We review here the mechanisms leading to two seizure-onset EEG patterns that occur in mesial temporal lobe epilepsy. Based on data obtained in epileptic patients and in animal models, we propose that the initiation of low-voltage fast-onset and hypersynchronous-onset seizures depends on the involvement of GABAergic interneuron and of principal (glutamatergic) networks, respectively, which in both cases rest on functional GABAA receptor signaling.
Keywords: high-frequency oscillations, hypersynchronous onset, low voltage fast onset, seizures
Abstract
Low-voltage fast (LVF) and hypersynchronous (HYP) patterns are the seizure-onset patterns most frequently observed in intracranial EEG recordings from mesial temporal lobe epilepsy (MTLE) patients. Both patterns also occur in models of MTLE in vivo and in vitro, and these studies have highlighted the predominant involvement of distinct neuronal network/neurotransmitter receptor signaling in each of them. First, LVF-onset seizures in epileptic rodents can originate from several limbic structures, frequently spread, and are associated with high-frequency oscillations in the ripple band (80–200 Hz), whereas HYP onset seizures initiate in the hippocampus and tend to remain focal with predominant fast ripples (250–500 Hz). Second, in vitro intracellular recordings from principal cells in limbic areas indicate that pharmacologically induced seizure-like discharges with LVF onset are initiated by a synchronous inhibitory event or by a hyperpolarizing inhibitory postsynaptic potential barrage; in contrast, HYP onset is associated with a progressive impairment of inhibition and concomitant unrestrained enhancement of excitation. Finally, in vitro optogenetic experiments show that, under comparable experimental conditions (i.e., 4-aminopyridine application), the initiation of LVF- or HYP-onset seizures depends on the preponderant involvement of interneuronal or principal cell networks, respectively. Overall, these data may provide insight to delineate better therapeutic targets in the treatment of patients presenting with MTLE and, perhaps, with other epileptic disorders as well.
NEW & NOTEWORTHY
We review here the mechanisms leading to two seizure-onset EEG patterns that occur in mesial temporal lobe epilepsy. Based on data obtained in epileptic patients and in animal models, we propose that the initiation of low-voltage fast-onset and hypersynchronous-onset seizures depends on the involvement of GABAergic interneuron and of principal (glutamatergic) networks, respectively, which in both cases rest on functional GABAA receptor signaling.
the eeg activity recorded during focal seizures in patients with mesial temporal lobe epilepsy (MTLE) is characterized by different levels of detail, depending on the recording approach used. Standard scalp electrode recordings may reveal, at the onset of a seizure, flattening of the EEG signals localized in the temporal lobe that is at times associated with the appearance of low-amplitude fast rhythms (Fisher et al. 1992; Gloor 1975; see also de Curtis and Gnatkovsky 2009). However, this straightforward diagnostic tool is not ideal for identifying the exact seizure initiation area that resides in mesial cortical structures such as the hippocampus or the amygdala; in addition, many seizures in MTLE do not show this pattern: some show other patterns, and many only show artifacts at onset. In contrast, intracranial EEG recordings obtained from deep limbic regions, which are the gold standard in presurgical evaluation of pharmacoresistant epileptic patients who are candidates for surgery, can lead to a more precise identification of the brain structures involved in seizure initiation while revealing in detail the features of discharge onset (Lieb et al. 1976; Pacia and Ebersole 1997).
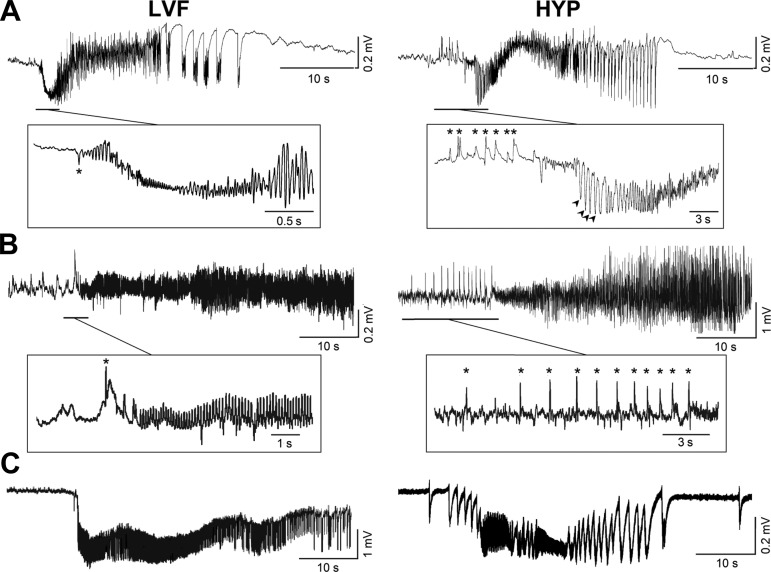
Seizures in MTLE patients studied with intracranial electrodes have variable EEG features, but evidence published during the last two decades indicates that two specific seizure-onset patterns may predominate in temporal lobe (limbic) structures (Ogren et al. 2009; Perucca et al. 2014; Singh et al. 2015; Spanedda et al. 1997; Spencer and Pappas 1992; Wendling et al. 2005). The most frequent onset pattern is characterized by “low-voltage fast” (LVF) activity in the gamma range, at times initiated by a single (also termed “sentinel”) spike (Fig. 1A, asterisk in the left panel); the second onset pattern, referred to as “hypersynchronous” (HYP), is associated with an initial series of large-amplitude spikes that occur at a frequency of ∼1 Hz (Fig. 1A, asterisks in the right panel) and can also reappear during the initial part of the seizure (Fig. 1A, arrowheads in the right panel). As illustrated in Fig. 1A, LVF- and HYP-onset seizures evolve into a phase of sustained oscillatory activity (also defined as “tonic”) and then into bursting rhythmic (“clonic”) discharges; in both cases, termination of seizure discharge is followed by a period of spontaneous electrical activity suppression that is termed “postictal depression.”
Fig. 1.
