Abstract
Background
Identifying differences and similarities between CLA+ polarized T-cell subsets in pediatric vs. adult atopic dermatitis (AD) is critical for directing new treatments towards children.
Objective
To compare activation markers and frequencies of skin-homing (CLA+) vs. systemic (CLA−) “polar” CD4 and CD8 T-cell subsets in early pediatric AD, adult AD and controls.
Methods
Flow cytometry was used to measure CD69/ICOS/HLA-DR frequency in memory subsets as well as IFN-γ, IL-13, IL-9, IL-17, and IL-22 cytokines, defining Th1/Tc1, Th2/Tc2, Th9/Tc9, Th17/Tc17, and Th22/Tc22 populations in CD4 and CD8 cells, respectively. We compared peripheral blood from 19 children <5 years and 42 adults with well-characterized moderate to severe AD, as well as age-matched controls (17 children and 25 adults).
Results
Selective ICOS activation (P<0.001) was seen in children. CLA+ Th2 T-cells were markedly expanded in both AD children and adults compared to controls, but decreases in CLA+ Th1 T-cells were greater in AD children (17% vs. 7.4%, P=0.007). Unlike in adults, no imbalances were detected in pediatric AD CLA− T-cells, nor were there altered frequencies of Th22 T-cells within CLA+ or CLA− compartments. Adults with AD had increased frequency of IL-22-producing CD4 and CD8 T-cells within the skin-homing population (9.5% vs. 4.5%; 8.6% vs. 2.4%, respectively; P<0.001) as well as increased HLA-DR activation (P<0.01).
Conclusions
These data suggest that Th2 activation within skin-homing T-cells may drive AD in children and that reduced counter-regulation by Th1 T-cells may contribute to excess Th2 activation. Th22 “spreading” of AD is not seen in young children and may be influenced by immune development, disease chronicity or recurrent skin infections.
Clinical Implications
Therapeutic approaches to treat AD in children may be best directed to correction of Th2/Th1 imbalance, while adults might also benefit from IL-22 targeted therapies.
Keywords: Atopic dermatitis, T-cell, CLA, IL-13, IL-22, IFN-γ, ICOS, CD69, HLA-DR
Capsule Summary
While AD in children is characterized by Th1/Th2 dysregulation, AD in adults is accompanied by higher chronic activation and a Th22/Tc22 component.
Introduction
Despite being one of the most common pediatric disorders,1, 2 atopic dermatitis (AD) harbors immunological alterations within the first 6 months of onset that are are largely unexplored in children.3, 4 Although 85% of AD cases present before 5 years old (y.o.) and immune polarization in children may differ from adults with long-standing disease, studies have largely been limited to adults.5–7
The percentage of CD4 memory T-cells (CD45RO+) in the circulation of newborns (5%-10%) is significantly lower than in 3–10 y.o. children (~15%) and adults (30–50%), while the percentage of naïve T-cells (CD45RA+) is highest in newborns (~90%).8, 9–11 Most T-cells in AD lesions are CD45RO+ memory cells that express the skin-homing receptor cutaneous lymphocyte antigen (CLA).12–14 Circulating CLA+ T-cells are expanded in AD, respond to skin-associated allergens, and play a role in disease pathogenesis.14–17 CLA+ T-cells have been also demonstrated to be peripheral biomarkers in several inflammatory skin diseases.18 The need for large biopsies to classify T-cell subsets limits the feasibility of intracellular cytokine staining (ICS) of skin from adults and even more so in children. Therefore, studying polarized, activated circulating CLA+ memory T-cell subsets may provide a surrogate to skin phenotyping.
Previous studies indicate low IFN-γ and high Th2 signals in cord blood of newborns that develop AD.19–21 Subsequent infant and toddler AD studies mostly demonstrate elevated Th2 signals22–24 with controversial data regarding Th1 frequency,23, 25–28 CD8 polar subsets23, 28 and levels of T-cell activation.29–31 Several studies include cohorts of adults and children, but most do not provide direct comparison between these groups.22, 23, 26, 32 While we have recently reported a detailed blood phenotype of adult AD,33 few pediatric studies differentiate cytokine expression in CLA subsets30 and virtually none compare adults to children.
Defining the similarities and differences between activated polarized T-cell subsets in adults and early onset pediatric AD is critical for understanding initial pathogenic immune mechanisms. Furthermore, linking IgE levels with T-cell polarization in newly diagnosed AD might help define the sequence of events that leads to AD development.
In this study, we compared differences between T-cell memory subset activation within the skin-homing/CLA+ and systemic/CLA− compartments, as well as frequencies of polarized CD4 and CD8 subsets in blood of pediatric and adult AD patients. We found that while AD onset in children is Th2-dominated and characterized by short-term activation, adult AD extends to additional Th subsets, particularly Th22.
Methods
Patients’ characteristics and blood samples
Blood was obtained (with patients’ or parents’ informed consent) from 19 children (11 females, 8 males; aged 5–70 months; mean 25 months) with moderate-to-severe AD as well as from 17 pediatric controls (7 females, 10 males; aged 6–67 months; mean 32 months) and from 42 adults (18 females, 24 males; aged 18–74; mean 42) with moderate-to-severe AD and 25 adult controls (12 females, 13 males; aged 19–66; mean 39) under Institutional Review Board-approved protocol. No age (P=0.3), ethnicity (P=0.8/P=0.055; children/adults) or gender (P=0.8/P=0.5; children/adults) disparities were observed between the groups. AD children were within 6 months from AD diagnosis.
AD adult serum IgE levels (normal <200 kU/L) ranged from 4–50000 kU/L (mean=6238 kU/L), and AD children (normal <100 kU/L) ranged from 23–1772 kU/L (mean=526 kU/L). Control children ranged 0–43 kU/L (mean=26 kU/L).
Scoring of AD/SCORAD was used to evaluate disease severity (adults: SCORAD range 32–97, mean=65; children: range 30–73, mean=54).
Controls had no personal history of inflammatory disease and no family history of atopy. Demographic and laboratory data are summarized in Table 1.
Table 1.
Epidemiological, laboratory and clinical data for AD patients and controls
| Adult AD |
Adult Controls |
P value | Children AD |
Children Controls |
P value | |
|---|---|---|---|---|---|---|
| Age (yrs for adults; mos for children) |
42 ± 2.1 (18–74) |
39 ± 2.8 (19–66) |
0.3 | 25 ± 1.9 (5–70) |
32 ± 1.8 (6–67) |
0.34 |
| Gender, no. |
0.8 | 0.51 | ||||
| Female | 18 | 12 | 11 | 7 | ||
| Male | 24 | 13 | 8 | 10 | ||
| Ethnicity, no. |
0.8 | 0.055 | ||||
| Asian | 3 | 3 | 5 | 0 | ||
| Black | 6 | 2 | 2 | 1 | ||
| White | 33 | 19 | 12 | 16 | ||
| SCORAD score |
64.5 ± 2.45 (32–97) |
NA | 53.5 ± 14.4 (30– 73) |
NA | ||
| IgEa (Ku/L) | 628.6 ± 114 (4– 50000) |
NA | 719.8 ± 318.3 (23– 1,772) |
28.8 ± 13.3 (0– 141) |
0.082 | |
| Eoshinophil countb (K/mcL) |
0.45 ± 0.05 (0– 1.2) |
NA | 0.71 ± 0.26 (0.20– 2.12) |
0.27 ± 0.088 (0– 1.12) |
0.14 |
Data presented as mean ± SEM unless otherwise specified. AD, atopic dermatitis; SCORAD, SCOring of AD index; SEM, Standard error of the mean
Reference range, 0–200 kU/L (adults), 0–100 kU/L (children)
Reference ranges, 0–0.8 K/mcL (absolute eosinophil count)
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from whole blood by Ficoll-Paque Plus (GE Healthcare, Sweden). Briefly, the blood was laid under Ficoll gradient; after spinning, PBMCs were collected at the interface between the plasma and the Ficoll gradient (see Methods in the Online Repository).
