Abstract
Background and Purpose
Long-term outcome information after TIA and stroke is required to help plan and allocate care services. We evaluated the impact of TIA and stroke on disability and institutionalisation over 5 years using data from a population-based study.
Methods
Patients from a UK population-based cohort study (Oxford Vascular Study) were recruited from 2002 to 2007, and followed-up to 2012. Patients were followed-up at 1, 6, 12, 24 and 60 months post-event and assessed using the modified Rankin Scale (mRS). A multivariate regression analysis was performed to assess the predictors of disability post-event.
Results
748 index stroke and 440 TIA cases were studied. For TIA patients, disability levels increased from 14% (63/440) pre-morbidly to 23% (60/256) at 5 years (p=0.002), with occurrence of subsequent stroke being a major predictor of disability. For stroke survivors, the proportion disabled (mRS>2) increased from 21% (154/748) pre-morbidly to 43% (273/634) at 1-month (p<0.001), with 39% (132/339) of survivors disabled 5 years post-stroke. 5 years post-event, 70% (483/690) of stroke patients and 48% (179/375) of TIA patients were either dead or disabled. The 5-year risk of care home institutionalisation was 11% after TIA and 19% after stroke. The average 5-year cost per institutionalised TIA patient was $99,831 (S.D. 67,020) and $125,359 (S.D. 91,121) for stroke patients.
Conclusions
Our results show that 70% of stroke patients are either dead or disabled 5 years after the event. There therefore remains considerable scope for improvements in acute treatment and secondary prevention to reduce post-event disability and institutionalisation.
Keywords: Stroke, Transient Ischemic Attacks, Long-Term Outcome, Nursing Home
Introduction
For many survivors, stroke exerts a negative effect on their lives by affecting many functions, including speech, swallowing, vision, ambulation, coordination, cognition, hampering their ability to perform usual activities.1,2 Therefore, a high proportion of stroke survivors will rely on health services, social services, and/or relatives and friends to provide care and assistance. Due to age-specific incidence changes, the ageing population and more events occurring at older ages,3 up-to-date information about stroke outcomes is needed to better understand disease impact, the impact of prevention and treatment strategies and to adequately plan and allocate health and social care services.
Large studies of stroke incidence with complete population-based ascertainment and long-term follow-up of cases are required to provide reliable information.4,5 Long-term health outcomes, including institutionalisation, after stroke have been examined in a number of population-based studies.6–10 However, although a number of studies have reported the proportion institutionalised in long-term care facilities, none has reported time to institutionalisation, or long-term institutionalisation risk, after stroke, making it difficult to estimate the costs associated with long-term institutionalisation. Rates of institutionalisation also vary between countries due to differences in healthcare systems and social norms.11 In addition, reliable evidence of long-term health outcome after transient ischaemic attack (TIA), a major risk factor for subsequent stroke, is lacking in general.12
Using data from patients in a population-based (Oxford Vascular Study - OXVASC) ascertained between 2002 and 2007, we sought to determine the frequency and predictors of disability and institutionalisation into long-term nursing or residential care over the short- and long-term after TIA or stroke.
Methods
The OXVASC study population comprises over 91,000 patients registered in 9 general practices across Oxfordshire, UK. The study methods have been described elsewhere.13 Briefly, patient registration began on April 2002 and is ongoing. Only consenting patients recruited from 1 April 2002 to 31 March 2007 were included in this analysis in order to allow minimum 5 years follow-up. Patients in whom TIA or stroke was suspected were ascertained using multiple overlapping methods of “hot” and “cold” pursuit and considered for inclusion,14 including:
-
1)
A daily (weekdays only), urgent open-access “TIA clinic” to which participating general practitioners (GPs) and the local accident and emergency department (A&E) send all individuals with suspected TIA or stroke whom they would not normally admit to hospital, with alternative on-call review provision at weekends;
-
2)
Daily searches of admissions to the medical, stroke, neurology and other relevant wards;
-
3)
Daily searches of the local A&E attendance register;
-
4)
Monthly computerized searches of GP diagnostic coding and hospital discharge codes;
-
5)
Monthly searches of all cranial and carotid imaging studies performed in local hospitals; and
-
6)
Monthly reviews of all death certificates and coroners reports.
Suspected TIA/stroke patients were assessed urgently by a study clinician. Stroke was defined according to World Health Organisation (WHO) definitions and included all ischaemic events, intracerebral haemorrhage (ICH), subarachnoid haemorrhage (SAH), and strokes of uncertain type. Informed consent was sought, and assessments of neurological impairment, history of presentation, medical and social history, and risk factors were performed. Impairment was measured using the National Institutes of Health Stroke Scale (NIHSS), which was used to categorise event severity. Minor events were defined as NIHSS scores≤3, moderate as scores from 4 to 10, and severe as scores>10. Although different study physicians were involved during the study, all cases were subsequently reviewed by the study senior neurologist (PMR) on a daily basis and imaging results were assessed by the same neuroradiologist, with the final classification as TIA, stroke or other condition being made by the same senior neurologist and neuroradiologist in all cases.
