TGM activity in human M2 macrophages requires the presence of a functional PLA2G5, generating PGE2.
Keywords: inflammation, eosinophil, nasal polyp, TGM2, phospholipase A2, asthma
Abstract
Phospholipases A2 are enzymes that liberate membrane-bound lipids in a tissue and cell-specific fashion. Group V secretory phospholipase A2 is necessary for the development of M2 macrophages and their effector functions in a mouse model of the T-helper-2 allergic airway inflammation. However, the function of group V phospholipase A2 in human M2 activation and T-helper-2 inflammation is ill-defined. Transglutaminase-2, a protein cross-linking enzyme, is a newly identified marker of both human and mouse interleukin-4-activated M2 macrophages and is also found in the lungs of patients with asthma. We report that group V phospholipase A2 and transglutaminase-2 colocalized in macrophages of human nasal polyp tissue obtained from patients with T-helper-2 eosinophilic inflammation, and their coexpression positively correlated with the number of eosinophils in each tissue specimen. We demonstrate that in human monocyte-derived macrophages activated by interleukin-4, group V phospholipase A2 translocated and colocalized with transglutaminase-2 in the cytoplasm and on the membrane of macrophages. Moreover, knocking down group V phospholipase A2 with small interfering ribonucleic acid reduced macrophage transglutaminase activity, whereas mass spectrometry analysis of lipids also showed reduced prostaglandin E2 production. Finally, exogenous prostaglandin E2 restored transglutaminase activity of group V phospholipase A2-small interfering ribonucleic acid–treated macrophages. Thus, our study shows a novel function of group V phospholipase A2 in regulating the transglutaminase activity of human interleukin-4–activated M2 macrophages through prostaglandin E2 generation and suggests that group V phospholipase A2 is a functionally relevant enzyme that may have therapeutic value for the treatment of human T-helper-2 inflammatory disorders.
Introduction
Phospholipases A2 (PLA2) are a family of structurally related enzymes that hydrolyze membrane phospholipids [1]. The largest group of the family is sPLA2. To access hydrophobic lipids, PLA2s need to bind the cell membrane. Group V sPLA2 (Pla2g5) can bind membrane heparan proteoglycans through cationic residues, like group IIA and group IID sPLA2 (Pla2g2a and Pla2g2d, respectively) [2–4]. In addition, Pla2g5 and group X sPLA2 (Pla2g10) have high affinity and activity toward membrane phosphatidylcholine [5–7]. The cleavage products of PLA2s are free fatty acids and lysophospholipids, both of which serve as second messengers. Moreover, cleavage of arachidonic acid initiates the biosynthesis of proinflammatory eicosanoids, including prostaglandins and leukotrienes. However, PLA2s have cell- and tissue-specific functions [8].
Animal models have helped define the functions of some PLA2s. Pla2g10 and Pla2g5 were shown to contribute to OVA-induced Th2 pulmonary inflammation [9, 10]. Using a model of house dust mite-induced lung Th2 inflammation, which relies on a robust innate immune response, we showed that Pla2g5 in DCs is essential for antigen presentation [11] and in M2 macrophages is necessary for the production of CCL22, a Th2-recruiting chemokine, and recruitment of T cells into the lung [12]. We also showed that a unique function of Pla2g5 is the conversion of mouse and human macrophages toward IL-4 activated M2 macrophages in vitro [12]. However, there are few studies investigating the function of human PLA2s. After the presence of extracellular PLA2 activity was found in the BAL fluid of patients with asthma [13, 14], only 2 PLA2s (PLA2G2A and PLA2G10) were found in the airways of patients with asthma [15–17]. Therefore, the expression and function of PLA2G5 in human cells in the context of Th2 inflammation is still largely unknown.
TGM2 is a ubiquitous protein cross-linking enzyme with multiple functions including posttranslational modification of proteins, cell adhesion and migration, and regulation of cell-extracellular matrix interactions [18, 19]. TGM2 is expressed in the cytoplasm of macrophages and epithelial cells and translocates through an unknown mechanism to the cell surface where it binds heparan proteoglycans (syndecan-4) [20, 21]. A recent study showed that TGM2 is consistently expressed in various models of human and mouse in vitro derived IL-4-activated M2 macrophages [22]. TGM2 was also found in macrophages of bronchial biopsies obtained from patients with asthma and in epithelial cells of patients with exercise-induced bronchoconstriction [22, 23].
Because Pla2g5 is critical in mice for development of M2 macrophages and Th2 pulmonary inflammation and given the presence of TGM2 in human and mouse IL-4-activated M2 macrophages and in specimens from humans with asthma, we hypothesized that PLA2G5 and TGM2 have related expression and function in human M2 macrophages.
Using specimens from patients with prototypical human Th2 inflammatory disorders, which are known to be rich in M2 macrophages [24, 25], we show that in human nasal polyp tissue, PLA2G5 and TGM2 colocalized in macrophages and their expression correlated with the extent of tissue eosinophilia. In human monocyte-derived macrophages, PLA2G5 and TGM2 translocated and colocalized in the cytoplasm and at the plasma membrane after IL-4 stimulation. Moreover, knocking down PLA2G5 in IL-4-activated M2 macrophages significantly decreased PGE2 production and TGM activity, which was restored by exogenous PGE2. Thus, in human M2 macrophages PLA2G5 is a functionally relevant enzyme that is likely involved in development of human Th2 disorders.
MATERIALS AND METHODS
Human monocyte–derived macrophages
Leukocyte-enriched buffy coat from healthy donors was overlaid on Ficoll-Paque Plus (GE Healthcare, Buckinghamshire, United Kingdom) and centrifuged at 600 g for 20 min. The mononuclear layer at the interface was collected, washed, and counted. Monocytes were isolated by negative selection (Miltenyi Biotec, Auburn, CA, USA) and plated at 2.2 × 105 cells/cm2 in 100 mm Petri dishes [12]. To derive macrophages, monocytes were cultured for 13 d in complete medium (RPMI 1640, 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptavidin, 10% nonessential amino acids, 1% HEPES, 1% sodium pyruvate, and 50 μM 2-ME) supplemented with 50 ng/ml human rGM-CSF (R&D Systems, Minneapolis, MN, USA) [22, 26]. To activate macrophages, cells were polarized for 6, 18, 24, or 48 h in complete medium, supplemented with 40 ng/ml human IL-4 (R&D Systems). To knock down PLA2G5, after the monocytes were cultured for 13 d in rGM-CSF, we transfected them with human PLA2G5 ON-TARGET Plus SMART Pool siRNA or nontargeting vector ON-TARGET Plus Control Pool (1000 nM; GE Dharmacon, Lafayette, CO, USA) using the Amaxa Human Macrophage Nucleofector kit (Amaxa, Lonza, Germany), according to the manufacturer’s instructions. After 24 h, the transfection medium was replaced by complete medium. Twenty-four hours later, cells were polarized with IL-4 for 6, 18, 24, or 48 h. In selected experiments PGE2 or human rPLA2G5 (Cayman, Ann Arbor, MI, USA) was added to cells [27].
Patient characterization and tissue allocation
Patients between the ages of 18 and 70 y were recruited at the Brigham and Women’s Hospital (Boston, MA, USA) and were classified according to their clinical characteristics. For the nasal polyp and nasal tissue studies, tissue was collected during surgical excision from subjects with eosinophilic sinusitis and nasal polyposis [28]. Control subjects were patients undergoing anatomic resection of concha bullosa tissue. The human subjects Institutional Review Board approved the study, and all participants provided written consent.