Stimulation of blood cell populations for cytokine responses
Whole blood was incubated with PMA (25ng/ml) plus ionomycin (2µg/ml) in the presence of brefeldin A (10µg/ml) for 4 hours at 37°C to induce T-cell cytokine responses. After stimulation, RBCs were lysed with FACS Lysing solution to obtain leukocytes (see Methods in the Online Repository).
Cell surface staining and intracellular staining on PBMCs and stimulated and non-stimulated CD4/CD8 T-cells
PBMCs were stained with fluorochrome-labeled Abs to cell surface markers (CD3, CD8, CD4, CD45RO, CCR7, ICOS, HLA-DR and CLA). Stimulated and non-stimulated blood cells were also stained for cell surface markers (CD3, CD4, CD69 and CLA) and then permeabilized with FACS/perm to stain for cytokines including IL-13, IL-22, IL-9, IFN-γ and IL-17 (see Methods in the Online Repository).
Statistical analysis
Data was analyzed using Student’s t-test to compare between variables. IgE values were log10-transformed before analyses. Variables were correlated using Pearson correlations. P<0.05 was considered significant.
Results
To examine immune activation in AD children vs. adults, surface staining was used to measure expression of early (CD69), mid (inducible co-stimulator molecule/ICOS) and late (HLA-DR) activation markers in central (Tcm/CCR7+CD45RO+) and effector memory (Tem/CCR7−CD45RO+) T-cells in skin homing/CLA+ and CLA− subsets. CD25+CD127−CCR4+ phenotype was used to exclude Tregs from all PBMCs analyses. Subsequently, ICS was used to measure frequencies of IFN-γ, IL-13, IL-22, IL-17A and IL-9 producing T-cells after PMA/ionomycin activation, defining Th1/Tc1, Th2/Tc2, Th22/Tc22, Th17/Tc17 and Th9/Tc9 subsets in CD4 and CD8 T-cells, respectively, in CLA+ and CLA− subsets. Gating strategy appears in Figure E1.
Effector T-cells are uniquely elevated in adult AD
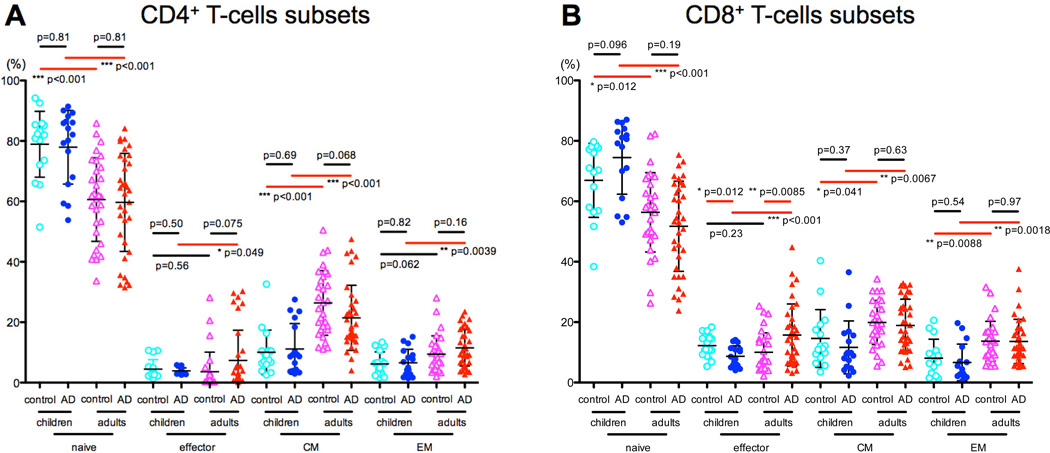
As previously described,11 our data shows that the proportion of naïve T-cells was higher in both control (CD4: 79% vs. 60%, P<0.001 and CD8: 68% vs. 56%, P=0.01; Fig 1A–B) and AD children vs. adults (CD4: 78% vs. 59%, P<0.001 and CD8: 77% vs. 55%, P<0.001; Fig 1A–B), whereas Tcm/Tem subsets were lower in control and AD children compared to adults (P=0.001; Fig 1A–B). No significant differences in memory subsets were observed between AD and controls (P>0.05; Fig 1A–B).
Figure 1. CD4 and CD8 T-cell subsets in children and adults with AD vs. controls.
A–B) Naïve T-cell frequency was higher in both control and AD children compared to adults, while Tcm/Tem subsets were lower in control and AD children than adults. Adults with AD had significantly higher effector cells compared with AD children.
While no significant differences in effector cell frequencies were observed among controls (CD4: 4.5% vs. 3.6%, P=0.5 and CD8: 12% vs. 10%, P=0.2; Fig 1A–B), adult AD had significantly higher effector cells compared with pediatric AD (CD4: 3.8% vs. 7.5%, P=0.049 and CD8: 8% vs. 15%, P<0.001; Fig 1A–B).
Memory subsets are positively correlated with IgE, but not with SCORAD, in AD children
Tcm and Tem are crucial components of the adaptive immune system, and expand upon antigenic stimulation.34 To evaluate potential associations between disease severity and antigenic stimulation, Tcm and Tem frequencies were correlated with IgE levels and disease severity/SCORAD.
Significant correlations were only observed between Tcm/Tem frequencies and IgE levels in AD children (CD4: Tcm: r=0.88, P=0.004 and Tem: r=0.82, P= 0.01; Fig E2B). Memory subset frequencies were neither correlated with SCORAD in AD populations, nor with IgE levels in control children (Fig E2A&C–E).
ICOS is highly expressed on CD4 Tcm and Tem memory subsets of AD patients
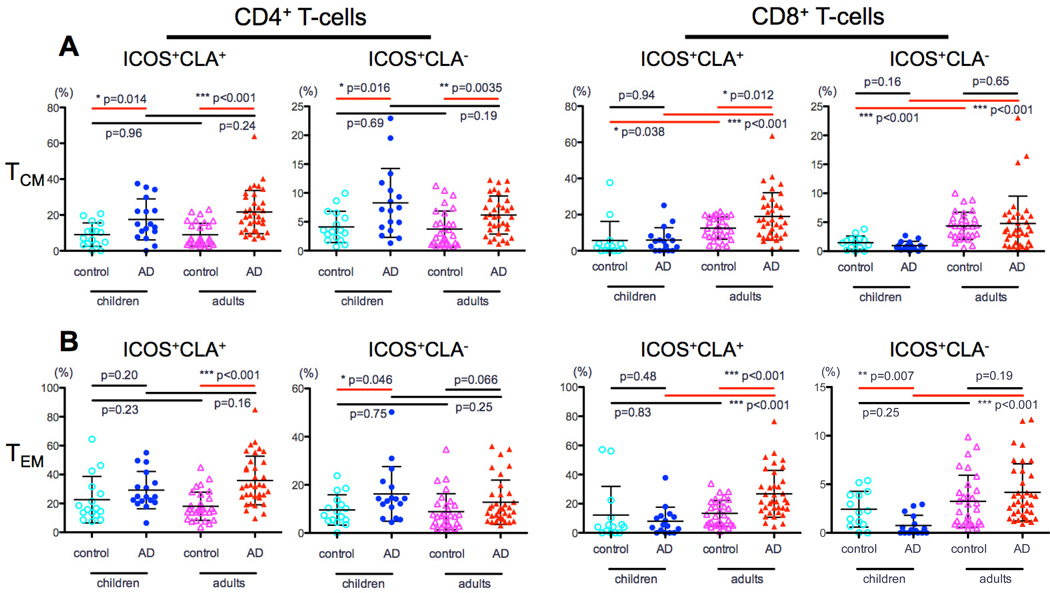
ICOS is a mid-activation surface marker that is expressed up to 24hrs after T-cell receptor stimulation and is essential for effective T-helper responses.35, 36 Comparing ICOS expression on CD4 T-cells between pediatric AD, adult AD and controls showed that although AD patients had increased ICOS expression in both CLA+ and CLA− memory subsets compared to their relative controls (Tcm CLA+: children 17% vs. 9%, P=0.01; adults 22% vs. 9%, P<0.001; Fig 2A), CLA+ ICOS levels were substantially higher (Tcm CLA−: children 8% vs. 4%, P=0.01 and adults 6% vs. 3.7%, P=0.003; Fig 2A). No significant CD4 ICOS expression differences were observed between AD groups in both CLA+/CLA− Tem/Tcm (P>0.16; Fig 2A–B).