Patients were followed-up from the first TIA or stroke in the study period for which the patient sought medical attention, referred to here as the index event. Surviving patients were followed-up by a research nurse at 1, 6, 12, 24 and 60 months after the event. Data were collected on patient’s living arrangements; risk factor changes; and disability (measured using the modified Rankin Scale - mRS). However, the 24-month follow-up was discontinued for all patients recruited on or after 1 April 2005, with all surviving patients still seen at 60 months. Patients were also followed-up via their general practitioner (GP) and hospital records, recurrent vascular events were identified by ongoing ascertainment, and all patients had mortality follow-up.
Disability was defined as mRS scores between 3 and 5. New disability was defined as patients who progressed from no disability before the event (mRS<3) to disability after the event.
At the time of follow-up, patients were asked if they lived in: their own home; with friends or relatives; warden housing (in which trained a warden is available 24 hours a day); care home (either residential or nursing care); hospital; or in intermediate care (e.g. community hospital). For the purposes of this study, long-term institutionalisation was defined as admission into a nursing or residential care home. As a result, we did not include post-acute care and rehabilitation stays in hospital, which, although in some cases might include stays of several months, are temporary in nature. 5-year hospitalisation, resource use and costs, including post-acute and rehabilitation stays, in stroke and TIA patients included in OXVASC are reported in a separate article.15
Statistical analyses
Disability was reported as a proportion, with differences between time periods and patient sub-groups being evaluated using chi-square tests. A logistic regression analysis was performed to determine the predictors of disability at 6 months post-index event, using the following variables: event type, severity, age and gender, past history of disease and disability, marital status, living arrangements before the event, deprivation as measured by postcode of residence, working status before the event, and subsequent vascular events between event onset and 6-month follow-up. We assessed the predictors of 6-month disability as no significant changes in disability levels were identified between the 6-month follow-up and that at 12, 24 and 60 months. A similar logistic regression was undertaken to determine the predictors of either death or disability 5 years post-index event, this time including subsequent vascular events between event onset and 5-year follow-up. Statistical significance was set at p<0.050. Goodness-of-fit was assessed using McFadden’s Adjusted R2. Model specification was tested using Preigbon’s link test, and multicollinearity was assessed using the tolerance value.
Time to institutionalisation was defined as the difference between the date in which the index event occurred and the date of admission into a nursing or residential care home. The 5-year risk of institutionalisation in long- term care home was evaluated using Kaplan-Meier techniques, adjusted for censoring due to mortality. Statistically significant differences in risk between subgroups were assessed using Cox’s proportional hazards model.16
We also evaluated the 5-year costs of institutionalisation into a nursing or residential care home following index stroke or TIA. We estimated the number of institutionalised days as the difference between either the: date of the 5-year follow-up or death, whichever was earliest, and the date of admission into the institution. All costs were converted from UK pounds sterling (£) to US dollars ($) and reported as means together with their standard deviation (S.D.). The currency conversion was based on the rate of purchasing power parities in 2011 ($1 is equal to £0.68).17 Institutionalisation was costed as the cost per week in a private nursing home, which in 2011 was $1,090 (£740) per week.18
Results
Patient sample
Between April 2002 and March 2007, 748 patients suffered a stroke and 440 patients a TIA as their index event for which medical attention was sought. Mean age was 75 (S.D. 12) years for stroke patients and 73 (S.D. 13) for TIA patients. Males accounted for 49% (n=370) of stroke cases and 44% (n=194) of TIA cases (Table 1). Of the 738 (99%) strokes with available baseline NIHSS scores, 436 (59%) were classified as minor, 169 (23%) as moderate, and 133 (18%) as severe.
Table 1. Study sample.
| Stroke (n=748) | TIA (n=440) | |
|---|---|---|
| Age (years), mean (SD) | 75 (12) | 73 (13) |
| Males | 370 (49) | 194 (44) |
| Previous MI* | 97 (13) | 51 (12) |
| Previous angina* | 119 (16) | 73 (17) |
| Previous stroke* | 150 (20) | 53 (12) |
| History of hypertension* | 450 (60) | 227 (52) |
| History of atrial fibrillation* | 144 (19) | 68 (15) |
| History of diabetes* | 78 (10) | 58 (13) |
| NIHSS score†, median (IQR) | 3 (0-7) | 0 (0) |
| Minor stroke (NIHSS 0 to 3) | 436 (59) | |
| Moderate stroke (NIHSS 4 to 10) | 169 (23) | |
| Severe stroke (NIHSS > 10) | 133 (18) | |
| Stroke type | ||
| Ischaemic | 618 (83) | |
| Intracerebral haemorrhage | 54 (11) | |
| Subarachnoid haemorrhage | 38 (5) | |
| Unknown stroke | 38 (3) | |
| Recurrent stroke after initial event at | ||
| 6 months | 83 (11) | 42 (10) |
| 5 years | 151 (20) | 70 (16) |
| Coronary event after initial event at | ||
| 6 months | 15 (2) | 10 (2) |
| 5 years | 47 (6) | 43 (10) |
All data expressed as no. (%), except were specified. IQR-Interquartile range.