Electrographic features of low-voltage fast (LVF) and hypersynchronous (HYP) seizure onsets in humans (A) as well as in in vivo (B) and in vitro (C) experimental models. Traces shown in A were obtained from hippocampal recordings performed during presurgical intracranial monitoring with depth macroelectrodes in a patient with a focal cortical dysplasia of the temporal lobe (trace on left) and in a patient with malformation (i.e., altered rotation) of the hippocampus that was associated with hippocampal sclerosis verified on magnetic resonance and histological examinations (trace on right). The asterisk in the LVF sample identifies the single, “sentinel” spike that can occur at onset, whereas asterisks in the HYP sample highlight the initial series of large-amplitude spikes at ∼1 Hz; note in this case that spikes at a similar frequency (arrowheads) reappear during the initial part of the seizure. Traces in B were recorded from the CA3 area of the hippocampus in a pilocarpine-treated epileptic rat; note that both types of seizure onsets could be recorded from the same animal; asterisks identify specific EEG events as described for the recordings shown in A. Traces in C were obtained from experiments performed with rat brain slices maintained in vitro and bathed in medium containing 4-aminopyridine; the LVF seizure-like onset shown on the left was recorded from the medial entorhinal cortex, whereas the HYP seizure on the right was obtained from the perirhinal cortex; these in vitro recordings were performed with DC amplifiers, and thus seizure-like discharges are associated with robust negative-going shifts. Traces shown in A were kindly provided by Dr. Stefano Francione and Dr. Laura Tassi of the Claudio Munari Epilepsy Surgery Center (Milan, Italy).
The clinical evidence for two distinct seizure-onset patterns has been confirmed in animal models of MTLE and of epileptiform synchronization in both in vivo (Fig. 1B) (Bragin et al. 2005; Grasse et al. 2013; Lévesque et al. 2012; Salami et al. 2015; Toyoda et al. 2015) and in vitro (Fig. 1C) (Avoli et al. 1996; Boido et al. 2014; Derchansky et al. 2008; Köhling et al. 2016; Lopantsev and Avoli 1998a and 1998b; Zhang et al. 2012; see for review Avoli and de Curtis 2011) preparations. Moreover, these fundamental studies have provided evidence suggesting that specific neuronal networks may contribute to LVF and HYP seizure-onset patterns. In this review, we will briefly analyze evidence obtained in patients suffering from MTLE; these clinical studies suggest that LVF and HYP seizure-onset patterns may reflect different epileptic conditions along with different degrees of brain damage, at least in MTLE. Next, we will consider experimental data obtained in vivo from the pilocarpine model of MTLE and in normal control rodents following acute diverse convulsive pharmacological manipulations. We will also summarize in vitro experiments indicating the predominant involvement of GABAA receptor signaling in initiating LVF seizure-like discharges and the dynamic changes in inhibition that accompany the onset of HYP seizure-like activity. Finally, we will analyze recent optogenetic in vitro findings; these results strongly suggest that the initiation of LVF- and HYP-onset seizures depends on the preponderant involvement of (GABAergic) interneuron and of principal (glutamatergic) cell networks, respectively, which in both cases rest on an operational GABAA receptor signaling.
Seizure-onset patterns in the clinical context.
Long-term intracranial-depth recordings obtained during presurgical stereo-EEG monitoring have revealed that LVF seizure onset represents the most common pattern of ictal discharge initiation. Indeed, it occurs across several neocortical focal epilepsies and is not restricted to MTLE (Gnatkovsky et al. 2011; Gotman et al. 1995; Perucca et al. 2014; Singh et al. 2015; Wu et al. 2014). In addition, MTLE patients presenting with LVF-onset seizures show diffuse neuronal loss that can comprise the CA2/CA4 regions (Ogren et al. 2009; Velasco et al. 2000). This pattern is also more frequent in patients showing amygdala atrophy in addition to hippocampal atrophy as well as in patients with normal mesial temporal structures (Spanedda et al. 1997).
In contrast, the HYP-onset pattern is most often, if not solely, observed in MTLE with hippocampal sclerosis, and it has never been reported in neocortical focal epilepsies (Ogren et al. 2009; Perucca et al. 2014; Spencer et al. 1992; Velasco et al. 2000). Magnetic resonance imaging studies and histopathological evaluation of postsurgical specimens have also demonstrated that MTLE patients presenting with HYP-onset seizures present with cell loss in all hippocampal areas with the exception of the CA2 subfield and the presubiculum (Spencer et al. 1992; Velasco et al. 2000). In addition, Ogren et al. (2009) have proposed that HYP-onset seizures occur only in MTLE patients presenting with pronounced but restricted hippocampal atrophy. Overall, evidence obtained in epileptic patients indicate that these two seizure-onset patterns often reflect different histopathological conditions, which in turn suggests the involvement of different types of neuronal networks and presumably a specific contribution of ligand-gated mechanisms.
Seizure-onset patterns in in vivo animal models of epilepsy and of epileptiform synchronization.
Bragin et al. (2005) were first in reporting that, in the “local” kainic acid model of MTLE, rats generate spontaneous seizures characterized by both LVF- and HYP-onset patterns; in these experiments, EEG recordings were obtained with depth electrodes positioned in several limbic structures. These researchers also found that HYP-onset seizures initiate most often from the hippocampus ipsilateral to the original kainic acid injection and that they are rarely accompanied by behavioral symptoms; therefore, these data suggest that these seizures remained focal. On the contrary, LVF-onset seizures occurring in these experiments originated from the hippocampus and from the entorhinal cortex, and they frequently propagated to other limbic and extralimbic areas (Bragin et al. 2005).
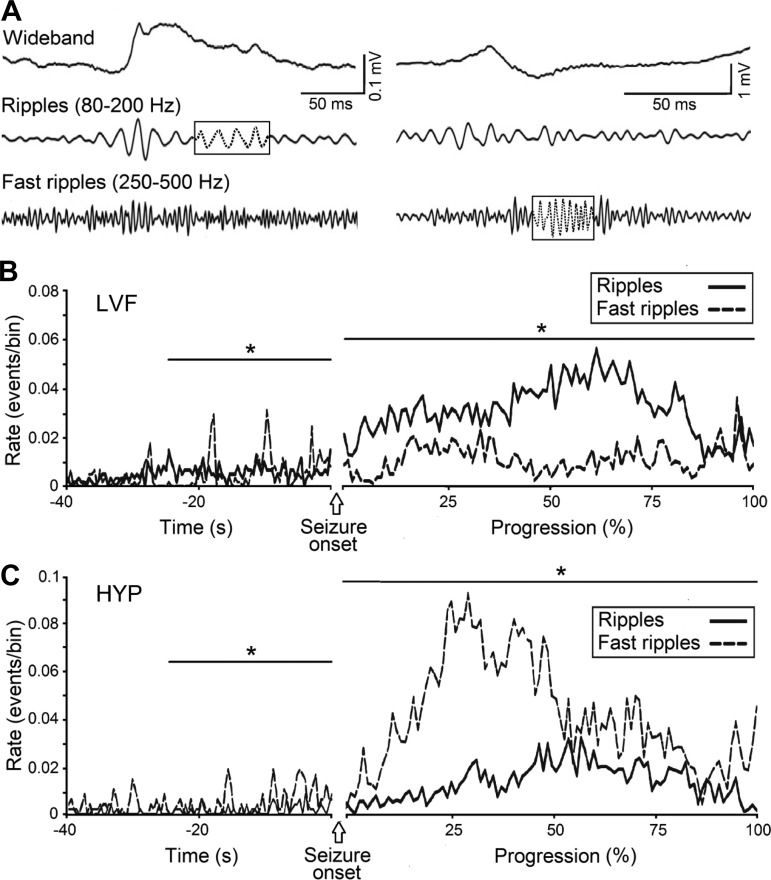
Similar results have been later obtained in the pilocarpine model of MTLE by Lévesque et al. (2012) who found that the majority of HYP-onset seizures originated from the hippocampal CA3 region, whereas LVF-onset seizures initiated in this hippocampal subfield and in the entorhinal cortex or even outside the hippocampal formation (Fig. 1B). An additional crucial difference between LVF and HYP seizures in the pilocarpine model rests on their association with specific types of pathological high-frequency oscillations (HFOs) at 80–500 Hz, which have been arbitrarily categorized into ripples (80–200 Hz) and fast ripples (250–500 Hz) (Fig. 2A) according to the original proposal made by Bragin et al. (1999). HFOs, which can only be extracted by amplifying the appropriately filtered EEG signal, have been recorded from patients presenting with MTLE and in animal models mimicking this neurological condition, and they have been proposed to represent better biomarkers than interictal spikes for identifying seizure-onset zones in focal epileptic disorders (Jacobs et al. 2008, 2010; see also Jacobs et al. 2012; Staba 2012).
