Review of connections between T1D genetic susceptibility and alterations of DC-mediated tolerance, and potential immunotherapy for autoimmunity.
Keywords: tolerance, insulin-dependent diabetes mellitus susceptibility, genetic polymorphism, antigen presentation, innate immunity
Abstract
Type 1 diabetes is an autoimmune disease that results from the defective induction or maintenance of T cell tolerance against islet β cell self-antigens. Under steady-state conditions, dendritic cells with tolerogenic properties are critical for peripheral immune tolerance. Tolerogenic dendritic cells can induce T cell anergy and deletion and, in some contexts, induce or expand regulatory T cells. Dendritic cells contribute to both immunomodulatory effects and triggering of pathogenesis in type 1 diabetes. This immune equilibrium is affected by both genetic and environmental factors that contribute to the development of type 1 diabetes. Genome-wide association studies and disease association studies have identified >50 polymorphic loci that lend susceptibility or resistance to insulin-dependent diabetes mellitus. In parallel, diabetes susceptibility regions known as insulin-dependent diabetes loci have been identified in the nonobese diabetic mouse, a model for human type 1 diabetes, providing a better understanding of potential immunomodulatory factors in type 1 diabetes risk. Most genetic candidates have annotated immune cell functions, but the focus has been on changes to T and B cells. However, it is likely that some of the genomic susceptibility in type 1 diabetes directly interrupts the tolerogenic potential of dendritic cells in the pathogenic context of ongoing autoimmunity. Here, we will review how gene polymorphisms associated with autoimmune diabetes may influence dendritic cell development and maturation processes that could lead to alterations in the tolerogenic function of dendritic cells. These insights into potential tolerogenic and pathogenic roles for dendritic cells have practical implications for the clinical manipulation of dendritic cells toward tolerance to prevent and treat type 1 diabetes.
Introduction
T1D is a complex, polygenic autoimmune disease that occurs when the immune-mediated destruction of the insulin-producing β cells in the islets of Langerhans causes hyperglycemia [1]. Both genetic and environmental risk factors influence T1D development [2, 3]. The NOD mouse is a well-established model of spontaneous T1D and is used extensively to investigate the molecular and cellular mechanisms for understanding the etiology, immunology, and genetics of T1D [4, 5]. The genetic loci that confer diabetes susceptibility have been identified in mouse and human and are called Idd loci and IDDM loci, respectively. Within each locus are sets of genes that are candidates for affecting diabetes pathogenesis. The initial stages of diabetes pathogenesis are triggered by the islet infiltration of innate immune cells, including macrophages and DCs [6, 7], which precede the priming and recruitment of β cell–specific lymphocytes into the islets. Initially, β cell–reactive lymphocytes are kept in check by immune regulatory mechanisms, but when regulation fails, a destructive insulitis phase ensues that is characterized by massive islet infiltration of activated β cell–specific T cells to mediate β cell destruction [8–10]. In NOD mice, initial T cell priming begins as early as 10 d of age, insulitis starts at 3–4 wk of age, and the onset of destructive insulitis and hyperglycemia is observed beginning at 12–16 wk of age.
Many studies have demonstrated the pathogenic roles of DCs in diabetes; the islet Ag-specific T cell priming in the pLN is mediated by presentation of apoptotic β cells by DCs [11], and inducible DC depletion blocks T cell-mediated diabetes development [12]. Although pancreatic β islet cells are the source of self-antigens targeted in diabetes, they do not normally display the signals needed for priming of diabetogenic T cells [13]. The initial APCs to break self-tolerance are most likely DCs present in the islet before disease. Even in nondiabetic-prone mice, islet DCs sample Ag from apoptotic β islet cells that arise via homeostatic turnover and migrate into pLN to present β cell–derived Ags [8]. This suggests that the ability of islet DCs to present self-antigen is not, in itself, pathogenic, but rather the DC interaction determines initiation of autoimmunity. This review considers the role of human and mouse T1D genetic susceptibility alleles in the dysregulation of either the development or function of tolerogenic DCs. Many genes in susceptibility loci have been shown to affect T cell function, and many of these genes are expressed by human pancreatic β cells, especially under inflammatory conditions [14]. Less focus has been given, however, to the ability of these same genes to affect APCs. Gene expression analysis in mice shows that T1D candidate genes are expressed in DCs and DC precursors (Fig. 1) (Immunologic Genome Project consortium, GSE15907 [75]). Better understanding of how genes associated with diabetes affect DCs may elucidate novel mechanisms of diabetes pathogenesis and help inform DC-based therapeutic strategies to induce self-antigen–specific tolerance.
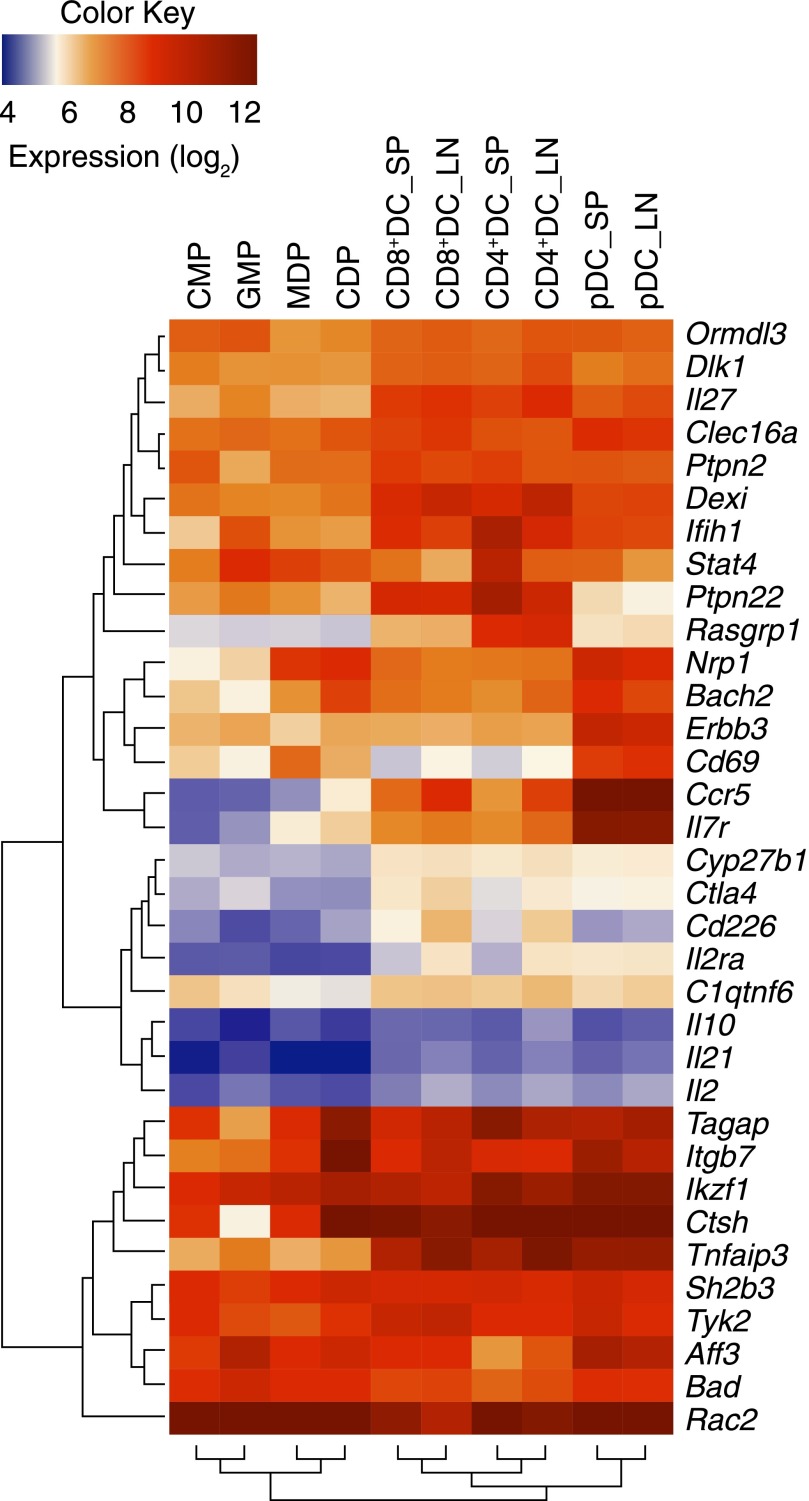
Figure 1. Mouse DC subsets and precursors express T1D candidate genes: data from the ImmGen Project.
Many T1D genetic-susceptibility candidate genes are expressed in DCs. The gene expression values in mouse DC precursors and DCs for the 34 T1D candidate genes shown in Tables 1 and 2 were extracted from the mouse Immunologic Genome Project database (http://www.immgen.org) and log-transformed to be represent as a blue–red color-map across DC precursors (CMP, GMP, MDP, CDP) and different DC populations. Only genes with expression above log24 in at least 1 DC cell type were included. Dark blue corresponds to low or no expression in that cell type, and dark red indicates high expression. The dendrograms present genes clustered by common patterns of gene expression. CMP, common myeloid progenitor; GMP, granulocyte-monocyte progenitor; MDP, macrophage dendritic cell progenitor; CDP, common dendritic cell progenitor; SP, spleen; LN, lymph node.
TOLEROGENIC DC SUBSETS IN NOD MICE
Since DCs were first identified by Ralph Steinman and Zanvil Cohn in 1973, many studies have shown that DCs employ diverse approaches to maintain T cell tolerance in peripheral tissues [76]. Although the genetic polymorphisms associated with T1D may contribute to a push of DCs away from tolerogenic features, DCs in NOD mice can also have a protective role in the development of diabetes [77]. The contribution of immune suppressive DCs to induce and maintain self-tolerance is conducted by DCs functionally defined as “tolerogenic.” Steady-state DCs remain largely immature under noninflammatory conditions and contribute to the maintenance of Ag-specific T cell peripheral tolerance through an induction of T cell unresponsiveness/anergic T cells and T cell depletion or an increase in Tregs and Bregs [78, 79]. Many phenotypic characteristics have been used to define tolerogenic DCs, for example, intermediate expression levels of maturation markers (CD80, CD86, CD40, and inducible costimulatory ligand), expression of IDO, or secretion of anti-inflammatory cytokines IL-10 and TGF-β [80]. The most important requirements for tolerogenic DCs appear to be their stage of maturation and their lineage affiliation. Numerous reports show that “semimature” or “alternatively activated” DCs can regulate autoreactive T cell responses and restore Ag-specific tolerance in experimental animal models [81]. Various DC subsets have been described based on their anatomic location and phenotypic or functional characteristics, and these subsets have differential roles in induction of tolerance, especially in autoimmune environments [79, 82]. Two conventional DC subsets as well as pDCs are the major DC subsets with distinct contributions to tolerance and pathogenesis in autoimmunity. T1D-associated genetic and environmental factors can alter the potent suppressive properties of tolerogenic DCs, highlighting potential candidates for therapeutic intervention in T1D.
DC subsets in NOD mice
Conventional tissue-resident CD8α+ DEC205+ DC1 (migratory CD103+ DC1).
cDCs use the transcription factor Zbtb46 and signals from the Flt3 ligand to develop from the common pre-cDC precursor and are subcategorized as cDC1 (either tissue-resident CD8α+ DCs or migratory CD103+ DCs) or cDC2 (CD11b+ DCs). cDC1s specifically arise from the newly identified pre-CD8 DCs [83] in an Irf8-Batf3–dependent manner. cDC1s are specialized to present exogenous Ags on their MHC class I molecules to CD8+ T cells, a process known as cross-presentation. In a steady state, the cross-presentation of self-antigens can stimulate deletion of self-reactive CD8+ T cells, termed cross-tolerance [84].
NOD mice have more Batf3-dependent CD103+ cDC1s in the islet at 3–4 wk old, before CD4+ T cell infiltration, and Batf3−/− NOD mice do not develop diabetes [7]. Three APC populations are absent in Batf3−/− NOD mice: islet CD103+ DCs, a population of islet CD11c+CD103− cells, and lymphoid organ-resident CD8α+ DCs; therefore, 1 or more of these APCs are necessary for initiation of diabetes pathogenesis. NOD mice, however, have fewer CD8α+ DCs in the spleen [85]. cDC1s are needed for cross-tolerance and are specialized to take up apoptotic cells for tolerance induction, and NOD CD8+ DCs are less effective for cross-tolerance [86] compared with diabetes-resistant mouse strains. In NOD mice, early treatment with Flt3 ligand increases the total number of DCs and can prevent diabetes development via increases in Tregs [3, 77, 87]. Thus, the impaired development of specific DC subsets can affect DC function and the balance between immunity and tolerance. To further understand the in vivo roles for the cDC1 subset in NOD mice, chimeric antibodies have been used to target Ags to these cells. Antibodies specific for DEC205 (CD205), a marker on cDC1, employ this C-type lectin endocytic receptor to internalize and deliver exogenous Ags to the intracellular Ag-processing pathway [88]. In vivo administration of anti-DEC205 chimeric antibody conjugated with a β cell–derived–peptide, enable delivery of the antigenic self-peptide to DEC205+ cDC1 in the absence of adjuvants or alteration of the DCs. In prediabetic NOD mice, DEC205+ cDC1s are capable of eliciting deletional CD8+ T cell tolerance but fail to establish peripheral CD4+ T cell tolerance because of defects in effector T cell depletion and Treg induction [89–91]. Therefore, cDC1s, both locally in the islets and systemically, contribute to autoimmune diabetes in NOD mice, and this may be in part due to DC-intrinsic genetic alterations.