Missing – Stroke: 4, TIA: 1
Missing (due to late presentation or events occurring out of area) – Stroke: 10, TIA: 2
Information on mRS scores was available for 99% (n=1,064), 97% (n=978), 95% (n=921) and 83% (n=595) of patients alive at the 1-, 6-, 12- and 60-month follow-up, respectively. Due to the 2-year follow-up being discontinued for patients recruited after 1 April 2005, 340 (37%) patients were not followed-up at that point, with outcome information missing in a further 35 (4%) patients. Detailed information on mRS scores is available in the online supplementary material (Supplementary Table I).
Disability
For stroke patients, there was a marked increase in disability levels from before the index event to 1-month after index stroke (21% vs. 43%, respectively; p<0.001 – Table 2). When compared to 1-month disability levels, the proportion of disabled stroke patients decreased to 37% (n=208) at 6 months (p=0.028), 36% (n=186) at 1 year (p=0.009), 38% (n=122) at 2 years (p=0.166), and 39% (n=132) at 5 years (p=0.214), reflecting the balance between death of disabled patients and development of new disability. Long-term outcome data by stroke type is available in the online supplementary material (Supplementary Table II). For TIA patients, disability levels did not differ pre-morbidly (n=63, 14%) and at the 1- (n=72, 17%, p=0.323) and 6-month follow-up (n=78, 19%, p=0.073). However, 1 year after TIA, disability levels had increased when compared to pre-morbid levels to 20% (n=79, p=0.033), increasing to 23% (n=60, p=0.002) by 5 years.
Table 2. Death, disability (mRS>2) and new disability following index TIA (n=440) or stroke (n=748).
| Death (%) | Survival (%) | Disabled (as % of survivors)† | Death or disability (%)† | Surviving cases with no pre-morbid disability | New disability n (as % surviving cases with no pre-morbid disability)** | |
|---|---|---|---|---|---|---|
| Stroke | ||||||
| Pre-morbid | 0 | 748 (100) | 154 (21) | 154 (21) | N/A | N/A |
| 1 month | 107 (14) | 641 (86) | 273 (43) | 380 (51) | 529 | 178 (34) |
| 6 months | 165 (22) | 583 (78) | 208 (37) | 373 (51) | 499 | 145 (30) |
| 1 year | 199 (27) | 549 (73) | 186 (36) | 385 (53) | 481 | 133 (29) |
| 2 years* | 232 (31) | 516 (69) | 122 (38) | 354 (64) | 457 | 92 (32) |
| 5 years | 351 (47) | 397 (53) | 132 (39) | 483 (70) | 367 | 110 (35) |
| TIA | ||||||
| Pre-morbid | 0 | 440 (100) | 63 (14) | 63 (14) | N/A | N/A |
| 1 month | 4 (1) | 436 (99) | 72 (17) | 76 (18) | 374 | 21 (6) |
| 6 months | 13 (3) | 427 (97) | 78 (19) | 91 (21) | 372 | 30 (8) |
| 1 year | 23 (5) | 417 (95) | 79 (20) | 102 (24) | 367 | 36 (10) |
| 2 years* | 48 (11) | 392 (89) | 46 (21) | 94 (36) | 356 | 26 (14) |
| 5 years | 119 (27) | 321 (73) | 60 (23) | 179 (48) | 300 | 43 (18) |
2 year follow-ups were discontinued for patients recruited on or after 1 April 2005. As a result, 2-year disability data was unavailable for 156 TIA patients and 184 stroke patients
Missing mRS data: TIA cases –1 month: 6; 6 months: 14; 1 year: 19; 2 years: 177; and 5 years: 65. Stroke cases – 1 month: 7; 6 months: 18; 1 year: 26; 2 years: 198; and 5 years: 58.
Missing mRS data: TIA cases –1 month: 6; 6 months: 14; 1 year: 17; 2 years: 164; and 5 years: 63. Stroke cases –1 month: 6; 6 months: 14; 1 year: 21; 2 years: 175; and 5 years: 54
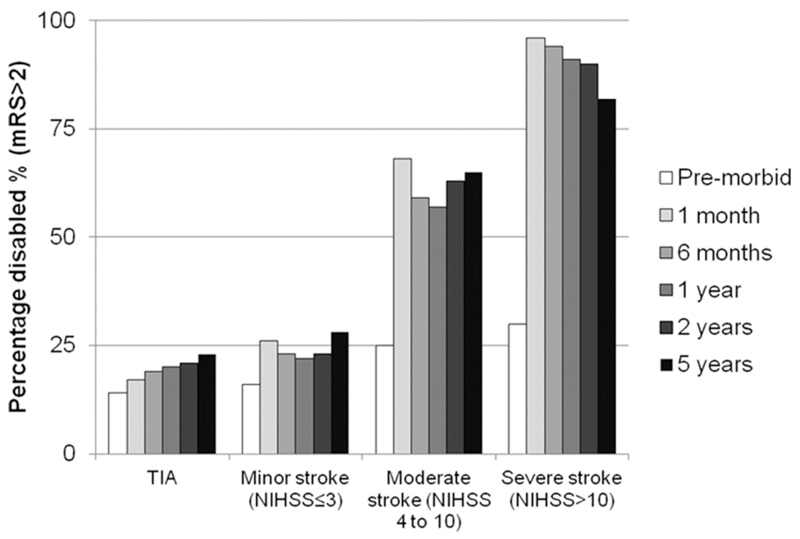
For minor stroke patients, the proportion of disabled patients significantly increased from 16% (68/436) pre-morbidly to 26% at 1 month (109/418; p<0.001 – Figure 1), with these differences maintained across subsequent follow-ups. For surviving severe stroke patients, 96% (66/69) were disabled at one month. Due to death of disabled patients over time, the proportion of disabled patients after severe stroke gradually decreased over the 5 years, with the proportion disabled significantly decreasing to 82% (23/28; p=0.030 vs. 1-month) at 5 years.