Fig. 2.
Analysis of high-frequency oscillations (HFOs) occurring before and during LVF and HYP seizures in pilocarpine-treated epileptic rats. A: left, recordings from the CA3 region of a pilocarpine-treated rat during an LVF seizure. The signal is shown in the wideband, ripple (80–200 Hz) and fast ripple (250–500 Hz) frequency range. Note the occurrence of a ripple (boxed dotted portion of the trace) with no co-occurrence of a fast ripple. Right, recording from CA3 during an HYP seizure in a pilocarpine-treated animal. Note in this case the occurrence of a fast ripple (boxed dotted portion of the trace). B: temporal distribution of HFOs recorded during 18 LVF seizures in pilocarpine-treated animals. Note that ripples predominate over fast ripples, especially after seizure onset. C: temporal distribution of HFOs recorded during 21 HYP seizures in pilocarpine-treated animals. Note the high occurrence of fast ripples compared with ripples. Rates of ripples and fast ripples were compared using nonparametric Wilcoxon signed-rank tests followed by Bonferroni-Holm corrections for multiple comparisons (*P < 0.05). Data were obtained during the experiments published by Lévesque et al. (2012)
Excitatory and inhibitory synaptic interactions along with intrinsic membrane oscillations, gap junctions, and ephaptic coupling have been considered to underlie physiological (Buzsáki 2015; Buzsáki and Chrobak 1995; Ylinen et al. 1995) and pathological (Jefferys et al. 2012a, 2012b) HFOs, and the contribution of these mechanisms to the generation of HFOs is under active examination. In addition, it has been reported that HFOs with similar frequency ranges can differ considerably in their physiological mechanisms (Jefferys et al. 2012a, 2012b). However, a convenient, although reductionist and, perhaps, simplistic, view is that pathological HFOs in the ripple band mainly represent population inhibitory postsynaptic potential (IPSP) generated by principal neurons that are entrained by synchronously active interneuron networks; in contrast, HFOs in the fast ripple band could be supported by the synchronous firing of abnormally active principal cells (Foffani et al. 2007) and could be independent of inhibitory neurotransmission (cf. Jefferys et al. 2012a). However, data obtained in some studies (e.g., Bragin et al. 2011; D'Antuono et al. 2005) have suggested that pathological ripples and fast ripples are both produced by pyramidal cell action potentials.
As shown in Fig. 2, B and C, HFO occurrence during preictal and ictal periods in pilocarpine-treated epileptic rats markedly differ between LVF- and HYP-onset seizures. During both the preictal and ictal period, LVF seizures were associated with a preponderant increase in ripple occurrence, whereas HYP seizures were mostly characterized by increased occurrence of fast ripples (Lévesque et al. 2012). Similar results had also been previously reported in kainic acid-treated epileptic rats (Bragin et al. 2005). Therefore, according to what was summarized in the previous paragraph, evidence obtained to date suggests that distinct transmitter signaling (and underlying neuronal network activity) predominates during LVF and HYP seizures recorded from epileptic animals; specifically, LVF onset should be mirrored by increased interneuron (GABAergic cell) activity while excitatory (glutamatergic) cells should be the main actors in the initiation of HYP-onset seizures (Lévesque et al. 2012).
This hypothesis is supported by a recent study in which multiunit activity recordings were obtained during spontaneous seizures in awake epileptic animals treated with kainic acid (Grasse et al. 2013). These investigators discovered that LVF seizures are associated with increased interneuron activity at onset followed by intense firing generated by principal cells. No in vivo study has yet investigated the activity of interneurons and principal cells during HYP-onset seizures in animal models of MTLE, although this aspect has been addressed in the feline neocortex in an in vivo acute preparation (Grenier et al. 2003; Timofeev et al. 2002).
The hypothesis that the initiation of LVF-onset seizures mainly rests on GABAergic function has been further tested in vivo by Salami et al. (2015); they induced seizures in normal control rats, which were chronically implanted with depth electrodes, through the systemic injection of the K+ channel blocker 4-aminopyridine, which is known to enhance both glutamatergic and GABAergic transmission (Buckle and Haas 1982; Perreault and Avoli 1991; Rutecki et al. 1987), or the GABAA receptor antagonist picrotoxin (De Groat et al. 1972). These experiments have shown that 4-aminopyridine causes seizures with LVF onset in the majority of cases, whereas seizures induced by picrotoxin are consistently characterized by HYP onset. In addition, HFO analysis revealed that 4-aminopyridine-induced LVF seizures are associated with higher ripple rates compared with fast ripples, whereas picrotoxin-induced seizures contained significantly higher rates of fast ripples compared with ripples (Salami et al. 2015).
Seizure-onset patterns in in vitro models of epileptiform synchronization.
