Subfornical organ (SFO) neurons play a pivotal role in body fluid homeostasis and autonomic function including neurogenic forms of hypertension and cardiovascular disease. In the present study, we provide direct evidence that DREADD technology is a useful tool to manipulate SFO neuronal activity. The experiments reported here indicate that acute or chronic activation of SFO neurons stimulates excessive fluid intake. Therefore, SFO neurons may be a potential therapeutic target for the treatment of body fluid homeostatic disorders. Future research is needed to identify the exact neuronal populations and signaling mechanisms that may underlie these responses during varying physiological challenges and diseases.
Keywords: hypothalamus, angiotensin II, sodium, Fos, electrophysiology
Abstract
The subfornical organ (SFO) plays a pivotal role in body fluid homeostasis through its ability to integrate neurohumoral signals and subsequently alter behavior, neuroendocrine function, and autonomic outflow. The purpose of the present study was to evaluate whether selective activation of SFO neurons using virally mediated expression of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) stimulated thirst and salt appetite. Male C57BL/6 mice (12–15 wk) received an injection of rAAV2-CaMKII-HA-hM3D(Gq)-IRES-mCitrine targeted at the SFO. Two weeks later, acute injection of clozapine N-oxide (CNO) produced dose-dependent increases in water intake of mice with DREADD expression in the SFO. CNO also stimulated the ingestion of 0.3 M NaCl. Acute injection of CNO significantly increased the number of Fos-positive nuclei in the SFO of mice with robust DREADD expression. Furthermore, in vivo single-unit recordings demonstrate that CNO significantly increases the discharge frequency of both ANG II- and NaCl-responsive neurons. In vitro current-clamp recordings confirm that bath application of CNO produces a significant membrane depolarization and increase in action potential frequency. In a final set of experiments, chronic administration of CNO approximately doubled 24-h water intake without an effect on salt appetite. These findings demonstrate that DREADD-induced activation of SFO neurons stimulates thirst and that DREADDs are a useful tool to acutely or chronically manipulate neuronal circuits influencing body fluid homeostasis.
NEW & NOTEWORTHY
Subfornical organ (SFO) neurons play a pivotal role in body fluid homeostasis and autonomic function including neurogenic forms of hypertension and cardiovascular disease. In the present study, we provide direct evidence that DREADD technology is a useful tool to manipulate SFO neuronal activity. The experiments reported here indicate that acute or chronic activation of SFO neurons stimulates excessive fluid intake. Therefore, SFO neurons may be a potential therapeutic target for the treatment of body fluid homeostatic disorders. Future research is needed to identify the exact neuronal populations and signaling mechanisms that may underlie these responses during varying physiological challenges and diseases.
the central nervous system plays a pivotal role in body fluid homeostasis through the ability of specialized neurons in the forebrain lamina terminalis to detect and integrate neurohumoral signals and subsequently alter behavior, neuroendocrine function, and autonomic outflow (Coble et al. 2015; McKinley et al. 2004; Toney and Stocker 2010). The lamina terminalis spans the rostral wall of the 3rd ventricle and includes two circumventricular organs: the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT). Neurons in these regions lack a complete blood-brain barrier and therefore can readily detect changes in circulating substances that other brain regions cannot (McKinley et al. 2003). For example, studies using in vivo single-unit recordings (Gutman et al. 1988; Tanaka et al. 1985) or immunocytochemical localization of Fos (Kinsman et al. 2014; Larsen and Mikkelsen 1995; Oldfield et al. 1994; Taylor et al. 2008) indicate that SFO neurons are responsive to either NaCl or the peptide hormone angiotensin II (ANG II). Activation of SFO neurons increases water intake (Simpson et al. 1978; Simpson and Routtenberg 1973, 1978; Smith et al. 1995), plasma vasopressin levels (Ferguson and Kasting 1986), and arterial blood pressure (Gutman et al. 1985). Furthermore, lesion or interruption of neurotransmission in the SFO attenuates thirst, vasopressin secretion, and/or changes in sympathetic nerve activity and blood pressure in response to multiple stimuli (Hosutt et al. 1981; Mangiapane et al. 1984; Osborn et al. 2012; Simpson et al. 1978; Simpson and Routtenberg 1973, 1978; Sunn et al. 2002; Thrasher et al. 1982; Thunhorst et al. 1999; Tiruneh et al. 2013). In addition to water homeostasis, SFO neurons have also been implicated in sodium balance. Experimental paradigms employed in rodents to produce a salt appetite increase Fos expression in SFO neurons (Thunhorst et al. 1998), and lesion of the SFO attenuates the ingestion of salt solutions (Thunhorst et al. 1999). Despite the evidence to support a role for SFO neurons in body fluid homeostasis, the neuronal populations or signaling mechanisms within SFO that contribute to thirst and salt appetite are not well understood.
Recent technological advances such as optogenetics permit a more detailed evaluation and dissection of various neuronal populations and circuits (Deisseroth 2015; Roth 2016). In regard to SFO neurons and body fluid homeostasis, Oka and colleagues (Oka et al. 2015) reported that optogenetic activation of SFO neurons by expression of channelrhodopsin under control of the CaMKII1α promoter produced an immediate increase in water, but not salt, intake in water-replete mice. On the other hand, activation of neurons expressing the vesicular GABA transporter inhibited thirst (Oka et al. 2015).
