We investigated how neural networks regulate their own output via their feedback synapses onto modulatory neurons that influence them. Many measures of network output were sensitive to the consequences of network feedback onto the identified modulatory neuron modulatory commissural neuron 1 (MCN1). However, for most parameters, MCN1 activity rate did not determine the extent to which network output was altered by network feedback acting on MCN1. Thus feedback can shape network output regardless of the extent of network modulation.
Keywords: stomatogastric nervous system, central pattern generator, pacemaker, neuropeptide
Abstract
Modulatory projection neurons alter network neuron synaptic and intrinsic properties to elicit multiple different outputs. Sensory and other inputs elicit a range of modulatory neuron activity that is further shaped by network feedback, yet little is known regarding how the impact of network feedback on modulatory neurons regulates network output across a physiological range of modulatory neuron activity. Identified network neurons, a fully described connectome, and a well-characterized, identified modulatory projection neuron enabled us to address this issue in the crab (Cancer borealis) stomatogastric nervous system. The modulatory neuron modulatory commissural neuron 1 (MCN1) activates and modulates two networks that generate rhythms via different cellular mechanisms and at distinct frequencies. MCN1 is activated at rates of 5–35 Hz in vivo and in vitro. Additionally, network feedback elicits MCN1 activity time-locked to motor activity. We asked how network activation, rhythm speed, and neuron activity levels are regulated by the presence or absence of network feedback across a physiological range of MCN1 activity rates. There were both similarities and differences in responses of the two networks to MCN1 activity. Many parameters in both networks were sensitive to network feedback effects on MCN1 activity. However, for most parameters, MCN1 activity rate did not determine the extent to which network output was altered by the addition of network feedback. These data demonstrate that the influence of network feedback on modulatory neuron activity is an important determinant of network output and feedback can be effective in shaping network output regardless of the extent of network modulation.
NEW & NOTEWORTHY
We investigated how neural networks regulate their own output via their feedback synapses onto modulatory neurons that influence them. Many measures of network output were sensitive to the consequences of network feedback onto the identified modulatory neuron modulatory commissural neuron 1 (MCN1). However, for most parameters, MCN1 activity rate did not determine the extent to which network output was altered by network feedback acting on MCN1. Thus feedback can shape network output regardless of the extent of network modulation.
central pattern generator (CPG) networks underlying rhythmic behaviors such as locomotion, respiration, and chewing are functionally flexible and produce multiple different outputs in response to modulatory inputs (Briggman and Kristan 2008; Doi and Ramirez 2008; Harris-Warrick 2011; Marder and Bucher 2007). One source of modulation is descending modulatory projection neurons that alter synaptic and intrinsic properties of network neurons to elicit distinct network outputs (Brodfuehrer et al. 2008; Jordan et al. 2008; Sharples et al. 2014; Stein 2009).
Modulatory inputs are active over a range of activity rates depending on their intrinsic properties and the strength and type of sensory stimuli (Blitz et al. 2004; Dubuc et al. 2008; Prisco et al. 1997; Rossignol et al. 2006; Velázquez-Ulloa et al. 2003). Additionally, network feedback to projection neurons results in phasic activity patterns time-locked to motor output (Arshavsky et al. 1988; Blitz and Nusbaum 2008; Buchanan and Einum 2008; Kozlov et al. 2014). Sensory and other stimuli can also modulate network feedback, altering its impact on projection neuron activity (Blitz 2015; Blitz and Nusbaum 2012). Despite the prevalence of network feedback eliciting phasic modulatory neuron activity, many studies utilize tonic activation of modulatory inputs to study their influence on neural networks (Blitz et al. 1999; Frost et al. 2001; Liu and Jordan 2005; Puhl et al. 2012). Thus there is a need to compare the influence of tonic and phasic modulatory neuron activity to understand the role of network feedback and its regulation of projection neuron activity.
Our understanding of how modulatory neuron activity is translated to network output across a range of activity is complicated by modulatory inputs using complements of modulatory and classical transmitters, as well as electrical synapses, in various combinations, to act on network targets (Kristan et al. 2005; Nusbaum 2002; Nusbaum et al. 2001). Each component is likely influenced in distinct ways by activity of the modulatory input (Cazalis et al. 1985; Peng and Horn 1991; Pereda et al. 2013; Vilim et al. 1996; Whim and Lloyd 1989). Thus it is not a simple task to predict the relationship between modulatory neuron activity and network output. The impact of modulatory neuron activity rate and/or phasic vs. tonic activity on some aspects of motor network output has been examined (Bartos et al. 1999; Hurwitz et al. 2005; Jing and Weiss 2005; Severi et al. 2014; Wood et al. 2004), yet the sensitivity of a CPG network to the effects of network feedback on modulatory neuron activity, across a physiological range of activity rates, has not been determined.
We address these issues using a leading model system for the study of neural network modulation, the stomatogastric nervous system (STNS) (Marder 2012; Marder and Bucher 2007; Stein 2009). The connectome of the related pyloric (filtering of food) and gastric mill (chewing) networks has been determined, and there are identified modulatory projection neuron inputs with known cotransmitters and network targets (Nusbaum et al. 2001; Stein 2009). In particular, modulatory commissural neuron 1 (MCN1) activates and modulates the pyloric and gastric mill networks and is activated in different ways in response to multiple inputs in vitro and in vivo (Bartos and Nusbaum 1997; Beenhakker and Nusbaum 2004; Blitz et al. 1999, 2004, 2008; Christie et al. 2004; Coleman and Nusbaum 1994; Hedrich et al. 2009, 2011; Stein et al. 2007). Previous studies used a limited range of MCN1 activity to explore the consequences for a subset of rhythm parameters (Bartos et al. 1999; Wood et al. 2000). Here, we examined responses of two networks that generate rhythmic activity through distinct cellular mechanisms and cycle at different frequencies, across a physiological range of modulatory projection neuron activity rates, with vs. without network feedback phase-locking modulatory neuron activity to motor activity.
We find similarities and differences in responses of the pyloric and gastric mill networks. This includes differences in how rhythm speed and neuronal activity levels of both networks are regulated by MCN1 activity rate and the presence vs. absence of network feedback. Additionally, there are differences in the range of MCN1 activity that reliably activates each rhythm. There was an interaction between the effects of feedback and MCN1 activity rate for network activation. However, for most other parameters MCN1 rate did not interact with the effects of network feedback on MCN1 in determining network output. Therefore, the control of modulatory projection neuron activity plays important roles in the regulation of motor output, with the influence of network feedback able to contribute across a physiological range of modulatory neuron activity.
METHODS
Animals.
Male C. borealis crabs were obtained from commercial suppliers (Fresh Lobster, Gloucester, MA; Ocean Resources, Sedgwick, ME) and maintained in recirculating filtered artificial seawater tanks at 10–12°C. Crabs were fed a diet of thawed squid and maintained in holding tanks for at least a week prior to use in an experiment. Animals were cold-anesthetized by packing in ice for 30–45 min prior to dissection.
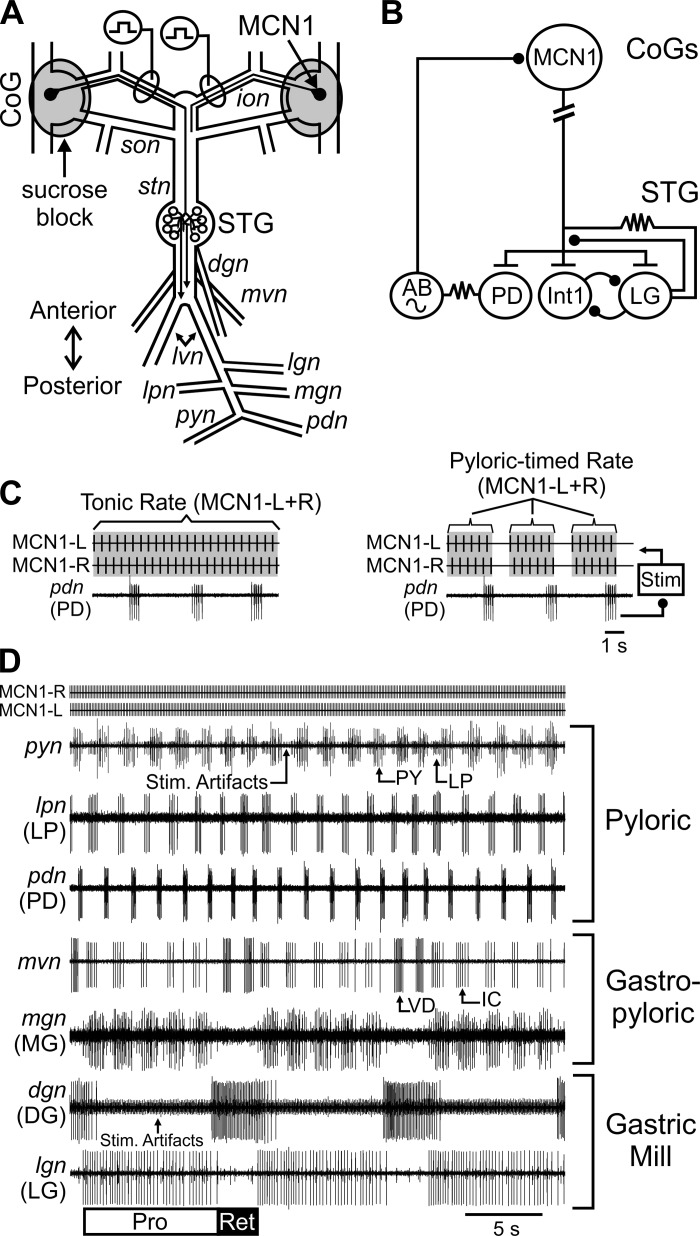
As detailed previously, the foregut was removed from the animal, bisected along the ventral surface, and pinned in a dark Sylgard-coated dish containing chilled C. borealis saline (∼4°C). The STNS (Fig. 1) was dissected free of surrounding tissue, removed from the foregut, and pinned in a clear Sylgard-lined petri dish for electrophysiological recordings (Blitz et al. 2008; Gutierrez and Grashow 2009). Preparations were continually superfused with C. borealis saline chilled (8–11°C) with Peltier wafers (Digi-Key Electronics) in a custom-made chamber (Miami University Instrumentation Laboratory) throughout electrophysiological experiments. In all experiments, the stomatogastric ganglion (STG) was isolated from descending commissural ganglion (CoG) inputs by building petroleum jelly (Vaseline, Amazon) wells around each CoG and replacing the saline with an isotonic sucrose (750 mM) solution (Fig. 1A) (Russell 1979).
Fig. 1.
The modulatory projection neuron modulatory commissural neuron 1 (MCN1) activates and modulates the pyloric and gastric mill rhythms. A: schematic illustrates the structure of the isolated stomatogastric nervous system and the projection pathway of MCN1. For clarity, the full axonal projection of only 1 MCN1 is shown. Gray circles indicate Vaseline wells in which commissural ganglia (CoGs) were sucrose blocked. Square pulses indicate sites of MCN1 axon stimulation within the inferior [o]esophageal nerve (ion). B: MCN1 acts on pyloric rhythm generator neurons [anterior burster (AB), pyloric dilator (PD)] and gastric mill rhythm generator neurons [interneuron 1 (Int1), lateral gastric (LG)]. T-bars indicate modulatory excitation, ball and stick represent inhibitory synapses, and resistors indicate electrical synapses. Break in MCN1 axon indicates additional distance between CoG and stomatogastric ganglion (STG). Sine wave indicates pacemaker properties. C: tick marks indicate an example of tonic (left) and “pyloric-timed” (right) stimulation. Stimulation of MCN1 left (MCN1-L) and MCN1 right (MCN1-R) was alternated. Activity rates refer to the combined MCN1-L and MCN1-R rates. To mimic the regulation of MCN1 activity by pyloric feedback, PD neuron activity was used to trigger pauses in MCN1 stimulation during pyloric-timed stimulations (right) without altering the firing rate between these pauses (see methods). The “pyloric-timed” stimulation rate refers to the rate between the PD-triggered pauses. D: example recordings demonstrate activity of pyloric rhythm generator (PD) and pattern generator [pyloric (PY), lateral pyloric (LP)] neurons, gastric mill rhythm generator (LG) and pattern generator [dorsal gastric (DG)] neurons, as well as neurons that have activity linked to both the pyloric and gastric mill rhythms [medial gastric (MG), inferior cardiac (IC), ventricular dilator (VD)]. dgn, Dorsal gastric nerve; lgn, lateral gastric nerve; lpn, lateral pyloric nerve; lvn, lateral ventricular nerve; mgn, medial gastric nerve; pdn, pyloric dilator nerve; pyn, pyloric nerve; son, superior [o]esophageal nerve; stn, stomatogastric nerve; Pro, gastric mill rhythm protraction phase; Ret, gastric mill rhythm retraction phase.
