Ruxolitinib blockade of STAT3 phosphorylation in IL-6/23- stimulated neutrophils, inhibits RORγt translocation, IL-1- induced production of ROS, MMP9, and elastase activity, and modulates Aspergillus fumigatus corneal infections.
Keywords: keratitis, RORγt, Ruxolitinb, Stattic, SR1001
Abstract
IL-6 and IL-23 (IL-6/23) induce IL-17A (IL-17) production by a subpopulation of murine and human neutrophils, resulting in autocrine IL-17 activation, enhanced production of reactive oxygen species, and increased fungal killing. As IL-6 and IL-23 receptors trigger JAK1, -3/STAT3 and JAK2/STAT3 phosphorylation, respectively, we examined the role of this pathway in a murine model of fungal keratitis and also examined neutrophil elastase and gelatinase (matrix metalloproteinase 9) activity by IL-6/23-stimulated human neutrophils in vitro. We found that STAT3 phosphorylation of neutrophils in Aspergillus fumigatus-infected corne as was inhibited by the JAK/STAT inhibitor Ruxolitinib, resulting in impaired fungal killing and decreased matrix metalloproteinase 9 activity. In vitro, we showed that fungal killing by IL-6/23-stimulated human peripheral blood neutrophils was impaired by JAK/STAT inhibitors Ruxolitinib and Stattic, and by the retinoic acid receptor-related orphan receptor γt inhibitor SR1001. This was also associated with decreased reactive oxygen species, IL-17A production, and retinoic acid receptor-related orphan receptor γt translocation to the nucleus. We also demonstrate that IL-6/23-activated neutrophils exhibit increased elastase and gelatinase (matrix metalloproteinase 9) activity, which is inhibited by Ruxolitinib and Stattic but not by SR1001. Taken together, these observations indicate that the regulation of activity of IL-17-producing neutrophils by JAK/STAT inhibitors impairs reactive oxygen species production and fungal killing activity but also blocks elastase and gelatinase activity that can cause tissue damage.
Introduction
Microbial infections of the cornea caused by bacteria and fungi are the second most common cause of blindness worldwide after cataracts. Fungal infections of the cornea (keratitis) manifest as purulent, ulcerative infections that are extremely painful and can result in severe loss of vision and even blindness. Infections caused by filamentous fungi, such as Aspergillus and Fusarium species are the leading causes of corneal ulcers in developing countries and in hot and humid regions of industrialized countries, most commonly following ocular trauma associated with agricultural work [1–3]. An additional risk factor is contact lens wear, and Fusarium species were the cause of an outbreak in the United States and worldwide [4] and remain an important risk factor.
In order to characterize the pathogenesis of this infection, we used murine models of Aspergillus and Fusarium keratitis in which live conidia are injected directly into the corneal stroma. These studies showed that conidia rapidly germinate and hyphae spread throughout the cornea within 24 h. We also showed that control of hyphal growth is regulated by proinflammatory and chemotactic cytokines produced by resident macrophages by activation of c-type lectins Dectin-1 and -2, which recruit neutrophils to the infected corneas [5–7]. Hyphal growth was found to be regulated by neutrophil-derived ROS and neutrophil iron and zinc chelators, including lactoferrin, transferrin, and calprotectin [8–10].
Analysis of corneas ulcers from acutely infected patients also showed that neutrophils were the predominant cell type but were also associated with increased production of the proinflammatory cytokine IL-17A (IL-17) in the cornea [11]. IL-17-producing neutrophils were prominent in peripheral blood, not only in fungal keratitis patients in south India but also in healthy individuals from this region, most likely as a consequence of inhaling airborne conidia [12]. That study showed a correlation between the percent of IL-17-producing neutrophils and plasma levels of IL-6 and IL-23 [12]. We also demonstrated that these cytokines directly stimulate not only IL-17A production but also expression of a functional IL-17RA/RC receptor, resulting in autocrine IL-17 activation of these neutrophils and elevated production of ROS [13]. In individuals exposed to high levels of airborne spores and in mice given spores subcutaneously, IL-6 and IL-23 are most likely produced by macrophages and dendritic cells at the site of initial exposure and activate neutrophils in the bone marrow [12, 13]. Neutrophils recognize hyphae through Dectin-1 and complement receptor 3 [8], and IL-6/23-activated neutrophils also express Dectin-2, which induces further up-regulation of IL-17RC [13].
Results from the current study extend these observations to show that IL-6 and IL-23 induce p-STAT3 in neutrophils and that blockade of this pathway using JAK/STAT inhibitors impairs ROS production and hyphal killing in vitro and in fungal keratitis. STAT3 signaling also inhibited elevated production of neutrophil elastase in vitro and MMP9 activity in vitro and in vivo, which can potentially limit gelatinase damage to the cornea and visual acuity. These findings support a combined therapeutic approach of anti-fungal agents with targeted blockade of neutrophil protease activity.
MATERIALS AND METHODS
Source and maintenance of mice
The Case Western Reserve University Institutional Animal Care and Use Committee approved the animal protocols used in this study. All of the mice used in these studies were C57BL/6J, which were obtained from The Jackson Laboratory (Bar Harbor, ME, USA).
JAK/STAT and RORγt inhibitors
The JAK1, -2 inhibitor Ruxolitinib (INC424, INCB18424) [14, 15] was purchased from Selleck Chemicals (Houston, TX, USA) and used at 20 μM final concentration. The STAT3-SH2 inhibitor Stattic was obtained from Tocris Bioscience (Bristol, United Kingdom) and used at 20 μM [16, 17]. The RORγt inhibitor SR1001 was purchased from Sigma (St. Louis, MO, USA) and used at 20 μM. SR1001 inhibits RORγt binding to the il17a promoter [18].
Aspergillus strains
The Af293 strain of Af-dsRed was used for corneal infections and in vitro fungal killing assays [8]. For subcutaneous “priming” injections, heat-killed, swollen conidia Aspergillus fumigatus strain Af-BP was used. A. fumigatus strain Af-BP is a clinical isolate from a fungal keratitis patient treated at Bascom Palmer Eye Institute (Miami, FL, USA), which was used in prior studies from our laboratory [8, 13].
For the AspHE used in ROS assay, A. fumigatus strain Af-BP hyphae were pulverized in liquid nitrogen and filtered through a 30 µm preseparation filter (Miltenyi Biotec, Auburn, CA, US) and protein measured by the BCA method (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA). AspHEs were stored at −20°C and used at a final concentration of 1 mg/ml.
Subcutaneous injection (immunization) with swollen, heat-killed conidia
Live, plate-grown A. fumigatus conidia from the Af-BP clinical isolate were harvested and incubated for 6 h in Sabouraud dextrose broth to allow germination to occur and for expression of β-glucan, which initiates the host response. Heat-killed, swollen (germinated) conidia (3 × 108/100 µl) were injected subcutaneously at the base of the tail. After 72 h, IL-17-producing neutrophils were present in bone marrow and spleen but not Th17 cells; this was confirmed by flow cytometry.
Isolation of murine bone marrow neutrophils
Bone marrow-derived cells were isolated from mouse femurs and tibias by flushing the bones through with RPMI using an 18-gauge needle. Erythrocytes were lysed with 1× lysis buffer (eBioscience, San Diego, CA, USA), and total bone marrow cells were separated on a Percoll (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) gradient by density centrifugation (52, 69, and 78%). Cells at the 69–78% interface were harvested, and neutrophil purity (>98%) was assessed by flow cytometry and Wright-Giemsa staining.
Murine model of A. fumigatus corneal infection
C57BL/6 mice were immunized subcutaneously, and after 3 d (before inducing a T cell response, "primed"), a single abrasion was made using a 30 gauge needle, and 1 × 105 live conidia were injected directly into the corneal stroma. Twenty-four hours postinfection (when IL-17-producing neutrophils are detected in the cornea), mice were euthanized, and fungal growth in the cornea was imaged by fluorescence stereoscopy and quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA USA). Infected corneas were also collected and digested with collagenase, and cells were incubated with NIMP-R14 to quantify the number of neutrophils. A sample size of 10 mice/group was analyzed.