Electrograhic activity closely resembling the ictal discharges recorded in epileptic patients, and in in vivo animal models, can also be reproduced in several in vitro preparations such as the brain slice (Fig. 1C), the isolated hippocampus, and the guinea pig whole brain (see, for review, Avoli and Jefferys 2016; de Curtis and Avoli 2016; de Curtis et al. 2015). In these studies, both LVF- and HYP-onset seizure-like discharges can be recorded from several limbic and extralimbic areas, and, to review in detail, these data are beyond the goal of this paper. However, a few remarks should be made. First, long-lasting periods of epileptiform synchronization, resembling ictal discharges, are rarely observed during pharmacological manipulations that fully antagonize GABAA receptor signaling, at least when employing adult brain tissue (see, for review, Avoli and de Curtis 2011; Avoli and Jefferys 2016). Second, HYP-onset ictal discharges are commonly seen when cortical tissue is perfused with medium containing low Mg2+ (Derchansky et al. 2008; Zhang et al. 2012) but are less frequently recorded during bath application of 4-aminopyridine (Avoli et al. 2013; Lopantsev and Avoli 1998b) with the notable exception of experiments carried out in the perirhinal cortex (Biagini et al. 2013; Köhling et al. 2016). In fact, as reviewed in detail by Avoli and de Curtis (2011), this K+ channel blocker readily and most often induces ictal discharges that closely resemble an LVF seizure onset in several limbic and extralimbic structures (Figs. 1C and 3, A and B).
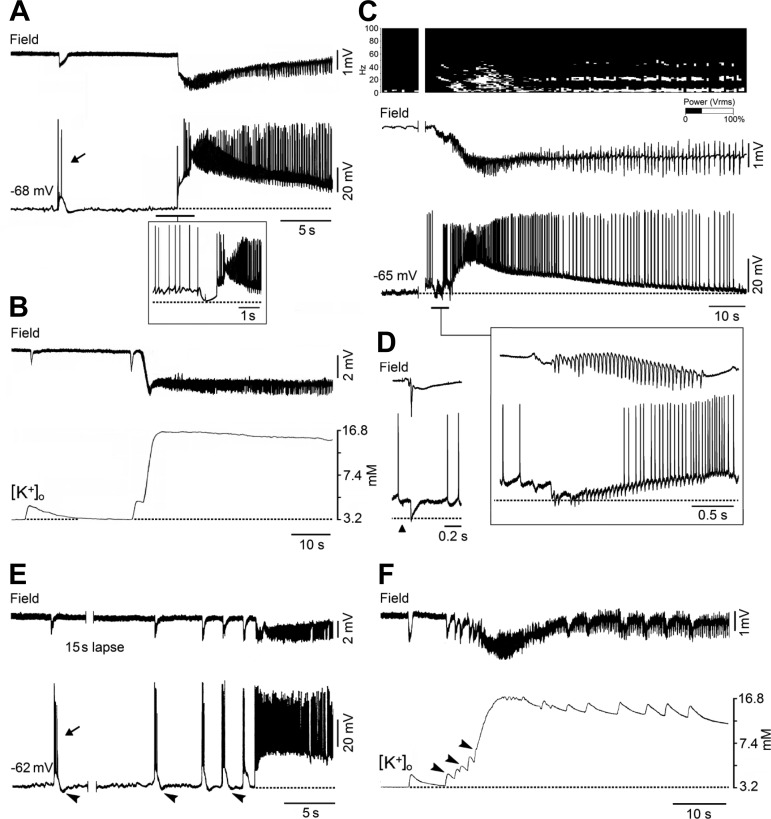
Fig. 3.
Field, extracellular K+ concentration ([K+]o), and intracellular recordings obtained in vitro during LVF-onset seizure-like dischargers. A: simultaneous field (Field) and intracellular (−68 mV) recordings obtained from the entorhinal cortex of a rat brain slice during bath application of 4-aminopyridine; in this and the following panels the dotted lines indicate the resting membrane potential of the neuron recorded intracellularly. Traces shown in the inset were obtained from a different neuron during active depolarization with intracellular current from a resting membrane potential at approximately −62 mV. B: field and [K+]o activities recorded during application of 4-aminopyridine from the deep layers of the rat entorhinal cortex; the dotted line indicates the [K+]o base level. C: simultaneous field and intracellular (−65 mV) recordings obtained from a principal cell in the entorhinal cortex during the perfusion of 50 μM bicuculline for 3 min in the in vitro isolated guinea pig brain. The spectrogram on the top illustrates the fast activity at around 20 Hz at the onset of the seizure-like event. The fast activity occurring at the onset of the seizure-like discharge is expanded in the inset where the principal cell was depolarized by intracellular injection of steady current. D: field and intracellular recordings of the response induced by lateral olfactory tract stimulation in an entorhinal cortex neuron with a resting membrane potential of −60 mV. Note that the brief inhibitory postsynaptic potentials (IPSPs) that correlate to the fast activity oscillations have reversal potentials similar to what is seen with the lateral olfactory tract-evoked IPSP. E: simultaneous field and intracellular recordings from a principal cell in the perirhinal cortex of a rat brain slice during bath application of 4-aminopyridine; note that ictal discharge onset is characterized by preictal spiking acceleration as well as that both interictal (arrow) and “preictal” discharges consist of depolarizations with action potential bursts followed by a hyperpolarizing potential that progressively decreases in amplitude (arrowheads) and coincides with more intense action potential bursting. F: simultaneous field and [K+]o recordings obtained in the deep layers of the perirhinal cortex during 4-aminopyridine application. Note the progressive increases in [K+]o that accompany the preictal spikes up to ictal discharge initiation that coincide with values larger than 6.3 mM. Data were obtained during experiments that have been published by Avoli et al. (1996, 2013), Biagini et al. (2013), Gnatkovsky et al. (2008), Köhling et al. (2016), Lopantsev and Avoli (1998a and b), Trombin et al. (2011), and Uva et al. (2015).
LVF seizure-like discharges recorded in vitro during application of 4-aminopyridine have been extensively studied in our laboratories, employing both rodent brain slices and the guinea pig whole brain preparation. When intracellularly recorded from principal (glutamatergic) cells of the entorhinal cortex in a brain slice preparation, the LVF onset coincides with a depolarization that is associated with few if any action potentials and becomes hyperpolarizing when the neuron membrane potential is brought to values less negative than −60 mV with steady injection of depolarizing current (Lopantsev and Avoli 1998a) (Fig. 3A). Therefore, the LVF onset of 4-aminopyridine-induced ictal discharges appears to be associated with a robust synchronous inhibitory event; this view is supported by studies in which powerful interneuron discharges could be recorded at the onset of these ictal discharges (de Curtis et al. 2015; Lévesque et al. 2016; Uva et al. 2015; Ziburkus et al. 2006) and by the ability of GABAA receptor antagonists to abolish this event along with ictal discharge occurrence (Avoli et al. 1996; Lopantsev and Avoli 1998a). It should be noticed that short-lasting depolarizations with similar pharmacological and electrophysiological (e.g., few action potentials and reversal potentials) characteristics are associated with the interictal-like discharges (Fig. 3A, arrow) that occur in vitro in the hippocampus and entorhinal cortex during 4-aminopyridine treatment (Avoli et al. 1996; Lopantsev and Avoli 1998; Uva et al. 2009).