Solutions.
C. borealis saline consisted of (in mM) 440 NaCl, 26 MgCl2, 13 CaCl2, 11 KCl, 10 Trizma base, and 5 maleic acid (pH 7.4–7.6).
Electrophysiology.
Custom-made extracellular electrodes were used to obtain recordings of motor and connecting nerves. For each recording one stainless steel wire of each pair was placed alongside a nerve and isolated from the main bath with Vaseline, while the other wire was placed in the main bath compartment. Extracellular signals were filtered and amplified with differential AC amplifiers (model 1700, A-M Systems), digitized at ∼5 kHz with a Micro 1401 data acquisition interface (Cambridge Electronic Design), and recorded with Spike2 software (v7, Cambridge Electronic Design) on a personal computer (Dell).
MCN1 left and right were activated through extracellular stimulation of their axon in the inferior [o]esophageal nerve (ion) (Fig. 1). MCN1 and modulatory commissural neuron 5 (MCN5) are the only two neurons projecting from the CoG to the STG through the ion. They have distinct effects on STG neurons, with the MCN1 voltage threshold typically lower than that of MCN5 (Bartos and Nusbaum 1997; Coleman et al. 1995; Norris et al. 1996). The voltage threshold of MCN1 left and right was independently determined in each preparation, and any preparation in which each MCN1 could not be stimulated in the absence of MCN5 was not used in this study.
Stimulations were performed with a Grass S88 and SIU5 stimulus isolation units (Natus Neurology-Grass Products). Stimulation of MCN1 left and right alternated with evenly spaced interstimulus intervals at 0.5 times the desired frequency (e.g., Fig. 1C). MCN1 was stimulated [1 ms, 5–35 Hz (2.5–17.5 Hz each MCN)] tonically or with pyloric-timed interruptions in the tonic stimulations, without otherwise altering the stimulation rate (Fig. 1C). Tonic stimulation refers to constant interstimulus intervals throughout the stimulation, whereas for pyloric-timed stimulations periodic pauses in MCN1 stimulation were triggered by bursts of activity in the pyloric dilator (PD) neurons (Fig. 1C) (Wood et al. 2004). This replicates the pauses in MCN1 activity elicited by rhythmic feedback from the pyloric pacemaker, anterior burster (AB) neuron (Blitz and Nusbaum 2008, 2012; Coleman and Nusbaum 1994). The AB and PD neurons fire together because of electrical coupling, and PD is a more readily recorded monitor of AB feedback timing (Blitz and Nusbaum 2008; Eisen and Marder 1982). Thus PD was used for timing interruptions in the MCN1 stimulations (Wood et al. 2004). PD burst detection and feedback to the MCN1 stimulation was performed with Spike2 software and a custom-written script (freely available at http://stg.rutgers.edu/resources/). Except where otherwise indicated, the reported MCN1 stimulation rate refers to the total frequency of MCN1 left plus right for tonic stimulations and the frequency of MCN1 left plus right between the PD-timed interruptions for pyloric-timed stimulations (Fig. 1C). In 20 of 23 experiments, a single tonic stimulation at each stimulation rate was bracketed by pyloric-timed stimulations at that rate. The pyloric-timed stimulation before or after the tonic stimulation was chosen at random for analysis. In the remaining 3 of 23 experiments, the order of a single tonic and a single pyloric stimulation at each rate was randomized. In all experiments, the order of stimulation rates was randomized.
Data analysis.
To determine whether a pyloric rhythm was activated by MCN1, we assayed whether regular triphasic alternation of the PD, lateral pyloric (LP), and pyloric (PY) neurons (e.g., Fig. 1) was elicited within 100 s of stimulation (Soofi et al. 2014). The gastric mill rhythm is defined as 1:1 alternation between the lateral gastric (LG) and dorsal gastric (DG) neurons (e.g., Fig. 1) (Heinzel et al. 1993; Marder and Bucher 2007; Norris et al. 1994). The criterion we used for gastric mill rhythm activation was whether a minimum of 10 consecutive cycles of 1:1 LG/DG alternation occurred, with an onset prior to 200 s of stimulation.
For quantification of pyloric and gastric mill rhythms, we used a length of activity that included 10 consecutive 1:1 alternating LG/DG gastric mill cycles and analyzed multiple parameters of gastric mill, pyloric, and gastro-pyloric neuron activity across this time period. Data were analyzed with custom-written scripts (available at http://stg.rutgers.edu/resources/) for use with Spike2 software. Although all gastric mill rhythms used for analysis began prior to 200 s of stimulation, because of the variable time at which a stable 1:1 alternating gastric mill rhythm was established and the large range of cycle periods, the time frame of the 10 consecutive gastric mill cycles varied across stimulations and preparations. However, we verified that the time of the stimulation did not impact the measured parameter. For each parameter measured for each neuron, the time of analysis relative to the onset of the stimulation accounted for <10% of the variance in the response (r2: 2.070 × 10−4-0.096, Pearson correlation; n = 2–21 for each parameter). Thus all data were grouped based on the stimulation type (with vs. without feedback) and the rate (tonic/between pyloric-timed interruptions; Fig. 1C), regardless of time after stimulation for the onset of analysis.
The MCN1-modulated pyloric rhythm is distinct during the two phases of the gastric mill rhythm (Bartos and Nusbaum 1997). These phases are protraction, during the LG burst, and retraction, during the LG interburst (Fig. 1D) (Diehl et al. 2013; Heinzel et al. 1993; Norris et al. 1994). Because of LG presynaptic inhibition of the MCN1 terminals, there is a reduction in MCN1 excitation to the pyloric network during each protraction phase (Fig. 1B) (Bartos and Nusbaum 1997; Coleman et al. 1995; Coleman and Nusbaum 1994; Nusbaum et al. 1992). Therefore, pyloric neuron activity was analyzed separately for pyloric cycles occurring during protraction and retraction. Some neurons are active in time with both the pyloric and gastric mill networks (Weimann et al. 1991). These neurons fire rhythmic bursts of action potentials timed to the pyloric rhythm, but primarily during one phase of the gastric mill rhythm (i.e., protraction or retraction). These neurons are silent or fire occasional action potentials during the other phase of the gastric mill rhythm. For these neurons, we analyzed their pyloric-timed activity only during the gastric mill phase in which they are primarily active [protraction: inferior cardiac (IC), medial gastric (MG); retraction: ventricular dilator (VD)] (Beenhakker and Nusbaum 2004; Norris et al. 1994) (e.g., Fig. 1).
Rhythm cycle period is defined as the duration from the start of a PD (pyloric) or LG (gastric mill) neuron burst to the start of the subsequent PD or LG neuron burst. For most neurons examined, burst duration (time from the first action potential to the last action potential in a burst), number of action potentials (spikes) per burst, and firing rate (number of spikes minus 1 divided by burst duration) were quantified. Most neurons analyzed in this study occur as a single copy, and their activity can be readily analyzed from extracellular recordings. As there are two PD neurons that cannot be analyzed individually from extracellular recordings, only burst duration of PD neurons was analyzed.
To determine the burst structure of the LG neuron, we quantified the percentage of LG action potentials per bin (20 bins per pyloric cycle) out of the total number of LG action potentials that occurred in each pyloric cycle. Each pyloric cycle was normalized to the duration from onset of a PD burst to onset of the subsequent PD burst. For each MCN1 stimulation, this analysis was performed for 10 LG bursts. The PD duty cycle (% of cycle in which PD neurons were active) and LG action potential timing were analyzed across all pyloric cycles during these 10 LG bursts. We summed the percentage of LG action potentials across the first 35% of the pyloric cycle (bins 1–7) in order to compare the extent of pyloric timing between different MCN1 stimulations. This duration is based on the previously measured time frame of pyloric-timed pauses in MCN1 and LG activity (Blitz et al. 2008; Blitz and Nusbaum 2012; White and Nusbaum 2011).
Figures were prepared with Spike2 (Cambridge Electronic Design), SigmaPlot (v13.0, SYSTAT Software), and CorelDraw (v16, Corel) software. In Fig. 3, an extracellular recording was duplicated with a single unit digitally removed with a custom-written script in Spike2. Manual inspection was used to ensure that only the single unit was removed. Pearson correlation (SigmaPlot) was used to examine correlations of network output parameters and analysis time windows or MCN1 activity rate. To compare correlation coefficients, Fisher r-to-z transformation was performed (http://vassarstats.net/rdiff.html). Binomial regression (R v3.1.2, R Core Team 2015) was used to assay dependence of rhythm activation on MCN1 activity rate and feedback effects on MCN1. Two-way within-subject analyses of variance (ANOVAs) were performed to investigate the influence of the feedback effects on MCN1 and the MCN1 activity rate on network output parameters with PROC MIXED in SAS for Windows version 9.4. Effects were considered to be significant at P < 0.05. Data are expressed as means ± SE.
Fig. 3.
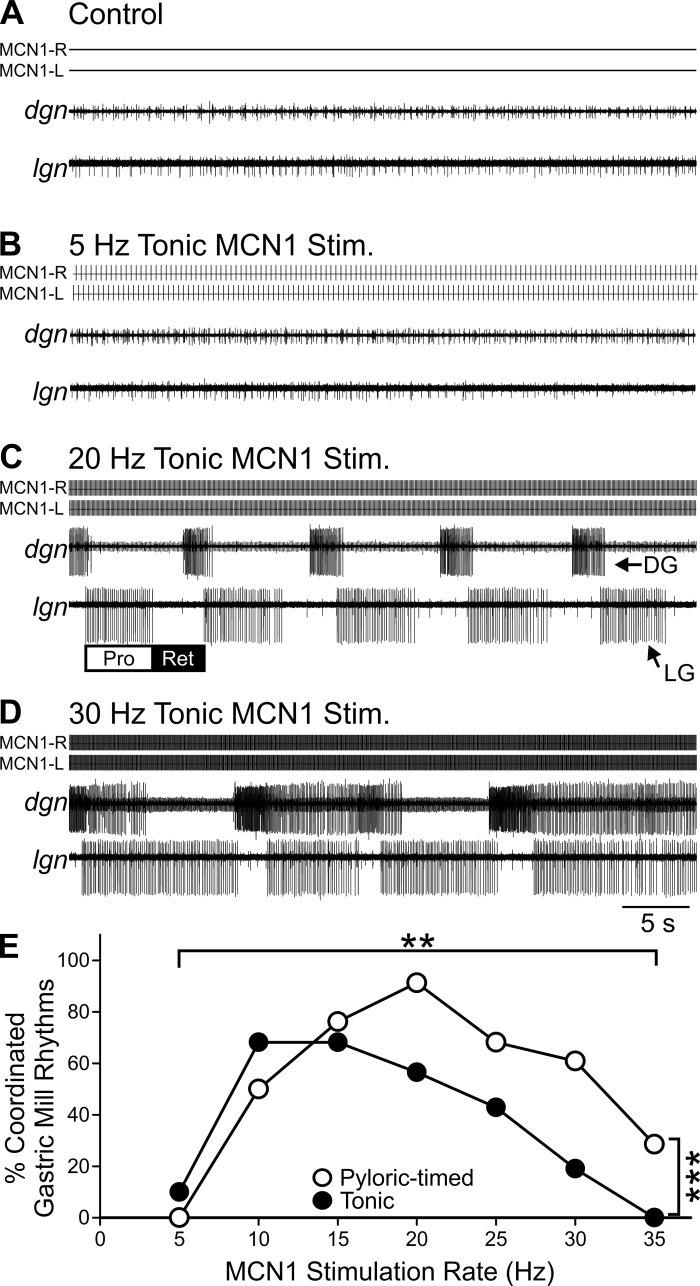
MCN1 stimulation elicits a gastric mill rhythm over a limited range of activity rates for both tonic and pyloric-timed stimulations. A: in control conditions, the gastric mill rhythm was always silent. B and C: in the example traces shown, 5-Hz tonic MCN1 stimulation (B) did not activate gastric mill neurons, while 20-Hz tonic MCN1 stimulation (C) elicited a coordinated gastric mill rhythm in which there was regular 1:1 alternating LG and DG activity. D: a 30-Hz tonic MCN1 stimulation elicited bursting in LG and DG that did not consistently alternate in a 1-for-1 manner. E: cumulative data illustrate that the middle of the MCN1 physiological range was most effective at activating a coordinated gastric mill rhythm. However, 10- to 15-Hz MCN1 stimulation elicited the highest percentage of coordinated gastric mill rhythms among tonic stimulations, while 20-Hz MCN1 stimulation elicited the highest % coordinated rhythms among pyloric-timed stimulations (n = 6–23). **P < 0.01, ***P < 0.001.