Figure 1.
Rates of disability (mRS>2) at follow-up stratified by severity of index event
A logistic regression was undertaken to assess the predictors of 6-month disability (Table 3). Significant predictors of increased disability included age, being disabled pre-morbidly, event severity (as measured using the NIHSS), and suffering one or more subsequent strokes or coronary events during the 6 months post-event. Surviving ischaemic stroke patients were significantly more likely to be disabled at 6 months than TIA patients. Marital status was also found to be a predictor of disability 6 months post-index event, with widowed and single patients more likely to be disabled.
Table 3. Predictors of disability (mRS>2) and death or disability after TIA or stroke.
| 6-month disability |
5-year disability or death |
|||
|---|---|---|---|---|
| Adj. OR (95% CI) |
p>|z| | Adj. OR (95% CI) |
p>|z| | |
| Age, years | 1.04 (1.01-1.07) | 0.005 | 1.13 (1.10-1.17) | <0.001 |
| Male | 0.81 (0.52-1.27) | 0.356 | 1.38 (0.91-2.08) | 0.128 |
| Previously disabled | 16.5 (9.12-29.8) | <0.001 | 14.5 (5.62-37.5) | <0.001 |
| History of: | ||||
| Hypertension | 0.92 (0.60-1.41) | 0.696 | 0.96 (0.65-1.42) | 0.824 |
| Stroke | 1.32 (0.78-2.22) | 0.301 | 1.45 (0.86-2.46) | 0.166 |
| Atrial fibrillation | 1.32 (0.77-2.26) | 0.305 | 1.70 (1.02-2.84) | 0.041 |
| Myocardial infarction | 0.87 (0.43-1.76) | 0.698 | 0.83 (0.44-1.57) | 0.563 |
| Angina | 0.92 (0.49-1.74) | 0.806 | 0.92 (0.52-1.64) | 0.780 |
| Diabetes | 1.50 (0.80-2.83) | 0.209 | 2.32 (1.22-4.41) | 0.010 |
| Peripheral vascular disease | 1.14 (0.54-2.43) | 0.724 | 0.75 (0.37-1.53) | 0.429 |
| Index event | ||||
| Ischaemic stroke | Reference case | Reference case | ||
| Intracerebral haemorrhage | 1.15 (0.34-3.92) | 0.825 | 1.23 (0.36-4.20) | 0.740 |
| Subarachnoid haemorrhage | 0.30 (0.01-9.24) | 0.488 | 1.53 (0.29-7.96) | 0.612 |
| Unknown stroke | 0.35 (0.06-2.25) | 0.271 | 1.04 (0.14-7.59) | 0.973 |
| TIA | 0.54 (0.34-0.88) | 0.013 | 0.64 (0.43-0.97) | 0.035 |
| Event severity (NIHSS score) | ||||
| 0 to 3 | Reference case | Reference case | ||
| 4 to 10 | 4.85 (2.77-8.47) | <0.001 | 3.19 (1.68-6.06) | <0.001 |
| >10 | 134 (35.0-511) | <0.001 | 24.7 (7.82-77.8) | <0.001 |
| Subsequent events | ||||
| Stroke | 4.27 (2.51-7.26) | <0.001 | 1.89 (1.35-2.62) | <0.001 |
| Coronary event | 5.70 (1.54-21.1) | 0.009 | 1.64 (1.00-2.70) | 0.052 |
| Peripheral vascular disease | 2.16 (0.16-30.1) | 0.566 | 1.39 (0.50-3.84) | 0.527 |
| Lived alone before event | 0.44 (0.23-0.85) | 0.014 | 0.47 (0.23-0.97) | 0.041 |
| Living arrangements before event | ||||
| Own home | Reference case | Reference case | ||
| With relative/friend | 0.27 (0.07-1.09) | 0.066 | 0.57 (0.13-2.52) | 0.454 |
| Warden housing | 0.45 (0.17-1.24) | 0.122 | 2.20 (0.67-7.15) | 0.192 |
| Care home | 1.80 (0.29-11.2) | 0.528 | 0.53 (0.04-6.45) | 0.619 |
| Hospital/intermediate care | 2.13 (0.49-9.23) | 0.312 | 1.20 (0.31-4.67) | 0.791 |
| Marital status before event | ||||
| Married | Reference case | Reference case | ||
| Widow | 3.17 (1.61-6.27) | 0.001 | 2.28 (1.07-4.84) | 0.032 |
| Single | 3.84 (1.47-10.0) | 0.006 | 8.71 (3.02-25.1) | <0.001 |