Conventional CD11b+DCIR2+ DC2.
Both immunogenic and tolerogenic roles have been ascribed for CD11c+CD11b+ cells in autoimmunity. As recently reviewed [79], the role of CD11b+ DCs in NOD mice is muddled because both monocyte-derived cells and CD8α− DCIR2+ cDCs express CD11b and CD11c, but these 2 populations are quite different functionally [82, 92, 93]. Importantly, we have reported a flow cytometric method that separates these 2 populations [94]. In addition, some articles have described a DC population, termed merocytic DCs, which share some properties with cDC2s but are CD11c+ and are negative for both CD8 and CD11b [95]. NOD mice have more of these merocytic DCs (this increase is linked to Idd13), and transfer of merocytic DCs accelerates diabetes development in NOD mice, suggesting a pathogenic role [96].
Chimeric antibodies specific for DCIR2 (clone 33D1) can efficiently deliver attached Ag to cDC2 in vivo. Our recent study has shown that CD11b+DCIR2+ cDC2s presenting β cell Ag are capable of inducing Ag-specific peripheral T cell tolerance in NOD mice with ongoing autoimmunity, whereas DEC205+ cDC1 fails to induce CD4+ T cell tolerance or inhibit diabetes. The administration of the anti-DCIR2+ chimeric antibody resulted in self-reactive T cell depletion and inhibition of diabetes. Comparative gene expression analysis of Ag-primed autoreactive T cells stimulated in vivo in NOD mice with either immunogenic cDC1 or tolerogenic cDC2 has revealed the early signature genes of CD4+ T cell tolerance induction in an autoimmune context. Some of these genes overlap with common signatures of anergy and exhaustion [97], including cell cycle regulators Cdk6 and Hells, costimulatory molecules Tnfrsr4 and Trl1, and cell signaling genes Tgtp1, Klh6, and Myb. One interesting gene identified as induced more in T cells after DCIR2+ DC stimulation is Zbtb32, a negative regulator of early T cell differentiation [98]. When Zbtb32 is overexpressed in β cell Ag-specific T cells, cell proliferation and IFN-γ production are inhibited [82]. Moreover, DCIR2, in addition to serving as a marker for cDC2s, might have a functional role in regulating DC-mediated immune homeostasis. DCIR2 is part of the mouse C-type lectin domain family 4, Clec4, that are all encoded within the Idd19 locus on mouse chromosome 6 and contain an immunoreceptor tyrosine–based inhibitory motif in the cytoplasmic tail. The related mDCIR1 negatively regulates DC expansion, and DCIR1 deficiency in mice is associated with autoimmune disease development, including joint abnormalities [99]. A single nucleotide polymorphism in the human DCIR gene increases susceptibility to RA [100]. Ligand or antibody binding to DCIR2 could possibly impart a negative or tolerogenic signal on the DC and needs to be studied further. Therefore, molecular analysis of the tolerogenic signals provided by the DCIR2+ cDC2 subset, and the resulting T cell unresponsiveness will help to unveil signaling pathways to target for increasing Ag-specific T cell suppression in autoimmune disease.
Plasmacytoid DCs.
The other major DC subset, pDCs, branch off developmentally from cDCs at the pre-DC stage, a process mediated by the transcription factors E2-2 and Spi-B [101]. pDCs are well known mediators of antiviral immunity through an ability to secrete large amounts of type I IFN [102]. In T1D autoimmunity, IFN-α may have a pathogenic role in T1D initiation in mouse and human because studies of IFN-α treatment in patients with viral infections (i.e., persistent hepatitis B and C infections) or with leukemia have been shown to have an increased incidence of T1D [103]. Moreover, type I IFN-inducible transcriptional signature genes were increased in individuals who later develop autoantibodies and overt diabetes, implicating type I IFN as a risk factor in preclinical diabetes in children genetically predisposed to T1D [104, 105]. Before insulitis, physiologic β cell death, coupled with impaired clearance of apoptotic cells, has been observed in NOD mice [106]; this may lead to secondary necrosis and signals that stimulate IFN-α production by pDCs through TLR9 [107]. The type I IFN response within the pancreas may not only initiate diabetogenic T cell responses but also boost resident cDCs and immunoglobulin-secreting B cells [108]. IFN-α–producing pDCs have been detected in the blood of patients with T1D at the time of diagnosis [109], and depletion of pDCs or blocking IFN-α receptor signals in NOD mice at the initiation of insulitis (15–25 d) significantly delays the onset and decreases the incidence of T1D [107, 110]. These findings indicate that pDC and IFN-α are important initiators of T1D pathogenesis in mouse and human.
However, type I IFN has been shown to be both immunogenic or tolerogenic depending on the context, and open questions remain regarding the contribution of pDCs and type I IFN to T1D pathogenesis. Some investigators argue that pDCs are not the initiating cell and are located in the pancreas but not in the islets [7]. IFN-α given to 5–9-wk-old NOD mice inhibited the development of diabetes, suggesting a potential regulatory role after initiation of disease, during chronic autoimmunity [111]. Fitting the evidence that infection of T1D patients with LCMV inhibits T1D progression, LCMV infection in NOD mice elicited alternative immune suppressive pathways in pDCs to prevent diabetes [112]. A study in NOD mice using depletion of CD11c+ cells and adding back pDCs suggested a protective role for pDCs and a concomitant involvement of IDO-mediated signaling [12]. As with other DC subsets, markers need to be chosen carefully, especially in the context of inflammation; the pDC marker, BST2 (also known as PDCA-1) is also expressed by inflamed monocytes [94]. Overall, pDCs may induce either Treg-mediated protection or type I IFN-dependent acceleration in T1D, depending on the specific stimulus, dose, and timing of activation. Further studies are required to define the role of pDCs in autoimmune diabetes and their mechanism of action.
Peripheral T cell tolerance in NOD mice: Tregs, T cell deletion, and anergy
Tolerance induction by DCs has been shown in earlier studies of NOD mice to occur through varied cellular mechanisms, such as T cell deletion [79, 82, 89] or induction/expansion of CD4+ Foxp3+ Tregs, which can induce dominant tolerance [113–115]. Treg development and function is impaired in NOD mice, especially in the inflamed islets but can be recovered with administration of a low dose of IL-2 [116, 117]. Adoptive transfer of islet Ag–specific Tregs expanded in vitro with DCs showed that Treg-mediated suppression is effective in blocking the activation of self-reactive effector T cells in an Ag-restricted manner and in reversing diabetes [112, 115]. Ag-specific Tregs have higher efficacy than polyclonal Tregs, likely because they home to the site of Ag exposure and can induce particular regulatory functions in this environment [118].
Naturally occurring Tregs are selected during thymic T cell development, whereas iTregs can be differentiated from naïve T cells in peripheral tissues under the influence of TGF-β and acquire expression of the Treg marker, Foxp3. An appropriate quality and quantity of costimulatory molecules on APCs, including an intermediate CD80/CD86 signal [119, 120], is required for stimulating Treg differentiation and proliferation and effector T cells [121]. In mice without autoimmunity, CD8+DEC205+ cDC1s can induce iTregs, likely because of local TGF-β production during Ag presentation [90]. However, NOD cDC1s express higher CD40 and are not able to increase Tregs [82, 91]; the proinflammatory context activates cDCs to up-regulate CD40 and drive T cell priming toward pathogenic effector differentiation. Thus, optimal tolerogenic DC phenotype for inducing Treg conversion is within a narrow window of activation and maturation status. In contrast to cDC1, CD11b+ cDC2s are not efficient inducers of new Tregs because they lack endogenous TGF-β production but can normally expand existing Tregs [90]. DCIR2+ cDC2s from NOD mice cannot increase Ag-specific Tregs [82]. pDCs may have a role in maintaining Treg function in the pancreas of NOD mice [122]. IL-10–manipulated tolerogenic DCs from human blood are capable of inducing an anergy state in CD4+ and CD8+ T cells against melanoma Ags [123]. Therefore, IL-10 can also be a tolerogenic signal for induction of regulation. In summary, DC subsets are critical for normal maintenance of T cell tolerance, and DCs from individuals with T1D have defects in the ability to induce tolerance.
DIABETES SUSCEPTIBILITY GENES AND DENDRITIC CELLS
GWAS have greatly expanded our knowledge of genetic variants associated with T1D. Currently, murine IDDM susceptibility studies has identified >27 Idd loci in the NOD strain (T1DBase, version 4.19). IDDM1/Idd1 is mapped to the MHC region and contributes most of the genetic susceptibility to T1D, which shows most significant evidence of linkage (LOD = 65.8), whereas the remaining loci have LOD scores under 5, with most under 2 [124]. Despite this, the non-MHC Idd loci are important contributors to IDDM development [125]. For some of these loci, the interval is small or evidence is strong for a particular candidate gene, but other loci are large, and it is not yet clear what the causative gene is [126]. These other genetic contributions have been studied in the immune system, primarily in B and T cells as well as in pancreatic β cells, to understand the cellular and molecular mechanisms of T1D etiology and progression. Functional abnormalities of NOD APCs have been described, including defective differentiation [127], maturation [128], and Ag presentation for T cell differentiation [127, 129]. Many of these diabetes susceptibility genes are expressed in DCs (Fig. 1) and may affect the balance between immunity and tolerance mediated by DC development and function (Fig. 2). Here, we will provide an overview of known and potential connections between the genetic alterations in NOD mice and individuals with T1D and the characteristics of aberrant DCs that may link candidate genes in these loci with development of autoimmune diabetes. Some of these genes have direct evidence for effects on DCs or monocytes in the context of patients with T1D or NOD mice (Table 1). Other genes from susceptibility loci are expressed in DCs or other mononuclear phagocytes with potential consequences for tolerance, but no direct link to DCs in autoimmune diabetes has yet been shown (Table 2).
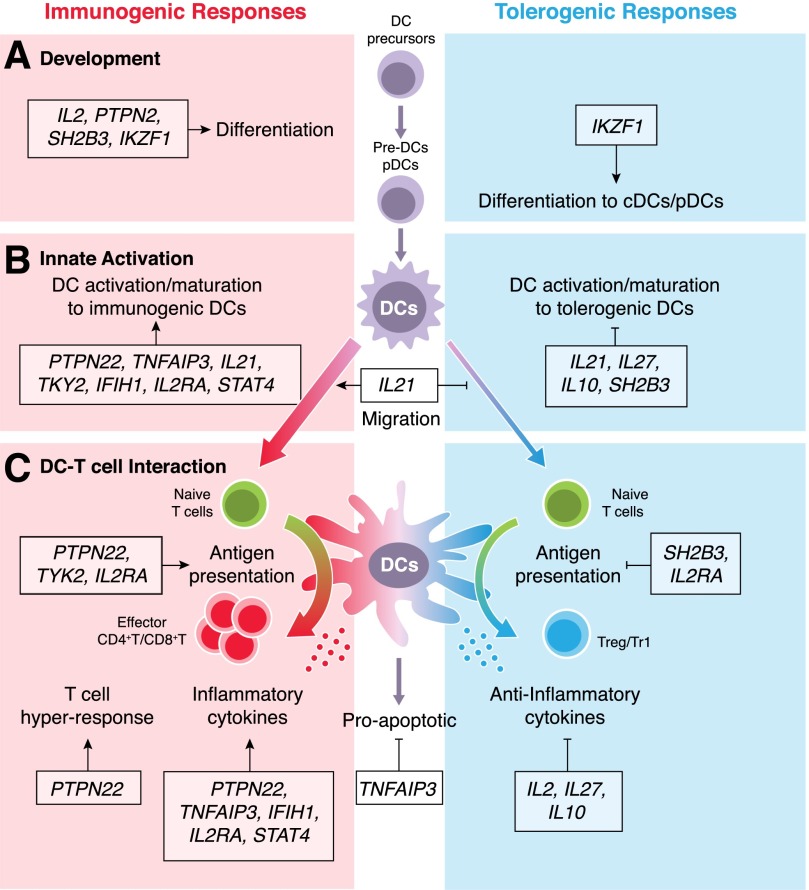
Figure 2. Potential roles for T1D susceptibility genes in DC development and their function that may alter tolerogenic balance.
T1D variant genes may affect multiple stages of DC development and function. It is not always clear whether the susceptible allele increases or decreases protein function, but the net result may be to either increase immunogenic responses or inhibit tolerogenic responses. Here, we classify 13 of the T1D susceptibility genes that are predicted to be expressed in DCs into 3 processes: DC development (A), innate activation (B), and DC-T cell interaction (C). Some key genes are discussed further in the text. (A) DC development in the bone marrow; Hematopoietic DC precursors differentiate into immature DC population. cDCs arise via pre-DCs, which migrate through the blood stream to lymphoid and nonlymphoid tissues, where they terminally differentiate. (B) DCs actively acquire either immunogenic or tolerogenic profiles under the control of distinct signature genes. The migration to lymph tissues also can promote immunity. (C) Antigen processing, presentation, and cytokine production in DCs and other APCs are also affected by T1D genes, which, in turn, affect T cell differentiation. DCs can undergo apoptosis to discontinue T cell activation.
TABLE 1.
