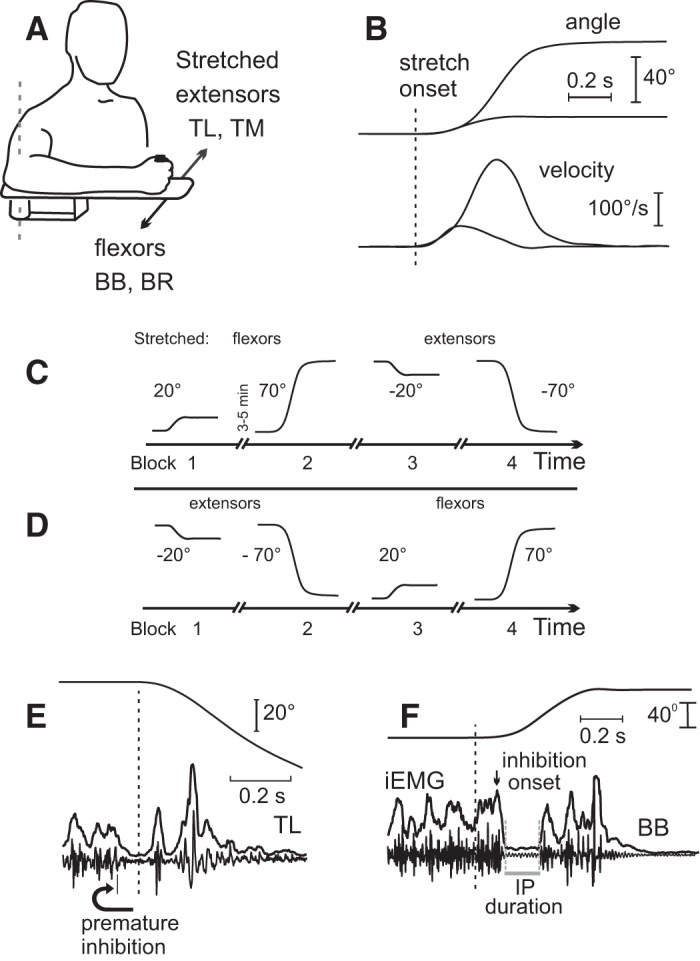
Methodology. A: experimental setup. Stretches of elbow flexors [biceps brachii (BB), brachioradialis (BR)] or extensors [triceps lateralis (TL), triceps medialis (TM)] were produced with a torque motor of the manipulandum. B: 20° and 70° stretches and velocity profiles. C: procedures: 4 blocks (40 trials in each) were done starting from stretching flexors (blocks 1 and 2) and then extensors (blocks 3 and 4). D: in different subjects chosen randomly, the experiments started from stretching extensors (blocks 1 and 2) and then flexors (blocks 3 and 4). E: example of a trial excluded from analysis: in 1 or 2 trials per subject, the muscle activity was reduced prematurely, before the stretch onset. F: identification of an inhibitory period (IP) in the integrated EMG (curve iEMG) and raw EMG. To minimize prediction of the stretch onset time, the latter was randomized within the range of 0.5–1 s after the initial position was stabilized, but in this and other figures only a segment of kinematic and EMG data just preceding the stretch onset is shown.
Muscle stretches produced by the torque motor had bell-shaped velocity profiles (Fig. 1B) that remained stable despite resistance from the stretched muscles. In eight subjects, four blocks of 40 trials each (8–10 s between each trial) in which 20° stretches of flexors (block 1) were done (Fig. 1C) were followed by longer stretches (70°) of the same muscles after a 3- to 5-min rest (block 2). In blocks 3 and 4, stretching of the antagonist (extensor) muscles that were shortened in the previous two blocks was produced. The same sequence of blocks was done in an additional seven subjects, but the order in which muscles were stretched was reversed, thus stretching the extensors in blocks 1 and 2 and the flexors in blocks 3 and 4 (Fig. 1D).
In the sequences described above, testing started with small, 20° stretches (block 1) and continued with longer, 70° stretches (block 2). To test whether or not the order influenced SR adaptation, in the remaining four subjects blocks 1 and 2 were rearranged.
Since adaptation of SR responses to stretching could occur from the first stretch, recording started without preliminary training. A custom-written program in LabVIEW (National Instruments, Austin, TX) was used to control the experiments and record elbow position and velocity as well as EMG data.
Recording of EMG activity and kinematics.
Surface EMG activity was recorded from two elbow flexors of the right (dominant) arm, biceps brachii (BB) and brachioradialis (BR), and two elbow extensors, lateral (TL) and medial (TM) heads of triceps brachii. After the skin was cleaned, pairs of 1-cm-diameter Ag-Ag Cl disk electrodes with interelectrode distances of ∼1.5–2 cm were placed on the bellies of the four elbow muscles. The chosen interelectrode distance is considered sufficient to pick up potentials of many motor units from the underlying muscle while minimizing cross-talk contamination (Hermens et al. 2000). EMG signals were monitored on a display and visually checked for minimal background noise level during full muscle relaxation. EMG signals were amplified with a wireless EMG system (Wave, Cometa Systems) and sampled at 2 kHz.
Data analysis.
Raw EMG signals were filtered (4th-order Butterworth filter, 20–400 Hz) and demeaned (nullifying a bias in the EMG amplifiers). If necessary, electrical noise components were removed with a notch filter at 60 Hz (bandwidth = 0.3 Hz). Joint angular displacement was low-pass filtered (2nd-order Butterworth, cutoff frequency 10 Hz).
