Abstract
Introduction
Relative to antiretroviral treatment (ART), early HIV cure-related trials (HCRTs) carry limited therapeutic benefits and unknown risks. In HCRTs requiring treatment interruption (TI) the health risks and burdens may create a barrier to study enrolment and increase the possibility for unintentional ethical violations in recruitment.
Methods
An online survey was administered to over 2,000 HIV-positive ART users in the US. Using multivariable ordinal regression we assessed effects of research participation attitudes, health and demographic traits on willingness to participate in treatment interruption studies (WtP-TI).
Results
WtP-TI was greatest among those who were highly motivated to participate in research studies for the benefit of science, society and, to a lesser extent, personal benefit. Personal benefit was less of an influence on WtP-TI among persons with higher viral loads or a history of multiple ART regimens. WtP-TI was greater among respondents who were more likely to consider personal health in making decisions about trial participation. WtP-TI had no association with perceptions of the importance of compensation to research participation. After accounting for attitudes, health status and demographic traits were generally not significantly related to WtP-TI. Notable exceptions included viral suppression status and race/ethnicity.
Conclusion
Recruitment strategies in TI studies can benefit from a focus on the long-term scientific and social benefits of study participation. Strategies targeted to particular demographic groups may have little impact on accrual, and in some cases will need to be accompanied by strategies to improve the quality of researcher–community relationships. Findings also suggest that informing communities about the health impacts of trial participation may positively impact participation decisions. However, more research is needed to interpret the impact of health messaging on recruitment and therapeutic expectations. Future work should explore the implications of altruism-based expectations on the strategic and ethical appropriateness of TI study recruitment efforts.
Keywords: HIV cure, clinical research, treatment interruption, patient attitudes, willingness to participate, altruism
Introduction
Antiretroviral treatments (ARTs) have substantially improved the health and wellbeing of persons living with HIV (PLWHs) [1–8]. Nevertheless, inadequate ART access, substantial viral resistance, long- and short-term side–effects, imperfect adherence and persistent high–risk behaviours all lead to incomplete viral suppression and demonstrate the growing need to identify an effective cure for HIV [9]. Cure strategies currently being explored may emphasise the complete elimination of HIV from the body, viral suppression and maintenance of HIV in the absence of ART and immunity from future HIV infection [10–14]. Early HIV cure-related trials (HCRTs) are likely to have limited therapeutic benefits while increasing susceptibility to severe or unknown health risks [11]. Moreover, most HCRTs will probably involve some degree of treatment interruption (discontinuation of ART use for a specified time period) to support proof of concepts, assess HIV clinical progression, and evaluate the safety and efficacy of novel modalities. Given the well-documented health benefits of ART adherence – including reduced drug resistance, decreased immune activation, fewer co-morbidities such as cardiovascular disease, lower mortality and less onward transmission [6–8,15–24] – HCRT studies involving treatment interruption (TI studies) will have a less favourable therapeutic risk–benefit profile compared to current treatment standards, particularly for virally suppressed PLWHs. The potentially high–risk profile of TI studies could present a significant obstacle to study accrual. The challenge to recruitment for TI studies may also be compounded by recent reports in mainstream media on viral rebound after ART discontinuation in persons previously considered to be ‘cured’ of HIV (e.g. the ‘Boston patients’ and ‘Mississippi baby’) [25,26]. Targeted or novel outreach approaches may be required to improve recruitment efficiency, equity and success.
Recruitment efforts may be enhanced by targeting outreach to particular study-eligible groups, or by working to improve attitudes about TI studies among harder-to-engage populations [27–33]. One goal of the present study is to provide preliminary evidence on associations between demographic and health traits, and willingness to participate in TI studies (WtP–TI). Targeted and non-targeted recruitment strategies may appeal to relevant concerns and attitudes about the benefits, burdens and risks of participation [27–33]. We focus on two classes of attitudes that may influence participation decision-making. One class of motivational attitudes reflects expectations about the immediate or long-term outcomes of trial participation. We refer to these as outcome attitudes, and these include attitudes about the personal, social or scientific benefits of participation. The latter two benefits are commonly considered altruistic motivations. A second class of motivational attitudes we call resource attitudes reflect perspectives about potential burdens of participation on important resources, with an emphasis on factors affecting daily functioning. Participation may impact therapeutic resources such as one's health, or non–therapeutic resources such as income, employment or social relationships. A second goal of our study is to explore the relative potential for these different attitudes to influence WtP-TI, and to assess whether personal (demographic and health) traits moderate the attitudinal effects.