Nasal tissue was excised at the time of surgery and placed into RPMI 1640 with 1% penicillin/streptomycin for transport to the laboratory. Within 2 h of surgery, the tissue was removed from RPMI 1640 and fixed in 4% paraformaldehyde, then embedded in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA, USA), and kept at −80°C until sectioning. Sections of 5 μm thickness were freshly cut, thaw mounted onto slides, and stained for confocal microscopy. Alternatively, in some samples, Congo red dye was used to highlight eosinophil infiltrates. A pathologist evaluated the extent of cellular infiltration of the tissue on 3 subepithelial areas of 2 mm.
Confocal microscopy
Monocyte-derived macrophages were collected and plated on glass coverslips before IL-4 stimulation. The cells were stained as has been described [29]. In brief, cells were fixed with 4% paraformaldehyde in PBS (Sigma-Albrich, St. Louis, MO, USA), permeabilized with 0.01% saponin (Sigma-Aldrich), and blocked for 1 h in HBSS containing 0.1% BSA (Sigma-Albrich) and 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA, USA). Rabbit anti-human PLA2G5 (Aviva Systems Biology, San Diego, CA, USA) and mouse anti-human TGM2 (Abcam, Cambridge, MA, USA) or mouse anti-human Golgin-97 (Thermo Scientific-Life Technologies, Carlsbad, CA, USA) were added to the cells (4°C, overnight) followed by secondary antibodies (Alexa Fluor 488 donkey anti-rabbit IgG and Cy3 donkey anti-mouse IgG (1:200; Jackson ImmunoResearch) and nuclear staining reagents Hoechst 33342 (Thermo Scientific-Invitrogen, Carlsbad, CA, USA) and ToPro-3 (Thermo Scientific-Life Technologies) for 1 h at room temperature. Cells were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Polyp-frozen sections were rehydrated for 1 h at room temperature then blocked with 10% donkey serum, followed by incubation with PLA2G5, TGM2, or CD68 (clone PG-M1; Dako, Glostrup, Denmark) Abs or appropriate isotypes controls at 4°C overnight. Samples were washed and incubated at room temperature for 1 h with appropriate secondary antibodies. The sections were washed and covered with Fluoroshield mounting medium (Electron Microscopy Sciences, Hatfield, PA, USA). Polyp sections and cells were imaged with a C1 plus laser scanning confocal system [29] with a ×40 oil Plan-Fluor NA1.3 or ×60 oil Plan Apo NA 1.4 objective lens (Nikon, Melville, NY, USA). Z-stack images of 0.5 μm (n = 8–10) were acquired through a small pinhole with Nikon EZ-C1 software. Images were stacked as 2D images by using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA).
FACS analysis
Monocyte-derived macrophages were collected and stained with CD64 (clone VI MA36), and CD209 (clone 9E9A8; BioLegend, San Diego, CA, USA), or corresponding isotypes as controls. Viability of transfected macrophages was assessed by staining with Zombie Aqua (BioLegend). Acquisition was performed on a FACSCanto flow cytometer with FACSDiva software (BD Biosciences, Franklin Lakes, NJ, USA), and data were analyzed with FlowJo (Tree Star, Ashland, OR, USA).
Western blot analysis
After stimulation, cells were collected with lidocaine/EDTA [29], lysed in RIPA buffer (Boston Bioproducts, Ashland, MA, USA) with protease inhibitors, and stored at 20°C. The protein concentration in the cell lysates was measured with the bicinchoninic acid assay (Thermo Scientific-Pierce, Rockford, IL, USA) and 20 μg protein from each sample was separated on a 10–20% Tris-Glycine gel (Novex, Thermo Scientific-Life Technologies) and transferred to a PVDF membrane. After blocking at 4°C overnight in 5% milk, the blots were incubated with TGM2 (1:500; Abcam) or GAPDH (1:1000; Cell Signaling Technology, Danvers, MA, USA) diluted in TBS at room temperature for 2 h, followed by appropriate secondary antibodies goat anti-mouse or goat anti-rabbit HRP (1:3000; Bio-Rad, Hercules, CA, USA), diluted in TBS at room temperature for 1 h. The blots were visualized by using Supersignal West Femto Chemiluminescent substrate (Thermo Scientific) and imaged by a M35A X-OMAT processor (Eastman Kodak, Rochester, NY, USA).
Quantification of TGM2 activity
After stimulation, cells were lysed in 10 mM DTT and 1 mM EDTA in PBS and stored at −20°C, and 5 μg protein was assayed. The TGM activity of the macrophages was quantified with a commercially available kit (Sigma-Aldrich) [22], according to the manufacturer’s instructions.
Real-time PCR
Total RNA was isolated from cell lysate with the RNeasy Mini Kit (Qiagen, Louisville, KY, USA), reverse transcribed into cDNA (High-Capacity cDNA Reverse Transcription Kit; Thermo Scientific-Applied Biosystems, Foster City, CA, USA) and measured by real-time qPCR for TGM2 and PLA2G5 with the use of SYBR Green/ROX master mix (SABiosciences, Frederick, MD, USA) on an Mx3005P thermal cycler (Stratagene, Santa Clara, CA, USA). The ratio of each mRNA relative to the GAPDH mRNA was calculated with the ΔΔCt threshold cycle method. The human primers used were TGM2 F: GGTGAGTGGCATGGTCAACT; R: GTCCCCGTAGTTGTTGTCCC. PLA2G5 F: CCCAAGGATGGCACCGATTT; R CGAATGTTGCAGCCCTTCTC; and GAPDH F: GATGACATCAAGAAGGTGGTGAA; R: GTCTTACTCCTTGGAGGCCATGT. CCL22 and CCL17 were from SABiosciences.
PGE2 analysis
Cell-free supernatants collected from vector and siRNA-treated macrophages activated by IL-4 were frozen and shipped for analysis by mass spectrometry. The amount of sample used was 200 μl, and 100 μl internal standard was added. Lipids were extracted with SPE: Strata-x polymeric reverse phase columns (8B-S100-UBJ; Phenomenex, Torrance, CA, USA). The following was added to each column: 100% MeOH, 100% H2O, sample, 10% MeOH, and 100% MeOH for elution. Samples were dried with a Speed-Vac (Thermo Scientific) and taken up in 100 μl buffer A (63% H2O, 37% acetonitrile, 0.02% acetic acid). Five microliters was injected into the ultra high-performance liquid chromatography system. Analysis was performed with a mass spectrometer (6500 Qtrap; Sciex, Framingham, MA, USA) [30].
Statistical analysis
Comparisons between 2 groups were made by using an unpaired Student’s t test; time course comparisons were made with 1-way ANOVA with Dunnett’s correction for multiple comparisons, and comparisons were performed with Prism software (GraphPad, San Diego, CA, USA). Data are expressed as mean ± sem; significance was set at P < 0.05.
