Review on MNT-comprised intercellular networks in immune cell function and dysfunction, with a focus on myeloid lineage APCs.
Keywords: CD40L, HIV-1, antigen transfer
Abstract
Membrane nanotubes, also termed tunneling nanotubes, are F-actin-based structures that can form direct cytoplasmic connections and support rapid communication between distant cells. These nanoscale conduits have been observed in diverse cell types, including immune, neuronal, stromal, cancer, and stem cells. Until recently, little was known about the mechanisms involved in membrane nanotube development in myeloid origin APCs or how membrane nanotube networks support their ability to bridge innate and adaptive immunity. New research has provided insight into the modes of induction and regulation of the immune process of "reticulation" or the development of multicellular membrane nanotube networks in dendritic cells. Preprogramming by acute type 1 inflammatory mediators at their immature stage licenses mature type 1-polarized dendritic cells to reticulate upon subsequent interaction with CD40 ligand-expressing CD4+ Th cells. Dendritic cell reticulation can support direct antigen transfer for amplification of specific T cell responses and can be positively or negatively regulated by signals from distinct Th cell subsets. Membrane nanotubes not only enhance the ability of immature dendritic cells to sense pathogens and rapidly mobilize nearby antigen-presenting cells in the peripheral tissues but also likely support communication of pathogen-related information from mature migratory dendritic cells to resident dendritic cells in lymph nodes. Therefore, the reticulation process facilitates a coordinated multicellular response for the efficient initiation of cell-mediated adaptive immune responses. Herein, we discuss studies focused on the molecular mechanisms of membrane nanotube formation, structure, and function in the context of immunity and how pathogens, such as HIV-1, may use dendritic cell reticulation to circumvent host defenses.
Introduction
Multicellular organisms require mechanisms of indirect and direct intercellular communication to coordinate complex cellular processes, such as the immune response. Cross-talk between adjacent and remote cells can occur via secreted proteins, including cytokines and chemokines that are recognized by specific target cell surface receptors for the initiation of signal transduction pathways involved in immune cell growth, differentiation, activation, and migration [1]. Additionally, extracellular membrane vesicles, e.g., exosomes, can transfer diverse proteins, lipids, and nucleic acids to target cells for the modulation of immune responses [2]. Whereas secreted proteins and exosomes are able to facilitate long- and short-range communication, they rely on random diffusion to reach their targets. Additional mechanisms, such as gap junctions, defined as narrow channels linking the cytosol of adjacent cells, have evolved specifically to facilitate cross-talk between cells in close contact [3]. Immunologic synapses, comprised of associated TCR and peptide-loaded MHC molecules, as well as adhesion molecules, can form at the junction between T cell and APC membranes and are required for initiation of adaptive immune responses [4].
The recently discovered MNTs, sometimes referred to as tunneling nanotubes, are F-actin-based extensions that establish membrane continuity between cells and combine various aspects of the aforementioned modes of cross-talk [5–10]. These structures can be open ended to support cytoplasm continuity or close ended with restricted ability to facilitate cytoplasmic molecule exchange. Mammalian cells have developed a number of distinctive plasma membrane protrusions to facilitate processes, e.g., probing of the extracellular environment, cell adhesion, and migration. APCs, such as DCs, display diverse membrane structures, including dendrites, veils, ruffles, stress fibers, and filopodia [11–14]. Although filopodia can serve as precursors of MNTs, these structures are adherent and relatively short in length, whereas MNTs are typically nonadherent and can span distances exceeding 100 µm [5, 9, 15]. Until recently, the role of these versatile conduits in the context of an immune response has been largely unappreciated.
DCs serve as critical bridges between innate and adaptive immunity by presenting processed antigens in the context of MHC I or II molecules, which are recognized by specific TCR molecules on cognate CD8+ or CD4+ T lymphocytes, respectively [16]. iDCs undergo a specific maturation program in response to pathogen and host environmental cues, resulting in expression of lymph node-homing chemokine receptors, costimulatory molecules, and cytokines that drive polarized adaptive immune responses [16]. Importantly, the peripheral preprogramming of DCs determines their subsequent responsiveness to the CD4+ Th cell-expressed molecule CD40L, which is critical for "licensing" DCs to promote cell-mediated immunity once they reach the lymphatic tissue [17–19]. DC1 [20], i.e., DCs matured by host- and pathogen-derived signals associated with acute viral infections, such as IFN-γ [21–24], respond to CD40L by producing enhanced levels of IL-12p70, the key factor promoting Th1-biased cellular immunity [25]. Exposure of iDCs to histamines or PGE2 generates DC2 [20], which drive Th2-biased responses and display a reduced ability to produce IL-12p70 upon CD40 ligation [21, 26]. New evidence suggests that the preprogramming of DCs by mediators of type 1 or 2 immunity dramatically impacts not only their ability to produce T cell-polarizing cytokines but also the formation of MNT networks in response to CD40L-mediated CD4+ T cell help, which may be important for intercellular exchange of antigens in lymph nodes [27].
In addition to their ability to present processed antigens to CD4+ T cells, DCs can "cross present" exogenous antigens from virus-infected or tumor cells as peptides associated with MHC I to cognate CD8+ T cells for subsequent initiation of CTL responses [28]. Whereas migratory DCs can present antigens and may be essential for their initial transportation to draining lymph nodes, the induction of effective CTL responses likely requires communication with a subset of lymph node-resident DCs that specialize in cross-presenting antigens to CD8+ T cells [29–32]. The precise mode of exchange is unclear, but the transfer of antigenic information between migratory and lymph node-residing DCs has been shown to be essential in models of immunity to viruses [32, 33]. Antigen acquisition by DCs through intercellular hand-off has been demonstrated via uptake of apoptotic cell debris [34] or DC-derived exosomes [35] and also direct membrane exchange between DCs [36]. Interestingly, in situ imaging studies have revealed that striking morphologic alterations occur in maturing migratory DCs upon entry into lymph nodes within 24–48 h, including the formation of extended membrane processes, as they are "linked in" or integrated into a network of lymphoid-residing DCs [11], thus supporting the notion of direct antigen transfer. PGE2 maturation, which until recently was considered the standard method for generating mature DCs in vitro, is typified by membrane ruffles and short hair-like protrusions, but the extended membrane processes described in vivo are rarely observed [13]. Consequently, in vitro culture systems have often failed to recapitulate this network and therefore, have conceptualized and interrogated individual DC function rather than that of a physically integrated system capable of orchestrating a coordinated multicellular response to a pathogen assault.
Although in vitro and in vivo evidence has recently emerged to support a role for MNTs in immune cell communication, transmission of certain pathogens, and chronic diseases, such as cancer [5, 8, 37, 38], little is known about the function of MNTs in the context of a coordinated immune response. For example, as the innate immune pathogen/tissue-sensing role of myeloid-derived DCs in the periphery is quite different from their antigen-presenting role within lymph nodes during the adaptive response, it is likely that their MNTs also serve distinct functions in these immune settings. In this review, we discuss the discovery of MNTs, the general molecular mechanisms of their formation, their structural and cellular diversity, the immunologic function of MNTs with a focus on myeloid origin APCs, and finally, their use by pathogens, i.e., HIV-1, for enhanced direct cell-to-cell spread. A summary of the modes of MNT induction, structure, and cargoes, as well as their proposed in vivo functions from the key primary studies described herein, is provided in Table 1.
TABLE 1.