T1D candidate genes expressed in mononuclear phagocytes with evidence for their potential roles in T1D pathogenesis
| Candidate genes |
Locationa |
Known roles in DCs or other mononuclear phagocytes | Known link to diabetes via mononuclear phagocytes | References | ||
|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Human T1D | Mouse NOD | |||
| CCR5 | Chemokine (C–C motif) receptor 5 | 3p21.31 | chr9 | Recruits DC precursor and immature DCs into inflammatory tissue via MIP/RANTES | Is reduced CCR5 on pancreatic CD8α+CD103+Langerin+ DCs in prediabetic NOD mice | [15, 16] |
| IDDM22 | ||||||
| CTLA4 | Cytotoxic T lymphocyte-associated protein 4 | 2q33.2 | chr1 | Inhibits DC maturation, Ag presentation, and IL-10/IDO production by monocyte-derived hDCs | Antagonistic anti-CTLA4–treated DCs prevent insulitis in NOD mice | [17–19] |
| IDDM12 | Idd5.1 | |||||
| CYP27B1 | Cytochrome P450, family 27, subfamily B, polypeptide 1 | 12q14.1 | chr10 | Regulates VitD3 activation and is predominantly expressed in monocyte-derived hDCs | CYP27B1 T1D variant may result in less active VitD3 in monocyte-derived hDCs | [20, 21] |
| ERBB3 | Erb-B2 receptor tyrosine kinase 3 | 12q13.2 | chr10 | Is upregulated by TLR ligands; affects cross-presentation by mBM-DCs; ERBB3+ hDCs increase T cell proliferation | T1D ERBB3 SNPs increase expression in hPBMC-DCs | [22, 23] |
| HLA-A | MHC, class I | chr6 | chr17 | Cross-presents exogenous Ag or presents endogenous Ag to stimulate CD8+ T cells | NOD MHC alters autoreactive CD8+ T cell selection | [24] |
| HLA-B | IDDM1 | Idd1 | ||||
| HLA-DQB1 | MHC, class II | chr6 | chr17 | Presents exogenous Ag to stimulate CD4+ T cells | NOD MHC alters autoreactive CD4+ T cell selection | [25, 26] |
| HLA-DRB1 | IDDM1 | Idd1 | ||||
| IFIH1 | Interferon-induced with helicase C domain 1, MDA5 | 2q24.2 | chr2 | Is a cytoplasmic dsRNA sensor in cDCs that induces type I IFN response for defense against viruses, including enteroviruses | Alleles that both increase and decrease diabetes risk have been identified; NOD mice with lower Ifih1 have less diabetes | [27, 28] |
| IKZF1 | IKAROS family zinc finger 1 (Ikaros) | 7p12.2 | chr11 | Ikaros deficiency results in the absence of most DCs; a hypomorphic mutation leads to a specific loss of pDCs | The minor allele (rs10272724) protects from T1D and is expressed in blood mononuclear cells | [29–31] |
| IL10 | IL-10 | 1q32.1 | chr1 | Suppresses DC maturation and IL-12 production and results in less Th1 response | IL-10–conditioned DCs induce islet-directed immune tolerance | [32, 33] |
| Idd5.4 | ||||||
| IL2 | IL-2 | 4q27 | chr3 | DC-derived IL-2 induces stimulation of NK cells and T cells, including Tregs; IL-2 inhibits DC development | For full protection from diabetes in NOD mice, the resistant IL-2 allele is needed in both APCs and T cells | [34–36] |
| Idd3 | ||||||
| IL21 | IL-21 | 4q27 | chr3 | Inhibits DC activation and maturation; IL-21R deficiency in DCs fails to acquire CCR7 and MHC II | Transfer of IL-21R+ DCs restores diabetes development in IL-21 RKO NOD mice | [37] |
| Idd3 | ||||||
| IL27 | IL-27 | 16p11.2 | chr7 | Modulates DCs to suppresses Th1/Th17 and increase Treg/Tr1 differentiation; inhibits HLA class I Ag presentation in immature DCs | IL-27-producing DCs induces IL-10+ T cells through anti-CD3 treatment in T1D mice | [38] |
| ITGB7 | Integrin, β-7 | 12q13.13 | chr15 | Migratory αE (CD103)+β7+ DCs cross-present Ags to CD8+ T cells; CD103+ DCs contribute to intestinal homeostasis by Treg conversion | CD103+ DCs presentation to CD4+ T cells and CD8+ T cells in pancreatic draining LNs is essential for diabetes development | [7, 39] |
| SH2B3 | SH2B adaptor protein 3, LNK | 12q24.12 | chr5 | Sh2b3 deficiency in mice increases DC number and Sh2b3- DCs induce more Th1 differentiation | The disease risk variant of human LNK increases proliferation of PBMCs in patients | [40, 41, 42] |
| IDDM20 | ||||||
| STAT4 | STAT4 | 2q32.3 | chr1 | Mediates IL-12-dependent IFN-γ production by activated DCs | STAT4 deficiency in NOD mice prevented the development of spontaneous diabetes | [43, 44] |
hDCs, human DCs; mBM-DCs; mouse bone marrow-derived DCs; hPBMC-DCs, human peripheral blood mononuclear cells-derived DCs.
Where applicable, the relevant human IDDM or mouse Idd locus is given.
TABLE 2.
T1D candidate genes expressed in mononuclear phagocytes with their functional roles that may potentially alter tolerance
| Candidate genes |
Locationb |
Known functions | Known roles in mononuclear phagocytes | References | ||
|---|---|---|---|---|---|---|
| Gene symbola | Gene name | Human T1D | Mouse NOD | |||
| AFF3 | AF4/FMR2 family, member 3 | 2q11.2 | chr1 | May function as nuclear transcriptional activator in lymphoid and brain development | Is expressed in pDCs | [45] |
| Idd26 | ||||||
| BACH2 | BTB and CNC homology 1, basic leucine zipper transcription factor 2 | 6q15 | chr4 | Is a transcriptional regulator that mediates B cell lineage development and represses CD4+ T cell differentiation | Regulates M1–M2 polarization; Bach2-deficient alveolar macrophages show enhanced cholesterol uptake and recruitment into lung | [46] |
| BAD | BCL2-associated agonist of cell death | 11q13.1 | chr19 | Mediates proapoptotic signaling by forming heterodimers with BCL-xL and BCL-2 | Mediates LPS/CpG-induced apoptosis in BM-DCs | [47] |
| C1QTNF6 | C1q and TNF-related protein 6, CTRP6 | 22q12.3 | chr15 | Is an adiponectin paralog that can modulate metabolism, inflammation, and angiogenesis | Treatment of CTRP6 induces IL-10 production in human macrophage cell lines | [48] |
| CD226 | CD226 molecule, DNAM-1 | 18q22.2 | chr18 | Stimulates NK cell-mediated cytotoxicity; expressed mainly on NK cells, platelets, T cells, and monocytes | Cross-linking CD226 mediates maturation of CD8α+ DCs | [49] |
| Idd21.1 | ||||||
| CD69 | CD69 molecule | 12p13.31 | chr6 | Regulates lymphocyte proliferation | Inhibits sphingosine-1-phosphate-induced skin DC migration; is expressed by pDCs | [50, 51] |
| CLEC16A | C-type lectin domain family 16, member A | 16p13.13 | chr16 | Predominately expressed in DCs, B cells, and NK cells; is involved in the costimulation and activation of T cells | Promotes late endosomal maturation to drive MHC class II expression in human monocyte DCs | [52] |
| CTSH | Cathepsin H | 15q25.1 | chr9 | Increases insulin and protects against cytokine-induced apoptosis in islet β cells | Is required for MHC-II antigen presentation in APCs; higher expression in hCD1c+ cDCs compared with hCD141+ cDCs | [53–55] |
| Idd2 | ||||||
| DEXI | Dexi homolog (mouse) | 16p13.13 | chr16 | Unknown function; induced by dexamethasone; risk allele corresponds with lower DEXI mRNA expression | Is highly expressed on DCs and monocytes | [56] |
| DLK1 | δ-like 1 homolog (Drosophila), δ-1 | 14q32.2 | chr12c | Mediates normal adipogenesis, muscular and neuronal differentiation, bone differentiation, and hematopoiesis | Promotes human mDC differentiation; promotes Th1 differentiation through interaction with Notch1 on T cells | [57] |
| IL2RA | IL-2 receptor, α | 10p15.1 | chr2 | Induces cell proliferation, differentiation, and survival/apoptosis | Blocking reduces LPS-stimulated inflammatory cytokines and Th1 priming by DCs; IL2RA+IDO+ DCs suppress T cell proliferation | [58] |
| IDDM21 | ||||||
| IL7R | IL-7 receptor | 5p13.2 | chr15 | IL-7 is a growth factor for lymphoid progenitors; induces homeostatic T cell proliferation | Is required for cDC and pDC development from CLPs; down-regulates MHC expression on DCs | [59, 60] |
| NRP1 | Neuropilin 1, BDCA-4 | 10p11.22 | chr8 | Is expressed in human pDCs, moDCs, and Tregs; is the receptor for Sema3A, VEGF and LAP-TGF-β1 | Increases contact between resting T cells/Tregs and DCs; is transferred from DCs to T cells by trogocytosis | [61, 62] |
| ORMDL3 | ORMDL sphingolipid biosynthesis regulator 3 | 17q12 | chr11 | Is involved in ER stress and inflammation; regulates eosinophil recruitment and degranulation in allergic asthma | Is induced on lung macrophages during allergic inflammation and increases chemokine expression | [63] |
| PTPN2 | Protein tyrosine phosphatase, nonreceptor type 2 | 18p11.21 | chr18 | Is a negative regulator of tyrosine kinases that signal downstream of cytokine and antigen receptors; inactivates JAK/STAT signal | Suppresses CSF-1–mediated mononuclear phagocyte development | [64, 65] |
| Idd21.2 | ||||||
| PTPN22 | Protein tyrosine phosphatase, nonreceptor type 22 | 1p13.2 | chr3 | Negatively regulates TCR signal in T cells; risk allele gives increase in memory/effector T cells | PTPN22 loss of function results in DC hyporesponsiveness | [66, 67] |
| Idd18.2 | ||||||
| RAC2 | Ras-related C3 botulinum toxin substrate 2 (Rho family, small GTP binding protein Rac2) | 22q12.3 | chr15 | Is a plasma membrane protein that regulates cell secretion, phagocytosis, and polarization | Rac2 deficiency in DCs impaired migration toward T cells and T cell priming; required for phagosomal pH regulation and cross-presentation in CD8+ DCs | [68, 69] |
| RASGRP1 | RAS guanyl releasing protein 1 (calcium and DAG-regulated) | 15q14 | chr2 | Regulates Ras activation in lymphocytes, a central determinant of Ag receptor signal strength | Is up-regulated by TLR agonists in macrophages and limits TLR-mediated inflammation | [70] |
| Idd13 | ||||||
| TAGAP | T cell activation Rho GTPase activating protein | 6q25.3 | chr17 | Is a negative regulator that mediates Rho GTPase signaling in activated T cells and other immune cells | Tagap deficiency in macrophages reduces inflammatory responses, including IFN-β, and increased virus replication | [71] |
| IDDM21 | Idd23 | |||||
| TNFAIP3 | TNF-α–induced protein 3, A20 | 6q23.3 | chr10c | Tnfaip3 deficient DCs are spontaneously activated and produce high levels of proinflammatory cytokines | Tnfaip3 deficiency in DCs causes systemic autoimmunity in mice | [72, 73] |
| TYK2 | Tyrosine kinase 2 | 19p13.2 | chr9 | Acts as JAK kinase for STAT signal for IFN-α, IL-12/IL-23, IL-10, GM-CSF stimulation | Tyk2 deficiency in DCs impaired Th1 differentiation | [94] |
BM-DCs, bone marrow-derived DCs; moDCs, monocyte-derived DCs.
Genes not in bold (BACH2 and C1QTNF6) show DC gene expression by ImmGen database, but no other publications confirm DC expression.
Where applicable, the relevant human IDDM or mouse Idd locus is given.
Mouse DlK1 and TNFAIP3 have not been shown to affect NOD diabetes susceptibility.
Disease risk variants affecting cytokine signaling in DCs
IL-2 signaling (IL-2, IL-2Rα, PTPN2).
IL2 and genes that affect IL-2 signaling are linked to many autoimmune diseases, including T1D, MS, and RA [130]. Idd3 on mouse chromosome 3 has a major effect on diabetes in NOD mice and mouse models of MS, disrupting the balance between the pathogenic autoreactive lymphocyte activation and Treg suppression [131]. The Idd3 region encodes 5 known candidates (Tenr, Il2, Il21, Cetn4, and Fgf2), but studies that recapitulate the reduced amount of IL-2 observed in NOD mice suggest reduced IL-2 impairs the maintenance of Treg cells, particularly in the inflamed pancreas [132]. Because IL-2 is required for the stability of Foxp3 expression, the neutralization of IL-2 exacerbates diabetes development in NOD mice because of fewer CD25+ Tregs [133]. IL-2 is also known to inhibit DC development, pushing cells away from the DC fate toward committed monocyte progenitors via decreased expression of CD135 (Flt3) and subsequent signaling [134, 135]. Overall, a reduction in IL-2 signaling in T1D may contribute to Treg decline and the emergence of DC-mediated effector phenotypes. Studies have shown the therapeutic potential of increased IL-2 signaling via enhanced development and the function of Tregs [136]. Treatment of NOD mice with low-dose IL-2 can prevent and reverse diabetes by increasing the Treg cells in the pancreas and by inducing expression of molecules associated with Treg suppressive function [137]. In NOD mice, this leads to an increase in Tregs without altering the number of effector T cells, and human trials have shown similar results [137, 138]. Tregs themselves cannot produce IL-2, and DCs can provide IL-2 signals that are important for regulating Treg development, expansion, and function [77, 139].
