Abstract
Afferent chorda tympani (CT) fibers innervating taste and somatosensory receptors in fungiform papillae have neuron cell bodies in the geniculate ganglion (GG). The GG/CT fibers branch in the tongue to innervate taste buds in several fungiform papillae. To investigate receptive field characteristics of GG/CT neurons, we recorded extracellular responses from GG cells to application of chemical and thermal stimuli. Receptive field size was mapped by electrical stimulation of individual fungiform papillae. Response latency to electrical stimulation was used to determine fiber conduction velocity. Responses of GG neurons to lingual application of stimuli representing four taste qualities, and water at 4°C, were used to classify neuron response properties. Neurons classified as SALT, responding only to NaCl and NH4Cl, had a mean receptive field size of six papillae. Neurons classified as OTHER responded to salts and other chemical stimuli and had smaller mean receptive fields of four papillae. Neurons that responded to salts and cold stimuli, classified as SALT/THERMAL, and neurons responding to salts, other chemical stimuli and cold, classified as OTHER/THERMAL, had mean receptive field sizes of six and five papillae, respectively. Neurons responding only to cold stimuli, categorized as THERMAL, had receptive fields of one to two papillae located at the tongue tip. Based on conduction velocity most of the neurons were classified as C fibers. Neurons with large receptive fields had higher conduction velocities than neurons with small receptive fields. These results demonstrate that GG neurons can be distinguished by receptive field size, response properties and afferent fiber conduction velocity derived from convergent input of multiple taste organs.
Keywords: taste, taste bud, geniculate ganglion, chemosensory, chorda tympani
primary afferent neurons of the chorda tympani nerve (CT) convey information to the central nervous system about sensory properties of food. CT fibers have cell bodies in the geniculate ganglion (GG) and respond to chemical, thermal and mechanical properties of oral stimuli (Finger et al. 2005; Fishman 1957; Kumari et al. 2015). Despite the obvious importance of their neurobiological and sensory roles, there is no comprehensive understanding of the full nature of the basic biology of GG neurons. As one example of the lack of information about GG/CT neuron biology, consider how primary afferents conveying skin sensation have been classified based on their peripheral targets (cutaneous, articular and visceral afferents), conduction velocity (afferent axon size and myelination), response properties (sensory modality and intensity thresholds) and neurochemical phenotype (such as peptide expression) (Lawson 2002; Le Pichon and Chesler 2014; McMahon and Priestley 2005). In contrast, we lack even very basic types of information for GG and associated CT afferent biology.
Taste receptor cells on the anterior tongue innervated by the CT are located in fungiform papillae. Single taste buds in the apex of the rodent papilla come in contact with food stimuli encountered during licking and food bolus manipulation in the oral cavity. In contrast, taste buds on the posterior tongue in the lining epithelium of the circumvallate and foliate papillae are exposed to chemical stimuli released during mastication (Bradley 1981). The contrasting taste bud locations suggest different roles in the initial stages of food evaluation. It has been proposed that the role of the anterior tongue in gustatory processing is stimulus identification (Spector and Glendinning 2009), similar to mechanoreceptors on the finger tips (Delmas et al. 2011). In fact, it has been known for some time that, like finger mechanoreceptors, single CT afferent fibers terminate in fungiform taste buds that are organized in receptive fields. Investigators using rat (Miller 1971), cat (Boudreau et al. 1971; Oakley 1975; Robinson 1988), goat (Boudreau et al. 1985) and sheep (Nagai et al. 1988) have shown that CT fiber receptive fields vary in size, ranging from 1 to 13 papillae. However, prior investigators have concentrated on the responses of GG neurons to chemical stimulation of the anterior tongue and omitted comprehensive study of the basic neurobiological properties of the GG neurons that include receptive field size, associated CT fiber properties and responses to nongustatory stimuli. In addition, there are no details about a possible relationship between receptive field characteristics and afferent fiber size and conduction velocity.
While there is some evidence that GG neurons are a heterogeneous population, by size, electrical properties and neurochemical signature (Grigaliunas et al. 2002; Kitamura et al. 1982; Lieberman 1976; Nakamura and Bradley 2011a, 2011b; Nawar et al. 1980), the nature and extent of this heterogeneity have not been comprehensively demonstrated. The receptive field of the GG neuron can convey a complex set of stimuli to each GG cell, resulting in different classifications of GG neurons.
In a previous developmental study, we used electrical stimulation of fungiform papillae to identify receptive field size and response properties of CT neurons in fetal, postnatal and adult sheep (Nagai et al. 1988; Vogt and Mistretta 1990). The method is reliable and reproducible and enables reasonably rapid quantification of receptive fields. We have used this method to characterize the receptive fields of CT afferent neurons in the rat.
MATERIALS AND METHODS
Animals.
Adult female Sprague-Dawley rats (Charles River Laboratories), weighing 270–360 g, were used. All surgical procedures were performed under National Institutes of Health guidelines and University of Michigan Animal Care and Use Committee-approved protocols.
Surgery.
Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) with supplemental doses given throughout the procedure to maintain surgical anesthesia. Animals were placed in a supine position and tracheotomized, and both hypoglossal nerves were cut to prevent tongue reflex movement. Rats were secured in a stereotaxic instrument with blunt ear bars and placed on a heating pad to maintain body temperature.
The GG was exposed using a dorsal approach (Lundy and Contreras 1999). Briefly, a midline incision was made on the occipital portion of the skull, and a portion of the left cranium between bregma and lambda cranial sutures was removed. Partial aspiration of the cortical tissue allowed access to the temporal bone. The petrous portion of the temporal bone overlying the GG was carefully removed by additional drilling. The exposed ganglion was covered with balanced salt solution saturated with oxygen during the experiment (Harper and Lawson 1985). The composition of the balanced salt solution was as follows (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 5 glucose, and 5 Tris·HCl (pH adjusted to 7.4 with NaOH). The tongue was extended from the oral cavity and stabilized by sutures through the ventral aspects of the tip and contralateral side of the tongue. To improve tongue access, the skin at the angle of the mouth was also cut.
Neurophysiology.
Extracellular action potentials were recorded from single GG neurons with tungsten microelectrodes (0.9–1.8 MΩ, FHC, ME or A-M Systems) mounted on a stereotaxic micromanipulator (Narishige) and advanced from the dorsal surface of the ganglion in 1-μm steps. Neural activity from the microelectrode was amplified using a Grass P511 preamplifier, displayed on an oscilloscope and monitored by an audio amplifier. The amplified neural activity was first digitized and then stored using the Spike 2 version 4 program (Cambridge Electronic Design). All recordings were maintained for at least 30 min.
Stimulus delivery and stimulation protocols.
Stimulus solutions were applied via syringe at 0.5 ml/s to the anterior portion of the tongue for 20 s, followed by a distilled water rinse for at least 30 s. Stimuli were 0.5 M NaCl, 0.5 M NH4Cl, 0.01 N HCl, 0.03 M citric acid, 0.02 M quinine HCl and 1.0 M sucrose dissolved in distilled water at room temperature. We also recorded responses to cold temperature on the tongue using distilled water at 4°C. All chemicals were reagent grade.
Single papilla receptive field mapping.