Figure 2. ICOS expression in CLA+ and CLA− memory T-cell subsets in adult and pediatric AD.
A) AD patients had increased ICOS expression in both CLA+ and CLA− memory subsets compared to their relative controls. No significant CD4 ICOS expression differences were observed between the AD groups in both CLA+/CLA− Tem/Tcm. B) Contrary to adults, in which higher CD8 ICOS expression was measured in CLA+ Tcm/Tem cells compared to their relative controls, no differences were observed between AD children and controls.
Contrary to adults, in which higher CD8 ICOS expression was measured in CLA+ Tcm/Tem cells compared to controls (Tcm: 19% vs. 12%; P=0.01 and Tem: 26.7% vs. 13.5%, P<0.001; Fig 2A–B), no parallel differences were observed between AD children and controls (P>0.48, Fig 2A–B). Among AD groups, adults had higher ICOS expression in CD8 Tcm/Tem compared to their pediatric counterparts (Tcm: CLA+ 19% vs. 6%; CLA− 4.7% vs. 1% and Tem: CLA+ 27% vs. 10%; CLA− 4% vs. 1.8%, P<0.001 for all; Fig 2A–B).
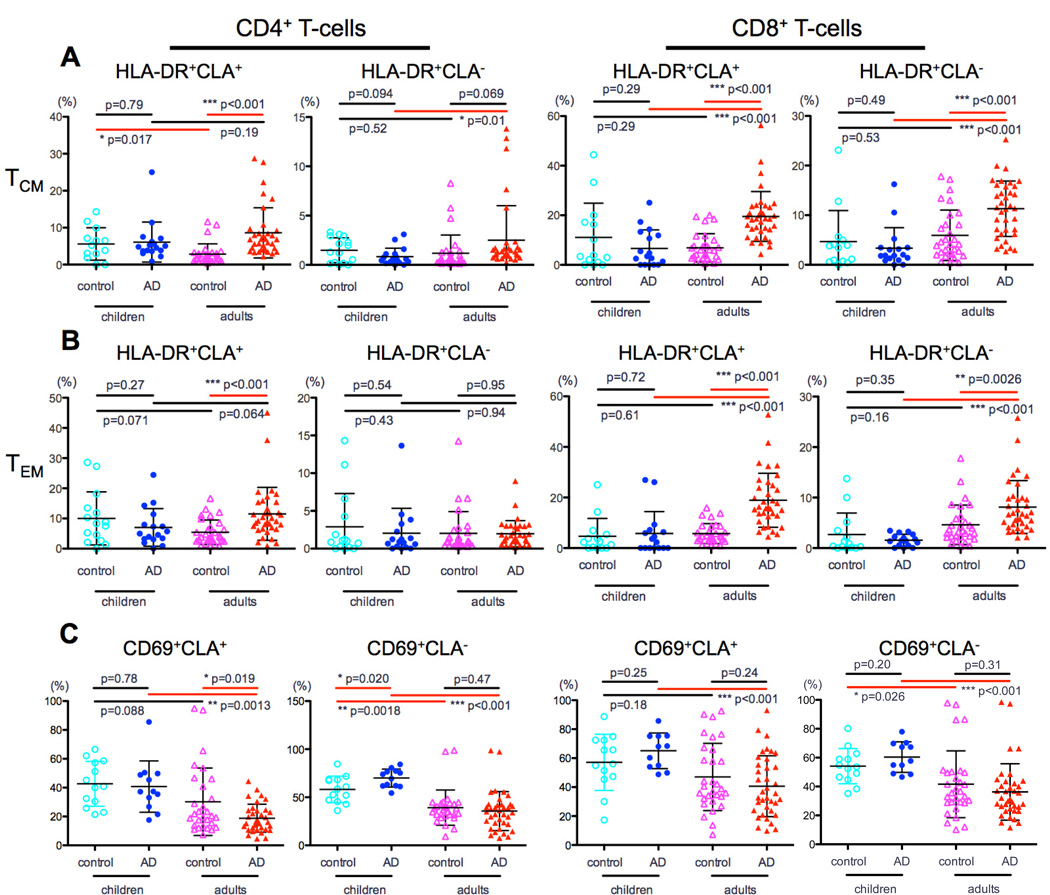
HLA-DR is preferentially expressed on Tcm/Tem in adult but not pediatric AD
HLA-DR is a human class II major histocompatibility complex (MHC) antigen that indicates chronic activation.37 While comparable levels of Tcm/Tem HLA-DR expression were observed in children regardless of AD status (P>0.1; Fig 3A–B), higher HLA-DR activation was found in adult AD compared to controls, particularly among CLA+ populations (P<0.001; Fig 3A–B). Adult AD also showed higher HLA-DR activation than pediatric AD, mainly among CD8 subsets (Tcm: CLA+ 20% vs. 6.5%; CLA− 11.5% vs. 3.3% and Tem: CLA+ 19% vs. 8%; CLA− 8% vs. 1.5%, P<0.001 for all; Fig 3A–B).
Figure 3. HLA-DR expression in in CLA+ and CLA− memory T-cell subsets in adult and pediatric AD.
A) Comparable levels of HLA-DR expression on Tcm/Tem cells between children with and without AD. B) AD adults had higher HLA-DR expression compared to controls, particularly among the CLA+ and CD8 populations. C) Lower CD69 in adults than in children with AD in both CD4 and CD8 subsets.
CD69 expression is higher in AD children compared to adults
CD69 is the earliest activation marker expressed on T-cells following CD4 and CD8 T-cell receptor activation.38 CD69 was the only activation marker that was higher in children, particularly prominent in AD for both CD4 (CLA+: 40% vs. 18%, P=0.0013 and CLA−: 70% vs. 35%, P<0.001, Fig 3C) and CD8 subsets (CLA+: 66% vs. 40%, P<0.001 and CLA−: 62% vs. 36%, P<0.001, Fig 3C).
Skin selective Th1/Th2 imbalance in pediatric AD
Because prior T-cell activation drives subset differentiation, we quantified polar T-cell subsets, including Th1/Tc1, Th2/Tc2, Th22/Tc22, Th17/Tc17 and Th9/Tc9 frequencies in CLA+/CLA− subsets in all children and adult populations.