In vivo application of Ruxolitinib
To inhibit p-JAK/STAT in vivo, 100 µl 0.8 mg Ruxolitinib (suspended in 20% DMSO and 80% PEG 300) was administered by oral gavage twice per day over a 5 d period, as described in other in vivo studies [19, 20]. Control mice were given the DMSO/PEG vehicle alone. C57BL/6 mice were primed 4 h after the final oral dose, and the corneal stroma were infected 3 d with 1 × 105 live RFP-expressing A. fumigatus conidia. Systemic inhibition of p-STAT3 was confirmed in bone marrow cells by flow cytometry.
Flow cytometry
Corneas were dissected and incubated for 1 h at 37°C in collagenase (80 U/ml; C0130; Sigma) and washed in FACS buffer (PBS + 1% FBS + 0.5% sodium azide). Ten corneas were pooled, and cells were analyzed by intracellular flow cytometry. Also, bone marrow cells were collected as described above and washed in FACS buffer. Cells were incubated (15 min) with anti-mouse CD16/32 antibody (Fc block, clone 93; eBioscience) or human FcR binding inhibitor (eBioscience), washed, and incubated (20 min) on ice with anti-mouse Ly6G/NIMP-R14 antibody (Abcam, Cambridge, MA, USA; in-house) or anti-human MMP9 antibody (R&D Systems, Minneapolis, MN, USA). For intracellular staining, cells were incubated (20 min) in Intracellular (IC) Fixation Buffer (eBioscience) with anti-mouse and human p-STAT3 Tyr705 (Cell Signaling Technology, Danvers, MA, USA), MMP9 (R&D Systems), STAT3, IL-17A, or RORγt antibodies (eBioscience) in the presence of Fc block (details of antibodies are in Supplemental Table 1). Cells were analyzed using a C6 Accuri flow cytometer (BD Biosciences, San Jose, CA, USA). Multispectral Imaging Flow Cytometry (ImageStream 100; Amnis, EMD Millipore, Billerica, MA, USA) was used for quantification of RORγt translocation to the nucleus. Settings for flow cytometry were based on isotype controls. We examined the anti-mouse Ly6G (NIMP-R14) mAb on several cell types, including macrophages, and showed that, neutrophils were the only cells found to be positive [13].
ImageStream analysis of nuclear translocation
Bone marrow neutrophils were isolated and RORγt was detected as described above, and nuclei were DAPI stained. Ten thousand events were acquired, and, the spatial relationship between RORγt and DAPI-stained nuclei was measured using the similarity feature of Integrated Design and Engineering Analysis Software (I-DEAS) software. The similarity score provides a measure of the degree of nuclear localization of RORγt by measuring the pixel intensity correlation between RORγt+ve and multilobed DAPI+ve images. Neutrophils with low similarity scores were scored as no translocation, whereas neutrophils with high similarity scores were recorded as translocated. The quantifications were then calculated and graphed using Prism (GraphPad Software, La Jolla, CA, USA).
Isolation of peripheral blood human neutrophils
Informed consent was obtained in accordance with the Declaration of Helsinki and the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH, USA). Neutrophils were isolated from peripheral blood of healthy donors followed by 3% Dextran and Ficoll gradient centrifugation (Thermo Fisher Scientific Life Sciences,), which yielded >95% neutrophils.
In vitro activation of murine and human neutrophils
Murine or human neutrophils were suspended at a density of 106 cells/ml and incubated with 20 μg/ml mouse rIL-6 or rhIL-6 and 2 μg/ml rhIL-23 in RPMI, and the cells were maintained for 1–3 h at 37°C in 5% CO2. All inhibitors were added to neutrophils 2 h before rIL-6 and rIL-23 stimulation. This concentration of cytokines was based on our prior studies [13].
In vitro fungal killing assay
A hyphal coincubation assay was used to study the ability of murine and human neutrophils to inhibit A. fumigatus hyphal growth as described [5, 13]. In brief, 12,500 strain Af-dsRed expressing conidia per 200 µl Sabouraud dextrose media were added to wells of black-walled, 96-well plates with an optically clear bottom (Costar 3720; Corning, Corning, NY, USA). After 6 h, the conidia had germinated, and the hyphae became adherent to the wells, at which time, the medium was removed.
Murine and human neutrophils were incubated with 20 μM Stattic, 20 μM Ruxolitinib, or 20 μM RORγt inhibitor SR1001 for 2 h. Cells were then stimulated with 20 μg/ml rhIL-6 and 2 μg/ml rhIL-23 for 3 h and washed, and 2 × 105 bone marrow murine neutrophils or 1 × 105 human peripheral blood neutrophils were added to each well with growing hyphae. RPMI media were used as a positive control (unimpaired growth), and PBS was used as a negative control [no growth (data not shown)]. After 16 h, the wells were washed with PBS, and the dsRed fungal mass was quantified in a 96-well fluorimeter at a 550/600 nm excitation/emission filter (The Synergy HT, BioTek Instruments, Winooski, VT, USA).
Production of ROS
In vitro ROS production was measured by CFDA, as described previously [13]. In brief, 100 μg/100 μl AspHE were incubated with 2 × 105 murine or human neutrophils. Neutrophils were treated for 1 h with 1 μM H2CFDA (Sigma), which fluoresces following ROS oxidation. Neutrophils were collected and analyzed by flow cytometry. H2CFDA-pulsed neutrophils that had not been incubated with AspHE were used to set the ROS histogram gate. The mean intensity was calculated by Accuri flow software and graphed in Prism (GraphPad Software).
Confocal imaging
Confocal images were collected using the UltraVIEW VoX spinning disk confocal system (PerkinElmer, Waltham, MA, USA), mounted on a Leica DMI6000 B microscope, equipped with HCX PL APO 100×/1.4 oil-immersion objective using a 0.2 µm step size. Confocal images were then imported into MetaMorph Image Analysis Software (Molecular Devices). Maximum projections were generated from the original stacks, which were subjected to "no neighbors" 2-dimensional deconvolution. We used the same anti-IL-17 and RORγt antibodies described above (eBioscience).
IL-17A ELISA
IL-17A protein was quantified by 2-site ELISA, according to the manufacturer’s directions (R&D Systems).
Western blot analysis
Neutrophils collected from primed or naïve C57BL/6 mice or unstimulated murine or human neutrophils were incubated with rIL-6 and rIL-23 for 60 min. After this time, cells were washed in PBS and lysed in ice-cold 1× lysis buffer (Cell Signaling Technology). Total protein was quantified using the BCA method, denatured with 2× Laemmli buffer (Sigma), and heated to 95°C for 5 min. Nuclear extracts were prepared with the Pierce nuclear extract kit, per the manufacturer’s directions (Thermo Fisher Scientific Life Sciences,). Total protein (10 μg) was analyzed on a 12% SDS polyacrylamide gel and transferred to nitrocellulose. Blots were probed with the following primary antibodies: total STAT3 (R&D Systems), p-STAT3 (R&D Systems), RORγt (eBioscience), TATA box binding protein [TBP (Abcam)], and β-actin (Cell Signaling Technology). (Details of antibody clones are in Supplemental Table 1.) HRP-conjugated secondary antibodies were used (Santa Cruz Biotechnology, Dallas, TX, USA) and Western blots developed with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific Life Sciences).
qPCR
Neutrophil and total corneal cell RNA was extracted using the RNeasy mini kit, according to the manufacturer's directions (Qiagen, Valencia, CA, USA). The quality of RNA was checked by spectrophotometry, and only samples with a 260/280 ratio of 2.0 were used to generate cDNA. The SuperScript First Strand synthesis system (Thermo Fisher Scientific Life Sciences) was used to generate cDNA, according to the manufacturer’s directions. This protocol also includes a DNAse step to avoid contamination with genomic DNA.
The SYBR Green system (Thermo Fisher Scientific Life Sciences) was used for qPCR using Thermo Fisher Scientific Life Sciences. Primer sequences are listed in Supplemental Table 2. PCR products were then run on a 2% agarose gel and visualized using ethidium bromide. PCR products were compared with Actb (encoding the β-actin gene) or GAPDH as the controls for gel loading.
