Abstract
Corticostriatal circuitry supports flexible reward learning and emotional behavior from the critical neurodevelopmental stage of adolescence through adulthood. It is still poorly understood how prescription drug exposure in adolescence may impact these outcomes in the long-term. We studied adolescent methylphenidate (MPH) and fluoxetine (FLX) exposure in rats and their impact on learning and emotion in adulthood. In Experiment 1, male and female rats were administered MPH, FLX, or saline (SAL), and compared with methamphetamine (mAMPH) treatment beginning in postnatal day (PND) 37. The rats were then tested on discrimination and reversal learning in adulthood. In Experiment 2, animals were administered MPH or SAL also beginning in PND 37 and later tested in adulthood for anxiety levels. In Experiment 3, we analyzed striatal dopamine D1 and D2 receptor expression in adulthood following either extensive learning (after Experiment 1) or more brief emotional measures (after Experiment 2). We found sex differences in discrimination learning and attenuated reversal learning after MPH and only sex differences in adulthood anxiety. In learners, there was enhanced striatal D1, but not D2, after either adolescent MPH or mAMPH. Lastly, also in learners, there was a sex x treatment group interaction for D2, but not D1, driven by the MPH-pretreated females, who expressed significantly higher D2 levels compared to SAL. These results show enduring effects of adolescent MPH on reversal learning in rats. Developmental psychostimulant exposure may interact with learning to enhance D1 expression in adulthood, and affect D2 expression in a sex-dependent manner.
Keywords: psychostimulants, dopamine receptors, sex, reversal learning, anxiety, striatum
Introduction
The adolescent period is characterized by increased risk-taking, reward-seeking, and an enhanced need for environmental stimulation (Kelley et al. 2004; Laviola et al. 2003; Marco et al. 2011); characteristics that likely evolved to promote skills for independence (Spear 2000). Changes in mesocorticolimbic dopamine (DA) signaling provide much of the basis for this behavioral phenotype. DA D1 receptor (D1) and D2 receptor (D2) densities in the striatum peak at the onset of the rat adolescent period (postnatal day, PND 28) but decrease with maturity (Tarazi and Baldessarini, 2000; Gelbard et al. 1989; Teicher et al. 1995). We recently reported reduced D1 expression, and unaltered D2 expression, in the striatum of animals that had experiences with reward learning during adolescence, when compared to animals that went through the same learning in adulthood (Stolyarova and Izquierdo 2015). This suggests that learning and the experience of cognitive training may interact with neural maturation processes to shape long-lasting expression profiles of, in particular, D1 receptors (Wass et al. 2013). Exposure to psychostimulants may also cause robust changes to DA receptors in the developing brain that manifest in long-lasting effects on learning and behavior in adulthood. Adolescent rats treated for 2 months with ADHD medication methylphenidate (MPH, 1 and 2 mg/kg) beginning on PND 30 show significantly reduced D2 binding compared to vehicle-treated rats, as measured by microPET (Thanos et al. 2007). This is likely meaningful to behavior since low striatal D2 availability has been associated with poor reversal learning and addiction vulnerability (Izquierdo and Jentsch 2012).
The administration of prescription drugs to adolescents is at an all time high (Zito et al. 2000; Miech et al. 2015). Some of the most commonly-prescribed are MPH for ADHD (Shanks et al. 2015; Caprioli et al. 2015; Crawford et al. 2011; Gray et al. 2007), and fluoxetine (FLX) for the treatment of Major Depression (Iñiguez et al. 2010; Iñiguez et al. 2014; Homberg et al. 2011). MPH exhibits a similar pharmacological profile to amphetamines and cocaine, may modulate neurodevelopment (Grund et al. 2007; Thanos et al. 2007; Adriani et al. 2006), and by extension, may impact learning and behavior mediated by corticostriatal circuitry. There is evidence for long-term effects of adolescent MPH on adult locomotor behavior and addiction vulnerability. These effects include increased sensitization to later mAMPH (Shanks et al. 2015), increased cocaine abuse risk (Jordan et al. 2014), increased alcohol intake (Gill et al. 2014), and increased cocaine-induced reward and behavioral sensitization (Achat-Mendes et al. 2003) in adulthood (cf. Gray et al. 2007). The effects of adolescent FLX, conversely, appear limited to significant increases in anxious responding to emotion-eliciting stimuli (Iñiguez et al. 2010; Iñiguez et al. 2014; Homberg et al. 2011; cf. Norcross et al. 2008). There is relatively little known about the long-lasting effects of adolescent FLX exposure on later adult learning and behavior, though in the adult, FLX results in fewer errors in the early phase of reversal learning (Brigman et al. 2010).
To our knowledge there has not yet been a systematic comparison of the long-term effects of MPH and FLX on adulthood learning flexibility and associated striatal D1 and D2 receptor expression. Additionally, females have not typically been included in these investigations. Therefore, in the present experiments we investigated the effects of adolescent MPH and FLX on later adulthood learning flexibility, and we compared these effects with those following treatment with escalating doses of illicit drug methamphetamine (mAMPH; Experiment 1). In a separate group of animals, we then studied the effects of MPH on anxiety in adulthood (Experiment 2), to ascertain differential effects of MPH on learning experiences vs. emotional reactivity. And finally, in Experiment 3, we assessed striatal dopamine D1 and D2 expression in adulthood following drug treatment and either extensive learning (late adulthood, PND 140) or a more brief emotion test (early adulthood, PND 64). We predicted that adolescent exposure to MPH and mAMPH, but not FLX, would interact with DA receptor expression, to produce enduring effects in learning flexibility measures in adulthood.
Methods
Subjects
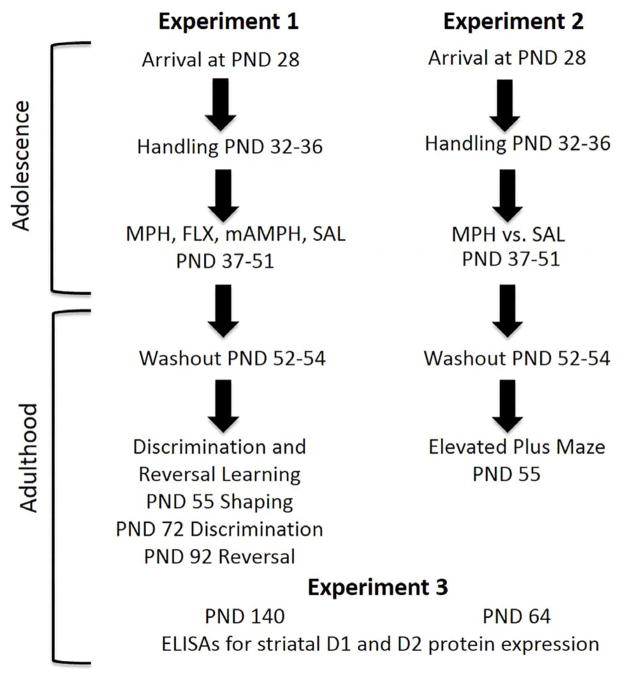
Male and female Long–Evans rats (Charles River Laboratories, Inc.) arrived at post-natal day (PND) 28, early rat adolescence (Spear 2000), weighing between 76 and 100g, and were socially housed two per cage with both males and females housed in the same room. All rats were habituated to the vivarium from PND 28 to 31. The rats had unrestricted access to food until behavioral testing began and were provided water ad libitum. Rats in Experiment 1 were maintained on a restricted diet during learning (85% of free-feeding weight). We have previously shown that this food scheduling does not compromise the healthy development of young animals: the rats stay within vendor-provided weight ranges for normal growth (Stolyarova and Izquierdo 2015). Rats in Experiment 2 did not undergo food restriction and were assessed for social play behavior during drug treatment (data not reported here). The vivarium maintained a 12-h light/12-h dark cycle, with the temperature held constant at 22 °C. All drug treatment and behavioral testing took place between 0700 and 0900 h. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Chancellor’s Animal Research Committee of UCLA. Order of drug treatment, testing, and euthanasia for Experiments 1–3 are outlined in Figure 1.
