Abstract
Personal battery-powered vaporizers or electronic cigarettes were developed to deliver a nicotine vapor such that smokers could simulate smoking tobacco without the inherent pathology of inhaled tobacco smoke. Electronic cigarettes and their e-cigarette liquid formulations are virtually unregulated. These formulations are typically composed of propylene glycol and/or glycerin, flavoring components and an active drug, such as nicotine. Twenty-seven e-cigarette liquid formulations that contain nicotine between 6 and 22 mg/L were acquired within the USA and analyzed by various methods to determine their contents. They were screened by Direct Analysis in Real Time™ Mass Spectrometry (DART-MS). Nicotine was confirmed and quantitated by high-performance liquid chromatography–tandem mass spectrometry, and the glycol composition was confirmed and quantitated by gas chromatography–mass spectrometry. The DART-MS screening method was able to consistently identify the exact mass peaks resulting from the protonated molecular ion of nicotine, glycol and a number of flavor additives within 5 mmu. Nicotine concentrations were determined to range from 45 to 131% of the stated label concentration, with 18 of the 27 have >10% variance. Glycol composition was generally accurate to the product description, with only one exception where the propylene glycol to glycerin percentage ratio was stated as 50:50 and the determined concentration of propylene glycol to glycerin was 81:19 (% v/v). No unlabeled glycols were detected in these formulations.
Introduction
Electronic cigarette devices have received widespread media attention and have rapidly increased in popularity across the USA and worldwide (1–3). They are known by a variety of names, including e-cigarettes, e-hookahs, vape pens, personal vaporizers, and electronic nicotine delivery systems (ENDS). All devices operate on the same basic principle: they produce a condensation aerosol for inhalation by the user. The devices have a liquid reservoir that is either a refillable tank system or a preassembled cartridge, with a wicking material that draws the liquid to the atomizer. The atomizer consists of a coil made of resistance wire that an electrical current is passed through, which quickly generates intense heat used to atomize the liquid. The current is produced by the activation of a battery, either manually by depressing a button or automatically by a sensor that detects negative pressure generated from the user inhaling. The liquid is vaporized and condenses into an aerosol, which is inhaled by the user through the mouthpiece. The first generation of e-cigarettes mimicked the appearance of a traditional cigarette, using a disposable cartridge system. Newer generations use refillable tanks, with a cartridge that houses the e-formulation liquid and the wicking material. These newer generations of e-cigarettes feature more customizable components, such as the ability to build custom coils, refill tanks and vary voltage output on batteries.
The e-cigarette liquid formulations are composed of a humectant, various flavoring components and an active drug or drugs. The humectants often consist of propylene glycol and/or vegetable glycerin. The flavorant compounds are added in combinations to create flavors that mimic anything from a traditional cigarette to candy. The advertised active ingredients may include nicotine, vitamins, caffeine, cannabidiol, tetrahydrocannabinol or any number of compounds. In the case of nicotine, the labeled concentration typically ranges between 0 and 36 mg/mL. The most commonly found active ingredient in these e-cigarette liquid formulations is nicotine.
Between 1 January 2015 and 31 October 2015, the American Association of Poison Control Centers has reported 2,689 exposures to e-cigarettes and liquid nicotine, compared with 3,783 in 2014, 1,543 in 2013, 460 in 2012, and 271 in 2011 (4). The Food and Drug Administration (FDA) has proposed that e-cigarettes are added to the Federal Food, Drug and Cosmetic Act. These products are presently unregulated. Additionally, the FDA has issued alerts in recent years to be wary of the contamination of glycols coming into the USA, especially from China, as they have been found to contain contaminants such as diethylene glycol (DEG) (5, 6). These glycols may find their way into e-cigarette liquid formulations.