MMP9 and neutrophil elastase activity assays
Active MMP9 was detected through activation of a modified prodetection peptide and cleavage of the chromogenic peptide substrate (S-2444) and quantified by spectrophotometry at excitation/emission wavelengths 485/525 nm. The neutrophil elastase activity assay uses a fluorescent substrate that is cleaved by active elastase to analyze elastase activity. Data were expressed as picograms per milliliter, according to the manufacturer’s instructions (MMP-9 Biotrak activity assay, GE Healthcare Bio-Sciences; Neutrophil Elastase Activity Assay Kit, Cayman Chemical, Ann Arbor, MI, USA).
Statistical analysis
Statistical analysis was performed for each experiment using an unpaired t-test and 1-way ANOVA analysis with Tukey’s post hoc analysis (Prism; GraphPad Software). P < 0.05 was considered significant. Correlation analyses were calculated using Spearman’s rank correlation coefficient, and P values and r2 analyses were used to determine statistical significance.
RESULTS
Neutrophils from A. fumigatus-infected corneas express p-STAT3
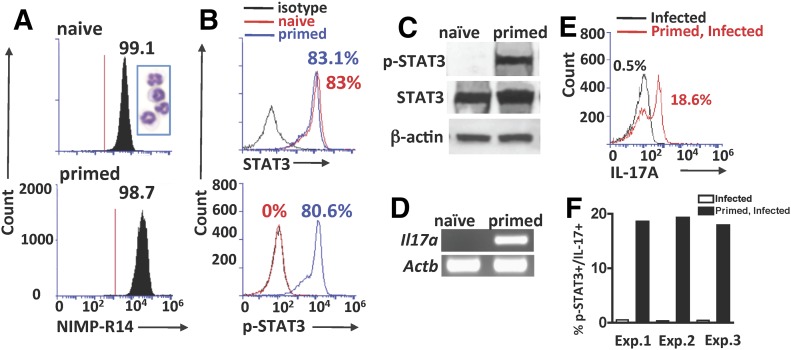
We demonstrated that a single subcutaneous injection of heat-killed, swollen A. fumigatus conidia induced elevated serum IL-6 and IL-23 and generated IL-17-producing neutrophils in the bone marrow after 72 h [13]. As we showed that this occurs before T cell activation, we used the term “primed” rather than "immunized" [13]. Bone marrow neutrophils from naïve and primed C57BL/6 mice were isolated by gradient centrifugation, which yielded >98% neutrophils (Fig. 1A), and antibodies targeted to the Tyr705 site of STAT3 were used to detect p-STAT3 by Western blot and intracellular flow cytometry. Corneas were examined 24 h postinfection as prior studies showed that IL-17 neutrophils are predominant at this time point; furthermore, IL-17-producing neutrophils and IL-23 were not detected in the corneas of nonprimed mice at any time point during a 72 h corneal infection [5].
Figure 1. p-STAT3 in vivo.
(A–D) Bone marrow neutrophils. (E, F) Neutrophils from infected corneas. (A) Representative histograms and Wright-Giemsa staining of murine NIMP-R14+ bone marrow neutrophils recovered 3 d after subcutaneous injection of heat-killed, swollen A. fumigatus conidia (primed) and from naïve C57BL/6 mice. (B) Percent intracellular STAT3 and p-STAT3 NIMP-R14+ bone marrow cells from naïve and primed C57BL/6 mice. (C) Western blot of total neutrophil lysates from the bone marrows of naïve and primed C57BL/6 mice. Membranes were probed with antibodies reactive with STAT3 (total STAT3), p-STAT3 (Tyr705), or β-actin. (D) Il17a gene expression in bone marrow neutrophils from naïve and primed C57BL/6 mice, showing products from qPCR. Actb (which encodes β-actin) served as the control for gel loading. (E) Representative flow cytometry of IL-17+ve Ly6G+ neutrophils from corneas 24 h after infection with live A. fumigatus conidia (cells were pooled from 10 corneas) and gated on p-STAT-3+ve cells. (F) Percent of p-STAT3+/IL-17+ neutrophils in infected and primed, infected corneas of C57BL/6 mice in 3 separate experiments (Exp.). CT scores from each experiment are in Supplemental Table 3.
We found that although ∼80% bone marrow-derived neutrophils from naïve and primed C57BL/6 mice had total STAT3, p-STAT3 was detected only in neutrophils from primed mice (Fig. 1B). Similar results are shown by Western blot analysis, where p-STAT3 was only detected in neutrophils from primed mice (Fig. 1C). Consistent with these data and with our previous studies [13], Il17a gene expression by qPCR was detected in neutrophils from primed mice only (Fig. 1D; agarose gels of qPCR products; CT scores are shown in Supplemental Table. 3).
To detect IL-17 in p-STAT3+ve neutrophils following fungal infection, corneas of naïve and 72 h primed mice were infected with live A. fumigatus conidia that express dsRed, and after 24 h, corneas were digested with collagenase, and p-STAT3 and IL-17 in infiltrating NIMP-R14+ve neutrophils were examined by flow cytometry. There were no p-STAT3+ve neutrophils in the cornea of infected mice that had not been primed; however, the neutrophils from primed, infected mice were mostly p-STAT3+ neutrophils that were gated for further IL-17 analysis by flow cytometer. Figure 1, E and F, shows no IL-17+ve neutrophils in infected mice that had not been primed, whereas 18.6% of neutrophils in the corneas of primed, infected mice were IL-17+ve. As shown previously [13, 21], there was no significant difference in the total number of neutrophils recruited to infected corneas of nonprimed compared with primed mice (data not shown).
These results indicate that STAT3 is phosphorylated in neutrophils in vivo following priming, and neutrophils are then recruited to the site of infection.
Impaired fungal clearance and MMP9 activity in infected corneas of Ruxolitinib-treated mice
STAT3 phosphorylation is mediated by activation of Jak1 and Jak2 by IL-6/gp130 and Jak2 by IL-23 [22–24]. Therefore, we examined the effect of blocking p-STAT3 in infected corneas using Ruxolitinib, which inhibits p-JAK1/2 and p-STAT3 [14, 15, 19].
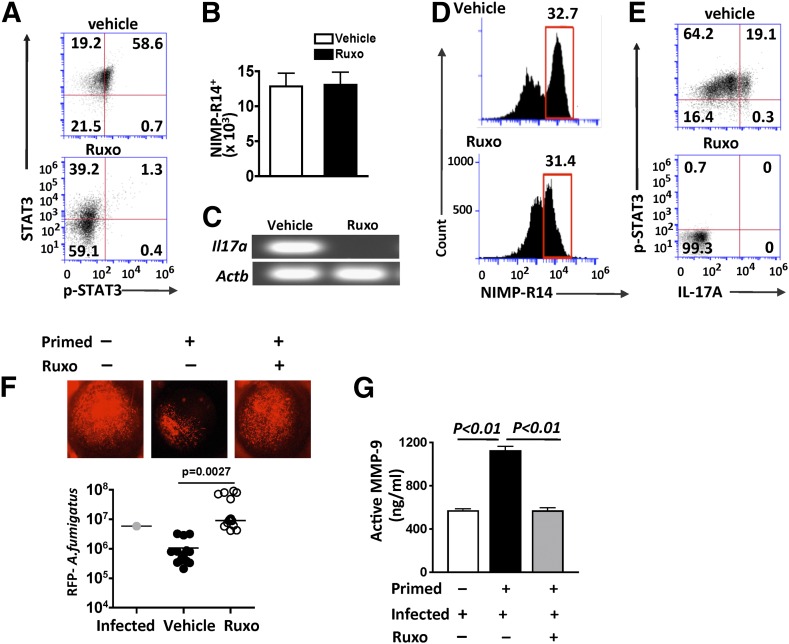
C57BL/6 mice were administered Ruxolitinib (0.8 mg/ml) or vehicle alone by oral gavage twice per day for 5 consecutive days before subcutaneous injection of heat-killed, swollen conidia (primed). After 72 h, the systemic effect of Ruxolitinib was examined in isolated bone marrow neutrophils. As shown in Fig. 2A, there was no p-STAT3 in neutrophils from mice given oral Ruxolitinib.