Figure 1. Order of drug treatment, behavioral testing, and tissue collection for Experiments 1, 2 and 3.
Animals for both experiments arrived from the vendor at postnatal day (PND) 28 and were allowed to acclimate undisturbed from PND 32–36. Drug treatment began on PND 37. For Experiment 1, rats were administered either methylphenidate (MPH), fluoxetine (FLX), methamphetamine (mAMPH) or saline (SAL) from PND 37–51. This was followed by a brief period of wash-out and subsequent testing on discrimination and reversal learning. The rate of learning differed between animals, therefore mean PNDs are provided for discrimination and reversal learning onset. For Experiment 2, rats were administered either MPH or SAL from PND 37–51. Treatment was followed by a brief wash-out period and subsequent testing on the elevated plus maze. Experiment 3 involved ELISA assays for striatal D1 and D2 expression for rats in Experiments 1 and 2.
Handling and Drug Treatment
Each rat was handled for a minimum of 10 minutes once per day for 5 consecutive days starting on PND 32, prior to drug treatment. Following handling, rats began treatment at PND 37. Injections were administered once per day for 15 consecutive days. For Experiment 1, drug treatments consisted of MPH (methylphenidate hydrochloride, Sigma, St. Louis, MO; 3 mg/kg, or 1 mg/kg, s.c.), FLX (fluoxetine hydrochloride, Sigma, St Louis, MO; 5 mg/kg or 10 mg/kg, s.c.), each dissolved in physiological saline (SAL) solution, mAMPH (d-methamphetamine hydrochloride, Sigma, St. Louis, MO; 0.1–3.0 mg/kg s.c., increasing in 0.3mg/kg increments between days) administered as an escalating dose to more closely resemble human recreational consumption, or SAL. The mAMPH group was treated until day 10 of injections and received SAL for the remaining 5 days of injections. This was done to ensure the treatment reached its maximum escalating dose (3 mg/kg) at day 10, to remain more consistent with the duration of treatment in our previous-published study (Ye et al. 2014). However, mAMPH treatment in the present experiment was initiated 5 d earlier than in Ye et al (2014). MPH doses were selected to remain consistent with the range of doses previously published, which are known to produce clinically relevant levels of drug in the plasma (Crawford et al. 2011; Gerasimov et al. 2000). For Experiment 2, MPH dosing was selected based on Experiment 1, which showed no significant differences between high and low dose MPH groups on learning. Rats for Experiment 2 were randomly assigned to one of two groups: MPH (3mg/kg, n=8) or SAL (n=8). Injections for both experiments began at 0700 h. The order of injections was counterbalanced by rat identification number, treatment, and sex, and left/right placements of injections were rotated daily. During drug or SAL treatment, rats had access to food and water ad libitum.
Experiment 1
Subjects
Thirty two male (n=16) and female (n=16) Long-Evans rats were randomly assigned to one of six groups: MPH high dose (3mg/kg, n=6; 3 male, 3 female), MPH low dose (1 mg/kg, n=4; 2 male, 2 female), FLX high dose (10 mg/kg, n=6; 3 male, 3 female), FLX low dose (5 mg/kg, n=4; 2 male, 2 female), mAMPH escalating dose (.3 mg–3 mg/kg, n=6; 3 male, 3 female) and SAL (n=6; 3 male, 3 female).
Behavioral Testing Apparatus
Behavioral testing was conducted in eight operant conditioning chambers (Model 80604 Lafayette Instrument Co., Lafayette, IN) that were housed within sound- and light- attenuating cubicles. Each chamber was equipped with a house light, tone generator, video camera, and LCD touchscreen opposing the pellet dispenser. The pellet dispenser delivered single 45-mg dustless precision sucrose pellets. Modified software (ABET II TOUCH) controlled touchscreen stimuli presentation, tone generation, tray- and house-light illumination and pellet dispensation.
Learning
Pre-training
The pre-training protocol, similar to previously-published methods (Izquierdo et al. 2010; Kosheleff et al. 2012; Ochoa et al. 2015), consisted of a series of phases: Habituation, Initial Touch training (IT), Must Touch training (MT), Must Initiate training (MI), and Punish Incorrect training (PI) designed to train rats to nose-poke, initiate a trial, and discriminate between stimuli.
Discrimination and Reversal Learning
Detailed methodological descriptions also appear in recent publications (Stolyarova et al. 2014; Ochoa et al. 2015; Stolyarova and Izquierdo, 2015). Rats were presented with two novel, white, equiluminescent stimuli that differed only in shape with predetermined reinforcement contingencies. The software enabled either a reward event in the form of sucrose pellet dispensation, paired with house-light illumination and an auditory feedback, as a result of nosepoking the correct stimulus, or a punishment as a result of nosepoking the incorrect stimulus; the latter followed by a 10 s “time out” wherein rats were unable to initiate the next trial. If the rat committed an error and received a punishment, a correction trial was administered: this consisted of the same spatial (left/right) presentation of the stimulus until the rat nosepoked correctly. Spatial configuration of stimuli presentation occurred pseudorandomly, the stimulus could not have appeared on the same side of the screen more than three times in a row except during a correction trial. Stimulus assignment was counterbalanced across treatment groups. Criterion for advancement was 60 rewards at 85% correct responses within 45 min, on each of two consecutive days. Upon reaching criterion on this phase, the rats were tested on a reversal of the reward contingencies.
After all rats completed the reversal phase, rats were humanly euthanized with an intraperitoneal injection of Euthasol, decapitated, and their brains dissected over wet ice, frozen by immersion in isopentane, and stored at −80°C before homogenization. Brains were dissected to analyze D1 and D2 receptor expression (details in Experiment 3).