Once chemical and thermal responses were recorded, receptive field characteristics were mapped by touching individual fungiform papillae with a monopolar platinum wire electrode connected to a stimulator (Grass S88). An indifferent electrode was placed on the contralateral tongue. To identify the fungiform papillae, the tongue was dyed by a topical application of a 0.5% aqueous solution of methylene blue (Fig. 1A). Each fungiform papilla was readily identified as a pale dot using a dissecting microscope. The receptive fields of single GG neurons were initially localized by the application of search stimulus of small droplets of a taste mixture or thermal stimuli to fungiform papillae via a Pasteur pipette. Then the receptive field was carefully delimited by touching individual fungiform papillae with the electrical stimulator probe. The electrical stimulation current was 1–5 μA for 1–2 s, and the minimum current intensity that evoked a response was used for each recording. The number of responsive papillae was counted to determine receptive field size, and the mapping was repeated 2–4 times. In addition, unresponsive adjacent papillae were also documented. After a map was established, a drawing was made of fungiform papillae in the field, and location of the field was noted on a standard diagram of the tongue (Fig. 1B). Finally, taste or thermal stimuli were reapplied to the receptive field to confirm reproducibility of neuron responsivity (Fig. 1C).
Fig. 1.
A: dorsal surface of tongue stained with an aqueous solution of methylene blue. The fungiform papillae are apparent as pale staining spots. The papilla-stimulating electrode is indicated in contact with a single papilla. B: standard diagram of the tongue fungiform papillae distribution with a receptive field of 3 papillae innervated by a single GG neuron. C: response of the receptive field to stimulation with 0.5 M NaCl and 1.0 M sucrose. Electrical stimulation identifies the three papillae that constitute the receptive field. Arrowheads and stimulus artifacts indicate the application (solid arrowhead) and removal (open arrowhead) of the electrical stimulation.
Response latency and conduction velocity.
Stimulus artifacts were evoked at application and removal of the electrical stimulator as well as application of chemical stimuli. Response latency was measured as the time from the initiation of the stimulus artifact to the beginning of the rise of the first action potential for each papilla evoked by electrical or chemical stimulation of the fungiform papilla. The mean latency for the papillae in each receptive field was calculated for each neuron from the latencies measured from all responsive papillae. Conduction velocity was calculated by dividing the distance between the receptive field and the GG cell bodies and neuron response latency. The mean distance between the GG cell bodies and the extended tongue tip, measured by dissecting the CT nerve in 17 animals, ranged from 60 to 69 mm with a mean of 63 mm (63.4 ± 0.5).
For each receptive field, the conductance distance was calculated by subtracting the distance between the center of the receptive field and the tongue tip. Fibers were classified into axon subgroups using standard descriptions of conduction velocities of different diameter afferent fibers (Harper and Lawson 1985).
Spike sorting.
The majority of the recordings originated from single GG neurons. The spike sorting function of Spike 2 was used to separate individual action potentials from few-unit recordings. Spike templates were formed from sampled data on the basis of amplitude and waveform. Spikes were characterized using measurements of 75 data points uniformly distributed over the waveform. Individual spikes were included in a template, only if >60% of the points matched the template and the amplitude differed by <15%. The spike templates obtained when sampling taste and thermal stimuli were used to ensure that response spikes evoked by electrical stimulation were derived from the same action potentials.
Statistical analysis.
Statistical analyses were conducted using SPSS (IBM) software. Receptive field sizes were compared across different neuron response categories using one-way ANOVA. Within each NaCl responsive neuron, the paired t-test was used to compare response latency (NaCl vs. electrical stimulation). Correlation (Pearson) and regression analysis were used to evaluate the relation between conduction velocity and receptive field sizes. Statistical significance for all tests was set at P < 0.05. All data are presented as means ± SE.
RESULTS
Receptive fields were characterized by extracellular recordings from 33 GG neurons.
Location and size of receptive fields.
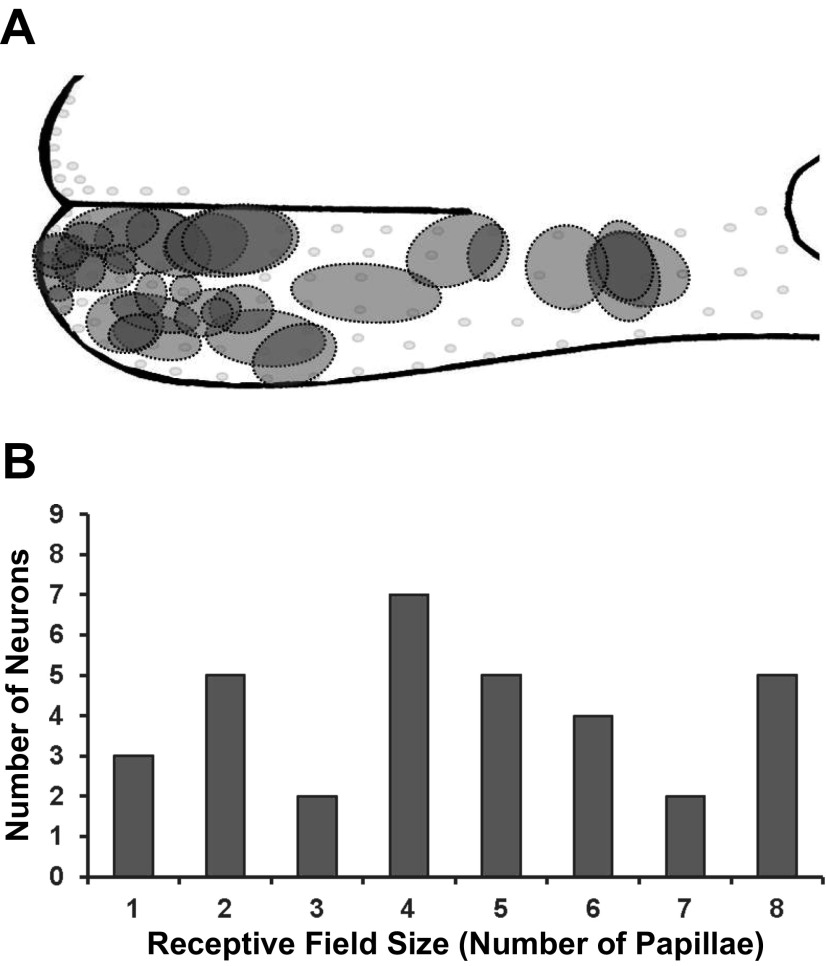
The location and overall outline of all 33 receptive fields are presented in Fig. 2A. Fields were sampled from an extensive CT distribution to the anterior tongue but tended to be concentrated toward the tongue tip, which has the highest density of fungiform papillae. In general, the smallest receptive fields are located on the tongue tip, and the larger fields are more posterior.
Fig. 2.
A: relative receptive field size and location of all receptive fields anterior to the intermolar eminence. B: distribution of the number of receptive fields of different sizes.
A distribution of the number of fungiform papillae in each receptive field for all 33 GG neurons is presented in Fig. 2B, demonstrating a wide range of field sizes from one to eight fungiform papillae. Mean receptive field size was four papillae.
Receptive field characteristics.