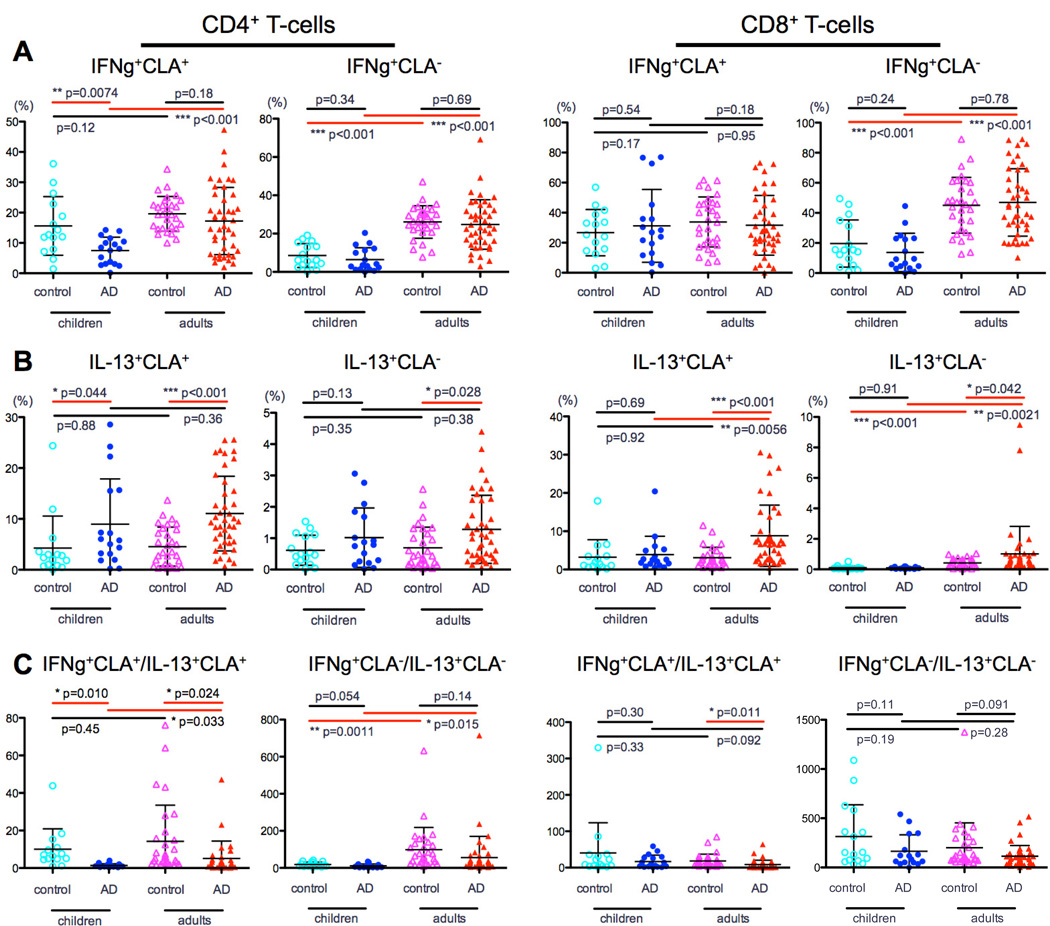
Adult AD patients had normal to slightly lower IFN-γ+CLA+ levels compared to healthy individuals (CD4: 17% vs. 20%, P=0.18; Fig 4A), and this difference is significant in severe patients, as we recently described.33 In contrast, AD children have significantly lower IFN-γ+CLA+ levels than control children (7.4% vs. 17%, P=0.007; Fig 4A) and lower levels of both CLA+ and CLA− CD4 T-cell subsets than AD adults (CLA+: 7.4% vs. 17%, P<0.001 and CLA−: 6.3% vs. 25%, P<0.001; Fig 4A).
Figure 4. IFN-γ and IL-13 polarization in CLA+ and CLA− CD4 and CD8 T-cells.
A) Compared to children, adults with AD had significantly higher levels of IFN-γ, particularly in CD4 T-cells. IFN-γ+CLA+ in AD children was significantly lower than control children. B) In adults IL-13 was higher in the AD group compared to controls in both CLA+/CLA− CD4/CD8 subsets. In children this difference was most prominent among CD4 CLA+ T-cells. C) AD children had markedly lower Th1:Th2 ratio compared with controls, particularly in CD4 CLA+ cells.
IL-13 was higher in adult AD compared to controls in CLA+/CLA− CD4/CD8 subsets (P<0.04; Fig 4B). In children this difference was most prominent among CD4 CLA+ T-cells (9% vs. 4%, P=0.04; Fig 4B). Unlike other cytokines, similar frequencies of CD4 IL-13+CLA+ were seen in pediatric and adult AD, highlighting Th2 dominance in early AD. Higher CD8 IL-13+CLA+ frequency was seen in adults compared to AD children (CLA+: 9% vs. 3.8%, P=0.006 and CLA−: 1% vs. 0.09%, P=0.002; Fig 4B), possibly suggesting a less prominent role of CD8 in AD children with early disease.
To further evaluate the Th1/Th2 imbalance in AD, we compared this ratio between groups. AD children had markedly lower Th1:Th2 compared to controls, particularly in CD4 CLA+ cells (1.2% vs. 12%, P=0.01; Fig 4C). This imbalance was less evident in CD8 T-cells. A significantly lower ratio was also observed in pediatric versus adult AD, particularly in CD4 cells (CLA+: 1.2% vs. 4%, P=0.03 and CLA−: 15% vs. 50%, P=0.01; Fig 4C).
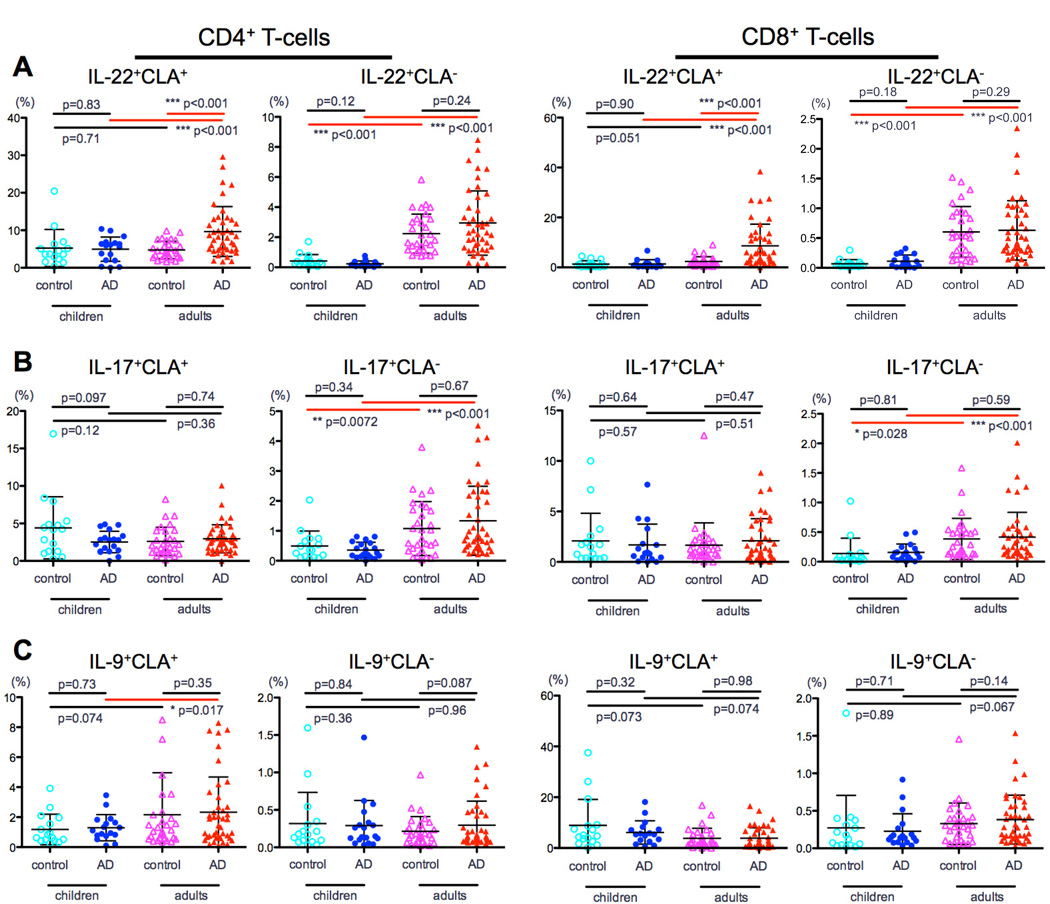
Since adult AD is characterized by Th22/Tc22 expansion in skin39, 40 and blood,33 these subsets were measured in children. No significant differences in IL-22 were observed between pediatric AD and controls (P>0.12; Fig 5A), whereas significantly higher IL-22 levels were observed in adult vs. pediatric AD (CD4: CLA+, 9.5% vs. 4.7%, and CLA−, 3% vs. 0.2%; CD8: CLA+, 8.6% vs. 4.8%, and CLA−, 0.6% vs. 0.1%, P<0.001 for all; Fig 5A). Th17/Tc17 levels were higher in adult versus pediatric AD, specifically in the CLA− subsets (CD4: 1.3% vs. 0.3%, P<0.001 and CD8: 0.4% vs. 0.1%, P<0.001; Fig 5B), with no difference compared to controls. Finally, while no IL-9 differences were observed between AD and controls, adults with AD showed higher Th9 levels in CLA+ cells compared to children with AD (2.3% vs. 1.3, P=0.01; Fig 5C).