Data Analysis
Data were analyzed using SPSS software. Statistical significance was noted when p-values were equal to or less than 0.05. ANOVA was used to assess treatment group and sex differences on the number of sessions to criterion. For data spanning sessions, only the days commonly-experienced by all animals in discrimination and reversal learning were included in a repeated-measures ANOVA (rmANOVA). A within (session) and between (treatment group, sex) factors rmANOVA was conducted on discrimination learning and reversal learning percent correct. Where the assumptions of sphericity were violated, Greenhouse-Geisser p-value corrections were applied and reported (Epsilon < 0.75). Post hoc Dunnett t-tests were used to assess treatment group differences in the means of follow-up measures, such as the microstructure of reversal learning, including within-session measures of time to collect reward, time to initiate a trial, and time to respond to stimuli. Following a multivariate ANOVA to assess treatment group differences in the number of sessions to reach 50% performance (“at chance”) and the number of sessions to reach criterion from “at chance” performance, post hoc Dunnett t-tests were used. High and low dose FLX and MPH treatment groups were collapsed because there were no significant dose differences in session to criterion in discrimination learning: FLX high dose vs. FLX low dose [t(8)=0.932, p=0.379] and MPH high dose vs. MPH low dose [t(8)=0.000, p=1.000]. Nor were there dose differences in sessions to reach criterion on reversal learning: FLX high dose vs. FLX low dose [t(8)=−.151, p=0.884] and MPH high dose vs. MPH low dose [t(8)=−0.062, p=0.952]. Similarly, there were no dose differences in the rmANOVA analyses of performance across sessions. Therefore, the total number of rats per treatment group was as follows, FLX (n=10), MPH (n=10), mAMPH (n=6) and SAL (n=6).
Experiment 2
Subjects
Sixteen male (n=8) and female (n=8) Long–Evans rats were randomly assigned to one of two groups: MPH (3mg/kg, n=8) or SAL (n=8).
Elevated Plus Maze
After 15 consecutive days of injections, the animals were given a 3 d washout period before assessment on the elevated plus maze (EPM). Animals were moved from their vivarium housing room to a separate testing room where they were placed on an EPM apparatus. The EPM was a dark grey nonreflective metal maze (#60250, Stoelting Co.), and consisted of two open and two closed arms (50 cm length × 10 cm width) as well as a central platform (13 × 13 cm). The closed arms had 40 cm tall grey walls. The primary measure on this test included the time spent in the open arms, an index of anxiety-related behavior, and the number (frequency) of arm entries. The rat was placed in the center of the maze with access to 2 open and 2 closed arms for 5 minutes. The test was video recorded to allow for later scoring. For scoring, arm entry frequency was counted when all 4 paws of the rat were in the (open or closed) arm. All testing occurred in bright fluorescent light (>100 lux). Behaviors were scored by two raters blind to treatment condition. The testing apparatus was wiped clean between subjects with 70% ethanol. High interrater reliability was established (r’s >0.802, p values <0.01), and raters’ means of duration and frequency were used for analyses.
Data Analysis
ANOVA was conducted to assess the effect of sex and treatment group on the duration in each arm and frequency of each arm entry. Where significant interactions were found, post-hoc simple main effects are reported.
Experiment 3
Tissue Dissection
Rats from Experiment 1 were euthanized 9–12 d after the last day of learning (late adulthood, PND 140) with a mean of 95 d post-treatment. Rats from Experiment 2 were euthanized 9 d after EPM (early adulthood, PND 64), with a mean of 13 d post-treatment. Therefore, rats from Experiments 1 and 2 were comparable in the elapsed time following their operant and maze experiences, but very different in the elapsed time following their drug treatment. Rats were given an overdose of Euthasol and decapitated. The brains were immediately extracted and two millimeter-thick coronal sections of striatum were rapidly dissected, using a brain matrix, over wet ice at 4°C. Following dissection, samples were then immersed in isopentane (surrounded by dry ice) and then stored at −80°C before homogenization. Striatal dissections included both dorsal and ventral subregions. Dissected tissue samples were maintained in −80°C before homogenization.
ELISA method
To prepare the tissues for the assays 0.3 mL of PBS (0.01mol/L, pH 7.2) containing a protease and phosphatase inhibitor cocktail (aprotinin, bestatin, E-64; leupeptin, NaF, sodium orthovanadate, sodium pyrophosphate, β-glycerophosphate; Thermo Scientific, Rockford, IL) was added to each sample. Each tissue was minced, homogenized, sonicated with an ultrasonic cell disrupter, and centrifuged at 5,000g at 4 °C for 10 min. Supernatants were removed and stored at −20 °C until ELISA assays were performed. Bradford protein assays were also performed to determine total protein concentrations in each sample. D1, D2 (Cat# SEB299Ra and SEA673Ra, Cloud-Clone Corp., Houston, TX) protein levels were determined using commercially-available ELISA kits. The assays were performed according to the manufacturer’s instructions. The sensitivity of the assays is 0.055ng/mL for D1 and 0.112ng/mL for D2, and the detection range is 0.156–10ng/mL for D1 and 0.312–20ng/mL for D2. The concentration of each protein is expressed as ng/mg of total protein accounting for dilution factor and presented as percent control.
Data Analysis
ANOVAs were conducted to assess the effect of treatment group and sex on striatal D1 and D2 expression. Where significant main effects or interactions were found, post-hoc simple main effects are reported. Pearson correlation coefficient matrices were generated to assess the relationship of striatal D1 and D2 expression with learning measures.
Results
Discrimination Learning
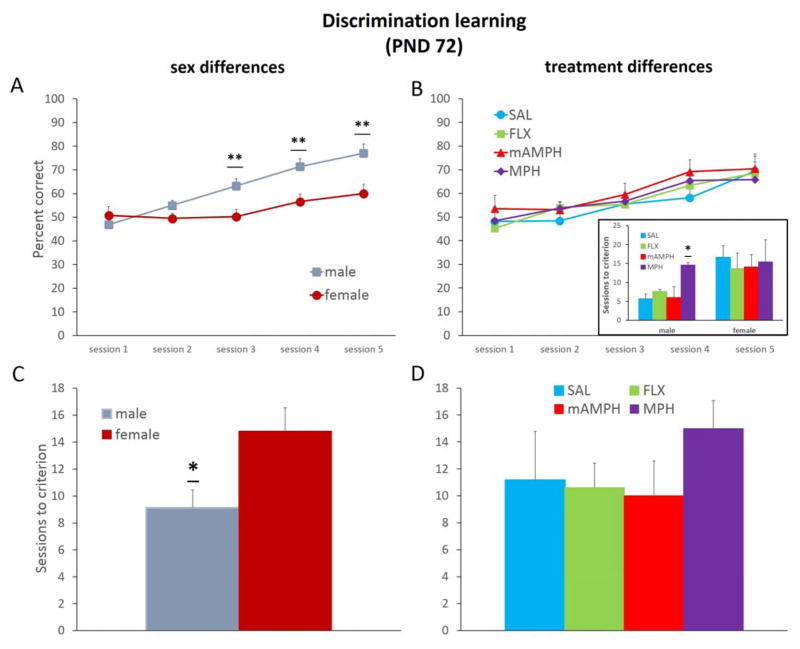
A rmANOVA with Greenhouse-Geisser correction was conducted with between-subject factors (sex and treatment) and within-subject factor (session) on discrimination learning accuracy (percent correct). Only the first 5 sessions commonly experienced by all rats were included (the quickest acquisition occurred in 5 sessions). We found a sex difference in rates of discrimination learning with female rats learning at a slower rate than male rats in this phase [F(2.589, 62.135)=5.165, p<0.01]. On discrimination sessions 3–5, females exhibited reduced accuracy compared to males [all p values <0.01], Figure 2a. A statistically significant within-subject effect of session was found [F(2.589,62.135)=18.82, p<0.001]: evidence that all rats improved performance across session in this phase, irrespective of drug treatment or sex. There was no significant effect of treatment [F(3,24)=0.421, p=0.740] or treatment x sex interaction [F(3,24)=2.296, p=0.103] (Figure 2b). An ANOVA on sessions to criterion resulted in a significant effect of sex [F(1,24)=8.156, p<0.01; mean sessions to criterion, male = 9.13 ± 1.33, female = 14.81 ± 1.76; Figure 2c], but no effect of treatment [F(3,24)=1.200, p=0.331] or interaction of treatment x sex [F(3,24)=0.961, p=0.427], Figure 2d. Given the sex difference, an ANOVA on sessions to criterion was conducted separately for males and revealed a significant effect of treatment [F(3,12)=4.555, p=0.024] but an analysis of only the females did not show an effect of treatment [F(3,12)=0.119, p=0.947], Figure 2b inset. In males, post hoc Dunnett t-tests on sessions to criterion revealed MPH > SAL (p=0.027).