Figure 2. p-STAT3 requirement to regulate Aspergillus hyphal growth in vivo.
Ruxolitinib (Ruxo) or vehicle control was administered by oral gavage twice/d for 5 d, primed with heat-killed, swollen conidia, and infected intrastromally with live A. fumigatus conidia. Bone marrow neutrophils and cells from infected corneas were examined after 24 h. (A) Flow cytometry of intracellular p-STAT3 and total STAT3 in bone marrow neutrophils. (B) Total NIMP-R14+ neutrophils in infected corneas of mice given either systemic Ruxolitinib or vehicle control (mean ± sd of 5 mice/group). (C) IL17a gene expression in total, homogenized corneas. (D) Total cells gated for NIMP-R14+ neutrophils in infected corneas. (E) Intracellular IL-17A and p-STAT3 in NIMP-R14+ neutrophils from infected corneas. (F) In vivo growth of RFP-expressing A. fumigatus hyphae in corneas of primed mice treated with Ruxolitinib. Total RFP was quantified by image analysis (data points represent individual corneas). Original magnification of representative, infected corneas, ×20. Results are combined from 3 separate experiments. (Individual experiments are shown in Supplemental Fig. 1). (G) MMP9 activity in infected corneas (mean ± sd) from 3 separate experiments.
To determine the effect of Ruxolitinib on fungal infection, live dsRed-expressing A. fumigatus conidia were injected into the corneal stroma, and after 24 h, Il17a gene expression and p-STAT3 and IL-17 in infiltrating neutrophils were examined, and fungal growth was quantified by image analysis.
There was no difference in the total number of neutrophils infiltrating the infected corneas (Fig. 2B); however, Il17a gene expression, which was detected in vehicle control corneas, was not detected in corneas from Ruxolitinib-treated mice (Fig. 2C; qualitative gels of qPCR products are shown in Fig. 2C, and CT scores are in Supplemental Table 3). Neutrophils in the cornea were gated from the total cells in infected corneas (Fig. 2D), and Fig. 2E shows p-STAT3/ IL-17+ve neutrophils in vehicle (control mice) but not in Ruxolitinib-treated mice. (Flow cytometry of total cells from infected corneas is shown in Supplemental Fig. 1.)
As shown in Fig. 2F, there was less RFP–A. fumigatus in infected corneas in primed, vehicle control compared with infected, nonprimed mice; however, hyphal growth was not impaired in Ruxolitinib-treated mice and was significantly higher than in primed; animals not given Ruxolitinib (quantification of RFP-expressing fungus shows combined data from 3 experiments; representative eyes from 2 other experiments are shown in Supplemental Fig. 2).
To determine if increased protease activity occurs in the corneas of primed, infected mice during fungal keratitis, corneas were dissected and homogenized 24 h postinfection from nonprimed, primed vehicle control, and primed/Ruxolitinib-treated mice. Active MMP9 was detected in lysates from all infected corneas; however, the level of MMP9 activity was significantly higher in the corneas of primed mice. When the mice were treated with STAT3 inhibitor Ruxolitinib before priming and infection, MMP9 activity was decreased significantly (Fig. 2G).
Together, these data show that in vivo, Ruxolitinib blocks p-STAT3 and IL-17 production by neutrophils in infected corneas, resulting in impaired control of hyphal growth but also less MMP9 activity that could cause tissue damage to the cornea.
Inhibition of p-STAT3 by murine and human neutrophils results in increased A. fumigatus hyphal growth and impaired ROS production
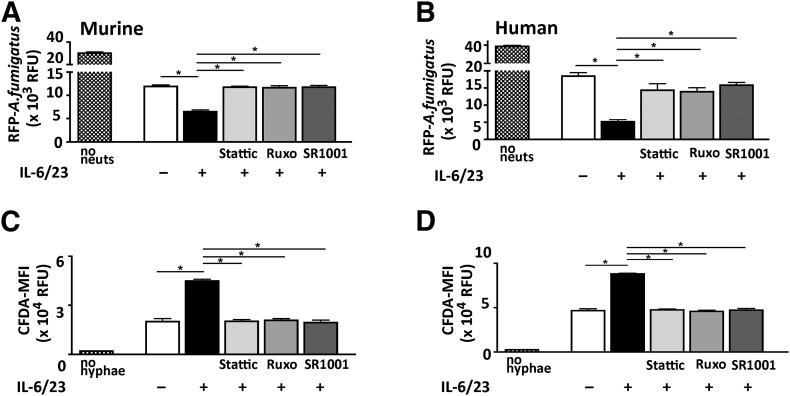
We reported an essential role for ROS in neutrophil killing of Aspergillus hyphae in vivo and in vitro and also reported that ROS production is elevated in IL-17-producing neutrophils generated in vitro or following in vivo priming [8, 13]. Therefore, to examine if p-STAT3 inhibition also impairs hyphal killing in vitro, bone marrow neutrophils from C57BL/6 mice and isolated human peripheral blood neutrophils were stimulated with IL-6/23 in the presence of Ruxolitinib or Stattic, which binds to the STAT3-SH2 domain and inhibits phosphorylation [16, 17], or with the SR1001, which blocks RORγt binding to the Il17a promoter [18]. Neutrophils were incubated with inhibitors for 2 h before adding to growing A. fumigatus hyphae, and hyphal mass was measured by fluorimetry and quantified by relative fluorescent units as we described [8, 13].
We found that hyphal mass was significantly lower after incubation with IL-6/23-treated compared with unstimulated murine or human neutrophils (Fig. 3A and B), indicating that these neutrophils have an enhanced ability to impair hyphal growth. However, in the presence of Ruxolitinib, Stattic, or SR1001, hyphal mass was significantly higher than with IL-6/23-stimulated neutrophils alone and similar to neutrophils not given IL-6/23.
Figure 3. Neutrophil regulation of hyphal growth and ROS production in vitro.
(A and B) Growth of dsRed expressing A. fumigatus after 18 h incubation with bone marrow neutrophils from C57BL/6 mice (A) or with human peripheral blood neutrophils (B) in the presence of Stattic, Ruxolitinib, or SR1001. Fungal mass was measured by fluorimetry of dsRed and represented as RFU. Controls are medium only [no neutrophils (no neuts)] and neutrophils that were not stimulated with IL-6/23. (C and D) ROS production (intracellular CFDA) by IL-6/23-stimulated murine and human neutrophils. MFI, Mean fluorescence intensity. Controls are unstimulated neutrophils (no hyphae). All wells are the means ± sd of 3 wells/experimental condition from neutrophils pooled from 3 mice/group (A and C) or from a single donor (B and D). Data are representative of 2 separate experiments. *P < 0.01.
As NADPH oxidase-generated ROS is important in hyphal killing by neutrophils and has elevated activity in IL-6/23-stimulated neutrophils [8, 13], we examined whether blockade of the JAK/STAT pathway inhibited elevated ROS production. Human and murine neutrophils were stimulated with IL-6 and IL-23 in the presence of p-STAT3 or RORγt inhibitors before adding them to wells with dsRed- expressing A. fumigatus hyphae, and after 16 h, intracellular ROS production was measured as described [8, 13]. As shown in Fig. 3C and D, ROS production by IL-6/23-stimulated neutrophils was significantly higher than unstimulated neutrophils. However, in the presence of Stattic, Ruxolitinib, or SR1001, IL-6/23-induced ROS production was significantly less, with levels similar to neutrophils not stimulated with IL-6/23. There was no effect of JAK/STAT inhibitors on fungal killing by neutrophils not activated by IL-6/23 (Supplemental Fig. 3).