Inducers of MNTs in different cell types and their structure and function
| Cell type |
Induction and formation |
Structure and morphology |
Labeled cargoes |
Proposed in vivo function |
|---|---|---|---|---|
| Cell lines, neuronal, and stem cells | ||||
| Rat PC12, HEK, NRK cells [39] | De novo in culture; formed by filopodia extension | F-actin+ MT−, 50–200 nm diameter (thin); longa, rarely branching, above substratum | Cytoplasmic LysoTracker+ lysosomes and EGFP-; SNP+ endosomes and MVs; membrane EGFP-fusion proteins, DiI, and DiO | Novel mode of intercellular communication for cytoplasmic vesicle and membrane cargo transfer |
| HeLa cells [40] | De novo in culture, LST-1 expression | ND | Transmembrane HLA-A2-EGFP | Exchange of antigen and MHC I-antigen complexes |
| Rat hippocampal astrocytes and neurons [41, 42] | H2O2- or serum depletion-induced cell stress; p53- and caspase 3-dependent; directionality determined by S100A4 gradient | F-actin+ MT− (indirect detection of MT by blocking); length range 21–30 µm, above substratum | Cytoplasmic AF488 dye, organelle light-tagged mitochondria, ER, Golgi, endosomes, and β-amyloid-EGFP (unidirectional toward unstressed cells) | Cellular response to deleterious signals for transfer of cellular components or energy |
| Mesenchymal stem cells to endothelial cells [43]; PC12 cells [44] | Endothelial cell injury induced by in vitro ischemia-reperfusion [43]; UV-induced PC12 cell stress [44] | F-actin+ (indirect detection by blocking) MT ND [43]; majority F-actin+ MT+ (thick) [44] | AcGFP1- and DsRed2- vector-labeled mitochondria [43]; MTDR+ and DsRed2− mitochondria (unidirectional toward stressed cells) [44] | Direct mitochondria transfer from healthy to unhealthy cells is a mechanism to rescue them from early apoptosis. |
| Lymphoid origin cells and granulocytes | ||||
| Human NK to target cells, human and murine macrophages [45] | De novo in coculture system; formed by cell divergence | F-actin ND MT ND, average length 30 µm, range 10 to >50 µm | Cytoplasmic DiO+ vesicles; DiD+ membrane lipids GPI-GFP, GFP-HLA-C | Novel mode of intercellular communication in immune cells |
| Human NK cells or human NK tumor cell line to target cells [46] | NK cell ligand-receptor pairing, NK-activating cytokines; cell divergence (contact >10 m) | Majority F-actin+ MT+ (thick), membrane junction present | Immunologic synapse proteins localized to MNT junction but were not transferred | Submicron scale long-range immunologic synapse formation leads to target lysis. |
| Human- and murine-activated CD4+ T cells, Jurkat T cells [47] | De novo in culture; cell divergence (contact >4 m) | F-actin+ MT− (thin), mean length 22 ± 3 µm, >100 µm maximum, above substratum, motile membrane junction | HIV-1 Gag-GFP proteins; membrane GFP-ICAM-1, HLA-C, and small cytoplasmic dyes localized to MNTs but were not transferred | Viruses, such as HIV-1, can exploit MNTs for rapid and direct cell-to-cell spread. |
| Human-activated CD4+ T cells, Jurkat T cells [48] | Fas/CD95 interaction with FasL, dependent on Rho GTPases | F-actin and MT ND, no membrane junction | Cytosolic YFP, CFSE, PKH67, caspase 3, TMRE+ mitochondria, IS-FasL; membrane IS-CD59 and -CD81 | Propagation of death signals between neighboring T cells |
| Murine mast cells [49] | Costimulation of FcεR1-CCR1 with antigen and MIP-1α | F-actin+ MT ND, ∼100 µm in length, cholesterol requirement | Fluo-3-AM-labeled Ca2+ flux | Induced during allergic and inflammatory response |
| Myeloid origin cells | ||||
| Human macrophages [50] | De novo in culture | F-actin+ MT−, diameter ≤0.7 µm (thin; close-ended?); F-actin+ MT+, diameter >0.7 µm (thick; open-ended?) | Thin: membrane-bound streptavidin beads and BCG-GFP (unidirectional); thick: cytoplasmic DiD+ vesicles, LTR+ and IS-lysosomes, MTDR+ mitochondria (bidirectional) | Distinct cytoskeletal composition of MNTs results in functional differences. |
| Human iDCs and monocytes [51] | Escherichia coli supernatants or mechanical stimulation | Thin connections ≤100 µm in length, not confined to substratum | Fura-2-labeled Ca2+ fluxes, small soluble marker (lucifer yellow), small particulate marker (Texas Red dextran) | Novel mechanism of direct Ca2+ exchange for multicellular response to inflammatory stimuli |
| Human iDCs, mature DC1 [27] | iDCs: IFN-γ + cytokines/PAMPs, activated CTLs, or NK cells; mature DC1: CD40L+ CD4+ T cells, rhCD40L | iDCs: long, thin, and nonbranching; DC1: F-actin+ MT ND, variable in diameter, length, and branching | iDCs: ND; DC1: cytoplasmic vesicles, EEA1+ endosomes, YG nanobeads, viral peptide, and bacterial protein antigens | CD40L+ CD4+ T cells induce MNTs uniquely in DC1 for antigen exchange and enhanced type 1-adaptive immune responses. |
| Murine corneal MHC II+-presumptive DCs [52] | Trauma or LPS in WT GFP and CX3CR1 chimeric mice | F-actin ND, MT ND, short (<60 µm) to long (≤333 µm) and thick; MNT+ cells in inflamed > naïve corneas | Focal bulges indicating vesicular transport | Integration of remote DCs across cornea for antigen transfer and response to inflammatory stimuli |
| Human macrophages [53–55] | HIV-1 infection | F-actin+ MT ND, average length 30 µm (short) and 150 µm (long) [53]; F-actin ND MT ND, length typically >100 µm [54, 55] | IS-HIV-1 Gag p24 in MNTs [53]; Rab9+ endosomes, IS-Golgi and -ER proteins in MNTs, transfer of ER- and Golgi-bearing IS-HIV-1 Env and Gag [54, 55] | HIV-1 infection induces MNTs for direct high-speed viral spread. |
| Human monocytes, macrophages to B cells [56, 57] | HIV-1 Nef [56]; HIV-1 Nef interaction with exocyst complex [57] | F-actin+ MT− (indirect measure by blocking) long and short [56]; F-actin+ MT ND [57] | Cytoplasmic HIV-1 Nef-EGFP and LysoTracker+ vesicles; membrane-bound Nef-EGFP [56]; ND [57] | HIV-1 infection induces MNTs via HIV-1 Nef interaction with the exocyst complex. |
| Human iDCs [58] | HIV-1-specific cross-reactive CTL-produced cytokines | F-actin ND MT ND, long | ND | CTL-programmed DC1 can facilitate direct, high-speed HIV-1 transfer. |
NRK, normal rat kidney; MT, microtubules; EGFP, enhanced GFP; SNP, synaptophysin; AF, Alexa Fluor MVs, microvesicles; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DiO, 3,3′-dioctadecyloxacarbocyanine perchlorate; ND, not determined; AcGFP1, Aequorea coerulescens GFP1; DsRed2, red fluorescent protein from Discosoma sp.; MTDR, MitoTracker Deep Red; DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt; FasL, Fas ligand; YFP, yellow fluorescent protein; TMRE, tetramethylrhodamine, ethyl ester, percholate; IS, immunostained; AM, acetoxymethyl ester; BCG, Mycobacterium bovis bacillus; LTR, LysoTracker Red; PAMPs, pathogen-associated molecular patterns; EEA1, early endosome antigen 1; YG, yellow green; WT, wild-type.
Greater than 1 cell diameter.
DISCOVERY OF MNTs
The initial in vitro observation of MNTs, defined as nonadherent, ultrafine cylindrical structures forming direct intercellular connections, was made by Gerdes and colleagues in 2004 [39]. These structures were visualized in rat PC12 cells, a commonly used embryonic neuronal cell model, in addition to HEK and normal rat kidney cells. The conduits were typically nonbranching, 50–200 nm in diameter, up to a few cell diameters in length, and could connect cells over long distances, resulting in the formation of complex intercellular networks. They supported the transfer of lysosomal and endosomal vesicles, presumably via the F-actin-associated molecular motor myosin-Va, as well as membrane proteins. Importantly, MNTs were readily disrupted by prolonged exposure to light, chemical fixation, or mechanical stress, explaining, in part, why the structures had been undiscovered previously. The authors also proposed a "filopodia extension model" of MNT formation, wherein an F-actin-driven protrusion from a donor cell is extended toward a target cell, presumably guided by chemotaxis, resulting in fusion with the target cell membrane.
Furthermore, in 2004, Davis and colleagues [45] first described MNTs in cultured myeloid- and lymphoid-origin immune cells, including human macrophages, murine macrophage J774 cells, and between human NK and target cells. These conduits similarly contained F-actin and supported the direct transfer of cytoplasmic vesicles and membrane components. Importantly, the authors also revealed an alternate mode of MNT formation, termed the “cell divergence model”, when they reported that MNTs were drawn out from NK and target cells that were in close contact as they moved apart. Although subsequent investigations of MNTs sometimes fail to describe the manner of MNT formation, this likely depends on the cell type involved, e.g., MNTs in lymphoid lineage immune cells are typically formed by cell divergence. Since these seminal discoveries, the conduits have been observed to form between a wide variety of immune cell types in vitro, including myeloid lineage monocytes, macrophages, and DCs, as well as lymphoid lineage T cells, and between NK cells or CTLs and target cells [5, 6]. However, MNT structure and function can differ greatly even between related cell types, and their modes of induction and in vivo functions are not fully understood.
MOLECULAR MECHANISMS OF MNT FORMATION
M-Sec, the RalA-exocyst complex, and LST-1
Recent studies have provided insight into the molecular cues involved in MNT formation in myeloid lineage and other cell types (Table 2). Hase et al. [59] revealed in late 2009 that functional de novo MNT formation in a murine macrophage line required interaction of the cytoplasmic protein M-Sec, also known as TNFaip2, with the active Ras-like GTPase RalA, which induces filopodia extensions by several mechanisms, including direct binding of filamin for actin filament cross-linking and the exocyst complex. Most important for MNT formation and MNT-mediated calcium flux was the upstream effect of M-sec on the RalA-exocyst effector complex. This composite consists of 8 protein subunits conserved in yeast and mammalian cells and participates in actin cytoskeleton remodeling and vesicle trafficking by tethering vesicles at the plasma membrane and regulating polarized exocytosis [57, 64]. Intriguingly, M-Sec mRNA was highly expressed in myeloid lineage cells and treatment of a macrophage line with LPS or IFN-γ induced M-Sec expression, hinting that MNT induction can occur in response to proinflammatory signals [59].
TABLE 2.
Molecular mechanisms of MNT formation
| Cell and MNT type | Molecule or nanodomain | Interacting partners | Role in MNT formation or maintenance |
|---|---|---|---|
| Macrophage cell line, intestinal M cells, HeLa cells; F-actin+ MT− (thin) [59] | M-Sec (also known as TNFaip2) | RalA GTPase, Cdc42, Sec5a | Inflammatory stimuli (LPS and IFN-γ) induce M-Sec expression, followed by MNTs via filopodia extension that support Ca2+ flux. |
| HeLa cells; F-actin+ MT− (thin), long; F-actin+ MT+ (thick), short [60] | LST-1 | RalA, filamin, M-Sec, myosin, and myoferlin | LST-1 acts as a scaffold, promoting assembly of the molecular machinery of MNT formation, which facilitates cytoplasmic vesicle transfer. |
| Murine neuronal cell line [61] | Myosin-X (also known as Myo10) | ND | Myosin-X-driven dorsal filopodia participate in forming MNTs that support cytoplasmic vesicle transfer. |
| Human urinary bladder cancer cell line [62] | Cholesterol-sphingomyelin membrane nanodomains | ND | Cholesterol-rich lipid rafts are necessary for MNT stability. |
| Human mesothelioma cells [10] | Cholesterol-rich membrane nanodomains | ND | Lipid rafts are enriched in MNT-expressing compared with MNT-deficient cells. |
| Urothelial cell line [63] | N-cadherin and β-catenin adhesion proteins | Target cell membrane proteins | Anchoring junction forms between patch of plasma membrane on tip of filopodia and target cell. |
Member of exocyst complex.