Figure 2. Sex differences but no treatment group differences in discrimination learning.
Session accuracy (percent correct) and rates of learning (sessions to criterion) in the discrimination phase. (A) Male rats showed quicker discrimination learning than female rats. Females exhibited lower percent correct on session 3–5, **p<0.01. (B) Drug-treated groups showed comparable discrimination learning compared to SAL, though an analysis of sessions to criterion in males vs. females revealed a slowing of males by MPH, *p<0.05 (inset). (C) Female animals required significantly more sessions to reach criterion-level performance compared to males, *p<0.05. (D) Drug-treated groups were not different overall in the number of sessions to reach criterion. Line and bar graphs represent group means + SEM. SAL, n=6, FLX, n=10, mAMPH, n=6, MPH, n=10. Effects of different doses were not statistically different and are therefore collapsed per group.
Reversal Learning
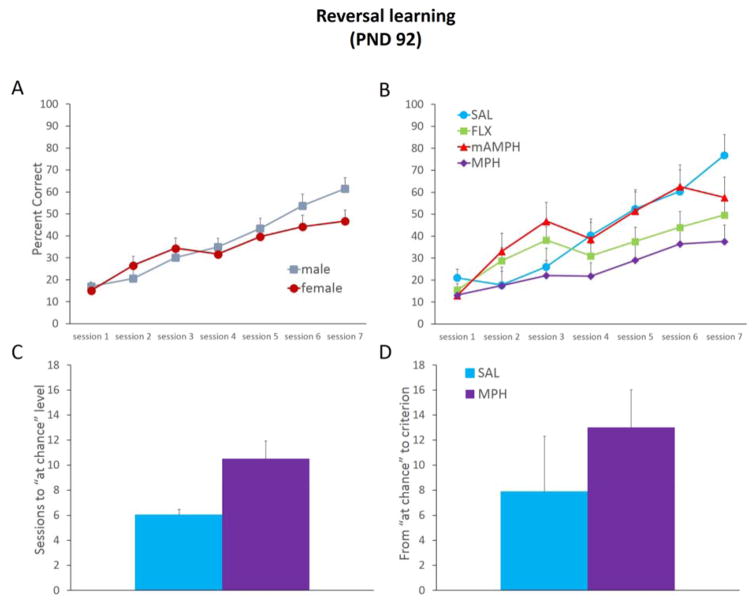
An ANOVA on sessions to criterion showed no significant effects of treatment [F(3,24)=2.312, p=0.102] or sex [F(1,24)=2.723, p=0.112] or treatment x sex interaction [F(3,24)=2.105, p=0.126]. A rmANOVA with Greenhouse-Geisser correction was conducted on reversal learning accuracy (percent correct) across sessions. Only the first 7 sessions commonly experienced by all rats were included (the quickest acquisition occurred in 7 sessions). For reversal learning accuracy, there was a significant within-subject effect of session [F(2.709,65.005)= 27.231, p<0.01], indicating that all rats similarly improved performance across sessions in this phase. We found no significant effect of sex [F(1,24)=0.758, p=0.393] or treatment by sex interaction [F(3,24)=0.531, p=0.665] (Figure 3a), but we did observe a main effect of treatment [F(3,24)= 5.310, p<0.01]; Figure 3b. A rmANOVA with Greenhouse-Geisser correction also revealed a trending, but nonsignificant session by treatment group interaction [F(8.126,65.005)= 1.783, p=0.095]. On reversal sessions 5–7, MPH treatment resulted in slower learning compared to SAL treatment [all p values <0.05]. Post hoc Dunnett t-test comparisons revealed a significant difference of MPH from SAL (p<0.01), with MPH scoring lower percent correct across these sessions [mean percent correct, MPH = 24.42 ± 3.29, SAL = 42.03 ± 4.24, mAMPH = 42.32 ± 4.24, FLX = 34.10 ± 3.29]. However, an ANOVA on correction trials resulted in no significant effect of treatment [F(3,24)=0.056, p=0.982] or sex [F(1,24)=0.001, p=0.980] or treatment x sex interaction [F(3,24)=0.315, p=0.814] over those first 7 sessions. Additionally, a micro-analysis of within-session performance revealed no significant treatment group differences on various measures: mean time to initiate a trial [F(1,14) = 1.718, p=0.211], mean time to stimulus on a correctly-performed trial [F(1,14) = 1.069, p=0.319], mean time to stimulus on an incorrectly-performed trial [F(1,14) = 1.869, p=0.193], or mean time to collect reward [F(1,14) = 2.203, p=0.160].
Figure 3. MPH effects on early phases of reversal learning.
Session accuracy (percent correct) and rates of learning (sessions to either “at chance” or criterion-level performance) in the reversal phase. (A) Male and female rats showed comparable reversal learning. (B) MPH rats exhibited lower percent correct in this phase compared to other treatment groups. (C) MPH-treated rats required more sessions to reach 50% (“at chance”) level performance than other groups, p=0.06. (D) There were no significant treatment group differences in the number of sessions to progress from “at chance” to criterion-level performance. Line and bar graphs represent group means + SEM. SAL, n=6, FLX, n=10, mAMPH, n=6, MPH, n=10. Effects of different doses were not statistically different and are therefore collapsed per group.
A multivariate ANOVA on number of sessions to reach the different stage of reversal was conducted, with ‘reaching at-chance’ and ‘above-chance’ as fixed factors, and with sex and treatment as between-subject factors. This analysis resulted in no significant effect of sex on either stage [F(1,24) < 1.962, p>0.174], no significant interaction of treatment x sex on either stage [F(3,24) < 1.631, p>0.209], but a marginally significant effect of treatment to reach “at chance” performance only [F(3,24) = 2.906, p=0.055]. A post hoc Dunnett t-test on the number of session to reach “at chance” performance resulted in a marginally significant treatment group difference, with MPH > SAL (p=0.06), Figure 3c. There was no significant difference between these groups in progressing from chance to criterion-level performance (all p’s> 0.57) (Figure 3d).