Integrals of the rectified EMG signals were computed over 25-ms windows (trapezoid method) and shifted with each EMG sample interval to obtain the EMG profiles (iEMG). This method has the advantage of being sensitive to changes in the density of EMG signals in response to perturbations.
The background (prestretch) EMG level was defined as the averaged iEMG signal computed over a period of 150 ms prior to stretch onset and used to normalize iEMG signals. Trials in which subjects started to change the EMG activity before stretch onset (e.g., Fig. 1E) were excluded from the analysis (1 or 2 trials per subject).
SR size was computed as the maximal magnitude of the iEMG exceeding the prestretch level in the range 20–110 ms after movement onset.
Inhibitory periods (IPs) in EMG response to stretches were defined as relatively large and long-lasting reductions in the ongoing EMG activity after muscle stretch onset (Fig. 1F). Specifically, IP onset was identified as the time when the averaged normalized iEMG patterns for both stretched muscles decreased below 25% of the prestretch EMG level. IP offset was identified as the time when iEMG began to exceed the same level. To distinguish real IPs from occasional short intervals between regular EMG spike discharges, only intervals exceeding 35 ms were considered as IPs. The time of reaching an IP was defined as the interval between the peak iEMG immediately preceding the IP and IP onset. Additionally, an interactive interface was used to verify IP identification in each trial.
The identification criteria for SR responses and IPs were chosen for the following reasons. Compared with simple averaging of rectified EMG responses, iEMG is more sensitive to an increase in the density of spikes of motor units in SR responses and less sensitive to occasional, isolated motor unit spikes. Because of broad variability in the IP onset and duration, the presence of IPs would not be apparent in averaged EMG signals, explaining why the IP occurrence was not noticed in previous SR studies. The duration of the sliding window for iEMG computations (35 ms) has been shown to be optimal in differentiating between periods of activity and inactivity of muscles in EMG signals (Bonato et al. 1998). The threshold for identification of IPs (25% of prestretch EMG level) was more stringent than that usually based on confidence intervals (i.e., mean + 2SD, e.g., Nilsson et al. 1997).
All computations were done with custom-made MATLAB programs. A custom graphical user interface was used to verify IP identification in each trial.
Statistical analysis.
Student t-tests and one-factor ANOVAs were used to compare SR size for individual subjects over muscle stretch repetitions. SR size (mean ± SD), IP onsets and latencies, and number of IPs in the group data were assessed with repeated-measures ANOVAs and Bonferroni post hoc tests. For this analysis, each block was divided into sequences of 10 trials (4 sequences per block of 40 trials) and the mean value for each sequence was computed, resulting in 16 repeated measures for each subject. Only IPs of finite duration (<0.4 s) were considered in this analysis. Correlation was assessed with the Pearson r coefficient. Differences in means were considered statistically significant if P < 0.05.
RESULTS
Attenuation of SR responses after several stretches.
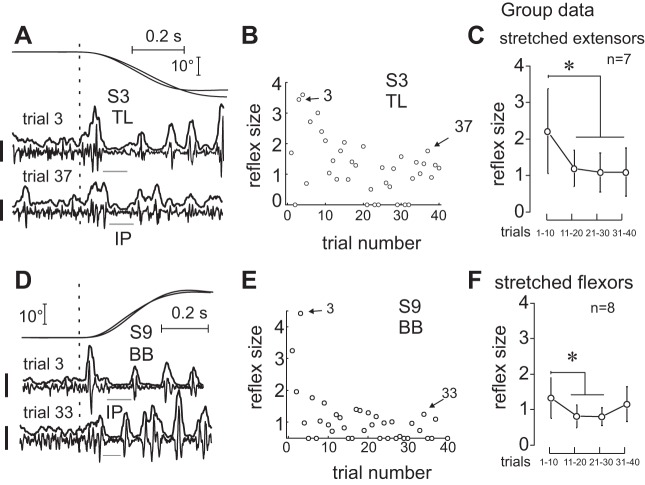
When experiments started by producing small 20° stretches, distinctive SR responses at latencies of 25–55 ms were observed (Fig. 2A). SR responses were also observed when experiments started by producing longer, 70° stretches (Fig. 2B). However, SR responses were attenuated after 5–12 stretches depending on the subject. Figure 2 shows typical examples of EMG recordings in later trials in which extensor (Fig. 2C) and flexor (Fig. 2D) SR responses were attenuated. Also note the presence of IPs in the EMG responses to muscle stretching. SR responses were attenuated by a factor of 3–4 depending on the subject (Fig. 3) in all but two subjects, whose flexor SR responses did not show significant changes during the 40 trials of block 1 (P > 0.134). Once attenuated, SR responses varied in subsequent trials but, on average, remained lower than in the first 10 trials, both in individual subjects (Fig. 3, B and E) and in two subgroups of subjects in one of which extensor (Fig. 3C) and in the other flexor (Fig. 3F) muscles were stretched. Considering only short-latency SR responses (25–55 ms), we found no effect of stretch repetition on SR size for every subject (P = 0.853 when stretching flexors and P = 0.746 when stretching extensors). We also analyzed the onset of short- and long-latency SRs for each muscle and found no significant changes with repetition (P > 0.069).
Fig. 2.