Collectively, these findings indicate that JAK/STAT and RORγt inhibitors block fungal killing by IL-6/23-stimulated neutrophils by impairing ROS production.
p-STAT3 mediates RORγt nuclear translocation and Il17a gene expression in IL-6/23-stimulated human neutrophils
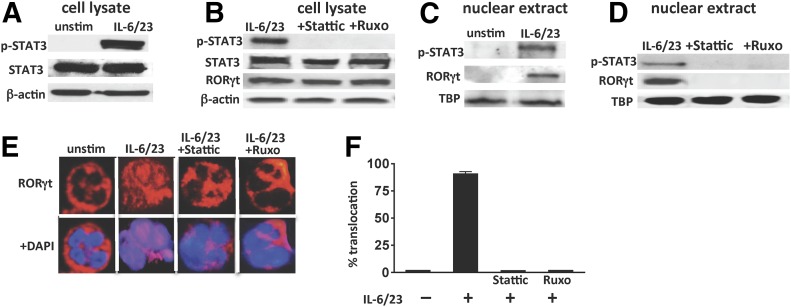
We reported that peripheral blood neutrophils from healthy individuals constitutively express RORγt in the cytoplasm and that following incubation with IL-6/23, RORγt is translocated to the nucleus and initiates Il17a transcription [13]. To examine the role of p-STAT3 in this process, neutrophils were stimulated with IL-6 and IL-23 in the presence of JAK/STAT inhibitors.
p-STAT3 was detected in human neutrophil cell lysates following IL-6/23 stimulation (Fig. 4A) but not in the presence of Stattic or Ruxolitinib (Fig. 4B). Likewise, p-STAT3 and RORγt were detected in nuclear extracts from IL-6/23-stimulated but not unstimulated neutrophils (Fig. 4C). However, when stimulated cells were also incubated with Stattic or Ruxolitinib, p-STAT3 and RORγt were not detected in nuclear extracts (Fig. 4D), indicating that these inhibitors block RORγt translocation to the nucleus. As a second approach to examine translocation, RORγt was visualized by confocal microscopy and found to be only in the cytoplasm of unstimulated cells, whereas RORγt was also in the nucleus of IL-6/23-stimulated human neutrophils. In contrast, RORγt was found only in the cytoplasm in IL-6/23-stimulated neutrophils incubated with Stattic or Ruxolitinib (Fig. 4E). Nuclear RORγt was quantified by multispectral image flow cytometry (ImageStream) of 50,000 cells. As shown in Fig. 4F, RORγt was detected in >80% of neutrophils incubated with IL-6/23 but not in the presence of Stattic or Ruxolitinib (representative ImageStream micrographs are shown in Supplemental Fig. 4).
Figure 4. p-STAT3 and RORγt nuclear translocation in human neutrophils.
(A–D) Western blots of total cell lysates and nuclear extracts from IL-6/23-stimulated peripheral blood human neutrophils in the presence of Stattic or Ruxolitinib. Blots were probed with antibodies to p-STAT3, STAT3, RORγt, and either β-actin as a loading control for total extracts or TBP for nuclear extracts. (E) Representative confocal images of intracellular RORγt (red) in unstimulated (unstim) and IL-6/23-stimulated human neutrophils in the presence of Stattic or Ruxolitinib. Cell nuclei were visualized using DAPI (blue). (F) Quantitative analysis (using ImageStream) showing percent of IL-6/23-stimulated neutrophils in which RORγt was detected in the nucleus. Original magnification, ×1000.
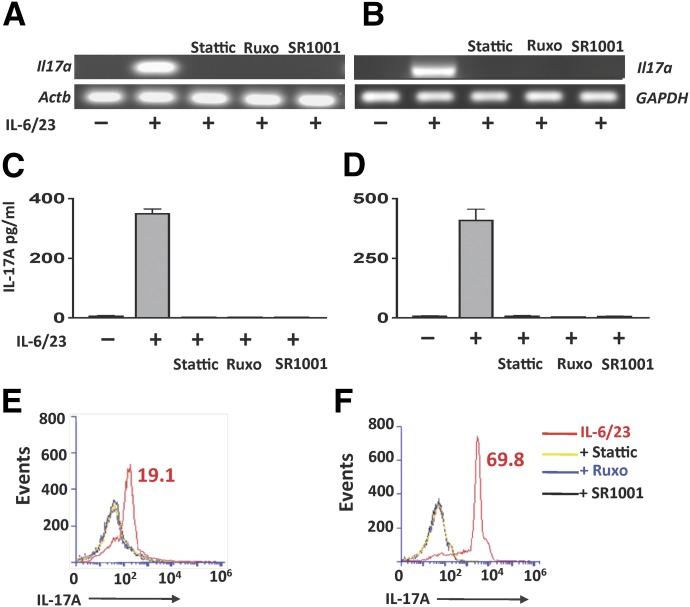
To examine the effect of STAT3 and RORγt inhibitors on IL-17 gene expression and protein, murine and human neutrophils were stimulated with IL-6/23 in the presence of these inhibitors. As shown in Fig. 5A and B, Il17a transcripts were detected in IL-6/23 but not unstimulated neutrophils; however, Il17a expression was completely inhibited when IL-6/23-stimulated cells were incubated with Stattic, Ruxolitinib, or SR1001. (qPCR data are shown in Supplemental Table 3.)
Figure 5. JAK/STAT and RORγt regulation of IL-17 production by murine and human neutrophils.
(A, C, and E) Bone marrow neutrophils from C57BL/6 mice. (B, D, and F) Human peripheral blood neutrophils. (A and B) Il17a gene expression in IL-6/23-stimulated neutrophils in the presence of Stattic, Ruxolitinib, or the RORγt inhibitor SR1001. Actb and GAPDH were loading controls. (C and D) Quantification of IL-17A protein in mouse and human neutrophils by ELISA. (E and F) Intracellular IL-17A production assessed by flow cytometry. Three experiments were performed with similar results.
Consistent with their effect on gene expression, IL-6/23-induced IL-17 protein was not detected when neutrophils were incubated with Stattic, Ruxolitinib, or SR1001 (Fig. 5C and D). Furthermore, flow cytometry analysis showed there were no IL-17+ve neutrophils in the presence of these inhibitors (Fig. 5E and F). Note that the concentrations of rIL-6/23 were based on titrations used previously that induced RNA expression within 1 h and protein within 3 h [13]. Also, the concentration of JAK/STAT inhibitors chosen was based on other reports [17] and on preliminary in vitro experiments (Supplemental Fig. 5).
p-STAT3 regulates elastase and MMP9 activity in IL-6/23-stimulated neutrophils
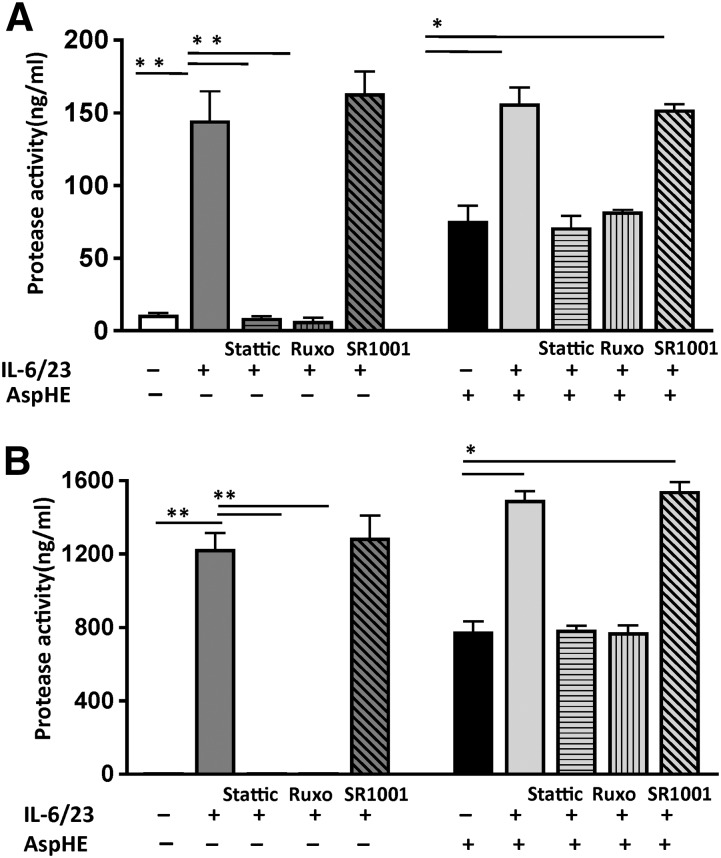
Neutrophil elastase and MMP9 are released from neutrophil granules when the cells are activated and have the potential to cause severe tissue damage. To determine if these proteases are activated in IL-17-producing human neutrophils, enzymatically active elastase and MMP9 were measured in culture supernatants following 3 h stimulation with IL-6/23 and a soluble extract of Aspergillus hyphae (AspHE).