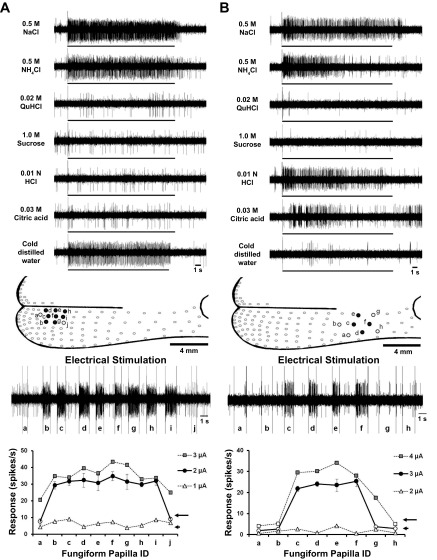
Two typical receptive field recordings are presented in Fig. 3, which illustrates differences in response characteristics and size of receptive fields. The recording in Fig. 3A was derived from a GG neuron that responds with high frequency to NaCl, NH4Cl and thermal stimuli. No responses were recorded to lingual application of the other test stimuli. Electrical stimulation resulted in a field of eight fungiform papillae (Fig. 3A, papillae b–i) that responded to 2-μA current intensity. Increasing the electrical current to 3 μA resulted in the same number of papillae in the receptive field, demonstrating the reliability of the stimulation procedure. A current of 1 μA was below the threshold of activation of the field. Note that stimulation of papillae adjacent to the receptive field (Fig. 3A, papillae a and j) did not elicit action potentials.
Fig. 3.
Recordings from GG neurons to chemical and thermal stimulation of the receptive fields. A, top: neuron responds to stimulation of the receptive field to 0.5 M NaCl, 0.5 M NH4Cl and 4°C water. The receptive field of this neuron did not respond to stimulation with other chemical stimuli. Middle: electrical stimulation of the fungiform papillae resulted in the identification of a receptive field made up of 8 papillae (b–i). Stimulation of papillae adjacent to this field confirmed the extent of the receptive field of this GG neuron. Bottom: electrical stimulation of 2 μA was used to define the field. Increasing current strength to 3 μA resulted in the same receptive field size. 1 μA was below the threshold to define receptive fields. B, top: neuron responds to 0.5 M NaCl, 0.5 M NH4Cl, 0.01 N HCl and 0.03 M citric acid, but not to the other stimuli and 4°C water. Middle: electrical stimulation of the fungiform papillae resulted in the identification of a receptive field made up of 4 papillae (c–f). Stimulation of papillae adjacent to this field confirmed the extent of the receptive field of this GG neuron. Bottom: electrical stimulation of 3 μA was used to define the field. Increasing current strength to 4 μA resulted in the same receptive field size. 2 μA was below the threshold to define receptive fields.
In contrast, the recording illustrated in Fig. 3B was derived from a GG neuron that not only responded to NaCl and NH4Cl, but also to HCl and citric acid, and not to the other chemical and thermal stimuli. The receptive field of this unit was smaller, consisting of four papillae (Fig. 3B, papillae c–f). Again, increasing stimulating current to 4 μA did not alter receptive field size.
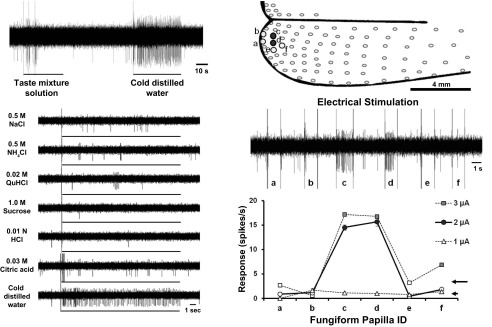
GG neurons that respond uniquely to cold stimuli have not been reported before. An example of one of these units is illustrated in Fig. 4. These units did not respond to lingual stimulation with any of the chemical stimuli, but responded with high frequency to cold water (Fig. 4A). The receptive field of this unit was small, consisting of two papillae located at the tongue tip (Fig. 4B, papillae c and d). Electrical stimulation with both 2 and 3 μA resulted in the identification of the same two fungiform papillae in the field. Stimulation of adjacent papillae with 2 and 3 μA did not evoke action potentials (Fig. 4B, papillae a, b, e, and f). A current of 1 μA was below threshold of activation of the field.
Fig. 4.
Recordings from a GG neurons that respond specifically to thermal stimulation of the receptive field. Left: neuron responds to stimulation of the receptive field with 4°C water. The receptive field of this neuron did not respond to stimulation with chemical stimuli. Right, top: electrical stimulation of the fungiform papillae resulted in the identification of a receptive field made up of 2 papillae (c and d). Stimulation of papillae adjacent to this field confirmed the extent of the receptive field of this GG neuron. Right, bottom: electrical stimulation of 2 μA was used to define the field. Increasing current strength to 3 μA resulted in the same receptive field size. 1 μA was below the threshold to define receptive fields.
Separation of units based on responses to chemical and thermal stimuli.
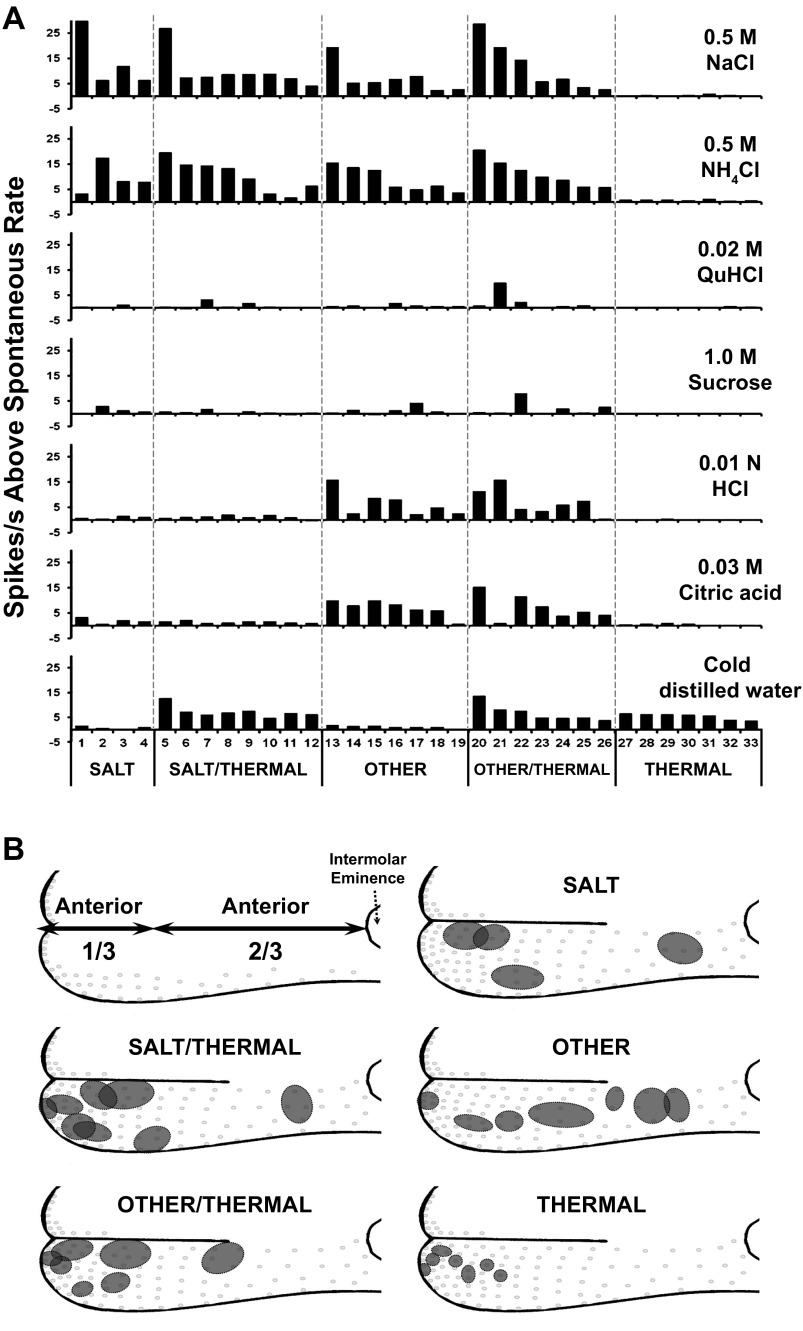
It is apparent from the data in Figs. 3 and 4 that GG neuron receptive fields differ in size and response characteristics. Based on responses to chemical and thermal stimuli, receptive fields were separated into five categories: SALT, SALT/THERMAL, OTHER, OTHER/THERMAL, and THERMAL. Thus the recording in Fig. 3A was classified as a SALT/THERMAL unit, whereas the unit in Fig. 3B was classified as an OTHER unit. The recording in Fig. 4 was classified as a THERMAL unit. Responses of all 33 GG neurons to the stimuli are presented in Fig. 5A.