Presented is the identification and quantitation of nicotine and glycols in 27 e-cigarette liquid formulations. All formulations used in this study were purportedly manufactured in the USA and were purchased from vendors within the USA. A Direct Analysis in Real Time™ ionization source coupled to a JEOL JMS-T100LC AccuTOF™ mass spectrometer (JEOL USA, Inc., Peabody, MA) (DART-MS) was used for the initial screening of these formulations for humectant and active ingredients. The DART-MS uses an open air ion source and is a rapid technique requiring little to no sample preparation.
Two previously validated methods were employed to confirm and quantitate nicotine and glycols in the liquid formulations. A 3200 Q Trap (Applied Biosystems, Foster City CA) attached to a SCL HPLC system (Shimadzu, Columbia, MD) high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS-MS) system was used for the confirmation and quantitation nicotine. A 6890 Gas Chromatograph coupled to a 5973 Mass Spectrometer Detector (Hewlett Packard, Santa Clara, CA) (GC–MS) was used to determine the composition of the humectants and the ratios of propylene glycol to glycerin, as well as to detect toxic glycols such as diethylene glycol.
Experimental
Reagents
Nicotine and nicotine-d4 reference standards were purchased from Sigma-Aldrich (St. Louis, MO). HPLC-grade methanol was purchased from Pharmco-Aaper (Brookfield, CT) and used for all dilutions and preparations of stock and working solutions. Polyethylene glycol (PEG) with an average molecular mass of 600 Da was used for DART-MS calibration and obtained from ULTRA Inc. (North Kingstown, RI). Nitrogen and helium gases were acquired from Praxair and Airgas (Richmond, VA). The HPLC-grade methanol, HPLC-grade water and ammonium formate comprising the HPLC mobile phase were purchased from Fisher Scientific (Pittsburgh, PA). Propylene glycol and glycerin used as primary reference materials were purchased from Wizard Labs (Orlando, FL). The 27 e-cigarette liquid formulations were randomly purchased in ‘vape shops’ located in the mid-Atlantic, the mid-West and West coast and via the Internet (Table I). Products were selected on the basis of being manufactured in the USA, as reported or suggested by the vendor.
Table I.
Vendors and Product Names of Twenty-Seven e-liquids
| Vendor | Flavor |
|---|---|
| Adirondack | Delta |
| Bombies | White Gummy Bear |
| Bryce's | Vanilla Cream Custard |
| Coval Vapes | Mayflower |
| Five Pawns | Grandmaster |
| Good Life Vapor | El Kamino |
| Gremlin Juice | Birthday Cake |
| Gremlin Juice | Kentucky Mint Julip |
| Gremlin Juice | Vanilla Custard |
| Indigo Vapor | Birthday Cake |
| Indigo Vapor | Captain Ron |
| Indigo Vapor | Sunset |
| Janty | FennetHIGH |
| Juice Mafia | Peach Tobacco |
| Juice Mafia | Turkish Tobacco |
| Mt. Baker | GWAR Spew |
| S&S Mods | Grumpy's Hooch |
| Seduce Juice | Jango |
| Seduce Juice | Pharaoh |
| Seduce Juice | Snake Eyes |
| Seduce Juice | Snake Oil |
| StLVapor | Spearmint |
| Supreme Nicotine | 258 Rally Squirrel |
| Top Vapor | Unflavored PG |
| VapeWell | Cheery |
| Velvet Cloud Vapor | Vanilla Tobacco |
| Wizard Labs | VG (12 mg/mL) |
Analysis of the electronic cigarette liquid formulations
The e-cigarette liquid formulations were screened using a DART-MS with a resolving power of 6000 FWHM operated at a helium gas temperature of 300°C with a flow rate of 2 L/min. The orifice 1 was operated in function switching mode, which alters the voltages from 20, 30, 60 and 90 V every 0.25 s. The orifice 2 voltage was fixed at 5 V. The ion guide voltage was set to 400 V, and the detector was set to 2,000 V. The system was operated in positive ion mode with a mass range of 40–1,000 m/z. The system was controlled using the Mass Center application for data acquisition. Samples were introduced into the DART-MS stream as previously described by Poklis et al. (7). In brief, the closed end of a melting point capillary tube was dipped into the sample and then wanded into the gas stream between the ionization source and the mass spectrometer. PEG was used as an internal calibration within each data file. This process was completed twice for each formulation, and the spectra were averaged and centroided. The peak on the total ion chromatogram with the greatest abundance was selected for analysis. The spectra were compared with the spectra of the primary references materials of propylene glycol, vegetable glycerin and nicotine. While flavorants were not specifically evaluated, several major compounds used to flavor formulations were noted. All compounds were identified if their masses were detected within 5 mmu of their calculated monoisotopic masses.