In 2013, Schiller et al. [60] demonstrated that assembly of the multiprotein complex required for MNT formation is facilitated by the transmembrane MHC III protein LST-1, which is also highly expressed on myeloid lineage cells, such as DCs and macrophages. In their model of MNT formation, LST-1 serves as a scaffold, first recruiting RalA and filamin to the plasma membrane for actin cross-linking and then facilitating the interaction of M-Sec, RalA, and the exocyst complex. Reports of high M-Sec mRNA expression in lymphoid tissues and M-Sec and LST-1 mRNAs in immune cells provide a rationale for further investigation of these conduits in an immunologic context [59, 60]. However, Zurzolo and colleagues [61] demonstrated that neurons fail to express this molecule, implying that M-Sec-mediated MNT formation is limited to immune cells. They also revealed a role for myosin-X-driven dorsal filopodia in MNT development and subsequent vesicle transfer in a mouse neuronal cell line. Whereas actin dynamics and the participation of accessory molecules, such as myosin-X, in MNT development remain to be explored, the aforementioned studies suggest a role for mediators of acute inflammation in the M-Sec- and LST-1-dependent induction of MNTs in myeloid origin cells.
Adhesion molecule and membrane lipid requirements
In addition to the described multimolecular MNT assembly complex, both adhesion molecules and cholesterol-sphingomyelin-rich membrane nanodomains are likely required for MNT formation (Table 2). Lokar et al. [62] showed that cholesterol-sphingomyelin nanodomains localize to the length of MNTs in human cancer cells. Depletion of membrane cholesterol resulted in the retraction of these structures, indicating a requirement for cholesterol-rich lipid rafts in the maintenance of MNT stability. Lou and colleagues [10] also reported that lipid rafts are 2-fold higher in MNT-expressing mesothelioma cells compared with MNT-deficient cells. Clearly, there is need to assess the effects of drugs acting on cholesterol biosynthesis pathways, such as 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins) on MNT formation in myeloid lineage cells. Another study in a human cancer cell line showed that the junction proteins N-cadherin and β-cetenin localize to MNTs and likely anchor the tip of the donor cell extension to the plasma membrane of the recipient cell [63]. Filopodia were defined as <10 µm in length and originated from the convex edge of the cell, whereas MNTs were ≥10 µm in length and typically extended from the upper rim of the cell. Intriguingly, extension of MNT-like protrusions required the presence of another cell within 100 µm, whereas filopodia formation occurred independently of cell proximity, indicating that the donor cell has a mechanism for sensing target cells before initiating MNT formation.
ENVIRONMENTAL SIGNALS DRIVING MNT DEVELOPMENT
In certain cell types, environmental cues, such as stress, a receptor-ligand interaction, and/or cytokine-induced activation, can initiate MNT formation. Zhang and colleagues [41] showed that rat hippocampal astrocytes and neurons develop MNTs in response to H2O2- or serum depletion-induced stress in a p53-dependent manner. Stressed cells consistently formed MNTs toward unstressed cells in cocultures, and these structures supported the unidirectional transfer of mitochondria, ER, Golgi, endosomes, and β-amyloid to unstressed cells. In a related study, cell stress resulted in cleavage of the small calcium (Ca2+)-binding protein S100A4 via a p53 and caspase 3 pathway [42]. A decrease in the concentration of this protein in astrocytes led to the establishment of a gradient, which presumably guided S100A4 receptor-bearing protrusions to extend toward healthy cells expressing higher S100A4 levels. Remarkably, astrocytes receiving MNT inputs were protected from later stress-induced apoptosis. MNT directionality in neurons also depended on an S100A4 gradient but was notably also activity dependent. In other studies, MNT directionality was observed exclusively from healthy toward stressed cells, i.e., from healthy mesenchymal stems cells to injured endothelial cells [43] and from healthy to UV-stressed PC12 cells [44]. The role of stress and small calcium-binding protein gradients in the induction and function of MNTs in other diverse cell types expressing MNTs remains to be investigated.
In immune cells, a specific receptor-ligand interaction can initiate MNT formation, although the intermediate molecular signaling pathways remain mysterious. For example, mast cell stimulation with chemokine receptor 1 and FcεR1, along with antigen and MIP-1α, induced MNTs, which grew out from cytoplasmic regions of Ca2+ accumulation and were also inhibited by membrane cholesterol depletion [49]. Furthermore, cytokine-induced activation and engagement of cognate receptor-ligand pairs enhanced MNT formation between NK and target cells [46]. Together, these data provide us with a partial understanding of the types of environmental signals involved in the induction of MNTs, but their structure and function in these various contexts warrant further elucidation.
DIVERSITY OF MNT STRUCTURE AND FUNCTION
Lymphoid lineage immune cells
MNTs display great diversity in composition and function, as they can support the intercellular transfer of vesicles, organelles, cytoplasmic and cell surface proteins, Ca2+ fluxes, microRNAs, electrical signals via interposed gap junctions, as well as some pathogens in various immune and nonimmune cells [5–10, 38, 65–67]. The diversity of MNT composition and function in the multitude of cell types capable of forming them has been reviewed extensively elsewhere [5–9, 37, 68]. In brief, the ability of MNTs to transmit different cargoes depends on their cytoskeletal composition and the presence or absence of a junction at the interface between a donor cell MNT and the target cell membrane. The cytoskeleton of thin, transient MNTs is comprised of F-actin alone, and these structures can readily transfer cytoplasmic small molecules, such as Ca2+ and vesicles, presumably via myosin motors, as well as surface molecules aided by constitutive flow (Fig. 1A). Thick MNTs, typically >0.7 µm in diameter, are more stable and contain F-actin and microtubules, which support the trafficking of large organelles, such as mitochondria and vesicles capable of transporting large particulates (Fig. 1B). Interestingly, conduits formed between activated CD4+ or Jurkat T cells were not open ended but displayed a dynamic membrane junction that limited the transfer of large transmembrane and cytoplasmic molecules (not depicted) [47]. The border found between activated human NK cell-derived MNTs and target cells enabled the formation of long-range immunologic synapses and killing of tethered target cells [46]. In contrast, engagement of the Fas/CD95 surface receptor, which mediates rapid apoptosis in diverse cell types, induced in CD4+ T or Jurkat T cells open ended MNTs capable of supporting the transfer of cytoplasmic components [48]. Together, these data highlight the emerging view that functionally distinctive MNTs can be induced even in the same cell type depending on the microenvironment and molecular signaling pathways involved in their formation.
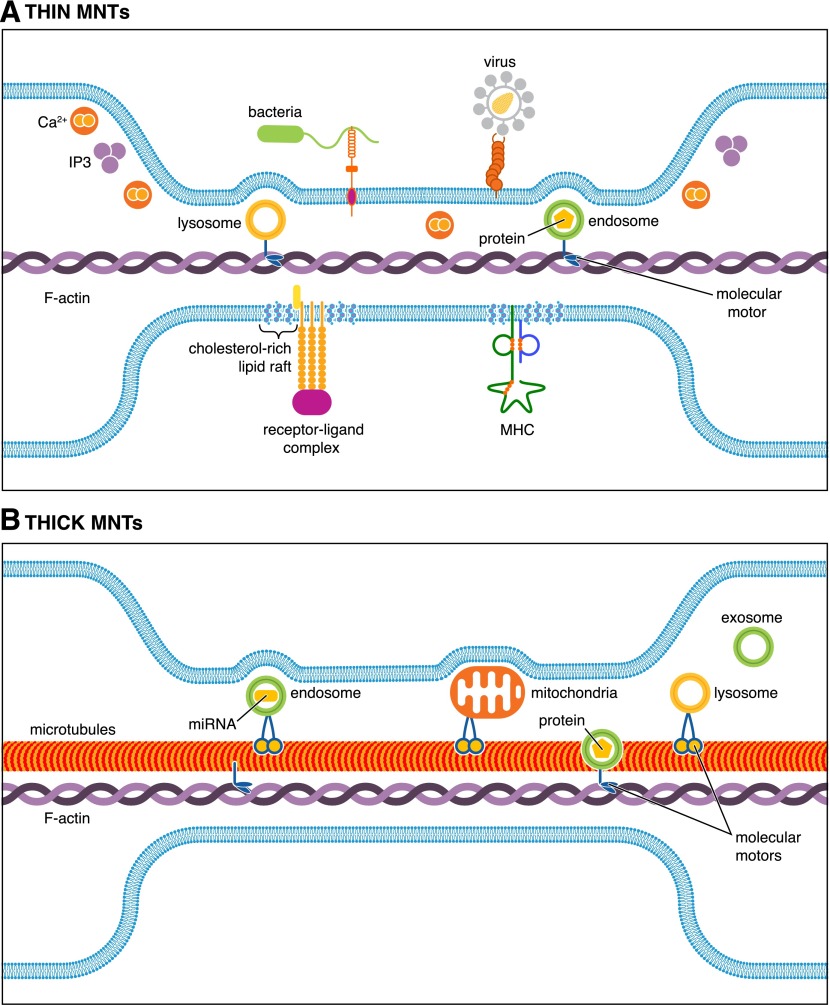
Figure 1. Thin and thick MNTs support intercellular exchange of diverse cargoes.
Representation of the various cytoplasmic and plasma membrane-associated cargoes that can be directly transported between interconnected cells via transient F-actin-comprised, thin MNTs compared with thick F-actin- and microtubule-containing stable MNTs,. The exchange of lysosome-, endosome-, and exosome-associated vesicles, which can contain a wide variety of molecules, such as proteins and microRNAs (miRNA), has been reported in thin and thick MNTs. MNTs can also be open ended or display a membrane junction between the donor cell MNT and the target cell, which limits cargo transfer but can facilitate long-range immunologic synapse formation or electrical signaling via interposed gap junctions (not depicted). (A) Membrane-associated receptor-ligand complexes and MHC molecules, pathogens bound to capture molecules, and cholesterol-rich membrane nanodomains can be transported along the outside of thin MNTs, aided by constitutive flow. Thin MNTs can also support intercellular Ca2+ flux over long distances. (B) The trafficking of large organelles, such as mitochondria, has been reported primarily in thick MNTs containing microtubules, in addition to F-actin.