Elevated Plus Maze after MPH treatment
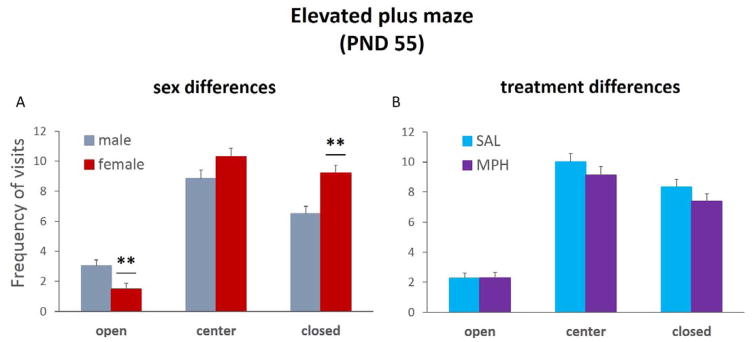
Pearson correlation coefficient matrices verified inter-rater reliability for frequency of entries and duration of arm visits (all r’s > 0.802, p values < 0.01). The duration and frequency of arm visits were analyzed using ANOVA with treatment group (MPH vs. SAL) and sex (male vs. female) as between-subject factors. There were sex differences in the frequency of arm visits [open: F(1,12)=8.399, p=0.01; closed: F(1,12)=14.162, p<0.01]. Post hoc analyses showed that males crossed into the open arms more frequently (p<0.01) and made fewer closed arm entries than female rats (p<0.01); Figure 4a. There were no sex differences in the time spent in open and closed arms, or treatment group differences in either duration or frequency of arm visits on the EPM; Figure 4b.
Figure 4. Sex differences in elevated plus maze activity in adulthood.
There were sex differences in the frequency of arm visits on the elevated plus maze. (A) Males crossed into the open arms more frequently and made fewer closed arm entries than female rats, **p<0.01. (B) There were no treatment group differences in open and closed arm entries. Bar graphs represent group means + SEM.
D1 and D2 receptor expression
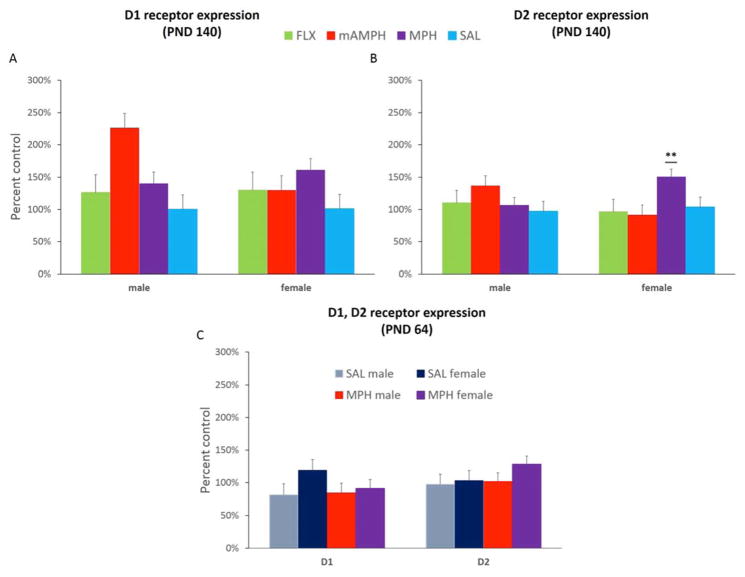
In older adults (PND 140, after learning, Experiment 1), ANOVA resulted in a main effect of treatment group [F(3,25)=3.308, p=0.039] on striatal D1 receptor expression. Post hoc tests revealed that mAMPH (p<0.01) and MPH (p<0.05) were significantly different from SAL-pretreated animals (results are shown for males and females separately, Figure 5a). There was no significant treatment group difference for D2 receptor expression. We did, however, observe a sex x treatment group interaction for D2 [F(3,18)=3.525, p=0.036], but not D1 receptors. Post hoc Dunnett tests revealed the interaction was driven by the MPH-pretreated group, with males exhibiting significantly lower expression of D2 receptors compared to females (p<0.05). Female (p<0.05), but not male, MPH rats showed significantly higher D2 levels compared to SAL rats (Figure 5b). There were no significant D1 or D2 correlations with any learning or anxiety measures. The results of the ELISA analysis from Experiment 2 (tissue collected at PND 64) resulted in no significant treatment group differences (MPH vs. SAL) in striatal D1 or D2 receptor expression (Figure 5c).
Figure 5. D1 and D2 receptor expression in adulthood.
(A) In older adults (PND 140, after learning, Experiment 1) mAMPH and MPH, not FLX, pretreatment resulted in significantly increased D1 expression in adulthood compared to SAL rats. (B) Though there were no significant treatment group differences for D2 receptor expression, there was a sex x treatment group interaction for D2, with MPH female, but not MPH male, rats showing significantly higher D2 levels compared to SAL rats, **p<0.01. (C) In younger adults (PND 64, after EPM, Experiment 2) there were no significant treatment group differences in striatal D1 or D2 receptor expression. Bar graphs represent group means + SEM.
Discussion
Reversal learning in adulthood after adolescent drug exposure
We report the novel finding that adolescent exposure to MPH has long-lasting consequences on flexible reward learning in adulthood. Acutely, MPH has been shown to enhance cognition by improving attention and memory performance (Mehta et al. 2004). An important distinction is that we did not study the acute effects of MPH here: rats underwent a wash-out period as well as an extended period off-drug during discrimination learning, before undergoing reversal learning- the timeframe during which the impairment was observed.
Previous studies have reported that MPH increases impulsivity in rats prescreened to be ‘low-impulsive’ before exposure (Caprioli et al. 2015). Accordingly, a subset of our adult rats pretreated with MPH in adolescence may have had difficulty attending to the task demands in reversal learning. In support of this, we demonstrate slower early reversal learning (i.e., “below chance”) when the levels of choice ambiguity and cognitive demand are high. This is similar to the long-lasting effects of mAMPH in adulthood (Stolyarova et al. 2014). In that study, we found an impairment in overcoming initial perseveration, but unaltered performance once the animals overcame the “at chance” level. Additionally, when males and females were analyzed separately for their overall learning in the discrimination phase, it was found that MPH treatment (not mAMPH or FLX) increased overall number of sessions to criterion in males, but not in females. Yet, despite this, female rats learned at a slower rate than males (discussed in Sex Differences, below). Long-lasting sensitization in adulthood following adolescent MPH exposure found by Shanks et al. (2015) may also partially explain the pattern of results we observe here. Rats may be more reward sensitive in adulthood (to natural reinforcers, such as food) following chronic developmental exposure to MPH, which may attenuate or alter learning from feedback when such learning matters most to accurate performance, in early reversal learning (Izquierdo et al. 2016).