RESULTS
PLA2G5 and TGM2 are coexpressed in human eosinophilic nasal polyps
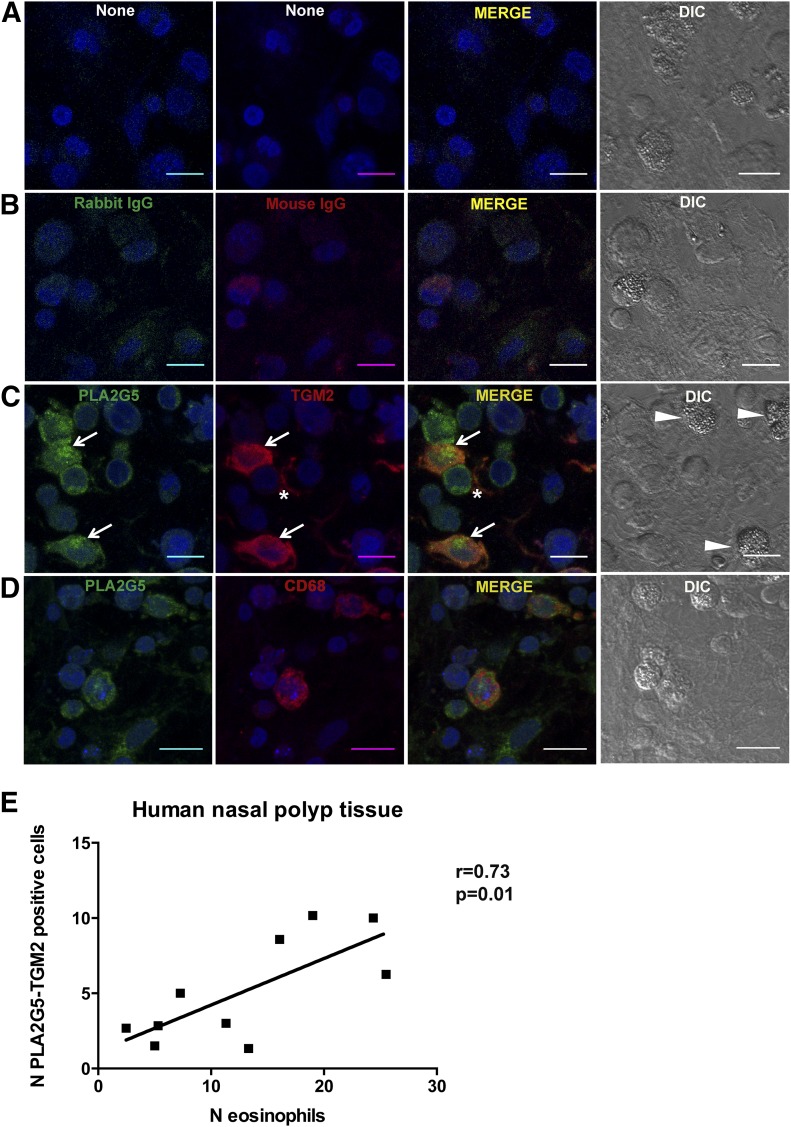
To investigate the expression of PLA2G5 and TGM2 in human Th2 inflammation, we used human nasal polyp tissue from patients with chronic eosinophilic sinusitis and nasal polyposis [28]. These Th2-type inflammatory disorders are associated with tissue eosinophilia (Fig. 1). Nonstained and isotype-stained tissue sections showed minimal fluorescence of frozen tissue sections (Fig. 1A, B). We used a combination of DIC imaging and fluorescence confocal microscopy to identify the eosinophils (cells with granulated cytoplasm) and macrophages (mononuclear cells expressing TGM2 and PLA2G5) (Fig. 1C). TGM2 was expressed in macrophages of patients with nasal polyposis and on structural elements. PLA2G5 was expressed in macrophages where it colocalized with TGM2, a marker of M2 macrophages, and in other cells. Staining of nasal polyp tissue sections with PLA2G5 and CD68, a pan-macrophage marker, showed that PLA2G5 was expressed by CD68+ macrophages, although PLA2G5 and CD68 did not colocalize (Fig. 1D). We compared the number of macrophages expressing both PLA2G5 and TGM2 with the number of eosinophils in the same tissue specimens. Colocalization of PLA2G5 and TGM2 was evident in samples with eosinophilia and correlated positively with the number of eosinophils (10 samples, r = 0.73, P = 0.01) (Fig. 1B). To stringently confirm our identification of eosinophils, in another set of samples from the same tissue specimens, eosinophils were visualized by Congo red staining. The number of eosinophils identified by Congo red staining also correlated positively with the number of macrophages coexpressing PLA2G5 and TGM2 (6 samples, r = 0.65, P = 0.1, and data not shown).
Figure 1. Coexpression of PLA2G5 and TGM2 in human nasal polyp tissue samples.
Representative immunofluorescent and DIC images of tissue samples from patients with eosinophilic nasal polyps (A) unstained; (B) stained with rabbit and mouse IgG and appropriate secondary antibodies; (C) stained with PLA2G5 (green, arrows) and TGM2 (red, arrows) and appropriate secondary antibodies; or (D) stained with PLA2G5 (green) and CD68 (red) and appropriate secondary antibodies. All cells are identified by nuclear staining (To-Pro3) and DIC images. Asterisk indicates expression of TGM2 on structural elements. (E) Association between the average number of eosinophils (DIC panels, arrowheads) and average number of cells expressing PLA2G5 and TGM2 (arrows) in 10 human nasal polyp tissue samples by Pearson correlation (r = 0.73, P = 0.01). At least 3 fields of view were analyzed (magnification ×40, NA 1.3), and average number of cells per sample is shown. (A–D) Magnification, ×60; NA1.4; scale bar, 10 μm.
IL-4 stimulation induces expression of PLA2G5 and TGM2 in human monocyte-derived macrophages
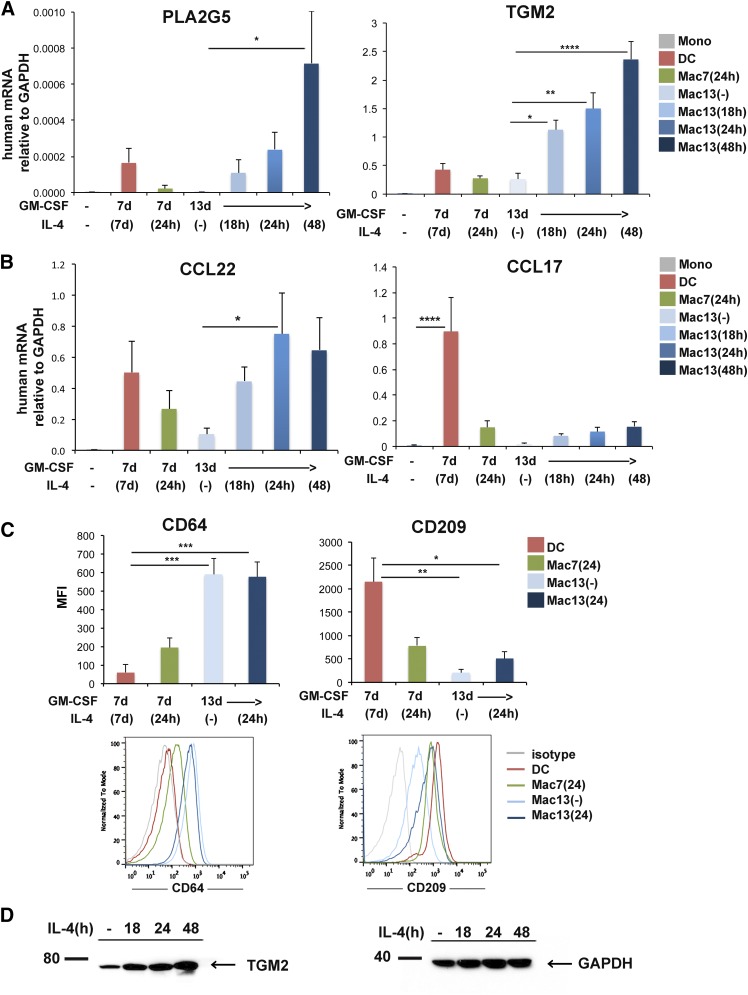
Because PLA2G5 and TGM2 colocalized in macrophages of human tissue samples, we speculated that they are functionally related. To start to address this hypothesis, we first wanted to confirm their expression in human monocyte-derived macrophages. Because PLA2G5 is reported to be induced by IL-4 but not LPS+IFNγ both in mouse and human macrophages [12] and TGM2 mRNA is induced by IL-4 in mouse and human macrophages [22], we used macrophages derived from monocytes, polarized with IL-4. We cultured monocytes for 13 d in GM-CSF (Mac13) and polarized with IL-4 for 18, 24, or 48 h. For comparison, macrophages were also derived from monocytes cultured in GM-CSF for 7 d (Mac7) and IL-4 for 24 h, which have been shown to express TGM2 [22]. Alternatively, monocytes were cultured in GM-CSF and IL-4 for 7 d to generate DCs [31]. We found that in macrophages cultured for 13 d, IL-4 induced a significant dose-dependent increase in PLA2G5 mRNA, (Fig. 2A). This induction was higher than in DCs and macrophages cultured in GM-CSF for 7 d and polarized for 24 h with IL-4. Similarly, TGM2 mRNA was significantly induced in macrophages cultured for 13 d in GM-CSF and polarized with IL-4 at any given time.