It has also been demonstrated that the negative shift of the local field potential observed at the onset of an LVF seizure correlates with elevations in extracellular K+ concentration ([K+]o; Fig. 3B) that rest on excessive GABAergic signaling (Avoli et al. 1996; reviewed by Avoli and de Curtis 2011; Avoli et al. 2013; de Curtis and Avoli 2016), leading to intracellular Cl− accumulation and subsequent activity of the KCl cotransporter 2 (KCC2) that extrudes both Cl− and K+ from the intraneuronal compartment (Viitanen et al. 2010). Such increases in [K+]o should depolarize neighboring neurons, thus causing hyperexcitability as suggested by the occurrence of small-amplitude presumably ectopic spikes (Kaila et al. 2014; Lopantsev and Avoli 1998a; Trombin et al. 2011; but also see Avoli et al. 1998). In addition, an elevation in [K+]o supports the emergence of neuronal network resonance, thus generating oscillatory patterns in the beta-gamma range (Bartos et al. 2007) (see also next paragraph), and should cause a positive shift of the membrane reversal of the GABAA receptor-mediated IPSP, therefore weakening inhibition (Jensen et al. 1993). The notion that elevations in [K+]o increase neuronal excitability and cause seizure activity has been firmly established over the last few decades in both in vivo (Zuckermann and Glaser 1968) and in vitro (Traynelis and Dingledine 1988) preparations. Moreover, the role played by KCC2 activity in LVF-onset seizure initiation and maintenance is supported by recent evidence showing that ictal discharges induced by 4-aminopyridine are abolished or facilitated by inhibiting or enhancing the activity of this cotransporter, respectively (Hamidi and Avoli 2015).
As shown in Fig. 3C, LVF ictal discharge in the entorhinal cortex of the isolated guinea pig brain can also be induced by a short-lasting arterial perfusion of bicuculline, a pharmacological procedure that reduces inhibition efficacy only by ∼30%, as also suggested by the ability of piriform or entorhinal cortical cells to generate a robust IPSP following electrical activation of the lateral olfactory tract under this experimental condition (Fig. 3D). In this specific in vitro model of ictogenesis, LVF seizures are initiated by fast-field oscillations in the beta-gamma range that are mirrored by intracellular hyperpolarizing potentials becoming of smaller amplitude as seizure progresses (Gnatkovsky et al. 2008) (Fig. 3C). In addition, similar to what was observed in the experiments performed with 4-aminopyridine, these LVF-onset events corresponded to elevation in [K+]o along with the occurrence of ectopic action potentials (Gnatkovsky et al. 2008; Trombin et al. 2011).
As already mentioned, HYP-onset ictal discharges have been analyzed in cortical tissue bathed with medium containing low Mg2+ (Derchansky et al. 2008; Zhang et al. 2012), an experimental manipulation that is known to weaken GABAA receptor signaling over time (Whittington et al. 1995). Interestingly, maintained GABAA receptor-mediated activity was initially reported by Derchansky et al. (2008) during the transition to HYP seizure activity in the isolated immature mouse hippocampus; however, further experiments carried out in the same laboratory have shown that HYP seizure onset is characterized by “exhaustion of presynaptic release of GABA, and unopposed increase in glutamatergic excitation” (Zhang et al. 2012). More recently, we have reported that ictal discharges with HYP-onset features can also occur in the perirhinal cortex in brain slices superfused with 4-aminopyridine medium (Fig. 3E) (Biagini et al. 2013; Köhling et al. 2015).
By employing intracellular recordings from principal cells of the perirhinal cortex, we have found that the recurrent field spikes typical of a HYP onset are characterized by intracellular depolarizations associated with action potential bursting as well as that the postburst hyperpolarizations (presumably caused by activation of postsynaptic GABAA receptors) gradually decrease in amplitude (Fig. 3E, arrowheads), a phenomenon that is characterized by a gradual positive shift in their reversal potential (Köhling et al. 2015). In addition, these changes were accompanied by a progressive increase in the associated transient elevations in [K+]o (Fig. 3F, arrowheads). While these data are in line with the conclusions proposed by Zhang et al. (2012), who identified a progressive impairment of inhibition at HYP onset with concomitant unrestrained enhancement of excitation, they also reveal an additional mechanism of inhibition weakening that may rest on progressively larger accumulations in [K+]o due to the postsynaptic activation of GABAA receptors.
Optogenetic approaches reveal the involvement of specific neuronal networks in different seizure-onset patterns.
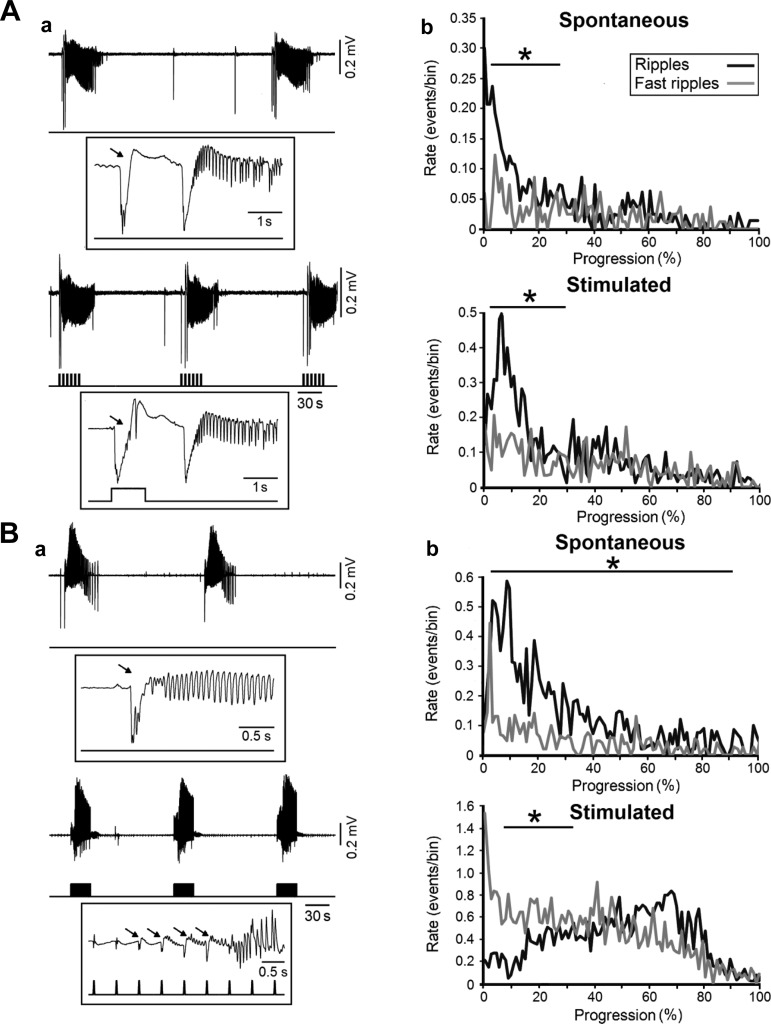
The pivotal role played by interneurons in the initiation of ictal discharges characterized by an LVF onset has recently been confirmed with optogenetic techniques in the entorhinal cortex during 4-aminopyridine treatment (Shiri et al. 2015a; Yekhlef et al. 2015). It was shown in these studies that optogenetic stimulation of parvalbumin- or somatostatin-positive interneurons can initiate ictal LVF-onset events similar to those occurring spontaneously (Fig. 4A). In addition, as shown in the expanded traces of Fig. 4Aa (arrows), the onset of an ictal discharge induced by optogenetic stimulation of parvalbumin-positive cells presented with the typical pattern consisting of one or two interictal-like transients leading to fast beta-gamma activity marking the beginning of the tonic phase. Interestingly, Shiri et al. (2015a) found that, during both spontaneous and stimulated LVF discharges, ripple rates predominated at ictal onset (Fig. 4Ab).