Adolescent FLX exposure resulted in a nonsignificant attenuation in later reversal learning when compared to SAL-pretreated rats. Previous studies have shown developmental FLX to engender an anxiogenic profile in adulthood (Iñiguez et al. 2010; Iñiguez et al. 2014; Homberg et al. 2011) and a cocaine preference later in life (Iñiguez et al. 2015). A slightly reduced learning flexibility in this group could be due to increased stress reactivity and a compromised ability to cope with changes in new task demands. Recent evidence shows that long-term effects may be different among different SSRIs paroxetine, citalopram, and FLX (Schaefer et al. 2013; Altieri et al. 2014; Amodeo et al. 2015), and furthermore, that long-term effects depend on age of exposure and whether animals are later tested on or off drug (Homberg et al. 2011; Vorhees et al. 2011). FLX exposure in adulthood, however, facilitates early reversal learning in mice tested on a touchscreen paradigm very similar to the present task wherein animals are presented with a concurrent pairwise discrimination problem (Brigman et al. 2010). Full engagement of the serotonin system in reversal learning may be most important in probabilistic tasks involving uncertainty about stimulus-reward contingencies (Rygula et al. 2014; Ochoa et al. 2015), not in deterministic choices in which one stimulus is always rewarded and the other, never rewarded (as in the present experiment).
Surprisingly, mAMPH pretreated rats did not display the discrimination or reversal learning impairments we previously observed when animals were treated in later adolescence, beginning in PND 41 (Ye et al. 2014). There is now evidence that there are differences in reward sensitivity and addiction vulnerability in early vs. late rat adolescence and that these effects are often sex dependent (Spear 2015). There may also be a difference in the aversive properties of mAMPH depending on age of exposure: in one study, a 9 d difference in adolescence had a significant impact on drug response (Vorhees et al. 2011). Importantly, Ye et al. (2014) only treated and tested male rats, different than in the present study, where male rats were single-housed in the same room as female rats, and tested in chambers that, despite thorough cleaning, frequently had female rats present in them before male testing. Future experiments should systematically control for the effects of these conditions in learning. Additionally, to account for differences in on- and off-drug effects, having a separate group of animals remain on drug treatment during learning would be an interesting comparison to those taken off the drug.
Sex differences
Female rats learned the initial discrimination at a slower rate than male rats. To our knowledge, a sex difference in visual discrimination learning in untreated rats has not been reported before. Sex differences in previous studies have been noted in the areas of increased addiction vulnerability in male rats (Crawford et al. 2011), higher levels of anxiety (Iñiguez et al. 2010), and higher levels of impulsivity in male vs. female rats (Caprioli et al. 2015). Rodent females, like human females, mature earlier than males: on average the development of genitalia and activation of sexual organs occurs 4–8 d faster (Spear 2015). In addition to this difference, hormone signaling and pharmacokinetic differences between the sexes (Shanks et al. 2015; Crawford et al. 2011) may also have contributed to the sex differences we report here. Another plausible explanation for the sex differences in discrimination learning is basal anxiety differences in novel environments that may have affected the level of exploration in the operant chambers. In support of this, we found that in a separate group of animals, later in adulthood at approximately the same timeframe of discrimination learning (Experiment 2), females engaged in fewer open arm entries and more closed arm entries than males. This indicates that there were sex differences in anxiety, irrespective of pretreatment group status.
It should also be noted that drug exposure using animal models of depression (Iñiguez et al. 2014) or ADHD (Vendruscolo et al. 2008; Baskin et al. 2015) may have yielded different results. Most groups, however, treat animals in development and assess long-term effects (as in the present study). Yet others have compared effects of developmental exposure with an adult-exposed group: van der Marel et al. (2014) reported small but significant age-dependent effects of MPH treatment in the striatum of healthy rats, with adolescent, but not adult, exposure reducing striatal volume and myelination. These changes likely have an impact on emotional behaviors and learning in adulthood. Additionally, it is possible that estrous cycle produced effects on learning. Current conventional methods to assess estrous cycle in intact females require obtaining vaginal smears, which would have introduced stress as a variable. Since there is evidence that even brief stressors affect learning flexibility (Izquierdo et al. 2006; George et al. 2015), we did not introduce this assessment in the present experiment. Future, adequately-powered experiments should systematically examine the relationship of hormonal influences on these measures.
Striatal D1 and D2 expression after adolescent psychostimulant exposure
Corticostriatal circuitry is critical to adaptive reward learning and motivation (Cagniard et al., 2006). D1 and D2 signaling in the striatum regulate overlapping yet dissociable aspects of reward learning and effort-based decision making (Izquierdo et al. 2006; Schweimer and Hauber, 2006; Groman et al. 2011; Stopper et al. 2013; Keeler et al. 2014; Yohn et al. 2015). The adolescent period is marked by decreased density of both types of receptors in the striatum (Gelbard et al. 1989; Teicher et al. 1995; Tarazi and Baldessarini 2000). In the present investigation, we found significantly increased D1 expression in adult learners that were previously exposed to MPH or mAMPH in adolescence. Interestingly, D1-mediated signaling has been previously linked to the behavioral phenotype of a rat model of ADHD (Ohno et al. 2012). The ability of MPH treatment to alter D1 expression following learning may contribute to its clinical efficacy in the adolescent population. Increased striatal D1 expression (and by extension, binding) would result in enhanced excitability of the pathway involved in learning about and responding to rewards (Cox et al. 2015). This result is consistent with what has been found for the role of D1 in prefrontal cortex: enhanced D1 in prefrontal cortex predicts general cognitive abilities and is modulated by working memory training (Wass et al. 2013). Similarly, developmental psychostimulant exposure may interact with later experience with reward (in discrimination and reversal learning) to upregulate D1 expression in the striatum, leading to an enduring reward sensitive phenotype. We note here that this important ‘later experience’ could simply be more environmental enrichment: It may not be the (food) reward exposure or learning per se, but rather the more complex environment and increased option space that crucially engage DA signaling at that later timepoint. In order to ascertain that such receptor expression changes are due to reward learning experience and not due simply to maturational changes, an appropriate age-matched homecage control group should be added to future investigations.
We propose here, as have others previously, that reduced or excessive (supranormal) DA activity can have different effects on cognitive processes, depending on region-specific receptor activation (e.g. whether D1 vs. D2 in prefrontal cortex; Floresco, 2013). For example, local infusions of MPH in the (baso)lateral amygdala (BLA) enhance cue-reward learning through a D1 mechanism and suppress task-irrelevant behaviors through a D2 mechanism (Tye et al. 2010; Larkin et al. 2015). Therefore, chronic administration of MPH may result in striatal downregulation of D1 receptors in the short term, but when assessed after prolonged drug-withdrawal and upon conditions of reward learning, may be upregulated in the long-term. We only assessed D1 and D2 receptor expression in the striatum in the present study, however DA signaling may be affected differently in another region where DA expression may correlate meaningfully with learning, such as the BLA (Wassum & Izquierdo, 2015). Additionally, since we collected striatal samples that included both dorsal and ventral regions of the striatum, it is possible that subregional differences may have masked receptor expression effects.