Multiple mechanisms decrease IL-2 signaling and the resulting phenotypes that may lead to loss of tolerance in diabetes. The reduced IL-2R signaling in patients with T1D is associated with the risk allele of IL2RA, rs2104286, and the rs1893217 SNP in PTPN2, in CD4+CD25+ T cells [140]. IL2RA is a well-established T1D susceptibility locus reported before GWAS [58]. IL-2RA is the high-affinity α-subunit of the IL-2 receptor complex and mediates IL-2 signaling in both host defense and self-specific Tregs, as described above. Apart from the role as a receptor to transduce IL-2 signals, IL-2RA may be a marker for a tolerogenic DC population; the treatment of human peripheral monocytes with prostaglandin E2 generates IL-2RA- and IDO-expressing tolerogenic DCs that can mediate immune tolerance against a tumor [141]. PTPN2 negatively regulates IL-2 signaling through its inhibition of JAK1 and JAK3. The PTPN2 risk allele rs1893217C is associated with reduced PTPN2 expression in CD4+ T cells and reduced pSTAT5 in response to IL-2 [142]. Further investigation is needed into the molecular mechanism of how IL-2RA and PTPN2 influences reduced IL-2 signaling and their roles in DC populations.
IL-21.
IL-21 is produced by activated CD4+ T cells, Th17 cells, and NKT cells and is known to act on both lymphoid and nonlymphoid cells. Although IL-21 increases immunity with effects such as enhanced proliferation of lymphocytes, increased cytotoxicity in T and NK cells, and differentiation of Th17 and plasma cells, it also has direct inhibitory effects on the Ag-presenting function of DCs [143]. The IL-21 gene is located near IL-2 on chromosomal region 4q27, which is associated with many autoimmune diseases, including T1D, Graves disease, SLE, arthritis, ulcerative colitis, CD, and celiac disease [144]. Levels of IL-21 are higher in NOD mice than in diabetes-resistant strains of mice, and NOD mice lacking IL-21 signals do not develop diabetes [145]. CD11c−CD11b+ APCs from diabetes-resistant NOD Idd3 mice or NOD Il21r-deficient mice displayed impaired ability to drive Th17 differentiation [146]. The transfer of IL-21R+ DCs into Il21r-knockout mice restores diabetes development because of reduced migration of pancreatic DCs carrying Ags to pLNs [37]. Neutralization of IL-21 successfully reversed diabetes in NOD mice, even at a late preclinical stage, when combined with islet transplant [147]. Thus, blocking IL-21 signals could have therapeutic benefit for treatment of autoimmune diseases but may be tricky because IL-21 drives both proinflammatory and regulatory responses.
IL-12 family member signaling (TYK2, STAT4, and IL-27).
STAT4 is a member of the STAT protein family, transcription factors that link the cytokine receptors with cytokine-induced gene transcription. STAT4 is expressed at sites of inflammation [148] and mediates IL-12, IL-23, and IL-35 signaling in DCs, monocytes, and macrophages and regulates Th1 differentiation and IFN-γ production [149]. The STAT4 rs7574865 (G>T) polymorphism is associated with susceptibility to T1D (odds ratio of 1.94) [150]. STAT4-deficient NOD mice have lower levels of both IFN-γ and IL-2 in serum and do not develop spontaneous diabetes [43].
TYK2 is a member of the Janus kinase-signal transduction family important for cytokine signaling by IFN-γ, IL-12, and IL-23 affects Th1 and Th17 lineage development. Tyk2-deficient DCs fail to produce IL-12- and IL-23 and do not induce Th1 cell differentiation, similar to STAT4-deficient DCs [74]. TYK2 is also important for TLR-mediated responses in DCs, including IFN-α/β, IFN-γ, IL-6, IL-10, IL-12, and IL-23 production. A meta-analysis [151] showed a protective association of the minor TYK2 allele rs2304256 (C>A) with T1D (odd ratio of 0.86) at chromosome band 19p13.2 and is also associated with SLE and MS; the protective allele has a missense mutation in the JAK-homology 4 region, which is predicted to reduce autophosphorylation and type 1 IFN signal. Mice lacking Tyk2 showed increased susceptibility to diabetes induced by a β cell tropic virus because of the inability of β cells to clear the virus [152]. This highlights the complex contribution of type 1 IFN to autoimmunity, which can directly induce inflammation that increases autoimmunity but is needed for protecting against viral-induced damage and infection-mediated autoimmunity [153, 154].
GWAS identified a T1D risk allele in IL27, rs4788084 (T>C) [155]. IL-27 is a member of the IL-12 family that is secreted by APCs, mainly activated DCs, and was shown to regulate the pathogenic activation of T and B cells by inhibiting differentiation and inducing IL-10 [156]. B6-H1, a costimulator associated with tolerance, is increased on DCs with IL-27 [157], which can generate IL-10–producing Tr1 cells in cooperation with TGF-β [158]. Administration of IL-27 suppresses some autoimmune diseases, and the absence of IL-27–mediated signaling induces hyperproduction of various proinflammatory cytokines and severe inflammation. IL-27 receptor (WSX-1)–deficient DCs were hyperresponsive to LPS stimulation and induced Th1 immune responses [159]. Notably, IL-10– and IL-27–producing DCs capable of enhancing IL-10 production of CD4+ T cells are induced in Peyer's patch in oral tolerance [160]. Consistent with this observation, the oral administration of anti-CD3 antibody can prevent spontaneous diabetes development through the induction of IL-10– and IL-27–producing DCs and the resultant IL-10–producing CD4+ T cells in the small intestine of NOD mice [38].
IL-10.
IL-10 is an anti-inflammatory cytokine, produced primarily by monocytes, lymphocytes, macrophages, and DCs, which has pleiotropic effects on both Ag presentation and subsequent release of proinflammatory cytokines. A meta-analysis [161] confirmed the protective association of IL10 SNP rs3024505 with T1D (odds ratio of 0.84, P = 1.9 × 10−9), and polymorphisms of IL10 gene promoter region are linked to T1D. Early systemic treatment of 2–3-wk-old NOD mice with high-dose IL-10 prevented spontaneous diabetes [162], and IL-10–conditioned, immature DCs prevented autoimmune diabetes in NOD mice, even when given later (7–10 wk old), through an induction of T cell hyporesponsiveness and Treg-mediated tolerance [32]. These results suggest that the IL10 gene itself and its promoter polymorphisms are associated with protection from T1D pathogenesis.
Intracellular signaling events affecting the development of DCs
IKAROS.
IKZF1, the gene encoding IKAROS, on chromosome band 7p12.2 is an essential transcriptional regulator of lymphopoiesis and immune homeostasis and is also required for terminal differentiation and maturation of DCs in mouse and human (Fig. 2). A minor allele of IKZF1 is protective against T1D [odds ratio of 0.87, rs10272724 (T>C)] [29], but it is not yet clear how this allele affects expression or function of IKAROS. Mice with a deletion of the DNA-binding domain of Ikaros lack both CD8α+ and CD8α− spleen cDCs, whereas mice with a null mutation of Ikaros fail to develop CD8α− cDCs but not CD8α+ cDCs [30]. Moreover, mice expressing low levels of Ikaros lack peripheral pDCs [31]. These data demonstrates that Ikaros drives development of the DC lineage, but further studies are needed to examine the roles of Ikaros in specific DC phenotypes and in autoimmunity.
SH2B3 (also known as LNK).
SH2B adaptor protein 3, SH2B3, has a pivotal role as a suppressor of T cell receptor-mediated immune signaling and is a negative regulator of lymphopoiesis and early hematopoiesis. Allele 784C of SH2B3 is related to a higher risk of T1D (odds ratio of 1.52, P = 1.2 × 10−12) on chromosome band 12q24 (IDDM20), and PBMCs carrying this allele have increased self-reactive T lymphocyte proliferation compared with PBMCs with the protective allele 784T [40]. Loss of SH2B3 increases hematopoietic stem cells, which display decelerated cell cycle kinetics and are kept in the quiescent phase [163]. SH2B3 has an inhibitory role on the expansion of DCs in spleen and lymph node and inhibits responses to IL-15 and GM-CSF, leading to less differentiation of IFN-γ–producing Th1 cells [41]. Therefore, alteration of SH2B3 in DCs could contribute to T1D risk.
Intracellular signaling events affecting the activation of DCs
PTPN22.
The PTPN22-encoded Lyp phosphatase allele (Lyp620W) confers a nearly 2-fold risk for developing RA or T1D, with odds ratios in the range of 3–4 for homozygous individuals. In terms of strength of association, PTPN22 is second in importance only to the HLA for these 2 diseases. PTPN22 is expressed in T cells, B cells, NK cells, and DCs but has mostly been studied for its role in negatively regulating TCR signaling, including ZAP70 and the Src family of kinases, leading to inhibition of TCR-driven proliferation and expansion of memory peripheral T cells. Consistent with this, mice deficient in Pep, the murine ortholog of PTPN22, have increased T cell memory [164]. In addition to these T cell phenotypes that could affect diabetogenic T cells, mice harboring Pep619W, the homolog of the human Lyp-susceptible allele, have more DCs in the spleen, and these DCs had an increased response to LPS (higher CD40 expression and IL-12 production) [66]. Therefore, the human PTPN22 risk allele may lead to impaired Src family kinase activity in both T cells and DCs, including Lck signaling in DCs, which could alter immune cell regulation.
TNFAIP3 (also known as A20).
TNFAIP3 negatively regulates proinflammatory signaling, driven by NF-κB, by deubiquitinating E3 ligase and ubiquitin-binding functions. A20 inhibits many inflammatory pathways that signal through NF-κB and is induced by a wide variety of inflammatory pathways, including TNF, TLR ligands, nucleotide-binding oligomerization domain protein, and CD40. Tnfaip3-deficient mice spontaneously develop severe inflammatory disease and perinatal death. Hence, A20 prevents lethal inflammation by restricting homeostatic MyD88-dependent signals [165]. Several SNPs in the TNFAIP3 genomic locus were found to be associated with chronic inflammation and increased susceptibility to various autoimmune diseases, including T1D, SLE, RA, CD, and MS [166]. A20-mediated suppression of DC activation is crucial for immune homeostasis and preventing inflammation that can lead to systemic autoimmunity. DCs lacking A20 show spontaneous and TLR-driven activation, which induces lymphocyte-dependent colitis, seronegative ankylosing arthritis, and enthesitis, conditions stereotypic of human inflammatory bowel disease [167]. Because of the role of A20 in preventing DC hyperresponsiveness, A20-deficient DCs stimulate pathogenic Th1 and Th17 cell immune responses and drive the differentiation of B cells into autoantibody-producing plasma cells [72]. Moreover, a binding partner of A20, ABIN-1 (encoded by TNIP1), cooperates with A20 to inhibit TLR or TNF-mediated NF-κB signals. Polymorphisms of TNIP1 are strongly linked with susceptibility to SLE and psoriatic arthritis [168]. This pathway is implicated in T1D because the Tnip1 gene is encoded in the mouse diabetes susceptibility locus Idd4.3. DC-specific deletion of ABIN-1 caused TLR-induced cDC activation and hyperproduction of IL-23, which exacerbated imiquimod-induced psoriasis [169]. Thus, these observations suggest both ABIN-1 and A20-mediated NF-κB signals are crucial for immune homeostasis, particularly for cDC regulation.
MDA5 (encoded by IFIH1).
Genetic studies have linked protective variants in IFIH1, encoding MDA5, a dsRNA virus sensor, with resistance to T1D. Although infection with viruses, such as enterovirus, has been hypothesized to have an etiologic role in human T1D, direct proof is difficult to obtain. A meta-analysis [170] of 26 studies showed a strong association between molecular evidence of enteroviral infection and developing T1D (odd ratio of 9.8). The protective allele of IFIH1 is thought to reduce expression of MDA5, and NOD mice heterozygous for Ifih1 express less than half the level of MDA5 protein in CD11c+ APCs and are protected from diabetes [171]. Signaling through MDA5 in DCs in response to dsRNA is critical for control of the type I IFN signature pathway that confers diabetes pathogenesis.
Vitamin D and vitamin D–metabolizing enzyme CYP27B1.
Although the correlation between the genetic polymorphisms in the vitamin D receptor and T1D is not yet clear [172, 173], recent studies identify human T1D-associated polymorphisms in genes encoding the enzyme CYP27B1, which is involved in VitD3 metabolism [174]. VitD3 signaling may affect insulin synthesis and sensitivity [175], and VitD3 supplements given in early childhood decrease the risk of developing T1D in patients and mice [174, 176]. In addition, VitD3 has been shown to promote regulatory immune responses and to inhibit T1D pathogenesis through enhancing tolerogenic DCs [104, 177] and Tregs [178] and impairing pDC-mediated T cell proliferation and IFN-γ secretion [179]. When GM-CSF–derived DCs from NOD mice were treated with VitD3 in vitro, these DCs promoted Treg proliferation and T cell IL-10 production [104]. VitD3 plasma levels were significantly less in patients with T1D at diagnosis [180], consistent with impaired DC-mediated immune regulation. Active VitD3, 1α,25-dihydroxyvitamin D3, is hydroxylated by CYP27B1 and potently inhibits DC priming of T cell activation [20]. The T1D risk allele of CYP27B1, rs10877012G, shows lower expression compared with the protective allele in healthy human CD14+ monocyte-derived DCs [21]. Thus, lower vitamin D signaling may lead to defects in tolerogenic DC development and its protection against autoimmunity. Of note, although therapeutic use of VitD3 metabolites in humans competes against the effects on calcium and bone metabolism, the treatment of T1D with VitD3 analogs still remains a promising future potential treatment. Together, these examples show that many genes with effects on diabetes pathogenesis can exert an effect on DC function, which may, in part, account for the altered susceptibility.