An alternative approach to manipulation of SFO neurons is chemogenetic tools such as Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) (Urban and Roth 2015). DREADDs may represent an advantage over optogenetic approaches, as neuronal activity can be easily manipulated acutely or chronically. Therefore, the purpose of the present study was to employ a DREADD-based approach to activate SFO neurons and examine the impact on thirst and salt appetite. We report that expression of hM3D(Gq) under the CaMKII promoter in SFO neurons acutely stimulated water intake and salt appetite after injection of clozapine N-oxide (CNO). Chronic activation of SFO neurons over several days nearly doubled 24-h water intakes without an effect on salt appetite. Additionally, we provide the first in vivo single-unit recordings of neuronal excitation using DREADDs.
MATERIALS AND METHODS
Animals.
All experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Penn State College of Medicine. Male C57BL/6 mice (12–15 wk of age; The Jackson Laboratory, Bar Harbor, ME; https://www.jax.org/strain/000664) were anesthetized with 2–2.5% isoflurane (in 100% O2) and placed into a stereotaxic frame. After a small craniotomy, rAAV2-CaMKII-HA-hM3D(Gq)-IRES-mCitrine (1 × 1012 molecules/ml, 50 nl over 5 min; UNC Gene Therapy Core) was injected into the SFO with glass micropipettes angled 87° to avoid the midsagittal sinus and at the following coordinates in reference to bregma: −0.4 mm caudal, 0.15 mm lateral, −2.8 mm ventral. Mice were treated with ampicillin (100 mg/kg sc), Buprenex (0.03 mg/kg sc), and carprofen (5 mg/kg sc). Animals were housed singly in a temperature-controlled room with a 12:12-h light-dark cycle, given access to distilled water and normal laboratory chow (Harlan Teklad no. 2018), and acclimated to the testing procedures daily.
Acute thirst and salt appetite studies.
Cumulative water intakes (±0.05 ml) were measured over 180 min with 10-ml graduated drinking tubes after injection of CNO (Tocris; 0.03, 0.06, 0.1, 0.3, 1.0, and 3.0 mg/kg sc) or vehicle (10 μl/g body wt). A CNO stock solution (20–40 mg/ml in DMSO) was made daily and subsequently diluted with isotonic saline. The various concentrations were tested in a randomized order separated by >3 days. After thirst studies were completed, mice were fed a low-sodium diet (0.01%, Research Diets D17010; New Brunswick, NJ) and given access to drinking tubes containing distilled water and 0.3 M NaCl for 1 wk. Cumulative water and 0.3 M NaCl intakes (±0.05 ml) were then measured after injection of CNO (3 mg/kg sc) or saline as described above. In every experiment, the drinking tubes were weighed before and at the end of the experiment to verify the volumetric measurement. We did not observe any differences (±0.1 g) between volume and mass.
Fos studies and immunocytochemistry.
After the completion of behavioral experiments, mice were given access to normal laboratory chow and distilled water for >1 wk. Then, animals were injected with CNO (3.0 mg/kg sc) or vehicle as described above but denied access to food or water. Ninety minutes later, mice were deeply anesthetized with isoflurane (3% in 100% O2) and perfused transcardially with isotonic saline and 4% paraformaldehyde. Brains were postfixed overnight at 4°C, sectioned at 30 μm with a vibratome, and processed immunocytochemically for Fos protein (Kinsman et al. 2014; Taylor et al. 2008) and/or hemagglutinin (HA)-Tag to assess DREADD expression. Sections were incubated in a rabbit anti-cFos antibody (1:4,000; EMD Biosciences Ab5-PC38) at 4°C for 48 h and subsequently visualized with an Alexa Fluor 594 goat anti-rabbit antibody (1:250; Molecular Probes). Sections were then incubated with a mouse anti-HA antibody (1:750; Cell Signaling no. 2367) at 4°C for 48 h and subsequently visualized with a biotin-XX goat anti-mouse IgG (1:250; Molecular Probes), avidin-biotin amplification (ABC VECTASTAIN Kit; Vector Laboratories), and streptavidin Alexa Fluor 488 (1:250; Molecular Probes). Sections were mounted onto slides and coverslipped with VECTASHIELD. Fos-positive nuclei and HA immunofluorescence were quantified in one representative section for each structure by two blinded individuals using a Nikon 90i microscope and NIS-Elements software.
In vivo and in vitro electrophysiology.