The nicotine concentrations of the e-cigarette liquid formulations were determined using HPLC–MS-MS with a Hypersil® Gold 3 × 50 mm, 5 µm column (Thermo Scientific, Waltham, MA). The mobile phase consisted of 1:9 DI water with 10 mmol ammonium acetate and 0.1% formic acid–methanol and was run at an isocratic flow rate of 0.5 mL/min. The injection volume was 10 µL. The ion spray voltage was 5,000 V with a declustering potential of 35 eV, and the source temperature was 600°C with 30 mL/min curtain gas flow. Ion source gas 1 was set at 50 mL/min, and ion source gas 2 was set at 30 mL/min. The instrument was operated in multiple reaction monitoring mode (MRM) monitoring the following m/z transitions: nicotine, 163 > 130 and 163 > 117 and nicotine-d4, 167 > 134. The total run time was 2 min. The e-cigarette liquid formulations were diluted 1:20,000 to 1:50,000 in 1:9 water–methanol to bring the nicotine concentration into the range of the seven-point nicotine calibration curve from 10 to 1,000 ng/mL (r2 > 0.9985 for all curves). Nicotine-d4 at a concentration of 250 ng/mL was used as an internal standard. A linear regression was plotted of the peak area ratio of nicotine to internal standard versus nicotine concentration. The limit of quantitation was administratively set at 10 ng/mL. Three sample batches were used to evaluate the accuracy and precision of the HPLC–MS-MS method. Six controls were included with each analytical batch: a blank, a double blank, LOQC (10 ng/mL), LQC (30 ng/mL), MQC (300 ng/mL) and HQC (900 ng/mL). Intraday (within-run) accuracy and precision were determined by taking the largest percent coefficient of variation (% CV) and most extreme accuracies for each control concentration out of each of the three runs (n = 6). Accuracy was determined to be between 96 and 103%, and interday precision was determined to be between 6 and 11% for all quality control samples. The intraday bias was determined to be 95–109% with a precision of 7–16% for all quality control samples. Carryover was assessed by running a nicotine-free negative control immediately following the highest concentration calibrator (1,000 ng/mL). Nicotine was not detected in the negative control. Additionally, injection of the LQC (30 ng/mL) always followed injection of the HQC (900 ng/mL), which resulted in no bias in the LQC. E-liquid cigarette formulations were diluted and analyzed on three separate days.
The glycol compositions of the e-cigarette liquid formulations were determined using a method for glycol analysis in toothpaste used by the FDA (8). In brief, a GC–MS with a 30 m Stabilwax of 0.25 mm id × 0.25 µm df (Restek, Bellefonte, PA) with a 5-m retention gap was used for this analysis. The GC–MS was operated in split injection mode at a ratio of 20:1. The carrier gas was helium at a constant flow of 35 cm/s. Oven temperature programming was 100°C (1 min) to 250°C (4 min) at a ramp of 10°C/min. The injection volume was 1 µL. The scan range for the mass spectrometer was 29–400 m/z. The total run time was 20 min. The e-cigarette liquid formulations were diluted 1:10 in deionized water and were then diluted 1:2 with 1 mg/mL of 2,3-butanediol as internal standard. Calibrators were prepared at 50, 100 and 200 mg/mL of propylene glycol and glycerin in water. A linear regression was plotted of the peak area ratio of glycols to internal standard versus glycol concentration. The limit of quantitation was administratively set at 1 ng/mL.