No elastase was detected in the supernatants of unstimulated neutrophils; however, following IL-6/23 stimulation, elastase activity was significantly elevated, and was blocked completely by Stattic or Ruxolitinib (Fig. 6A). Neutrophils incubated with AspHE also had elevated elastase; however, cells incubated with AspHE and IL-6/23 did not produce more elastase than IL-6/23 stimulation alone. Furthermore, in the presence of Stattic or Ruxolitinib, elastase production was at the level shown for neutrophils incubated with AspHE alone (Fig. 6A), indicating that AspHE-induced elastase is independent of STAT3. RORγt inhibition had no effect on elastase activity in any of the stimulations. Similar results were found for MMP9 following IL-6/23 stimulation, which was completely inhibited by Stattic and Ruxolitinib but not SR1001 (Fig. 6B). However, protease secretion by neutrophils stimulated with AspHE alone was not inhibited by Ruxolitinib or Stattic (data not shown).
Figure 6. JAK/STAT-dependent elastase and MMP9 activity in human neutrophils.
Human peripheral blood neutrophils were incubated with AspHE or IL-6/23 in the presence of Stattic, Ruxolitinib, or SR1001. Supernatants were assayed for elastase (A) and MMP9 (B) activity. *P < 0.01; **P < 0.001.
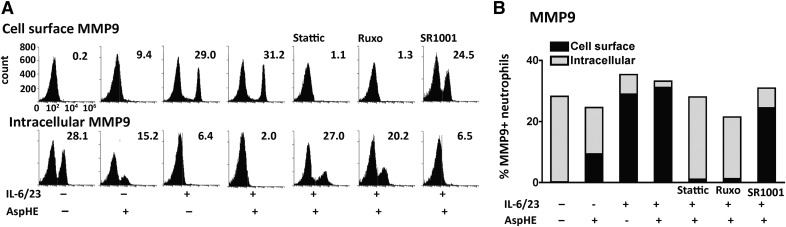
The inactive proform of MMP9 is mostly located in the tertiary granules but is transported and expressed on the cell membrane of activated neutrophils, where it is enzymatically active [25]. Therefore, we examined intracellular and extracellular MMP9 on AspHE and IL-6/23-stimulated neutrophils by flow cytometry.
As shown in Fig. 7A, there was no cell-surface MMP9 in unstimulated or AspHE-stimulated neutrophils; however, MMP9 was present on the cell surface of IL-6/23-stimulated neutrophils. Following incubation with Stattic or Ruxolitinib but not SR1001, there was no extracellular MMP9. Approximately 9% of neutrophils stimulated with AspHE alone expressed cell-surface MMP9. Conversely, intracellular MMP9 was measured in neutrophils following cell permeabilization. Quantification shows ∼30% unstimulated neutrophils with intracellular MMP9, which was reduced following stimulation with AspHE, IL-6/23, and 2% IL-6/23/AspHE-treated cells (Fig. 7B). As with cell-surface MMP9, the effect of IL-6/23 was completely reversed by Stattic or Ruxolitinib but not SR1001.
Figure 7. JAK/STAT-dependent cell-surface expression of MMP9 in human neutrophils.
Neutrophils were incubated with anti-human MMP9 antibody to detect extracellular MMP9 or were permeabilized to detect intracellular MMP9. (A) Representative flow cytometry profiles and (B) combined results. Data are representative of 2 repeat experiments.
These data show that cell-surface MMP9 and secreted, enzymatically active neutrophil elastase and MMP9 can be blocked by JAK/STAT inhibitors following induction by IL-6/23, whereas AspHE-stimulated proteases are independent of this pathway. These findings also indicate that IL-6/23-induced protease activity is independent of RORγt.
DISCUSSION
Individuals with mutations in STAT3 exhibit increased susceptibility to fungal infections [26–29]. STAT3 also regulates (RORγt) expression and Th17 cell differentiation [30, 31]. Although Th17 cells are generated in response to fungal infections, their development requires several days; therefore, Th17 cells have a limited role in the early stages of a rapidly progressing infection with bacteria or fungi [32]. In contrast, IL-17-producing NK–T cells and γδ−T cells constitutively express RORγt and can rapidly produce IL-17 in response to cytokine stimulation [33]. We reported that a subset of human and murine neutrophils constitutively expresses IL-23R and RORγt and produces IL-17 following stimulation IL-6 and IL-23 (IL-6/23) [13]. Furthermore, in contrast to IL-17-producing lymphocytes, this population of neutrophils also expresses a functional IL-17R (IL-17RA/RC), enabling autocrine IL-17 activity, increased production of ROS, and increased hyphal killing in vitro and in a mouse model of A. fumigatus corneal infection [13].
IL-17 stimulates an inflammatory response by activating epithelial cells and fibroblasts, which constitutively express the IL-17RA and IL-17RC subunits of the receptor to produce neutrophil chemokines [34]. The resulting neutrophil influx is required for microbial killing, although release of granule proteases and ROS also causes tissue damage [34, 35].
In the current study, we show that JAK/STAT inhibitors abrogate RORγt translocation to the nucleus of human and murine neutrophils, inhibit IL-17 production, and reduce elastase and MMP9 activity in vitro and in a mouse model of A. fumigatus keratitis.
Given that neutrophils are a predominant source of IL-17 in patients with fungal keratitis [11, 12] and have been reported as a source of IL-17 in fungal and bacterial infections [36–39], in autoimmune diseases, including psoriasis and rheumatoid arthritis [40–42], and in cystic fibrosis [43, 44], findings from the current study are also relevant to these other conditions. IL-17 is reported to stimulate MMP9 release and activity in the bronchial alveolar lavage fluids of COPD patients [45]. Proinflammatory mediators, such as TNF-α and LPS, can activate MMP9 to migrate to the cell surface or cause the cleaved MMP9 to migrate into the extracellular space as an active, soluble form of MMP9 [25].
Neutrophil elastase is in azurophilic (primary) granules, whereas MMP9 is in specific (secondary) and gelatinase (tertiary) granules. Furthermore, exocytosis of each granule type is differentially regulated, including by small GTPases such as RAB27a [46, 47]. IL-8-mediated MMP9 release is dependent on protein kinase C and ERK1/2 but not p38 MAPK [48], whereas PI3K activity is essential for LPS-induced exocytosis of primary but not secondary or tertiary granules. Also, p38 MAPK regulates mobilization of all granules [49]. IL-6, together with GM-CSF, inhibits neutrophil elastase release in the context of tumor growth, in part, by blocking activation of RAB27a, and the increased MMP9 release was associated with increased p-STAT3 [50]. The difference between those findings and ours regarding the inhibitory versus the stimulatory role of IL-6 on elastase activity has yet to be determined but may relate to the combined stimulation with IL-6 and GM-CSF compared with IL-6/23 stimulation in the current study, which may affect the activity of small GTPases that regulate granule mobilization.
Exocytosis of neutrophil granules generally requires incubation with agonists, such as LPS or formyl peptides, before stimulation with cytokines; however, in the current study, we clearly demonstrated that incubation with only IL-6 and IL-23 induced MMP9 expression on the cell surface and secretion of bioactive MMP9 and elastase. We also showed that MMP9 and elastase activity are induced by hyphal extracts, although this was independent of the JAK/STAT pathway and is more likely to be a consequence of activating c-type lectins, such as Dectin-1 and -2, which are expressed on IL-17-producing neutrophils [13, 51]. The increased MMP9 and elastase activity in IL-17-producing neutrophils was blocked by the JAK/STAT inhibitors but not by SR1001, indicating that the elevated activity of these proteases is regulated by IL-6/23 and STAT3 but through a pathway that is independent of RORγt and IL-17.