Mice received an SFO injection of rAAV2-CaMKII-HA-hM3D(Gq)-IRES-mCitrine at least 3 wk before experiments. In vivo single-unit recordings of SFO neurons were then performed in mice anesthetized with isoflurane with glass electrodes (10–30 MΩ) angled 86° from the midsagittal sinus and filled with 4% Neurobiotin dissolved in 0.5 M sodium acetate (pH 7.4) (Stocker et al. 2015; Stocker and Toney 2005). A multibarrel glass pipette was lowered into the lateral ventricle to test SFO neuronal responsiveness to 0.5 M NaCl (100 nl over 5 s) or ANG II (20 ng in 100 nl over 5 s). Then, CNO (0.3 mg/kg iv) was injected through a femoral venous catheter (100 μl). CNO was tested once per animal. At the end of recordings, cells were juxtacellularly labeled (Pinault 1996) and animals perfused transcardially with 4% paraformaldehyde. Neurobiotin-filled cells and HA-Tag expression were visualized by overnight incubations with Alexa Fluor 594 and mouse anti-HA antibody and goat anti-mouse Alexa Fluor 488, respectively.
For in vitro patch-clamp experiments, mice were injected with Fluoro-Gold (0.2 ml of 3.75 mg/ml ip) to facilitate identification of SFO neurons, as Fluoro-Gold will also label areas lacking a complete blood-brain barrier. Approximately 3 days later, mice were deeply anesthetized with isoflurane and decapitated and the brain was harvested into ice-cold Krebs solution (in mM: 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 11 dextrose, maintained at pH 7.4 by bubbling with 95% O2-5% CO2). Coronal slices (250 μm) containing the SFO were cut, and slices were incubated in Krebs solution at 30 ± 1°C for at least 90 min prior to use. A single slice was placed in a custom-made perfusion chamber (500-μl volume) mounted onto the stage of a Nikon E600FN microscope equipped with UV epifluorescent filters. Slices were maintained at 32 ± 1°C by continuous perfusion with warmed Krebs solution. Fluoro-Gold-containing SFO neurons were identified under UV epifluorescence, and electrophysiological recordings were made under brightfield illumination with DIC (Nomarski) optics. Whole cell patch-clamp recordings were made with pipettes (4–5 MΩ tip resistance) filled with a potassium gluconate solution (in mM: 28 K-gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 HEPES, 1 EGTA, 2 Na2ATP, 0.25 NaGTP, adjusted to pH 7.36 with KOH) and a single-electrode voltage-clamp amplifier (Axoclamp 200B; Molecular Devices, Union City, CA). Data were filtered at 2 kHz, digitized via a Digidata 1400 interface, stored on a computer, and analyzed with pCLAMP 10 software (Molecular Devices). Recordings with series resistance >20 MΩ were eliminated from the study. Neurons were current clamped at approximately −60 mV. Electrotonic potentials sufficient to hyperpolarize the membrane ∼10 mV were applied to the neuron every 5 s. CNO (10 μM) was applied via superfusion for a period of time sufficient for the response to reach plateau or for 2 min if no response was noted. The CNO-induced response was measured as the peak change in membrane potential (Vm) relative to baseline. At the conclusion of the recording, Neurobiotin (2.5%) included in the recording pipette was injected into the neuron by passing subthreshold depolarizing current pulses (400-ms duration, 0.8 Hz for 20 min). After removal of the pipette, the membrane was allowed to reseal for 5–10 min before the slice was fixed in Zamboni's fixative at 4°C for at least 24 h. Neurobiotin-filled cells and HA-Tag were visualized as described above.
Chronic thirst and salt appetite studies.
A final set of experiments was performed to determine whether chronic activation of SFO neurons with DREADDs altered thirst and salt appetite. Mice were injected with rAAV2-CaMKII-HA-hM3D(Gq)-IRES-mCitrine and given access to water, 0.3 M NaCl, and Na+-deficient chow as described above for at least 2 wk. Twenty-four-hour intakes were recorded for several days. Then, CNO was administered through both drinking tubes for 3 successive days. The initial CNO concentration was 0.025 mg/ml and adjusted daily based on the 24-h intake of the previous day to yield ∼3 mg/kg.
Statistical analysis.
All data were analyzed by an ANOVA with repeated measures (when appropriate) followed by a layered Bonferroni with correction (Systat Software v10.2). Cell discharge was averaged in 1-min bins; baseline discharge was calculated by a 3-min average. In vitro experiments calculated baseline and 30-s peak responses in Vm or discharge. A P < 0.05 was statistically significant.
RESULTS
CNO increases water and 0.3 M NaCl intake.