Myeloid lineage macrophages
In 2006, Davis and colleagues [50] reported the existence of functionally distinct types of MNTs in human monocyte-derived macrophages. Thin MNTs were described as those with a diameter ≤0.7 µm that contained only F-actin and participated in capture and "surfing" of bacteria along the length of the structures for internalization by phagocytosis at the cell body. Furthermore, streptavidin-coated beads traveled along the outside of thin MNTs, aided by unidirectional constitutive flow at a rate of 0.13 µm/s. On the other hand, thick MNTs (>0.7 µm) were comprised of F-actin and microtubules and supported the bidirectional trafficking of vesicles at a rate of ∼1 µm/s. Importantly, vesicles and mitochondria were observed trafficking between cells solely via thick MNTs, implying that the conduits were open ended, but they failed to support bacteria surfing on the surface. Whereas the previously described ultrafine MNTs were relatively transient, thick MNTs could persist for a few hours. Both thin and thick MNTs were typically nonbranching, and the 2:1 ratio of thin:thick MNTs was relatively unchanged by culture on glass, fibronectin, or collagen.
Interestingly, recent studies suggest a protective effect on cells receiving healthy mitochondria via microtubule-containing MNTs. Cell-to-cell transfer of mitochondria is thought to be a general mechanism for rescuing stressed or damaged cells from apoptosis, and MNTs can provide a direct pathway for such exchanges, as shown in the mitochondrial rescue of injured vascular endothelia cells by mesenchymal stems cells [43] and in the rescue of UV-stressed PC12 cells by healthy PC12 cells [44]. Unlike healthy cells, MNTs formed by stressed cells contained microtubule tracks that facilitated mitochondria transport [44]. With the consideration of the existence of thick MNTs in myeloid origin APCs, it is conceivable that they could be utilized as a future therapeutic delivery mechanism for mitochondria-mediated rescue of stressed or damaged cells, particularly those that do not regenerate or proliferate slowly, e.g., cardiomyocytes and neurons.
Cell-to-cell propagation of Ca2+ fluxes in iDCs
Critical insight into inducible MNT function in myeloid cells was provided by Watkins and Salter [51] in 2005, when they revealed how novel, MNT-like extensions participate in transmitting pathogen-inducible activation signals. The authors showed that these conduits mediate the transmission of Ca2+ fluxes, a critical early step in cell activation, between human monocyte-derived iDCs and THP-1 monocytes in response to mechanical stimulation or E. coli supernatants. Gap junction and extracellular ATP inhibitors failed to affect MNT-mediated flux transmission, whereas physical disruption of MNTs abolished flux transmission. Furthermore, a small soluble marker transferred more readily than larger particulates via the formations, which resembled previously described thin MNTs. With the consideration that MNT-mediated Ca2+ fluxes were actively propagated by IP3 in nonimmune cell lines, and IP3 receptors localized along the length of MNTs [69], IP3 could also play a role in this process in myeloid cells. Existing data suggest that MNT-mediated Ca2+ signaling can initiate a rapid, coordinated, multicellular response to pathogen-related signals in iDCs, which may assist in an acute proinflammatory response to injury or infection.
CD4+ Th cell-mediated induction and regulation of MNTs in mature DCs
Whereas MNT participation in the transmission of signaling fluxes in response to pathogen sensing has been well described in iDCs [51], until recently, little information existed regarding MNT induction and function in maturing migratory DCs. Our recent investigation revealed that the key DC-activating CD4+ Th cell signal, CD40L, is critical for initiating MNT development in DCs matured in the presence of type 1 mediators of acute inflammation [27]. With DC1, CD40L activation resulted in reticulation or the rapid formation of an intricate and extensive network of MNTs (Fig. 2), whereas PGE2-programmed DC2 failed to form these networks, and mature, nonpolarized DC0 displayed an intermediate response. Furthermore, costimulation with IFN-γ dramatically enhanced CD40L-induced reticulation in DC0, suggesting an IFN-γ-driven, positive-feedback loop, wherein IFN-γ-producing effector NK cells, CTLs, and Th1 cells can promote further DC1 polarization and MNT-mediated intercellular communication. Conversely, the Th2 cytokine, IL-4, negatively regulated the reticulation process in DC0 and DC1, suggesting a negative-feedback mechanism for dampening this inflammatory process. Importantly, preprogrammed DC2 were completely refractory to IFN-γ- and CD40L-induced reticulation, indicating an inability of this cell type to be reprogrammed once terminally differentiated.
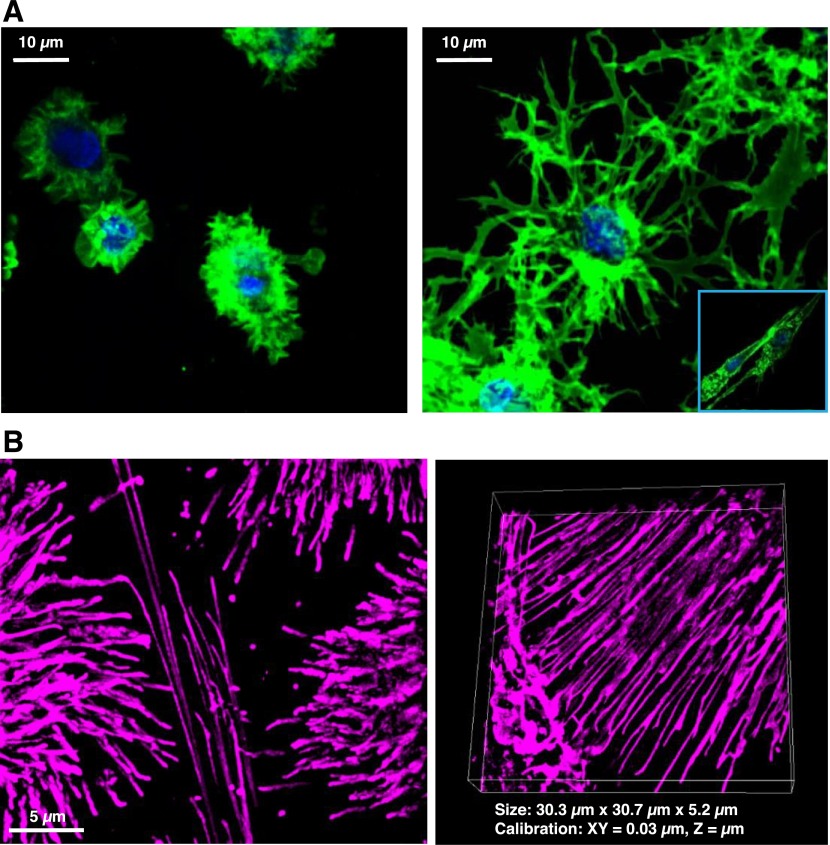
Figure 2. Visualization of CD40L-inducible reticulation in DC1.
(A) Maximum intensity Z-slice reconstruction images (600×) of PGE2-programmed DC2 (left) or IFN-γ programmed DC1 (right), imaged by confocal after 18–20 h stimulation with rhCD40L. (Inset) Morphology of resting DC1 exposed to media alone. (B) Live-cell super-resolution SIM (1000×) of CD40L-activated DC1 labeled with cyanine 5 in a maximum projection Z-slice reconstruction (left) or volume view Z-slice reconstruction (right).
CD40L induced extensive MNTs in DC1, and these conduits displayed a high degree of variability in diameter, length, and complexity of branching [27]. CD40L-activated DC2 morphology differed dramatically from DC1, as these cells were typically smaller, more rounded, and exhibited membrane ruffling or short hair-like projections, as opposed to MNT-like extensions. Interestingly, approximately 25% of resting DC1 also displayed MNTs, which were long, ultrafine, and nonbranching, similar to those described previously in iDCs [51]. CD40L-activated DC1 also consistently displayed thick MNTs that resembled those described in macrophages, hinting that these thicker MNTs were comprised of F-actin and microtubules and could have a distinct function compared with thin MNTs.
Further study is required to determine the exact cytoskeleton composition and dynamics, as well as downstream signaling molecules involved in the formation of CD40L-induced MNTs. The role of the CD40L-induced DC1 reticulation process in vivo also remains to be explored. However, these data suggest that reticulation could facilitate the "linking in" or integration of migratory DCs into the pre-existing resident DC network, which has been observed previously in lymph nodes [11], to facilitate the direct transfer of antigen from migratory to resident DCs for enhanced cellular immune responses (Fig. 3). In addition to de novo production by naïve CD4+ T cells in lymph nodes [70], preformed CD40L can be stored by effector and memory CD4+ T cells in lysosomal compartments for rapid delivery to the cell surface upon secondary antigen stimulation [71]. Therefore, CD40L-mediated induction of MNTs in myeloid cells could also occur at sites of inflammation in the periphery.
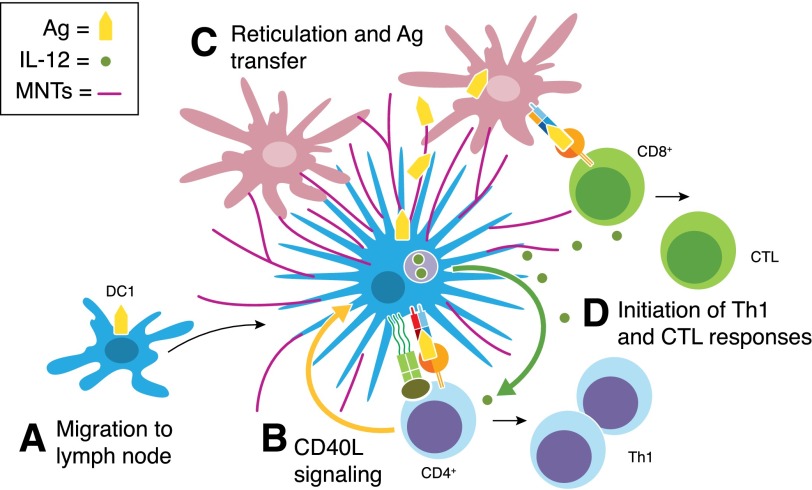
Figure 3. CD40L-induced DC1 reticulation and antigen transfer.