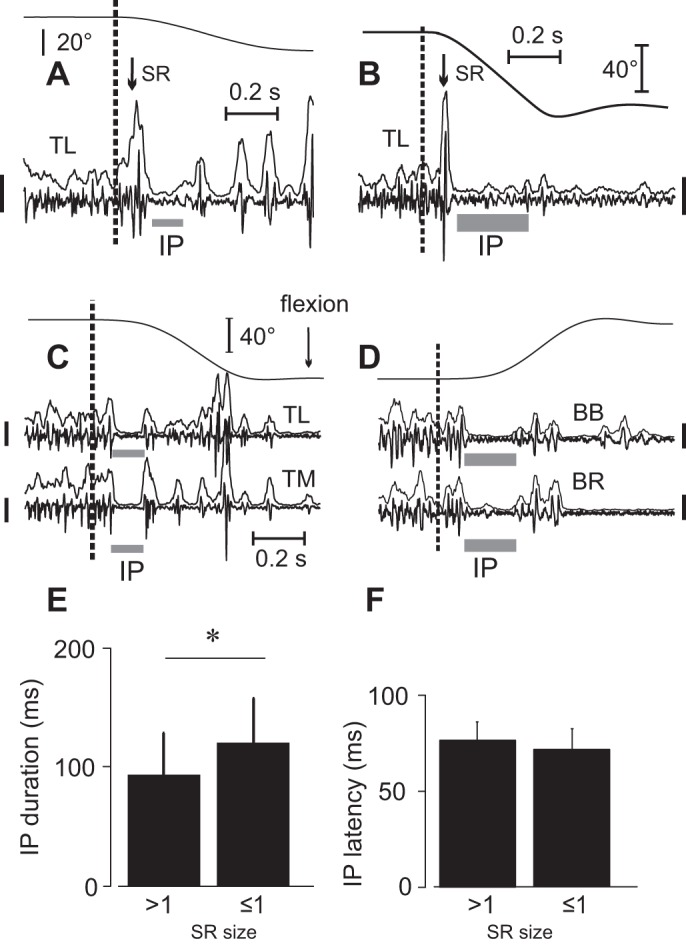
Typical responses to muscle stretching. A and B: substantial stretch reflexes (SRs) were evoked in response to both short- and long-amplitude stretches if they began to be applied to muscles that were previously not stretched in subjects 3 and 14, respectively. C and D: after several trials, SRs were attenuated but the IP remained in each stretched muscle. Calibration bars for EMG signals: 200 μV. E and F: IP duration and latency for large and small SR sizes. SR size = 1 when it exceeds the mean prestretch EMG level by 100% (group data) *P < 0.05.
Fig. 3.
Stretch reflex attenuation. Reflex responses were attenuated after several trials of stretching extensors (A–C) or flexors (D–F). In C and F, SR responses were normalized with respect to the mean rectified EMG activity before the stretch onset; *Significant difference: P < 0.001 in C and P = 0.002 in F. Calibration bars for EMG signals: 200 μV in A and 100 μV in D.
Attenuation of SRs could be explained if subjects increased the level of tonic coactivation of agonist and antagonist muscles before the stretch onset in sequential trials. However, we did not find any significant changes in the prestretch activity of the four muscles in the last compared with the first trials (P > 0.392).
Temporary suppression of background muscle activity.
In all but one subject, background EMG activity was transiently suppressed after SR responses in stretched muscles—an IP—to a level below the mean prestretch EMG activity, such that the muscles were practically silent. IPs were observed in both stretched elbow flexors and stretched extensors and remained visible even if phasic SR responses were previously attenuated (Fig. 2 and Fig. 3).
EMG suppression was initiated at a minimal latency of ∼35 ms (35.0 ± 9.2 ms for the group), although on average IPs started later (72.2 ± 12.3 ms for the group). Once started, the EMG suppression rapidly reached its maximum in 23–26 ms (approximately the noise level in EMG recordings).
The IP duration was highly variable (minimal duration for the group was 79.2 ± 29.4; maximal duration was 159.4 ± 50.0 ms). Longer IP durations in 5 of 14 subjects were associated with lower sizes of SR responses. However, although statistically significant, the correlation between IP duration and phasic SR size was low (mean r = −0.331; P < 0.001 to P = 0.040). In only two subjects was a higher SR size associated with a later IP onset, but the correlation was also low (mean r = 0.342, P < 0.022). For the group, the IP duration increased with the decreasing SR size (P = 0.002; Fig. 2E) while the IP onset was not related to the SR size (P = 0.157; Fig. 2F). No specific trend of IP duration (P = 0.423) or onset (P = 0.781) with stretch repetition was found.
In six subjects for stretched extensor trials and in three subjects for stretched flexor trials, the EMG activity remained suppressed until the end of muscle lengthening (Fig. 2B). In the remaining cases, once reactivated, muscles could be silenced again and later reactivated, thus generating multiple IPs while the muscles continued to be stretched (Fig. 2, A and C; Fig. 3, A and D). The number of IPs was 4.3 ± 2.3 per subblock of 10 trials but was also independent of the number of stretches (P = 0.136).
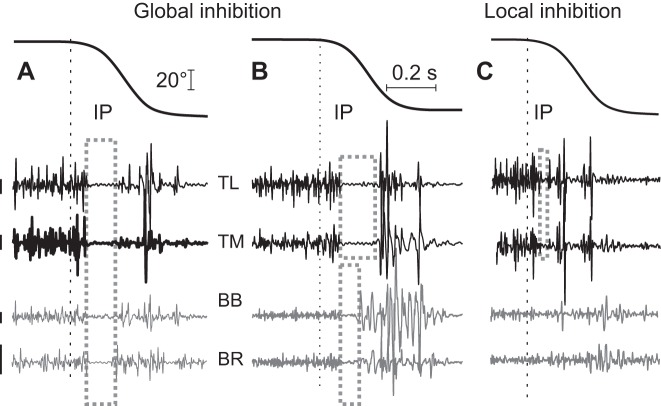
Muscle stretching could also affect the EMG activity of shortening antagonist muscles (Fig. 4). Most often (in 11 subjects when flexors were stretched and in 9 subjects when extensors were stretched), there was a common IP with subsequent reactivation of both stretched and shortened muscles (Fig. 4A). Less often, IPs in EMG activity of stretched muscles were associated with short-duration IPs (Fig. 4B) or with the absence of changes in antagonist EMG activity of shortened antagonist muscles (Fig. 4C).