Results
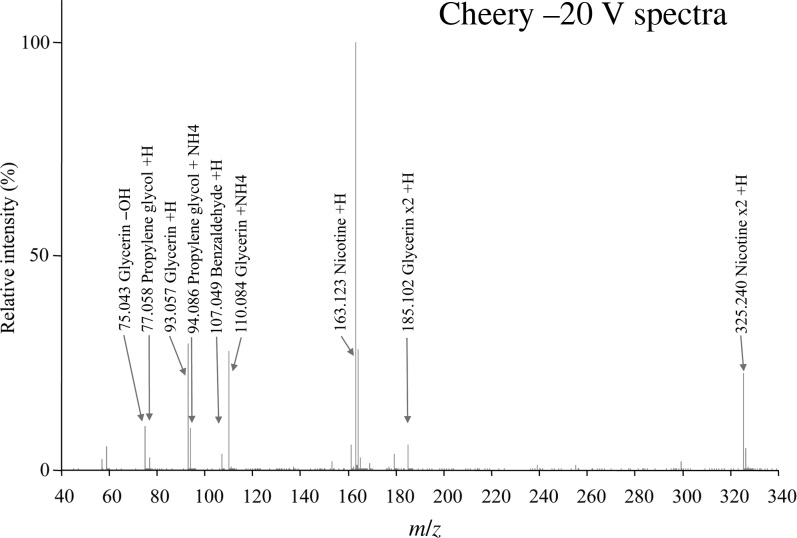
The analysis of the e-cigarette liquid formulations resulted in the identification of propylene glycol, glycerin, nicotine and a number of flavorant compounds by using DART-MS (Figure 1). The protonated nicotine molecule was always present as the base peak in these spectra. Nicotine formed a dimer, which was prominent in these spectra, and dimers were also present for propylene glycol and glycerin. The adduct ions used to help identify propylene glycol, glycerin and nicotine are given in Table II. The e-cigarette liquid formulations were determined to have 45–131% of their labeled nicotine concentration. Of 27 formulations, 18 were run in triplicate and the remainder in duplicate. All samples had a %CV value of ≤16. Of the 27, 18 had a greater than or equal to a 10% difference from their labeled concentrations, with 9 being greater than a 20% difference (Table III). The glycol composition of the e-liquid formulations ranged from pure propylene glycol to pure glycerin, with the most common ratio being an approximate 1:1 mixture of both. Of the 27 e-cigarette liquid formulations, 6 did not describe the contents of the humectant, whereas the remaining 21 formulations listed propylene glycol and/or glycerin contents. Seventeen of these were within ±10% of the product description (% v/v). The remaining four were determined to have a >10% difference from the 50:50 listed propylene glycol-to-glycerin ratio. The greatest difference in concentration of propylene glycol to glycerin was reported as 50:50 (% v/v) but determined to be 81:19 (Table IV). No other glycols were detected other than propylene glycol and glycerin in the 27 e-cigarette liquid formulations. Eight flavorants, benzaldehyde, carvone, ethyl homovanillate, methyl anthranilate, methyl salicylate/vanillin and ethyl vanillin, were easily identified.
Figure 1.
DART-MS 20 V spectra of the e-cigarette liquid formulations named Cheery.
Table II.