RESULTS
The pyloric CPG is pacemaker driven with a cycle period in vivo and in vitro of ∼1–2.5 s (Clemens et al. 1998; Hamood and Marder 2015a; Hedrich et al. 2011). The AB neuron is a pacemaker and thus rhythm generator neuron for the pyloric network (Marder and Bucher 2007). Because of strong electrical coupling, the PD neurons are active in time with AB and are part of the pacemaker ensemble for the pyloric rhythm (Fig. 1) (Eisen and Marder 1982). As the PD neurons are motor neurons, they are readily recorded in a peripheral nerve and were used throughout this study to monitor the rhythm generator component of the pyloric network.
In contrast to the pyloric CPG, the gastric mill CPG is network driven with a cycle period of 10–50 s (Blitz et al. 2008; Clemens et al. 1998; Diehl et al. 2013; Hamood and Marder 2015b). The core rhythm generator consists of reciprocal inhibition between the LG neuron and interneuron 1 (Int1) (Marder and Bucher 2007; White and Nusbaum 2011). Throughout this study, we used the LG motor neuron as a monitor of rhythm generation for the gastric mill network.
We also examined the sensitivity of network neurons that are not required for generation of the rhythm but contribute to other aspects of the motor pattern (i.e., pattern generator neurons), including a pyloric neuron (LP), a gastric mill neuron (DG), and three neurons considered gastro-pyloric because of both pyloric and gastric mill components of their activity (Weimann et al. 1991), the MG, IC, and VD neurons (Fig. 1D).
To examine the effects of only the MCN1 modulatory input in the absence of other modulatory inputs, we eliminated activity in the CoGs by replacing the saline in wells around the CoGs with isotonic (750 mM) sucrose (Fig. 1A) (Russell 1979). Under these conditions, the gastric mill rhythm remained off and the pyloric rhythm slowed or occasionally stopped completely as is typical for these networks with descending inputs removed (Hamood et al. 2015; Marder and Bucher 2007; Zhang et al. 2009). Thus the baseline conditions for all MCN1 stimulations consisted of the absence of a gastric mill rhythm and either a slow or an absent pyloric rhythm.
MCN1 can be activated in vitro and in vivo from ∼5 to 35 Hz and with different activity patterns (Beenhakker and Nusbaum 2004; Blitz et al. 2004, 2008; Christie et al. 2004; Hedrich et al. 2009, 2011). One means by which the MCN1 activity pattern is controlled is through regulation of CPG feedback (Blitz 2015; Blitz and Nusbaum 2008, 2012). For instance, inhibitory feedback from the pyloric pacemaker AB neuron can time-lock MCN1 activity to ongoing pyloric activity (i.e., pyloric timed) (Fig. 1). Eliminating the AB feedback synapse eliminates the pyloric-timed gaps in MCN1 activity but does not otherwise alter MCN1 interspike intervals. That is, interspike intervals in the absence of AB feedback are the same as those occurring between the pyloric-timed gaps with AB feedback (Blitz et al. 2008; Blitz and Nusbaum 2012; DM Blitz, unpublished observation). Thus, to compare the impact of MCN1 activity without vs. with network feedback, we used “tonic” MCN1 stimulations (constant interstimulus interval, 5–35 Hz) or “pyloric-timed” stimulations (periodic interruptions time-locked to AB/PD activity with all other interstimulus intervals matched to the comparable tonic stimulation; see methods) (Fig. 1C) and examined responses of the pyloric and gastric mill networks across this activity range of the modulatory projection neuron MCN1.
Activation.
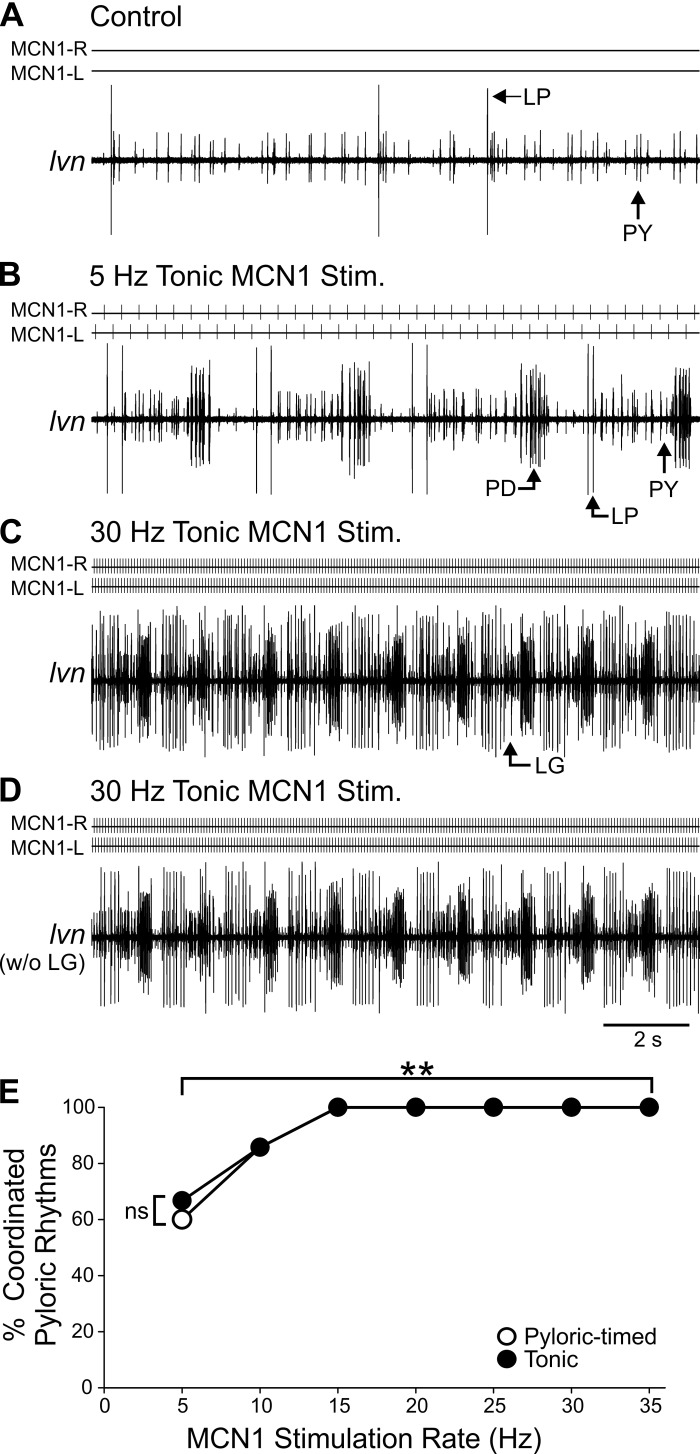
In the majority of preparations, after inputs from the CoGs were inactivated the pyloric cycle period increased (n = 16/23). However, in a subset of preparations the pyloric rhythm turned off in baseline conditions (n = 7/23). A complete pyloric rhythm is defined as triphasic bursting of the PD, LP, and PY neurons (Fig. 1) (Marder and Bucher 2007). Qualitatively, MCN1 modulatory actions on the pyloric network are evident within seconds of stimulation (Bartos et al. 1999; Coleman and Nusbaum 1994; Stein et al. 2007). We determined whether tonic or pyloric-timed MCN1 stimulation at frequencies of 5–35 Hz elicited a regular triphasic pyloric rhythm within 100 s of stimulation in preparations in which the pyloric rhythm was inactive at baseline: 5-Hz MCN1 pyloric-timed stimulations elicited a complete pyloric rhythm in three of five (60%) preparations, while tonic 5-Hz MCN1 stimulations did so in four of six (67%) preparations tested (Fig. 2). Above 5 Hz, however, there was only one experiment in which a complete pyloric rhythm was not activated in response to MCN1 stimulation at 10 Hz [tonic and pyloric timed; n = 6/7 (86%)], while 15–35 Hz activated complete pyloric rhythms in all preparations [15 Hz pyloric timed: n = 6/6 (100%); 35 Hz: n = 2/2 (100%); all others n = 7/7 (100%)] (Fig. 2). Thus pyloric CPG activation was influenced by MCN1 activity rate (df = 6, χ2 = 17.33, P = 0.008; binomial regression) but not by the effects of network feedback on MCN1 activity (df = 1, χ2 = 0.03, P = 0.860) or the interaction of rate and feedback effects (df = 6, χ2 = 0.02, P = 1.00).
Fig. 2.
MCN1 activates a pyloric rhythm across a large range of activity rates. A: in a subset of preparations, the pyloric rhythm was off in control conditions. B and C: in the example shown, a coordinated pyloric rhythm with regular triphasic (PD, LP, PY) activity was elicited by 5-Hz (B) and 30-Hz (C) tonic MCN1 stimulation. D: same recording as in C is shown with the gastric mill neuron, LG, digitally removed (see methods). E: cumulative data indicate that 5- and 10-Hz MCN1 activity rates activated a coordinated pyloric rhythm in some preparations, but 15-Hz and higher stimulation rates activated a coordinated pyloric rhythm in all preparations regardless of tonic or pyloric-timed MCN1 activation (15 Hz pyloric timed: n = 6; 35 Hz: n = 2; all others n = 7). **P < 0.01. ns, Nonsignificant.