(A) Following their entry into the regional lymph node, migratory DC1 (blue) interact with cognate naïve CD4+ T cells. (B) CD40–CD40L signaling mediates bidirectional cross-talk between DC1 and interacting T cells. In addition to producing IL-12p70, DC1 uniquely respond to the CD4+ T cell signal CD40L by undergoing the process of reticulation, wherein they develop an extensive network of MNT-like processes. (C) Reticulation likely allows migratory DC1 to integrate in the multicellular resident DC network (red). (D) The direct hand-off of antigen to interconnected DC could facilitate the amplification of antigen-specific Th1 and CTL responses by migratory and resident DCs. Effector T cells are programmed to migrate from the lymph node to the initial site of infection, where they produce key inflammatory mediators, such as IFN-γ and TNF-α, upon secondary antigen stimulation, thereby promoting the polarization of additional DC1 in a positive-feedback loop (not shown).
Direct MNT-mediated antigen and MHC molecule exchange
Recent studies suggest that MNT networks can serve as a direct pathway for antigen exchange, either through the transfer of processed antigens associated with MHC molecules or as unprocessed or partially processed antigens. Weiss and colleagues [40] demonstrated the intercellular transfer of vesicles containing MHC I molecules and to a lesser extent, surface-bound MHC I molecules in myeloid origin cells and HeLa cells. Inflammatory stimuli increased LST-1 protein expression in TNF-α- and IFN-γ-exposed mature DCs and HeLa cells, confirming a role for these mediators in MNT induction. Transfected HeLa cells readily transferred the transmembrane fusion protein HLA-A2-EGFP to target HeLa cells in an exchange that primarily involved cargo-carrying endocytic vesicles, and this phenomenon was effectively blocked by disruption of actin polymerization and enhanced by overexpression of LST-1. This study provides indirect evidence for MHC molecule exchange in myeloid origin APCs and highlights a possible role for MNT networks in the facilitation of "cross-dressing" or the exchange of MHC-antigen complexes between remote APCs.
The selective induction of reticulation in DC1 represents a novel helper function of CD40L and likely provides a pathway for intercellular transfer of activation signals or antigens from donor to recipient DC. Our recent study demonstrated direct, contact-dependent antigen transfer from donor to recipient CD40L-activated DC1 during reticulation for the enhancement of specific T cell responses to viral and bacterial antigens [27]. Furthermore, live-cell imaging enabled the tracking of beads representing antigens as they traveled from donor to recipient DC via MNTs, and flow cytometric quantitation revealed that CD40L activation dramatically enhanced bead acquisition in DC1 compared with reticulation-refractory DC2. Together, these data suggest that inducible reticulation in migratory, proinflammatory DC1 represents a novel mechanism of direct antigen exchange, which could be important for antigen hand-off to resident DCs in lymph nodes. This process could, in part, explain the recent findings of how spatially distinct interactions between CD4+ and CD8+ T cells with different DC subsets in the lymph node are facilitated for the efficient induction of an adaptive antiviral, cellular immune response [72].
Evidence of MNTs in vivo
The function of MNTs in iDCs in peripheral tissues likely involves the early sensing of pathogen- and/or host-derived danger signals resulting from tissue assault. In support of this supposition, in 2008, Chinnery et al. [52] provided the first in vivo evidence of a network of MNTs connecting remote DCs in inflamed mouse corneas. Until this point, a paucity of evidence for the existence and function of MNTs in vivo led some members of the scientific community to question whether these conduits were merely an in vitro artifact as opposed to a versatile, ubiquitous, and previously unknown mode of intercellular communication. Whereas shorter MNTs were expressed by GFP+ MHC II+-putative DCs in the periphery of inflamed and to a lesser extent, in naïve corneas, DCs of the central region of inflamed corneas uniquely formed long and highly curved or winding MNTs. The diameter of the structures found in corneas was equivalent to that of thick MNTs described in vitro [5], and localized beading or bulging along their length and base—a marker of vesicular trafficking [50]—was also observed. Interestingly, MNTs were not detected in CD11b-, CD68-, or CD69-positive macrophages [52]. Both LPS and injury significantly increased MNT expression in MHC II+ DCs in the central compared to the naïve cornea, highlighting a potential link between inflammation and MNT formation for the first time in vivo. Furthermore, the predominance of extremely long and curved MNTs in the central region of the cornea, where DCs are sparse, indicates that these conduits likely pass messages in the setting of inflammation to induce a rapid and coordinated response by resident DCs. Importantly, this group also observed MNT-like structures that appeared unconnected to a second cell, which perhaps represented disrupted MNTs, those seeking target cells, or those connected to an unlabeled cell type, such as stromal cells or neurons.
In vivo evidence of MNT-like extensions exists for a number of other cell types and tissues. For example, Lou and colleagues [67] observed MNTs ex vivo in both human ovarian adenocarcinoma tumor explants and demonstrated MNT-mediated exchange of oncogenic microRNAs between ovarian cancer cells and also between osteosarcoma and stromal cells in vitro. Long and short membrane bridges were also observed in live mouse embryo culture during neural tube closure [73]. Similar studies have reported these formations in mouse, chick, zebrafish, and sea urchin embryos at various developmental stages, and the implications of MNT-mediated communication during development have been expertly reviewed elsewhere [74]. Finally, intravital imaging of lymph nodes in a humanized mouse model revealed the presence of long MNT-like tethers in HIV-1-infected CD4+ T cells [75]. These in vivo observations support the extensive in vitro evidence of a role for MNTs in HIV-1 disease, which we discuss in detail in the following section.
HIV-1 USE OF MNTs IN MYELOID LINEAGE CELLS
MNT-facilitated HIV-1 infection of monocytes and macrophages
Whereas MNTs likely serve a variety of important functions in the context of health, these conduits have also been implicated in chronic diseases, such as cancer and neurodegenerative disorders involving prions [38, 76, 77]. For example, Langevin et al. [77] demonstrated that bone marrow-derived DCs can rapidly internalize prions upon exposure to infected brain homogenates and transfer them directly to cerebellar neurons via MNTs in a coculture system. These structures have also been detected in vitro in cancer cell lines and ex vivo in tumors, and their function in supporting the tumor microenvironment has been expertly reviewed elsewhere [38]. Outside of cancer, probably the most well-studied example of a detrimental role for MNTs in humans involves HIV-1, which can hijack MNTs to spread rapidly to distant cells and circumvent the inhospitable extracellular milieu [5]. In addition to CD4+ T cells, the virus is capable of productively infecting myeloid-derived macrophages [78] and to a limited extent DCs, depending on their relative state of maturation [79]. Importantly, APCs are probably among the earliest targets of HIV-1 infection, as a result of their localization to mucosal tissues and participation in antigen acquisition at these sites.
Monocytes and macrophages are considered to be important targets of HIV-1 infection in vivo and likely provide a reservoir for the virus during antiretroviral therapy [80]. Eugenin et al. [53] showed in 2009 that HIV-1 infection increases the frequency of MNTs in human monocyte-derived macrophages and that HIV-1 particles localize to these structures. The authors categorized "short" MNTs with an average length of 30 µm and "long" MNTs with an average length of 150 µm, and both of these types were distinct from filopodia, which were typically 5 µm in length. They observed immunolabeled HIV-1 p24 in both intracellular vesicles, as well as MNT-like processes, and infected macrophages displayed a marked increase in short MNTs compared with that of uninfected macrophages. Furthermore, the peak of MNT formation was 2–3 d postinfection, which correlated with active virus replication. Finally, virus particles appeared to be inside of short MNTs but surfed along the outside of narrower, long-range MNTs, hinting that the mechanism of virus transfer may differ depending on the type of MNT involved.
In 2011, Kadiu and Gendelman [54] published an extensive proteomic, biochemical, and imaging study showing that endocytic trafficking drives intercellular HIV-1 spread in human monocyte-derived macrophages through MNT-like connections, termed bridging conduits, which are the equivalent of thick MNTs described previously in this cell type [50]. They confirmed that HIV-1 infection increases MNT formation in macrophages, identified endosome, Golgi, and ER proteins in the proteome of the conduits, and established the presence of these markers, as well as ER- and Golgi-associated HIV-1 envelope and capsid core proteins, within the structures [54]. Infectious HIV-1 particles underwent retrograde transport from early/recycling endosomes to the trans-Golgi following endocytosis. In a related study, the same group showed that these conduits indeed facilitated HIV-1 spread to neighboring, uninfected macrophages [55]. The authors noted the absence of mature HIV-1 virions within endosomes in MNTs and postulated that conduit-mediated HIV-1 spread could involve shuttling of disassembled viral cargoes, which are still capable of initiating infection, to uninfected cells.
Whereas the aforementioned studies clearly demonstrate HIV-1 use of endocytic vesicular trafficking for cell-to-cell spread in macrophages, monocyte-derived iDCs are relatively resistant to HIV-1 cis-infection, and maturation further limits their susceptibility [81]. Therefore, the mechanisms of intercellular viral transfer by DCs could also conceivably differ from macrophages. DCs are thought to carry intact the virus to regional lymph nodes, where they transmit virions in trans to CD4+ T cells [82]. Although the precise mechanisms underlying this process are unclear, MNTs could provide a direct route of transfer from DCs to T cells during antigen presentation. Interestingly, Rappocciolo et al. [83], from our laboratory, recently reported an intriguing association between transinfection and HIV-1 disease progression, in that iDCs and activated B cells, which can bind HIV-1 via DC-SIGN [84] from HIV-1-infected, long-term nonprogressors, failed to transinfect CD4+ T cells as a result of abnormalities in iDC and B cell cholesterol metabolism. Another recent study indicated that cholesterol-containing membrane nanodomains are necessary for MNT stability [62] and enriched in cells connected by MNTs [10], but the specific role that MNT abnormalities play in inhibiting HIV-1 disease progression is yet to be determined.