Our study is also concerned with ethical considerations in TI studies; in particular, unrealistic expectations, therapeutic misconception, coercion and exploitation [34–38]. Participation in high-risk, low-benefit trials is not necessarily unethical, even when effective alternatives (i.e. ART) are widely available [38,39]. However, phrases such as ‘cure’ can lead to unrealistic expectations, or therapeutic misconceptions about the benefits of participation. Coercion and exploitation in clinical trials reflect both intentional/unintentional behaviours of researchers (or influential others), and perceptions of potential volunteers [37,40]. Resource disadvantage may compel people to participate in a trial they otherwise would not have joined in the hopes of acquiring needed supports. Such motivations can increase the potential for exploitation or coercion in recruitment of volunteers. Although our study does not directly explore these ethical issues, we intend our analyses to highlight potential factors for future explorations of ethical recruitment strategies in TI studies.
Methods
Sample and recruitment
From December 2011–January 2012, 2,262 HIV-positive individuals completed an uncompensated online survey exploring HCRT participation attitudes. Participants were recruited through popular HIV educational and community websites and list-serves. Eligibility was restricted to those who were HIV-positive and over the age of 16. Survey participants were provided with a brief overview of HCRTs. Our analyses are restricted to respondents who reported current ART use (n=2,100), and who had non–missing data on the measured variables (n=2,094; 92% of original respondent sample).
WtP-TI measure
Our primary outcome measure is the willingness of respondents to participate in treatment interruption studies (WtP-TI). This was assessed through a single item: ‘If a study would require you to go off of your HIV medication for a period of time, which might carry health risk, how willing would you be to participate?’ A 4–point Likert response scale was used with responses ranging from ‘not at all willing’ to ‘very willing’.
Outcome attitudes
In this study attitudinal measures reflect attitudes about participation in HCRTs in general and are not limited to TI studies specifically. Among our outcome attitude measures, the personal benefit item asked respondents ‘Assuming that entering a study might pose health problems and other risks, how much would the chance to benefit yourself by participating in a study motivate you to join the study?’ Social benefit was measured with the statement: ‘Assuming that entering a study might pose health problems and other risks, how much would the chance to benefit others by participating in the study motivate you to join the study?’ Scientific benefit was measured as: ‘Assuming that entering a study might pose health problems and other risks, if you were aware that you would probably not benefit from a new drug or procedure being studied, but that your participation in the study might advance the field of HIV research, how willing would you be to participate?’ All items were rated on a 4-point Likert scale with responses ranging from ‘not at all motivated’ to ‘very motivated’. The statement ‘potential health risks and other harms’ included in each of the above measures does not specify precise harms or risks. Thus, individuals may differ in how they interpret this statement (see Discussion below).
Resource attitudes
Resource attitude measures focus on the importance or influence of a given resource to participation decisions. Similar to outcome attitude measures, our resource attitude measures focus on participation in HCRTs in general. We focus on two resource attitudes: perceived health influence (‘How much would your current health affect your willingness to participate in studies that may eventually lead to a cure for HIV?’) and the importance of compensation to trial participation (‘Assuming that entering a study might pose health problems and other risks, how important would it be to compensate you for your time and discomfort?’). Both items were rated on 4-point scales ranging from ‘very reluctant’ to ‘very motivated’ (health influence), or ‘not at all important’ to ‘very important’ (compensation importance).
Health traits
HIV diagnostic measures were self-reported and include current viral load [<50 copies/mL, i.e. suppressed, 50+ copies/mL, or ‘Don't know’(DK)], and CD4 cell count (>500, 351–500, <351, or ‘Don't know’). HIV experience includes ‘years HIV positive’ (categorical) and the ‘number of ART regimens since first diagnosis’ (categorical). Perceived current health was rated on a 4-point response scale ranging from ‘very poor’ to ‘excellent’.