Fig. 4.
Major patterns of responses of agonist and antagonist muscles to stretching of extensor muscles. A: the most frequent pattern—after SR responses of stretched muscles (elbow extensors in this example), a common inhibitory period (IP, rectangular box) with subsequent reactivation of all muscles occurred. B: as in A, common extensor IP was associated with a shorter-duration IP in the shortened flexor muscles. C: only stretched extensors had a common IP without detectable changes in the EMG activity of shortening flexors. Calibration bars for EMG signals: 200 μV for preloaded extensors and 25 μV for flexors (antagonists).
History-dependent changes in EMG responses to muscle stretching.
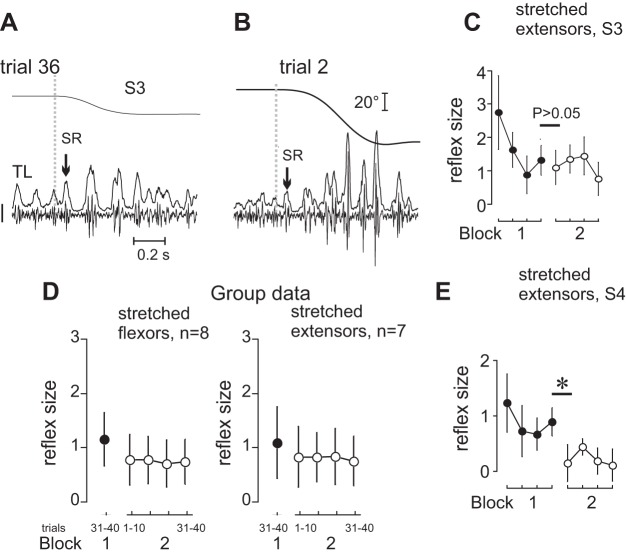
SR responses preliminarily attenuated by 20° stretches in block 1 remained attenuated when SR responses were tested in block 2 after a 3- to 5-min rest and longer stretches (70°) were produced (Fig. 5, A–C). This effect was observed when either flexors or extensors were stretched in block 1 (Fig. 5D). In 11 subjects, the mean size of SR responses in the first 10 trials of block 2 did not significantly differ from that in the last 10 trials from the preceding block (P = 0.058 to 0.631). In four subjects, the 3- to 5-min rest period between the blocks was associated with a significant decrease in the SR responses (i.e., by −58.9 ± 15.9%) despite an increase in the stretch magnitude from 20° to 70° (P < 0.001 to 0.005; Fig. 5E).
Fig. 5.
Persistence of SR attenuation after 3- to 5-min rest. A–C: after attenuation in trials with 20° stretches, the SR size was not restored when stretches were resumed at a high (70°) magnitude after 3–5 min of rest (data for subject S3 in C, P = 0.322). D: the difference between the mean SR responses during the last ten 20° stretches and subsequent 70° stretches was insignificant (P > 0.05). E: the transition from block 1 to block 2 could be associated with a significant decrease in the SR responses (example for subject 4, *P < 0.001). Calibration bar for EMG signals in A and B: 200 μV.
Ability to minimize muscle stretch resistance was generalized to previously nonstretched muscles.
Figure 6 shows the effects of stretching of antagonists 3–5 min after agonist SR responses were attenuated in the previous block of trials. Regardless of whether flexor (Fig. 6A) or extensor (Fig. 6C) phasic SRs were initially attenuated, only modest stretch responses could be observed in the originally nonstretched antagonist muscles in first and subsequent trials (Fig. 6, B and D). This was characteristic of the group (Fig. 6, E and F). With the transition from stretching extensors to stretching flexors or vice versa, SR size did not change significantly (P = 0.438 and P = 0.506, respectively), showing that adaptive changes in EMG stretch responses of one group of muscles were generalized to previously nonstretched antagonist muscles of the joint.
Fig. 6.