We previously found that D1 expression decreases in adolescent animals that had prior experience with (food) reward learning, compared to animals that had the same experience in adulthood (Stolyarova & Izquierdo, 2015). Decreased D1 expression at the onset of adulthood is predicted to render animals less reward sensitive, whereas increased D1 expression in adolescence may help to establish this reward-sensitive phenotype. Of course, there are differences in measures of DA receptor expression, availability, and binding, and with our current methods, we are unable to detect differences in functionality. For example, there is the possibility that there may be D1 turnover changes, trafficking and insertion of receptors “on demand,” or silent synapses (Dong & Nestler, 2014) that we are unable to capture with our protein assays. However, protein expression assessment via ELISA provides an advantage over binding studies, as it allows the distinction of D1 and D5 subpopulations of D1-like family of receptors. Taken together with results from untreated adolescent vs. adult animals (Stolyarova & Izquierdo, 2015), our findings are consistent with the ‘prepare and select’ model of striatal D1 and D2, respectively (Keeler et al. 2014). Increased striatal D1 expression and/or availability would be expected to engender a readiness to respond to reward in animals pretreated with MPH or mAMPH. Noteworthy is that food restriction on its own has been previously shown to upregulate D1 receptors in ventral striatum (Carr et al. 2009). Since all of the groups in the present study experienced identical food restriction conditions, MPH and mAMPH pretreatment during adolescence may have rendered striatal D1 transcription machinery more responsive to environmental changes.
Two months of treatment with MPH beginning in PND 30, similar to our timepoint, results in decreases in D2 availability assessed with in vivo microPET (Thanos et al. 2007), suggesting an addiction-vulnerable profile. Ontogenetic changes in D2 receptors may be partially responsible for differences in psychostimulant sensitivity (McDougall et al. 2015) since functionality of the D2 receptor continues to mature beyond the preweanling period (Der-Ghazarian et al. 2014) and likely through adolescence. Other groups, assessing D2 density via autoradiography after early MPH exposure (PND 21) do not report enduring effects of the drug on either D1 or D2 density (Gill et al. 2013). MPH may have long lasting effects on the functionality and expression of D2 depending on early vs. late adolescent exposure, however, in the present study we did not observe robust MPH effects on the expression of D2 receptors in the striatum. Interestingly, however, we did find a sex x MPH interaction for D2 but not D1 receptors: Female MPH but not male MPH rats showed higher D2 expression compared to SAL rats. These results are particularly intriguing when compared to those from Experiment 2 (PND 64, early adulthood) wherein animals had not engaged in learning and no significant treatment effect on striatal D1 or D2 receptor expression was found. Striatal D1 or D2 expression was not correlated with any learning or anxiety measures, indicating that expression of these receptors was not tightly linked to performance. Sex differences in response to developmental psychostimulant exposure and subsequent study of D1 and D2 expression should be explored further. If lower D2 expression is associated with addiction vulnerability, our results suggest juvenile MPH exposure may have efficacy in reducing this risk in females. This should also be explored. Lastly, an important area for investigation is to determine how the pharmacodynamics and plasma half-lives of MPH and mAMPH may differentially contribute to selective receptor expression and learning effects.
Conclusions
In the present report, we show sex differences in visual discrimination learning and anxiety, assessed in novel environments. Though estrous cycle was not measured in the current experiments, our data provide a basis for future systematic inquiry into sex differences on these outcomes. Here we also report the first evidence of enduring effects of adolescent MPH on adulthood reversal learning in rats. These findings have limited implications for learning flexibility and adaptive decision-making in a human clinical population (those diagnosed with ADHD), but may have the most relevance to an adolescent recreational user population. To that end, our results show that developmental psychostimulant exposure may interact with reward learning experience to boost D1 expression in adulthood, and D2 expression in a sex-dependent manner later in life. This may be particularly analogous to the young human recreational user that consumes psychostimulants as cognitive enhancers to boost academic performance. These findings also contribute to a larger literature that reduced or excessive DA activity may have different effects on cognitive processes, depending on region-specific receptor activation.
Acknowledgments
This work was supported by UCLA’s Division of Life Sciences Recruitment and Retention fund (Izquierdo) and by UCLA CARE, PROPS, IMSD Scholars, and PEERS programs.
Footnotes
Conflict of Interest
Authors report no conflict of interest.
Author contributions
AI, HP, AD, and SD designed the research; HP, AD, AS, SD, JC, and JR performed the research; AS, HP, AD, SD, and AI analyzed the data; AI, HP, and AS wrote the paper.
References
- Achat-Mendes C, Anderson KL, Itzhak Y. Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology. 2003;45(1):106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, Perrone-Capano C. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31(9):1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- Altieri SC, Yang H, O’Brien HJ, Redwine HM, Senturk D, Hensler JG, Andrews AM. Perinatal vs. genetic programming of serotonin states associated with anxiety. Neuropsychopharmacology. 2015;40(6):1456–1470. doi: 10.1038/npp.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo LR, Greenfield VY, Humphrey DE, Varela V, Pipkin JA, Eaton E, Johnson JD, Plant CP, Harmony ZR, Wang L, Crawford CA. Effects of acute or repeated paroxetine and fluoxetine treatment on affective behavior in male and female adolescent rats. Psychopharmacology. 2015 doi: 10.1007/s00213-015-4003-1. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin BM, Dwoskin LP, Kantak KM. Methylphenidate treatment beyond adolescence maintains increased cocaine self-administration in the spontaneously hypertensive rat model of attention deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2015;131:51–56. doi: 10.1016/j.pbb.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cerebral Cortex. 2010;20(8):1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51(5):541–7. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Jupp B, Hong YT, Sawiak SJ, Ferrari V, Wharton L, Williamson DJ, McNabb C, Berry D, Aigbirhio FI, Robbins TW, Fryer TD, Dalley JW. Dissociable Rate-Dependent Effects of Oral Methylphenidate on Impulsivity and D2/3 Receptor Availability in the Striatum. The Journal of Neuroscience. 2015;35:3747–3755. doi: 10.1523/JNEUROSCI.3890-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Cabeza de Vaca S, Sun Y, Chau LS. Reward-potentiating effects of D-1 dopamine receptor agonist and AMPAR GluR1 antagonist in nucleus accumbens shell and their modulation by food restriction. Psychopharmacology (Berl) 2009;202(4):731–743. doi: 10.1007/s00213-008-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Frank MJ, Larcher K, Fellows LK, Clark CA, Leyton M, Dagher A. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage. 2015;109:95–101. doi: 10.1016/j.neuroimage.2014.12.070. [DOI] [PubMed] [Google Scholar]
- Der-Ghazarian T, Widarma CB, Gutierrez A, Amodeo LR, Valentine JM, Humphrey DE, Gonzalez AE, Crawford CA, McDougall SA. Behavioral effects of dopamine receptor inactivation in the caudate-putamen of preweanling rats: role of the D2 receptor. Psychopharmacology. 2014;231(4):651–662. doi: 10.1007/s00213-013-3280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35(8):374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Res Dev Brain Res. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- George SA, Rodriguez-Santiago M, Riley J, Abelson JL, Floresco SB, Liberzon I. Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav Brain Res. 2015;286:256–64. doi: 10.1016/j.bbr.2015.02.051. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295(1):51–57. [PubMed] [Google Scholar]