Fig. 5.
A: chemical and thermal response patterns of single GG neurons. Based on individual response characteristics, the neurons have been categorized. From left to right: SALT neurons respond to NaCl and NH4Cl; SALT/THERMAL neurons respond to NaCl, NH4Cl and to 4°C water; OTHER neurons respond to NaCl, NH4Cl and to HCl and citric acids; OTHER/THERMAL neurons respond to NaCl, NH4Cl and to HCl and citric acids, as well as 4°C water; and THERMAL respond only to 4°C water. B: arbitrary division of anterior tongue into anterior one-third and anterior two-thirds relative to the intermolar eminence and location of the receptive fields of the GG neuron response categories.
SALT neurons responded with high frequency to NaCl and/or NH4Cl and little, if at all, to any other stimuli. Two neurons responded with highest frequency to NaCl, and the other neurons responded with highest frequency to NH4Cl. The average response frequency of the SALT units was 14 ± 6 impulses/s for NaCl and 9 ± 3 impulses/s for NH4Cl (Fig. 5A).
SALT/THERMAL neurons responded to both salts (NaCl and/or NH4Cl) and cold water (Fig. 5A). Five neurons responded with highest frequency to NH4Cl, and the other neurons in this group responded with highest frequency to NaCl. The average response frequency of SALT/THERMAL neurons was 10 ± 2 action potentials/s in response to NH4Cl, 10 ± 3 action potentials/s resulting from stimulation with NaCl and 7 ± 1 action potentials/s for thermal stimulation (Fig. 5A).
OTHER neurons responded to salts as well as at least one other chemical stimulus (sour, bitter and sweet) (Fig. 5A). Four neurons responded with highest frequency to NH4Cl, two neurons responded highest to NaCl and one neuron responded most to citric acid. The average response rate to stimulation with salts was 9 ± 2 spikes/s for NH4Cl and 7 ± 2 impulses/s for NaCl. The response frequency to citric acid was 7 ± 1 spikes/s.
OTHER/THERMAL neurons responded to salts, other chemical stimuli and cold water (Fig. 5A). Three neurons responded with the highest frequency to NaCl, three neurons responded highest to NH4Cl and one neuron responded most to HCl. The average response rate to stimulation with salts was 12 ± 4 spikes/s for NaCl and 11 ± 2 spikes/s for NH4Cl, and to acids was 7 ± 2 spikes/s for citric acid and 7 ± 2 spikes/s for HCl, respectively. The average cold water response was 7 ± 2 spikes/s, a frequency similar to thermal response rates of SALT/THERMAL neurons.
THERMAL neurons responded almost exclusively to cold water (Fig. 5A). The average response frequency to a cold stimulus was 5 ± 1 spikes/s, and responses to other chemical stimuli, when they occurred, were all <1.0 spikes/s.
Receptive field locations.
Receptive fields were distributed over the entire anterior tongue. Location of receptive fields defined by response to chemicals and thermal stimuli are diagrammed in Fig. 5B. We have arbitrarily divided the dorsal tongue into anterior one-third and two-thirds divisions relative to the intermolar eminence, as diagrammed in Fig. 5B and presented in Table 1. In general, the receptive fields of SALT, SALT/THERMAL and OTHER/THERMAL neurons were principally localized on the anterior one-third of the tongue. THERMAL neurons receptive fields were localized only on the anterior one-third around the tongue tip. Receptive fields of OTHER neurons were evenly distributed over the entire anterior tongue. THERMAL responses distinguish anterior one-third tongue receptive fields whether responses are specific to the cold stimulus or combined with responses to chemical stimuli, suggesting a unique distribution of cold receptors in anterior one-third tongue fungiform papillae.
Table 1.
Receptive field distribution by response category on the anterior one-third and two-thirds of the tongue
| Response Category | Anterior 1/3, no. | Anterior 2/3, no. |
|---|---|---|
| THERMAL | 7 | 0 |
| SALT | 3 | 1 |
| SALT/THERMAL | 7 | 1 |
| OTHER | 3 | 4 |
| OTHER/THERMAL | 6 | 1 |
| All | 26 | 7 |
Analysis of receptive field size.
Mean receptive field sizes were six for SALT, six for SALT/THERMAL, four for OTHER, five for OTHER/THERMAL and two for THERMAL neurons (Fig. 6). A one-way ANOVA indicated that receptive field size was significantly difference among all categories [F(4,28) = 11.14, P < 0.001]. The receptive field size of THERMAL was significantly smaller than all of the chemically responsive receptive fields (post hoc Bonferroni multiple-comparison test: all P values <0.05). However, there were no significant differences between chemical responsiveness and receptive field size (Fig. 6).
Fig. 6.
Distribution of receptive field size and response category. Except for the THERMAL category, there are no significant differences between receptive field size and response category. **Significant differences (P values < 0.05 or higher) by ANOVA, followed by Bonferroni post hoc tests.
Response latency.