Demographic traits
Gender was reported as male, female, transgender [separately for male-to-female (MTF) and female-to-male (FTM)], or transitioning (separately by MTF and FTM). Very few respondents (<1%) identified as transgender or transitioning (and only as MTF). We include these women within the ‘female’ designation. Latino/Hispanic ethnicity was asked separately from racial identity. We combined these variables to obtain the following racial/ethnic designations: white (not Latino), Latino (alone or in combination), black and ‘other race’. We also measured age, annual income, employment status and highest level of educational attainment.
Analyses
In addition to standard univariate descriptive analyses, we conducted bivariate and multivariate analyses. First, we conducted chi-squared analyses exploring differences in the distributions of outcome and resource attitudes about HCRT participation. Second, the health and compensation resource attitude items reflect the importance of these resource considerations to HCRT participation decision-making. We conducted supplemental multivariable ordinal regression analyses of these resource attitudes to assess associations between (a) health resource attitudes and health traits, and (b) compensation resource attitudes and demographic traits. These supplemental analyses are intended to highlight personal traits that might influence responsiveness to resource attitude-based recruitment strategies. Third, we employed ordinal logistic regression to estimate the effects of our attitudinal, health and demographic factors on WtP-TI. This analysis provides insight into the potential impact of trait-targeted or attitude-focused recruitment strategies on TI study accrual. We also tested a series of interaction terms between the significant trait and attitudinal measures in our model in order to assess the extent to which the relative importance of attitudinal motivators of WtP-TI differ by personal traits. Proportional odds models included a logit link and unstructured thresholds, and Wald-type confidence intervals were estimated. Analyses were conducted in the R statistical software and proportional odds models were estimated using the Ordinal package [41,42].
Results
Sample demographic and health traits
The sample largely comprised older, college-exposed white men with low to moderate income. Men accounted for 83% of the sample, and 74% of the sample identified as white. Only about 10% of the sample identified as Latino (alone or in combination) and 10% identified as black. Of respondents, 44% were over the age of 50, and 34% were between the ages of 41and 50. Nearly 50% of respondents reported income below $25,000 per year (22% <$10,000, 27% $10,000–24,999), 27% had income between $25,000 and $49,999, 15% had income between $50,000 and $74,999, and roughly 20% had income at or above $75,000. Respondents were generally either employed full time (48%) or on disability (28%), and 55% had at least a college degree compared to 15% with only a high school degree/equivalent.
With respect to self-reported health, 36% of respondents stated that they were in excellent health, and an additional 45% stated that their health was ‘good’. Nearly 87% of respondents reported suppressed viral loads (<50 copies per mL), and a small majority (55%) had CD4 cell counts above 500 cells/μL. For both the viral load and CD4 cell items, roughly 2% of the sample (n=45 and 48, respectively) were unaware of their current status. We make the assumption that persons reporting unknown HIV diagnostics are not likely to be engaged in regular HIV monitoring and include them with those reporting unsuppressed viral loads and lower CD4 cell counts. Roughly 30% of respondents were on their first regimen, 46% were on second, third or fourth regimens, and 23% were on their fifth or higher regimen. Over half of respondents (57%) received an HIV diagnosis over 10 years prior, and very few (17%) had been diagnosed in the previous 3 years.