Nef-mediated HIV-1 modulation of MNTs in immune cells
Nef, a membrane-associated accessory protein conserved in HIV-1, HIV-2, and SIV, plays a central role in viral pathogenesis and disease progression as it modulates cell surface receptors to escape immune detection, regulates T cell activation, and enhances virus infectivity, replication, and transmission [57, 85]. In addition to down-regulation of CD4, CD3, and MHC I and modulation of T cell signaling pathways, this protein has been shown to facilitate viral transmission by inducing multivesicular bodies and MNT formation via interaction with the exocyst complex [57]. Although HIV-1 does not productively infect B cells [84], untreated infection indirectly leads to a multitude of B cell-intrinsic humoral defects, in part, by Nef-mediated interference with CD40L-driven CD4+ T cell help [86]. In 2009, Cerutti and colleagues [56] revealed an abundance of CD68+ macrophages containing capsid p24 and Nef proteins, often proximal to preclass-switched Nef+ IgD+ B cells, in follicular and extrafollicular regions of infected lymphoid tissues from chronically infected patients. In a Nef-dependent manner, HIV-1-infected macrophages inhibited B cell IgG2 and IgA class switching via Nef and induced long-range MNT development between infected THP-1 cells or primary macrophages and B cells in vitro. In addition to Nef+ vesicles budding from the membrane of THP-1 cells, Nef localized along the length of MNTs or within bulges indicative of MNT-mediated vesicular trafficking. Examination of systemic and intestinal follicles in the context of HIV-1 infection additionally revealed in vivo evidence of a role for MNT-mediated Nef transfer from infected macrophages to B cells, promoting their dysfunction.
Davis and colleagues [47] showed that MNTs formed between HIV-1-infected Jurkat and uninfected Jurkat T cells, and whereas infection did not induce MNT formation, their existing MNTs supported the direct receptor-dependent transfer of nascent virions to uninfected T cells over long distances. Colocalized Gag and Env proteins, suggestive of late-stage virus, were detected in MNTs connecting infected and uninfected Jurkat cells after only 1 h of coincubation. Furthermore, colocalized HIV-1 proteins were also detected in uninfected target cells that were physically connected to infected cells by MNTs but not in unconnected target cells. Laser-scanning confocal microscopy revealed trafficking of Gag-GFP recombinant HIV-1 virions between infected and uninfected Jurkat cells at a speed that is consistent with actin-mediated movement but considerably faster than filopodia-driven intercellular trafficking of murine leukemia virus. The role for Nef in MNT-mediated HIV-1 transfer between T cells was not explored, but another study demonstrated that Nef is necessary for optimum virus replication in iDC-CD4+ T cell cocultures and in T cells alone [87]. Furthermore, Gabuzda and colleagues [57] shed light on the mechanisms of Nef-mediated MNT induction when they identified an association between Nef and 5 components of the exocyst complex, EXOC1–4 and EXOC6, in Jurkat T cells. The exocyst complex plays a central role in MNT formation and vesicle secretion at the plasma membrane, so it follows that direct interaction between Nef and this multiprotein complex could facilitate direct spread of HIV-1 virions, as well as HIV-1-associated, immunosuppressive proteins via MNTs and vesicles.
MNT-facilitated HIV-1 transinfection from DC to CD4+ T cells
The ability of HIV-1 to circumvent CD8+ CTL-mediated recognition and elimination by frequently mutating and generating variant strains in response to host immune-selective pressure is well known [88]. Intriguingly, our research group recently showed that CTL-epitope divergence in surviving HIV-1 variants can result in the selective induction of CTL cytokine production in the absence of cytotoxic function [58]. As a result, this HIV-1 variant-induced, dysfunctional CTL activity can be used by HIV-1 to promote the programming of proinflammatory DC1 with an enhanced capacity to mediate HIV-1 transinfection of CD4+ T cells [58, 89]. In addition to expressing high levels of CCL4, CCL5, CXCL9, and CXCL10, a chemokine profile preferentially likely to attract activated CD4+ Th cells, these CTL-programmed DC1 possessed a heightened ability to develop far-reaching networks of MNT-like extensions (schematically shown in Fig. 4).
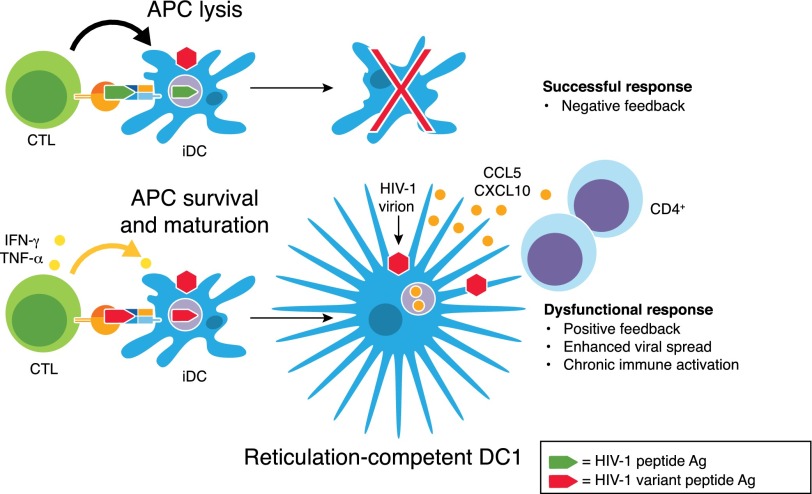
Figure 4. CTL-epitope divergence in surviving HIV-1 variants can facilitate the selective induction of DC1.
As a negative-feedback mechanism, CTL-driven, successful responses result in CTL-mediated killing of the peptide APC. In contrast, during chronic HIV-1 infection, DC presentation of HIV-1 epitope variants can induce dysfunctional CTL memory responses, resulting in their selective production of cytokines in the absence of killing. This positive-immune feedback, in turn, drives DC differentiation into reticulation-competent DC1 that possess a superior ability to attract and facilitate HIV-1 transinfection of Th cells.
These data demonstrate a scenario in which HIV-1 could perpetuate a positive-feedback loop that promotes acute inflammation and the development of MNT networks to facilitate high-speed, cell-to-cell viral transmission. Such proinflammatory DC1 develop an even more dramatic network of MNTs upon interaction with CD40L-expressing CD4+ T cells, which could provide a direct route for transmission of virus from the DC1 to CD4+ T cells or facilitate viral spread to other DCs within lymph node regions. MNTs have been observed to form between lymphoid lineage antigen-presenting B cells and Jurkat T cells in vitro for the exchange of small membrane-localized proteins [90], but direct MNT-mediated communication between myeloid lineage APCs and T cells requires additional study. These conduits likely provide competing advantages for the host and virus during an immune response to HIV-1 infection. Further investigation is needed to confirm this supposition and to determine ways to target these formations safely and effectively to disrupt HIV-1 transmission in vivo.
CONCLUDING REMARKS
MNTs represent the most mechanistically diverse form of intercellular communication yet discovered, as mounting evidence suggests that they can work cooperatively with numerous other modes of cross-talk, including receptor-ligand signaling, immunologic synapse formation, vesicular trafficking, and gap junction-coupled electrical signaling [5–9]. The outcome of MNT-mediated cross-talk, i.e., help or harm to the host, likely depends on the cell types involved, the surrounding microenvironment and disease setting, and the nature of the cargo. MNT formation may also represent a general protective response to cell stress. In some cases, stressed cells develop MNTs unidirectionally toward unstressed cells [41], likely facilitating the rapid transfer of energy, organelles, or pathogenic information to neighboring healthy cells as the "final act" of the dying cell. Other recent investigations have shown that healthy cells, e.g., stem cells, preferentially develop microtubule-containing MNTs toward injured or stressed cells for the unidirectional transfer of mitochondria, which rescues them from early apoptosis [43, 44]. Therefore, direct delivery of protective cargoes via MNTs represents a promising future tool of regenerative medicine that warrants further investigation, particularly for the salvaging of cell types with limited ability to regenerate, such as neurons.
Membrane conduits developed in response to mediators of inflammation, activation, and/or death signals can display distinct structure and function, even in the same cell type. Importantly, proinflammatory and pathogen-derived signals can induce MNTs in iDCs in the periphery, whereas secondary CD40L activation initiates more extensive reticulation responses, i.e. conduit formation, in mature proinflammatory DC1 [27]. We postulate that MNT-mediated communication between myeloid origin DCs during acute inflammation serves to activate adjacent and remote cells for a rapid, coordinated response to a pathogen assault in the periphery, whereas the linking in of migratory DC1 to resident DC networks supports antigen exchange for the amplification of cellular immune responses in lymph nodes. Interestingly, MNTs formed between activated CD4+ T cells contain a membrane junction that limits cargo transfer [47], whereas those developed between CD4+ T cells in response to death signals are open ended and support transfer of diverse cargoes, including death signals [48]. MNTs formed between NK and target cells also contain a membrane junction, which supports long-range functional immunologic synapse formation [46], but it remains to be determined whether conduits formed between lymphocytes and APCs similarly facilitate immunologic synapse establishment. This ability could represent another means by which MNTs facilitate the initiation of adaptive immune responses during acute inflammation by increasing the probability of migratory DC1 finding and presenting antigen to cognate T cells.
Conversely, chronic inflammation plays an integral role in HIV-1 pathogenesis, as well as in neurodevelopmental and neurodegenerative diseases associated with aging [91–95], and may contribute to the dysregulation of MNT-mediated communication. CD40L-inducible DC1 reticulation, for example, likely benefits the pathogen during HIV-1 infection by facilitating viral spread, highlighting this process as a potential therapeutic target for slowing disease progression. Further study is urgently required to determine if MNTs represent a common immune evasion strategy exploited by other intracellular pathogens. Importantly, strategies for shutting down this versatile communication system could benefit the host in not only HIV-1 but also cancer and Alzheimer’s disease by limiting MNT-mediated spread of microRNAs encoding drug-resistance genes or amyloid-β, respectively.