Fig. 4.
Optogenetic activation of interneurons or principal cells leads to LVF or HYP ictal discharges, respectively. A: a, LVF-onset ictal discharges occurring spontaneously during bath application of 4-aminopyridine (top) and during parvalbumin-positive interneuron light stimulation (bottom); one event under each condition is further expanded to reveal the onset pattern. Stimuli were 1 s long and delivered at 0.2 Hz for 30 s. Note that, in both spontaneous and stimulated events, the ictal discharge is preceded by one or two negative-going interictal field potentials (arrows). b, Plots of the average rate of ripples and fast ripples occurring during spontaneous (top) and optogenetically stimulated (bottom) ictal discharges (n = 10 events were used for both plots). Note that ripple rates are significantly higher than fast ripple rates at the onset of both LVF discharges (*P < 0.01). B: a, spontaneous LVF ictal discharges occurring during bath application of 4-aminopyridine are shown in the top while those induced by optogenetic stimulation of calcium/calmodulin-dependent protein kinase II-positive principal cells are shown on the bottom; light pulses were 20 ms long and were delivered at 2 Hz for 30 s; note that this procedure triggers ictal discharges preceded by repeated spiking (arrows), a pattern that is characteristic of HYP-onset events. b, Plots of the average rate of ripples and fast ripples occurring during spontaneous (top) and optogenetically stimulated (bottom) ictal discharges; note that the stimulated HYP events are characterized by higher fast ripple rates at onset. Ten events were used for both plots (*P < 0.01). Data were obtained from the experiments published by Shiri et al. (2015, 2016).
However, as shown in Fig. 4B, LVF ictal events that occurred spontaneously during 4-aminopyridine application virtually switched to HYP-onset events when the calcium/calmodulin-dependent protein kinase II-positive principal cells were optogenetically stimulated in the entorhinal cortex (Shiri et al. 2016). Specifically, the onset of ictal discharges triggered by principal cell activation was characterized by repeated field spikes, thus closely resembling a HYP-onset pattern. In addition, these optogenetically induced HYP-onset ictal events were found to be associated with higher fast ripple rates at onset (Fig. 4Bb). Therefore, these optogenetic experiments demonstrate that, under identical conditions (i.e., during application of 4-aminopyridine), activation of each specific cell population can generate ictal discharges with a different onset pattern: LVF-onset events, similar to those occurring spontaneously, depended on the optogenetic activation of interneuronal networks, whereas HYP-onset discharges rest on the optogenetic activation of glutamatergic principal cells.
Concluding remarks.
The studies reviewed here underscore the concept that the “excessive” activity of interneurons (and the resulting activation of postsynaptic GABAA receptors) can be sufficient to disrupt the excitation/inhibition balance within forebrain neuronal networks, thus leading to ictal activity (Avoli and de Curtis 2011; de Curtis and Avoli 2016). This conclusion is in line with in vivo data obtained from epileptic patients (Schevon et al. 2012; Truccolo et al. 2011) and animal models (Grasse et al. 2013; Toyoda et al. 2015) in which seizure onset was shown to be associated with sustained firing of interneurons or with depressed or consistent firing activity of principal cells.
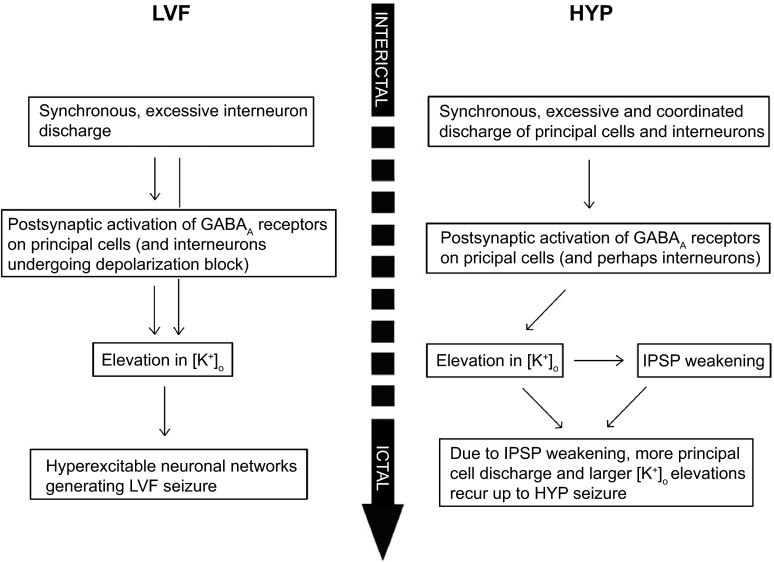
We also propose that different types of neuronal networks (i.e., interneurons and principal glutamatergic cells) and thus different neurotransmitter receptor signaling predominate in MTLE at the onset of LVF and HYP ictal discharges. As summarized in the diagrams shown in Fig. 5, two different sets of cellular events/interactions are indeed likely to be at work during these two focal seizure-onset patterns. Moreover, in light of the evidence obtained in human epileptic patients (Ogren et al. 2009; Spencer et al. 1992; Velasco et al. 2000) and of the structure-dependent (Köhling et al. 2015) and optogenetic (Shiri et al. 2016) findings identified in vitro, it is reasonable to hypothesize that the preponderant involvement of interneuronal or principal cell networks in LVF- and HYP-onset seizures, respectively, may reflect some distinctive features in network connectivity and/or in brain state excitability.