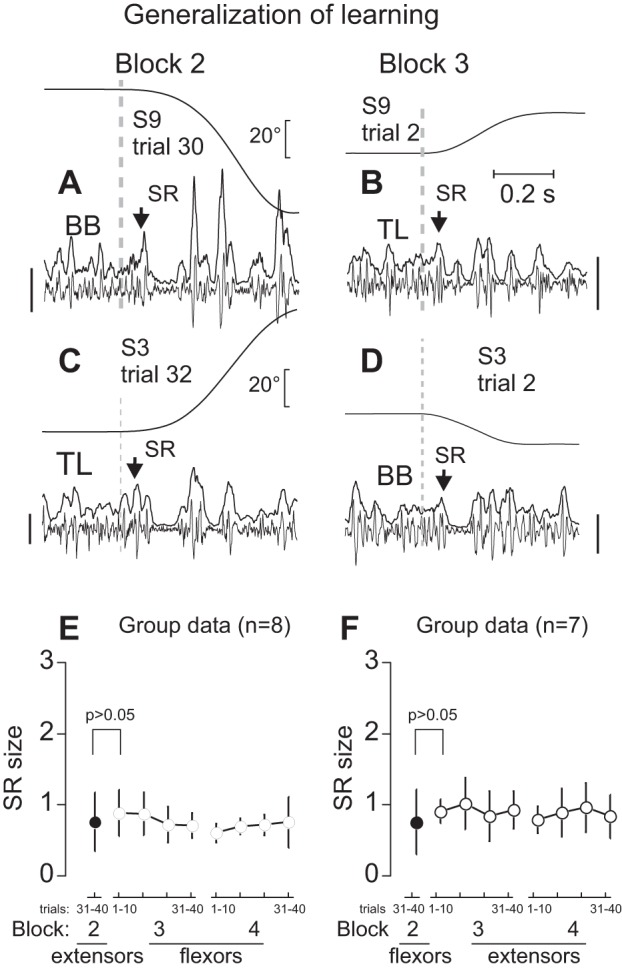
Generalization of SR attenuation. A: example of flexor SR response (arrow) and IP from one of the trials in which SRs were attenuated. B: after a 3 to 5-min rest period, originally nonstretched extensors began to be stretched. Extensor SR was immediately suppressed (arrow) without preliminary trials of stretching. C and D: a similar effect of flexor SR suppression was observed after previous suppression of extensor SRs. E and F: group data—antagonist SRs were suppressed without preliminary attenuation if SRs of agonist muscles were suppressed during repeated stretches before a 3- to 5-min rest period. Calibration bars for EMG signals: 100 μV (A and B) and 200 μV (C and D).
DISCUSSION
Our study showed that subjects learned to minimize resistance to muscle stretching in sequential trials and preserved this strategy after a 3- to 5-min rest. They were unaware of this behavior (implicit learning). Moreover, once succeeding in achieving such a strategy, they used it, without learning anew, to immediately minimize stretch resistance of the other, originally nonstretched muscles (generalization of learning).
Minimizing SR responses and ongoing EMG activity.
Attenuation of SR responses was observed in finger and wrist muscles during repetitive stretches at a constant frequency (Rothwell et al. 1986) such that subjects could predict the stretch onsets. In our study, stretch effects of elbow muscles were analyzed and stretch onset predictability was minimized. Our results show that SR attenuation can be observed even in these cases.
Subjects maintained the prestretch levels of agonist and antagonist EMG activity in sequential trials, which makes it unlikely that SR attenuation resulted from an increase in coactivation of agonist and antagonist muscles with repeated stretching.
The finding of the immediate transfer of SR attenuation to originally nonstretched antagonist muscles makes it unlikely that changes in intrinsic muscle properties during stretch repetition (Avela et al. 1999) were responsible for SR attenuation. This, as well as the finding that, once attenuated, the SR was not restored after comparatively long resting periods (3–5 min), suggests that depression of neurotransmitter release from afferent terminals on MNs during stretch repetition (Stein et al. 2007) was not a factor responsible for SR attenuation in our study.
Postspike hyperpolarization and recurrent inhibition of MNs could contribute to IP when SR responses were present. The duration of these postspike effects is usually <50 ms (Kudina and Pantseva 1988; Turker and Powers 1999) and could not be responsible for longer IPs (>70 ms). IPs could be observed even in the absence of SR responses, when the effects of postspike hyperpolarization or recurrent inhibition of MNs were minimized. Tendon organ afferents could be activated by muscle stretching and initiate IPs, but since they reflect only momentary changes in active muscle tension (Jami 1992), it is also unlikely that they could maintain these IPs when active tension decreased. Widespread presynaptic inhibition elicited by signals from skin and subcutaneous receptors (Pierrot-Deseilligny and Burke 2005) could be responsible for IPs in both agonist (stretched) and antagonist muscles. In addition, there are spinal inhibitory neurons that, unlike Ib interneurons, receive inputs from free intramuscular terminals (Cleland et al. 1982, 1990). Activated by large muscle stretches, these interneurons produce long-lasting inhibition of MNs and could be responsible for IPs in our study.
IPs were initiated very early after the stretch onset, at a minimal latency of ∼35 ms. It is possible that subjects could prepare an inhibitory response prior to and trigger it with the onset of perturbation presumably via pre- and postsynaptic inputs to MNs to attenuate phasic SR responses (Colebatch et al. 1979; Rothwell et al. 1986). The interplay between facilitatory afferent feedback eliciting SR responses and central inhibition of these responses triggered by this feedback could result in a comparatively broad range of SR latencies (25–55 ms).
History-dependent effects.
History-dependent behavior is illustrated by the observation that SR attenuation was accomplished gradually, over several trials. Such behavior is also illustrated by the finding that, attenuated in the 20° stretch trials, SRs remained attenuated after a 3- to 5-min rest when stretching was prolonged from 20° to 70°. Moreover, in some subjects, SRs decreased after the first rest period, suggesting that neural processes associated with learning implicitly continued during the pause in the task repetition. “Off-line” performance gains are thought to occur during learning in which task solutions are reinforced due to experience-driven neural plasticity and memory consolidation (Bourne and Archer 1956; Karni et al. 1998; Korman et al. 2003; Meital et al. 2013).