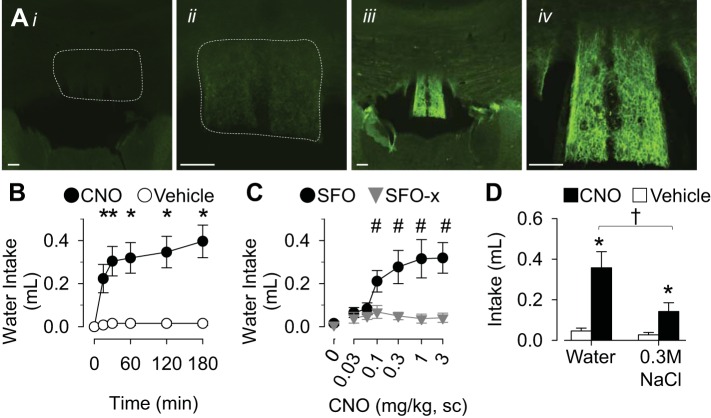
Post hoc analysis of HA immunofluorescence identified two primary groups of mice based on the absence or presence of DREADD expression in the SFO (Fig. 1A); these mice are referred to as SFO-x or SFO mice, respectively. SFO mice displayed strong HA immunofluorescence localized to the SFO without expression dorsal or rostral in the hippocampal commissure, caudal in the hippocampus, or ventral in the thalamus. In these animals, the immunofluorescence was present throughout the rostral-caudal, dorsal-ventral, and medial-lateral extent of SFO (Fig. 1A, iii and iv). In contrast, the majority of SFO-x mice had no detectable expression in any brain structure and a few SFO-x mice had a small amount of HA immunofluorescence present in the surrounding structures such as the hippocampus or ventral in the thalamus. Finally, there were two mice that displayed HA immunofluorescence in SFO, but the expression was limited to 10–15 SFO neurons. These animals did not respond to CNO (data not shown) and were not included in the analysis because of the clear delineation in expression between SFO vs. SFO-x mice.
Fig. 1.
A: low- and high-power digital images of HA immunofluorescence in SFO-x (i and ii) and SFO (iii and iv) mice. Scale bars, 100 μm. B: cumulative water intake of SFO mice (n = 13) after injection of CNO (3.0 mg/kg sc) or vehicle (0.25 ml sc). C: 60-min cumulative water intake of SFO (n = 13) or SFO-x (n = 12) mice plotted as a function of CNO dose. D: 120-min water and 0.3 M NaCl intakes of SFO mice after injection of CNO (3.0 mg/kg sc) or vehicle (0.25 ml sc). All values are means ± SE. *P < 0.01 vs. vehicle, #P < 0.05 vs. 0 mg/kg CNO or SFO-x, †P < 0.05, water vs. 0.3 M NaCl.
For acute experiments, body weight did not differ between SFO and SFO-x mice (26.8 ± 1.4 g vs. 27.4 ± 0.3 g; n = 13 and 12 per group, respectively). Injection of 3.0 mg/kg CNO significantly increased water intake of SFO mice within 15 min (Fig. 1B). In fact, the dipsogenic response in SFO mice was observed over a range of CNO doses (0.1–3.0 mg/kg sc; Fig. 1C). In marked contrast, CNO did not stimulate water intake in SFO-x mice (Fig. 1C). To assess whether DREADD-induced activation of SFO neurons stimulated salt appetite, SFO mice were given access to both water and 0.3 M NaCl. Injection of CNO significantly increased the ingestion of water and 0.3 M NaCl (Fig. 1D); however, CNO stimulated a significantly greater increase in water vs. 0.3 M NaCl intake.
CNO increases Fos immunoreactivity in SFO neurons.
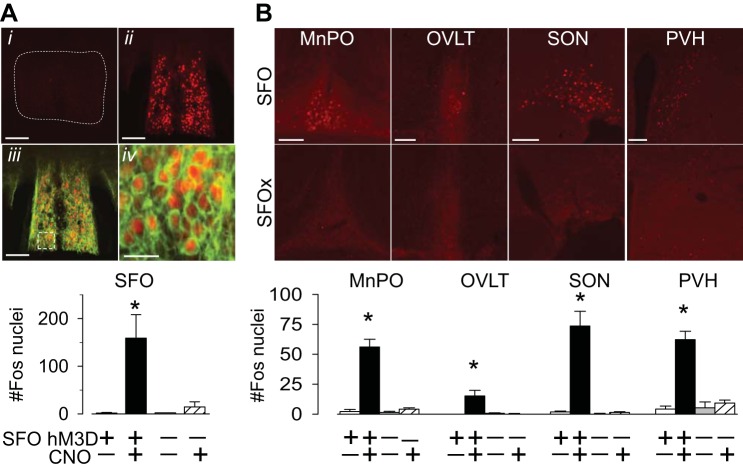
To determine whether CNO activates SFO neurons, Fos immunoreactivity was assessed in SFO and SFO-x mice. Injection of CNO vs. vehicle in SFO mice significantly increased the number of Fos-positive nuclei in the SFO (Fig. 2A). Fos-positive nuclei were observed throughout the rostral-caudal, dorsal-ventral, and medial-lateral extent of the SFO. In SFO mice, the Fos-positive nuclei were associated with strong HA immunofluorescence. Interestingly, injection of CNO also increased the number of Fos-positive nuclei in several efferent targets including the median preoptic nucleus, OVLT, supraoptic nucleus, and hypothalamic paraventricular nucleus (Fig. 2B). It is noteworthy that injection of CNO in SFO-x mice did not statistically increase Fos expression in any of the above structures.
Fig. 2.