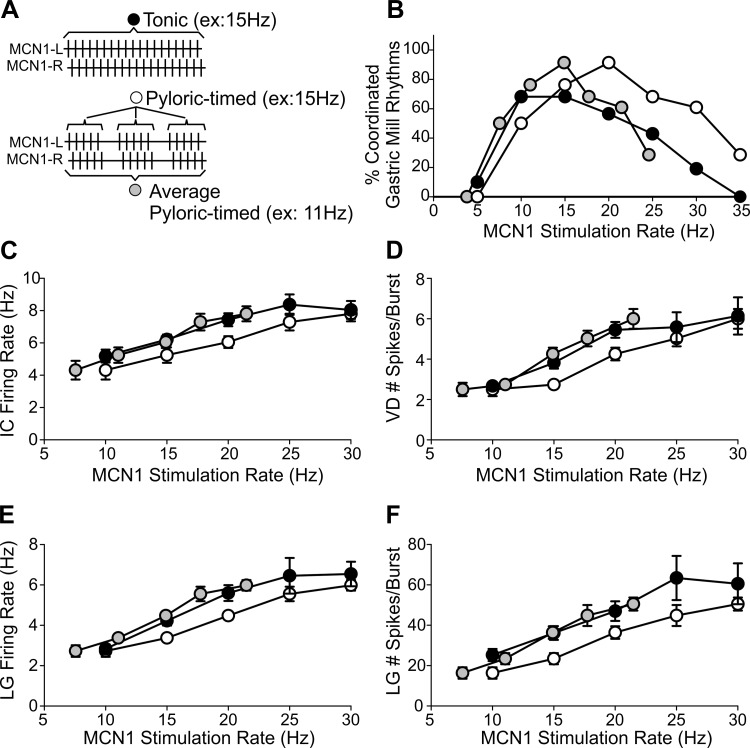
We next asked whether the distinct cellular mechanisms of rhythm generation in the gastric mill network resulted in a different activation range. Unlike the pyloric rhythm, the gastric mill rhythm is network driven and produces oscillations at a 10-fold-slower frequency. A gastric mill rhythm consists of alternating bursts in the LG and DG motor neurons, controlling the lateral and medial teeth inside the foregut (Heinzel et al. 1993; Marder and Bucher 2007). We defined MCN1 activation of the gastric mill rhythm as 10 consecutive alternating (1:1) LG and DG bursts that initiated within the first 200 s of MCN1 stimulation (see methods). Unlike the pyloric CPG, in most preparations 5-Hz MCN1 stimulation was unable to activate a gastric mill rhythm [pyloric timed: n = 0/8 (0%); tonic: n = 1/10 (10%)] (Fig. 3). Further supporting this distinction between the pyloric and gastric mill networks, in an additional 18 preparations there was no activation of the gastric mill rhythm during 5-Hz stimulations lasting at least 100 s. Faster MCN1 firing rates, however, did activate regular gastric mill rhythms. For instance, at 10 Hz a regular gastric mill rhythm was elicited in 15/22 (68%) preparations in response to tonic stimulation and in 10/20 (50%) with pyloric-timed stimulations. We found that the range over which MCN1 stimulations were most effective at eliciting a gastric mill rhythm differed for pyloric-timed vs. tonic stimulations. Tonic stimulations elicited the highest percentage of regular gastric mill rhythms at 10 and 15 Hz [n = 15/22 (68%)], while pyloric-timed stimulations elicited the highest percentage of regular gastric mill rhythms at 20 Hz [n = 21/23 (91%)]. At higher MCN1 stimulation rates, although the LG and DG neurons were activated they often did not maintain a regular 1:1 alternation (Fig. 3D). At these high rates, regardless of whether feedback was regulating MCN1 activity, stimulations were not very effective at eliciting a regular gastric mill rhythm [30 Hz: pyloric timed n = 14/23 (61%), tonic n = 4/21 (19%); 35 Hz: pyloric timed n = 2/7 (39%); tonic n = 0/6 (0%)]. Unlike the pyloric CPG, in which its activation was only sensitive to MCN1 rate, the gastric mill CPG was sensitive to both MCN1 activity rate (df = 6, χ2 = 45.55, P = 3.63 × 10−8; binomial regression) and the effects of network feedback on MCN1 activity (df = 1, χ2 = 9.08, P = 0.003), as well as the interaction between rate and feedback effects (df = 6, χ2 = 15.55, P = 0.016) (Fig. 3E). This indicates that for gastric mill CPG activation but not pyloric CPG activation the influence of network feedback, through its regulation of projection neuron activity, differs depending on the modulatory neuron activity rate. Additionally, the ability of MCN1 to activate the pyloric vs. the gastric mill CPG differed for tonic stimulations (H = 16.495, df = 3, P = 0.009; Kruskal-Wallis 1-way ANOVA on ranks, Tukey post hoc) but not for pyloric-timed stimulations (H = 16.495, df = 3, P = 0.079).
Rhythm generator neurons.
Within the activation range for both networks, we sought to determine whether there were similarities or differences in how parameters of network output were regulated by the presence vs. absence of network feedback to MCN1 across a range of MCN1 activity rates. Because of the low number of regular gastric mill rhythms at 5 and 35 Hz, we restricted our analysis to MCN1 stimulations from 10 to 30 Hz and stimulations that activated both the pyloric and gastric mill rhythms. During an ongoing gastric mill rhythm, presynaptic inhibition from LG onto the terminals of MCN1 (Fig. 1) rhythmically decreases MCN1 transmitter release, thereby decreasing the MCN1 modulation of the pyloric rhythm. As a result, pyloric CPG activity differs during the two phases of the gastric mill rhythm (i.e., protraction vs. retraction; Fig. 1) (Bartos and Nusbaum 1997). Thus for pyloric cycle period and other measures of the pyloric rhythm, we separated our analyses into protraction and retraction phases (see methods).
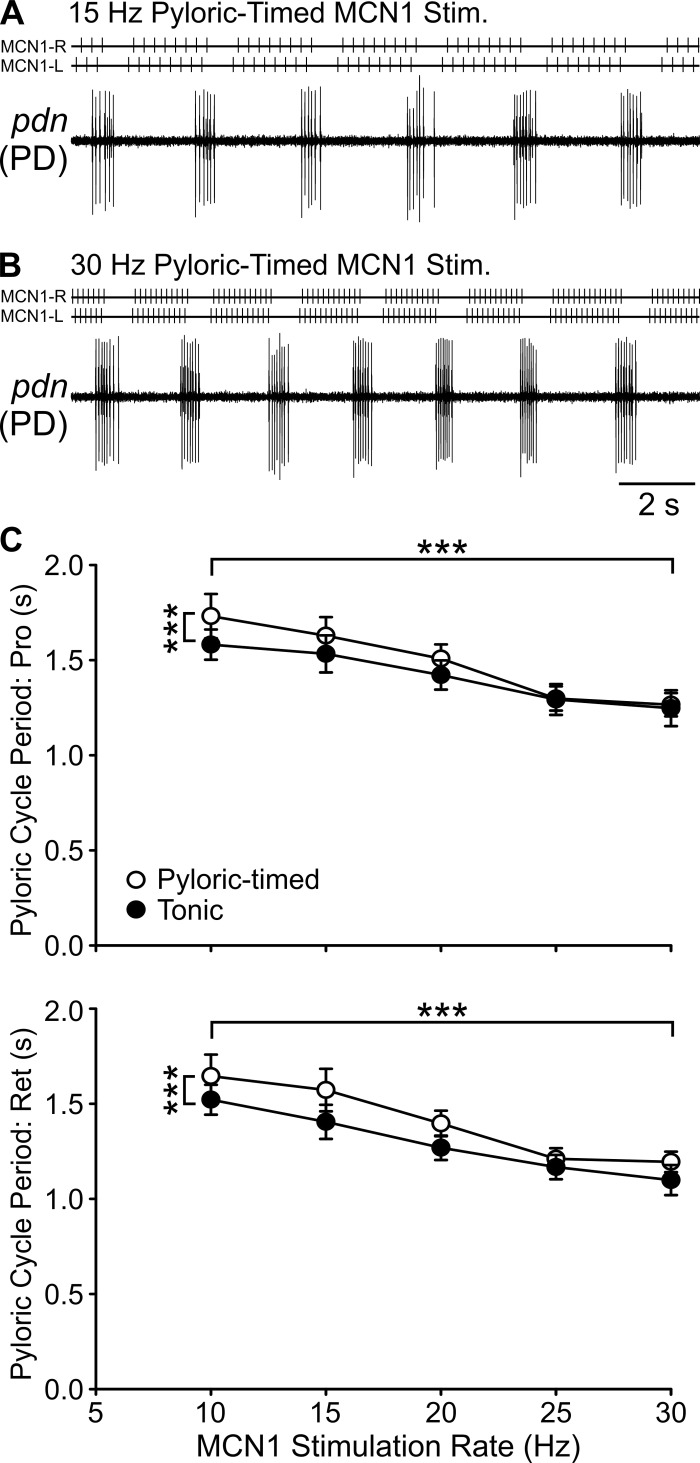
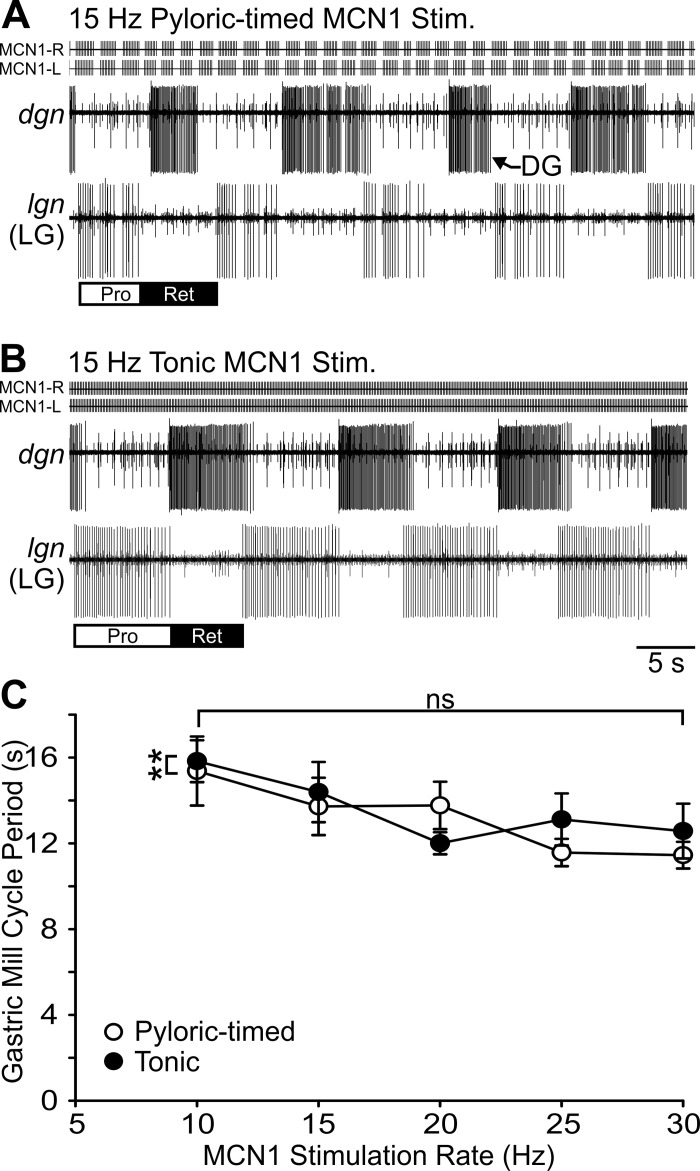
We first examined cycle period, the average duration of a single cycle of each rhythm. In the example traces shown in Fig. 4, the pyloric cycle period was shorter in response to a 30-Hz pyloric-timed MCN1 stimulation compared with a 15-Hz pyloric-timed stimulation. During both protraction and retraction phases of the gastric mill rhythm, for both tonic and pyloric-timed stimulations, pyloric cycle period decreased with increasing MCN1 stimulation rate (Fig. 4) (protraction: F4,105 = 24.87, P < 0.0001; retraction: F4,105 = 27.78, P < 0.0001; 2-way within-subject ANOVA). There was also a difference in cycle period between pyloric-timed and tonic stimulation (protraction: F1,105 = 12.90, P = 0.0005; retraction: F1,105 = 23.29, P < 0.0001). There was, however, no interaction between MCN1 activity rate and the effects of network feedback (protraction: F4,105 = 0.10, P = 0.9819; retraction: F4,105 = 0.30, P = 0.8790).
Fig. 4.
Pyloric cycle period decreases with increasing MCN1 activity rate. A and B: pyloric cycle period was shorter in response to 30-Hz pyloric-timed stimulation than to 15-Hz pyloric-timed MCN1 stimulation. C: pyloric cycle period is plotted as a function of MCN1 activity rate for pyloric-timed and tonic stimulations during the protraction (top) and retraction (bottom) phases of gastric mill rhythms (n = 4–21). ***P < 0.001.