Another type of history-dependent behavior was manifested by the finding that once EMG responses to muscle stretching became adapted they were already adapted in the responses to the first stretches of the antagonists, a sign of generalization due to implicit learning. How could such generalization be achieved? Figure 4 shows that before muscle stretching subjects usually activated not only stretched muscles to compensate for the initial load but also, to a lesser degree, antagonist muscles (coactivation). In most subjects, after the stretch onset, both agonist and antagonist muscles were deactivated and reactivated in parallel (global inhibition during a common IP; Fig. 4A). Indeed, antagonist muscles could be deactivated because of a decrease in afferent feedback to α-MNs during muscle shortening, but this effect could be compensated by the simultaneous reduction in inhibition from agonist Ia interneurons of reciprocal inhibition during agonist IP. In most subjects, however, pauses in the activity of stretched and shortened muscles occurred simultaneously (Fig. 4A), suggesting that common central signals could initially suppress and then restore EMG activity of both stretched and shortened muscles. If so, the central inhibition suppressing SRs and background EMG activity was not local but global and could be employed when antagonist muscles began to be stretched, explaining the effect of learning generalization.
Considering results in general context of posture and movement control.
We continue to discuss our findings in reference to the spatial nature of the central influences on reflexes and MNs via shifts in the threshold positions of body segments at which muscles begin to be activated (see introduction).
On the basis of experimental findings (summarized in Feldman 2015), the following condition of muscle activation has been formulated. If x is the current muscle length, then the muscle is active if
| (1) |
where λ* is the dynamic threshold muscle length:
| (2) |
which means that λ* decreases with stretch velocity; μ is the sensitivity of threshold to velocity (v > 0 for muscle stretching and v < 0 for shortening). The dynamic threshold also depends on central influences on λ and on heteronymous reflexes such as reflex reciprocal inhibition and crossed-extensor reflexes (Feldman and Orlovsky 1972; Matthews 1959) as well as on the history-dependent intrinsic state of α-MNs (e.g., associated with the plateau potentials). Taken together, these factors influence term ρ in the equation for λ*.
These formulas account for different ways by which muscles can be activated or deactivated. In particular, reflex gating and gain modulation can be considered as caused by shifts in the spatial threshold (λ) of reflexes (Feldman 2015).
In our study, muscles to be stretched were preactivated to counteract the initial load. This can be achieved by setting λ below the muscle length at the initial elbow position (x − λ > 0; Fig. 7). Anticipating muscle stretching, the system could prepare to increase and trigger these threshold shifts as soon as stretching started. Prepared at the level of spinal interneurons responsible, in particular, for presynaptic inhibition of MNs, the threshold shifts could be produced sufficiently early to attenuate phasic SR responses to muscle stretching. Produced rapidly, shifts in λ could overrun the ongoing muscle stretching, suppressing not only SR responses but the ongoing EMG activity and thus eliciting an IP.
Fig. 7.
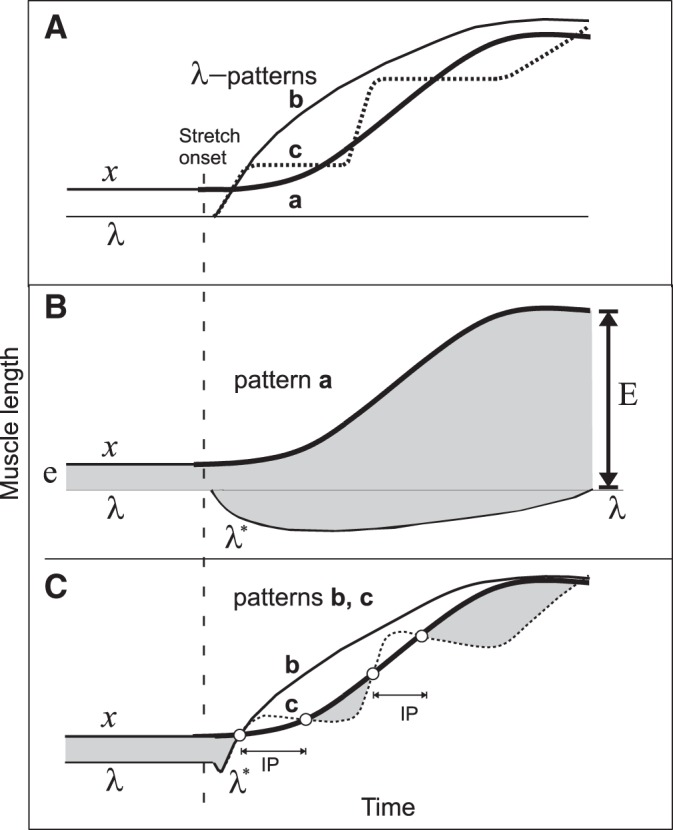
Qualitative explanations of results in terms of threshold position control. A: 3 possible patterns (a–c) of central changes in the threshold muscle length (λ) in response to passive changes in muscle length (x) are shown. Pattern a: λ is constant, i.e., the control system tolerates the muscle stretching without intervening. Pattern b: the system continuously increases λ to make it overrun and remain ahead of x during the entire muscle stretching. Pattern c: in response to the stretch onset, the system triggers a set of discrete ramp-and-hold increases in λ, each time overrunning the muscle stretching. B: dynamics of muscle activation during pattern a. Muscle activation is roughly proportional to the difference between x and λ* (vertical distance between upper and lower curves). Before the stretch onset, the muscle activation is defined by distance e. Although λ remains constant, the dynamic threshold (λ*) decreases with stretch velocity (i.e., deviates down from λ line). After the stretch onset, the shaded area below λ line represents the phasic, velocity-dependent SR component; the shaded area above λ line is the tonic, length-dependent SR component. After the end of stretching, the phasic component vanishes but the tonic component (E) of muscle activation remains and substantially exceeds the initial, prestretch activation level (E >> e). C: during pattern b, the phasic component of the SR is suppressed and the muscle becomes deactivated, thus ceasing active muscle resistance during the entire muscle stretching. During pattern c, the phasic SR component and the ongoing muscle activation are also suppressed, producing an IP. During the holding phase of each λ ramp, the continuing stretching reaches threshold λ* and the muscle is reactivated but generates much less activity (defined by the small shaded areas below curve x) than during pattern a, thus minimizing active resistance to muscle stretching. After learning to generate pattern c, the system can use it, without further learning, to minimize resistance to stretching of preliminary not stretched muscles (generalization of learning).