POTENTIAL DC-BASED THERAPIES FOR AUTOIMMUNITY
Understanding how, in an autoimmune environment, DCs override their tolerogenic state to become inflammatory can lead to solutions for manipulating DCs toward stable tolerogenic states for treatment of autoimmune diseases. For the goal of Ag-driven tolerance, one consideration is how best to induce dominant tolerance, namely, the ability of tolerance directed at a single Ag specificity to promote tolerance to other Ags expressed in the same tissue. Induction of Tregs against one β cell specificity can inhibit other β cell–specific pathogenic T cells and reverse diabetes [181]. Therefore, manipulating DCs to induce Tregs is one desired therapeutic outcome. However, a recent report [182] shows that deletional tolerance is more durable than regulation so manipulating autoimmune DCs to induce both forms of tolerance may provide the optimal therapeutic benefit.
A wide range of immunosuppressive agents have been investigated for their effects on DC maturation and function in vivo and in vitro. Despite convincing preclinical evidence supporting DC-based prevention or reversal of autoimmunity, to date, few clinical trials have assessed the safety and potential benefits of in vitro manipulation to generate therapeutic tolerogenic DCs for treatment of autoimmunity [183]. Most preclinical studies and ex vivo human assays have used monocyte-derived DCs, but new methods for generation of Flt3 ligand-based human cDCs may broaden the types of DCs available [184]. The first phase I clinical testing of ex vivo tolerogenic DCs used autologous monocyte-derived DCs treated with antisense oligonucleotides to down-regulate the costimulatory molecules CD40, CD80, and CD86 and demonstrated that these DCs were well tolerated and safe in human patients with T1D [185]. This study was designed to test safety and because the patients had long-standing diabetes, no therapeutic benefit was expected. Curiously, patients given these “tolerogenic” DCs had an increased frequency of Breg-like cells. This type of cell therapy approach has the advantage of control over the in vitro conditions for generation of the DCs and will likely yield new information about human tolerogenic DCs. However, such manipulation of cells requires high resources per patient and limits implementation.
An alternative approach to in vitro generation of tolerogenic DCs is to deliver agents that manipulate DCs toward tolerance induction in vivo. One component of such a therapy would be the use of drugs that modulate DC maturation, and we can learn from the application of pharmacologic agents to generate ex vivo tolerogenic DCs (as reviewed in Creusot et al. [183]). Some of these immune-modulating signals have safety profiles that would allow systemic in vivo use, but the balance of effect size with toxicity may be improved by targeting them to DCs. For example, rapamycin is a potent immunosuppressive drug that can induce tolerogenic DCs [186, 187] and Tregs [188, 189], but it also may have negative effects on β cells that may preclude systemic use in patients with T1D [190]. These immunomodulatory agents could potentially be used alone for treatment, but that would depend on the presence of endogenous self-antigens presented to the autoreactive T cells. Alternatively, including a second Ag-specific component would likely increase efficacy [113]. For example, when NOD mice were given either JAK inhibitors that block cytokine signals or tyrosine kinase inhibitors, such as imatinib, fewer mice developed diabetes, potentially because of effects on DCs [191, 192]. Adding self-antigen to these small-molecule treatments may improve outcomes. Effectively, this would create a “tolerogenic vaccine,” delivering an Ag-specific ally in the context of drugs that modulate DCs back toward tolerogenic Ag presentation.
For any of these treatments, defining reliable biomarkers is critical [193]. Biomarkers could potentially identify the subset of patients with T1D who have particular tolerance defects and who may respond to a particular treatment. Baseline alterations in DC phenotype may be useful because the genetic and environmental modulators of DC function will vary among individuals with T1D. Biomarkers can also identify efficacy and responsiveness to specific modulations, and this type of marker will likely include T cell readouts, but assessing the function and stability of tolerogenic DCs after treatment would also be informative.
CONCLUDING REMARKS
Many autoimmune diseases, including T1D, are T cell–mediated, and research has focused on how genetic susceptibility affects T cells. Indeed, autoimmune susceptibility alleles have clear effects on T cell phenotypes, but APCs in general, and especially DCs, are also altered by these susceptibility alleles and are critical for maintaining T cell tolerance. DCs comprise potent multifunctional subsets that determine whether a T cell differentiates into an executioner or guardian, such as effector T cells vs. Tregs, and when this process is askew for self-specific cells, autoimmunity ensues. DCs respond to environmental cues, such as viral infection and/or proinflammatory feedback from self-derived signals, and genetic susceptibility to autoimmunity can alter the ability of DCs to maintain homeostasis. Therefore, to implement Ag-specific immune therapies to correct the pathogenic T cell self-attack, it will be necessary to consider the central role of DCs in both connecting innate and adaptive immunity and in controlling the adaptive immune responses. The application of DCs to treat patients with autoimmune disease may be complicated, both because of the plasticity of DCs in directing effector responses or suppressive response and because of the disease-susceptibility gene variants that impair the ability of the DCs to push self-tolerance. In the worst-case scenario, signals that may enhance tolerance induction in normal individuals could instead exacerbate autoimmune disease progression. However, understanding the effects of genomic polymorphisms on specific DC subsets that influence autoimmune disease can lead us to a better definition of “tolerogenic” DCs in the context of autoimmunity and improve the desired therapeutic effects. Tolerance therapies designed to target Ag to particular DC subsets, such as our current protocol in NOD mice, using DC subset-specific chimeric antibodies [82, 89, 91], are potentially more beneficial than untargeted delivery of Ags to immune cells with varying activation phenotypes. Likewise, addition of agents to enhance tolerance may allow combined DC-based therapies that could enhance immune tolerance by increasing Ag-specific Foxp3+ Tregs quantitatively and qualitatively and by engineering the stable therapeutic properties of DCs.
AUTHORSHIP
C.H.-I. and K.V.T. designed and wrote the review.
Acknowledgments
This work was supported by the U. S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Division of Intramural Research. We would like to thank Drs. Jeffrey Price, Jubayer Rahman, and William Coley for critical reading of this manuscript, and the ImmGen consortium for providing gene expression data.
Glossary
- ABIN1
A20-binding inhibitor of NF-κB activation 1
- Breg
regulatory B cell
- CD
Crohn's disease
- cDC
conventional DC
- DC
dendritic cell
- DCIR2
dendritic cell inhibitory receptor 2
- dsRNA
double-stranded RNA
- Flt3
fms-related tyrosine kinase 3
- GWAS
genome-wide association studies
- Idd
insulin-dependent diabetes
- IDDM
insulin-dependent diabetes mellitus
- IFIH1
IFN induced with helicase C domain 1
- IKZF1
IKAROS family zinc finger 1
- ImmGen
Immunological Genome Project
- iTreg
induced regulatory T cell
- JAK
Janus kinase
- LCMV
lymphocytic choriomeningitis virus
- LNK
lymphocyte-specific adaptor protein
- LOD
logarithm of odds
- MDA5
melanoma differentiation-associated protein 5
- MS
multiple sclerosis
- NK
natural killer
- PTPN
protein tyrosine phosphatase, non-receptor type
- RA
rheumatoid arthritis
- SH2B3
SH2B adaptor protein 3
- SLE
systemic lupus erythematosus
- SNP
single-nucleotide polymorphisms
- T1D
type I diabetes
- Treg
regulatory T cells
- TYK2
tyrosine kinase 2
- VitD3
vitamin D3
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Lehuen A., Diana J., Zaccone P., Cooke A. (2010) Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10, 501–513. [DOI] [PubMed] [Google Scholar]
- 2.Todd J. A. (2010) Etiology of type 1 diabetes. Immunity 32, 457–467. [DOI] [PubMed] [Google Scholar]
- 3.Van Belle T. L., Juntti T., Liao J., von Herrath M. G. (2010) Pre-existing autoimmunity determines type 1 diabetes outcome after Flt3-ligand treatment. J. Autoimmun. 34, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. (1980) Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 29, 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Anderson M. S., Bluestone J. A. (2005) The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485. [DOI] [PubMed] [Google Scholar]
- 6.Jansen A., Homo-Delarche F., Hooijkaas H., Leenen P. J., Dardenne M., Drexhage H. A. (1994) Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 43, 667–675. [DOI] [PubMed] [Google Scholar]
- 7.Ferris S. T., Carrero J. A., Mohan J. F., Calderon B., Murphy K. M., Unanue E. R. (2014) A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 41, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathis D., Vence L., Benoist C. (2001) β-Cell death during progression to diabetes. Nature 414, 792–798. [DOI] [PubMed] [Google Scholar]
- 9.Fu W., Wojtkiewicz G., Weissleder R., Benoist C., Mathis D. (2012) Early window of diabetes determinism in NOD mice, dependent on the complement receptor CRIg, identified by noninvasive imaging. Nat. Immunol. 13, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnuson A. M., Thurber G. M., Kohler R. H., Weissleder R., Mathis D., Benoist C. (2015) Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc. Natl. Acad. Sci. U. S. A. 112, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turley S., Poirot L., Hattori M., Benoist C., Mathis D. (2003) Physiological β cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 198, 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena V., Ondr J. K., Magnusen A. F., Munn D. H., Katz J. D. (2007) The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol. 179, 5041–5053. [DOI] [PubMed] [Google Scholar]
- 13.De Jersey J., Snelgrove S. L., Palmer S. E., Teteris S. A., Mullbacher A., Miller J. F., Slattery R. M. (2007) β cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 104, 1295–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eizirik D. L., Sammeth M., Bouckenooghe T., Bottu G., Sisino G., Igoillo-Esteve M., Ortis F., Santin I., Colli M. L., Barthson J., Bouwens L., Hughes L., Gregory L., Lunter G., Marselli L., Marchetti P., McCarthy M. I., Cnop M. (2012) The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 8, e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B., Sharron M., Montaner L. J., Weissman D., Doms R. W. (1999) Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. U. S. A. 96, 5215–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welzen-Coppens J. M., van Helden-Meeuwsen C. G., Leenen P. J., Drexhage H. A., Versnel M. A. (2012) Reduced numbers of dendritic cells with a tolerogenic phenotype in the prediabetic pancreas of NOD mice. J. Leukoc. Biol. 92, 1207–1213. [DOI] [PubMed] [Google Scholar]
- 17.Karumuthil-Melethil S., Perez N., Li R., Prabhakar B. S., Holterman M. J., Vasu C. (2010) Dendritic cell-directed CTLA-4 engagement during pancreatic β cell antigen presentation delays type 1 diabetes. J. Immunol. 184, 6695–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent S., Carrega P., Saverino D., Piccioli P., Camoriano M., Morabito A., Dozin B., Fontana V., Simone R., Mortara L., Mingari M. C., Ferlazzo G., Pistillo M. P. (2010) CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum. Immunol. 71, 934–941. [DOI] [PubMed] [Google Scholar]
- 19.Han Y., Chen Z., Yang Y., Jiang Z., Gu Y., Liu Y., Lin C., Pan Z., Yu Y., Jiang M., Zhou W., Cao X. (2014) Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 59, 567–579. [DOI] [PubMed] [Google Scholar]
- 20.Kundu R., Chain B. M., Coussens A. K., Khoo B., Noursadeghi M. (2014) Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur. J. Immunol. 44, 1781–1790. [DOI] [PubMed] [Google Scholar]
- 21.Shahijanian F., Parnell G. P., McKay F. C., Gatt P. N., Shojoei M., O’Connor K. S., Schibeci S. D., Brilot F., Liddle C., Batten M., Stewart G. J., Booth D. R.; ANZgene Multiple Sclerosis Genetics Consortium (2014) The CYP27B1 variant associated with an increased risk of autoimmune disease is underexpressed in tolerizing dendritic cells. Hum. Mol. Genet. 23, 1425–1434. [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Jin Y., Reddy M. V., Podolsky R., Liu S., Yang P., Bode B., Reed J. C., Steed R. D., Anderson S. W., Steed L., Hopkins D., Huang Y., She J. X. (2010) Genetically dependent ERBB3 expression modulates antigen presenting cell function and type 1 diabetes risk. PLoS One 5, e11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moita C. F., Chora Â., Hacohen N., Moita L. F. (2012) RNAi screen for kinases and phosphatases that play a role in antigen presentation by dendritic cells. Eur. J. Immunol. 42, 1843–1849. [DOI] [PubMed] [Google Scholar]
- 24.DiLorenzo T. P., Serreze D. V. (2005) The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol. Rev. 204, 250–263. [DOI] [PubMed] [Google Scholar]