Decision-making outcome and resource attitudes
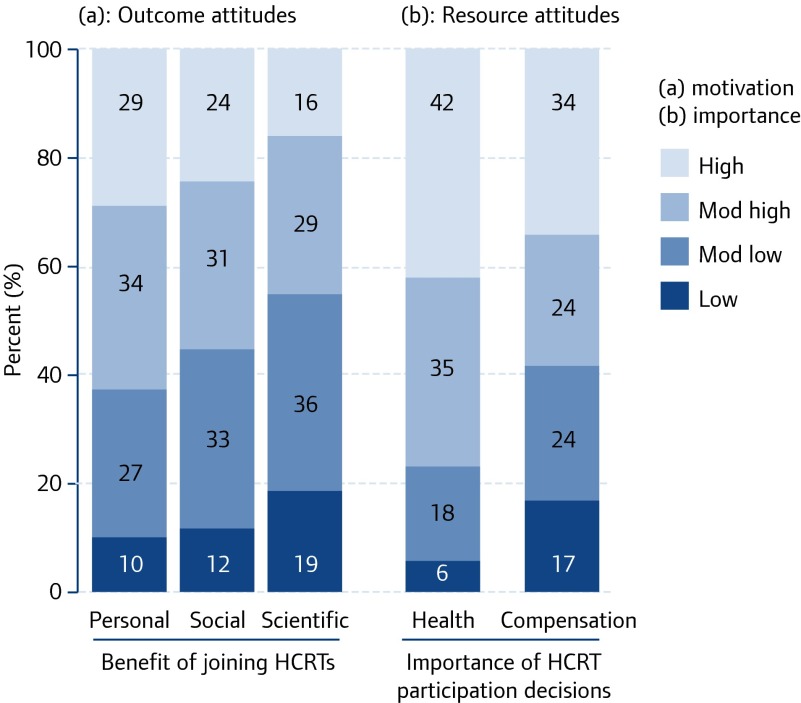
We hypothesise that willingness to participate in TI studies will vary according to perceived motivation to participate in any HIV cure-related trials. We explored five potential motivators: personal benefit; social benefit; scientific benefit; health influence; and financial compensation. Figure 1 highlights the distribution of responses to each of these motivators. For each item, the 4-point motivational attitude responses ranged from low (e.g. ‘not at all motivated’ to participate for personal benefit) to high (e.g. ‘very motivated’). Most respondents stated that they were motivated or very motivated to participate in any HCRTs for personal (62%) or social (56%) benefits, whereas less than half of the sample was motivated or very motivated to participate for scientific (45%) benefit. Current health was reported as an important influence on participation, with 42% of respondents stating that their current health was a significant motivator (‘very motivated’), and another 35% stating that it was ‘somewhat’ of a motivator. Only 23% of respondents expressed reluctance to participate due to current health. Although 34% of respondents noted that financial compensation was ‘very important’ to HCRT participation, the remaining respondents were nearly equally likely to state that compensation was not important (17%), somewhat important (24%) or important (24%).
Figure 1.
Distribution of outcome and resource attitudes about participation in HCRTs (n=2,094)
We conducted chi-squared tests of homogeneity to assess the degree of difference in the distributions of the attitudinal variables presented in Figure 1. All chi-squared analyses were significant at P<0.001 (Table 1). We classify distributions as meaningfully different if they lie above the median χ2 of 293. Respondents were much more likely to be motivated to participate in HCRTs for personal as opposed to scientific benefits (χ2=338). Social benefit motivations mirrored perspectives on both personal (χ2=47) and scientific (χ2=168) benefit. Respondents’ views on the relevance of health and compensation considerations to participation decisions most closely reflected personal benefit motivations (χ2<248) and differ substantially from social or scientific benefit motivations.
Table 1.
Chi–squared comparisons of outcome and resource attitudes about HCRT participation (n=2,094)*
| Social | Scientific | Health | Compensation | |
|---|---|---|---|---|
| Personal | 47.3 | 338.3 | 247.2 | 169.9 |
| Social | 167.5 | 493.0 | 208.7 | |
| Scientific | 1275.9 | 495.8 | ||
| Health | 638.3 |
All chi–squared values significant at P<0.001; df=3; values in bold reflect χ2 values above the median value.
In order to better interpret the distributional meanings of health and compensation resource attitudes we regressed these items on health and demographic traits, respectively. For brevity we present only those variables demonstrating significance in each of the models in Table 2. The relationship between personal traits and resource attitudes about trial participation is complex and multidimensional. Persons reporting greater influence of health considerations to trial participation (Table 2a) had higher viral loads and lower CD4 cell counts compared to others, but (perhaps paradoxically) rated their self-reported health as ‘excellent’. Years HIV positive and number of ART regimens since diagnosis were unrelated to health resource attitudes. Persons reporting greater importance of compensation to trial participation (Table 2b) were black or Latino, and had low incomes (<$10,000). However, unemployed individuals were far less likely to state that compensation was important to their decision-making. Age, sex and education were not significantly related to compensation importance.
Table 2.