A, top: Fos immunoreactivity in the SFO after injection of CNO in SFO-x (i) or SFO (ii) mice. The Fos-immunoreactive nuclei overlapped with HA-positive cells in SFO mice (iii and iv). Bottom: mean ± SE number of Fos-positive nuclei in the SFO of mice injected with CNO or vehicle. B, top: digital images of Fos immunoreactivity in median preoptic nucleus (MnPO), organum vasculosum of the lamina terminalis (OVLT), supraoptic nucleus (SON), and hypothalamic paraventricular nucleus (PVH) of SFO and SFO-x mice after injection of CNO. Bottom: mean ± SE number of Fos-positive nuclei in the SFO of mice injected with CNO or vehicle. *P < 0.01 vs. vehicle, n = 4 for all groups. Scale bars, 100 μm for all images except Aiv (25 μm).
CNO excites SFO neurons.
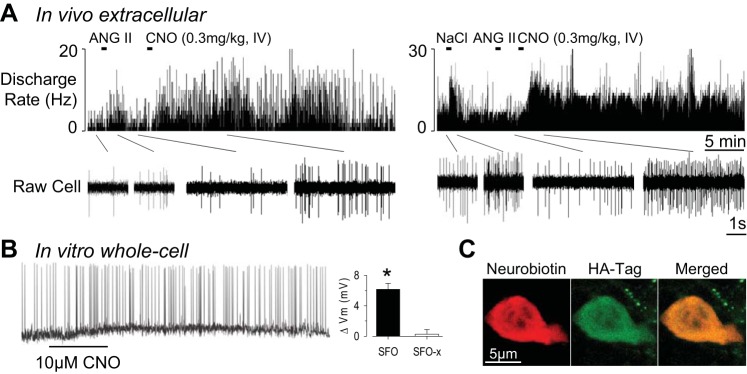
In vivo single-unit recordings demonstrate that systemic injection of CNO increases the discharge of SFO neurons (n = 6) responsive to either intracerebroventricular ANG II (n = 3) or 0.5 M NaCl (n = 3) (Fig. 3A). CNO significantly increased the discharge of all six SFO neurons from 6.2 ± 2.2 Hz to 22.0 ± 7.2 Hz (P < 0.05). The response occurred within 1–2 min, and discharge remained elevated for >45 min. It is noteworthy that CNO did not increase the firing rate of three HA-negative neurons located ∼400 μm dorsal to the SFO in SFO mice (data not shown). Moreover, CNO did not alter neuronal discharge in SFO-x mice (baseline 9.4 ± 1.8 Hz vs. peak 10.7 ± 2.4 Hz; n = 5). A final set of in vitro current-clamp recordings revealed that bath application of 10 μM CNO in SFO vs. SFO-x mice produced significant membrane depolarization (SFO 6.2 ± 0.8 mV vs. SFO-x 0.2 ± 0.6 mV, n = 4 per group; P < 0.01) and increase in action potential frequency (Fig. 3B).
Fig. 3.
A: 2 examples of single-unit recordings in SFO mice. SFO neurons were responsive to either intracerebroventricular ANG II (left) or 0.5 M NaCl (right). Injection of CNO (0.3 mg/kg iv) significantly increased SFO neuronal discharge. B: whole cell patch-clamp recording of SFO neuron demonstrates that bath application of CNO depolarizes Vm and increases cell discharge. Note that hyperpolarizing potentials are not readily visible in this example because of higher baseline noise. C: confocal image of Neurobiotin-filled SFO neuron and HA-Tag immunofluorescence. *P < 0.05 SFO vs. SFO-x.
Chronic activation of SFO neurons stimulates thirst.
Chronic administration of CNO in the water and 0.3 M NaCl drinking tubes significantly increased 24-h water intake but did not affect the ingestion of 0.3 M NaCl in SFO mice (Fig. 4). In fact, CNO doubled 24-h water intakes on day 2 (112 ± 34%) and day 3 (117 ± 26%). The excessive water intake persisted for 2 days after CNO administration was stopped (day 4 107 ± 28%, day 5 51 ± 12% vs. baseline intakes). Interestingly, the body weight of SFO mice increased during chronic CNO administration (day 0 26.6 ± 1.0 g vs. day 4 27.0 ± 1.0 g, P < 0.05; Δbody wt: 0.4 ± 0.1 g). In contrast, chronic CNO administration to SFO-x mice did not alter water and 0.3 M NaCl intake (Fig. 4) or body weight (day 0 26.2 ± 0.9 g vs. day 4 26.1 ± 1.0 g, P > 0.6; Δbody wt: −0.1 ± 0.1 g). The average daily dose of CNO was 3.8 ± 0.4 and 3.1 ± 0.2 mg/kg in SFO vs. SFO-x mice, respectively.
Fig. 4.