DART-MS Commonly Seen Ions at 20 V
| Compound | Adduct | Formula | Monoisotopic Mass |
|---|---|---|---|
| Propylene glycol | −OH | C3H7O | 59.050 |
| Propylene glycol | +H | C3H9O2 | 77.060 |
| Propylene glycol | +NH4 | C3H12O2N | 94.087 |
| Propylene glycol | x2 − OH | C6H15O3 | 135.102 |
| Propylene glycol | x2 + H | C6H17O4 | 153.113 |
| Propylene glycol | x2 + NH4 | C6H20O4N | 170.139 |
| Propylene glycol | x3 − OH | C9H24O5 | 211.155 |
| Glycerin | −OH | C3H7O2 | 75.045 |
| Glycerin | +H | C3H9O3 | 93.055 |
| Glycerin | +NH4 | C3H12O3N | 110.082 |
| Glycerin | x2 + H | C6H17O6 | 185.103 |
| Glycerin | x2 + NH4 | C6H20O6N | 202.129 |
| Nicotine | +H | C10H15N2 | 163.124 |
| Nicotine | x2 + H | C20H29N4 | 325.239 |
The –OH ‘adduct’ is characterized by a neutral loss of water followed by protonation.
Table III.
Nicotine Concentrations of e-liquid Formulations
| Brand/flavor | Label (mg/mL) | Actual (mg/mL) | Percent Difference |
|---|---|---|---|
| 258 Rally Squirrel | 16 | 10.3 | −36 |
| Captain Ron | 12 | 11.0 | −8 |
| Cheery | 18 | 14.7 | −18 |
| Delta | 12 | 7.7 | −36 |
| El Kamino | 12 | 8.7 | −27 |
| FennetHIGH | 12 | 12.4 | 3 |
| Grandmaster | 6 | 7.8 | 31 |
| Gremlin Juice Birthday Cake | 12 | 11.6 | −3 |
| Grumpy's Hooch | 12 | 9.0 | −25 |
| GWAR Spew | 12 | 13.3 | 11 |
| Indigo Birthday Cake | 12 | 10.8 | −10 |
| Jango | 12 | 12.6 | 5 |
| Kentucky Mint Julip | 6 | 6.3 | 5 |
| Mayflower | 6 | 4.3 | −18 |
| Peach Tobacco | 12 | 8.9 | −26 |
| Pharaoh | 12 | 10.7 | −11 |
| Snake Eyes | 12 | 10.1 | −16 |
| Snake Oil | 12 | 10.5 | −13 |
| Spearmint | 22 | 10 | −55 |
| Sunset | 6 | 6.0 | 0 |
| Turkish Tobacco | 12 | 11.2 | −7 |
| Unflavored PG | 6 | 8.3 | 39 |
| Vanilla Cream Custard | 6 | 5.9 | −1 |
| Vanilla Custard | 12 | 9.1 | −24 |
| Vanilla Tobacco | 6 | 6.5 | 8 |
| VG (12 mg/mL) | 12 | 10.3 | −15 |
| White Gummy Bear | 6 | 5.0 | −17 |
Table IV.
Glycol Composition of e-liquids
| E-liquid |
Measured
|
Labeled
|
||
|---|---|---|---|---|
| % PG | % G | % PG | % G | |
| 258 Rally Squirrel | 60 | 40 | ||
| Captain Ron | 45 | 55 | 50 | 50 |
| Cheery | 33 | 67 | ||
| Delta | 28 | 72 | 50 | 50 |
| El Kamino | 46 | 54 | ||
| FennetHIGH | 100 | 0 | ||
| Grandmaster | 67 | 33 | 50 | 50 |
| Gremlin Juice Birthday Cake | 46 | 54 | 50 | 50 |
| Grumpy's Hooch | 55 | 45 | 50 | 50 |
| GWAR Spew | 48 | 52 | 50 | 50 |
| Indigo Birthday Cake | 46 | 54 | 50 | 50 |
| Jango | 49 | 51 | 50 | 50 |
| Kentucky Mint Julip | 54 | 46 | 50 | 50 |
| Mayflower | 22 | 78 | 30 | 70 |
| Peach Tobacco | 43 | 57 | 50 | 50 |
| Pharaoh | 61 | 39 | 50 | 50 |
| Snake Eyes | 47 | 53 | 50 | 50 |
| Snake Oil | 54 | 46 | 50 | 50 |
| Spearmint | 51 | 49 | ||
| Sunset | 43 | 57 | 50 | 50 |
| Turkish Tobacco | 81 | 19 | 50 | 50 |
| Unflavored PG | 100 | 0 | 100 | 0 |
| Vanilla Cream Custard | 42 | 58 | ||
| Vanilla Custard | 48 | 52 | 50 | 50 |
| Vanilla Tobacco | 100 | 100 | ||
| VG (12 mg/mL) | 100 | 100 | ||
| White Gummy Bear | 100 | |||
PG, propylene glycol; G, glycerin.
