Abstract
Background
In 2013, an estimated 686,000 people died from hepatitis B virus (HBV) infection worldwide. Mass treatment programmes for hepatitis B will require very low drug costs. International treatment guidelines recommend first-line monotherapy with either entecavir or tenofovir disoproxil fumarate (TDF). While the basic patent on TDF expires in 2017/8, entecavir is already generic in several countries, including the US. The chemical structure of entecavir is related to abacavir, which costs <$200 per person-year in low-income countries.
Methods
The clinical efficacy, chemical structures, daily doses, routes of chemical synthesis, costs of raw materials and patent expiry dates were analysed for entecavir and TDF. Costs of sustainable, generic production were calculated for entecavir, and compared with published originator and generic prices in high- and low-income countries.
Results
With a daily dose of 0.5 mg, one year's supply of entecavir treatment requires <0.2 g of active pharmaceutical ingredient (API) per person, estimated to cost $4/year, based on quotations of API production from generic suppliers. With an additional $20 per year for formulation/packaging and a 50% profit margin, entecavir was estimated to cost a minimum of $36/person-year, substantially lower than current originator and generic prices. Entecavir is no longer under patent protection in the USA, China, Brazil and South Africa, with European expiry in 2017. Given differences in daily dosing, production volumes for entecavir would be 600 times lower than TDF (300 mg once daily) for treating the same numbers of patients.
Conclusions
Mass treatment for hepatitis B with generic entecavir could be achieved at very low cost in all countries, provided that important projections can be met in terms of pricing for the API and finished dosage form.
Keywords: hepatitis B, entecavir, tenofovir, lamivudine
Introduction
Epidemiology
Currently, more than 350 million people are chronically infected with hepatitis B (HBV)[1]. In recognition of the large global burden of disease, the World Health Organization established the Global Hepatitis Programme in 2010 [2].
Prevalence rates are highest in Central and Southeast Asia including China, central areas of South America and sub-Saharan Africa, where most transmission is perinatal [1,3]. Approximately 686,000 deaths annually are due to HBV [4]. Hepatitis B causes over half of all primary liver cancers in Africa, China and Southeast Asia [5]. The majority of chronic hepatitis B (CHB) cases are HBeAg-positive, although a growing proportion is HBeAg-negative, making up 15% of patients in Asia and the Pacific, and one-third of patients in the Mediterranean [5,6].
Between 5% and 20% of HBV carriers are co-infected with hepatitis C (HCV)[7], and 10–25% of HIV-positive people are co-infected with HBV [8]. Co-infection with HCV is associated with increased risks of liver cirrhosis and hepatocellular carcinoma (HCC)[7]. Co-infection with HIV leads to higher levels of HBV viraemia, increased risk of progression from acute to chronic HBV infection, and higher rates of cirrhosis and HCC [6,8].
Treatment guidelines
Current guidelines outline three different approaches to treatment: finite-duration treatment with pegylated interferon or nucleos(t)ide analogue (NA), or long-term treatment with NAs. Monotherapy with either entecavir (ETV) or tenofovir disoproxil fumarate (TDF) is recommended as first-line treatment in international treatment guidelines, including new WHO guidelines published in March 2015 [6,9–11]. The UK's National Institute for Health and Care Excellence (NICE) recommends initial treatment with peginterferon alpha-2a before offering entecavir or TDF monotherapy [12]. Both entecavir and TDF are highly effective and pose high genetic barriers to resistance, with <1.2% and 0% resistance, respectively, at 5 years [6]. Entecavir has a low risk of adverse effects [9], while TDF poses some risk in terms of nephrotoxicity, and decreases in bone mineral density in HIV-positive patients [6,9] and in selected HBV mono-infected patients [13]. Entecavir is not recommended in patients previously exposed to lamivudine [6,9], as lamivudine resistance reduces the genetic barrier to resistance to entecavir [14]. In these patients, treatment with TDF is indicated instead. Entecavir is listed by the US Food and Drug Administration (FDA) as a pregnancy Category C drug (evidence for teratogenicity in animals, no trials in humans). TDF is listed as Category B (no evidence for teratogenicity in animals, no trials in humans)[6]. TDF would thus be preferred in treating pregnant patients. However, a recent, large randomised trial has found that a TDF-based antiretroviral therapy administered to HIV-positive mothers resulted in more deaths of infants within 2 weeks of birth than a zidovudine-based regimen [15].