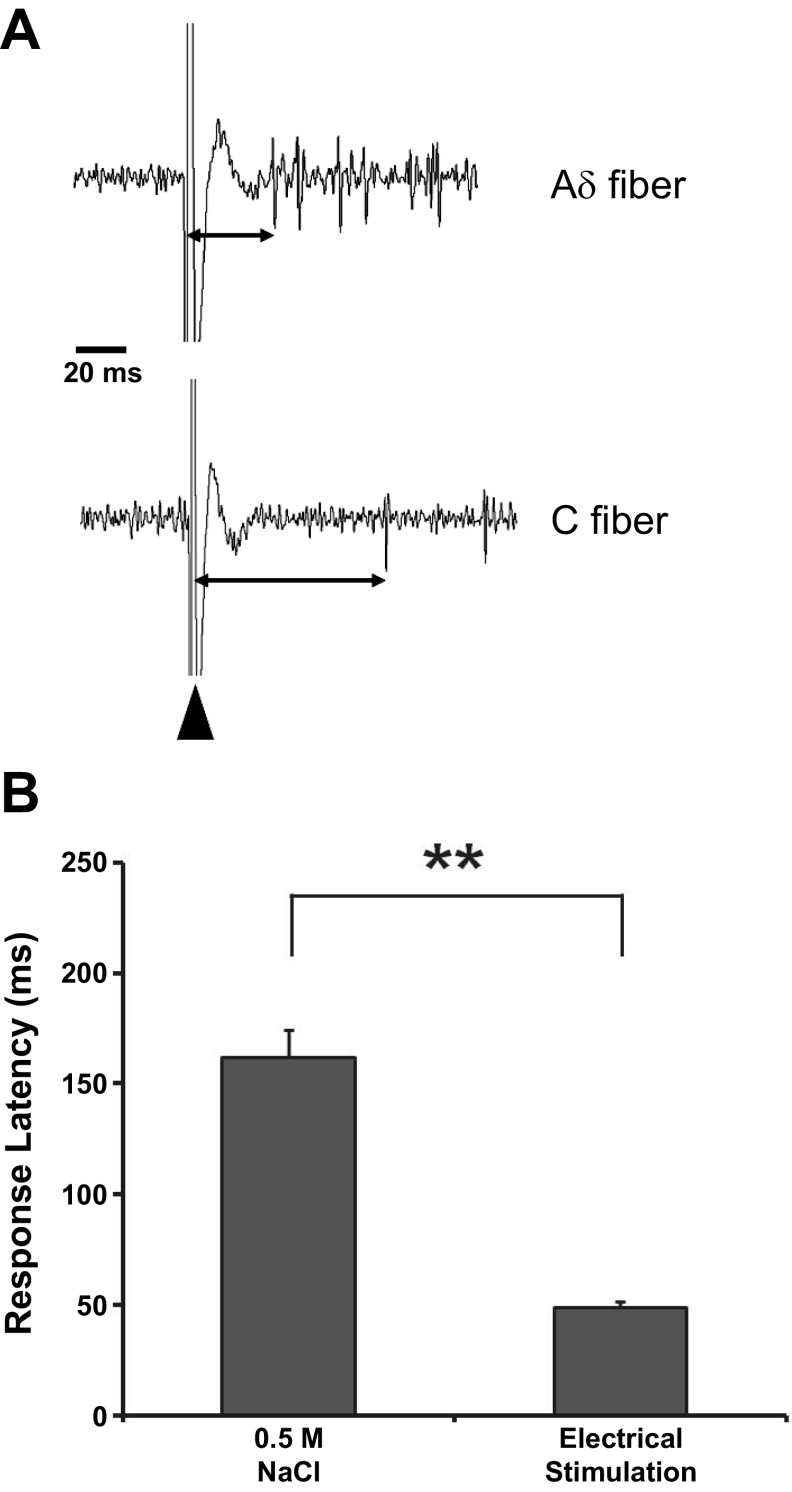
Examples of response latency measures are illustrated in Fig. 7A. The time from the electrical stimulus resulting in a large artifact to the first spike varies, indicating different conduction times; however, we also measured the latency to stimulation of the receptive field with 0.5 M NaCl. Response latency to electrical stimulation was shorter than response latency to chemical stimulation when measured from the same neuron (Fig. 7B). Average latency for response was 49 ± 3 ms for electrical stimulation and 162 ± 13 ms for 0.5 M NaCl; a paired t-test revealed a significant difference [t(25) = 10.43, P < 0.001]. These differences in latency presumably result from time for transduction in the taste bud because electrical stimulation bypasses the transduction process and results from direct stimulation of the CT innervation of the taste bud.
Fig. 7.
A: examples of latency measures (double arrows) and based on time between stimulus artifact (large arrowhead) and the initiation of the first action potential. B: when latency is measured in the same GG neuron to electrical and chemical stimulation, electrical stimulation results in a significantly shorter latency. Values are means ± SE. **P < 0.001 indicates significant difference by paired t-test.
Separation of units based on fiber conduction velocity.
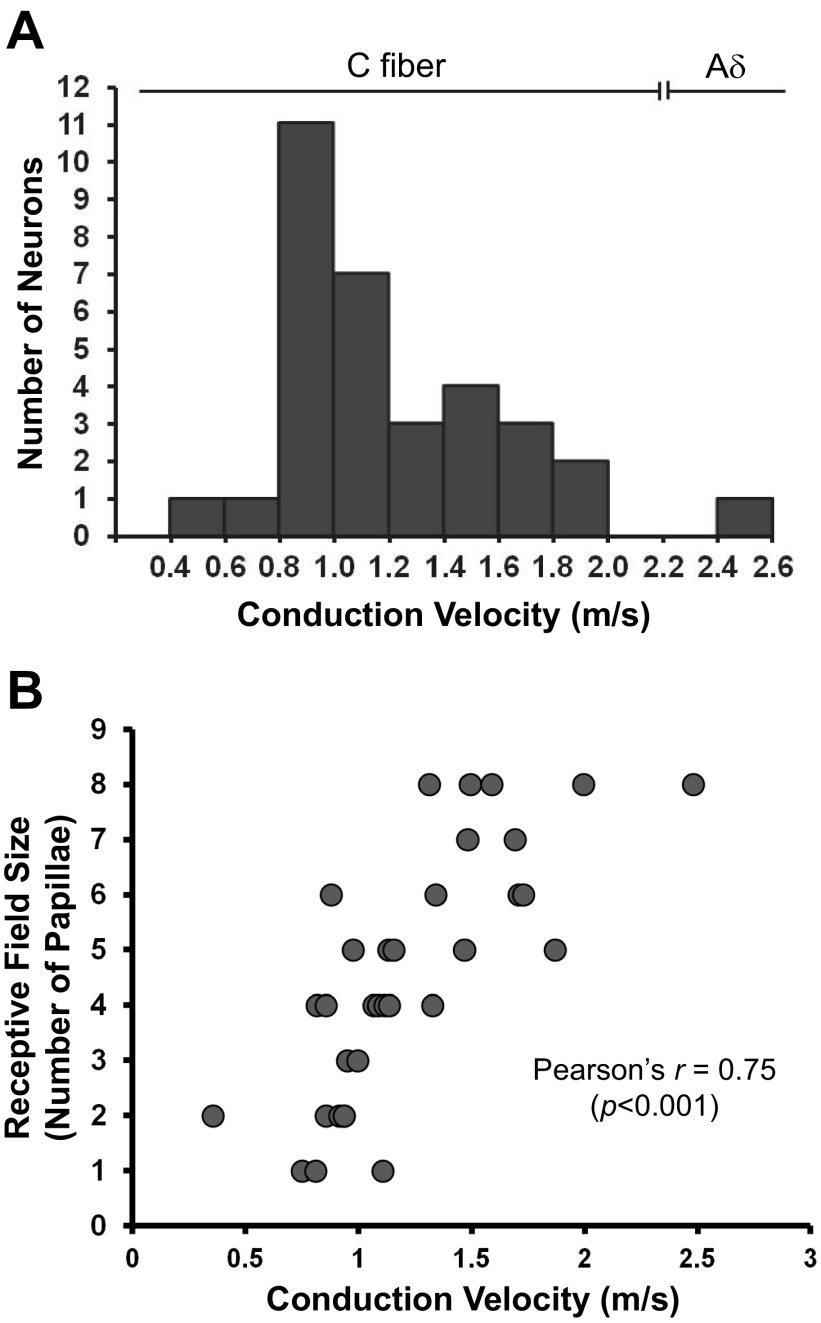
Based on latency measure to electrical stimulation of the papillae and the measured conduction distance between the papilla and the GG, conduction velocity for all 33 neurons was calculated (Fig. 8A). We categorized fiber types based on measures of the relationship between conduction velocity and fiber diameter of dorsal root ganglion neurons (Aδ, 2.2–8 m/s; C, <1.4 m/s) (Harper and Lawson 1985).
Fig. 8.