Figure 7 shows three patterns of threshold shift timing. In pattern a, descending systems maintain the same value of λ and let the peripheral mechanisms generate phasic and tonic SR responses. In pattern b, descending systems accomplish a rapid and large monotonic increase in λ as soon as stretching starts, thus attenuating SR responses and suppressing the ongoing muscle activity during the entire stretch. Pattern c consists of several ramp-and-hold-like increases in λ, resulting in attenuation of phasic SR responses and several IPs. During each holding phase of λ, the stretched muscle reaches and exceeds the activation threshold and the muscle is reactivated. Multiple IPs thus allow the system gradually, in several steps, to yield the muscle stretching while minimizing muscle active resistance during and at the end of stretching. This qualitatively explains the occurrence of a single prolonged IP and multiple IPs with muscle activation between them in our study. The multistep pattern of shifts in the activation thresholds could be applied simultaneously to MNs of both stretched and shortened muscles. This common pattern could be used to produce adaptive responses to the first and subsequent stretches of previously nonstretched muscles (generalization of learning).
Without central modulation of spatial thresholds, transient inhibition of muscle activity would not prevent accumulation of SR resistance in proportion to the difference between x and λ at the end of stretching (Fig. 7B, arrow E). In contrast, SR threshold modulation (patterns b and c) allows the system to minimize reflex resistance to stretching. Note that, while shifting the spatial threshold, the system does not eliminate the SR as such—it just resets (“readdresses”) it to a new range of arm positions where the SR remains fully functional. This central strategy has been shown to underlie the control of posture, movement, isometric torque, and motor learning. It also solves several classical problems in motor control (Feldman 2015; Feldman et al. 2011). It was found that the corticospinal system is directly involved in feedforward setting and resetting of the spatial thresholds in the control of wrist position and movement (Ilmane et al. 2013; Raptis et al. 2010; Sangani et al. 2011). Thus regulation of stretch responses is explained in the context of threshold position control that also underlies control of posture and movement.
Relation to neurological motor deficits.
There is certain resemblance between the inhibitory reactions of stretched preactivated muscles observed in healthy subjects and the clasp-knife phenomenon, i.e., sudden suppression of muscle resistance during stretching of spastic or rigid muscles in some poststroke and Parkinson subjects (Burke et al. 2013; Xia et al. 2011) or in rigid muscles in decerebrated cats (Burke et al. 1972; Cleland et al. 1990). It may be suggested that the phenomenon results from resetting of SR thresholds prepared in advance at a spinal level and triggered in response to muscle stretching. Some patients may thus have a neural means of suppressing spasticity or rigidity, which can be verified in future studies and exploited in rehabilitation of these deficits.
Although facilitated in pathology, the phenomenon may represent a normal physiological mechanism of dealing with muscle resistance to stretching. For example, it might be used in situations when stretching of active muscles can exceed the force generation capacity of sarcomeres or permissible excursions of body segments.
Conclusions.
The present study showed that when active muscles undergo anticipated stretching subjects learn to minimize resistance to it by attenuating both SRs and ongoing EMG. This process is well explained by resetting of the spatial thresholds of muscle activation prepared prior to and triggered after stretching onset at a short latency comparable with that of the SR, possibly via pre- and postsynaptic inputs to MNs. The study advances the understanding of the fundamental role of threshold position control of posture, movement, and motor learning in the healthy and damaged nervous system.
GRANTS
This work was supported by the National Science and Engineering Research Council and Heart and Stroke Foundation, Canada. M. F. Levin holds a Canada Research Chair in Motor Recovery and Rehabilitation. Postdoctoral studies of N. A. Turpin are supported by program Mentor (REPAR/FRSC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.A.T., M.F.L., and A.G.F. conception and design of research; N.A.T. and A.G.F. performed experiments; N.A.T., M.F.L., and A.G.F. analyzed data; N.A.T., M.F.L., and A.G.F. interpreted results of experiments; N.A.T., M.F.L., and A.G.F. prepared figures; N.A.T., M.F.L., and A.G.F. drafted manuscript; N.A.T., M.F.L., and A.G.F. edited and revised manuscript; N.A.T., M.F.L., and A.G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rim Rahal and Sandeep Subramanian for help in data collection for this project.