- Gill KE, Beveridge TJR, Smith HR, Porrino LJ. The effects of rearing environment and chronic methylphenidate administration on behavior and dopamine receptors in adolescent rats. Brain Research. 2013;1527:67–78. doi: 10.1016/j.brainres.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Chappell AM, Beveridge TJR, Porrino LJ, Weiner JL. Chronic Methylphenidate Treatment During Early Life Is Associated with Greater Ethanol Intake in Socially Isolated Rats. Alcoholism: Clinical and Experimental Research. 2014;38:2260–2268. doi: 10.1111/acer.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, Zupan B, Menzer D, Rice J, Drake CT, Romeo RD, Brake WG, Torres-Reveron A, Milner TA. Methylphenidate Administration to Juvenile Rats Alters Brain Areas Involved in Cognition, Motivated Behaviors, Appetite, and Stress. The Journal of Neuroscience. 2007;27:7196–7207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund T, Teuchert-Noodt G, Busche A, Neddens J, Brummelte S, Moll GH, Dawirs RR. Administration of oral methylphenidate during adolescence prevents suppressive development of dopamine projections into prefrontal cortex and amygdala after an early pharmacological challenge in gerbils. Brain Res. 2007;1176:124–132. doi: 10.1016/j.brainres.2007.06.107. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31(20):7291–9. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Blom T, Arentsen T, van Brunschot C, Schipper P, Korte-Bouws G, van Luijtelaar G, Reneman L. Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One. 2011;6(1):e16646. doi: 10.1371/journal.pone.0016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, Wright KN, Dayrit G, Nieto SJ, Wilkinson MB, Lobo MK, Neve RL, Nestler EJ, Bolaños-Guzman CA. Fluoxetine Exposure during Adolescence Alters Responses to Aversive Stimuli in Adulthood. The Journal of Neuroscience. 2014;34:1007–1021. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17(3):247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Wright KN, Zamora NN, Cruz B, Zavala AR, Robison AJ, Mazei-Robison MS. Fluoxetine exposure during adolescence increases preference for cocaine in adulthood. Sci Rep. 2015;5:15009. doi: 10.1038/srep15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Bolaños-Guzman CA. Short- and Long-Term Functional Consequences of Fluoxetine Exposure During Adolescence in Male Rats. Biological Psychiatry. 2010;67:1057–1066. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;25(2):505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: An updated perspective. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219(2):607–20. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26(21):5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171(2):181–8. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Jordan CJ, Harvey RC, Baskin BB, Dwoskin LP, Kantak KM. Cocaine-seeking behavior in a genetic model of attention-deficit/hyperactivity disorder following adolescent methylphenidate or Atomoxetine treatments. Drug and Alcohol Dependence. 2014;140:25–32. doi: 10.1016/j.drugalcdep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler JF, Pretsell DO, Robbins TW. Functional implications of dopamine D1 vs. D2 receptors: A ‘prepare and select’ model of the striatal direct vs. indirect pathways. Neuroscience. 2014;282C:156–175. doi: 10.1016/j.neuroscience.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A. Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology. 2012;224(3):459–467. doi: 10.1007/s00213-012-2774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JD, Jenni NL, Floresco SB. Modulation of risk/reward decision making by dopaminergic transmission within the basolateral amygdala. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4094-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macrì S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Ruocco LA, Canese R, Sadile AG, Laviola G. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci Biobehav Rev. 2011;35(8):1722–1739. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Eaton SE, Mohd-Yusof A, Crawford CA. Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2(High) receptors. Psychopharmacology. 2015;232(13):2287–2301. doi: 10.1007/s00213-014-3860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: Relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45(2):293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2014: Volume I, Secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey-White J, Holmes A. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology. 2008;200(3):413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JG, Stolyarova A, Kaur A, Hart EE, Bugarin A, Izquierdo A. Post-training depletions of basolateral amygdala serotonin fail to disrupt discrimination, retention, or reversal learning. Front Neurosci. 2015;9:155. doi: 10.3389/fnins.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Okano M, Masui A, Imaki J, Egawa M, Yoshihara C, Tatara A, Mizuguchi Y, Sasa M, Shimizu S. Region-specific elevation of D1 receptor-mediated neurotransmission in the nucleus accumbens of SHR, a rat model of attention deficit/hyperactivity disorder. Neuropharmacology. 2012;63(4):547–554. doi: 10.1016/j.neuropharm.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Rygula R, Clarke HF, Cardinal RN, Cockcroft GJ, Xia J, Dalley JW, Robbins TW, Roberts AC. Role of central serotonin in anticipation of rewarding and punishing outcomes: Effects of selective amygdala or orbitofrontal 5-HT depletion. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Grace CE, Braun AA, Amos-Kroohs RM, Graham DL, Skelton MR, Williams MT, Vorhees CV. Cognitive impairments from developmental exposure to serotonergic drugs: citalopram and MDMA. Int J Neuropsychopharmacol. 2013;16(6):1383–1394. doi: 10.1017/S1461145712001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn Mem. 2006;13(6):777–82. doi: 10.1101/lm.409306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RA, Ross JM, Doyle HH, Helton AK, Picou BN, Schulz J, Tavares C, Bryant S, Dawson BL, Lloyd SA. Adolescent exposure to cocaine, amphetamine, and methylphenidate cross-sensitizes adults to methamphetamine with drug- and sex-specific effects. Behavioural Brain Research. 2015;281:116–124. doi: 10.1016/j.bbr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stolyarova A, O’Dell SJ, Marshall JF, Izquierdo A. Positive and negative feedback learning and associated dopamine and serotonin transporter binding after methamphetamine. Behav Brain Res. 2014;271:195–202. doi: 10.1016/j.bbr.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarova A, Izquierdo A. Distinct patterns of outcome valuation and amygdala-prefrontal cortex synaptic remodeling in adolescence and adulthood. Front Behav Neurosci. 2015 doi: 10.3389/fnbeh.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology. 2013;38(5):715–28. doi: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18(1):29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87(4):426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Tye KM, Tye LD, Cone JJ, Hekkelman EF, Janak PH, Bonci A. Methylphenidate facilitates learning-induced amygdala plasticity. Nat Neurosci. 2010;13(4):475–81. doi: 10.1038/nn.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel K, Klomp A, Meerhoff GF, Schipper P, Lucassen PJ, Homberg JR, Dijkhuizen RM, Reneman L. Long-term oral methylphenidate treatment in adolescent and adult rats: differential effects on brain morphology and function. Neuropsychopharmacology. 2014;39(2):263–273. doi: 10.1038/npp.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Izídio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behavioural Pharmacology. 2008;19(1):21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Morford LR, Graham DL, Skelton MR, Williams MT. Effects of periadolescent fluoxetine and paroxetine on elevated plus-maze, acoustic startle, and swimming immobility in rats while on and off-drug. Behavioral and Brain Functions. 2011;7:41. doi: 10.1186/1744-9081-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015 doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass C, Pizzo A, Sauce B, Kawasumi Y, Sturzoiu T, Ree F, Otto T, Matzel LD. Dopamine D1 sensitivity in the prefrontal cortex predicts general cognitive abilities and is modulated by working memory training. Learn Mem. 2013;20(11):617–627. doi: 10.1101/lm.031971.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Pozos H, Phillips TJ, Izquierdo A. Long-term effects of exposure to methamphetamine in adolescent rats. Drug Alcohol Depend. 2014 doi: 10.1016/j.drugalcdep.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M, Salamone JD. The role of dopamine D1 receptor transmission in effort-related choice behavior: Effects of D1 agonists. Pharmacol Biochem Behav. 2015;135:217–26. doi: 10.1016/j.pbb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283(8):1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]