A: distribution of conduction velocities calculated from response latency to electrical stimulation of the fungiform papillae. The majority of conduction velocities correspond to published values for C fibers. Only one fiber had a conduction velocity of an Aδ fiber. B: relationship between conduction velocity and receptive field size. GG/CT neurons with faster conduction velocities innervate larger receptive fields of fungiform papillae.
Conduction velocity for most of the neurons (32 neurons) ranged from 0.4 to 2.0 m/s (1.2 ± 0.07), and these were classified as C fibers. One neuron (categorized as OTHER/THERMAL) was classified as an Aδ fiber. Mean conduction velocity in each response category was similar.
In Fig. 8B, the relationship between conduction velocity and receptive field size, with regression analysis to fit a linear relation with a correlation coefficient of 0.75 (r = 0.75, P < 0.001), indicates that neurons with larger receptive fields have higher conduction velocities than those with smaller receptive fields.
DISCUSSION
Prior studies of GG neurons innervating fungiform taste buds on the anterior tongue have reported a highly heterogeneous population, differing in size, neurochemical signature, biophysical and functional properties (Grigaliunas et al. 2002; Kitamura et al. 1982; Lieberman 1976; Nakamura and Bradley, 2011a, 2011b; Nawar et al. 1980). However, these studies categorized separate properties of GG neuron neurobiology (e.g., chemical responses or neurochemistry). We have recorded in vivo from GG neurons during natural stimulation of the tongue with a wide battery of stimuli and measured receptive field size, sensory response properties, and fiber latency and conduction velocity. Our results add to demonstrated heterogeneous GG properties while detailing differences in receptive field characteristics, conduction velocity of the afferent fibers and additional response properties that include sensitivity to cooling stimuli. It is thus apparent that the afferent input to the brain stem taste relay is multifaceted and complex in taste and lingual sensory circuits that contribute to coding.
Receptive field characteristics of GG/CT neurons.
Most electrophysiological investigations of the response properties of taste buds innervated by GG/CT neurons have used whole tongue stimulation by flowing the stimuli over the tongue. Thus, even though recordings were made from single afferent fibers or GG/CT neurons, the whole tongue was stimulated, and, therefore, the responses were complicated by possible interactions between adjacent papillae innervated by the same afferent input. These interactions were first reported in recordings from a single fungiform papilla achieved using a suction device (Miller 1971). Using this device, Miller reported that several papillae were innervated by a single CT afferent fiber, and that, when recording from one papilla, stimulation of adjacent papillae could enhance or diminish the response. On the other hand, in recordings from cat fungiform receptive fields, investigators had attempted to determine the response characteristics of taste buds innervated by the same CT fiber (Oakley 1975; Robinson 1988). Results suggested that all fungiform taste buds in a receptive field respond similarly when individually stimulated, indicating that connections are defined and do not occur randomly. However, cat fungiform papillae each contain an average of four taste buds, and this complicates the interpretation (Hayes and Elliot 1942).
Receptive field size.
Using electrical stimulation, we have shown that the receptive field size of single GG/CT neurons responding to chemical stimulation varies from one to eight fungiform papillae. Papilla field sizes of GG neurons responding only to a cold stimulus are small. No correlations were found between receptive field size and response to chemical stimuli. Mean receptive field sizes were six for SALT, six for SALT/THERMAL, four for OTHER, and five for OTHER/THERMAL neurons.
Receptive fields at the tongue tip were generally smaller than those closer to the intermolar eminence. The largest receptive fields were those classified as responding to SALT or SALT/THERMAL stimuli. Whereas THERMAL receptive fields are significantly smaller than all of the other categories of receptive field, there is wide variability, ranging from small to large fields (Fig. 2B). We have shown a significant relationship between receptive field size and conduction velocity. Thus fast conducting large fibers innervate more fungiform papillae, possibly relating to the relationship between taste bud size and the number of innervating GG neurons (Krimm and Hill 1998).
Responses to lingual application of chemical stimuli.
Previous investigators recording from single CT fibers have grouped afferent responses to chemical stimuli in attempts to understand how taste stimuli are encoded. Beginning with Pfaffmann's pioneering study (1941) of cat CT responses and similar later investigations in rat and hamster (Frank 1973; Frank et al. 1983; Hill et al. 1982; Smith et al. 2000), it was reported that fibers do not respond specifically to a single taste quality, but respond “best” to one quality. Arguing that, because single-axon recordings from the CT involve sectioning of the nerve that results in injury and limits recording time, Boudreau recorded extracellularly from GG neurons while stimulating the tongue with a range of chemical stimuli (Boudreau et al. 1971, 1985). The Boudreau approach was later used by the laboratories of Contreras and Hill to reexamine responses of rat GG neurons and determine their sensitivity to chemical stimulation of the tongue (Lundy and Contreras 1999) and soft palate (Sollars and Hill 2005). The overall goal was to examine sensory coding of the afferent input and not the basic biology of the GG neurons. Similar to earlier investigations, GG/CT neuron responses can be “grouped” as NaCl specialist, NaCl generalist and HCl generalist (Lundy and Contreras 1999; Sollars and Hill 2005). When additional stimuli were added, the resulting classification of GG neuron responses changed (Sollars and Hill 2005), so that many of the GG neurons that were classified as salt (NaCl) specialist in fact responded to NH4Cl also. Not surprisingly, in data from responses of GG/CT single neurons, a similar distribution of broad response profiles has been demonstrated (Lundy and Contreras 1999; Sollars and Hill 2005).
We did not follow the classification of chemosensory responses identified by prior investigations of GG/CT neurons (Lundy and Contreras 1999; Sollars and Hill 2005). In particular, we did not identify the “salt specialist” classification of response sensitivity. Perhaps the missing “salt specialist” would appear if we studied more GG neuron chemical response characteristics. In fact, if we had not used NH4Cl as a stimulus, Fig. 5A would be dramatically different. The “salt (i.e., NaCl) specialist” would emerge, and our classification of SALT would be the salt specialist. In addition, if we had not used a thermal stimulus as well, the “salt specialist” group would be even larger and the thermal specialist group would be entirely missing. We feel that our use of two chloride salts, two acids, and a cold stimulus strengthens our approach to defining receptive field size, response latency and differing response characteristics of single GG neurons.
Recently, investigators (Wu et al. 2015) have used transgenic mice expressing a calcium indicator that permits visualization of the activity of populations of GG neurons, allowing separation of GG ensembles into functional classes that receive input from both the tongue and soft palate. The investigators concluded that, although some neurons respond to a single taste quality, others are classified as generalists, responding to more than one taste quality. While this technique is focused on providing new information on cross-neuron response patterns to taste qualities, it does not provide information on the basic biophysical properties and receptive field characteristics. Furthermore, the focus has been on taste only without associated definition of lingual touch and temperature modalities, and, while salt specialist neurons were identified, no other salt stimuli were used, so it is not clear if the GG neurons responding to NaCl respond to other salts as well.
Unlike the prior studies, we based response classification not on what was a “best” stimulus or on how the neurons were “tuned”, but on the range of stimuli eliciting responses from each neuron. Thus the SALT grouping in the current experiments is similar to the salt specialist described by other investigators responding specifically to NaCl and NH4Cl stimuli only. However, we found a second group of salt-sensitive neurons that also respond to tongue cooling (SALT/THERMAL). If the cooling stimulus had not been used, these neurons would have been classified as salt specialists. A further group of neurons responded to salt and acid stimuli but not to a cooling stimulus, but a second similar group responded to salt, acid and thermal stimuli. These results differ from prior studies and reflect how the GG/CT neurons connect to the taste buds in their receptive field.