Figure 5. IL-22, IL-17 and IL-9 frequency in CLA+ and CLA− CD4 and CD8 T-cells.
A) No significant IL-22 differences were measured between children with and without AD. B) Th17/Tc17 levels were higher in AD adults versus children, specifically in the CLA− CD4/CD8 subsets. C) AD adults had higher Th9 levels in CLA+ cells than AD children.
Discussion
While initial pathogenic events in AD development remain elusive, IgE switching, T-cell activation and cytokine polarization have been suggested as key players.41, 42 Our current understanding of AD pathogenesis derives mostly from studies in adults with long-standing disease. However, only by studying young children with new-onset AD can we gain insight into primary pathogenic events. This is the first comprehensive report characterizing T-cell polarization and activation within skin homing and systemic memory subsets in early onset disease in young children with well-characterized disease severity, in comparison with age-matched pediatric controls and severity-matched AD adults.
At birth, T-cell repertoire consists mostly of naïve cells. With antigenic exposure, memory cells comprise ~35% of circulating T-cells by the second decade of life.43 Our data on memory T-cell subsets corroborates this naïve-memory CD4/CD8 T-cell shift. Furthermore, it shows that CD4/CD8 effector cells are the only consistently higher subset in adult vs. pediatric AD with no parallel differences in their controls. Normally, effector cell proliferation is part of the ‘expansion phase’ of the immune reaction, which is replaced shortly after by a ‘death phase’ of greater than 90% of these effectors, with remaining cells entering a ‘memory phase’.44 Since antigenic exposure and microbial phenotype play central roles in shaping T-cell effector profiles,44 cumulatively more antigenic exposure and increased infection rates may contribute to increased effector cells in adult AD.45–48
T-cell activation through the T-cell receptor leads to a specific sequence of surface activation marker expression. CD69 is a very early activation marker (upregulated within 1hr from cell activation and peaking by 16–24hrs). ICOS is an intermediate activation marker, appearing within 24hrs, while HLA-DR is a late marker indicating chronic activation.49–51
The ICOS co-stimulatory signal is required for T-cell activation and plays a role in shaping adaptive immunity.52–54 Mouse studies show that ICOS is vital in T-cell/B-cell interaction, immunoglobulin isotype switching (IgE production), and germinal center formation.55, 56 Here, we show that AD children have higher ICOS expression, particularly among CD4 cells. These results may relate to the role of ICOS in Th2 immunity expansion and IgE switching,57 supporting an IgE-Th2 mechanism in pediatric AD. The only parameter that showed a trend of higher ICOS activation in AD children compared to adults was the CLA− CD4 population, which further supports the role of ICOS in promoting non-cutaneous atopy in AD children. One possible implication is that ICOS suppression might be used as a target in patients with extrinsic AD accompanied by a central Th2 component.
These ICOS differences were not observed among CD8 cells in pediatric AD. Besides quantitative CD8 decreases relative to CD4 in infants,10, 29 an alternative explanation may be that antigenic stimulation of AD in young populations is not presented by MHC-I molecule.
HLA-DR expression on T-cells indicates a state of chronic activation.50 While Bunikowski et al.58 have previously reported high HLA-DR+CD3+ T-cells in AD children above 2 years of age compared to controls, another blood study showed no difference.29 Antunez et al.30 subsequently showed no difference between AD children and controls in CD4/CD8 HLA-DR expression, but results were not stratified to CLA+/CLA− subsets and all children were above 7 years. While we59 and others60, 61 reported high CD4/CD8 CLA+/CLA− Tcm/Tem HLA-DR activation in circulating skin homing/CLA+ T-cells of adult AD, parallel pediatric data has been unavailable. Our current data indicate that all memory subsets show low HLA-DR activation in children, both in controls and AD. For most subsets, HLA-DR activation is greater in adults versus pediatric AD, but not in normal adults vs. children. This may result from the long-term, chronic activation in adults vs. young children with newly diagnosed AD. Furthermore, differences between the adult groups were more striking in CD8 subsets (with higher values seen in CLA+ cells), indicating a possible role for CD8 in the pathogenesis of chronic, long-standing AD.
CD69 also functions as an immunoregulatory receptor,62 and has been reported to have opposing functional roles in inflammatory responses.62 In AD, its expression on eosinophils was associated with high IgE levels and active inflammation,63 and data from other allergic diseases indicate that CD69 promotes the inflammatory process.64 Our data show that CD69 is the only activation marker higher in children vs. adults with AD. This is in line with prior reports showing that CD69 was virtually absent on CLA+ T-cells from adults with AD, probably due to its transient nature as a very early activation marker.60, 65 Thus, not surprisingly it is highest in early developmental stages of AD in young children in whom inflammation is peaking.
Since conversion of naïve to cytokine-producing memory subsets is an indication of T-cell activation, we have also assessed cytokine production.
In adults, Th2/Th22 profile predominates the skin phenotype with Th1/Th17 components in chronic lesions.39 We have recently shown that the blood profile to a large extent resembles the skin phenotype, with skin-selective Th2/Tc2/Th22/Tc22 dominance and a Th1/Tc1 defect.33 ICS data in children is limited and mostly confined to total cytokine measurements (as opposed to CLA+/CLA−), largely showing high Th2 frequency with conflicting results regarding Th1/Tc1.20, 23, 24, 26, 28, 32 The sole pediatric paper including CLA cytokine data highlights only the IL-13+CLA+ predominance in CD4 cells.30 Our results in AD children display a skin-selective Th1 defect, accompanied by skin-selective increases in Th2 and an imbalanced CLA+ Th1:Th2 ratio. While similar trends were observed in adults, levels and differences from controls were more striking in children. Increased migration to skin,39, 66 Th1 exhaustion67 and high susceptibility to apoptosis68 are all plausible explanations for the low IFN-γ in children. However, in light of the very high Th2 signal and high ICOS activation, we suggest that the main cause for decreased Th1 is suppression by Th2 cells.69, 70
Current IgE models suggest that Th2 T-cell differentiation is required to promote IgE class switching, with a suppressive effect of IFN-γ.71, 72 Thus, increased ICOS expression that contributes to Th2 differentiation, and in turn to Th1 suppression, may all drive the allergic phenotype observed in young children with AD, including high IgE levels, increased food allergy and other atopic diseases. Indeed, our data show that Tcm/Tem subsets in adults with AD do not correlate with disease severity or IgE levels, but in AD children they strongly correlate with IgE levels. This data supports the notion that Th2-dependent IgE class switching occurs early in the disease and persists into adulthood. Yet, the pathogenic role of high IgE in early-onset AD (seen in approximately 70% of affected children)29, 73, 74 remains unclear. Recent reports in adults suggest that IgE might serve to amplify immune activation rather than having a primary pathogenic role.75
Our results show that while adult AD demonstrates significantly higher IL-22+CLA+ than adult controls or pediatric AD, no differences in IL-22 are observed between healthy and AD children. AD in infants and young children tends to be characterized by acute lesions with erythema and dryness, whereas adults with long-standing disease have marked thickening or lichenification of lesions. Our data, together with the previously recognized role of IL-22 in inducing epidermal hyperplasia,76 support a role for IL-22 in disease chronicity and not in disease initiation. Immune reactions tend to have ‘epitope’ spreading over time, and in a similar way may involve T-helper subset expansion to Th22 seen in chronic AD in adults.