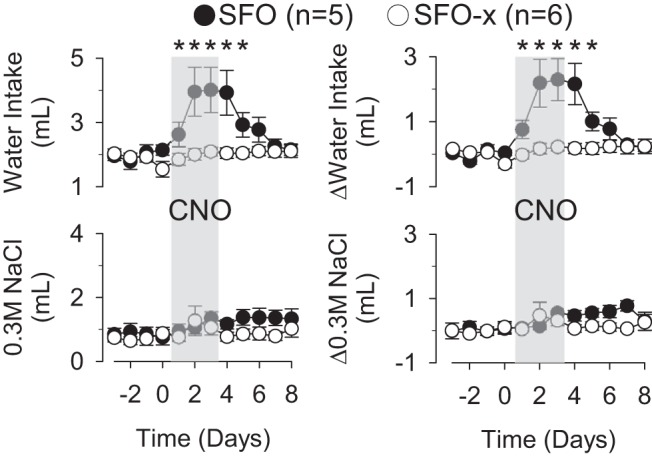
Chronic administration of CNO through drinking tubes significantly increased 24-h water intake but not 0.3 M NaCl intake of SFO mice. CNO did not affect water or 0.3 M NaCl intake of SFO-x mice. Values are means ± SE. ΔWater or 0.3 M NaCl intakes were calculated by the difference between the 24-h intake and the average baseline intake (day −3 to 0). *P < 0.05 vs. baseline or SFO-x mice.
DISCUSSION
The present study employed a chemogenetic approach using DREADDs to acutely and chronically activate SFO neurons and assess the impact on thirst and salt appetite. There are several novel findings, including 1) acute CNO injection dose-dependently stimulated water intake in SFO mice; 2) acute CNO produced a small but significant increase in salt appetite; 3) acute CNO injection significantly increased Fos expression in SFO neurons (and downstream efferent targets); 4) in vivo single-unit recordings demonstrate that CNO produced an immediate and sustained increase in cell discharge that persisted for >45 min; 5) in vitro patch-clamp recordings demonstrate that CNO depolarized neurons and increased action potential frequency; and 6) chronic administration of CNO doubled 24-h water intake without an effect on salt appetite. Collectively, these findings indicate that DREADDs can be used to acutely and chronically manipulate SFO neuronal activity and alter body fluid homeostasis.
A myriad of studies have reported that numerous dipsogenic stimuli (i.e., ANG II, hypertonic NaCl) increase cell discharge or Fos expression in SFO neurons (Anderson et al. 2000; Gutman et al. 1988; Kinsman et al. 2014; Larsen and Mikkelsen 1995; Oldfield et al. 1994; Sunn et al. 2002; Tanaka et al. 1985; Taylor et al. 2008). In many cases, SFO lesions or interruption of SFO neurotransmission attenuates thirst responses to these stimuli (Hosutt et al. 1981; Mangiapane et al. 1984; Osborn et al. 2012; Simpson et al. 1978; Simpson and Routtenberg 1973, 1978; Sunn et al. 2002; Thrasher et al. 1982; Thunhorst et al. 1999; Tiruneh et al. 2013). In the present study, DREADD-induced excitation of SFO neurons produced a dose-dependent increase in water intake of water-replete mice. The effect was immediate and largely observed in the first 15–30 min. One remarkable finding in the present study was that the minimum dose of CNO to produce a dipsogenic response was ∼0.1 mg/kg (Fig. 1C). This dose is lower than those commonly used in experiments employing DREADDs to manipulate neuronal activity and behavior.
Two other studies have used optogenetic or chemogenetic approaches to manipulate SFO neurons and thirst (Betley et al. 2015; Oka et al. 2015). These studies reported that optogenetic activation of SFO neurons through CaMKII promoter or mice expressing Cre under the vesicular glutamate transporter-2 significantly increased the number of licks of water. Furthermore, Oka et al. (2015) reported that mice drank 8% of body weight in 15 min (∼1.7 ml) during optogenetic activation of SFO neurons at 20 Hz. There was no effect on salt appetite. This level of water intake is much higher than the levels reported here or those of mice deprived of water for 48 h (Oka et al. 2015) or having substantial plasma hypernatremia (Kinsman et al. 2014; Taylor et al. 2008). On the other hand, our findings suggest that injection of CNO produced an increase in water intake comparable to that evoked by moderate plasma hypernatremia (∼5–10 mM) (Kinsman et al. 2014). The discrepancies in thirst responses between these studies may be attributed to the manipulated cell population (CaMKII vs. glutamatergic neurons) or differences in cellular mechanisms of activation between optogenetics and DREADDs. For example, optogenetic activation of neurons with channelrhodopsin is mediated by a nonselective cation conductance (Deisseroth 2015), whereas DREADD-induced activation through hM3D(Gq) increases intracellular calcium (Roth 2016). However, there are limited data that directly compare the effect of both approaches on a behavioral outcome while directly measuring changes in neuronal activity. Therefore, it remains unclear whether these different mechanisms of neuronal depolarization and excitation impact behavior differently.