Fig. 5.
Flow diagram of the dysfunctional mechanisms leading to LVF and HYP seizure onset. Experimental evidence taken into account for providing the mechanisms involved in LVF seizure onset was provided in the following studies: Avoli et al. 1996, Barbarosie et al. 2000, Gnatkovsky et al. 2008, Trombin et al. 2011, Grasse et al. 2013, Lévesque et al. 2016, Lopantsev et al. 1998a, Uva et al. 2015, and Ziburkus et al. 2006. Data identifying the mechanisms leading to HYP seizure onset originate from the following studies: Lopantsev and Avoli 1998b, Derchanski et al. 2006, Zhang et al. 2012, and Köhling et al. 2016.
The evidence reviewed here may provide insight to delineate better therapeutic targets in the treatment of patients presenting with MTLE and suggest that (theoretically) seizure-onset patterns and associated HFOs should be taken into consideration to implement optimal pharmacological therapies. However, this last aspect may suffer a practical drawback since both types of seizure onset can also coexist in the same experimental condition both in vivo (Lévesque et al. 2012) and in vitro (Köhling et al. 2016) as well as in patients with MTLE (B. Frauscher, F. Dubeau, and J. Gotman, personal communication). Finally, it should be emphasized that the findings summarized in this review focus on determinants of seizure-onset types (i.e., on the mechanisms underlying ictogenesis), not on epileptogenesis. Therefore, the impact of different seizure-onset patterns on the development of MTLE remains to be defined.
GRANTS
The original work reviewed here was supported by the Canadian Institutes of Health Research (Grants 8109 and 74609 to M. Avoli, 143208 to J. Gotman, and 119340 to S. Williams), Citizens United for Research in Epilepsy (M. Avoli), the Fondazione Banca del Monte di Lombardia (2014-15 to M. de Curtis), the Italian Health Ministry (Ricerca Corrente 2014; Grants RF-2010-2304417 and RF-2007-GR141 to M. de Curtis), the Savoy Foundation (M. Avoli), and the Telethon Foundation (Grant GGP12265 to M. de Curtis).
DISCLOSURES
None of the authors have any conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
M.A., M.D.C., M.L., and Z.S. conception and design of research; M.A., V.G., M.L., and Z.S. analyzed data; M.A., M.D.C., V.G., J.G., R.K., M.L., and Z.S. interpreted results of experiments; M.A., M.D.C., J.G., and R.K. prepared figures; M.A., M.D.C., J.G., R.K., M.L., and Z.S. drafted manuscript; M.A., M.D.C., J.G., R.K., M.L., and Z.S. edited and revised manuscript; M.A., M.D.C., V.G., J.G., R.K., M.L., F.M., Z.S., and S.W. approved final version of manuscript; V.G. and Z.S. performed experiments.
ACKNOWLEDGMENTS
We thank Drs. M. Barbarosie, R. Benini, G. Biagini, M. D'Antuono, P. de Guzman, S. Hamidi, V. Lopantsev, J. Louvel, R. Pumain, P. Salami, and L. Uva as well as I. Kurcewicz for contributing to some of the original experiments that were reported in this review.
REFERENCES
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci 16: 3912–3924, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol 95: 104–132, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M, Köhling R. Does interictal synchronization influence ictogenesis? Neuropharmacology 69: 37–44, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Jefferys JGR. Models of drug-induced epileptiform synchronization in vitro. J Neurosci Methods 260: 26–32, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Methot M, Kawasaki H. GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci 10: 2714–2722, 1998. [DOI] [PubMed] [Google Scholar]
- Avoli M, Panuccio G, Herrington R, D'Antuono M, de Guzman P, Lévesque M. Two different interictal spike patterns anticipate ictal activity in vitro. Neurobiol Dis 52: 168–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, Kurcewicz I, Avoli M. CA3-released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol 83: 1115–1124, 2000. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007. [DOI] [PubMed] [Google Scholar]
- Biagini G, D'Antuono M, Benini R, de Guzman P, Longo D, Avoli M. Perirhinal cortex and temporal lobe epilepsy. Front Cell Neurosci 7: 130, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boido D, Jesuthasan N, de Curtis M, Uva L. Network dynamics during the progression of seizure-like events in the hippocampal-parahippocampal regions. Cereb Cortex NYN 1991 24: 163–173, 2014. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J Jr. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia 46: 1592–1598, 2005. [DOI] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J Jr. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia 52: 45–52, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel JJ, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 40: 127–137, 1999. [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol 326: 109–122, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25: 1073–1188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol 5: 504–510, 1995. [DOI] [PubMed] [Google Scholar]
- D'Antuono M, de Guzman P, Kano T, Avoli M. Ripple activity in the dentate gyrus of dishinibited hippocampus-entorhinal cortex slices. J Neurosci Res 80: 92–103, 2005. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avoli M. GABAergic networks jump-start focal seizures. Epilepsia 2016 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Reevaluating the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia 50: 2514–2525, 2009. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Librizzi L, Uva L. The in vitro isolated whole guinea pig brain as a model to study epileptiform activity patterns. J Neurosci Methods 260: 83–90, 2016. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Lalley PM, Saum WR. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res 44: 273–277, 1972. [DOI] [PubMed] [Google Scholar]
- Derchansky M, Jahromi SS, Mamani M, Shin DS, Sik A, Carlen PL. Transition to seizures in the isolated immature mouse hippocampus: a switch from dominant phasic inhibition to dominant phasic excitation. J Physiol 586: 477–494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol 9: 441–448, 1992. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55: 930–941, 2007. [DOI] [PubMed] [Google Scholar]
- Gloor P. Contributions of electroencephalography and electrocorticography to the neurosurgical treatment of the epilepsies. Adv Neurol 8: 59–105, 1975. [PubMed] [Google Scholar]
- Gnatkovsky V, Francione S, Cardinale F, Mai R, Tassi L, Lo Russo G, de Curtis M. Identification of reproducible ictal patterns based on quantified frequency analysis of intracranial EEG signals. Epilepsia 52: 477–488, 2011. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol 64: 674–686, 2008. [DOI] [PubMed] [Google Scholar]
- Gotman J, Levtova V, Olivier A. Frequency of the electroencephalographic discharge in seizures of focal and widespread onset in intracerebral recordings. Epilepsia 36: 697–703, 1995. [DOI] [PubMed] [Google Scholar]
- Grasse DW, Karunakaran S, Moxon KA. Neuronal synchrony and the transition to spontaneous seizures. Exp Neurol 248: 72–84, 2013. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J Neurophysiol 89: 841–852, 2003. [DOI] [PubMed] [Google Scholar]