Adjusted relative odds ratios for significant associations between health and demographic traits and resource attitudes about HCRT participation (n=2,094)
| Variable | αROR (95% CI) |
|---|---|
| (a) Health resources attitudes in HCRT participation decision–making | |
| Viral load (copies/μL) | |
| <50 | ref |
| 50+/DK | 1.32* (1.04, 1.69) |
| CDF | |
| >500 | ref |
| 351–500 | 0.98 (0.81, 1.19) |
| <351/DK | 1.28* (1.04, 1.59) |
| Health | |
| Excellent | ref |
| Good | 0.69† (0.58, 0.83) |
| Fair/Poor/Very poor | 0.48† (0.38, 0.61) |
| (b) Compensation resource attitudes in HCRT decision–making | |
| Race/ethnicity | |
| White | ref |
| Latino | 1.54‡ (1.17, 2.02) |
| Black | 2.21‡ (1.66, 2.94) |
| Other | 1.19 (0.83, 1.71) |
| Income | |
| <$10,000 | ref |
| $10,000–24,999 | 0.71† (0.52, 0.96) |
| $25,00–49,999 | 0.47* (0.34, 0.64) |
| $50,000–74,999 | 0.32‡ (0.22, 0.46) |
| $75,000–100,000 | 0.27‡ (0.18, 0.41) |
| >$100,000 | 0.18‡ (0.12, 0.26) |
| Employment status | |
| Full time | ref |
| Part time | 0.91 (0.69, 1.20) |
| Unemployed | 0.548* (0.54, 0.97) |
| Disability (temporary or permanent) | 0.96 (0.76, 1.22) |
P<0.05,
P<0.01,
P<0.001.
Determinants of willingness to participate in treatment interruption studies (WtP-TI)
Most respondents expressed ambivalence about participating in TI studies. Only a third (34%) of respondents stated that they would be ‘willing’ or ‘very willing’ to participate in HCRTs that involve TI, compared to 34% who agreed that they would be ‘somewhat willing’, and 32% reporting that they would be ‘not at all willing’ to participate in TI studies. Table 3 lists the adjusted odds ratios of our attitudinal (outcome and resource) and personal (health and demographic) trait measures on WtP–TI. A significant positive gradient relationship with WtP-TI was evident for personal, social and scientific benefit motivators and for health influence. In ANOVA stepwise exclusion analyses of each of the attitudinal items in a main effects model (i.e. excluding interaction terms) removal of the scientific benefit measure yielded the highest change in the likelihood ratio (LR) statistic (LR=101.3), followed by health resource attitude (LR=55.6), social benefit (LR=32.1) and personal benefit (LR=19.0). All likelihood ratio statistics were significant at P<0.001. Interestingly, persons who considered their own health as important to participation decision-making were up to six times more likely to express higher levels of WtP–TI compared to peers who did not consider health as important to participation decision-making. As noted in Table 2 (panel a) whether these derive from challenges to managing HIV (e.g. unsuppressed viral load) or feeling healthy enough to participate in such studies remains to be seen. The absence of interaction effects between health resource attitudes and health traits further complicates interpretation. With respect to health traits, persons with unsuppressed (>50/DK) viral loads, and persons with a treatment history of two or more ART regimens were significantly more likely than others to express higher WtP–TI. However, among persons who reported high levels of motivation to participate in HCRTs for personal benefit, those with higher viral loads (>50/DK) had lower WtP-TI scores [interaction (a) in Table 3]. Additionally, among persons who reported moderately low personal benefit motivations for trial participation, those who had a history of two or more ART regimens were less likely than their counterparts to express high levels of WtP-TI [interactions (b)–(d)]. Black respondents and those 60 years or older reported lower WtP-TI relative to others irrespective of outcome or resource attitudes. Latino respondents reported higher WtP-TI scores relative to others.
Table 3.