Responses to cold stimuli.
A unique finding of the present study was revealed by the use of cold stimuli. Other reports that the rat CT responds to tongue cooling but not warming (Ogawa et al. 1968) were confirmed in our preliminary experiments. We, therefore, used a cool stimulus. Two groupings of neurons, SALT/THERMAL and OTHER/THERMAL, responded to both chemical and cold stimulation of the receptive fields. A further group responded only to cooling. The cooling-specific group also was characterized by having the smallest receptive fields. Other investigators have reported on thermal responses of GG/CT neurons. However, these investigations are based on varying the temperature of the chemical stimuli (Breza et al. 2006; Lundy and Contreras 1999). Thermal responses measured by altering the temperature of chemical stimuli would activate not only cold-sensitive receptors, but influence the thermal binding characteristics of the taste receptor channels.
The discovery of small GG/CT neuron receptive fields that respond specifically to cold stimulation adds a further complication to the characteristics of sensory input to the GG. It is generally accepted that all fungiform papillae contain taste buds (Miller and Preslar 1975). However, taste buds are not required for the sensitivity of the CT to thermal stimulation of the anterior tongue (Kumari et al. 2015). Thus cold-specific responses quite possibly do not originate in taste receptors, but in separate terminal endings of the CT.
Cutaneous cold sensitivity is mediated by transient receptor potential melastatin 8 (TRPM8) receptors (Patapoutian et al. 2003). Using both a genetically engineered mouse and histochemical staining of the TRPM8 receptors, cold-sensitive fibers have not been demonstrated in taste buds but in fibers lateral to the taste buds (Abe et al. 2005; Dhaka et al. 2008). However, whether these TRPM8-positive fibers originated in the lingual or CT nerves was not determined.
Afferent fiber characteristics.
The majority of the afferent fibers isolated in the present study were classified as C fibers. Investigators have counted the axons in the rat CT (Beidler 1969; Farbman and Hellekant 1978) and report that it consists of both 641 myelinated and 455 unmyelinated fibers. A proportion of these axons are efferent parasympathetic fibers innervating the submandibular salivary gland. When the efferent fibers are eliminated surgically, the CT contains 484 myelinated and 122 unmyelinated axons. In rat CT, the myelinated axon diameters range from 2.0 to 4.0 μm (Beidler 1969). Unfortunately, the distribution of fiber diameters has not been published for the rat CT, but is available for the hamster (Jang and Davis 1987). The hamster CT has a bimodal distribution of fiber diameters, with the unmyelinated ranging from 0.2 to 0.8 μm (mean 0.5) and the myelinated range from 0.8 to 1.6 μm (mean 1.2). Based on the measured conduction velocity, all but one of the GG/CT fibers recorded from the present study are C fibers.
Complexity of fungiform papilla receptive field innervation.
The simplest description of the relationship between an afferent fiber of a GG/CT and the fungiform papillae that it innervates is that the axon travels to the tongue and then divides to innervate from one to eight fungiform papillae, forming a complex taste end organ. However, a number of investigators have demonstrated that characteristics of fungiform receptive field innervation are highly complex (Mistretta et al. 1988; Nagai et al. 1988).
At present, there are no detailed anatomical descriptions of the course and branching innervation pattern of a single GG/CT afferent fiber, but, based on the present experiments, a single GG/CT neuron innervates an average of five fungiform papillae. Since there are a total of about 90 fungiform papillae on one lateral half of the anterior tongue (Farbman and Hellekant 1978; Krimm 2007; Miller and Preslar 1975), only 18 ganglion cells would be required to innervate the 90 fungiform papillae. By applying a retrograde label to the central cut end of the CT, 506 ganglion cells (Gomez 1978) were labeled in the GG, resulting in the conclusion that more than a single ganglion cell innervates the fungiform papillae making up a GG/CT receptive field. Thus, by simple calculation, each taste bud is innervated by about six GG cells.
In fact, investigators have determined the number of GG/CT cells innervating a rat fungiform papilla by injecting single taste buds with a fluorescent tracer that then transports from the taste bud to the cell bodies of the GG/CT neuron. Single rat fungiform papillae are innervated by 3–14 GG neurons (Krimm and Hill 1998), and single hamster taste buds are innervated by an average of 15 GG neurons (Whitehead et al. 1999). Thus GG/CT receptive fields are innervated by more than one ganglion cell, further contributing to the complexity of the afferent input. Interestingly, larger volume taste buds are innervated by larger numbers of GG/CT neurons (Krimm and Hill 1998), suggesting a relationship between numbers of ganglion cells that synapse with taste bud cells. Furthermore, it is not known whether the several ganglion cells that innervate a single fungiform papilla also innervate other papillae in the receptive field, and whether a single ganglion cell innervating a receptive field synapses with taste bud cells expressing a similar taste receptor. The extent of overlap between the receptive fields of GG/CT neurons remains to be determined, although tracer injected into a single taste bud spreads to nearby noninjected papillae, and activity recorded from a single taste bud is influenced by stimulation of the surrounding tongue area (Miller 1971; Whitehead et al. 1999).
A further complication of the receptive field innervation is revealed by study of the number of CT axons in the papilla core. The branching pattern of the single GG afferent fiber is extensive (Beidler 1969; Miller 1974). The GG/CT afferent fibers ascending the papilla core consist of a large number of both myelinated and unmyelinated fibers with a mean of 54 fibers that enter the base of the taste bud. In the taste bud, some fibers have synaptic terminals with taste cells, while other fibers ascend between the taste bud cells (Kinnamon et al. 1985; Murray 1971). Thus branches of the same GG neuron that distribute to several taste buds in the receptive field probably innervate taste cells with different receptor specificity, resulting in nonspecific responses recorded in the ganglion cell.
Fungiform taste buds are complex sensory organs consisting of several cell types, including supporting and basal progenitor cells (Delay et al. 1986; Yee et al. 2001) with synaptic connections to GG/CT neurons. A further characteristic of taste buds is that they turn over with a half-life of 10 days (Beidler and Smallman 1965), resulting in alterations in terminal fiber connection patterns. It is apparent, therefore, that, even though taste bud cells express some membrane receptors that respond specifically to one of the five classic taste qualities, the complexity of the innervation pattern and receptor cell turnover accounts for loss of this specificity recorded from the GG/CT neurons.
Finally, terminal fields of GG central processes in the brain stem taste relay nucleus, the rostral nucleus of the solitary tract, have been demonstrated using anterograde labeling of the whole CT (May and Hill 2006). Thus most current information on termination patterns describes a broad pattern of the terminal fields of gustatory nerves. Detailed connection pattern of GG/CT central terminations with single first-relay neurons has been investigated in rat and sheep (Hill et al. 1983; Vogt and Mistretta 1990). In both rat and sheep, there is considerable convergence between the CT and second-order rostral nucleus of the solitary tract neurons, resulting in amplification of the peripheral neural input.
In conclusion, the receptive field characteristics of GG/CT neurons is variable, consisting of single fungiform papillae, as well as large fields of eight papillae. The smaller receptive fields often respond specifically to a cool tongue stimulus, whereas the larger fields respond to a number of chemical stimuli, as well as a cooling. Not all multimodal responding receptive fields are the same, varying in field size and response to different combinations of test stimuli. Based on conduction velocity, most receptive fields are innervated by C fibers. However, the larger receptive fields are innervated by fibers with faster conduction velocities.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-014428.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.Y. performed experiments; Y.Y. and R.M.B. analyzed data; Y.Y. interpreted results of experiments; Y.Y. and R.M.B. prepared figures; Y.Y. and R.M.B. edited and revised manuscript; R.M.B. conception and design of research; R.M.B. drafted manuscript; R.M.B. approved final version of manuscript. jane.