Previous cytokine data in children is discordant regarding relative CD4/CD8 immune dysregulation.20, 23, 25, 30, 32 Contrary to adults, and similar to our observations with ICOS, Th1/Th2 imbalance is not evident in CD8 T-cells.
We have shown that CD8 T-cells are a major source of IL-22 in adult AD skin lesions, and that CD8+IL-22+ T-cells are correlated with disease severity.40 Although AD was historically considered a CD4-driven disease, a pathogenic role has recently been elucidated for CD8 cells.77–79 Thus, in infants and young children CD8 T-cell immaturity,80–82 low counts,11, 82 exhaustion from super/antigenic stimulation (environmental, food, bacterial etc.) seen in AD46, 83–86 or dysfunctional states87 are all putative explanations for the low IL-22 signal in pediatric AD.
In addition to the Th2 bias at birth, bias towards Th17 T-cell development has recently been described in normal infants.88 According to our results, AD and control adults had higher IL-17 only among CLA− subsets compared to their respective pediatric counterparts. This may be due to a selectively higher IL-17 signal in CLA+ cells in children, but the trend of lower Th17 in AD children compared to controls suggests that AD children are failing to properly build this axis within the cutaneous compartment.
During the naïve-to-memory cell conversion in infancy and early childhood, there is a physiological increased susceptibility to infections.44, 89 In addition, increased S. aureus colonization, as evidenced by increased T-cell receptor (TCR) Vβ expression on CLA+ T-cells,90 underdeveloped IL-22, reduced IL-17 and suppressed IFN-γ, all considered protective cytokines against pathogens,91–98 might orchestrate the immune milieu, rendering AD children more susceptible to skin infections.99, 100
Interestingly, IL-9 is higher in adult versus pediatric AD, but only in the skin homing compartment. Ma et al.101 have previously shown that Th9 frequencies were higher in AD compared to controls and that frequency correlated with SCORAD and IgE levels. Furthermore, we showed increased IL-9 in chronic AD lesional skin.39 Alternately, higher Th9 frequency in adults versus children with AD may be attributed to higher allergic contact dermatitis seen in this group, though this is speculative.102
Studies have shown that approximately 75% of children "outgrow" their AD by 10 years of age.42, 103–105 One possibility, based on our data, is that age-related disease resolution in children may reflect increased differentiation of Th1 T-cells to counter-regulate the increased Th2, as well as reciprocal up-regulation of Th1/Th17 cells, which synergistically suppress Th2 production or Th22 development.
Finally, these results may have relevant therapeutic implications. While targeted anti-IL-22 treatment may be beneficial only in adults (ILV-094; ClinicaTrial.gov identifier: NCT01941537), investigating therapeutics that target Th2 cytokines, such as anti IL-13 mAb (Tralokinumab; ClinicaTrial.gov identifier: NCT02347176) and anti-IL-4R alpha mAb (Dupilumab [REGN668/SAR231893]; ClinicaTrial.gov identifier: NCT01979016) may be particularly valuable in the pediatric AD population.
Supplementary Material
Acknowledgments
Funding: This work was funded by a research grant to Drs. Guttman-Yassky and Paller from the LEO Foundation, research grant # 0266-2572, GCO # 13-1310. TC was co-sponsored by the Center for Basic and Translational Research on Disorders of the Digestive System through the generosity of the Leona M. and Harry B. Helmsley Charitable Trust. JG was supported in part by grant # UL1TR0000 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. DM received funding from the American Dermatological Association Medical Student Fellowship. JGK was supported by grant number 5UL1RR024143-02 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. Blood acquisition was supported by the Northwestern University Skin Disease Research Center (NIAMS P30AR057216).
Abbreviations
- AD
Atopic dermatitis
- CD69
Cluster of Differentiation 69
- CLA
Cutaneous Lymphocyte Antigen
- HLA-DR
human leukocyte antigen DR
- IFN-γ
Interferon gamma
- IL
Interleukin
- ICOS
Inducible co-stimulator molecule
- MHC
Major histocompatibility complex
- PBMC
Peripheral Blood Mononuclear Cell
- SCORAD
SCORing of Atopic Dermatitis
- Tc1
Type 1 Cytotoxic T-cell
- Tc17
Type 17 cytotoxic T-cell
- Tc2
Type 2 cytotoxic T-cell
- Tc22
Type 22 cytotoxic T-cell
- Th
T helper
- Th1
Type 1 helper T-cell
- Th17
Type 17 helper T-cell
- Th2
Type 2 helper T-cell
- Th22
Type 22 helper T-cell
- Y.O.
Years old
Footnotes
Disclosures: The authors have declared that they have no conflict of interest.
References
- 1.Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am. 2015;35:161–183. doi: 10.1016/j.iac.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3–16. doi: 10.1111/all.12270. [DOI] [PubMed] [Google Scholar]
- 3.von Kobyletzki LB, Svensson A, Apfelbacher C, Schmitt J. Which factors predict remission of infant atopic dermatitis? A systematic review. British Journal of Dermatology. 2014;170:E52–E53. doi: 10.2340/00015555-1941. [DOI] [PubMed] [Google Scholar]
- 4.Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, Irwin McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006;118:152–169. doi: 10.1016/j.jaci.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraja, Kanwar AJ, Dhar S, Singh S. Frequency and significance of minor clinical features in various age-related subgroups of atopic dermatitis in children. Pediatric Dermatology. 1996;13:10–13. doi: 10.1111/j.1525-1470.1996.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 7.Folster-Holst R. Management of atopic dermatitis: are there differences between children and adults? J Eur Acad Dermatol Venereol. 2014;28(Suppl 3):5–8. doi: 10.1111/jdv.12481. [DOI] [PubMed] [Google Scholar]
- 8.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton CA, Upham JW, Wikstrom ME, Holt BJ, White GP, Sharp MJ, et al. Functional maturation of CD4+CD25+CTLA4+CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol. 2004;173:3084–3092. doi: 10.4049/jimmunol.173.5.3084. [DOI] [PubMed] [Google Scholar]
- 10.Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, et al. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 11.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Ferran M, Santamaria-Babi LF. Pathological mechanisms of skin homing T cells in atopic dermatitis. World Allergy Organ J. 2010;3:44–47. doi: 10.1097/WOX.0b013e3181d675f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–1068. [PMC free article] [PubMed] [Google Scholar]
- 14.Jung T, Schulz S, Zachmann K, Neumann C. Expansion and proliferation of skin-homing T cells in atopic dermatitis as assessed at the single cell level. Int Arch Allergy Immunol. 2003;130:143–149. doi: 10.1159/000069010. [DOI] [PubMed] [Google Scholar]
- 15.Babi LFS, Picker LJ, Soler MTP, Drzimalla K, Flohr P, Blaser K, et al. Circulating Allergen-Reactive T-Cells from Patients with Atopic-Dermatitis and Allergic Contact-Dermatitis Express the Skin-Selective Homing Receptor, the Cutaneous Lymphocyte-Associated Antigen. Journal of Experimental Medicine. 1995;181:1935–1940. doi: 10.1084/jem.181.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akdis M, Akdis CA, Weigl L, Disch R, Blaser K. Skin-homing, CLA+ memory T cells are activated in atopic dermatitis and regulate IgE by an IL-13-dominated cytokine pattern: IgG4 counter-regulation by CLA-memory T cells. J Immunol. 1997;159:4611–4619. [PubMed] [Google Scholar]
- 17.Abernathycarver KJ, Sampson HA, Picker LJ, Leung DYM. Milk-Induced Eczema Is Associated with the Expansion of T-Cells Expressing Cutaneous Lymphocyte Antigen. Journal of Clinical Investigation. 1995;95:913–918. doi: 10.1172/JCI117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferran M, Romeu ER, Rincon C, Sagrista M, Arnau AMG, Celada A, et al. Circulating CLA+ T lymphocytes as peripheral cell biomarkers in T-cell-mediated skin diseases. Experimental Dermatology. 2013;22:439–442. doi: 10.1111/exd.12154. [DOI] [PubMed] [Google Scholar]