Unlike the pyloric cycle period, the gastric mill cycle period was only regulated by the influence of feedback on MCN1 activity. For instance, the gastric mill cycle period was shorter in response to a 15-Hz pyloric-timed MCN1 stimulation (Fig. 5A) than a 15-Hz tonic MCN1 stimulation (Fig. 5B). The population data indicate a difference in gastric mill cycle period during pyloric-timed compared with tonic stimulations (Fig. 5C) (F1,100 = 7.22, P = 0.0084; 2-way within-subject ANOVA); however, there was no significant effect of MCN1 stimulation rate on gastric mill cycle period (F4,100 = 1.92, P = 0.1135). Although in the average data it appears there was a dependence of gastric mill cycle period on MCN1 stimulation rate, there was no consistent relationship in individual experiments. There was also no interaction of MCN1 rate with the feedback regulation of MCN1 activity in determining gastric mill cycle period (F4,100 = 1.24, P = 0.2981). Thus another distinction between the pyloric and gastric mill networks is that MCN1 activity rate regulates pyloric cycle period but not gastric mill cycle period.
Fig. 5.
Gastric mill cycle period is sensitive only to the effects of network feedback on MCN1 activity. A and B: during MCN1 stimulation a gastric mill rhythm is evident in the 1:1 alternating bursting of the DG and LG neurons. The gastric mill cycle period was shorter during 15-Hz pyloric-timed (A) than 15-Hz tonic (B) MCN1 stimulation. Across preparations cycle period was shorter during pyloric-timed than tonic stimulation. C: although on average across preparations gastric mill cycle periods are shorter at higher MCN1 activity rates, within individual experiments this relationship was not evident and thus gastric mill cycle period was not dependent upon MCN1 activity rate (n = 4–20). **P < 0.01. ns, Nonsignificant.
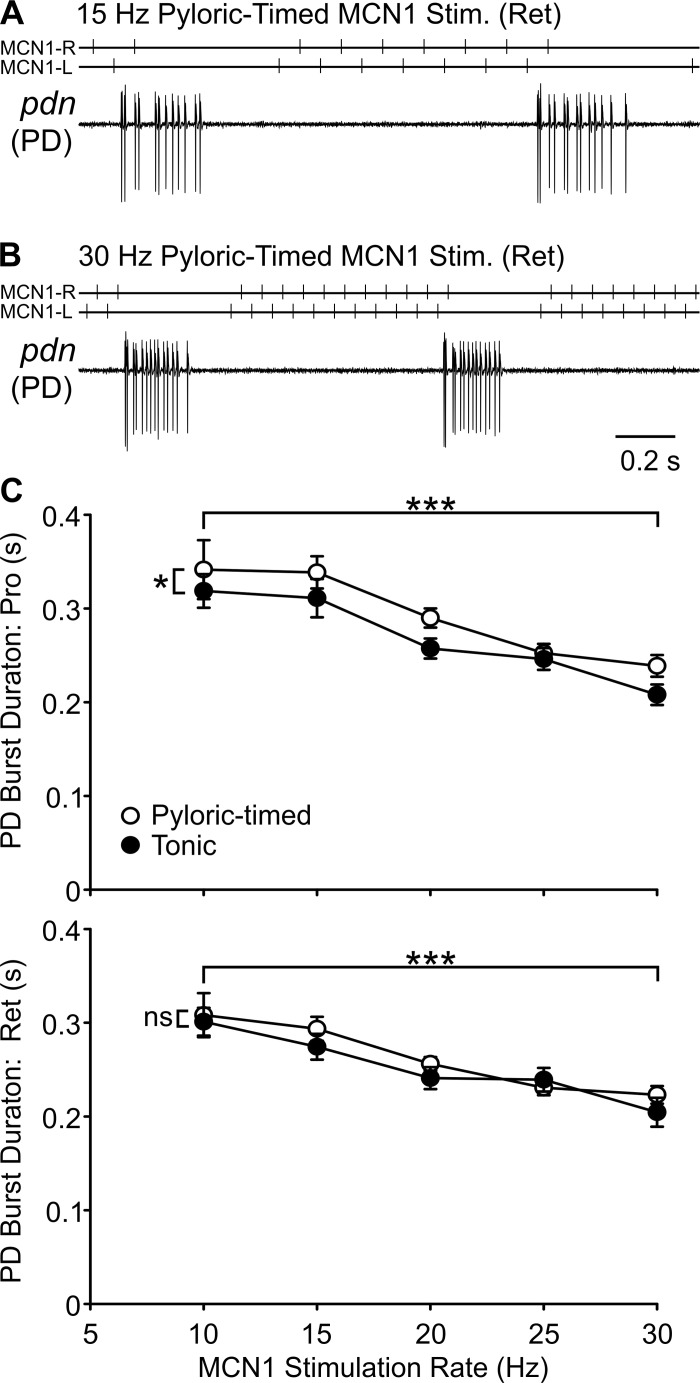
The durations of rhythm generator neuron bursts are important, as they provide electrical and chemical synaptic input to other rhythm generator as well as pattern generator neurons. As evident in example traces (Fig. 6, A and B) and average data (Fig. 6C), the PD burst duration decreased with increasing MCN1 stimulation rate during both the protraction (n = 4–21, P < 0.0001; Table 1) and retraction (n = 4–21, P < 0.0001; Table 1) phases of the gastric mill rhythm. PD burst duration was also longer during pyloric-timed stimulations than tonic stimulations, but only during the protraction phase (protraction: P = 0.0116, retraction: P = 0.0598; Table 1). The manner in which network feedback effects on MCN1 altered PD burst duration during protraction were not dependent on MCN1 rate (P = 0.8276; Table 1).
Fig. 6.
Burst duration of pyloric rhythm generator neuron PD decreases with increasing MCN1 stimulation rate. Example traces display the activity of the two PD neurons recorded extracellularly in the pdn. At 30 Hz (B), the PD burst duration was shorter than at 15 Hz (A) MCN1 pyloric-timed stimulation. C: across preparations, there was a decrease in PD burst duration with increasing MCN1 activity rate for both tonic and pyloric-timed stimulations, during both protraction (top) and retraction (bottom) phases of the gastric mill rhythm. During the protraction phase of the gastric mill rhythm, the burst duration was also longer during pyloric-timed than tonic stimulation (n = 4–21). *P < 0.05, ***P < 0.001. ns, Nonsignificant.
Table 1.
Network neuron activity parameters in response to MCN1 activity with or without feedback across a range of activity rates
| Burst Duration |
Spikes per Burst |
Firing Rate |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neurons | F | df | P | F | df | P | F | df | P | |
| Pyloric | ||||||||||
| PD: Pro | Rate | 19.64 | 4,105 | <0.0001 | ND | ND | ||||
| Feedback* | 6.59 | 1,105 | 0.0116 | |||||||
| Interaction | 0.37 | 4,105 | 0.8276 | |||||||
| PD: Ret | Rate | 24.40 | 4,105 | <0.0001 | ND | ND | ||||
| Feedback* | 2.05 | 1,105 | 0.0598 | |||||||
| Interaction | 0.93 | 4,105 | 0.4495 | |||||||
| LP: Pro | Rate | 0.36 | 4,71 | 0.8355 | 11.49 | 4,71 | <0.0001 | 42.56 | 4,71 | <0.0001 |
| Feedback* | 0.49 | 1,71 | 0.4873 | 5.86 | 1,71 | 0.0182 | 11.08 | 1,71 | 0.0014 | |
| Interaction | 0.17 | 4,71 | 0.9509 | 0.59 | 4,71 | 0.6690 | 0.74 | 4,71 | 0.5699 | |
| LP: Ret | Rate | 3.75 | 4,71 | 0.0080 | 7.28 | 4,71 | <0.0001 | 76.32 | 4,71 | <0.0001 |
| Feedback* | 0.09 | 1,71 | 0.7603 | 7.12 | 1,71 | 0.0094 | 23.94 | 1,71 | <0.0001 | |
| Interaction | 0.83 | 4,71 | 0.5116 | 0.08 | 4,71 | 0.9884 | 1.18 | 4,71 | 0.3291 | |
| Gastro-pyloric | ||||||||||
| IC | Rate | 0.71 | 4,80 | 0.5893 | 8.15 | 4,80 | <0.0001 | 28.04 | 4,80 | <0.0001 |
| Feedback* | 10.01 | 1,80 | 0.0022 | 48.02 | 1,80 | <0.0001 | 31.55 | 1,80 | <0.0001 | |
| Interaction | 0.89 | 4,80 | 0.4712 | 1.64 | 4,80 | 0.1727 | 0.69 | 4,80 | 0.6025 | |
| VD | Rate | 29.95 | 4,88 | <0.0001 | 57.44 | 4,88 | <0.0001 | 82.78 | 4,88 | <0.0001 |
| Feedback* | 26.96 | 1,88 | <0.0001 | 22.76 | 1,88 | <0.0001 | 7.22 | 1,88 | 0.0086 | |
| Interaction | 1.77 | 4,88 | 0.1421 | 2.36 | 4,88 | 0.0594 | 3.04 | 4,88 | 0.0213 | |
| MG | Rate | 0.63 | 4,25 | 0.6468 | 6.81 | 4,25 | 0.0008 | 17.26 | 4,25 | <0.0001 |
| Feedback* | 3.17 | 1,25 | 0.0870 | 8.24 | 1,25 | 0.0082 | 5.20 | 1,25 | 0.0314 | |
| Interaction | 0.45 | 4,25 | 0.7712 | 0.73 | 4,25 | 0.5806 | 0.83 | 4,25 | 0.5164 | |
| Gastric mill | ||||||||||
| LG | Rate | 12.21 | 4,100 | <0.0001 | 66.38 | 4,100 | <0.0001 | 90.41 | 4,100 | <0.0001 |
| Feedback* | 38.60 | 1,100 | <0.0001 | 79.60 | 1,100 | <0.0001 | 46.58 | 1,100 | <0.0001 | |
| Interaction | 1.66 | 4,100 | 0.1659 | 1.15 | 4,100 | 0.3394 | 1.97 | 4,100 | 0.1054 | |
| DG | Rate | 2.32 | 4,103 | 0.0616 | 4.64 | 4,103 | 0.0017 | 27.26 | 4,103 | <0.0001 |
| Feedback* | 9.82 | 1,103 | 0.0022 | 23.98 | 1,103 | <0.0001 | 9.20 | 1,103 | 0.0031 | |
| Interaction | 1.77 | 4,103 | 0.1404 | 2.47 | 4,103 | 0.0491 | 0.99 | 4,103 | 0.4162 | |
Two-way within-subject ANOVA (PD: n = 4–21; LP: n = 3–15; IC: n = 3–16; VD: n = 4–18; MG: n = 2–6; LG: n = 4–20; DG: n = 4–20). Pro, gastric mill protraction phase; Ret, gastric mill retraction phase; ND, not determined.
Comparing responses to MCN1 stimulation with feedback (pyloric timed) to without feedback (tonic) stimulations. Significant P values are in bold.