In contrast to IL-6/23-induced elastase and MMP9 production, SR1001 and JAK/STAT inhibitors blocked IL-6/23-induced ROS production and hyphal killing by human neutrophils in vitro but could be rescued by adding rhIL-17. These findings demonstrated that JAK/STAT activation of ROS is dependent on IL-17, most likely through the autocrine activation of IL-17RC [13]. Likewise, in vivo hyphal killing was impaired in primed mice given Ruxolitinib, in which IL-17 production was inhibited. Although we cannot eliminate the possibility that Ruxolitinib also inhibited STAT3 activation of other cells, such as resident or infiltrating macrophages, there was no difference in neutrophil recruitment to infected corneas in vehicle compared with Ruxolitinib-treated mice. Furthermore, the in vitro effect of Ruxolitinib on ROS-production and fungal killing by murine and human neutrophils support the concept that the major effect of Ruxolitinib on infected corneas is to inhibit neutrophil activation.
ROS production by neutrophils is mediated primarily by NADPH oxidase reduction of O2 to the superoxide radical O2−, and we showed that IL-17-producing neutrophils from mice lacking the NADPH oxidase GP91 subunit have impaired fungal clearance [13]. Hence, it is likely that the mechanism involving Ruxolitinib inhibition of fungal killing occurs by blocking assembly of the NADPH oxidase and subsequent ROS production. As ROS production was blocked by all 3 inhibitors, it is therefore dependent on RORγt and IL-17, possibly by blocking assembly of the NADPH oxidase complex.
We also showed that IL-17-producing neutrophils predominate in individuals who are exposed to high levels of airborne spores and in patients with corneal ulcers caused by the filamentous molds Aspergillus and Fusarium [11, 12]. Therefore, it is likely that the increased proteases contribute to the tissue damage and blindness associated with this infection. Neutrophil elastase and MMP9 are also elevated in the lungs of patients with cystic fibrosis or COPD [45, 52–54]. As IL-17-producing neutrophils have been reported in lungs of patients with cystic fibrosis [43, 44], findings from the current study suggest that targeting IL-17-producing neutrophils could potentially limit the severity of tissue damage. However, as neutrophil ROS has an important role in host defense against bacteria and fungi, the possibility that JAK/STAT inhibitors could abrogate microbial clearance by neutrophils obviates their sole use during infection. If given together with antibiotics or antifungal agents, JAK/STAT inhibitors, such as Ruxolitinib, which has been used in human trials of myelofibrosis [55, 56], would also have the potential to block tissue damage caused by IL-17-producing neutrophils during infection.
AUTHORSHIP
P.R.T., S.R., C.J.M., and E.P. designed the experiments, analyzed the data, and prepared the manuscript. P.R.T., S.R., E.C.M., Y.S., and S.J.H. performed the experiments.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants F32 EY022278 (to P.R.T.), R01 EY018612 (to E.P.), and P30 EY011373 (to E.P.); and by the Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation (to the Department of Ophthalmology).
Glossary
- Actb
actin, β
- Af-BP
Aspergillus fumigatus strain from Bascom Palmer Eye Institute
- Af-dsRed
Aspergillus fumigatus-expressing Discosoma red
- AspHE
Aspergillus hyphal extract
- BCA
bicinchoninic acid
- CFDA
6-carboxyfluorescein diacetate
- COPD
chronic obstructive pulmonary disease
- CT
comparative threshold
- dsRed
Discosoma red
- MMP
matrix metalloproteinase
- p-JAK/STAT
phosphorylated JAK/STAT
- PEG
polyethylene glycol
- qPCR
quantitative PCR
- RFP
red fluorescent protein
- rh
recombinant human
- RORγt
retinoic acid receptor-related orphan receptor γt
- ROS
reactive oxygen species
- SH2
Src homology 2
- TBP
telomerase binding protein
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Lalitha P., Prajna N. V., Manoharan G., Srinivasan M., Mascarenhas J., Das M., D’Silva S. S., Porco T. C., Keenan J. D. (2015) Trends in bacterial and fungal keratitis in South India, 2002–2012. Br. J. Ophthalmol. 99, 192–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L., Sun S., Jing Y., Han L., Zhang H., Yue J. (2009) Spectrum of fungal keratitis in central China. Clin. Experiment. Ophthalmol. 37, 763–771. [DOI] [PubMed] [Google Scholar]
- 3.Xie L., Zhong W., Shi W., Sun S. (2006) Spectrum of fungal keratitis in north China. Ophthalmology 113, 1943–1948. [DOI] [PubMed] [Google Scholar]
- 4.Fusarium Keratitis Investigation Team (2006) Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 296, 953–963. [DOI] [PubMed] [Google Scholar]
- 5.Leal S. M. Jr., Cowden S., Hsia Y. C., Ghannoum M. A., Momany M., Pearlman E. (2010) Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 6, e1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarabishy A. B., Aldabagh B., Sun Y., Imamura Y., Mukherjee P. K., Lass J. H., Ghannoum M. A., Pearlman E. (2008) MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J. Immunol. 181, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrion Sde. J., Leal S. M. Jr., Ghannoum M. A., Aimanianda V., Latgé J. P., Pearlman E. (2013) The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 191, 2581–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leal S. M. Jr., Vareechon C., Cowden S., Cobb B. A., Latgé J. P., Momany M., Pearlman E. (2012) Fungal antioxidant pathways promote survival against neutrophils during infection. J. Clin. Invest. 122, 2482–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark H. L., Jhingran A., Sun Y., Vareechon C., de Jesus Carrion S., Skaar E. P., Chazin W. J., Calera J. A., Hohl T. M., Pearlman E. (2016) Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J. Immunol. 196, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal S. M. Jr., Roy S., Vareechon C., Carrion Sd., Clark H., Lopez-Berges M. S., Di Pietro A., Schrettl M., Beckmann N., Redl B., Haas H., Pearlman E. (2013) Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog. 9, e1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karthikeyan R. S., Leal S. M. Jr., Prajna N. V., Dharmalingam K., Geiser D. M., Pearlman E., Lalitha P. (2011) Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J. Infect. Dis. 204, 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karthikeyan R. S., Vareechon C., Prajna N. V., Dharmalingam K., Pearlman E., Lalitha P. (2015) Interleukin 17 expression in peripheral blood neutrophils from fungal keratitis patients and healthy cohorts in southern India. J. Infect. Dis. 211, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor P. R., Roy S., Leal S. M. Jr., Sun Y., Howell S. J., Cobb B. A., Li X., Pearlman E. (2014) Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat. Immunol. 15, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstovsek S. (2013) Ruxolitinib: an oral Janus kinase 1 and Janus kinase 2 inhibitor in the management of myelofibrosis. Postgrad. Med. 125, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintás-Cardama A., Vaddi K., Liu P., Manshouri T., Li J., Scherle P. A., Caulder E., Wen X., Li Y., Waeltz P., Rupar M., Burn T., Lo Y., Kelley J., Covington M., Shepard S., Rodgers J. D., Haley P., Kantarjian H., Fridman J. S., Verstovsek S. (2010) Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115, 3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Y., Zhou F., Zhang R., Claret F. X. (2013) Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PLoS One 8, e54565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schust J., Sperl B., Hollis A., Mayer T. U., Berg T. (2006) Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13, 1235–1242. [DOI] [PubMed] [Google Scholar]