Figure 2. Characterization of human monocyte-derived macrophages.
(A, B) Expression of PLA2G5, TGM2, CCL22, and CCL17 mRNA relative to GAPDH measured by qPCR in human monocytes (Mono), DCs (monocytes cultured in GM-CSF and IL-4 for 7 d), and macrophages: Mac7 (24 h) (monocytes cultured in GM-CSF for 7 d and IL-4 for 24 h), Mac13 [monocytes cultured in GM-CSF for 13 d, and no IL-4 (−)] or IL-4 for 18, 24, or 48 h. (C) Analysis of the pan-macrophage marker CD64 and DC marker CD209 by flow cytometry in DCs; macrophages cultured in GM-CSF for 7 d and IL-4 24 h, Mac7(24); macrophages cultured with GM-CSF for 13 d without IL-4, Mac13(−), or IL-4 24 h, Mac13(24). (D) Expression of TGM2 protein analyzed by Western blot in human macrophages cultured for 13 d in GM-CSF and stimulated with IL-4 for 0, 18, 24, or 48 h. Equivalent loading was confirmed by immunoblot analysis for GAPDH. (A–D) Data are from at least 3 independent experiments. (A–C) Values are expressed as means ± sem and were compared by 1-way ANOVA with Dunnett’s correction for multiple comparisons. *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.0001.
We next investigated the expression of CCL22 mRNA, a Th2 chemokine produced by mouse macrophages in a Pla2g5-dependent fashion [12]. We found that in macrophages cultured for 13 d in GM-CSF, IL-4 significantly increased the expression of CCL22 mRNA (Fig. 2B). CCL17, a Th2 chemokine produced by DCs, was expressed by DCs at a higher level than macrophages.
Next, we wanted to better characterize in macrophages their expression of CD64, a pan-macrophage marker highly expressed by human monocyte-derived macrophages irrespective of macrophage polarization [31], and CD209, a molecule highly expressed by monocyte-derived DC. FACS analysis (Fig. 2C) showed that CD64 expression was significantly higher on macrophages, irrespective of their activation state, than on DCs. In contrast, CD209 was significantly higher on DCs than on macrophages. Because TGM2 is considered a marker of human IL-4-activated M2 macrophages, we wanted to confirm the expression of TGM2 protein in macrophages. Western blot analysis (Fig. 2D) showed an IL-4-dependent increase in TGM2 protein in macrophages cultured for 13 d in GM-CSF and polarized with IL-4 for 0, 18, 24, or 48 h.
IL-4 stimulation induces translocation of PLA2G5 and colocalization with TGM2
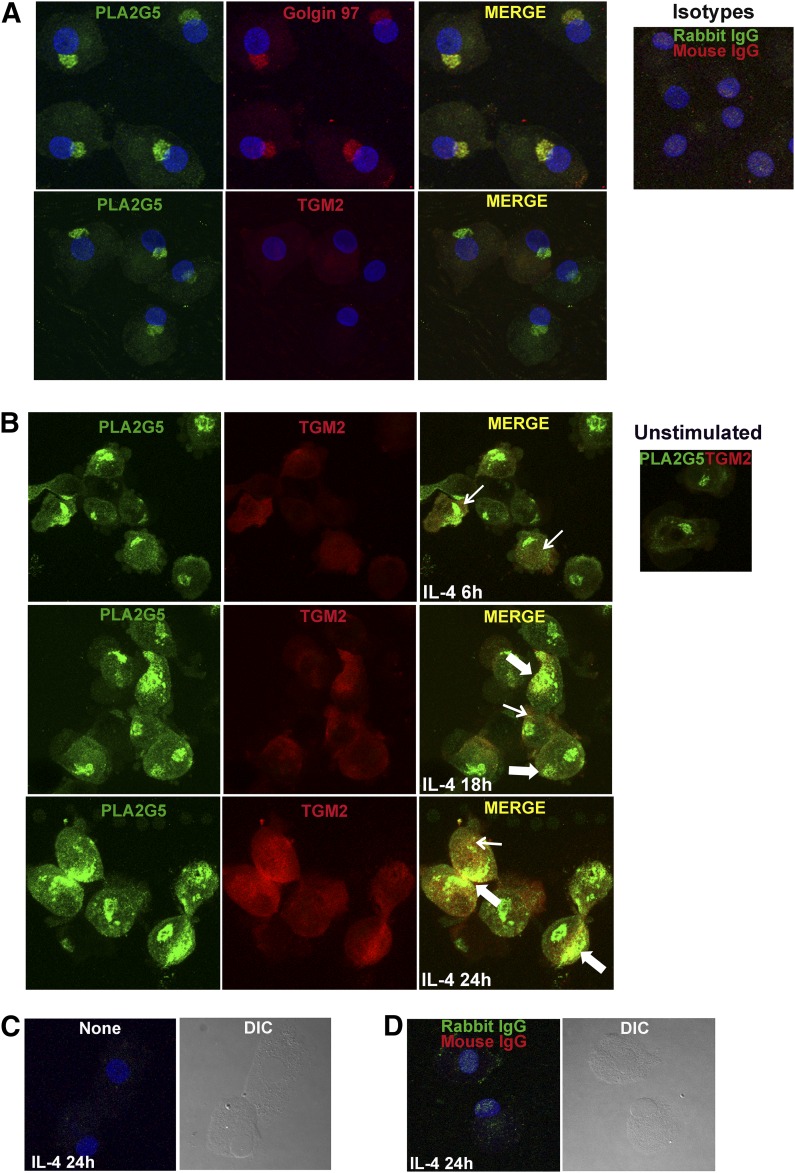
To determine whether cultured macrophages coexpress PLA2G5 and TGM2 protein, we used confocal microscopy. PLA2G5 in resting human macrophages was expressed in the cytoplasm and robustly expressed in a juxtanuclear region suggestive of Golgi (Fig. 3A), which is a pattern similar to that in mouse macrophages [32]. To ascertain the location of PLA2G5, cells were stained with Golgin 97 (a marker of the Golgi apparatus). PLA2G5 colocalized with Golgin 97, confirming that PLA2G5 localizes in the Golgi of human macrophages. Double staining of resting monocyte-derived macrophages with TGM2 and PLA2G5 showed that the expression of TGM2 in resting macrophages was very low; therefore, it was difficult to determine colocalization of PLA2G5 and TGM2 in nonactivated macrophages. Because PLA2G5 and TGM2 colocalized in tissue macrophages of patients with Th2 eosinophilic nasal polyposis, which are most likely activated macrophages, we wanted to ascertain whether PLA2G5 and TGM2 could colocalize in IL-4 activated monocyte-derived macrophages. Therefore, we followed the expression of PLA2G5 and TGM2 during IL-4 activation. After 6 h of IL-4 stimulation, the expression of PLA2G5 and TGM2 increased in the cytoplasm of macrophages (Fig. 3B). By 18 h, both PLA2G5 and TGM2 translocated to the same region of the plasma membrane, with some areas of colocalization on the membrane and in the cytoplasm. At 24 h, PLA2G5 and TGM2 were increasingly induced and robustly colocalized in the cytoplasm and at the plasma membrane in most cells. To account for autofluorescence, macrophages activated with IL-4 for 24 h, were imaged unstained (only counterstained with the blue nuclear dye To-Pro3) or stained with mouse and rabbit IgG isotype controls and appropriate secondary antibodies (Fig. 3C, D), showing minimal autofluorescence.