- 25.Cucca F., Lampis R., Congia M., Angius E., Nutland S., Bain S. C., Barnett A. H., Todd J. A. (2001) A correlation between the relative predisposition of MHC class II alleles to type 1 diabetes and the structure of their proteins. Hum. Mol. Genet. 10, 2025–2037. [DOI] [PubMed] [Google Scholar]
- 26.Tsai S., Santamaria P. (2013) MHC class ii polymorphisms, autoreactive T-cells, and autoimmunity. Front. Immunol. 4, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105. [DOI] [PubMed] [Google Scholar]
- 28.Looney B. M., Xia C. Q., Concannon P., Ostrov D. A., Clare-Salzler M. J. (2015) Effects of type 1 diabetes-associated IFIH1 polymorphisms on MDA5 function and expression. Curr. Diab. Rep. 15, 96. [DOI] [PubMed] [Google Scholar]
- 29.Swafford A. D., Howson J. M., Davison L. J., Wallace C., Smyth D. J., Schuilenburg H., Maisuria-Armer M., Mistry T., Lenardo M. J., Todd J. A. (2011) An allele of IKZF1 (Ikaros) conferring susceptibility to childhood acute lymphoblastic leukemia protects against type 1 diabetes. Diabetes 60, 1041–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L., Nichogiannopoulou A., Shortman K., Georgopoulos K. (1997) Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity 7, 483–492. [DOI] [PubMed] [Google Scholar]
- 31.Allman D., Dalod M., Asselin-Paturel C., Delale T., Robbins S. H., Trinchieri G., Biron C. A., Kastner P., Chan S. (2006) Ikaros is required for plasmacytoid dendritic cell differentiation. Blood 108, 4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai N., Yasuda H., Xiang Y., Zhang L., Rodriguez-Pinto D., Yokono K., Sherwin R., Wong F. S., Nagata M., Wen L. (2011) IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clin. Immunol. 139, 336–349. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H., Qiu W., Lei P., Zhou W., Wen X., He F., Li L., Dai H., Shen G., Gong F. (2008) IL-10 gene modified dendritic cells inhibit T helper type 1-mediated alloimmune responses and promote immunological tolerance in diabetes. Cell. Mol. Immunol. 5, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sgouroudis E., Albanese A., Piccirillo C. A. (2008) Impact of protective IL-2 allelic variants on CD4+ Foxp3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice. J. Immunol. 181, 6283–6292. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton-Williams E. E., Cheung J., Rainbow D. B., Hunter K. M., Wicker L. S., Sherman L. A. (2012) Cellular mechanisms of restored β-cell tolerance mediated by protective alleles of Idd3 and Idd5. Diabetes 61, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulme M. A., Wasserfall C. H., Atkinson M. A., Brusko T. M. (2012) Central role for interleukin-2 in type 1 diabetes. Diabetes 61, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Belle T. L., Nierkens S., Arens R., von Herrath M. G. (2012) Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity 36, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu C., Ding H., Zhang X., Wong F. S., Wen L. (2013) Combination treatment with anti-CD20 and oral anti-CD3 prevents and reverses autoimmune diabetes. Diabetes 62, 2849–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Rio M. L., Bernhardt G., Rodriguez-Barbosa J. I., Förster R. (2010) Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 234, 268–281. [DOI] [PubMed] [Google Scholar]
- 40.Lavrikova E. Y., Nikitin A. G., Kuraeva T. L., Peterkova V. A., Tsitlidze N. M., Chistiakov D. A., Nosikov V. V. (2011) The carriage of the type 1 diabetes-associated R262W variant of human LNK correlates with increased proliferation of peripheral blood monocytes in diabetic patients. Pediatr. Diabetes 12, 127–132. [DOI] [PubMed] [Google Scholar]
- 41.Mori T., Iwasaki Y., Seki Y., Iseki M., Katayama H., Yamamoto K., Takatsu K., Takaki S. (2014) Lnk/Sh2b3 controls the production and function of dendritic cells and regulates the induction of IFN-γ-producing T cells. J. Immunol. 193, 1728–1736. [DOI] [PubMed] [Google Scholar]
- 42.Li Y. J., Li X. Y., Guo X. R., Li Y., Shen B. F., Shi Y. C., Zhang J. Y. (2012) Absence of SH2B3 mutation in nonobese diabetic mice. Genet. Mol. Res. 11, 1266–1271. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z., Chen M., Ellett J. D., Fialkow L. B., Carter J. D., McDuffie M., Nadler J. L. (2004) Autoimmune diabetes is blocked in Stat4-deficient mice. J. Autoimmun. 22, 191–200. [DOI] [PubMed] [Google Scholar]
- 44.Fukao T., Frucht D. M., Yap G., Gadina M., O’Shea J. J., Koyasu S. (2001) Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J. Immunol. 166, 4446–4455. [DOI] [PubMed] [Google Scholar]
- 45.Miller J. C., Brown B. D., Shay T., Gautier E. L., Jojic V., Cohain A., Pandey G., Leboeuf M., Elpek K. G., Helft J., Hashimoto D., Chow A., Price J., Greter M., Bogunovic M., Bellemare-Pelletier A., Frenette P. S., Randolph G. J., Turley S. J., Merad M.,; Immunological Genome Consortium (2012) Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura A., Ebina-Shibuya R., Itoh-Nakadai A., Muto A., Shima H., Saigusa D., Aoki J., Ebina M., Nukiwa T., Igarashi K. (2013) Transcription repressor Bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function. J. Exp. Med. 210, 2191–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M., Huang L., Wang J. (2007) Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood 109, 4360–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M. J., Lee W., Park E. J., Park S. Y. (2010) C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol. Cells 30, 59–64. [DOI] [PubMed] [Google Scholar]
- 49.Tahara-Hanaoka S., Shibuya K., Kai H., Miyamoto A., Morikawa Y., Ohkochi N., Honda S., Shibuya A. (2006) Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood 107, 1491–1496. [DOI] [PubMed] [Google Scholar]
- 50.Lamana A., Martin P., de la Fuente H., Martinez-Muñoz L., Cruz-Adalia A., Ramirez-Huesca M., Escribano C., Gollmer K., Mellado M., Stein J. V., Rodriguez-Fernandez J. L., Sanchez-Madrid F., del Hoyo G. M. (2011) CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J. Invest. Dermatol. 131, 1503–1512. [DOI] [PubMed] [Google Scholar]
- 51.Alari-Pahissa E., Vega-Ramos J., Zhang J. G., Castaño A. R., Turley S. J., Villadangos J. A., Lauzurica P. (2012) Differential effect of CD69 targeting on bystander and antigen-specific T cell proliferation. J. Leukoc. Biol. 92, 145–158. [DOI] [PubMed] [Google Scholar]
- 52.Van Luijn M. M., Kreft K. L., Jongsma M. L., Mes S. W., Wierenga-Wolf A. F., van Meurs M., Melief M. J., der Kant Rv., Janssen L., Janssen H., Tan R., Priatel J. J., Neefjes J., Laman J. D., Hintzen R. Q. (2015) Multiple sclerosis-associated CLEC16A controls HLA class II expression via late endosome biogenesis. Brain 138, 1531–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudziak D., Kamphorst A. O., Heidkamp G. F., Buchholz V. R., Trumpfheller C., Yamazaki S., Cheong C., Liu K., Lee H. W., Park C. G., Steinman R. M., Nussenzweig M. C. (2007) Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111. [DOI] [PubMed] [Google Scholar]
- 54.Jongbloed S. L., Kassianos A. J., McDonald K. J., Clark G. J., Ju X., Angel C. E., Chen C. J., Dunbar P. R., Wadley R. B., Jeet V., Vulink A. J., Hart D. N., Radford K. J. (2010) Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207, 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fløyel T., Brorsson C., Nielsen L. B., Miani M., Bang-Berthelsen C. H., Friedrichsen M., Overgaard A. J., Berchtold L. A., Wiberg A., Poulsen P., Hansen L., Rosinger S., Boehm B. O., Ram R., Nguyen Q., Mehta M., Morahan G., Concannon P., Bergholdt R., Nielsen J. H., Reinheckel T., von Herrath M., Vaag A., Eizirik D. L., Mortensen H. B., Størling J., Pociot F. (2014) CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc. Natl. Acad. Sci. U. S. A. 111, 10305–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davison L. J., Wallace C., Cooper J. D., Cope N. F., Wilson N. K., Smyth D. J., Howson J. M., Saleh N., Al-Jeffery A., Angus K. L., Stevens H. E., Nutland S., Duley S., Coulson R. M., Walker N. M., Burren O. S., Rice C. M., Cambien F., Zeller T., Munzel T., Lackner K., Blakenberg S., Fraser P., Gottgens B., Todd J. A., Attwood T., Belz S., Braund P., Cambien F., Cooper J., Crisp-Hihn A., Diemert P., Deloukas P., Foad N., Erdmann J., Goodall A. H., Gracey J., Gray E., Williams R. G., Heimerl S., Hengstenberg C., Jolley J., Krishnan U., Lloyd-Jones H., Lugauer I., Lundmark P., Maouche S., Moore J. S., Muir D., Murray E., Nelson C. P., Neudert J., Niblett D., O’Leary K., Ouwehand W. H., Pollard H., Rankin A., Rice C. M., Sager H., Samani N. J., Sambrook J., Schmitz G., Scholz M., Schroeder L., Schunkert H., Syvannen A. C., Tennstedt S., Wallace C.; Cardiogenics Consortium (2012) Long-range DNA looping and gene expression analyses identify DEXI as an autoimmune disease candidate gene. Hum. Mol. Genet. 21, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng P., Gabrilovich D. (2008) Notch signaling in differentiation and function of dendritic cells. Immunol. Res. 41, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vella A., Cooper J. D., Lowe C. E., Walker N., Nutland S., Widmer B., Jones R., Ring S. M., McArdle W., Pembrey M. E., Strachan D. P., Dunger D. B., Twells R. C., Clayton D. G., Todd J. A. (2005) Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am. J. Hum. Genet. 76, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogt T. K., Link A., Perrin J., Finke D., Luther S. A. (2009) Novel function for interleukin-7 in dendritic cell development. Blood 113, 3961–3968. [DOI] [PubMed] [Google Scholar]
- 60.Guimond M., Veenstra R. G., Grindler D. J., Zhang H., Cui Y., Murphy R. D., Kim S. Y., Na R., Hennighausen L., Kurtulus S., Erman B., Matzinger P., Merchant M. S., Mackall C. L. (2009) Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat. Immunol. 10, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O., Roméo P. H. (2002) A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3, 477–482. [DOI] [PubMed] [Google Scholar]
- 62.Sarris M., Andersen K. G., Randow F., Mayr L., Betz A. G. (2008) Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller M., Tam A. B., Cho J. Y., Doherty T. A., Pham A., Khorram N., Rosenthal P., Mueller J. L., Hoffman H. M., Suzukawa M., Niwa M., Broide D. H. (2012) ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc. Natl. Acad. Sci. U. S. A. 109, 16648–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.You-Ten K. E., Muise E. S., Itié A., Michaliszyn E., Wagner J., Jothy S., Lapp W. S., Tremblay M. L. (1997) Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simoncic P. D., Bourdeau A., Lee-Loy A., Rohrschneider L. R., Tremblay M. L., Stanley E. R., McGlade C. J. (2006) T-cell protein tyrosine phosphatase (Tcptp) is a negative regulator of colony-stimulating factor 1 signaling and macrophage differentiation. Mol. Cell. Biol. 26, 4149–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J., Zahir N., Jiang Q., Miliotis H., Heyraud S., Meng X., Dong B., Xie G., Qiu F., Hao Z., McCulloch C. A., Keystone E. C., Peterson A. C., Siminovitch K. A. (2011) The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat. Genet. 43, 902–907. [DOI] [PubMed] [Google Scholar]
- 67.Rawlings D. J., Dai X., Buckner J. H. (2015) The role of PTPN22 risk variant in the development of autoimmunity: finding common ground between mouse and human. J. Immunol. 194, 2977–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benvenuti F., Hugues S., Walmsley M., Ruf S., Fetler L., Popoff M., Tybulewicz V. L., Amigorena S. (2004) Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 305, 1150–1153. [DOI] [PubMed] [Google Scholar]
- 69.Savina A., Peres A., Cebrian I., Carmo N., Moita C., Hacohen N., Moita L. F., Amigorena S. (2009) The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8+ dendritic cells. Immunity 30, 544–555. [DOI] [PubMed] [Google Scholar]
- 70.Tang S., Chen T., Yu Z., Zhu X., Yang M., Xie B., Li N., Cao X., Wang J. (2014) RasGRP3 limits Toll-like receptor-triggered inflammatory response in macrophages by activating Rap1 small GTPase. Nat. Commun. 5, 4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao J., Jijon H. B., Kim I. R., Goel G., Doan A., Sokol H., Bauer H., Herrmann B. G., Lassen K. G., Xavier R. J. (2014) An image-based genetic assay identifies genes in T1D susceptibility loci controlling cellular antiviral immunity in mouse. PLoS One 9, e108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kool M., van Loo G., Waelput W., De Prijck S., Muskens F., Sze M., van Praet J., Branco-Madeira F., Janssens S., Reizis B., Elewaut D., Beyaert R., Hammad H., Lambrecht B. N. (2011) The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35, 82–96. [DOI] [PubMed] [Google Scholar]
- 73.Song X. T., Evel-Kabler K., Shen L., Rollins L., Huang X. F., Chen S. Y. (2008) A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat. Med. 14, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tokumasa N., Suto A., Kagami S., Furuta S., Hirose K., Watanabe N., Saito Y., Shimoda K., Iwamoto I., Nakajima H. (2007) Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-γ production and the induction of Th1 cell differentiation. Blood 110, 553–560. [DOI] [PubMed] [Google Scholar]
- 75.Heng T. S., Painter M. W.; Immunological Genome Project Consortium (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094. [DOI] [PubMed] [Google Scholar]
- 76.Steinman R. M., Hawiger D., Liu K., Bonifaz L., Bonnyay D., Mahnke K., Iyoda T., Ravetch J., Dhodapkar M., Inaba K., Nussenzweig M. (2003) Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. N. Y. Acad. Sci. 987, 15–25. [DOI] [PubMed] [Google Scholar]