REFERENCES
- Asatryan DG, Feldman AG. Functional tuning of the nervous system with control of movement or maintenance of a steady posture. I. Mechanographic analysis of the work of the joint or execution of a postural task. Biophysics 10: 925–934, 1965. [Google Scholar]
- Avela J, Kyröläinen H, Komi PV. Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J Appl Physiol 86: 1283–1291, 1999. [DOI] [PubMed] [Google Scholar]
- Bonato P, Alessio TD, Knaflitz M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Trans Biomed Eng 45: 287–299, 1998. [DOI] [PubMed] [Google Scholar]
- Bourne LE Jr, Archer EJ. Time continuously on target as a function of distribution of practice. J Exp Psychol 51: 25–33, 1956. [DOI] [PubMed] [Google Scholar]
- Burke D, Knowles L, Andrews C, Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain 95: 31–48, 1972. [DOI] [PubMed] [Google Scholar]
- Burke D, Wissel J, Donnan GA. Pathophysiology of spasticity in stroke. Neurology 80: S20–S26, 2013. [DOI] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol 440: 243–255, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland CL, Hayward L, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. J Neurophysiol 64: 1319–1330, 1990. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Rymer W, Edwards FR. Force-sensitive interneurons in the spinal cord of the cat. Science 217: 652–655, 1982. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC, McCloskey DI, Potter EK. Subject instruction and long latency reflex responses to muscle stretch. J Physiol 292: 527–534, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Gating of motor cortex reflexes by prior instruction. Brain Res 71: 479–494, 1974. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Referent Control of Action and Perception: Challenging Conventional Theories in Behavioral Neuroscience. New York: Springer, 2015. [Google Scholar]
- Feldman AG, Krasovsky T, Banina MC, Lamontagne A, Levin MF. Changes in the referent body location and configuration may underlie human gait, as confirmed by findings of multi-muscle activity minimizations and phase resetting. Exp Brain Res 210: 91–115, 2011. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Orlovsky GN. The influence of different descending systems on the tonic stretch reflex in the cat. Exp Neurol 37: 481–494, 1972. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. [DOI] [PubMed] [Google Scholar]
- Horak F, Diener H, Nashner L. Influence of central set on human postural responses. J Neurophysiol 62: 841–853, 1989. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Spinal reflexes, mechanisms and concepts: from Eccles to Lundberg and beyond. Prog Neurobiol 78: 215–232, 2006. [DOI] [PubMed] [Google Scholar]
- Ilmane N, Sangani S, Feldman AG. Corticospinal control strategies underlying voluntary and involuntary wrist movements. Behav Brain Res 236: 350–358, 2013. [DOI] [PubMed] [Google Scholar]
- Jankowska E, McCrea D, Mackel R. Oligosynaptic excitation of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol 316: 411–425, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72: 623–666, 1992. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA 100: 12492–12497, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudina LP, Pantseva RE. Recurrent inhibition of firing motoneurones in man. Electroencephalogr Clin Neurophysiol 69: 179–185, 1988. [DOI] [PubMed] [Google Scholar]
- Lewis GN, MacKinnon CD, Perreault EJ. The effect of task instruction on the excitability of spinal and supraspinal reflex pathways projecting to the biceps muscle. Exp Brain Res 174: 413–425, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CD, Tolhurst SA, Bawa P. Proprioceptive reaction times and long-latency reflexes in humans. Exp Brain Res 221: 155–166, 2012. [DOI] [PubMed] [Google Scholar]
- Matthews PB. A study of certain factors influencing the stretch reflex of the decerebrate cat. J Physiol 147: 547–564, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meital N, Korinth SP, Karni A. Plasticity in the adult oculomotor system: offline consolidation phase gains in saccade sequence learning. Brain Res 1528: 42–48, 2013. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res 26: 59–72, 1976. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Steeves JD. Resetting of resultant stiffness in ankle flexor and extensor muscles in the decerebrate cat. Exp Brain Res 62: 401–410, 1986. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Panizza M, Arieti P. Computer-aided determination of the silent period. J Clin Neurophysiol 14: 136–140, 1997. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge Univ. Press, 2005, p. 355. [Google Scholar]
- Raptis H, Burtet L, Forget R, Feldman AG. Control of wrist position and muscle relaxation by shifting spatial frames of reference for motoneuronal recruitment: possible involvement of corticospinal pathways. J Physiol 588: 1551–1570, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Berardelli A, Marsden CD. Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res 63: 197–204, 1986. [DOI] [PubMed] [Google Scholar]
- Sangani SG, Raptis HA, Feldman AG. Subthreshold corticospinal control of anticipatory actions in humans. Behav Brain Res 224: 145–154, 2011. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Time course of “set”-related changes in muscle responses to stance perturbation in humans. J Physiol 487: 787–796, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol 121: 1680–1689, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale Univ. Press, 1906. [Google Scholar]
- Sillar KT. Spinal pattern generation and sensory gating mechanisms. Curr Opin Neurobiol 1: 583–589, 1991. [DOI] [PubMed] [Google Scholar]
- Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci 11: 328–332, 1988. [DOI] [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res 182: 309–319, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker KS, Powers RK. Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol 82: 829–840, 1999. [DOI] [PubMed] [Google Scholar]
- Turpin NA, Rahal R, Subramanian K, Levin MF, Feldman AG. Threshold position resetting suppresses both stretch reflexes and background muscle activity in arm muscles in response to prolonged muscle lengthening (Abstract). Neuroscience Meeting Planner 2015: 67–22., 2015. [Google Scholar]
- Wei K, Glaser JI, Deng L, Thompson CK, Stevenson IH, Wang Q, Hornby TG, Heckman CJ, Kording KP. Serotonin affects movement gain control in the spinal cord. J Neurosci 34: 12690–12700, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Segal RL. Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol 75: 1637–1646, 1996. [DOI] [PubMed] [Google Scholar]
- Xia R, Powell D, Rymer WZ, Hanson N, Fang X, Threlkeld AJ. Differentiation between the contributions of shortening reaction and stretch-induced inhibition to rigidity in Parkinson's disease. Exp Brain Res 209: 609–618, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58 185–205, 1999. [DOI] [PubMed] [Google Scholar]