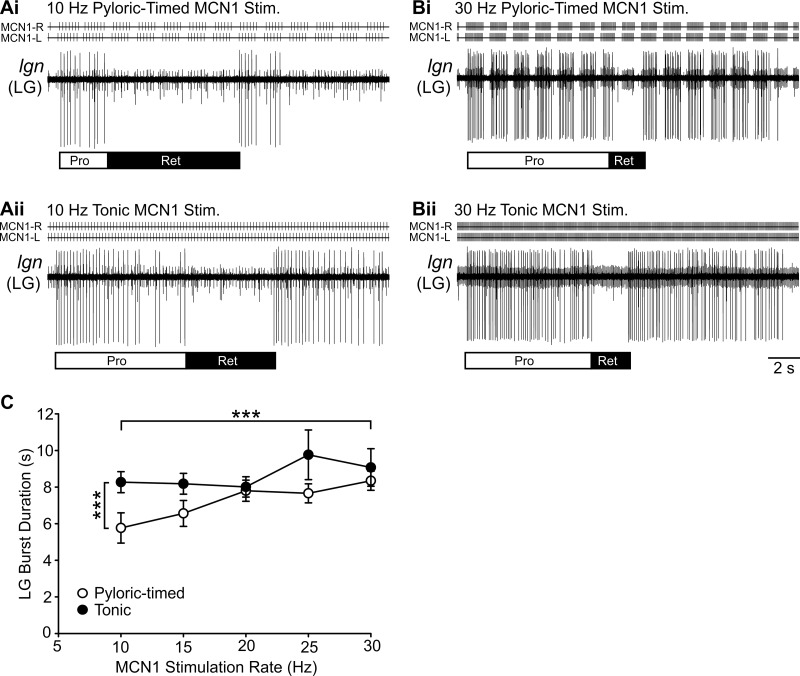
The burst duration of the gastric mill rhythm generator neuron LG was regulated by both MCN1 activity rate and feedback effects on MCN1 activity (n = 4–20, P < 0.0001; Table 1). For instance, at 10 Hz the LG burst duration was shorter during pyloric-timed stimulation than during tonic stimulation (Fig. 7A), but at 30 Hz LG burst duration was longer and was similar during pyloric-timed and tonic stimulation (Fig. 7B). However, there was no significant interaction between MCN1 activity rate and feedback effects on MCN1 in regulating LG burst duration (P = 0.1659; Table 1).
Fig. 7.
Burst duration of gastric mill rhythm generator neuron LG is regulated by MCN1 activity rate and the effects of network feedback on MCN1. A: a 10-Hz pyloric-timed MCN1 stimulation (Ai) elicited shorter LG neuron bursts than a 10-Hz tonic MCN1 stimulation (Aii). B: at 30 Hz, the LG burst duration was longer than at 10 Hz for both pyloric timed (Bi) and tonic (Bii) MCN1 stimulations. C: across multiple preparations, the LG bust duration was longer during tonic MCN1 stimulations than pyloric-timed stimulations and increased with higher MCN1 stimulation rates (n = 4–20). ***P < 0.001.
There was a positive correlation between gastric mill rhythm generator (LG) burst duration and the rate of pyloric-timed MCN1 stimulations (r = 0.313, P = 0.007; Pearson correlation) but no significant correlation for tonic stimulations (r = 0.152, P = 0.259). This is in contrast to the pyloric rhythm generator (PD) burst duration, which was negatively correlated with MCN1 stimulation rate in response to tonic and pyloric-timed stimulations (tonic: protraction r = −0.500, P = 4.8 × 10−5, retraction r = −0.501, P = 4.6 × 10−5; pyloric timed: protraction r = −0.551, P = 1.9 × 10−7, retraction r = −0.566, P = 8.1 × 10−9; Pearson correlation) (Fig. 4). The correlation coefficients for LG and PD burst durations were different for both pyloric-timed MCN1 stimulations (protraction: z = 5.68, P = 0; retraction: z = 5.77, P = 0; Fisher r-to-z transformation) and tonic MCN1 stimulations (protraction: z = 4.2, P = 0; retraction: z = 4.21, P = 0). Thus burst durations of rhythm generator neurons in the pyloric and gastric mill networks are distinctly regulated by MCN1 activity.
Pattern generator neurons.
We aimed to determine whether network neurons beyond those necessary for rhythm generation in the distinct pyloric and gastric mill networks were sensitive to how network feedback regulates MCN1 activity across the range of MCN1 activity rate. Neuronal activity levels (burst duration, number of action potentials per burst, and firing rate) of pattern generator neurons for both networks (pyloric: LP; gastric mill: DG) were measured. Additionally, we examined the activity of gastro-pyloric neurons (IC, VD, MG), which have activity linked to both the pyloric and gastric mill rhythms (Beenhakker and Nusbaum 2004; Weimann et al. 1991). Despite the 10-fold difference in their cycle periods, for pyloric and gastric mill network neurons as well as gastro-pyloric neurons many activity parameters were different in response to pyloric-timed compared with tonic MCN1 activity while some were sensitive to MCN1 rate only and some to only the effects of feedback on MCN1 activity (Table 1). With the exception of the firing rate of the VD neuron (P = 0.0213; Table 1) and the number of DG spikes per burst (P = 0.0491; Table 1), MCN1 activity rate did not interact with feedback effects on MCN1 to regulate network neuron activity (interaction P values 0.0594–0.9884; Table 1).
Network feedback effects on burst structure.
The impact of network feedback on MCN1 activity, that is, pyloric-timed stimulations, regulated many parameters in the pyloric and gastric mill networks differently than tonic MCN1 stimulations. However, most of the measured parameters were not influenced by interactions between feedback effects on MCN1 activity and the MCN1 activity rate (Table 1; see above).
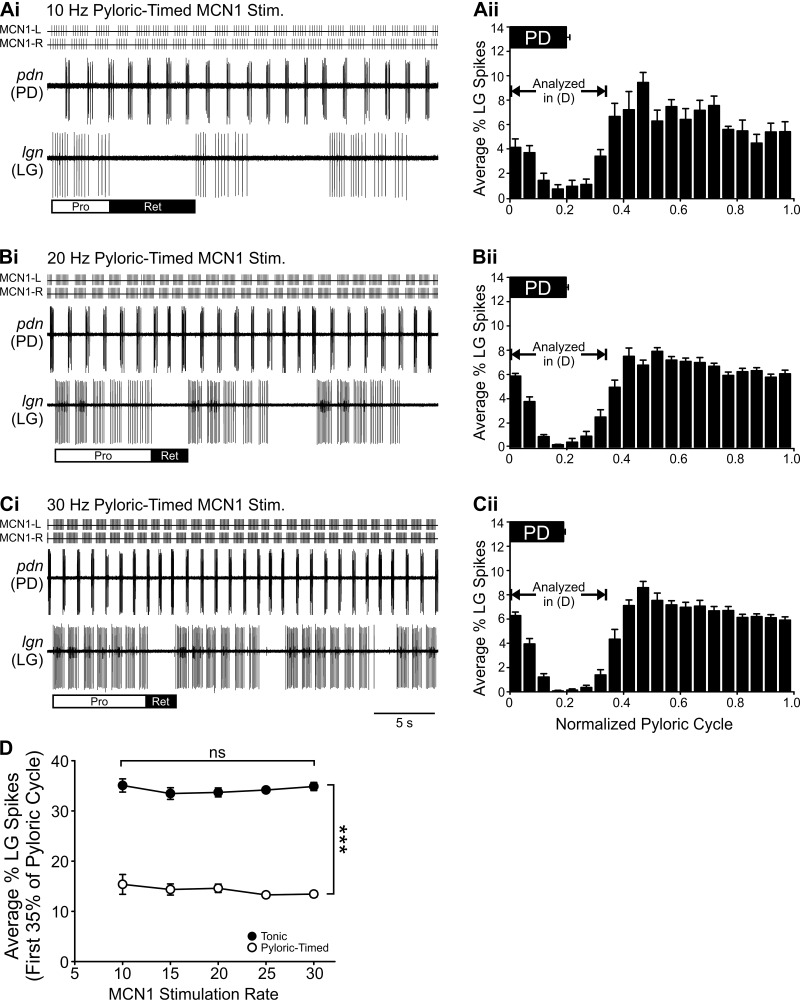
In addition to the parameters measured above, network feedback to MCN1 also has consequences for the gastric mill motor pattern in terms of the burst structure of the gastric mill neuron LG. Specifically, pyloric-timed MCN1 activity elicits pyloric-timed action potentials across the LG bursts (Blitz and Nusbaum 2012; White and Nusbaum 2011; Wood et al. 2004) (Fig. 5A; Fig. 7, A and B). MCN1 acts on LG via electrical coupling and activation of a modulator-activated inward current (IMI) by one of its peptide transmitters (Coleman et al. 1995; DeLong et al. 2009b; Stein et al. 2007). We hypothesized that because of the properties of these MCN1 synapses onto LG, the LG burst structure would have a different dependence on how feedback affected MCN1 activity at low vs. high MCN1 activity rates. For instance, at high MCN1 activity rates an increased modulator-activated current could maintain the LG membrane potential above action potential threshold for a longer duration after MCN1 action potentials paused and raise LG membrane potential above threshold more quickly when MCN1 resumed firing after a pyloric-timed gap. If so, these actions would serve to decrease the extent of the pyloric-timed gaps in LG at high MCN1 rates compared with those occurring during lower MCN1 activity rates.
Contrary to our hypothesis, recordings of LG in response to pyloric-timed MCN1 activity at three MCN1 activity rates (10 20, 30 Hz) suggest that, as with other parameters measured in this study, the impact of network feedback on MCN1 activity does not depend on MCN1 activity rate. Specifically, similar gaps in LG action potentials are evident in time with PD bursts at all three MCN1 activity rates (Fig. 8, Ai–Ci). To quantify the LG burst structure, we measured the distribution of LG spikes across a normalized pyloric cycle at each stimulation rate (see methods). A decrease in the average percentage of LG action potentials per bin is evident during and shortly after the PD burst (Fig. 8, Aii–Cii). The average percentage of LG action potentials during the initial 35% of the pyloric cycle (Blitz and Nusbaum 2012; see methods) was lower during pyloric-timed MCN1 stimulations compared with tonic stimulations (Fig. 8D) (F4,99 = 1,020.08, P < 0.0001; 2-way within-subject ANOVA). However, there was no difference in the degree of pyloric timing in the LG burst structure during pyloric-timed stimulations from 10 Hz to 30 Hz (Fig. 8) (F4,99 = 0.94, P = 0.4433). Thus the ability of pyloric-timed MCN1 activity to activate the gastric mill network depended on the MCN1 activity rate. However, for almost all other parameters quantifying the output of the pyloric and gastric mill networks in this study, the impact of network feedback, through its regulation of projection neuron activity, was consistent across the full physiological range of modulatory neuron activity rate.
Fig. 8.
Network neuron burst structure is regulated by feedback effects on MCN1 activity but not by MCN1 activity rate. Ai–Ci: in traces from an example experiment, rhythmic interruptions in the LG neuron bursts time-locked to the pyloric rhythm (PD: pdn) are evident during pyloric-timed MCN1 stimulation at 10 (Ai), 20 (Bi), and 30 (Ci) Hz. Aii–Cii: the LG burst structure was quantified by binning the timing of all LG action potentials during 10 gastric mill cycles in each condition relative to the pyloric cycle (normalized to 1.0; see methods). This analysis demonstrates that there is a smaller % of LG action potentials during and for a short period after the PD neuron burst at the 3 example MCN1 activity rates. D: the average % of LG action potentials occurring during the initial 35% of the pyloric cycle (Blitz and Nusbaum 2012; see methods) was greater during tonic stimulations than during pyloric-timed stimulations. However, there was no dependence of burst structure on MCN1 stimulation rate (n = 4–20). ***P < 0.001. ns, Nonsignificant.
Feedback regulation of MCN1 activity.
As discussed above, pyloric network feedback elicits pyloric-timed gaps in MCN1 activity but does not otherwise alter MCN1 interspike intervals (Blitz et al. 2008; Blitz and Nusbaum 2012; DM Blitz, unpublished observations). Thus we compared the effects of tonic stimulations to pyloric-timed stimulations that had the same interspike intervals except for pyloric-timed longer duration gaps (Fig. 1C; Fig. 9A), to replicate the effects of network feedback on MCN1 activity. This approach allowed identification of parameters that were sensitive to the influence of network feedback on MCN1 activity. However, because we examined network responses over the course of 10 gastric mill cycles, there were multiple pyloric-timed gaps per MCN1 stimulation. This resulted in network feedback not only altering the pattern of MCN1 action potentials but also lowering the average rate. In particular, for tonic MCN1 rates of 5, 10, 15, 20, 25, 30, and 35 Hz, the average pyloric-timed MCN1 rates (Fig. 9A) were 3.8, 7.6, 11.0, 14.9, 17.7, 21.4, and 23.9 Hz (n = 5–21). Therefore, pyloric-timed MCN1 activity might have different effects on network output than tonic MCN1 activity for multiple reasons: the neurons may be sensitive to the pattern of MCN1 activity, to the average MCN1 activity rate, or to both pattern and average rate.