- 77.Darrasse-Jèze G., Deroubaix S., Mouquet H., Victora G. D., Eisenreich T., Yao K. H., Masilamani R. F., Dustin M. L., Rudensky A., Liu K., Nussenzweig M. C. (2009) Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 206, 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J. V., Steinman R. M., Nussenzweig M. C. (2001) Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price J. D., Tarbell K. V. (2015) The role of dendritic cell subsets and innate immunity in the pathogenesis of type 1 diabetes and other autoimmune diseases. Front. Immunol. 6, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomson A. W., Robbins P. D. (2008) Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann. Rheum. Dis. 67(Suppl 3), iii90–iii96. [DOI] [PubMed] [Google Scholar]
- 81.Morelli A. E., Thomson A. W. (2007) Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 7, 610–621. [DOI] [PubMed] [Google Scholar]
- 82.Price J. D., Hotta-Iwamura C., Zhao Y., Beauchamp N. M., Tarbell K. V. (2015) DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T cell tolerance and inhibit diabetes. Diabetes 64, 3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grajales-Reyes G. E., Iwata A., Albring J., Wu X., Tussiwand R., Kc W., Kretzer N. M., Briseño C. G., Durai V., Bagadia P., Haldar M., Schönheit J., Rosenbauer F., Murphy T. L., Murphy K. M. (2015) Batf3 maintains autoactivation of Irf8 for commitment of a CD8α(+) conventional DC clonogenic progenitor. Nat. Immunol. 16, 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belz G. T., Behrens G. M., Smith C. M., Miller J. F., Jones C., Lejon K., Fathman C. G., Mueller S. N., Shortman K., Carbone F. R., Heath W. R. (2002) The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vasquez A. C., Feili-Hariri M., Tan R. J., Morel P. A. (2004) Qualitative and quantitative abnormalities in splenic dendritic cell populations in NOD mice. Clin. Exp. Immunol. 135, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee C. N., Lew A. M., Shortman K., Wu L. (2015) NOD mice are functionally deficient in the capacity of cross-presentation. Immunol. Cell Biol. 93, 548–557. [DOI] [PubMed] [Google Scholar]
- 87.O’Keeffe M., Brodnicki T. C., Fancke B., Vremec D., Morahan G., Maraskovsky E., Steptoe R., Harrison L. C., Shortman K. (2005) Fms-like tyrosine kinase 3 ligand administration overcomes a genetically determined dendritic cell deficiency in NOD mice and protects against diabetes development. Int. Immunol. 17, 307–314. [DOI] [PubMed] [Google Scholar]
- 88.Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M. C., Steinman R. M. (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mukhopadhaya A., Hanafusa T., Jarchum I., Chen Y. G., Iwai Y., Serreze D. V., Steinman R. M., Tarbell K. V., DiLorenzo T. P. (2008) Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 105, 6374–6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamazaki S., Dudziak D., Heidkamp G. F., Fiorese C., Bonito A. J., Inaba K., Nussenzweig M. C., Steinman R. M. (2008) CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181, 6923–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Price J. D., Beauchamp N. M., Rahir G., Zhao Y., Rieger C. C., Lau-Kilby A. W., Tarbell K. V. (2014) CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L. J. Leukoc. Biol. 95, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guilliams M., Ginhoux F., Jakubzick C., Naik S. H., Onai N., Schraml B. U., Segura E., Tussiwand R., Yona S. (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kasahara S., Clark E. A. (2012) Dendritic cell-associated lectin 2 (DCAL2) defines a distinct CD8α− dendritic cell subset. J. Leukoc. Biol. 91, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong M. B., Rahman M. J., Tarbell K. V. (2015) Flow cytometric gating for spleen monocyte and DC subsets: differences in autoimmune NOD mice and with acute inflammation. J. Immunol. Methods [published online ahead of print September 5, 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katz J. D., Ondr J. K., Opoka R. J., Garcia Z., Janssen E. M. (2010) Cutting edge: merocytic dendritic cells break T cell tolerance to β cell antigens in nonobese diabetic mouse diabetes. J. Immunol. 185, 1999–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pelletier A. N., Lesage S. (2013) The Idd13 congenic interval defines the number of merocytic dendritic cells, a novel trait associated with autoimmune diabetes susceptibility. J. Autoimmun. 43, 70–77. [DOI] [PubMed] [Google Scholar]
- 97.Crawford A., Angelosanto J. M., Kao C., Doering T. A., Odorizzi P. M., Barnett B. E., Wherry E. J. (2014) Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang B. Y., Miaw S. C., Ho I. C. (2005) ROG negatively regulates T-cell activation but is dispensable for Th-cell differentiation. Mol. Cell. Biol. 25, 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujikado N., Saijo S., Yonezawa T., Shimamori K., Ishii A., Sugai S., Kotaki H., Sudo K., Nose M., Iwakura Y. (2008) Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat. Med. 14, 176–180. [DOI] [PubMed] [Google Scholar]
- 100.Lorentzen J. C., Flornes L., Eklöw C., Bäckdahl L., Ribbhammar U., Guo J. P., Smolnikova M., Dissen E., Seddighzadeh M., Brookes A. J., Alfredsson L., Klareskog L., Padyukov L., Fossum S. (2007) Association of arthritis with a gene complex encoding C-type lectin-like receptors. Arthritis Rheum. 56, 2620–2632. [DOI] [PubMed] [Google Scholar]
- 101.Cisse B., Caton M. L., Lehner M., Maeda T., Scheu S., Locksley R., Holmberg D., Zweier C., den Hollander N. S., Kant S. G., Holter W., Rauch A., Zhuang Y., Reizis B. (2008) Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lande R., Gilliet M. (2010) Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. N. Y. Acad. Sci. 1183, 89–103. [DOI] [PubMed] [Google Scholar]
- 103.Devendra D., Eisenbarth G. S. (2004) Interferon α—a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin. Immunol. 111, 225–233. [DOI] [PubMed] [Google Scholar]
- 104.Ferreira G. B., Gysemans C. A., Demengeot J., da Cunha J. P., Vanherwegen A. S., Overbergh L., Van Belle T. L., Pauwels F., Verstuyf A., Korf H., Mathieu C. (2014) 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J. Immunol. 192, 4210–4220. [DOI] [PubMed] [Google Scholar]
- 105.Kallionpää H., Elo L. L., Laajala E., Mykkänen J., Ricaño-Ponce I., Vaarma M., Laajala T. D., Hyöty H., Ilonen J., Veijola R., Simell T., Wijmenga C., Knip M., Lähdesmäki H., Simell O., Lahesmaa R. (2014) Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes 63, 2402–2414. [DOI] [PubMed] [Google Scholar]
- 106.O’Brien B. A., Geng X., Orteu C. H., Huang Y., Ghoreishi M., Zhang Y., Bush J. A., Li G., Finegood D. T., Dutz J. P. (2006) A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J. Autoimmun. 26, 104–115. [DOI] [PubMed] [Google Scholar]
- 107.Diana J., Simoni Y., Furio L., Beaudoin L., Agerberth B., Barrat F., Lehuen A. (2013) Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 19, 65–73. [DOI] [PubMed] [Google Scholar]
- 108.Swiecki M., Wang Y., Vermi W., Gilfillan S., Schreiber R. D., Colonna M. (2011) Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J. Exp. Med. 208, 2367–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allen J. S., Pang K., Skowera A., Ellis R., Rackham C., Lozanoska-Ochser B., Tree T., Leslie R. D., Tremble J. M., Dayan C. M., Peakman M. (2009) Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 58, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Q., McDevitt H. O. (2011) The role of interferon-α in initiation of type I diabetes in the NOD mouse. Clin. Immunol. 140, 3–7. [DOI] [PubMed] [Google Scholar]
- 111.Sobel D. O., Ahvazi B. (1998) Alpha-interferon inhibits the development of diabetes in NOD mice. Diabetes 47, 1867–1872. [DOI] [PubMed] [Google Scholar]
- 112.Ghazarian L., Diana J., Simoni Y., Beaudoin L., Lehuen A. (2013) Prevention or acceleration of type 1 diabetes by viruses. Cell. Mol. Life Sci. 70, 239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarbell K. V., Yamazaki S., Olson K., Toy P., Steinman R. M. (2004) CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 199, 1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tarbell K. V., Yamazaki S., Steinman R. M. (2006) The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin. Immunol. 18, 93–102. [DOI] [PubMed] [Google Scholar]
- 115.Tarbell K. V., Petit L., Zuo X., Toy P., Luo X., Mqadmi A., Yang H., Suthanthiran M., Mojsov S., Steinman R. M. (2007) Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Z., Herman A. E., Matos M., Mathis D., Benoist C. (2005) Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202, 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang Q., Adams J. Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E., Piccirillo C. A., Salomon B. L., Bluestone J. A. (2008) Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belkaid Y., Tarbell K. V. (2009) Arming Treg cells at the inflammatory site. Immunity 30, 322–323. [DOI] [PubMed] [Google Scholar]
- 119.Salomon B., Lenschow D. J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J. A. (2000) B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12, 431–440. [DOI] [PubMed] [Google Scholar]
- 120.Yamazaki S., Iyoda T., Tarbell K., Olson K., Velinzon K., Inaba K., Steinman R. M. (2003) Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198, 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bour-Jordan H., Esensten J. H., Martinez-Llordella M., Penaranda C., Stumpf M., Bluestone J. A. (2011) Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol. Rev. 241, 180–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beaudoin L., Diana J., Ghazarian L., Simoni Y., Boitard C., Lehuen A. (2014) Plasmacytoid dendritic cells license regulatory T cells, upon iNKT-cell stimulation, to prevent autoimmune diabetes. Eur. J. Immunol. 44, 1454–1466. [DOI] [PubMed] [Google Scholar]
- 123.Steinbrink K., Jonuleit H., Müller G., Schuler G., Knop J., Enk A. H. (1999) Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood 93, 1634–1642. [PubMed] [Google Scholar]
- 124.Cox N. J., Wapelhorst B., Morrison V. A., Johnson L., Pinchuk L., Spielman R. S., Todd J. A., Concannon P. (2001) Seven regions of the genome show evidence of linkage to type 1 diabetes in a consensus analysis of 767 multiplex families. Am. J. Hum. Genet. 69, 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wicker L. S., Todd J. A., Peterson L. B. (1995) Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 13, 179–200. [DOI] [PubMed] [Google Scholar]
- 126.Concannon P., Rich S. S., Nepom G. T. (2009) Genetics of type 1A diabetes. N. Engl. J. Med. 360, 1646–1654. [DOI] [PubMed] [Google Scholar]
- 127.Serreze D. V., Gaskins H. R., Leiter E. H. (1993) Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J. Immunol. 150, 2534–2543. [PubMed] [Google Scholar]
- 128.Strid J., Lopes L., Marcinkiewicz J., Petrovska L., Nowak B., Chain B. M., Lund T. (2001) A defect in bone marrow derived dendritic cell maturation in the nonobesediabetic mouse. Clin. Exp. Immunol. 123, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Salomon B., Bluestone J. A. (2001) Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19, 225–252. [DOI] [PubMed] [Google Scholar]
- 130.Vandenbroeck K. (2012) Cytokine gene polymorphisms and human autoimmune disease in the era of genome-wide association studies. J. Interferon Cytokine Res. 32, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.D’Alise A. M., Ergun A., Hill J. A., Mathis D., Benoist C. (2011) A cluster of coregulated genes determines TGF-β–induced regulatory T-cell (Treg) dysfunction in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 108, 8737–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamanouchi J., Rainbow D., Serra P., Howlett S., Hunter K., Garner V. E., Gonzalez-Munoz A., Clark J., Veijola R., Cubbon R., Chen S. L., Rosa R., Cumiskey A. M., Serreze D. V., Gregory S., Rogers J., Lyons P. A., Healy B., Smink L. J., Todd J. A., Peterson L. B., Wicker L. S., Santamaria P. (2007) Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat. Genet. 39, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Setoguchi R., Hori S., Takahashi T., Sakaguchi S. (2005) Homeostatic maintenance of natural Foxp3+ CD25+ CD4+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201, 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lau-Kilby A. W., Kretz C. C., Pechhold S., Price J. D., Dorta S., Ramos H., Trinchieri G., Tarbell K. V. (2011) Interleukin-2 inhibits FMS-like tyrosine kinase 3 receptor ligand (flt3L)-dependent development and function of conventional and plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 108, 2408–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guerrero A. D., Dong M. B., Zhao Y., Lau-Kilby A., Tarbell K. V. (2014) Interleukin-2-mediated inhibition of dendritic cell development correlates with decreased CD135 expression and increased monocyte/macrophage precursors. Immunology 143, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klatzmann D., Abbas A. K. (2015) The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 15, 283–294. [DOI] [PubMed] [Google Scholar]
- 137.Grinberg-Bleyer Y., Baeyens A., You S., Elhage R., Fourcade G., Gregoire S., Cagnard N., Carpentier W., Tang Q., Bluestone J., Chatenoud L., Klatzmann D., Salomon B. L., Piaggio E. (2010) IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 207, 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V., Sene D., Cacoub P., Klatzmann D. (2011) Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077. [DOI] [PubMed] [Google Scholar]