Relative odds ratios (ROR) from final ordinal logistic regression model of willingness to participate in treatment interruption studies (WtP–TI) (n=2,094)
| Variable1 | αROR2 (95% CI) |
|---|---|
| Personal benefit motivation | |
| Low | ref |
| Moderate–low | 2.87† (1.38, 5.98) |
| Moderate–high | 4.88‡ (2.38, 9.98) |
| High | 4.69‡ (2.25, 9.77) |
| Social benefit motivation | |
| Low | ref |
| Moderate–low | 1.57* (1.05, 2.37) |
| Moderate–high | 2.38‡ (1.52, 3.75) |
| High | 3.53‡ (2.16, 5.78) |
| Scientific benefit motivation | |
| Low | ref |
| Moderate–low | 3.76‡ (2.08, 6.82) |
| Moderate–high | 5.00‡ (2.78, 8.98) |
| High | 6.69‡ (3.72, 12.03) |
| Viral load (copies/μL) | |
| <50 | ref |
| 50+/DK | 4.10† (1.63, 10.32) |
| ART regimens | |
| 1 | ref |
| 2 | 3.17* (1.20, 8.40) |
| 3 | 3.51* (1.35, 9.13) |
| 4 | 4.13* (1.37, 12.48) |
| 5+ | 3.42* (1.28, 9.09) |
| Race/ethnicity | |
| White | ref |
| Latino | 1.36* (1.02, 1.83) |
| Black | 0.70* (0.52, 0.93) |
| Other | 0.80 (0.55, 1.16) |
| Age | |
| <31 | ref |
| 31–40 | 0.99 (0.67, 1.44) |
| 41–50 | 1.01 (0.70, 1.44) |
| 51–60 | 0.81 (0.56, 1.17) |
| >60 | 0.54† (0.35, 0.82) |
| Significant interaction | |
| (a) Personal benefit=High/Viral load=50+/DK | 0.32* (0.11, 0.87) |
| (b) Personal benefit=Mod–low/ART regimens=2 | 0.34* (0.12, 0.99) |
| (c) Personal benefit=Mod–low/ART regimens=4 | 0.22* (0.07, 0.72) |
| (d) Personal benefit=Mod–low/ART regimens=5+ | 0.25† (0.09, 0.69) |
Other variables tested but not significant: importance of financial compensation; current CD4; years HIV positive, perceived current health; gender; income; employment; and educational attainment.
Adjusted relative odds of expressing greater support for participation in HIV cure related trials (HCRTs).
P<0.05,
P<0.01,
P<0.001.
Discussion
Our findings suggest that appealing to scientific altruism for TI study participation may have the highest impact on accrual, irrespective of demographic or health traits. Perhaps paradoxically, we found that persons for whom health resource considerations were highly relevant to study participation decision-making were more likely to express support for TI study participation compared to others, even after accounting for health traits and personal benefit motivators. We also found an ambiguous relationship between health resource attitudes about participating in HCRTs and self-reported health traits, which suggests that the health risks and benefits of participation may be complexly related to decision-making. Moreover, to the extent that our findings support demographic-targeting in TI recruitment strategies to increase accrual or diversity, they suggest that developing positive researcher–community relationships may be more important than identity-focused marketing. Among black respondents, in particular, the lower levels of WtP-TI and greater importance of compensation to participation decision-making irrespective of attitudes, health, and economic considerations may reflect greater distrust for high-risk/low-benefit trial participation among blacks. Demographically targeted TI recruitment strategies will likely only succeed where the legacy of government- and academic-sponsored abuses based on identity and group affiliations are openly addressed, and direct researcher–community trust is actively promoted.
Our results suggest that future ethical research should explore the implications of recruitment strategies that heavily appeal to scientific and social altruism or health resource attitudes. These considerations have a strong impact on WtP-TI such that unrealistic expectations or therapeutic misconceptions may unduly sway participation decision making. Appeals to personal benefit, while affecting WtP-TI, did not carry as much weight as the other motivational attitudes.
The absence of WtP-TI associations with compensation resource attitudes, gender, income, employment and education may either be a consequence of the sample recruited for the study, or indicate that these factors do not substantially influence TI participation decision making. More in-depth qualitative and quantitative work is needed to explore influence of structural factors such as social organisation, cultural/community norms, and differential access on participation decision making.
Among our study limitations, survey recruitment and completion was conducted entirely online through diverse yet select websites. Thus, our sample excludes PLWHs who never or rarely access these sites. Survey respondents did not receive financial compensation, which may have affected motivations to complete the survey. However, lack of compensation would also reduce the incentive for respondents to complete these anonymous surveys multiple times, or provide socially desirable responses. In some cases (especially our attitudinal and WtP items) the complexity of survey wording may have limited full understanding of items and responses. Future work should continue to assess facilitators of risk, benefit, burden and procedural understanding among PLWHs interested in participating in TI studies.