Figure 3. IL-4 induced expression and colocalization of PLA2G5 and TGM2 in human macrophages.
(A) Immunofluorescence microscopy was used to visualize PLA2G5 (green) and Golgin or TGM2 (red) proteins in unstimulated macrophages. Blue: nuclei. Inset: macrophages stained with rabbit and mouse isotype controls and appropriate secondary antibodies. (B) Macrophages were stimulated with IL-4 for 6, 18, or 24 h and stained for PLA2G5 and TGM2. Inset: unstimulated macrophages stained with PLA2G5 and TGM2. Thin and thick arrows: areas of colocalization in the cytoplasm or at the plasma membrane, respectively. (C) Macrophages stimulated with IL-4 for 24 h were not stained or (D) were stained with isotype controls and appropriate secondary antibodies to account for autofluorescence. Blue: nuclei identified by nuclear staining (To-Pro3). Images are 2D reconstructions of Z-stack files and are representative of at least 3 experiments with similar results.
PLA2G5 regulates TGM activity in human macrophages
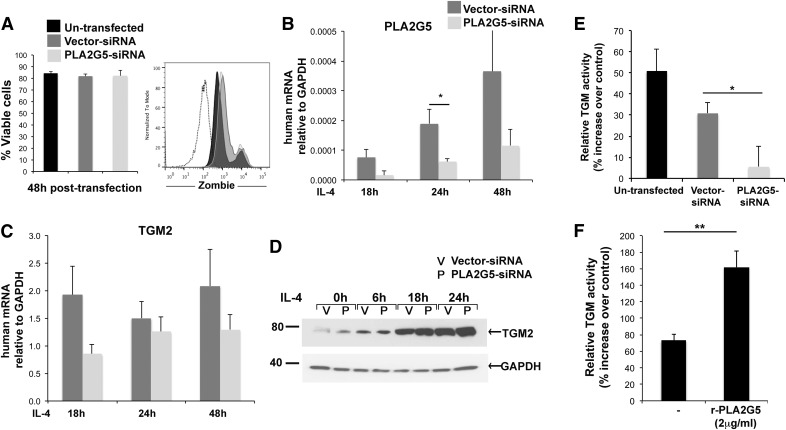
The colocalization of PLA2G5 and TGM2 in activated human macrophages prompted us to investigate whether PLA2G5 and TGM2 are functionally related. We used siRNA to knock down human PLA2G5. Vital staining showed that both vector- and PLA2G5-siRNA–transfected cells were >80% viable 48 h after transfection, which was comparable to the viability of nontransfected cells (Fig. 4A). Compared to transfected vector controls, PLA2G5-siRNA-transfected cells showed a significant reduction in PLA2G5 mRNA at 24 h of IL-4 stimulation, suggesting high efficacy of transfection (Fig. 4B). Because PLA2G5 was necessary for the expression of selected markers of M2 macrophages [12], we investigated the effect of knocking down PLA2G5 on TGM2 mRNA and protein expression. PLA2G5 knockdown did not affect TGM2 mRNA expression or protein expression at any time point (Fig. 4C, D). However, TGM2 is an enzyme whose TGM activity is also increased by IL-4 in macrophages [22]. We therefore measured TGM activity in macrophages stimulated with IL-4 (24 h) that were not transfected or were transfected with vector- or PLA2G5-siRNA and in corresponding unstimulated controls. TGM activity was significantly reduced in IL-4-stimulated human macrophages transfected with PLA2G5-siRNA compared to IL-4-stimulated macrophages transfected with vector-siRNA (Fig. 4E). To confirm the effect of PLA2G5 on TGM activity, we added human rPLA2G5 to IL-4-activated nontransfected macrophages. R-PLA2G5 significantly increased TGM activity in human macrophages stimulated with IL-4 (Fig. 4F).
Figure 4. PLA2G5 regulates TGM activity of macrophages.
(A) Viability of nontransfected, vector- and PLA2G5-siRNA–transfected macrophages was assessed 48 h after transfection by staining with Zombie Aqua. Dead cells were significantly brighter for Zombie staining (Zombiehigh), compared with viable cells (Zombielow). Viable cells are expressed as a percentage of Zombielow cells relative to total cells analyzed. Graph shows unstained cells (dotted line), nontransfected and vector- and PLA2G5-siRNA–transfected cells (black, dark gray, and light gray histograms, respectively). (B, C) Expression of PLA2G5 and TGM2 mRNA relative to GAPDH measured by qPCR in vector- and PLA2G5-siRNA–transfected human macrophages stimulated with IL-4 for 18, 24, or 48 h. (D) Expression of TGM2 protein analyzed by Western blot in vector- and PLA2G5-siRNA–transfected human macrophages, unstimulated (0) or stimulated with IL-4 for 6, 18, or 24 h. Equivalent loading was confirmed by immunoblotting for GAPDH. (E) Percentage increase in TGM activity measured in nontransfected and vector- and PLA2G5-siRNA–transfected macrophages stimulated with IL-4 (24 h) relative to TGM activity in equally transfected unstimulated controls. (F) Percentage increase in TGM activity measured in nontransfected macrophages activated for 24 h with IL-4 or IL-4+human rPLA2G5 (2 μg/ml). Values are means ± sem of 3 (A–C, F), or 5 (E) independent experiments. (D) Images are from 1 experiment representative of 3 experiments with similar results. *P ≤ 0.05; **P < 0.02.
PLA2G5 enhances TGM activity through PGE2 generation
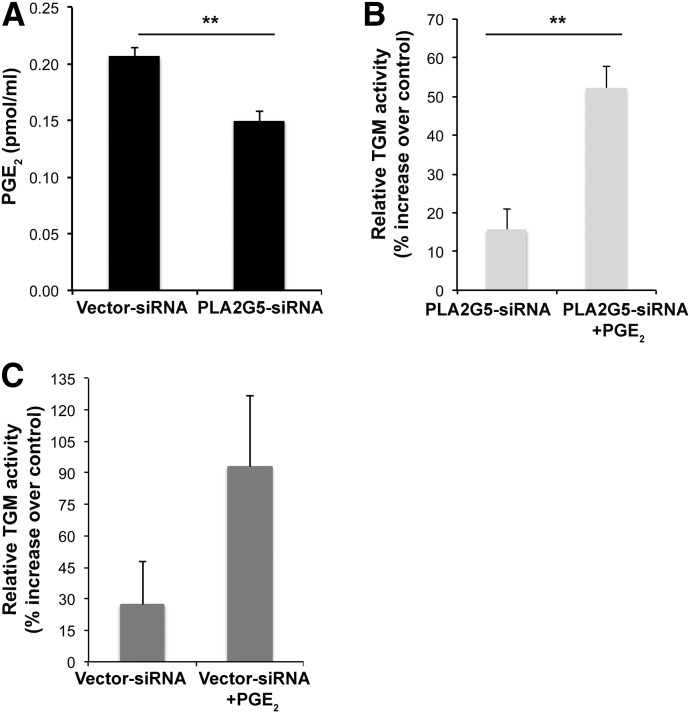
It has been reported that certain lipids, in particular PGE2, enhance TGM activity in mouse peritoneal macrophages [27]. Because PLA2G5 is an enzyme that contributes to PGE2 generation in mouse peritoneal macrophages [33], we next asked whether PLA2G5 could regulate TGM activity through PGE2 generation. First, we used mass spectrometry to analyze PGE2 generated in IL-4-activated human macrophages treated with vector- or PLA2G5-siRNA. The production of PGE2 in IL-4-activated M2 macrophages transfected with PLA2G5-siRNA, was robustly reduced compared to equally stimulated vector controls (Fig. 5A). Then, to verify whether exogenous PGE2 could restore TGM activity, we added PGE2 back to PLA2G5-siRNA–transfected macrophages. As shown in Fig. 5B, exogenous PGE2 (10−7 M) significantly increased TGM activity of PLA2G5-siRNA–transfected macrophages. Addition of PGE2 to vector-siRNA–treated macrophages (Fig. 5C) increased TGM activity, but not significantly, likely reflecting the effect of macrophage-endogenous PLA2G5 and PGE2 on TGM2 activity.