Fig. 9.
Network neuron activity parameters that differ in response to tonic vs. pyloric-timed MCN1 activity may be sensitive to average MCN1 activity rate. A: the “pyloric-timed” MCN1 rate refers to the rate between the pyloric-timed gaps (open circle), while the “average pyloric-timed” rate (gray circle) is the rate throughout the stimulation, including the longer-duration gaps due to network feedback. Thus tonic (filled circle) and pyloric-timed MCN1 rates MCN1 are the same, while the average rates during pyloric-timed stimulations are lower than the comparable tonic stimulation. All stimulation rates are the cumulative rate of MCN1-Left and MCN1-Right alternating (see methods). B–F: when plotted against the tonic and pyloric-timed stimulation rates (filled and open circles, respectively), there is separation between the tonic and pyloric-timed data for the % of coordinated gastric mill rhythms elicited (B), IC firing rate (C), VD number of spikes per burst (D), and LG firing rate (E) and number of spikes per burst (F). When the data in response to pyloric-timed stimulations are instead plotted against the average pyloric-timed rate (gray circles), there is overlap with the tonic stimulation data (filled circles). n = 6–23 (B), 3–16 (C), 4–18 (D), 4–20 (E and F).
To examine whether there may be an influence of average rate we plotted the data for parameters that were distinct between tonic and pyloric-timed stimulations, i.e., distinct in response to MCN1 activity with vs. without network feedback in two ways. For five example parameters, when the data were plotted against tonic and pyloric-timed (i.e., excluding the pyloric-timed gaps, Fig. 9A) MCN1 rates, separation between the data sets was evident (Fig. 9, B–F). However, when data from pyloric-timed stimulations were instead plotted against average pyloric-timed rates (i.e., including the pyloric-timed gaps, Fig. 9A), the pyloric-timed plots aligned closely with the tonic stimulation plots (Fig. 9, B–F). The convergence of the curves when plotted against average rate suggests that network parameters are sensitive to the lower average activity rate elicited by network feedback, albeit without ruling out contributions from network feedback altering the MCN1 activity pattern. However, we did not perform statistical analyses because of the nonmatching data points, particularly at the extremes, when the pyloric-timed plots are shifted in this manner (Fig. 9). Furthermore, as pyloric-timed stimulations included changes in both activity pattern and average rate, dissecting the relative contributions of feedback effects on MCN1 pattern and rate in regulating network output awaits future study (see discussion). Yet, regardless of which feedback-elicited changes in MCN1 activity the network neurons are sensitive to, the control of modulatory neuron activity by network feedback has consequences for the output of distinct networks across a range of network modulation.
DISCUSSION
Activity of modulatory inputs can vary across a range of rates and patterns because of the type and strength of sensory input, interactions of intrinsic properties with inputs, and network feedback (Blitz et al. 2004; Blitz and Nusbaum 2008; Dubuc et al. 2008; Prisco et al. 1997; Rossignol et al. 2006; Velázquez-Ulloa et al. 2003). In this study, we used a well-characterized modulatory neuron to ask how network output is regulated across a physiological modulatory neuron activity range. Furthermore, we asked whether the changes in modulatory neuron activity due to network feedback have different effects on network output, depending on the level of modulatory neuron activity. We find that there are both similarities and distinctions in how network activation, frequency of rhythmic output, and activity levels of rhythm generator and pattern generator neurons in the distinct pyloric and gastric mill networks are controlled across a range of MCN1 activity. However, with a few exceptions such as activation of the gastric mill network, the consequences of network feedback shaping MCN1 activity are similar for network output regardless of the level of MCN1 activity.
Activity parameters of modulatory neurons.
MCN1 activity ranges from ∼5 to 35 Hz in response to chemosensory, proprioceptive, mechanosensory, and neuroendocrine inputs in vitro and in vivo (Beenhakker and Nusbaum 2004; Blitz et al. 2004, 2008; Christie et al. 2004; Hedrich et al. 2009, 2011). Yet it is not a simple translation from activity rate to transmitter release, as different transmitters may have distinct relationships with firing rate (Peng and Horn 1991; Vilim et al. 1996; Whim and Lloyd 1989). Even if there is a linear relationship across a physiological activity range, synaptic and cellular properties of network neurons may additionally shape network responses to modulatory inputs (Marder 2012; Nadim and Bucher 2014).
The pyloric and gastric mill cycle periods have distinct sensitivity to MCN1 activity rate that may reflect differences in cellular mechanisms of rhythm generation. MCN1 is thought to activate a single current (IMI) in one or more of the five pyloric pacemaker ensemble neurons, which are electrically coupled and oscillate together (Swensen and Marder 2000; Wood et al. 2000). Although the pacemaker ensemble receives a feedback synapse, it appears to primarily function to reduce variability without impacting cycle period duration (Thirumalai et al. 2006; Zhao et al. 2011). Thus the linear relationship between MCN1 activity rate and pyloric cycle period likely reflects a graded increase in transmitter release and amount of IMI controlling the frequency of intrinsic oscillations, whereas for the gastric mill network MCN1-activated IMI in LG and ionotropic excitation of Int1 are integrated into a network oscillator comprised of chemical and electrical synapses, of which the synaptic strengths and dynamics might also be modulated (Bartos et al. 1999; Bartos and Nusbaum 1997; Coleman et al. 1995; Nadim and Bucher 2014; Stein et al. 2007; Zhao et al. 2011). Therefore, increasing activation of IMI is only one aspect of increased MCN1 rate, and the gastric mill cycle period appears to be more constrained by intrinsic and synaptic properties of the network. To the extent to which it has been examined in other systems, there also appears to be variability in relationships between activity rate of modulatory inputs and cycle period including relatively linear relationships (Severi et al. 2014), no impact (Larimer and Moore 2003), and selective regulation by some modulatory inputs but not others (Jing and Weiss 2005; Nakamura and Katakura 1995).
In addition to rate, pattern can be an important aspect of modulatory neuron activity. In rhythmic motor systems, descending modulatory inputs often fire rhythmically during motor activity because of network feedback (Arshavsky et al. 1988; Blitz and Nusbaum 2008; Buchanan and Einum 2008; Hedwig 2000; Kozlov et al. 2014; Nusbaum 1986). The synaptic strength of this feedback can be modulated by extrinsic inputs (Blitz 2015; Blitz and Nusbaum 2012), or stronger network activity could lead to enhanced feedback. Therefore, the pattern of modulatory neuron activity is a dynamic component of a motor pathway. Although tonic activity of modulatory inputs can readily activate and/or modulate networks, there are often differences in the responses to tonic vs. rhythmic activity patterns (Abbott et al. 2011; Blitz and Nusbaum 2012; Hedwig 2000; Kozlov et al. 2014). We found that a number of parameters in this study were different during tonic vs. pyloric-timed MCN1 activity. The differences tended to be weaker responses such as shorter burst durations or fewer action potentials per burst in response to pyloric-timed vs. tonic MCN1 activity. However, pyloric network feedback to MCN1 elicits pyloric-timed longer interspike intervals without any compensatory increase in intervening interspike intervals. Thus not only does network feedback shape the pattern of MCN1 spiking, it also decreases the average MCN1 firing rate across multiple pyloric cycles (Fig. 9). Therefore, weaker responses to pyloric-timed stimulations may simply be a consequence of the lower average MCN1 firing rate during pyloric-timed activity compared with tonic activity (Fig. 9). Given the modulatory transmission from MCN1 to many of its targets, a lower average firing rate may explain many consequences of network feedback on MCN1 for network output. However, additional experiments manipulating average rate separately from pattern would be necessary to determine the extent to which average MCN1 rate, MCN1 pattern, and the combination, regulate network parameters. Although not systematically explored, both activity rate and pattern can contribute to shaping the effects of modulatory inputs (Bartos et al. 1999; Hurwitz et al. 2005; Jing and Weiss 2005; Severi et al. 2014; Wood et al. 2004). Activity rate can determine the amount of transmitter released, including potentially varying the relative amounts of cotransmitters released (Kupfermann 1991; Vilim et al. 1996). Bursting presynaptic activity regulates transmitter release differently than tonic activity in some neurons, while in others release does not depend on activity pattern (Cazalis et al. 1985; Peng and Horn 1991; Vilim et al. 1996). Thus further investigation will be necessary to better understand the relationships between activity rate and pattern and the release of transmitter and subsequent postsynaptic effects, to more fully appreciate how modulatory inputs determine network output.
Pyloric-timed interruptions in MCN1 activity elicit pyloric-timed pauses in LG activity that are retained in muscle responses and tooth movements, eliciting a chattering mode of chewing. Thus the timing of MCN1 action potentials is behaviorally relevant (Diehl et al. 2013). The regulation of LG burst structure was found to be consistent across MCN1 activity rates, indicating that this particular mode of chewing would be conserved across a range of strengths of network activity. The lack of sensitivity of LG burst structure to MCN1 firing rate suggests that although the occurrence of LG bursts relies on peptidergic activation of IMI (Coleman et al. 1995; DeLong et al. 2009b), the detailed structure within the burst might be more dependent on electrical coupling between MCN1 and LG, or the time constant of IMI modulation may be sufficiently fast to allow for pyloric-timed gaps in LG spiking. The contributions of different synaptic components by which MCN1 regulates LG burst structure and whether the contributions might differ across the range of MCN1 activity remain undetermined.
Activation of distinct networks.
One potential mechanism underlying distinct regulation of the pyloric and gastric mill networks is differential use of cotransmitters. MCN1 transmitters include GABA and two peptides, proctolin and Cancer borealis tachykinin peptide Ia (CabTRP Ia) (Blitz et al. 1999; Stein et al. 2007; Wood et al. 2000). Within the gastric mill rhythm generator, MCN1 uses CabTRP Ia to act on LG and GABA to act on Int1. The pyloric rhythm generator neurons, however, are influenced by proctolin and CabTRP Ia (Stein et al. 2007; Wood et al. 2000). It is possible that the lower pyloric threshold for reliable activation is due to the cotransmitters proctolin and CabTRP Ia converging on the same current (IMI), which enables bursting properties in neurons (Swensen and Marder 2000; Zhao et al. 2010). Because MCN1 uses only CabTRP Ia to activate IMI in the LG neuron (DeLong et al. 2009b; Stein et al. 2007), a higher MCN1 rate may be necessary to enable burst generation.
The limited ability of higher MCN1 rates to elicit a coordinated gastric mill rhythm may reflect differences in the extent to which the two networks rely on sensory feedback more so than differences in cotransmitter actions. An in vitro system often lacks components such as sensory feedback loops that might stabilize the in vivo rhythm, or recruit additional neurons. For instance, the projection neuron CPN2 when recruited by sensory and other inputs is coactive with MCN1 and through inhibition of DG (Beenhakker and Nusbaum 2004; Blitz et al. 2008; Christie et al. 2004; Norris et al. 1994) may help maintain 1:1 LG and DG coordination. Additionally, proprioceptors activated during the retraction phase (DeLong et al. 2009a; Katz et al. 1989) might stabilize the gastric mill rhythm. The robustness of the pyloric rhythm across the MCN1 activity range and its lower variability compared with the gastric mill rhythm suggest that it is less dependent on external inputs (Hamood and Marder 2015b; Marder and Bucher 2007). CPGs producing motor output that must adapt to a continually changing interaction with the external world, such as locomotion over varied terrain, are thought to be more dependent upon sensory feedback for appropriate timing such as phase transitions (Büschges et al. 2011; Stein 2014). Chewing, in which the textures of food can change because of variety in the diet, may similarly rely more on sensory feedback than the filtering of chewed food mediated by the pyloric rhythm (Donahue et al. 2009; Lund and Kolta 2006; Stehlik 1993).