Some key questions remain to be investigated to understand better the role of MNT-mediated communication in stress, inflammation, and immunity, as well as certain chronic disease states. These conduits are currently distinguished from other similar F-actin-based structures primarily based on their morphology, as a specific molecular marker for MNTs has not yet been identified. Importantly, MNTs can be formed by filopodia extension or cell-divergence mechanisms. They can also be open or close ended, and their cytoskeleton can be comprised of F-actin alone or F-actin and microtubules in different or the same cell type depending on the activation state and microenvironment. Whereas these observations attest to the considerable heterogeneity in MNT structure and function, the underlying molecular mechanisms have not been fully elucidated. Finally, further exploration of the existence and function of the conduits in specific organ settings is required if we are one day to translate our growing knowledge of MNTs into medicine.
AUTHORSHIP
C.R.Z. and R.B.M. conceived of the study, C.R.Z. and R.B.M. wrote the manuscript, and C.R.R. edited the manuscript.
Acknowledgments
This study was funded, in part, by the U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases (Grants U01 AI-35041, R37 AI-41870, and T32 AI-065380). Furthermore, SIM images were generated using an AAA Nikon SIM, which is supported by the 1S10OD10625-0A1 grant. The authors acknowledge the staff at the University of Pittsburgh’s Center for Biologic Imaging, and in particular, Dr. Simon Watkins, the imaging center’s director, for his insights regarding the relationship between structure and function of MNTs in myeloid cells and for providing the imaging resources used in Fig. 2.
Glossary
- CD40L
CD40 ligand
- DC
dendritic cell
- DC0/1/2
type 0/1/2 polarized dendritic cells
- ER
endoplasmic reticulum
- HEK
human embryonic kidney
- iDC
immature dendritic cell
- IP3
inositol triphosphate
- LST-1
leukocyte-specific transcript 1
- MHC I/II/III
MHC class I/II/III
- MNT
membrane nanotube
- Nef
negative regulatory factor
- PC12
pheochromocytoma
- rhCD40L
recombinant human CD40 ligand
- SIM
structured illumination microscopy
- TNFaip2
TNF-α-induced protein 2
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Commins S. P., Borish L., Steinke J. W. (2010) Immunologic messenger molecules: cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 125 (2, Suppl 2) S53–S72. [DOI] [PubMed] [Google Scholar]
- 2.Robbins P. D., Morelli A. E. (2014) Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neijssen J., Pang B., Neefjes J. (2007) Gap junction-mediated intercellular communication in the immune system. Prog. Biophys. Mol. Biol. 94, 207–218. [DOI] [PubMed] [Google Scholar]
- 4.Dustin M. L. (2005) A dynamic view of the immunological synapse. Semin. Immunol. 17, 400–410. [DOI] [PubMed] [Google Scholar]
- 5.Davis D. M., Sowinski S. (2008) Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 9, 431–436. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes H. H., Carvalho R. N. (2008) Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 20, 470–475. [DOI] [PubMed] [Google Scholar]
- 7.Austefjord M. W., Gerdes H. H., Wang X. (2014) Tunneling nanotubes: diversity in morphology and structure. Commun. Integr. Biol. 7, e27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marzo L., Gousset K., Zurzolo C. (2012) Multifaceted roles of tunneling nanotubes in intercellular communication. Front. Physiol. 3, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abounit S., Zurzolo C. (2012) Wiring through tunneling nanotubes--from electrical signals to organelle transfer. J. Cell Sci. 125, 1089–1098. [DOI] [PubMed] [Google Scholar]
- 10.Thayanithy V., Babatunde V., Dickson E. L., Wong P., Oh S., Ke X., Barlas A., Fujisawa S., Romin Y., Moreira A. L., Downey R. J., Steer C. J., Subramanian S., Manova-Todorova K., Moore M. A., Lou E. (2014) Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp. Cell Res. 323, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousso P. (2008) T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat. Rev. Immunol. 8, 675–684. [DOI] [PubMed] [Google Scholar]
- 12.Salter R. D., Tuma-Warrino R. J., Hu P. Q., Watkins S. C. (2004) Rapid and extensive membrane reorganization by dendritic cells following exposure to bacteria revealed by high-resolution imaging. J. Leukoc. Biol. 75, 240–243. [DOI] [PubMed] [Google Scholar]
- 13.Swetman C. A., Leverrier Y., Garg R., Gan C. H., Ridley A. J., Katz D. R., Chain B. M. (2002) Extension, retraction and contraction in the formation of a dendritic cell dendrite: distinct roles for Rho GTPases. Eur. J. Immunol. 32, 2074–2083. [DOI] [PubMed] [Google Scholar]
- 14.Mattila P. K., Lappalainen P. (2008) Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454. [DOI] [PubMed] [Google Scholar]
- 15.Bukoreshtliev N. V., Wang X., Hodneland E., Gurke S., Barroso J. F., Gerdes H. H. (2009) Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 583, 1481–1488. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J., Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 17.Bennett S. R., Carbone F. R., Karamalis F., Flavell R. A., Miller J. F., Heath W. R. (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393, 478–480. [DOI] [PubMed] [Google Scholar]
- 18.Schoenberger S. P., Toes R. E., van der Voort E. I., Offringa R., Melief C. J. (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483. [DOI] [PubMed] [Google Scholar]
- 19.Ridge J. P., Di Rosa F., Matzinger P. (1998) A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478. [DOI] [PubMed] [Google Scholar]
- 20.Kaliński P., Hilkens C. M., Wierenga E. A., Kapsenberg M. L. (1999) T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20, 561–567. [DOI] [PubMed] [Google Scholar]
- 21.Kapsenberg M. L. (2003) Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3, 984–993. [DOI] [PubMed] [Google Scholar]
- 22.Mailliard R. B., Wankowicz-Kalinska A., Cai Q., Wesa A., Hilkens C. M., Kapsenberg M. L., Kirkwood J. M., Storkus W. J., Kalinski P. (2004) alpha-Type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 64, 5934–5937. [DOI] [PubMed] [Google Scholar]
- 23.Mailliard R. B., Son Y. I., Redlinger R., Coates P. T., Giermasz A., Morel P. A., Storkus W. J., Kalinski P. (2003) Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171, 2366–2373. [DOI] [PubMed] [Google Scholar]
- 24.Kalinski P., Moser M. (2005) Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat. Rev. Immunol. 5, 251–260. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146. [DOI] [PubMed] [Google Scholar]
- 26.Vieira P. L., de Jong E. C., Wierenga E. A., Kapsenberg M. L., Kaliński P. (2000) Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164, 4507–4512. [DOI] [PubMed] [Google Scholar]
- 27.Zaccard C. R., Watkins S. C., Kalinski P., Fecek R. J., Yates A. L., Salter R. D., Ayyavoo V., Rinaldo C. R., Mailliard R. B. (2015) CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. J. Immunol. 194, 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joffre O. P., Segura E., Savina A., Amigorena S. (2012) Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12, 557–569. [DOI] [PubMed] [Google Scholar]
- 29.Jongbloed S. L., Kassianos A. J., McDonald K. J., Clark G. J., Ju X., Angel C. E., Chen C. J., Dunbar P. R., Wadley R. B., Jeet V., Vulink A. J., Hart D. N., Radford K. J. (2010) Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207, 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villadangos J. A., Shortman K. (2010) Found in translation: the human equivalent of mouse CD8+ dendritic cells. J. Exp. Med. 207, 1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Den Haan J. M., Lehar S. M., Bevan M. J. (2000) CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allan R. S., Waithman J., Bedoui S., Jones C. M., Villadangos J. A., Zhan Y., Lew A. M., Shortman K., Heath W. R., Carbone F. R. (2006) Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162. [DOI] [PubMed] [Google Scholar]
- 33.Qu C., Nguyen V. A., Merad M., Randolph G. J. (2009) MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J. Immunol. 182, 3650–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert M. L., Sauter B., Bhardwaj N. (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89. [DOI] [PubMed] [Google Scholar]
- 35.André F., Chaput N., Schartz N. E., Flament C., Aubert N., Bernard J., Lemonnier F., Raposo G., Escudier B., Hsu D. H., Tursz T., Amigorena S., Angevin E., Zitvogel L. (2004) Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172, 2126–2136. [DOI] [PubMed] [Google Scholar]
- 36.Harshyne L. A., Zimmer M. I., Watkins S. C., Barratt-Boyes S. M. (2003) A role for class A scavenger receptor in dendritic cell nibbling from live cells. J. Immunol. 170, 2302–2309. [DOI] [PubMed] [Google Scholar]
- 37.Gurke S., Barroso J. F., Gerdes H. H. (2008) The art of cellular communication: tunneling nanotubes bridge the divide. Histochem. Cell Biol. 129, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou E., Fujisawa S., Barlas A., Romin Y., Manova-Todorova K., Moore M. A., Subramanian S. (2012) Tunneling nanotubes: a new paradigm for studying intercellular communication and therapeutics in cancer. Commun. Integr. Biol. 5, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rustom A., Saffrich R., Markovic I., Walther P., Gerdes H. H. (2004) Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. [DOI] [PubMed] [Google Scholar]
- 40.Schiller C., Huber J. E., Diakopoulos K. N., Weiss E. H. (2013) Tunneling nanotubes enable intercellular transfer of MHC class I molecules. Hum. Immunol. 74, 412–416. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Cui J., Sun X., Zhang Y. (2011) Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 18, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Wang Y., Zhang J., Tu J., Wang X. J., Su X. D., Wang L., Zhang Y. (2012) Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death Dis. 3, e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu K., Ji K., Guo L., Wu W., Lu H., Shan P., Yan C. (2014) Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92, 10–18. [DOI] [PubMed] [Google Scholar]
- 44.Wang X., Gerdes H. H. (2015) Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onfelt B., Nedvetzki S., Yanagi K., Davis D. M. (2004) Cutting edge: membrane nanotubes connect immune cells. J. Immunol. 173, 1511–1513. [DOI] [PubMed] [Google Scholar]