Discussion
A number of studies have tested the nicotine concentrations in cartridges and e-cigarette liquid formulations for e-cigarettes (9–13). This study looked at products obtained solely in the USA. Previously published studies evaluated e-cigarette liquid formulations predominantly obtained and manufactured in other counties. Etter et al. analyzed electronic cigarette refill liquids for nicotine, nicotine degradation products, diethylene glycol and ethylene glycol on a number of formulations from five different countries (9). Two studies by Goniewicz et al. (10, 11) explore the nicotine concentration of electronic cigarette liquids in cartridges and refill formulations from the UK, US and Poland. Trehy et al. (12) analyzed a variety of cartridges for both nicotine and impurities related to nicotine. Lisko et al. (13) analyzed e-cigarette refill liquids and cartridges for not only nicotine but also nicotine-related impurities and a handful of flavorant compounds. A recent study by Kavvalakis et al. (14) analyzed multiple components, including nicotine, flavors and glycols, of a large number of e-liquids from the Greek market. Pagano et al. evaluated products solely acquired in the USA. They evaluated five disposable electronic cigarettes obtained in the USA, one of which was a product manufactured in the USA and four in China (15).
The correlation between the labeled and measured nicotine concentrations is similar to the reported results by Kavvalakis et al., who studied 263 e-liquids purchased in Greece (14). The variability observed in this study was also consistent with the other studies on the subject (9–13), with most formulations having slightly less nicotine than stated on the product label. Pagano et al. determined the concentration of nicotine as a function of the disposable electronic cigarette (mg/E-Cig), and they conclude that their findings for products obtained in the USA are similar to those of the international survey of cartridges and refill formulations performed by Goniewicz et al. as this study has done. While a substantially higher nicotine concentration from the stated label concentration was found in some of the formulations, this is not enough in itself to make them significantly more dangerous when used as intended. However, from the perspective of e-cigarettes being used as a potential nicotine replacement therapy, those differences in concentration may be important to the consumer.
Previously, reports of the analysis of e-cigarette liquid formulations have noted that the humectants consist of propylene glycol and/or vegetable glycerin. All 27 e-cigarette liquid formulations of the analyzed contained propylene glycol and/or vegetable glycerin. Of the 27 e-cigarette liquid formulations with identified flavorants, 12 included at one or more of the following vanillin/methyl salicylate, ethyl vanillin and ethyl homovanillate. Of the 27 e-cigarette liquid formulations in this study, 26% contained ethyl vanillin compared with the studies of Kavvalakis et al. who reported 18.6% in 263 e-cigarette liquid formulations and Hutzler et al. who reported 141 different identified flavorants in 28 e-cigarette liquid formulations with 50% ethyl vanillin in their formulations (14, 16).
Conclusion
A greater than 20% difference in labeled versus actual nicotine concentrations was found in 9 of the 27 e-cigarette liquid formulations and 4 listed the propylene glycol to glycerin percentage ratio as 50:50 and were determined to have greater than 10% expected difference. These findings agree with previously published nicotine and glycol variations between labeled and measured concentrations. Given that the e-cigarette industry is unregulated, it should not be unexpected to find nicotine or glycol concentrations different from the labeled concentrations on e-cigarette liquid formulations. However, it is still expected that propylene glycol and/or glycerin in the presence of drug and/or flavorants would be present in an e-cigarette liquid formulation.