Conclusion
Our results indicate that support for participation in TI studies will probably be predicated on altruistic and health resource considerations, irrespective of therapeutic risks, personal benefits, and health and demographic traits. Future work should explore these considerations in greater detail. Such work can aid HCRT TI researchers in their goal to improve effective outreach, raise awareness, assess community support and gaps, and increase accrual rates and diversity. Additionally, more research is needed to understand the ethical implications of these studies for persons who decide to participate. These ethical considerations will require attention to social, cultural, and structural components of altruistic motivations for participation. Qualitative and quantitative work in this area should better explicate and calibrate the importance of specific study designs, risks, burdens, and benefits to WtP-TI. The views of providers, educators and advocates should also be explored as these may be essential to promoting participation and developing acceptable research and engagement strategies for HCRTs. Future work should explore the ethical implications of altruism-based recruitment strategies on participation expectations, and of researcher–community trust on coercion and exploitation in demographically targeted recruitment efforts.
Acknowledgements
We would like to thank members of the International AIDS Society (IAS) Psychosocial Study Section for their feedback and discussion of previous analyses of this data, and Dr Joseph Turner for his review of earlier versions of our manuscript. Most importantly, we would like to thank the thousands of individuals who completed the uncompensated survey. The study received approval through the Institutional Review Board at Fred Hutchinson Cancer Research Center (Seattle, WA).
Authors’ contributions
DE and NV led survey creation and dissemination and data collection. MA conducted analyses and led manuscript development. All authors reviewed and edited the final manuscript.
Competing interests
No competing interests exist for the authors.
References
- 1. Palella FJ Jr, Baker RK, Moorman AC et al. . Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43: 27– 34. [DOI] [PubMed] [Google Scholar]
- 2. Kohli R, Klein RS, Schoenbaum EE et al. . Aging and HIV infection. J Urban Health 2006; 83: 31– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cain LE, Cole SR, Chmiel JS et al. . Effect of highly active antiretroviral therapy on multiple AIDS-defining illnesses among male HIV seroconverters. Am J Epidemiol 2006; 163: 310– 315. [DOI] [PubMed] [Google Scholar]
- 4. Sterne JAC, Hernan MA, Ledergerber B et al. . Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet 2005; 366: 378– 384. [DOI] [PubMed] [Google Scholar]
- 5. Nash D, Katyal M, Shah S.. Trends in predictors of death due to HIV-related causes among persons living with AIDS in New York City: 1993–2001. J Urban Health 2005; 82: 584– 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El-Sadr WM, Affrunti M, Gamble T, Zerbe A.. Antiretroviral therapy: a promising HIV prevention strategy? J AIDS 2010; 55: S116– S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Sadr WM, Coburn BJ, Blower S.. Modeling the impact on the HIV epidemic of treating discordant couples with antiretrovirals to prevent transmission. AIDS 2011; 25: 2295– 2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Sadr WM, Lundgren J, Neaton JD et al. . CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355: 2283– 2296. [DOI] [PubMed] [Google Scholar]
- 9. Dieffenbach CW, Fauci AS.. Thirty years of HIV and AIDS: future challenges and opportunities. Ann Intern Med 2011; 154: 766– 771. [DOI] [PubMed] [Google Scholar]
- 10. Chun T-W, Fauci AS.. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS 2012; 26: 1261– 1268. [DOI] [PubMed] [Google Scholar]
- 11. Deeks S, Autran B, Berkhout B et al. . Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 2012; 12: 607– 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston R, Barre-Sinoussi F.. Controversies in HIV cure research. J Int AIDS Soc 2012; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewin SR, Evans VA, Elliott JH et al. . Finding a cure for HIV: will it ever be achievable? J Int AIDS Soc 2011; 14: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewin SR, Rouzioux C.. HIV cure and eradication: how will we get from the laboratory to effective clinical trials? AIDS 2011; 25: 885– 897. [DOI] [PubMed] [Google Scholar]
- 15. Ickovics JR, Cameron A, Zackin R et al. . Consequences and determinants of adherence to antiretroviral medication: results from Adult AIDS Clinical Trials Group protocol 370. Antivir Ther 2002; 7: 185– 193. [DOI] [PubMed] [Google Scholar]