- 19.van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy. 2001;31:997–1006. doi: 10.1046/j.1365-2222.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- 20.Herberth G, Heinrich J, Roder S, Figl A, Weiss M, Diez U, et al. Reduced IFN-gamma- and enhanced IL-4-producing CD4+ cord blood T cells are associated with a higher risk for atopic dermatitis during the first 2 yr of life. Pediatr Allergy Immunol. 2010;21:5–13. doi: 10.1111/j.1399-3038.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 21.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–985. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaminishi K, Soma Y, Kawa Y, Mizoguchi M. Flow cytometric analysis of IL-4, IL-13 and IFN-gamma expression in peripheral blood mononuclear cells and detection of circulating IL-13 in patients with atopic dermatitis provide evidence for the involvement of type 2 cytokines in the disease. Journal of Dermatological Science. 2002;29:19–25. doi: 10.1016/s0923-1811(01)00174-8. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto N, Kaneko H, Takemura M, Seishima M, Sakurai S, Fukao T, et al. Age-related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol. 2006;17:125–133. doi: 10.1111/j.1399-3038.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 24.La Grutta S, Richiusa P, Pizzolanti G, Mattina A, Pajno GB, Citarrella R, et al. CD4(+)IL-13(+) cells in peripheral blood well correlates with the severity of atopic dermatitis in children. Allergy. 2005;60:391–395. doi: 10.1111/j.1398-9995.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DE, Fryga AS, Bol S, Kemp AS. Intracellular interferon-gamma (IFN-gamma) production in normal children and children with atopic dermatitis. Clin Exp Immunol. 1999;115:377–382. doi: 10.1046/j.1365-2249.1999.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsunuma T, Kawahara H, Yuki K, Akasawa A, Saito H. Impaired interferon-gamma production in a subset population of severe atopic dermatitis. Int Arch Allergy Immunol. 2004;134:240–247. doi: 10.1159/000078772. [DOI] [PubMed] [Google Scholar]
- 27.Machura E, Mazur B, Kwiecien J, Karczewska K. Intracellular production of IL-2, IL-4, IFN-gamma, and TNF-alpha by peripheral blood CD3+ and CD4+ T cells in children with atopic dermatitis. Eur J Pediatr. 2007;166:789–795. doi: 10.1007/s00431-006-0319-5. [DOI] [PubMed] [Google Scholar]
- 28.Antunez C, Torres MJ, Corzo JL, Pena RR, Mayorga C, Jurado A, et al. Different lymphocyte markers and cytokine expression in peripheral blood mononuclear cells in children with acute atopic dermatitis. Allergol Immunopathol (Madr) 2004;32:252–258. doi: 10.1016/s0301-0546(04)79251-4. [DOI] [PubMed] [Google Scholar]
- 29.Leonardi S, Rotolo N, Vitaliti G, Spicuzza L, La Rosa M. IgE values and T-lymphocyte subsets in children with atopic eczema/dermatitis syndrome. Allergy Asthma Proc. 2007;28:529–534. doi: 10.2500/aap2007.28.3038. [DOI] [PubMed] [Google Scholar]
- 30.Antunez C, Torres MJ, Mayorga C, Corzo JL, Jurado A, Santamaria-Babi LF, et al. Cytokine production, activation marker, and skin homing receptor in children with atopic dermatitis and bronchial asthma. Pediatr Allergy Immunol. 2006;17:166–174. doi: 10.1111/j.1399-3038.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 31.Chernyshov PV. Expression of activation inducer molecule (CD69) on CD3+CD8+ T lymphocytes in children with atopic dermatitis correlates with SCORAD but not with the age of patients. J Eur Acad Dermatol Venereol. 2009;23:462–463. doi: 10.1111/j.1468-3083.2008.02909.x. [DOI] [PubMed] [Google Scholar]
- 32.Jung T, Lack G, Schauer U, Uberuck W, Renz H, Gelfand EW, et al. Decreased frequency of interferon-gamma- and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–527. doi: 10.1016/s0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 33.Czarnowicki T, Shemer A, Malajian D, Xu H, Zheng X, Khattri S, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, Krueger JG, Guttman-Yassky E. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. Journal of Allergy and Clinical Immunology. 2015 doi: 10.1016/j.jaci.2015.01.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 35.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 36.Beier KC, Hutloff A, Dittrich AM, Heuck C, Rauch A, Buchner K, et al. Induction, binding specificity and function of human ICOS. European Journal of Immunology. 2000;30:3707–3717. doi: 10.1002/1521-4141(200012)30:12<3707::AID-IMMU3707>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM. CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: Blood and skin comparisons by flow cytometry. Journal of Autoimmunity. 2000;14:63–78. doi: 10.1006/jaut.1999.0343. [DOI] [PubMed] [Google Scholar]
- 38.Biselli R, Matricardi PM, Damelio R, Fattorossi A. Multiparametric Flow Cytometric Analysis of the Kinetics of Surface-Molecule Expression after Polyclonal Activation of Human Peripheral-Blood T Lymphocytes. Scandinavian Journal of Immunology. 1992;35:439–447. doi: 10.1111/j.1365-3083.1992.tb02879.x. [DOI] [PubMed] [Google Scholar]
- 39.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQF, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. Journal of Allergy and Clinical Immunology. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing T(H)17 T cells. Journal of Allergy and Clinical Immunology. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371–379. doi: 10.1016/j.jaip.2014.03.006. quiz 80–1. [DOI] [PubMed] [Google Scholar]
- 42.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 43.Cossarizza A, Ortolani C, Paganelli R, Barbieri D, Monti D, Sansoni P, et al. CD45 isoforms expression on CD4(+) and CD8(+) T cells throughout life, from newborns to centenarians: Implications for T cell memory. Mechanisms of Ageing and Development. 1996;86:173–195. doi: 10.1016/0047-6374(95)01691-0. [DOI] [PubMed] [Google Scholar]
- 44.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nature Reviews Immunology. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boguniewicz M, Leung DYM. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunological Reviews. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol. 2000;105:814–819. doi: 10.1067/mai.2000.105528. [DOI] [PubMed] [Google Scholar]
- 47.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 48.Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Invest Dermatol. 2012;132:933–939. doi: 10.1038/jid.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maino VC, Suni MA, Ruitenberg JJ. Rapid Flow Cytometric Method for Measuring Lymphocyte Subset Activation. Cytometry. 1995;20:127–133. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- 50.Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. Journal of Immunological Methods. 2004;293:127–142. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Caruso A, Licenziati S, Corulli M, Canaris AD, DeFrancesco MA, Fiorentini S, et al. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry. 1997;27:71–76. doi: 10.1002/(sici)1097-0320(19970101)27:1<71::aid-cyto9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4(+) and CD8(+) T cells. Immunological Reviews. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 53.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 54.Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, et al. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. Journal of Experimental Medicine. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 56.Mages HW, Hutloff A, Heuck C, Buchner K, Himmelbauer H, Oliveri F, et al. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. European Journal of Immunology. 2000;30:1040–1047. doi: 10.1002/(SICI)1521-4141(200004)30:4<1040::AID-IMMU1040>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Tesciuba AG, Shilling RA, Agarwal MD, Bandukwala HS, Clay BS, Moore TV, et al. ICOS costimulation expands Th2 immunity by augmenting migration of lymphocytes to draining lymph nodes. Journal of Immunology. 2008;181:1019–1024. doi: 10.4049/jimmunol.181.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bunikowski R, Staab D, Kussebi F, Brautigam M, Weidinger G, Renz H, et al. Low-dose cyclosporin A microemulsion in children with severe atopic dermatitis: Clinical and immunological effects. Pediatric Allergy and Immunology. 2001;12:216–223. doi: 10.1034/j.1399-3038.2001.012004216.x. [DOI] [PubMed] [Google Scholar]