- Hamidi S, Avoli M. KCC2 function modulates in vitro ictogenesis. Neurobiol Dis 79: 51–58, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 49: 1893–1907, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, Mohamed I, Kahane P, Dubeau F, Navarro V, Gotman J. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol 98: 302–315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol 67: 209–220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Jasper's Basic Mechanisms of the Epilepsies, edited by Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Bethesda, MD: National Center for Biotechnology Information, 2012. [PubMed] [Google Scholar]
- Jefferys JGR, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol 98: 250–264, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Cherubini E, Yaari Y. Opponent effects of potassium on GABAA-mediated postsynaptic inhibition in the rat hippocampus. J Neurophysiol 69: 764–771, 1993. [DOI] [PubMed] [Google Scholar]
- Kaila K, Ruusuvuori E, Seja P, Voipio J, Puskarjov M. GABA actions and ionic plasticity in epilepsy. Curr Opin Neurobiol 26: 34–41, 2014. [DOI] [PubMed] [Google Scholar]
- Köhling R, D'Antuono M, Benini R, de Guzman P, Avoli M. Hypersynchronous ictal onset in the perirhinal cortex results from dynamic weakening in inhibition. Neurobiol Dis 87: 1–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci 32: 13264–13272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Herrington R, Hamidi S, Avoli M. Interneurons spark seizure-like activity in the entorhinal cortex. Neurobiol Dis 87: 91–101, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb JP, Walsh GO, Babb TL, Walter RD, Crandall PH. A comparison of EEG seizure patterns recorded with surface and depth electrodes in patients with temporal lobe epilepsy. Epilepsia 17: 137–160, 1976. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictal-like discharges in the rat entorhinal cortex. J Neurophysiol 79: 352–360, 1998a. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Laminar organization of epileptiform discharges in the rat entorhinal cortex in vitro. J Physiol 509: 785–796, 1998b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren JA, Bragin A, Wilson CL, Hoftman GD, Lin JJ, Dutton RA, Fields TA, Toga AW, Thompson PM, Engel J, Staba RJ. Three-dimensional hippocampal atrophy maps distinguish two common temporal lobe seizure-onset patterns. Epilepsia 50: 1361–1370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia 38: 642–654, 1997. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol 65: 771–785. 1991. [DOI] [PubMed] [Google Scholar]
- Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain 137: 183–196, 2014. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol 57: 1911–1924, 1987. [DOI] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Gotman J, Avoli M. Distinct EEG seizure patterns reflect different seizure generation mechanisms. J Neurophysiol 113: 2840–2844, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G, Goodman RR, Yuste R, Emerson RG, Trevelyan AJ. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun 3: 1060, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol 77: 541–546, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Activation of specific neuronal networks leads to different seizure onset types. Ann Neurol 79: 354–365, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sandy S, Wiebe S. Ictal onset on intracranial EEG: Do we know it when we see it? State of the evidence. Epilepsia 56: 1629–1638, 2015. [DOI] [PubMed] [Google Scholar]
- Spanedda F, Cendes F, Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia 38: 1300–1314, 1997. [DOI] [PubMed] [Google Scholar]
- Spencer DD, Pappas CT. Surgical decisions regarding medically intractable epilepsy. Clin Neurosurg 38: 548–566, 1992. [PubMed] [Google Scholar]
- Spencer SS, Guimaraes P, Katz A, Kim J, Spencer D. Morphological patterns of seizures recorded intracranially. Epilepsia 33: 537–545, 1992. [DOI] [PubMed] [Google Scholar]
- Staba RJ. Normal and Pathologic high-frequency oscillations. In: Jasper's Basic Mechanisms of the Epilepsies, edited by Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Bethesda, MD: National Center for Biotechnology Information, 2012. [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. The role of chloride-dependent inhibition and the activity of fast-spiking neurons during cortical spike-wave electrographic seizures. Neuroscience 114: 1115–1132, 2002. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Fujita S, Thamattoor AK, Buckmaster PS. Unit activity of hippocampal interneurons before spontaneous seizures in an animal model of temporal lobe epilepsy. J Neurosci 35: 6600–6618, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol 59: 259–276, 1988. [DOI] [PubMed] [Google Scholar]
- Trombin F, Gnatkovsky V, de Curtis M. Changes in action potential features during focal seizure discharges in the entorhinal cortex of the in vitro isolated guinea pig brain. J Neurophysiol 106: 1411–1423, 2011. [DOI] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nat Neurosci 14: 635–641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, Avoli M, de Curtis M. Synchronous GABA-receptor-dependent potentials in limbic areas of the in-vitro isolated adult guinea pig brain. Eur J Neurosci 29: 911–920, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, Breschi GL, Gnatkovsky V, Taverna S, de Curtis M. Synchronous inhibitory potentials precede seizure-like events in acute models of focal limbic seizures. J Neurosci 35: 3048–3055, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J Jr. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast 7: 49–63, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol 588: 1527–1540, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Hernandez A, Bellanger JJ, Chauvel P, Bartolomei F. Interictal to ictal transition in human temporal lobe epilepsy: insights from a computational model of intracerebral EEG. J Clin Neurophysiol 22: 343–356, 2005. [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Erosion of inhibition contributes to the progression of low magnesium bursts in rat hippocampal slices. J Physiol 486: 723–734, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Kunhi Veedu HP, Lhatoo SD, Koubeissi MZ, Miller JP, Lüders HO. Role of ictal baseline shifts and ictal high-frequency oscillations in stereo-electroencephalography analysis of mesial temporal lobe seizures. Epilepsia 55: 690–698, 2014. [DOI] [PubMed] [Google Scholar]
- Yekhlef L, Breschi GL, Lagostena L, Russo G, Taverna S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J Neurophysiol 113: 1616–1630, 2015. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neuirosci 15: 30–46, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Koifman J, Shin DS, Ye H, Florez CM, Zhang L, Valiante TA, Carlen PL. Transition to seizure: ictal discharge is preceded by exhausted presynaptic GABA release in the hippocampal CA3 region. J Neurosci 32: 2499–2512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol 95: 3948–3954, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann EC, Glaser GH. Hippocampal epileptic activity induced by localized ventricular perfusion with high-potassium cerebrospinal fluid. Exp Neurol 20: 87–110, 1968. [DOI] [PubMed] [Google Scholar]