- 16. Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT.. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharmaceut Des 2005; 11: 1805– 1843. [DOI] [PubMed] [Google Scholar]
- 17. Thompson MA, Aberg JA, Cahn P et al. . Antiretroviral treatment of adult HIV infection 2010 recommendations of the International AIDS Society-USA Panel. JAMA 2010; 304: 321– 333. [DOI] [PubMed] [Google Scholar]
- 18. Sterne JAC, May M, Costagliola D et al. . Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 2009; 373: 1352– 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rieder P, Joos B, Wyl V et al. . HIV-1 transmission after cessation of early antiretroviral therapy among men having sex with men. AIDS 2010; 24: 1177– 1183. [DOI] [PubMed] [Google Scholar]
- 20. Ray M, Logan R, Sterne JAC et al. . The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24: 123– 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montaner JSG, Lima VD, Barrios R et al. . Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376: 532– 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitahata MM, Gange SJ, Abraham AG et al. . Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360: 1815– 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasse B, Ledergerber B, Furrer H et al. . Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53: 1130– 1139. [DOI] [PubMed] [Google Scholar]
- 24. Granich R, Crowley S, Vitoria M et al. . Highly active antiretroviral treatment as prevention of HIV transmission: review of scientific evidence and update. Curr Opin HIV AIDS 2010; 5: 298– 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelland K. Relapse of ‘cured’ HIV patients spurs AIDS science on. Reuters 2014. Available at: http://www.reuters.com/article/2014/01/02/us-aids-cure-idUSBREA010B520140102 ( accessed November 2014). [Google Scholar]
- 26. Henrich TJ, Hanhauser E, Sirignano MN et al. . HIV-1 Rebound Following Allogeneic Stem Cell Transplantation and Treatment Interruption. 21st Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA. March 2014. Abstr. 144LB.
- 27. Slomka J, Kypriotakis G, Atkinson J et al. . Factors associated with past research participation among low-income persons living with HIV. AIDS Patient Care STD 2012; 26: 496– 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone VE, Mauch MY, Steger K, Janas SF, Craven DE.. Race, gender, drug use, and participation in AIDS clinical trials – Lessons from a municipal hospital cohort. J Gen Intern Med 1997; 12: 150– 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sengupta S, Strauss RP, DeVellis R et al. . Factors affecting African-American participation in AIDS research. J AIDS 2000; 24: 275– 284. [DOI] [PubMed] [Google Scholar]
- 30. Mills E, Wilson K, Rachlis B et al. . Barriers to participation in HIV drug trials: a systematic review. Lancet Infect Dis 2006; 6: 32– 38. [DOI] [PubMed] [Google Scholar]
- 31. Gifford AL, Cunningham WE, Heslin KC et al. . Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med 2002; 346: 1373– 1382. [DOI] [PubMed] [Google Scholar]
- 32. Wolak C, Bass SB, Tedaldi E et al. . Minority HIV patients’ perceptions of barriers and facilitators to participation in clinical research. Curr HIV Res 2012; 10: 348– 355. [DOI] [PubMed] [Google Scholar]
- 33. Adeyemi OF, Evans AT, Bahk M.. HIV-infected adults from minority ethnic groups are willing to participate in research if asked. AIDS Patient Care STD 2009; 23: 859– 865. [DOI] [PubMed] [Google Scholar]
- 34. Appelbaum PS, Anatchkova M, Albert K et al. . Therapeutic misconception in research subjects: development and validation of a measure. Clin Trials 2012; 9: 748– 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Appelbaum PS, Lidz CW.. The mismeasure of therapeutic misconception. Clin Trials 2012; 9: 765– 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hawkins JS, Emanuel EJ.. Clarifying confusions about coercion. Hastings Center Rep 2005; 35: 16– 19. [PubMed] [Google Scholar]
- 37. Kirkwood K. On the exploitation of research subjects. J Clin Res Bioeth 2012; 3: 1– 3. [Google Scholar]
- 38. Jansen LA, Appelbaum PS, Klein WM et al. . Unrealistic optimism in early-phase oncology trials. IRB. 2011; 33: 1– 8. [PMC free article] [PubMed] [Google Scholar]
- 39. Jansen LA. The ethics of altruism in clinical research. Hastings Center 2009; 39: 26– 36. [DOI] [PubMed] [Google Scholar]
- 40. MalmqVist E. ( Mis)understanding exploitation. IRB 2011; 33: 1– 5. [PubMed] [Google Scholar]
- 41. R Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 42. Christensen RHB. Ordinal: Regression Models for Ordinal Data: R package version 2013.9-30 2013.