Figure 5. PLA2G5 regulates TGM activity through PGE2 generation.
(A) Mass spectrometry analysis of PGE2 in the supernatant collected from vector- and PLA2G5-siRNA–transfected macrophages stimulated with IL-4. Percentage increase in TGM activity measured in PLA2G5-siRNA-transfected macrophages (B) or vector-siRNA transfected macrophages (C) stimulated with IL-4 or IL-4+PGE2 for 24 h relative to equally transfected unstimulated controls. Values are mean ± sem of 5 independent macrophage cultures from 2 donors (A) or 4 independent experiments (B, C). **P < 0.005.
DISCUSSION
We found PLA2G5 to be essential in IL-4 activation of M2 macrophages and in macrophage effector functions in a Th2 mouse model of pulmonary inflammation induced by the house dust mite Dermatophagoides farinae [12]. In the current study, we extended those studies to characterize the expression and function of PLA2G5 in human macrophages in the context of Th2 inflammation by studying its interaction with TGM2, a protein cross-linking enzyme that is expressed by human and mouse IL-4-activated M2 macrophages and that is found in macrophages and epithelial cells of asthmatic patients [22, 23]. We report that PLA2G5 is expressed in TGM2-positive tissue macrophages of nasal polyp tissue from patients with Th2 eosinophilic inflammation, and this coexpression is absent in the noninflamed sinus tissue from healthy controls. In monocyte-derived macrophages PLA2G5 translocated and colocalized with TGM2 after IL-4 activation. Interestingly, human M2 macrophage TGM activity required the presence of functional PLA2G5, apparently because of the need for PLA2G5-dependent PGE2 generation. Thus, PLA2G5 may be considered a potential therapeutic target in human Th2 inflammatory disorders.
The distinction between M1 and M2 macrophage activation is now expanded to reflect a broad spectrum of activated macrophages in each group [34, 35]. In vivo, the function and expression of markers of various activated macrophages depend on the environment in which macrophages develop and are activated [34–36]. TGM2 is a protein cross-linking enzyme involved in a variety of functions, including matrix deposition, cell migration, and eosinophil recruitment, in a Th2 model of pulmonary inflammation [37, 38]. All of these functions have been linked to M2 macrophages. Furthermore TGM2 has recently been found in tissue macrophages of specimens obtained from patients with asthma [22]. We have reported that Pla2g5 is necessary in macrophages for CCL22 production and recruitment of T cells and eosinophils in a model of Th2 pulmonary inflammation and that mouse macrophages lacking Pla2g5 have reduced expression of selective markers of mouse M2-activated macrophages (including Relm-α, Arg-1, and CCL22) [12]. Therefore, we hypothesized that PLA2G5, and TGM2 may have related expression and function in human M2 macrophages and Th2 inflammation. We focused on human cells because the function of PLA2G5 in human M2 activation and Th2 settings was still ill defined.
Because tissue macrophages in nasal polyps of patients with eosinophilic inflammation and nasal polyposis express molecules related to M2 macrophages [24, 25], we used nasal polyps as a source of tissue macrophages to ascertain the presence of PLA2G5 in macrophages of patients with Th2 eosinophilic inflammation. We found that PLA2G5 was expressed in TGM2-expressing M2 macrophages and in other cells (Fig. 1D). Within each tissue sample, the number of macrophages coexpressing PLA2G5 and TGM2 positively correlated with the number of eosinophils (Fig. 1E), a cardinal feature of Th2 inflammation, and these macrophages were nearly absent in the noninflamed sinus tissue (data not shown). These data suggest a correlation between the expression of PLA2G5 in human M2 macrophages and the development of Th2 inflammatory disorders, further supporting the relevance of PLA2G5 in Th2/M2 settings reported in studies using various mouse models [12, 39, 40].
Because PLA2G5 and TGM2 colocalized in tissue macrophages, we wanted to investigate whether, under Th2 conditions, PLA2G5 and TGM2 are functionally related. In vitro macrophages can be activated by several cytokines to mimic the in vivo phenotype, and the Th2 cytokines IL-4 and -13 are the ones most commonly used for M2 activation. However, depending on the culture conditions, also in vitro IL-4-activated macrophages may have variable expression of markers. Recently, Martinez et al. [22] reported that TGM2 was the only molecule expressed at the mRNA and protein levels in M2 macrophages derived from human monocytes under different culture conditions (including M-CSF or GM-CSF). In this study, we used human monocyte-derived macrophages cultured for 13 d in GM-CSF and polarized with IL-4, which showed high expression of PLA2G5 and TGM2 mRNA compared to monocytes, DCs, or monocyte-derived macrophages cultured for 7 d in GM-CSF and 24 h in IL-4 (Fig. 2A). To better characterize these macrophages, we investigated the production of CCL22, a chemokine produced by M2 macrophages, which we had shown to be produced by mouse macrophages in a Pla2g5-dependent fashion and CCL17, a chemokine produced by DC and to some extent by M2 macrophages. As expected, CCL22 mRNA was highly produced by macrophages cultured for 13 d in GM-CSF and polarized with IL-4, whereas CCL17 was highly expressed by DCs and only modestly induced by IL-4 on macrophages (Fig. 2B). To further confirm the phenotype of the macrophages employed in this study before and after addition of IL-4, we used FACS analysis to investigate the expression of CD64, a pan-macrophage marker, and CD209, a DC marker. CD64 also was highly expressed on macrophages cultured for 13 d in GM-CSF, before and after addition of IL-4, whereas CD209 was highly expressed on DCs (Fig 2C). Altogether, these data support the notion that monocytes cultured for 13 d in GM-CSF are macrophages that can be polarized toward an M2 phenotype by IL-4.
PLA2G5 mRNA is also expressed by both mouse and human IL-4-activated M2 macrophages [12] (Fig. 2A). However, PLA2G5 mRNA induced by IL-4 is low relative to other M2 genes, including TGM2. A possible explanation for this is that in type 2 immunity, PLA2G5 may also be regulated at the protein level. Indeed, our immunofluorescence analysis showed that although PLA2G5 was already expressed in unstimulated macrophages and had a prevalent Golgi location (Fig. 3A), as in mouse macrophages [32], IL-4 also induced its translocation. These data suggest that IL-4 could regulate PLA2G5 protein and mRNA most likely through 2 independent mechanisms.