| Separated | 1.15 (0.34-3.88) | 0.821 | 2.97 (1.05-8.38) | 0.040 |
| Partnered | 0.14 (0.01-1.53) | 0.107 | 1.45 (0.33-6.34) | 0.624 |
| Employment status before event | ||||
| Retired | Reference case | Reference case | ||
| Full-time worker | 0.36 (0.13-0.97) | 0.043 | 0.61 (0.28-1.35) | 0.221 |
| Part-time worker | 0.59 (0.23-1.51) | 0.270 | 0.60 (0.28-1.27) | 0.178 |
| Home carer | 0.42 (0.09-2.06) | 0.285 | 0.62 (0.13-3.06) | 0.557 |
| Unemployed | 1.37 (0.23-8.13) | 0.728 | 0.31 (0.03-2.78) | 0.295 |
| Cannot work | 2.05 (0.44-9.46) | 0.360 | 1.70 (0.39-7.39) | 0.480 |
| Age left education | 0.98 (0.92-1.04) | 0.524 | 0.96 (0.92-1.02) | 0.181 |
| Deprivation by area of residence | ||||
| Quintile 5 (least deprived) | Reference case | Reference case | ||
| Quintile 4 | 1.57 (1.00-2.49) | 0.052 | 1.45 (0.94-2.24) | 0.095 |
| Quintile 3 | 0.74 (0.35-1.56) | 0.426 | 1.06 (0.57-1.98) | 0.851 |
| Quintile 2 | 0.97 (0.32-2.98) | 0.964 | 0.97 (0.37-2.54) | 0.951 |
| Quintile 1 (most deprived) | 2.06 (0.09-48.6) | 0.655 | 6.57 (0.51-84.7) | 0.149 |
| No. | 915 | No. | 946 | |
| p>X2 | <0.001 | p>X2 | <0.001 | |
| Adj. R2 | 0.353 | Adj. R2 | 0.375 | |
Adj. OR – Adjusted Odds Ratio
Death or disability
Of the 748 stroke patients, 47% (n=351) died within 5 years (Table 2). 119 (27%) of the 440 TIA patients died by the end of the 5-year follow-up.
At 1-month follow-up, over half of stroke patients (n=380) were either dead or disabled (Table 2), with a similar proportion at 6 months (51%, n=730; p=0.943) and at 1 year (53%, n=385; p=0.434). However, by two years the proportion of dead or disabled stroke patients had risen to 64% (n=354; p<0.001 vs. 1-month), and 70% (n=690) by 5 years post-index event (p<0.001 vs.1-month). For TIA patients, the proportion of patients dead or disabled after index event increased gradually over the 5 years, with 18% (n=76) being dead or disabled at 1 month and 48% (n=179) 5 years post-event (p<0.001).
A logistic regression was used to determine the predictors of death or disability 5-years post-event (Table 3). The results from this analysis were broadly similar to those from the analysis evaluating the predictors of disability at 6 months. However, we found that previous history of atrial fibrillation (p=0.041) or diabetes (p=0.010) were significant predictors of death or disability at 5 years post-index event.
Institutionalisation in nursing or residential care settings
Information on living arrangements at 5 years was available for 395 (99%) surviving stroke and 317 (99%) TIA patients. Of these, 45 (11%) stroke and 28 (9%) TIA patients were institutionalised in nursing or residential care home settings. These findings, however, do not take into account those patients that were institutionalised after their index event and died before the end of the 5-year follow-up. As a result, we evaluated the temporal patterns of institutionalisation after index TIA or stroke using Kaplan-Meier survival techniques. For this analysis, we excluded a total of 31 (12 TIA and 19 stroke - 3%) patients who were living in a nursing or residential care home before their index event.