- 59.Czarnowicki T, Fuentes-Duculan J, Gonzalez J, Suárez-Fariñas M, Krueger JG, Guttman-Yassky E. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. Journal of Allergy and Clinical Immunology. 2015 doi: 10.1016/j.jaci.2015.03.032. In Press. [DOI] [PubMed] [Google Scholar]
- 60.Seneviratne SL, Black AP, Jones L, Bailey AS, Ogg GS. The role of skin-homing T cells in extrinsic atopic dermatitis. QJM. 2007;100:19–27. doi: 10.1093/qjmed/hcl132. [DOI] [PubMed] [Google Scholar]
- 61.Antunez C, Torres MJ, Mayorga C, Cornejo-Garcia JA, Santamaria-Babi LF, Blanca M. Different cytokine production and activation marker profiles in circulating cutaneous-lymphocyte-associated antigen(+) T cells from patients with acute or chronic atopic dermatitis. Clinical and Experimental Allergy. 2004;34:559–566. doi: 10.1111/j.1365-2222.2004.1933.x. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Amaro R, Cortes JR, Sanchez-Madrid F, Martin P. Is CD69 an effective brake to control inflammatory diseases? Trends in Molecular Medicine. 2013;19:625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toma T, Mizuno K, Okamoto H, Kanegane C, Ohta K, Ikawa Y, et al. Expansion of activated eosinophils in infants with severe atopic dermatitis. Pediatrics International. 2005;47:32–38. doi: 10.1111/j.1442-200x.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 64.Thunberg S, Gafvelin G, Nord M, Gronneberg R, Grunewald J, Eklund A, et al. Allergen provocation increases TH2-cytokines and FOXP3 expression in the asthmatic lung. Allergy. 2010;65:311–318. doi: 10.1111/j.1398-9995.2009.02218.x. [DOI] [PubMed] [Google Scholar]
- 65.Werfel T, Boeker M, Kapp A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy. 1997;52:465–469. doi: 10.1111/j.1398-9995.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 66.Grewe M, Gyufko K, Schopf E, Krutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994;343:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 67.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 68.Zhang XH, Brunner T, Carter L, Dutton RW, Rogers P, Bradley L, et al. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. Journal of Experimental Medicine. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biedermann T, Rocken M, Carballido JM. TH1 and TH2 lymphocyte development and regulation of TH cell-mediated immune responses of the skin. J Investig Dermatol Symp Proc. 2004;9:5–14. doi: 10.1111/j.1087-0024.2004.00829.x. [DOI] [PubMed] [Google Scholar]
- 70.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 71.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 72.Kabashima-Kubo R, Nakamura M, Sakabe J, Sugita K, Hino R, Mori T, et al. A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: Possible immunological state of the intrinsic type. Journal of Dermatological Science. 2012;67:37–43. doi: 10.1016/j.jdermsci.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Fuiano N, Incorvaia C. Dissecting the causes of atopic dermatitis in children: less foods, more mites. Allergol Int. 2012;61:231–243. doi: 10.2332/allergolint.11-RA-0371. [DOI] [PubMed] [Google Scholar]
- 74.Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1–7. doi: 10.1016/j.jdermsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Suarez-Farinas M, Dhingra N, Gittler J, Shemer A, Cardinale I, Strong CD, et al. Intrinsic atopic dermatitis shows similar T(H)2 and higher T(H)17 immune activation compared with extrinsic atopic dermatitis. Journal of Allergy and Clinical Immunology. 2013;132:361–370. doi: 10.1016/j.jaci.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 77.Hennino A, Vocanson M, Toussaint Y, Rodet K, Benetiere J, Schmitt AM, et al. Skin-infiltrating CD8(+) T cells initiate atopic dermatitis lesions. Journal of Immunology. 2007;178:5571–5577. doi: 10.4049/jimmunol.178.9.5571. [DOI] [PubMed] [Google Scholar]
- 78.Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973–979. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akdis M, Simon HU, Weigl L, Kreyden O, Blaser K, Akdis CA. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8(+) T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. Journal of Immunology. 1999;163:466–475. [PubMed] [Google Scholar]
- 80.McDonagh M, Bell EB. The survival and turnover of mature and immature CD8 T cells. Immunology. 1995;84:514–520. [PMC free article] [PubMed] [Google Scholar]
- 81.Weerkamp F, de Haas EF, Naber BA, Comans-Bitter WM, Bogers AJ, van Dongen JJ, et al. Age-related changes in the cellular composition of the thymus in children. J Allergy Clin Immunol. 2005;115:834–840. doi: 10.1016/j.jaci.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 82.Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, Debruyere M, et al. Age-Related-Changes in Human Blood Lymphocyte Subpopulations .2. Varying Kinetics of Percentage and Absolute Count Measurements. Clinical Immunology and Immunopathology. 1994;70:152–158. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 83.Lezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 84.Schade RP, Van Ieperen-Van Dijk AG, Van Reijsen FC, Versluis C, Kimpen JLL, Knol EF, et al. Differences in antigen-specific T-cell responses between infants with atopic dermatitis with and without cow's milk allergy: Relevance of T(H)2 cytokines. Journal of Allergy and Clinical Immunology. 2000;106:1155–1162. doi: 10.1067/mai.2000.110802. [DOI] [PubMed] [Google Scholar]
- 85.Higaki S, Morohashi M, Yamagishi T, Hasegawa Y. Comparative study of staphylococci from the skin of atopic dermatitis patients and from healthy subjects. Int J Dermatol. 1999;38:265–269. doi: 10.1046/j.1365-4362.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 86.Agner T. Staphylococcal-mediated worsening of atopic dermatitis: many players involved. Br J Dermatol. 2010;163:1147. doi: 10.1111/j.1365-2133.2010.10114.x. [DOI] [PubMed] [Google Scholar]
- 87.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends in Immunology. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol. 2012;42:311–319. doi: 10.1002/eji.201141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–1035. doi: 10.1086/605562. [DOI] [PubMed] [Google Scholar]
- 90.Torres MJ, Gonzalez FJ, Corzo JL, Giron MD, Carvajal MJ, Garcia V, et al. Circulating CLA(+) lymphocytes from children with atopic dermatitis contain an increased percentage of cells bearing staphylococcal-related T-cell receptor variable segments. Clinical and Experimental Allergy. 1998;28:1264–1272. doi: 10.1046/j.1365-2222.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 91.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerging Microbes & Infections. 2013;2 doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nature Immunology. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 93.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. Journal of Clinical Investigation. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential Roles of Interleukin-17A and-17F in Host Defense against Mucoepithelial Bacterial Infection and Allergic Responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Liu SY, Sanchez DJ, Aliyari R, Lu S, Cheng GH. Systematic identification of type I and type II interferon-induced antiviral factors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4239–4244. doi: 10.1073/pnas.1114981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 98.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 99.Leung DYM. Atopic dermatitis: New insights and opportunities for therapeutic intervention. Journal of Allergy and Clinical Immunology. 2000;105:860–876. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 100.Leung DYM, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. Journal of Allergy and Clinical Immunology. 2011;127:U965–U210. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175:25–31. doi: 10.1111/cei.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J, Harberts E, Tammaro A, Girardi N, Filler RB, Fishelevich R, et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J Invest Dermatol. 2014;134:1903–1911. doi: 10.1038/jid.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burr ML, Dunstan FD, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol. 2013 doi: 10.1111/bjd.12216. [DOI] [PubMed] [Google Scholar]
- 105.Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. 2013;110:24–28. doi: 10.1016/j.anai.2012.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.