- 139.Granucci F., Vizzardelli C., Pavelka N., Feau S., Persico M., Virzi E., Rescigno M., Moro G., Ricciardi-Castagnoli P. (2001) Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2, 882–888. [DOI] [PubMed] [Google Scholar]
- 140.Cerosaletti K., Schneider A., Schwedhelm K., Frank I., Tatum M., Wei S., Whalen E., Greenbaum C., Kita M., Buckner J., Long S. A. (2013) Multiple autoimmune-associated variants confer decreased IL-2R signaling in CD4+ CD25(hi) T cells of type 1 diabetic and multiple sclerosis patients. PLoS One 8, e83811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Von Bergwelt-Baildon M. S., Popov A., Saric T., Chemnitz J., Classen S., Stoffel M. S., Fiore F., Roth U., Beyer M., Debey S., Wickenhauser C., Hanisch F. G., Schultze J. L. (2006) CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood 108, 228–237. [DOI] [PubMed] [Google Scholar]
- 142.Long S. A., Cerosaletti K., Wan J. Y., Ho J. C., Tatum M., Wei S., Shilling H. G., Buckner J. H. (2011) An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun. 12, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brandt K., Bulfone-Paus S., Foster D. C., Rückert R. (2003) Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102, 4090–4098. [DOI] [PubMed] [Google Scholar]
- 144.Todd J. A., Walker N. M., Cooper J. D., Smyth D. J., Downes K., Plagnol V., Bailey R., Nejentsev S., Field S. F., Payne F., Lowe C. E., Szeszko J. S., Hafler J. P., Zeitels L., Yang J. H., Vella A., Nutland S., Stevens H. E., Schuilenburg H., Coleman G., Maisuria M., Meadows W., Smink L. J., Healy B., Burren O. S., Lam A. A., Ovington N. R., Allen J., Adlem E., Leung H. T., Wallace C., Howson J. M., Guja C., Ionescu-Tîrgovişte C., Simmonds M. J., Heward J. M., Gough S. C., Dunger D. B., Wicker L. S., Clayton D. G.; Genetics of Type 1 Diabetes in Finland; Wellcome Trust Case Control Consortium (2007) Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 39, 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spolski R., Leonard W. J. (2008) Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26, 57–79. [DOI] [PubMed] [Google Scholar]
- 146.Liu S. M., Lee D. H., Sullivan J. M., Chung D., Jäger A., Shum B. O., Sarvetnick N. E., Anderson A. C., Kuchroo V. K. (2011) Differential IL-21 signaling in APCs leads to disparate Th17 differentiation in diabetes-susceptible NOD and diabetes-resistant NOD.Idd3 mice. J. Clin. Invest. 121, 4303–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McGuire H. M., Vogelzang A., Ma C. S., Hughes W. E., Silveira P. A., Tangye S. G., Christ D., Fulcher D., Falcone M., King C. (2011) A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity 34, 602–615. [DOI] [PubMed] [Google Scholar]
- 148.Frucht D. M., Aringer M., Galon J., Danning C., Brown M., Fan S., Centola M., Wu C. Y., Yamada N., El Gabalawy H., O’Shea J. J. (2000) Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J. Immunol. 164, 4659–4664. [DOI] [PubMed] [Google Scholar]
- 149.Stamm L. M., Satoskar A. A., Ghosh S. K., David J. R., Satoskar A. R. (1999) STAT-4 mediated IL-12 signaling pathway is critical for the development of protective immunity in cutaneous leishmaniasis. Eur. J. Immunol. 29, 2524–2529. [DOI] [PubMed] [Google Scholar]
- 150.Zervou M. I., Mamoulakis D., Panierakis C., Boumpas D. T., Goulielmos G. N. (2008) STAT4: a risk factor for type 1 diabetes? Hum. Immunol. 69, 647–650. [DOI] [PubMed] [Google Scholar]
- 151.Wallace C., Smyth D. J., Maisuria-Armer M., Walker N. M., Todd J. A., Clayton D. G. (2010) The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat. Genet. 42, 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Izumi K., Mine K., Inoue Y., Teshima M., Ogawa S., Kai Y., Kurafuji T., Hirakawa K., Miyakawa D., Ikeda H., Inada A., Hara M., Yamada H., Akashi K., Niho Y., Ina K., Kobayashi T., Yoshikai Y., Anzai K., Yamashita T., Minagawa H., Fujimoto S., Kurisaki H., Shimoda K., Katsuta H., Nagafuchi S. (2015) Reduced Tyk2 gene expression in β-cells due to natural mutation determines susceptibility to virus-induced diabetes. Nat. Commun. 6, 6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCartney S. A., Vermi W., Lonardi S., Rossini C., Otero K., Calderon B., Gilfillan S., Diamond M. S., Unanue E. R., Colonna M. (2011) RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J. Clin. Invest. 121, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Swiecki M., McCartney S. A., Wang Y., Colonna M. (2011) TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes. J. Leukoc. Biol. 90, 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang K., Baldassano R., Zhang H., Qu H. Q., Imielinski M., Kugathasan S., Annese V., Dubinsky M., Rotter J. I., Russell R. K., Bradfield J. P., Sleiman P. M., Glessner J. T., Walters T., Hou C., Kim C., Frackelton E. C., Garris M., Doran J., Romano C., Catassi C., Van Limbergen J., Guthery S. L., Denson L., Piccoli D., Silverberg M. S., Stanley C. A., Monos D., Wilson D. C., Griffiths A., Grant S. F., Satsangi J., Polychronakos C., Hakonarson H. (2010) Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects. Hum. Mol. Genet. 19, 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hunter C. A., Kastelein R. (2012) Interleukin-27: balancing protective and pathological immunity. Immunity 37, 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karakhanova S., Bedke T., Enk A. H., Mahnke K. (2011) IL-27 renders DC immunosuppressive by induction of B7-H1. J. Leukoc. Biol. 89, 837–845. [DOI] [PubMed] [Google Scholar]
- 158.Awasthi A., Carrier Y., Peron J. P., Bettelli E., Kamanaka M., Flavell R. A., Kuchroo V. K., Oukka M., Weiner H. L. (2007) A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389. [DOI] [PubMed] [Google Scholar]
- 159.Wang S., Miyazaki Y., Shinozaki Y., Yoshida H. (2007) Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J. Immunol. 179, 6421–6428. [DOI] [PubMed] [Google Scholar]
- 160.Shiokawa A., Tanabe K., Tsuji N. M., Sato R., Hachimura S. (2009) IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol. Lett. 125, 7–14. [DOI] [PubMed] [Google Scholar]
- 161.Barrett J. C., Clayton D. G., Concannon P., Akolkar B., Cooper J. D., Erlich H. A., Julier C., Morahan G., Nerup J., Nierras C., Plagnol V., Pociot F., Schuilenburg H., Smyth D. J., Stevens H., Todd J. A., Walker N. M., Rich S. S.; Type 1 Diabetes Genetics Consortium (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goudy K. S., Burkhardt B. R., Wasserfall C., Song S., Campbell-Thompson M. L., Brusko T., Powers M. A., Clare-Salzler M. J., Sobel E. S., Ellis T. M., Flotte T. R., Atkinson M. A. (2003) Systemic overexpression of IL-10 induces CD4+CD25+ cell populations in vivo and ameliorates type 1 diabetes in nonobese diabetic mice in a dose-dependent fashion. J. Immunol. 171, 2270–2278. [DOI] [PubMed] [Google Scholar]
- 163.Bersenev A., Wu C., Balcerek J., Tong W. (2008) Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J. Clin. Invest. 118, 2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hasegawa K., Martin F., Huang G., Tumas D., Diehl L., Chan A. C. (2004) PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 303, 685–689. [DOI] [PubMed] [Google Scholar]
- 165.Lee E. G., Boone D. L., Chai S., Libby S. L., Chien M., Lodolce J. P., Ma A. (2000) Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289, 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Musone S. L., Taylor K. E., Nititham J., Chu C., Poon A., Liao W., Lam E. T., Ma A., Kwok P. Y., Criswell L. A. (2011) Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes Immun. 12, 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hammer G. E., Turer E. E., Taylor K. E., Fang C. J., Advincula R., Oshima S., Barrera J., Huang E. J., Hou B., Malynn B. A., Reizis B., DeFranco A., Criswell L. A., Nakamura M. C., Ma A. (2011) Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 12, 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ma A., Malynn B. A. (2012) A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 12, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Callahan J. A., Hammer G. E., Agelides A., Duong B. H., Oshima S., North J., Advincula R., Shifrin N., Truong H. A., Paw J., Barrera J., DeFranco A., Rosenblum M. D., Malynn B. A., Ma A. (2013) Cutting edge: ABIN-1 protects against psoriasis by restricting MyD88 signals in dendritic cells. J. Immunol. 191, 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yeung W. C., Rawlinson W. D., Craig M. E. (2011) Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342, d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lincez P. J., Shanina I., Horwitz M. S. (2015) Reduced expression of the MDA5 Gene IFIH1 prevents autoimmune diabetes. Diabetes 64, 2184–2193. [DOI] [PubMed] [Google Scholar]
- 172.Guo S. W., Magnuson V. L., Schiller J. J., Wang X., Wu Y., Ghosh S. (2006) Meta-analysis of vitamin D receptor polymorphisms and type 1 diabetes: a HuGE review of genetic association studies. Am. J. Epidemiol. 164, 711–724. [DOI] [PubMed] [Google Scholar]
- 173.Kahles H., Morahan G., Todd J. A., Badenhoop K.; Type I Diabetes Genetics Consortium (2009) Association analyses of the vitamin D receptor gene in 1654 families with type I diabetes. Genes Immun. 10(Suppl 1), S60–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mathieu C., Badenhoop K. (2005) Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol. Metab. 16, 261–266. [DOI] [PubMed] [Google Scholar]
- 175.Mitri J., Dawson-Hughes B., Hu F. B., Pittas A. G. (2011) Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 94, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hyppönen E., Läärä E., Reunanen A., Järvelin M. R., Virtanen S. M. (2001) Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358, 1500–1503. [DOI] [PubMed] [Google Scholar]
- 177.Adorini L. (2003) Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann. N. Y. Acad. Sci. 987, 258–261. [DOI] [PubMed] [Google Scholar]
- 178.Gregori S., Giarratana N., Smiroldo S., Uskokovic M., Adorini L. (2002) A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51, 1367–1374. [DOI] [PubMed] [Google Scholar]
- 179.Karthaus N., van Spriel A. B., Looman M. W., Chen S., Spilgies L. M., Lieben L., Carmeliet G., Ansems M., Adema G. J. (2014) Vitamin D controls murine and human plasmacytoid dendritic cell function. J. Invest. Dermatol. 134, 1255–1264. [DOI] [PubMed] [Google Scholar]
- 180.Littorin B., Blom P., Schölin A., Arnqvist H. J., Blohmé G., Bolinder J., Ekbom-Schnell A., Eriksson J. W., Gudbjörnsdottir S., Nyström L., Ostman J., Sundkvist G. (2006) Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 49, 2847–2852. [DOI] [PubMed] [Google Scholar]
- 181.Tang Q., Adams J. Y., Tooley A. J., Bi M., Fife B. T., Serra P., Santamaria P., Locksley R. M., Krummel M. F., Bluestone J. A. (2006) Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 7, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Legoux F. P., Lim J. B., Cauley A. W., Dikiy S., Ertelt J., Mariani T. J., Sparwasser T., Way S. S., Moon J. J. (2015) CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity 43, 896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Creusot R. J., Giannoukakis N., Trucco M., Clare-Salzler M. J., Fathman C. G. (2014) It’s time to bring dendritic cell therapy to type 1 diabetes. Diabetes 63, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee J., Breton G., Oliveira T. Y., Zhou Y. J., Aljoufi A., Puhr S., Cameron M. J., Sékaly R. P., Nussenzweig M. C., Liu K. (2015) Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J. Exp. Med. 212, 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Giannoukakis N., Phillips B., Finegold D., Harnaha J., Trucco M. (2011) Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 34, 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Turnquist H. R., Raimondi G., Zahorchak A. F., Fischer R. T., Wang Z., Thomson A. W. (2007) Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 178, 7018–7031. [DOI] [PubMed] [Google Scholar]
- 187.Turnquist H. R., Cardinal J., Macedo C., Rosborough B. R., Sumpter T. L., Geller D. A., Metes D., Thomson A. W. (2010) mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 115, 4758–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Monti P., Scirpoli M., Maffi P., Piemonti L., Secchi A., Bonifacio E., Roncarolo M. G., Battaglia M. (2008) Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes 57, 2341–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Long S. A., Rieck M., Sanda S., Bollyky J. B., Samuels P. L., Goland R., Ahmann A., Rabinovitch A., Aggarwal S., Phippard D., Turka L. A., Ehlers M. R., Bianchine P. J., Boyle K. D., Adah S. A., Bluestone J. A., Buckner J. H., Greenbaum C. J.; Diabetes TrialNet and the Immune Tolerance Network (2012) Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61, 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Barlow A. D., Nicholson M. L., Herbert T. P. (2013) Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes 62, 2674–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cetkovic-Cvrlje M., Dragt A. L., Vassilev A., Liu X. P., Uckun F. M. (2003) Targeting JAK3 with JANEX-1 for prevention of autoimmune type 1 diabetes in NOD mice. Clin. Immunol. 106, 213–225. [DOI] [PubMed] [Google Scholar]
- 192.Louvet C., Szot G. L., Lang J., Lee M. R., Martinier N., Bollag G., Zhu S., Weiss A., Bluestone J. A. (2008) Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 105, 18895–18900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Odegard J. M., Nepom G. T., Wambre E. (2015) Biomarkers for antigen immunotherapy in allergy and type 1 diabetes. Clin. Immunol. 161, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]