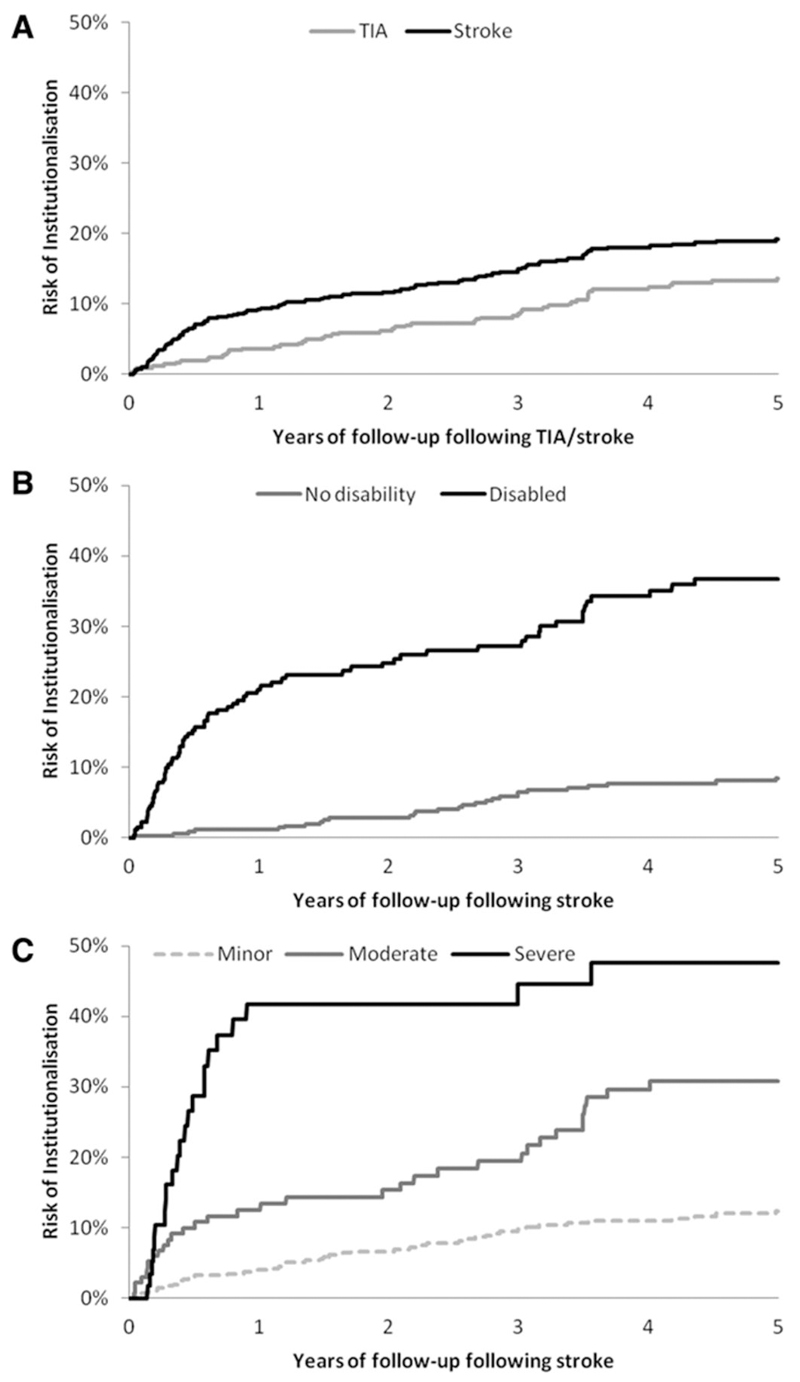
Figure 2 describes the proportion of patients institutionalised in a nursing or residential care home over the 5-year follow-up. A total of 102 stroke patients were institutionalised during the 5-year follow-up period compared with 51 TIA patients (hazard ratio (HR) 1.51, 95% CI: 1.08 to 2.12; p=0.016). Institutionalisation also occurred later for TIA patients (mean: 2.06 years, median: 2.03 years) than for stroke patients (mean: 0.86 years, median: 1.44 years). Over the 5 years the mean number of days spent in a nursing or residential care home for the 102 institutionalised stroke and 51 TIA patients was 774 (S.D. 562) and 616 (S.D. 414), respectively. Over the 5 years of follow-up, the average cost per institutionalised patient was $125,359 (S.D. 91,121) for stroke patients and $99,831 (S.D. 67,020) for TIA patients. Averaged across all patients in the study, the mean 5-year costs of institutionalisation were $17,093 (S.D. 54,551) for stroke patients and $11,572 (S.D. 39,181) for TIA patients.
Figure 2.
5-year risk of institutionalisation in: (A) all patients with TIA or stroke; (B) stroke patients disabled and not disabled at 1 month post-index event; and (C) minor, moderate, and severe stroke patients.
For stroke patients we also assessed the impact of disability (as measured at 1 month) on time to institutionalisation (Figure 2). By the end of the 5-year follow-up, over 35% (n=73) of stroke patients disabled at 1 month had been institutionalised compared with less than 10% (n=28) of non-disabled stroke patients (HR 5.92, 95% CI: 3.80 to 9.23; p<0.001). For those institutionalised, mean time to institutionalisation was also shorter in those disabled at 1 month (1.13 years; median: 0.49 years) than in those who were not (mean: 2.28 years; median: 2.46 years). Stroke severity was also found to be a significant univariate predictor of time to institutionalisation. The cumulative proportion of minor stroke patients institutionalised by the end of the 5-year follow-up was 12% (n=45), compared with 31% (n=33) for moderate stroke patients and 48% (n=23) for severe stroke patients. The risk of institutionalisation was significantly higher after moderate stroke than for minor stroke (HR 2.75, 95% CI: 1.75 to 4.33; p<0.001), and likewise for severe stroke when compared with moderate stroke (HR 2.11, 95% CI: 1.24 to 3.61; p=0.006).