- 46.Chauveau A., Aucher A., Eissmann P., Vivier E., Davis D. M. (2010) Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc. Natl. Acad. Sci. USA 107, 5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sowinski S., Jolly C., Berninghausen O., Purbhoo M. A., Chauveau A., Köhler K., Oddos S., Eissmann P., Brodsky F. M., Hopkins C., Onfelt B., Sattentau Q., Davis D. M. (2008) Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10, 211–219. [DOI] [PubMed] [Google Scholar]
- 48.Arkwright P. D., Luchetti F., Tour J., Roberts C., Ayub R., Morales A. P., Rodríguez J. J., Gilmore A., Canonico B., Papa S., Esposti M. D. (2010) Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res. 20, 72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fifadara N. H., Beer F., Ono S., Ono S. J. (2010) Interaction between activated chemokine receptor 1 and FcepsilonRI at membrane rafts promotes communication and F-actin-rich cytoneme extensions between mast cells. Int. Immunol. 22, 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onfelt B., Nedvetzki S., Benninger R. K., Purbhoo M. A., Sowinski S., Hume A. N., Seabra M. C., Neil M. A., French P. M., Davis D. M. (2006) Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177, 8476–8483. [DOI] [PubMed] [Google Scholar]
- 51.Watkins S. C., Salter R. D. (2005) Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 23, 309–318. [DOI] [PubMed] [Google Scholar]
- 52.Chinnery H. R., Pearlman E., McMenamin P. G. (2008) Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J. Immunol. 180, 5779–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eugenin E. A., Gaskill P. J., Berman J. W. (2009) Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell. Immunol. 254, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadiu I., Gendelman H. E. (2011) Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J. Proteome Res. 10, 3225–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadiu I., Gendelman H. E. (2011) Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J. Neuroimmune Pharmacol. 6, 658–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu W., Santini P. A., Sullivan J. S., He B., Shan M., Ball S. C., Dyer W. B., Ketas T. J., Chadburn A., Cohen-Gould L., Knowles D. M., Chiu A., Sanders R. W., Chen K., Cerutti A. (2009) HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 10, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukerji J., Olivieri K. C., Misra V., Agopian K. A., Gabuzda D. (2012) Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mailliard R. B., Smith K. N., Fecek R. J., Rappocciolo G., Nascimento E. J., Marques E. T., Watkins S. C., Mullins J. I., Rinaldo C. R. (2013) Selective induction of CTL helper rather than killer activity by natural epitope variants promotes dendritic cell-mediated HIV-1 dissemination. J. Immunol. 191, 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hase K., Kimura S., Takatsu H., Ohmae M., Kawano S., Kitamura H., Ito M., Watarai H., Hazelett C. C., Yeaman C., Ohno H. (2009) M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432. [DOI] [PubMed] [Google Scholar]
- 60.Schiller C., Diakopoulos K. N., Rohwedder I., Kremmer E., von Toerne C., Ueffing M., Weidle U. H., Ohno H., Weiss E. H. (2013) LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. J. Cell Sci. 126, 767–777. [DOI] [PubMed] [Google Scholar]
- 61.Gousset K., Marzo L., Commere P. H., Zurzolo C. (2013) Myo10 is a key regulator of TNT formation in neuronal cells. J. Cell Sci. 126, 4424–4435. [DOI] [PubMed] [Google Scholar]
- 62.Lokar M., Kabaso D., Resnik N., Sepčić K., Kralj-Iglič V., Veranič P., Zorec R., Iglič A. (2012) The role of cholesterol-sphingomyelin membrane nanodomains in the stability of intercellular membrane nanotubes. Int. J. Nanomedicine 7, 1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lokar M., Iglic A., Veranic P. (2010) Protruding membrane nanotubes: attachment of tubular protrusions to adjacent cells by several anchoring junctions. Protoplasma 246, 81–87. [DOI] [PubMed] [Google Scholar]
- 64.Kimura S., Hase K., Ohno H. (2013) The molecular basis of induction and formation of tunneling nanotubes. Cell Tissue Res. 352, 67–76. [DOI] [PubMed] [Google Scholar]
- 65.Wang X., Gerdes H. H. (2012) Long-distance electrical coupling via tunneling nanotubes. Biochim. Biophys. Acta 1818, 2082–2086. [DOI] [PubMed] [Google Scholar]
- 66.Mineo M., Garfield S. H., Taverna S., Flugy A., De Leo G., Alessandro R., Kohn E. C. (2012) Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis 15, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thayanithy V., Dickson E. L., Steer C., Subramanian S., Lou E. (2014) Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl. Res. 164, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerdes H. H., Bukoreshtliev N. V., Barroso J. F. (2007) Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 581, 2194–2201. [DOI] [PubMed] [Google Scholar]
- 69.Smith I. F., Shuai J., Parker I. (2011) Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys. J. 100, L37–L39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma D. Y., Clark E. A. (2009) The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 21, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koguchi Y., Thauland T. J., Slifka M. K., Parker D. C. (2007) Preformed CD40 ligand exists in secretory lysosomes in effector and memory CD4+ T cells and is quickly expressed on the cell surface in an antigen-specific manner. Blood 110, 2520–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hor J. L., Whitney P. G., Zaid A., Brooks A. G., Heath W. R., Mueller S. N. (2015) Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4 and CD8 T cell activation to localized viral infection. Immunity 43, 554–565. [DOI] [PubMed] [Google Scholar]
- 73.Pyrgaki C., Trainor P., Hadjantonakis A. K., Niswander L. (2010) Dynamic imaging of mammalian neural tube closure. Dev. Biol. 344, 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerdes H. H., Rustom A., Wang X. (2013) Tunneling nanotubes, an emerging intercellular communication route in development. Mech. Dev. 130, 381–387. [DOI] [PubMed] [Google Scholar]
- 75.Murooka T. T., Deruaz M., Marangoni F., Vrbanac V. D., Seung E., von Andrian U. H., Tager A. M., Luster A. D., Mempel T. R. (2012) HIV-infected T cells are migratory vehicles for viral dissemination. Nature 490, 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gousset K., Schiff E., Langevin C., Marijanovic Z., Caputo A., Browman D. T., Chenouard N., de Chaumont F., Martino A., Enninga J., Olivo-Marin J. C., Männel D., Zurzolo C. (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11, 328–336. [DOI] [PubMed] [Google Scholar]
- 77.Langevin C., Gousset K., Costanzo M., Richard-Le Goff O., Zurzolo C. (2010) Characterization of the role of dendritic cells in prion transfer to primary neurons. Biochem. J. 431, 189–198. [DOI] [PubMed] [Google Scholar]
- 78.Campbell J. H., Hearps A. C., Martin G. E., Williams K. C., Crowe S. M. (2014) The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 28, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piguet V., Steinman R. M. (2007) The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbas W., Tariq M., Iqbal M., Kumar A., Herbein G. (2015) Eradication of HIV-1 from the macrophage reservoir: an uncertain goal? Viruses 7, 1578–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Izquierdo-Useros N., Lorizate M., McLaren P. J., Telenti A., Kräusslich H. G., Martinez-Picado J. (2014) HIV-1 capture and transmission by dendritic cells: the role of viral glycolipids and the cellular receptor Siglec-1. PLoS Pathog. 10, e1004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rinaldo C. R. (2013) HIV-1 trans infection of CD4(+) T cells by professional antigen presenting cells. Scientifica (Cairo) 2013, 164203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rappocciolo G., Jais M., Piazza P., Reinhart T. A., Berendam S. J., Garcia-Exposito L., Gupta P., Rinaldo C. R. (2014) Alterations in cholesterol metabolism restrict HIV-1 trans infection in nonprogressors. MBio 5, e01031–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rappocciolo G., Piazza P., Fuller C. L., Reinhart T. A., Watkins S. C., Rowe D. T., Jais M., Gupta P., Rinaldo C. R. (2006) DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2, e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landi A., Iannucci V., Nuffel A. V., Meuwissen P., Verhasselt B. (2011) One protein to rule them all: modulation of cell surface receptors and molecules by HIV Nef. Curr. HIV Res. 9, 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudnicka D., Schwartz O. (2009) Intrusive HIV-1-infected cells. Nat. Immunol. 10, 933–934. [DOI] [PubMed] [Google Scholar]
- 87.Petit C., Buseyne F., Boccaccio C., Abastado J. P., Heard J. M., Schwartz O. (2001) Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286, 225–236. [DOI] [PubMed] [Google Scholar]
- 88.Goulder P. J., Brander C., Tang Y., Tremblay C., Colbert R. A., Addo M. M., Rosenberg E. S., Nguyen T., Allen R., Trocha A., Altfeld M., He S., Bunce M., Funkhouser R., Pelton S. I., Burchett S. K., McIntosh K., Korber B. T., Walker B. D. (2001) Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412, 334–338. [DOI] [PubMed] [Google Scholar]
- 89.Zaccard C. R., Watkins S. C., Ayyavoo V., Rinaldo C. R., Mailliard R. B. (2014) HIV’s ticket to ride: cytotoxic T-lymphocyte-activated dendritic cells exploited for virus intercellular transfer. AIDS Res. Hum. Retroviruses 30, 1023–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rainy N., Chetrit D., Rouger V., Vernitsky H., Rechavi O., Marguet D., Goldstein I., Ehrlich M., Kloog Y. (2013) H-Ras transfers from B to T cells via tunneling nanotubes. Cell Death Dis. 4, e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appay V., Sauce D. (2008) Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214, 231–241. [DOI] [PubMed] [Google Scholar]
- 92.Estes M. L., McAllister A. K. (2015) Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 16, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amor S., Puentes F., Baker D., van der Valk P. (2010) Inflammation in neurodegenerative diseases. Immunology 129, 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singhal G., Jaehne E. J., Corrigan F., Toben C., Baune B. T. (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front. Neurosci. 8, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franceschi C., Campisi J. (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69 (Suppl 1), S4–S9. [DOI] [PubMed] [Google Scholar]