The most well-known code for inputs to elicit particular network outputs is population coding, in which the subset of active descending inputs determines network output (Georgopoulos 1995; Jing and Weiss 2005; Lewis and Kristan 1998; Morgan et al. 2002; Zelenin 2005). However, an alternative “activity code” in which multiple stimuli activate the same subsets of descending projection neuron inputs, but trigger different activity patterns and rates, is used in some systems to select distinct versions of motor output (Beenhakker and Nusbaum 2004; Blitz et al. 2004; Blitz and Nusbaum 2008; Kohashi and Oda 2008). Therefore it becomes more important to understand how the activity of modulatory projection neurons is regulated, including by network feedback, and how their activity is translated to network output in rhythmic motor systems. Even in the small pyloric and gastric mill networks, sensitivity to the activity range of a single modulatory input is complicated by cotransmission, the particular cellular and synaptic mechanisms of rhythm generation, and local and long-distance feedback pathways. Thus it is important to continue to explore how modulatory signals regulate output from different networks to increase our understanding of physiological flexibility of network function and as a baseline from which to investigate the impact of dysregulation of modulatory systems (Berridge and Waterhouse 2003; Clemens et al. 2006; Katz et al. 2009).
GRANTS
This work was supported by National Science Foundation grant IOS-1153417 (D. M. Blitz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.M.S. performed experiments; R.M.S. and D.M.B. analyzed data; R.M.S. and D.M.B. interpreted results of experiments; R.M.S. and D.M.B. prepared figures; R.M.S. and D.M.B. drafted manuscript; R.M.S. and D.M.B. edited and revised manuscript; R.M.S. and D.M.B. approved final version of manuscript; D.M.B. conception and design of research.
ACKNOWLEDGMENTS
We thank Paul Schaeffer, Aaron Cook, Jessica Peebles-Spencer, and Michael Hughes (Statistical Consulting Center, Miami University) for assistance with statistical analyses.
REFERENCES
- Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci 31: 16410–16422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Orlovsky GN, Perret C. Activity of rubrospinal neurons during locomotion and scratching in the cat. Behav Brain Res 28: 193–199, 1988. [DOI] [PubMed] [Google Scholar]
- Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci 19: 6650–6660, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Nusbaum MP. Intercircuit control of motor pattern modulation by presynaptic inhibition. J Neurosci 17: 2247–2256, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci 24: 6741–6750, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42: 33–84, 2003. [DOI] [PubMed] [Google Scholar]
- Blitz DM. Differential modulation of circuit feedback determines activity rate of a circuit input (Abstract). Neuroscience Meeting Planner 2015: 798–04, 2015. [Google Scholar]
- Blitz DM, Beenhakker MP, Nusbaum MP. Different sensory systems share projection neurons but elicit distinct motor patterns. J Neurosci 24: 11381–11390, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci 19: 5449–5463, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. State-dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs. J Neurosci 28: 9564–9574, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Modulation of circuit feedback specifies motor circuit output. J Neurosci 32: 9182–9193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol 211: 1000–1011, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci 31: 271–294, 2008. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, McCormick K, Tapyrik L, Albano AM, Graybeal C. Activation of two forms of locomotion by a previously identified trigger interneuron for swimming in the medicinal leech. Invert Neurosci 8: 31–39, 2008. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Einum JF. The spinobulbar system in lamprey. Brain Res Rev 57: 37–45, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol 21: R513–R524, 2011. [DOI] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol 369: 45–60, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE, Stein W, Quinlan JE, Beenhakker MP, Marder E, Nusbaum MP. Actions of a histaminergic/peptidergic projection neuron on rhythmic motor patterns in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol 469: 153–169, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Combes D, Meyrand P, Simmers J. Long-term expression of two interacting motor pattern-generating networks in the stomatogastric system of freely behaving lobster. J Neurophysiol 79: 1396–1408, 1998. [DOI] [PubMed] [Google Scholar]
- Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology 67: 125–130, 2006. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Meyrand P, Nusbaum MP. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378: 502–505, 1995. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP. Functional consequences of compartmentalization of synaptic input. J Neurosci 14: 6544–6552, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ND, Beenhakker MP, Nusbaum MP. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol 102: 3492–3504, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ND, Kirby MS, Blitz DM, Nusbaum MP. Parallel regulation of a modulator-activated current via distinct dynamics underlies comodulation of motor circuit output. J Neurosci 29: 12355–12367, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, White RS, Stein W, Nusbaum MP. Motor circuit-specific burst patterns drive different muscle and behavior patterns. J Neurosci 33: 12013–12029, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Nichols A, Santamaria CA, League-Pike PE, Krediet CJ, Perez KO, Shulman MJ. Predation risk, prey abundance, and the vertical distribution of three brachyuran crabs on Gulf of Maine shores. J Crustacean Biol 29: 523–531, 2009. [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fenelon K, Gariepy JF, Smetana R, Menard A, Le Ray D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Rev 57: 172–182, 2008. [DOI] [PubMed] [Google Scholar]
- Eisen JS, Marder E. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol 48: 1392–1415, 1982. [DOI] [PubMed] [Google Scholar]
- Frost WN, Hoppe TA, Wang J, Tian LM. Swim initiation neurons in Tritonia diomedea. Am Zool 41: 952–961, 2001. [Google Scholar]
- Georgopoulos AP. Current issues in directional motor control. Trends Neurosci 18: 506–510, 1995. [DOI] [PubMed] [Google Scholar]
- Gutierrez GJ, Grashow RG. Cancer borealis stomatogastric nervous system dissection. J Vis Exp 25: 1–5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood AW, Haddad SA, Otopalik AG, Rosenbaum P, Marder E. Quantitative reevaluation of the effects of short- and long-term removal of descending modulatory inputs on the pyloric rhythm of the crab, Cancer borealis. eNeuro 2: 1–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood AW, Marder E. Animal-to-animal variability in neuromodulation and circuit function. Cold Spring Harb Symp Quant Biol 79: 21–28, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood AW, Marder E. Consequences of acute and long-term removal of neuromodulatory input on the episodic gastric rhythm of the crab Cancer borealis. J Neurophysiol 114: 1677–1692, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM. Neuromodulation and flexibility in central pattern generator networks. Curr Opin Neurobiol 21: 685–692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich UB, Diehl F, Stein W. Gastric and pyloric motor pattern control by a modulatory projection neuron in the intact crab Cancer pagurus. J Neurophysiol 105: 1671–1680, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich UB, Smarandache CR, Stein W. Differential activation of projection neurons by two sensory pathways contributes to motor pattern selection. J Neurophysiol 102: 2866–2879, 2009. [DOI] [PubMed] [Google Scholar]
- Hedwig B. Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 83: 712–722, 2000. [DOI] [PubMed] [Google Scholar]
- Heinzel HG, Weimann JM, Marder E. The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J Neurosci 13: 1793–1803, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz I, Susswein AJ, Weiss KR. Transforming tonic firing into a rhythmic output in the Aplysia feeding system: presynaptic inhibition of a command-like neuron by a CPG element. J Neurophysiol 93: 829–842, 2005. [DOI] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15: 1712–1721, 2005. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008. [DOI] [PubMed] [Google Scholar]
- Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol 168: 101–108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Eigg MH, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol 62: 558–570, 1989. [DOI] [PubMed] [Google Scholar]
- Kohashi T, Oda Y. Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci 28: 10641–10653, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AK, Kardamakis AA, Hellgren Kotaleski J, Grillner S. Gating of steering signals through phasic modulation of reticulospinal neurons during locomotion. Proc Natl Acad Sci USA 111: 3591–3596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan WB, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol 76: 279–327, 2005. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Functional studies of cotransmission. Physiol Rev 71: 683–732, 1991. [DOI] [PubMed] [Google Scholar]
- Larimer JL, Moore D. Neural basis of a simple behavior: abdominal positioning in crayfish. Microsc Res Tech 60: 346–359, 2003. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Kristan WB. A neuronal network for computing population vectors in the leech. Nature 391: 76–79, 1998. [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94: 1392–1404, 2005. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 21: 167–174, 2006. [DOI] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol 87: 49–61, 2002. [DOI] [PubMed] [Google Scholar]
- Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol 29: 48–56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res 23: 1–19, 1995. [PubMed] [Google Scholar]
- Norris BJ, Coleman MJ, Nusbaum MP. Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis. J Neurophysiol 72: 1451–1463, 1994. [DOI] [PubMed] [Google Scholar]
- Norris BJ, Coleman MJ, Nusbaum MP. Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system. J Neurophysiol 75: 97–108, 1996. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP. Synaptic basis of swim initiation in the leech. III. Synaptic effects of serotonin-containing interneurones (cells 21 and 61) on swim CPG neurones (cells 18 and 208). J Exp Biol 122: 303–321, 1986. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol 60: 378–387, 2002. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci 24: 146–154, 2001. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Weimann JM, Golowasch J, Marder E. Presynaptic control of modulatory fibers by their neural network targets. J Neurosci 12: 2706–2714, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YY, Horn JP. Continuous repetitive stimuli are more effective than bursts for evoking LHRH release in bullfrog sympathetic ganglia. J Neurosci 11: 85–95, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochim Biophys Acta 1828: 134–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 278: 1122–1125, 1997. [DOI] [PubMed] [Google Scholar]
- Puhl JG, Masino MA, Mesce KA. Necessary, sufficient and permissive: a single locomotor command neuron important for intersegmental coordination. J Neurosci 32: 17646–17657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- Russell DF. CNS control of pattern generation in the lobster stomatogastric ganglion. Brain Res 177: 598–602, 1979. [DOI] [PubMed] [Google Scholar]
- Severi KE, Portugues R, Marques JC, O'Malley DM, Orger MB, Engert F. Neural control and modulation of swimming speed in the larval zebrafish. Neuron 83: 692–707, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8: 1–16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi W, Goeritz ML, Kispersky TJ, Prinz AA, Marder E, Stein W. Phase maintenance in a rhythmic motor pattern during temperature changes in vivo. J Neurophysiol 111: 2603–2613, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik LL. Diets of the brachyuran crabs Cancer irroratus, C. borealis, and Ovalipes ocellatus in the New York Bight. J Crustacean Biol 13: 723–735, 1993. [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 989–1009, 2009. [DOI] [PubMed] [Google Scholar]
- Stein W. Sensory input to central pattern generators. In: Encyclopedia of Computational Neuroscience. New York: Springer, 2014. [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci 26: 1148–1165, 2007. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci 20: 6752–6759, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai V, Prinz AA, Johnson CD, Marder E. Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol 95: 1762–1770, 2006. [DOI] [PubMed] [Google Scholar]
- Velázquez-Ulloa N, Blackshaw SE, Szczupak L, Trueta C, García E, De-Miguel FF. Convergence of mechanosensory inputs onto neuromodulatory serotonergic neurons in the leech. J Neurobiol 54: 604–617, 2003. [DOI] [PubMed] [Google Scholar]
- Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Release of peptide cotransmitters in Aplysia: regulation and functional implications. J Neurosci 16: 8105–8114, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol 65: 111–122, 1991. [DOI] [PubMed] [Google Scholar]
- Whim MD, Lloyd PE. Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in Aplysia. Proc Natl Acad Sci USA 86: 9034–9038, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RS, Nusbaum MP. The same core rhythm generator underlies different rhythmic motor patterns. J Neurosci 31: 11484–11494, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Manor Y, Nadim F, Nusbaum MP. Intercircuit control via rhythmic regulation of projection neuron activity. J Neurosci 24: 7455–7463, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J Neurosci 20: 8943–8953, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV. Activity of individual reticulospinal neurons during different forms of locomotion in the lamprey. Eur J Neurosci 22: 2271–2282, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Khorkova O, Rodriguez R, Golowasch J. Activity and neuromodulatory input contribute to the recovery of rhythmic output after decentralization in a central pattern generator. J Neurophysiol 101: 372–386, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Golowasch J, Nadim F. Pacemaker neuron and network oscillations depend on a neuromodulator-regulated linear current. Front Behav Neurosci 4: 1–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci 31: 13991–14004, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]