Discussion
Our results show that at 5 years post stroke, approximately 47% of patients are dead, over one third of survivors are left disabled, leaving 70% of patients either dead or disabled 5 years after the index stroke. Our results are similar to those observed in previous population studies assessing long-term outcomes after stroke in Australia and New Zealand.6–8 Results from the Auckland Stroke Outcomes study showed that 5 years post-event 31% of stroke survivors had poor overall outcome (defined as mRS>2),8 and those from the Perth Community Study, which showed that 36% of stroke patients surviving to 5 years were disabled (also defined as mRS>2).6 Our results are also similar to those observed in the Oxford Community Stroke Project (OCSP),19 which was conducted in the same population as that in OXVASC during 1981-1984 but only included incident strokes. In OCSP disability levels at 1 and 12 months among stroke survivors were 44% and 36% respectively, compared to 43% and 37% in OXVASC.
Results from OXVASC show that there was little improvement in patient outcome during follow-up after stroke. Although disability levels fell significantly from 43% one month post-stroke to 37% at 6 months, this observed improvement was mainly due to death of the most disabled cases, with disability levels of over 35% being observed at the 1-, 2-, and 5-year follow-ups. For TIA patients, disability levels rose gradually over time in part because of patient aging and subsequent stroke and coronary events, with approximately one quarter of surviving TIA patients disabled at 5 years.
We found that, after controlling for other characteristics, being previously disabled, event severity, age, subsequent strokes and coronary events, employment status and marital status predicted 6-month disability. These findings further highlight the importance of timely secondary stroke prevention strategies, which not only reduce stroke recurrence but prevent a proportion of patients from becoming disabled.20 In addition, the finding that employed patients before the stroke and those living alone were significantly less likely to become disabled, after controlling for other factors including age, would suggest that encouraging patients to keep active might also reduce the progression to disability post-event.
Given the impact of stroke on a patient’s ability to conduct their activities of daily life, either through physical disability or cognitive impairment, stroke is a major cause of institutionalisation following acute care, 6–10 with patients in many cases requiring long periods of post-acute care or rehabilitation and then placement in long-term nursing or residential care. As opposed to other countries, such as the USA, where post-acute care and rehabilitation following acute stroke are provided in short-term nursing or rehabilitation facilities,21 in the UK this type of care is provided, in the vast majority of cases, in hospitals publicly funded by the National Health Service.11 As a result, nursing and residential care home admission in the UK is permanent in nature and commensurate to long-term institutionalisation in countries such as the USA. Although over 90% of this care is provided by private or voluntary organisations in the UK, around 60% of care home provision is funded by government either through local government authorities or the National Health Service.22
In OXVASC we found that for 5-year survivors, 11% of stroke and 9% of TIA patients were institutionalised in nursing or residential care settings. For stroke patients, these results are similar to those from other population-based studies, conducted in different countries, who have also evaluated the proportion of stroke survivors institutionalised in nursing and residential care home settings: 15% at 10 years post-event in Perth, Australia;7 15% at 5 years in Auckland, New Zealand;8 13% at 5 years in Erlangen, Germany;9 and 9% at 4 years in London, UK.10 Results from these population-based studies, including ours, have found lower proportions of stroke survivors in institutionalised care settings than those studies based in hospitalised stroke patients only,23–25 with some reporting proportions of over 40% of survivors living in institutionalised settings four years post-stroke.25
However, in contrast to other population-based studies, we also estimated the cumulative risk of institutionalisation during the 5 years after index stroke or TIA using Kaplan-Meier survival techniques. We found that the 5-year risk of institutionalisation after stroke was 19%, with an estimated average 5-year cost of $125,000 per institutionalised stroke patient. The risk of institutionalisation was especially high in patients disabled at 1 month, who faced a 37% 5-year risk of institutionalisation.
Despite the advantages of OXVASC, its limitations should also be noted. Firstly, the multivariate analyses performed to determine the predictors of 6-month disability and 5-year death or disability included a large number of covariates. With statistical significance being set at p<0.050, there was a 5% chance of a positive result when there was no real difference. Therefore, some of the significant results observed in these analyses might have occurred by chance. Finally, stroke and TIA are associated with old age and often occur in patients with other comorbidities.13 Therefore, it is likely that a proportion of patients would have been institutionalised regardless of event onset. Without a control group, we were therefore unable to estimate the true impact of stroke and TIA on institutionalisation.
In summary, our study reports up-to-date estimates of the long-term outcome after stroke and TIA using data from a population-based study. We show that long-term disability is common among survivors of stroke, and because of the risk of suffering subsequent strokes, also common for TIA patients. Our results also highlight the cumulative risk of institutionalisation following stroke, especially after disabling stroke. Therefore, efforts to prevent first and recurrent stroke are likely to generate substantial health and financial benefits.
Supplementary Material
Acknowledgements
We are grateful to all patients who took part in the study. We thank all primary care practices and physicians who collaborate with OXVASC.13 The comments from two anonymous referees are also acknowledged.