DREADD-induced excitation by CNO in SFO mice was demonstrated with multiple approaches including 1) increase in the number of Fos-immunoreactive nuclei, 2) membrane depolarization and increased action potential frequency in vitro, and 3) increase in neuronal discharge via in vivo single-unit recordings. Numerous studies have employed the first two approaches to confirm the actions of CNO on various neuronal systems. However, there are limited data from the use of direct cell recordings in vivo to demonstrate the effect and time course of CNO on neuronal activity. The present findings provide such evidence, for the first time, as intravenous injection of 0.3 mg/kg CNO produced an immediate (<2 min) increase in cell discharge that persisted for >45 min. Interestingly, mice did not ingest significant amounts of water after the initial 15 min, and this may be attributed to the generation of inhibitory signals on thirst such as gastric distension, activation of gut osmoreceptors, or osmotic dilution (Stricker and Sved 2000). The in vivo single-unit recordings also indicated that CNO affected both ANG II- and NaCl-responsive neurons in the SFO. Although there are limited data in mice, prior Fos and in vivo electrophysiological studies in rats suggest that ANG II and NaCl largely affect different populations of SFO neurons. ANG II activates SFO neurons located in the central core, whereas hypertonic NaCl activates neurons located in the lateral margins (McKinley et al. 1998; Rosas-Arellano et al. 1996). Unfortunately, there is no evidence for a topographical distribution of functionally distinct neurons in the SFO of mice. Nevertheless, the present findings demonstrate that CNO produced a prolonged excitation of SFO neurons in vivo.
Several studies have reported that SFO neurons play an important role in salt appetite (Hosutt et al. 1981; Thunhorst et al. 1999). This idea is supported by studies that have observed a reduction in the ingestion of a salt solution after SFO lesion. Although excitation of SFO neurons by acute injection of CNO did produce a statistically significant increase in both water and 0.3 M NaCl intake, the effect on 0.3 M NaCl intake (salt appetite) was much less. Indeed, the volume of 0.3 M NaCl was very small and unlikely to have a physiological impact. Surprisingly, chronic CNO administration over 3 days did not alter 0.3 M NaCl intake. Optogenetic activation of SFO also failed to stimulate salt appetite (Oka et al. 2015). There are two potential explanations for the small or absent effect on salt appetite in these experiments. First, salt appetite is usually observed in volume-depleted animals, but the present experiments were performed in volume-replete mice. Second, as noted above, hM3D(Gq) expression affected both ANG II- and NaCl-responsive SFO neurons. Thus injection of CNO may activate several populations of SFO neurons that both stimulate thirst and generate opposing signals for salt appetite. That is, ANG II stimulates salt appetite, but plasma hypernatremia likely opposes the ingestion of 0.3 M NaCl. Subsequent studies that selectively target neurochemically or topographically distinct populations of SFO neurons may yield different results.
Chemogenetic and optogenetic tools represent unique approaches to manipulate neuronal activity and elucidate neural circuits and function. However, each approach has distinct advantages and disadvantages. For example, optogenetics permits second-to-second control of cellular activity, whereas DREADDs activate or inhibit neurons for much longer periods of time without any need for instrumentation. With this chemogenetic approach, chronic CNO administration through the drinking tubes to activate SFO neurons approximately doubled water intake and produced a small increase in body weight. Interestingly, the polydipsia observed in SFO mice persisted for 1 day after the CNO administration ceased and may be attributed to the pharmacokinetics of CNO. Although we did not directly assess the underlying mechanisms for the increase in body weight, a plausible explanation is the polydipsia and resultant volume expansion. SFO neurons directly innervate magnocellular vasopressin neurons of the hypothalamic paraventricular and supraoptic nuclei (McKinley et al. 2003). Therefore, activation of SFO neurons should increase plasma vasopressin levels and stimulate renal water reabsorption. These neuroendocrine effects on kidney function combined with the polydipsia may underlie the increase in body weight. Altogether, these findings highlight the pivotal role of SFO neurons in the acute and chronic regulation of thirst. In addition, the present findings highlight the potential utility of DREADD-based approaches to acutely or chronically manipulate body fluid homeostasis.
A distinct advantage of chemogenetic and optogenetic approaches is the ability to selectively manipulate neurochemically or anatomically distinct populations of cells through cre-lox-based approaches. For example, SFO neurons express a number of receptors (ANG II type 1A, atrial natriuretic peptide, relaxin, estrogen receptor α) and densely innervate several hypothalamic nuclei (McKinley et al. 2003). Although the present study did not investigate the relative contributions of these specific neuronal populations in body fluid homeostasis, future studies can directly address these questions using transgenic animals, anatomical tracers (WGA-Cre), or constructs with unique promoters. Thus these approaches will not only permit a selective control of neuronal activity but also provide unique insight into the contribution of specific neuronal populations to these responses on an unprecedented level.
GRANTS
The research was supported by National Heart, Lung, and Blood Institute Grant HL-113270 (S. D. Stocker), an American Heart Association Established Investigator Grant (S. D. Stocker), and Great Rivers Predoctoral Fellowship 14PRE19530001 (B. J. Kinsman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.L.N., M.N., B.J.K., K.N.B., and S.D.S. conception and design of research; H.L.N., M.N., B.J.K., K.N.B., and S.D.S. performed experiments; H.L.N., M.N., B.J.K., K.N.B., and S.D.S. analyzed data; H.L.N., M.N., B.J.K., K.N.B., and S.D.S. interpreted results of experiments; H.L.N., M.N., K.N.B., and S.D.S. prepared figures; H.L.N. and S.D.S. drafted manuscript; H.L.N., M.N., B.J.K., K.N.B., and S.D.S. edited and revised manuscript; H.L.N., M.N., B.J.K., K.N.B., and S.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Bryan Roth and the UNC Viral Core for the rAAV2-CaMKII-HA-hM3D(Gq)-IRES-mCitrine.