Because TGM2 was only modestly expressed in the cytoplasm of unstimulated macrophages, colocalization of PLA2G5 and TGM2 proteins could not be assessed in resting macrophages (Fig. 3A, B). As PLA2G5 and TGM2 share the ability to bind membrane proteoglycans [4, 20], we suspected that they would translocate and colocalize in macrophages after IL-4 activation. Indeed, PLA2G5 and TGM2 did translocate and colocalize in both the cytosol and on the membrane of IL-4-activated macrophages, in a clearly time-dependent manner (Fig. 3B). These data confirm that, in macrophages activated in vitro under Th2 conditions, PLA2G5 and TGM2 are regulated by IL-4 at both mRNA and protein level and also that they colocalize as in tissue macrophages of human nasal polyps. Moreover, these data strongly suggest that PLA2G5 and TGM2 are functionally related.
As a consequence of our findings that PLA2G5 and TGM2 colocalize in macrophages and because mouse M2 macrophages lacking Pla2g5 have reduced expression and function of selected M2-related molecules [12], it was important to ascertain whether PLA2G5 knockdown would reduce the expression and function of TGM2. Knocking down PLA2G5 did not significantly affect IL-4-induced TGM2 mRNA or protein expression at any time point analyzed (Fig. 4C, D).
However, TGM2 is a protein cross-linking enzyme whose TGM activity is induced by IL-4 [22]. Therefore, our next step was to ascertain whether knocking down PLA2G5 would reduce TGM activity. Indeed, PLA2G5 knockdown significantly reduced macrophage TGM activity (Fig. 4E). The effect of PLA2G5 on TGM activity was further confirmed by a second approach using rPLA2G5, which significantly increased the TGM activity of macrophages (Fig. 4F). PLA2G5 is an enzyme that generates lipids, and we suspected that the mechanism by which PLA2G5 regulate TGM activity is through PLA2G5-depedent generation of lipids. A previous study reported that certain arachidonate-derived lipids, particularly PGE2, increased TGM2 activity in mouse peritoneal macrophages [27]. Therefore, we analyzed PGE2 produced in vector- and PLA2G5-siRNA–treated IL-4-activated macrophages and found that knocking down PLA2G5 reduced the generation of PGE2 (Fig. 5A). To confirm the role of PGE2 in this system, we then reintroduced exogenous PGE2 to PLA2G5-siRNA–transfected macrophages and showed that it restored TGM activity (Fig. 5B). Although the details of this regulatory system are currently under investigation, our data revealed that in human IL-4-activated M2 macrophages, PLA2G5 regulates the protein cross-linking activity of TGM2 through PGE2 generation.
Our study does not exclude the possibility that TGM2 also influences the expression and function of PLA2G5. A previous report showed that TGM2 post-translationally modifies molecules with PLA2 activity [41]. Furthermore, Hallstrand et al. [23] showed that TGM2 regulates the activity of PLA2G10. PLA2G10 is mainly produced by epithelial cells and secreted in the alveolar space where it can be activated by extracellular TGM2 [9, 42]. In our study, TGM2 and PLA2G5 first interact intracellularly in macrophages and then on the surface of macrophages, suggesting that the ability of PLA2G5 to modulate TGM2 protein cross-linking activity may be an early event in the inflammatory response. Altogether, these data support the notion that PLA2G5 and PLA2G10 have synergistic, nonredundant roles in the development of human Th2 inflammation [43].
Our study has some limitations, as the mechanism by which PLA2G5 increases TGM activity through PGE2, and possibly other lipids, warrants further investigation. However, that PLA2G5 and TGM2 colocalize after IL-4 stimulation suggests that locally generated PGE2 could regulate TGM2 activity. Furthermore, the possibility that TGM2 in turn influences the function of PLA2G5 in macrophages adds more complexity to the interaction between PLA2G5 and TGM2, which would be better explored in studies in vivo.
In conclusion, we have uncovered a novel function of PLA2G5 in human M2 macrophages, the ability to influence TGM2 cross-linking activity through PLA2G5-dependent PGE2 generation. Our study also shows for the first time that PLA2G5 and TGM2 colocalize in vitro in IL-4 activated monocyte-derived macrophages and in vivo in human tissue macrophages, and that their coexpression is related to the extent of tissue eosinophilia, a hallmark of Th2 inflammation. Therefore, human PLA2G5 may be considered a potential therapeutic target in certain human Th2 inflammatory disorders in which M2 macrophages have a role.
AUTHORSHIP
M.Y. and J.Z. performed experiments, analyzed the data, and wrote the manuscript. T.M.L. provided human samples, analyzed the data, and wrote the manuscript. B.B. designed the study, performed experiments, analyzed the data, and wrote the manuscript.
Acknowledgments
This work was supported by an ALA/AAAAI Allergic Respiratory Diseases Award and by the U.S. National Institutes of Health National Heart, Lung and Blood Institute Grants R01HL113071 (to B.B.) and K23HL111113 (to T.M.L.).
Glossary
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- DIC
differential interference contrast
- F
forward
- PGE2
prostaglandin E2
- PLA2
phospholipase A2
- PLA2G5
group V phospholipase A2
- qPCR
quantitative PCR
- rGM-CSF
recombinant GM-CSF
- rPLA2G5
recombinant PLA2G5
- siRNA
small interfering RNA
- sPLA2
secretory PLA2
- TGM
transglutaminase
DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Dennis E. A., Cao J., Hsu Y. H., Magrioti V., Kokotos G. (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111, 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami M., Kambe T., Shimbara S., Higashino K., Hanasaki K., Arita H., Horiguchi M., Arita M., Arai H., Inoue K., Kudo I. (1999) Different functional aspects of the group II subfamily (Types IIA and V) and type X secretory phospholipase A(2)s in regulating arachidonic acid release and prostaglandin generation. Implications of cyclooxygenase-2 induction and phospholipid scramblase-mediated cellular membrane perturbation. J. Biol. Chem. 274, 31435–31444. [DOI] [PubMed] [Google Scholar]
- 3.Murakami M., Koduri R. S., Enomoto A., Shimbara S., Seki M., Yoshihara K., Singer A., Valentin E., Ghomashchi F., Lambeau G., Gelb M. H., Kudo I. (2001) Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 276, 10083–10096. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M., Shimbara S., Kambe T., Kuwata H., Winstead M. V., Tischfield J. A., Kudo I. (1998) The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 273, 14411–14423. [DOI] [PubMed] [Google Scholar]
- 5.Han S. K., Kim K. P., Koduri R., Bittova L., Munoz N. M., Leff A. R., Wilton D. C., Gelb M. H., Cho W. (1999) Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2. J. Biol. Chem. 274, 11881–11888. [DOI] [PubMed] [Google Scholar]
- 6.Lambeau G., Gelb M. H. (2008) Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 77, 495–520. [DOI] [PubMed] [Google Scholar]
- 7.Singer A. G., Ghomashchi F., Le Calvez C., Bollinger J., Bezzine S., Rouault M., Sadilek M., Nguyen E., Lazdunski M., Lambeau G., Gelb M. H. (2002) Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277, 48535–48549. [DOI] [PubMed] [Google Scholar]
- 8.Murakami M., Taketomi Y., Miki Y., Sato H., Yamamoto K., Lambeau G. (2014) Emerging roles of secreted phospholipase A enzymes: the 3rd edition. Biochimie, 107:105-113. [DOI] [PubMed] [Google Scholar]
- 9.Henderson W. R. Jr., Chi E. Y., Bollinger J. G., Tien Y. T., Ye X., Castelli L., Rubtsov Y. P., Singer A. G., Chiang G. K., Nevalainen T., Rudensky A. Y., Gelb M. H. (2007) Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204, 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz N. M., Meliton A. Y., Arm J. P., Bonventre J. V., Cho W., Leff A. R. (2007) Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J. Immunol. 179, 4800–4807. [DOI] [PubMed] [Google Scholar]