Funding
This project was supported by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice (award no. 2014-R2-CX-K010) and the National Institutes of Health (award no. P30DA033934). The opinions, findings and conclusions or recommendations expressed in this publication/program/exhibition are those of the author(s) and do not necessarily reflect those of the Department of Justice.
References
- 1.Adkison S.E., O'Connor R.J., Bansal-Travers M., Hyland A., Borland R., Yong H.H. et al. (2013) Electronic nicotine delivery systems: international tobacco control four-country survey. American Journal of Preventive Medicine, 44, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi K., Fabian L., Mottey N., Corbett A., Forster J. (2012) Young adults’ favorable perceptions of snus, dissolvable tobacco products, and electronic cigarettes: findings from a focus group study. American Journal of Public Health, 102, 2088–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flouris A.D., Oikonomou D.N. (2010) Electronic cigarettes: miracle or menace. British Medical Journal, 340, c311. [DOI] [PubMed] [Google Scholar]
- 4.American Association of Poison Control Centers Electronic Cigarettes and Liquid Nicotine Data (2015). http://www.aapcc.org/alerts/e-cigarettes/ (accessed November 20, 2015).
- 5.Lang L. (2007) FDA issues statement on diethylene glycol and melamine food contamination. Gastroenterology, 133, 5–6. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration Import Alert 55–02: Increased Surveillance of Bulk Ingredients Susceptible To Contamination with Diethylene Glycol (2013). http://www.accessdata.fda.gov/cms_ia/importalert_145.html (accessed April 10, 2015).
- 7.Poklis J.L., Raso S.A., Alford K.N., Poklis A., Peace M.R. (2015) Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and other dimethoxyphenyl-N-[(2-methoxyphenyl) methyl]ethanamine derivatives on blotter paper. Journal of Analytical Toxicology, 39, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration, Gas Chromatography–Mass Spectrometry (GC–MS) Screening Procedure for the Presence of Diethylene Glycol and Ethylene Glycol in Toothpaste (2007). http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm113209.htm (accessed October 16, 2014).
- 9.Etter J.F., Zather E., Svensson S. (2013) Analysis of refill liquids for electronic cigarettes. Addiction, 108, 1671–1679. [DOI] [PubMed] [Google Scholar]
- 10.Goniewicz M.L., Hajek P., McRobbie H. (2014) Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction, 109, 500–507. [DOI] [PubMed] [Google Scholar]
- 11.Goniewicz M.L., Kuma T., Gawron M., Knysak J., Kosmider L. (2013) Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research, 15, 158–166. [DOI] [PubMed] [Google Scholar]
- 12.Trehy M.L., Ye W., Hadwiger M.E., Moore T.W., Allgire J.F., Woodruff J.T. et al. (2011) Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography & Related Technologies, 34, 1442–1458. [Google Scholar]
- 13.Lisko J.G., Tran H., Stanfill S.B., Blount B.C., Watson C.H. (2015) Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in E-cigarette cartridges and refill solutions. Nicotine & Tobacco Research, 17, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavvalakis M.P., Stivaktakis P.D., Tzatzarakis M.N., Kouretas D., Liesivuori J., Alegakis A.K. et al. (2015) Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. Journal of Analytical Toxicology, 39, 262–269. [DOI] [PubMed] [Google Scholar]
- 15.Pagano T., DiFrancesco A.G., Smith S.B., George J., Wink G., Rahman I. et al. (2016) Determination of nicotine content and delivery in disposable electronic cigarettes available in the United States by gas chromatography–mass spectrometry. Nicotine & Tobacco Research, 18, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutzler C., Paschke M., Kruchinskis S., Henkler F., Hahn J., Luch A. (2014) Chemical hazards presented in liquids and vapors of electronic cigarettes. Archives of Toxicology, 88, 1295–1308. [DOI] [PubMed] [Google Scholar]