Sources of funding
RL-F is funded from an Economic and Social Research Council/Medical Research Council/National Institute for Health Research (NIHR) early career fellowship in economics of health. AMG is a NIHR Senior Investigator. PMR is a NIHR and Wellcome Trust Senior Investigator. The research was supported by the NIHR Biomedical Research Centre Programme, Oxford. OXVASC is funded by the UK Medical Research Council, the Dunhill Medical Trust, the Stroke Association and the Wellcome Trust. HERC obtains part of its funding from the NIHR.
Footnotes
Disclosures: None.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Epidemiology of Stroke. Cerebrovasc Dis. 1999;9:1–68. [Google Scholar]
- 3.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK, from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Van der Hoorn S. How to study stroke incidence. Lancet. 2004;363:1920. doi: 10.1016/S0140-6736(04)16436-2. [DOI] [PubMed] [Google Scholar]
- 5.Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 6.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-Term Disability After First-Ever Stroke and Related Prognostic Factors in the Perth Community Stroke Study 1989-1990. Stroke. 2002;33:1033–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- 7.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-Year Risk of First Recurrent Stroke and Disability After First-Ever Stroke in the Perth Community Stroke Study. Stroke. 2004;35:731–735. doi: 10.1161/01.STR.0000116183.50167.D9. [DOI] [PubMed] [Google Scholar]
- 8.Feigin VL, Barker-Collo S, Parag V, Senior H, Lawes CM, Ratnasabapathy Y, et al. Auckland Stroke Outcomes Study - Part 1: Gender, stroke types, ethnicity, and functional outcomes 5 years poststroke. Neurology. 2010;75:1597–1607. doi: 10.1212/WNL.0b013e3181fb44b3. [DOI] [PubMed] [Google Scholar]
- 9.Liman TG, Heuschmann PU, Endres M, Floel A, Schwab S, Kolominsky-Rabas PL. Impact of low mini-mental status on health outcome up to 5 years after stroke: the Erlangen Stroke Project. J Neurol. 2012;259:1125–1130. doi: 10.1007/s00415-011-6312-6. [DOI] [PubMed] [Google Scholar]
- 10.Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA. Natural history, predictors, and associations of depression 5 years after stroke: The South London stroke register. Stroke. 2011;42:1907–1911. doi: 10.1161/STROKEAHA.110.605808. [DOI] [PubMed] [Google Scholar]
- 11.Ribbe MW, Ljunggren G, Steel K, Topinková E, Hawes C, Ikegami N, et al. Nursing homes in 10 nations: a comparison between countries and settings. Age Ageing. 1997;26(Suppl 2):3–12. doi: 10.1093/ageing/26.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 12.Hankey GJ. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16(suppl 1):14–19. doi: 10.1159/000069936. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories. Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 14.Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM. Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke. 2004;35:2041–2047. doi: 10.1161/01.STR.0000137605.48864.2f. [DOI] [PubMed] [Google Scholar]
- 15.Luengo-Fernandez R, Gray AM, Rothwell PM. A population-based study of hospital care costs during five years after TIA and stroke. Stroke. 2012;43:3343–3351. doi: 10.1161/STROKEAHA.112.667204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox D. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:184–220. [Google Scholar]
- 17.Organisation for Economic Co-operation and Development (OECD) PPPs and Exchange Rates. [Accessed May 17, 2013]; http://stats.oecd.org/Index.aspx?DatasetCode=SNA_TABLE4
- 18.Curtis L. Personal Social Services Research Unit; University of Kent: 2011. Unit costs of health and social care 2011. [Google Scholar]
- 19.Bamford J, Sandercock P, Dennis M, Warlow C, Burn J. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 20.Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol. 2009;8:235–243. doi: 10.1016/S1474-4422(09)70019-5. [DOI] [PubMed] [Google Scholar]
- 21.Rundek T, Mast H, Hartmann A, Boden-Albala B, Lennihan L, Lin IF, et al. Predictors of resource use after acute hospitalization: The Northern Manhattan Stroke Study. Neurology. 2000;55:1180–1187. doi: 10.1212/wnl.55.8.1180. [DOI] [PubMed] [Google Scholar]
- 22.Forder J, Allan S. Competition in the care homes market. Office for Health Economics; London: 2011. [Google Scholar]
- 23.Walsh T, Donnelly S, Carew S, O'Connor C, O'Riordan R, Lyons D. Stroke unit care: recurrence, mortality and institutionalisation rates: a four year follow-up study. Ir J Med Sci. 2008;177:135–139. doi: 10.1007/s11845-007-0110-2. [DOI] [PubMed] [Google Scholar]
- 24.Portelli R, Lowe D, Irwin D, Pearson M, Rudd AG. Institutionalization after stroke. Clin Rehabil. 2005;19:97–108. doi: 10.1191/0269215505cr822oa. [DOI] [PubMed] [Google Scholar]
- 25.Brodaty H, Altendorf A, Withall A, Sachdev PS. Mortality and Institutionalization in Early Survivors of Stroke: The Effects of Cognition, Vascular Mild Cognitive Impairment, and Vascular Dementia. J Stroke Cerebrovasc Dis. 2010;19:485–493. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.