REFERENCES
- Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Mol Brain Res 136: 91–98, 2005. [DOI] [PubMed] [Google Scholar]
- Beidler LM. Innervation of rat fungiform papilla. In: Olfaction and Taste III, edited by Pfaffmann C. New York: Rockefeller University Press, 1969, p. 352–369. [Google Scholar]
- Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol 27: 263–272, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau JC, Bradley BE, Bierer PR, Kruger S, Tsuchitani C. Single unit recordings from the geniculate ganglion of the facial nerve of the cat. Exp Brain Res 13: 461–488, 1971. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Sivakumar L, Do LT, White TD, Oravec J, Hoang NK. Neurophysiology of geniculate ganglion (facial nerve) taste systems: species comparisons. Chem Senses 10: 89–128, 1985. [Google Scholar]
- Bradley RM. Basic Oral Physiology. Chicago, IL: Year Book Medical, 1981. [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006. [DOI] [PubMed] [Google Scholar]
- Delay RJ, Kinnamon JC, Roper SD. Ultrastructure of mouse vallate taste buds. II. Cell types and cell lineage. J Comp Neurol 253: 242–252, 1986. [DOI] [PubMed] [Google Scholar]
- Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci 12: 139–153, 2011. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28: 566–575, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman AI, Hellekant G. Quantitative analyses of the fiber population in rat chorda tympani nerves and fungiform papillae. Am J Anat 153: 509–522, 1978. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005. [DOI] [PubMed] [Google Scholar]
- Fishman IY. Single fiber gustatory impulses in rat and hamster. J Cell Comp Physiol 49: 319–334, 1957. [DOI] [PubMed] [Google Scholar]
- Frank M. An analysis of hamster afferent taste nerve response functions. J Gen Physiol 61: 588–618, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol 50: 941–960, 1983. [DOI] [PubMed] [Google Scholar]
- Gomez MM. Afferent soma populations within the geniculate ganglion (Abstract). Abst Soc Neurosci 4: 87, 1978. [Google Scholar]
- Grigaliunas A, Bradley RM, Maccallum DK, Mistretta CM. Distinctive neurophysiological properties of embryonic trigeminal and geniculate neurons in culture. J Neurophysiol 88: 2058–2074, 2002. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol 359: 47–63, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes ER, Elliott R. Distribution of the taste buds on the tongue of the kitten, with particular reference to those innervated by the chorda tympani branch of the facial nerve. J Comp Neurol 76: 227–238, 1942. [Google Scholar]
- Hill DL, Bradley RM, Mistretta CM. Development of taste responses in rat nucleus of solitary tract. J Neurophysiol 50: 879–895, 1983. [DOI] [PubMed] [Google Scholar]
- Hill DL, Mistretta CM, Bradley RM. Developmental changes in taste response characteristics of rat single chorda tympani fibers. J Neurosci 2: 782–790, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang T, Davis BJ. The chorda tympani and glossopharyngeal nerves in the adult hamster. Chem Senses 12: 381–395, 1987. [Google Scholar]
- Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol 253: 48–60, 1985. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kimura RS, Schuknecht HF. The ultrastructure of the geniculate ganglion. Acta Otolaryngol (Stockh) 93: 175–186, 1982. [DOI] [PubMed] [Google Scholar]
- Krimm RF. Factors that regulate embryonic gustatory development. BMC Neurosci 8: S4, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol 398: 13–24, 1998. [PubMed] [Google Scholar]
- Kumari A, Ermilov AN, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol 113: 1034–1040, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ- or Aα/β-fibres. Exp Physiol 87: 239–244, 2002. [DOI] [PubMed] [Google Scholar]
- Le Pichon CE, Chesler AT. The functional anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 8: 1–18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AR. Sensory ganglia. In: The Peripheral Nerve, edited by Landon DN. London: Chapman and Hall, 1976, p. 188–278. [Google Scholar]
- Lundy RF, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 82: 2970–2988, 1999. [DOI] [PubMed] [Google Scholar]
- May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J Comp Neurol 497: 658–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Priestley JV. Nociceptor plasticity. In: The Neurobiology of Pain, edited by Hunt SP, Koltzenburg M. Oxford, UK: Oxford University Press, 2005, p. 35–64. [Google Scholar]
- Miller IJ., Jr Branched chorda tympani neurons and interactions among taste receptors. J Comp Neurol 158: 155–166, 1974. [DOI] [PubMed] [Google Scholar]
- Miller IJ., Jr Peripheral interactions among single papilla inputs to gustatory nerve fibers. J Gen Physiol 57: 1–25, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IJ Jr, Preslar AJ. Spatial distribution of rat fungiform papillae. Anat Rec 181: 679–684, 1975. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Gurkan S, Bradley RM. Morphology of chorda tympani fiber receptive fields and proposed neural rearrangements during development. J Neurosci 8: 73–78, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RG. The ultrastructure of taste buds. In: The Ultrastructure of Sensory Organs, edited by Friedmann I. Amsterdam: North-Holland, 1971, p. 1–81. [Google Scholar]
- Nagai T, Mistretta CM, Bradley RM. Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity. J Neurosci 8: 64–72, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Bradley RM. Characteristics of calcium currents in rat geniculate ganglion neurons. J Neurophysiol 105: 224–234, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Bradley RM. Characteristics of sodium currents in rat geniculate ganglion neurons. J Neurophysiol 106: 2982–2991, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar NNY, Mikhail Y, Ibrahim KA. Quantitative and histomorphological studies on the geniculate ganglion of the facial nerve in man. Acta Anat (Basel) 106: 57–62, 1980. [DOI] [PubMed] [Google Scholar]
- Oakley B. Receptive fields of cat taste fibers. Chem Senses 1: 431–442, 1975. [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chorda tympani fibers of the rat and hamster to gustatory and thermal stimuli. J Physiol 199: 223–240, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. Thermotrp channels and beyond: mechanisms of temperture sensation. Nat Rev Neurosci 4: 529–539, 2003. [DOI] [PubMed] [Google Scholar]
- Pfaffmann C. Gustatory afferent impulses. J Cell Comp Physiol 17: 243–258, 1941. [Google Scholar]
- Robinson PP. The characteristics and regional distribution of afferent fibres in the chorda tympani of the cat. J Physiol 406: 345–359, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV St John SJ, Boughter JD Jr. Neuronal cell types and taste quality coding. Physiol Behav 69: 77–85, 2000. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones. J Physiol 564: 877–893, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Glendinning JI. Linking peripheral taste processes to behavior. Curr Opin Neurobiol 19: 370–377, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt MB, Mistretta CM. Convergence in mammalian nucleus of solitary tract during development and functional differentiation of salt taste circuits. J Neurosci 10: 3148–3157, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC, Ganchrow JR, Ganchrow D, Yao B. Organization of geniculate and trigeminal ganglion cells innervating single fungiform taste papillae: A study with tetramethylrhodamine dextran amine labeling. Neuroscience 93: 931–941, 1999. [DOI] [PubMed] [Google Scholar]
- Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun 6: 8171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yang RB, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: Immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 95, and serotonin. J Comp Neurol 440: 97–108, 2001. [DOI] [PubMed] [Google Scholar]