REFERENCES
- Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience 100: 539–547, 2000. [DOI] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521: 180–185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble JP, Grobe JL, Johnson AK, Sigmund CD. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: importance of the subfornical organ. Am J Physiol Regul Integr Comp Physiol 308: R238–R249, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18: 1213–1225, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV, Kasting NW. Electrical stimulation in subfornical organ increases plasma vasopressin concentrations in the conscious rat. Am J Physiol Regul Integr Comp Physiol 251: R425–R428, 1986. [DOI] [PubMed] [Google Scholar]
- Gutman MB, Ciriello J, Mogenson GJ. Effect of paraventricular nucleus lesions on cardiovascular responses elicited by stimulation of the subfornical organ in the rat. Can J Physiol Pharmacol 63: 816–824, 1985. [DOI] [PubMed] [Google Scholar]
- Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 254: R746–R754, 1988. [DOI] [PubMed] [Google Scholar]
- Hosutt JA, Rowland N, Stricker EM. Impaired drinking responses of rats with lesions on the subfornical organ. J Comp Physiol Psychol 95: 104–113, 1981. [DOI] [PubMed] [Google Scholar]
- Kinsman B, Cowles J, Lay J, Simmonds SS, Browning KN, Stocker SD. Osmoregulatory thirst in mice lacking the transient receptor potential vanilloid type 1 (TRPV1) and/or type 4 (TRPV4) receptor. Am J Physiol Regul Integr Comp Physiol 307: R1092–R1100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci 15: 2609–2627, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiapane ML, Thrasher TN, Keil LC, Simpson JB, Ganong WF. Role for the subfornical organ in vasopressin release. Brain Res Bull 13: 43–47, 1984. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl 25: S61–S67, 1998. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: III–XII, 1–122, 2003. [DOI] [PubMed] [Google Scholar]
- Oka Y, Ye M, Zuker CS. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520: 349–352, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience 60: 255–262, 1994. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin II-salt hypertension in the rat. Exp Physiol 97: 80–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods 65: 113–136, 1996. [DOI] [PubMed] [Google Scholar]
- Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Arcuate nucleus inputs onto subfornical organ neurons that respond to plasma hypernatremia and angiotensin II. Brain Res 707: 308–313, 1996. [DOI] [PubMed] [Google Scholar]
- Roth BL. DREADDs for neuroscientists. Neuron 89: 683–694, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JB, Epstein AN, Camardo JS Jr. Localization of receptors for the dipsogenic action of angiotensin II in the subfornical organ of rat. J Comp Physiol Psychol 92: 581–601, 1978. [DOI] [PubMed] [Google Scholar]
- Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science 181: 1172–1175, 1973. [DOI] [PubMed] [Google Scholar]
- Simpson JB, Routtenberg A. Subfornical organ: a dipsogenic site of action of angiotensin II. Science 201: 379–381, 1978. [DOI] [PubMed] [Google Scholar]
- Smith PM, Beninger RJ, Ferguson AV. Subfornical organ stimulation elicits drinking. Brain Res Bull 38: 209–213, 1995. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 66: 1184–1190, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568: 599–615, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker EM, Sved AF. Thirst. Nutrition 16: 821–826, 2000. [DOI] [PubMed] [Google Scholar]
- Sunn N, Egli M, Burazin TC, Burns P, Colvill L, Davern P, Denton DA, Oldfield BJ, Weisinger RS, Rauch M, Schmid HA, McKinley MJ. Circulating relaxin acts on subfornical organ neurons to stimulate water drinking in the rat. Proc Natl Acad Sci USA 99: 1701–1706, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Kaba H, Saito H, Seto K. Electrophysiological evidence that circulating angiotensin II sensitive neurons in the subfornical organ alter the activity of hypothalamic paraventricular neurohypophyseal neurons in the rat. Brain Res 342: 361–365, 1985. [DOI] [PubMed] [Google Scholar]
- Taylor AC, McCarthy JJ, Stocker SD. Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol 294: R1285–R1293, 2008. [DOI] [PubMed] [Google Scholar]
- Thrasher TN, Simpson JB, Ramsay DJ. Lesions of the subfornical organ block angiotensin-induced drinking in the dog. Neuroendocrinology 35: 68–72, 1982. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Beltz TG, Johnson AK. Effects of subfornical organ lesions on acutely induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol 277: R56–R65, 1999. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Xu Z, Cicha MZ, Zardetto-Smith AM, Johnson AK. Fos expression in rat brain during depletion-induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol 274: R1807–R1814, 1998. [DOI] [PubMed] [Google Scholar]
- Tiruneh MA, Huang BS, Leenen FH. Role of angiotensin II type 1 receptors in the subfornical organ in the pressor responses to central sodium in rats. Brain Res 1527: 79–86, 2013. [DOI] [PubMed] [Google Scholar]
- Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 55: 399–417, 2015. [DOI] [PubMed] [Google Scholar]