- 11.Giannattasio G., Fujioka D., Xing W., Katz H. R., Boyce J. A., Balestrieri B. (2010) Group V secretory phospholipase A2 reveals its role in house dust mite-induced allergic pulmonary inflammation by regulation of dendritic cell function. J. Immunol. 185, 4430–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta S., Imamura M., Xing W., Boyce J. A., Balestrieri B. (2013) Group V secretory phospholipase A2 is involved in macrophage activation and is sufficient for macrophage effector functions in allergic pulmonary inflammation. J. Immunol. 190, 5927–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowton D. L., Seeds M. C., Fasano M. B., Goldsmith B., Bass D. A. (1997) Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am. J. Respir. Crit. Care Med. 155, 421–425. [DOI] [PubMed] [Google Scholar]
- 14.Chilton F. H., Averill F. J., Hubbard W. C., Fonteh A. N., Triggiani M., Liu M. C. (1996) Antigen-induced generation of lyso-phospholipids in human airways. J. Exp. Med. 183, 2235–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallstrand T. S., Chi E. Y., Singer A. G., Gelb M. H., Henderson W. R. Jr (2007) Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am. J. Respir. Crit. Care Med. 176, 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallstrand T. S., Lai Y., Ni Z., Oslund R. C., Henderson W. R. Jr., Gelb M. H., Wenzel S. E. (2011) Relationship between levels of secreted phospholipase A₂ groups IIA and X in the airways and asthma severity. Clin. Exp. Allergy 41, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeds M. C., Jones K. A., Duncan Hite R., Willingham M. C., Borgerink H. M., Woodruff R. D., Bowton D. L., Bass D. A. (2000) Cell-specific expression of group X and group V secretory phospholipases A(2) in human lung airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 23, 37–44. [DOI] [PubMed] [Google Scholar]
- 18.Király R., Demény M., Fésüs L. (2011) Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 278, 4717–4739. [DOI] [PubMed] [Google Scholar]
- 19.Belkin A. M. (2011) Extracellular TG2: emerging functions and regulation. FEBS J. 278, 4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpellini A., Germack R., Lortat-Jacob H., Muramatsu T., Billett E., Johnson T., Verderio E. A. (2009) Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J. Biol. Chem. 284, 18411–18423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Griffin M. (2013) The role of TG2 in regulating S100A4-mediated mammary tumour cell migration. PLoS One 8, e57017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez F. O., Helming L., Milde R., Varin A., Melgert B. N., Draijer C., Thomas B., Fabbri M., Crawshaw A., Ho L. P., Ten Hacken N. H., Cobos Jiménez V., Kootstra N. A., Hamann J., Greaves D. R., Locati M., Mantovani A., Gordon S. (2013) Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 121, e57–e69. [DOI] [PubMed] [Google Scholar]
- 23.Hallstrand T. S., Wurfel M. M., Lai Y., Ni Z., Gelb M. H., Altemeier W. A., Beyer R. P., Aitken M. L., Henderson W. R. (2010) Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS One 5, e8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng J., Zhou P., Liu Y., Liu F., Yi X., Liu S., Holtappels G., Bachert C., Zhang N. (2013) The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS One 8, e82373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plager D. A., Kahl J. C., Asmann Y. W., Nilson A. E., Pallanch J. F., Friedman O., Kita H. (2010) Gene transcription changes in asthmatic chronic rhinosinusitis with nasal polyps and comparison to those in atopic dermatitis. PLoS One 5, e11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer M., Mallmann M. R., Xue J., Staratschek-Jox A., Vorholt D., Krebs W., Sommer D., Sander J., Mertens C., Nino-Castro A., Schmidt S. V., Schultze J. L. (2012) High-resolution transcriptome of human macrophages. PLoS One 7, e45466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishitani K., Ogawa S., Suzuki M. (1988) Influence of arachidonate metabolism on enhancement of intracellular transglutaminase activity in mouse peritoneal macrophages. J. Biochem. 104, 397–402. [DOI] [PubMed] [Google Scholar]
- 28.Laidlaw T. M., Kidder M. S., Bhattacharyya N., Xing W., Shen S., Milne G. L., Castells M. C., Chhay H., Boyce J. A. (2012) Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 119, 3790–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balestrieri B., Maekawa A., Xing W., Gelb M. H., Katz H. R., Arm J. P. (2009) Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J. Immunol. 182, 4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Armando A. M., Quehenberger O., Yan C., Dennis E. A. (2014) Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J. Chromatogr. A 1359, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue J., Schmidt S. V., Sander J., Draffehn A., Krebs W., Quester I., De Nardo D., Gohel T. D., Emde M., Schmidleithner L., Ganesan H., Nino-Castro A., Mallmann M. R., Labzin L., Theis H., Kraut M., Beyer M., Latz E., Freeman T. C., Ulas T., Schultze J. L. (2014) Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balestrieri B., Hsu V. W., Gilbert H., Leslie C. C., Han W. K., Bonventre J. V., Arm J. P. (2006) Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J. Biol. Chem. 281, 6691–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satake Y., Diaz B. L., Balestrieri B., Lam B. K., Kanaoka Y., Grusby M. J., Arm J. P. (2004) Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J. Biol. Chem. 279, 16488–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez F. O., Gordon S. (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines (published correction available at http://dx.doi.org/10.1016/j.immuni.2014.07.009). Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosselin D., Link V. M., Romanoski C. E., Fonseca G. J., Eichenfield D. Z., Spann N. J., Stender J. D., Chun H. B., Garner H., Geissmann F., Glass C. K. (2014) Environment drives selection and function of enhancers controlling tissue-specific macrophage identities (published correction available at http://dx.doi.org/10.1016/j.cell.2014.12.024). Cell 159, 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gundemir S., Colak G., Tucholski J., Johnson G. V. (2012) Transglutaminase 2: a molecular Swiss army knife. Biochim. Biophys. Acta 1823, 406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh K., Seo M. W., Lee G. Y., Byoun O. J., Kang H. R., Cho S. H., Lee D. S. (2013) Airway epithelial cells initiate the allergen response through transglutaminase 2 by inducing IL-33 expression and a subsequent Th2 response. Respir. Res. 14, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson W. R. Jr., Ye X., Lai Y., Ni Z., Bollinger J. G., Tien Y. T., Chi E. Y., Gelb M. H. (2013) Key role of group v secreted phospholipase A2 in Th2 cytokine and dendritic cell-driven airway hyperresponsiveness and remodeling. PLoS One 8, e56172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato H., Taketomi Y., Ushida A., Isogai Y., Kojima T., Hirabayashi T., Miki Y., Yamamoto K., Nishito Y., Kobayashi T., Ikeda K., Taguchi R., Hara S., Ida S., Miyamoto Y., Watanabe M., Baba H., Miyata K., Oike Y., Gelb M. H., Murakami M. (2014) The adipocyte-inducible secreted phospholipases PLA2G5 and PLA2G2E play distinct roles in obesity. Cell Metab. 20, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordella-Miele E., Miele L., Beninati S., Mukherjee A. B. (1993) Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J. Biochem. 113, 164–173. [DOI] [PubMed] [Google Scholar]
- 42.Lai Y., Oslund R. C., Bollinger J. G., Henderson W. R. Jr., Santana L. F., Altemeier W. A., Gelb M. H., Hallstrand T. S. (2010) Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group X. J. Biol. Chem. 285, 41491–41500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami M., Lambeau G. (2013) Emerging roles of secreted phospholipase A(2) enzymes: an update. Biochimie 95, 43–50. [DOI] [PubMed] [Google Scholar]