- 18.Solt L. A., Kumar N., Nuhant P., Wang Y., Lauer J. L., Liu J., Istrate M. A., Kamenecka T. M., Roush W. R., Vidović D., Schürer S. C., Xu J., Wagoner G., Drew P. D., Griffin P. R., Burris T. P. (2011) Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L. Y., Abou-Ghazal M. K., Wei J., Chakraborty A., Sun W., Qiao W., Fuller G. N., Fokt I., Grimm E. A., Schmittling R. J., Archer G. E. Jr., Sampson J. H., Priebe W., Heimberger A. B. (2008) A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin. Cancer Res. 14, 5759–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiguchi A., Asano T., Kuroda K., Sato A., Asakuma J., Ito K., Hayakawa M., Sumitomo M., Asano T. (2010) STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br. J. Cancer 102, 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor P. R., Leal S. M. Jr., Sun Y., Pearlman E. (2014) Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J. Immunol. 192, 3319–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malemud C. J., Pearlman E. (2009) Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr. Signal Transduct. Ther. 4, 201–221. [Google Scholar]
- 23.Garbers C., Aparicio-Siegmund S., Rose-John S. (2015) The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr. Opin. Immunol. 34, 75–82. [DOI] [PubMed] [Google Scholar]
- 24.Watford W. T., Hissong B. D., Bream J. H., Kanno Y., Muul L., O’Shea J. J. (2004) Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 202, 139–156. [DOI] [PubMed] [Google Scholar]
- 25.Owen C. A., Hu Z., Barrick B., Shapiro S. D. (2003) Inducible expression of tissue inhibitor of metalloproteinases-resistant matrix metalloproteinase-9 on the cell surface of neutrophils. Am. J. Respir. Cell Mol. Biol. 29, 283–294. [DOI] [PubMed] [Google Scholar]
- 26.Milner J. D., Brenchley J. M., Laurence A., Freeman A. F., Hill B. J., Elias K. M., Kanno Y., Spalding C., Elloumi H. Z., Paulson M. L., Davis J., Hsu A., Asher A. I., O’Shea J., Holland S. M., Paul W. E., Douek D. C. (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma C. S., Chew G. Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D. A., Tangye S. G., Cook M. C. (2008) Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205, 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Jannière L., Fieschi C., Stéphan J. L., Boileau C., Lyonnet S., Jondeau G., Cormier-Daire V., Le Merrer M., Hoarau C., Lebranchu Y., Lortholary O., Chandesris M. O., Tron F., Gambineri E., Bianchi L., Rodriguez-Gallego C., Zitnik S. E., Vasconcelos J., Guedes M., Vitor A. B., Marodi L., Chapel H., Reid B., Roifman C., Nadal D., Reichenbach J., Caragol I., Garty B. Z., Dogu F., Camcioglu Y., Gülle S., Sanal O., Fischer A., Abel L., Stockinger B., Picard C., Casanova J. L. (2008) Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205, 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puel A., Cypowyj S., Bustamante J., Wright J. F., Liu L., Lim H. K., Migaud M., Israel L., Chrabieh M., Audry M., Gumbleton M., Toulon A., Bodemer C., El-Baghdadi J., Whitters M., Paradis T., Brooks J., Collins M., Wolfman N. M., Al-Muhsen S., Galicchio M., Abel L., Picard C., Casanova J. L. (2011) Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda K., Clausen B. E., Kaisho T., Tsujimura T., Terada N., Förster I., Akira S. (1999) Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49. [DOI] [PubMed] [Google Scholar]
- 31.Yang X. O., Panopoulos A. D., Nurieva R., Chang S. H., Wang D., Watowich S. S., Dong C. (2007) STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363. [DOI] [PubMed] [Google Scholar]
- 32.Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517. [DOI] [PubMed] [Google Scholar]
- 33.Cua D. J., Tato C. M. (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489. [DOI] [PubMed] [Google Scholar]
- 34.Iwakura Y., Ishigame H., Saijo S., Nakae S. (2011) Functional specialization of interleukin-17 family members. Immunity 34, 149–162. [DOI] [PubMed] [Google Scholar]
- 35.Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182. [DOI] [PubMed] [Google Scholar]
- 36.Werner J. L., Gessner M. A., Lilly L. M., Nelson M. P., Metz A. E., Horn D., Dunaway C. W., Deshane J., Chaplin D. D., Weaver C. T., Brown G. D., Steele C. (2011) Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect. Immun. 79, 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L., Huang L., Vergis A. L., Ye H., Bajwa A., Narayan V., Strieter R. M., Rosin D. L., Okusa M. D. (2010) IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S. Y., Yu J. H., Liu F. T., Miaw S. C., Wu-Hsieh B. A. (2013) Galectin-3 negatively regulates dendritic cell production of IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum. J. Immunol. 190, 3427–3437. [DOI] [PubMed] [Google Scholar]
- 39.Cai S., Batra S., Langohr I., Iwakura Y., Jeyaseelan S. (2015) IFN-γ induction by neutrophil-derived IL-17A homodimer augments pulmonary antibacterial defense. Mucosal Immunol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin A. M., Rubin C. J., Khandpur R., Wang J. Y., Riblett M., Yalavarthi S., Villanueva E. C., Shah P., Kaplan M. J., Bruce A. T. (2011) Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187, 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keijsers R. R., Hendriks A. G., van Erp P. E., van Cranenbroek B., van de Kerkhof P. C., Koenen H. J., Joosten I. (2013) In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORγt and IL-17. J. Invest. Dermatol. 134, 1276–1284. [DOI] [PubMed] [Google Scholar]
- 42.Katayama M., Ohmura K., Yukawa N., Terao C., Hashimoto M., Yoshifuji H., Kawabata D., Fujii T., Iwakura Y., Mimori T. (2013) Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One 8, e62231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan H. L., Regamey N., Brown S., Bush A., Lloyd C. M., Davies J. C. (2011) The Th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 184, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brodlie M., McKean M. C., Johnson G. E., Anderson A. E., Hilkens C. M., Fisher A. J., Corris P. A., Lordan J. L., Ward C. (2011) Raised interleukin-17 is immunolocalised to neutrophils in cystic fibrosis lung disease. Eur. Respir. J. 37, 1378–1385. [DOI] [PubMed] [Google Scholar]
- 45.Prause O., Bozinovski S., Anderson G. P., Lindén A. (2004) Increased matrix metalloproteinase-9 concentration and activity after stimulation with interleukin-17 in mouse airways. Thorax 59, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catz S. D. (2013) Regulation of vesicular trafficking and leukocyte function by Rab27 GTPases and their effectors. J. Leukoc. Biol. 94, 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catz S. D. (2014) The role of Rab27a in the regulation of neutrophil function. Cell. Microbiol. 16, 1301–1310. [DOI] [PubMed] [Google Scholar]
- 48.Chakrabarti S., Patel K. D. (2005) Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J. Leukoc. Biol. 78, 279–288. [DOI] [PubMed] [Google Scholar]
- 49.Brzezinska A. A., Johnson J. L., Munafo D. B., Ellis B. A., Catz S. D. (2009) Signalling mechanisms for Toll-like receptor-activated neutrophil exocytosis: key roles for interleukin-1-receptor-associated kinase-4 and phosphatidylinositol 3-kinase but not Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-beta (TRIF). Immunology 127, 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan B., Wei J. J., Yuan Y., Sun R., Li D., Luo J., Liao S. J., Zhou Y. H., Shu Y., Wang Q., Zhang G. M., Feng Z. H. (2013) IL-6 cooperates with G-CSF to induce protumor function of neutrophils in bone marrow by enhancing STAT3 activation. J. Immunol. 190, 5882–5893. [DOI] [PubMed] [Google Scholar]
- 51.Underhill D. M., Pearlman E. (2015) Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 43, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruce M. C., Poncz L., Klinger J. D., Stern R. C., Tomashefski J. F. Jr., Dearborn D. G. (1985) Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am. Rev. Respir. Dis. 132, 529–535. [DOI] [PubMed] [Google Scholar]
- 53.Cantin A. M., Hartl D., Konstan M. W., Chmiel J. F. (2015) Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J. Cyst. Fibros. 14, 419–430. [DOI] [PubMed] [Google Scholar]
- 54.Power C., O’Connor C. M., MacFarlane D., O’Mahoney S., Gaffney K., Hayes J., FitzGerald M. X. (1994) Neutrophil collagenase in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 150, 818–822. [DOI] [PubMed] [Google Scholar]
- 55.Becker H., Engelhardt M., von Bubnoff N., Wäsch R. (2014) Ruxolitinib. Recent Results Cancer Res. 201, 249–257. [DOI] [PubMed] [Google Scholar]
- 56.Bhagwat N., Levine R. L., Koppikar P. (2013) Sensitivity and resistance of JAK2 inhibitors to myeloproliferative neoplasms. Int. J. Hematol. 97, 695–702. [DOI] [PubMed] [Google Scholar]