Adefovir and lamivudine are less effective than TDF or entecavir, and associated with higher rates of resistance, and are thus less recommended as first-line treatments in adults [6,9,10]. In addition, adefovir shows a significant level of nephrotoxicity [9,16]. In the UK, NICE guidelines do not recommend the use of adefovir in the treatment of CHB in adults [12]. Emtricitabine has efficacy against HBV, but has a similar resistance profile to lamivudine, and is not recommended as monotherapy for CHB [6,9,10,12]. Pegylated interferon alpha-2b is not approved for CHB treatment in the US [9], most European countries [6] or most Asian countries [10].
In HBV/HIV coinfection, treating both infections concurrently is recommended [6]. A combination of TDF with one of emtricitabine, lamivudine or other antiretrovirals is recommended as treatment against both infections [6,9]. Entecavir can be used as an alternative to suppress HBV if antiretrovirals are provided separately [17]. However, entecavir without suppressive alternative nucleoside analogues may lead to nucleoside mutations in HIV [18], as may TDF monotherapy. HIV tests are therefore necessary before initiation of entecavir and/or TDF treatment.
Treatment of HBV with potent NAs results in a reduction in the incidence of HCC [19].
Number on treatment
Treatment with recommended NAs is unavailable to the majority of patients in developing countries, often due to the current high price of therapy [3,20,21]. Approximately 40% of those diagnosed with CHB in Europe are not on treatment [22]. In Greece, it is estimated that only 34% of people diagnosed with CHB are on treatment [23], in Australia, this figure is 9% [24], and in the US, it is between 8% and 13% [25]. One study found that only 18% of those on treatment for CHB in the UK were receiving the recommended therapies [26].
In countries where health systems in general reach a smaller proportion of the population, the proportion of those with CHB receiving treatment may be lower. The proportion receiving the newest, recommended treatments is likely to be lower still.
Patent status and prices
Table 1 gives an overview of patent status and prices for drugs used in the treatment of HBV. Entecavir and adefovir are now off patent in the US, and lamivudine is off patent both in the US and the European Union (EU). Of the newer NAs, entecavir is the most expensive both in the US – a developed country with historically high drug prices – and globally. For all five drugs, a large difference between originator and generic prices can be seen, for example $15,111 versus $427 for entecavir and $10,718 versus $38 for tenofovir. The lowest generic price for entecavir ($427) is noticeably higher than those for lamivudine ($10), emtricitabine ($62) and TDF ($38), a result of the fact that the other drugs have been in use for longer, have multiple generic manufacturers, and are used in first-line treatment of HIV and thus under great demand from international agencies.
Table 1.
Expiry dates for basic patents, and global price overview for HBV drugs
| Drug | Expiry datesa | US lowest priceb | Global lowest price (US$ pppy) | |
|---|---|---|---|---|
| USA | EU | |||
| Entecavir (ETV) | 2015 (invalidated 2014) | 2017 | $15,111** originator
$6,127** generic |
$427c |
| Adefovir (ADV) | 2014 | 2016 | $13,480** originator | $133c |
| Emtricitabine (FTC) | 2021 | 2016 | $6,203** originator | $62d* |
| Tenofovir disoproxil fumarate (TDF) | 2018 | 2018 | $10,718** originator | $38e*** |
| Lamivudine (3TC) | 2010 | 2010 | $2,627** originator
$1,047** generic |
$10e* |
For lamivudine, all prices are reported for doses used in treating HIV, as comparable information is only available for this dose – 150 mg. For adefovir, no global price overview exists, but the lowest price of generic adefovir in India is shown to give an impression
All patent dates from Gilead 2012 Form 10-K Annual Report [48];
goodrx.com lowest price of authorized pharmacy, including coupon discount [55]
Lowest price as reported by MSF[35];
Lowest ‘incoterms’ price as reported in the WHO's Global Price Reporting Mechanism in 2014 [33]
WHO prequalified;
USFDA approved;
WHO prequalified and USFDA approved; pppy: per person per year
A recent study on the minimum target prices of directly acting antiretrovirals (DAAs) in hepatitis C (HCV) therapy estimated the potential costs of manufacture for the treatment of millions (i.e. 1–5 million) of patients per year to be hundreds of times lower than the current retail prices, for example $68–136 for the manufacture of sofosbuvir, compared with a price of $84,000 in the US. This highlights the potential for widespread generic production to enable scale-up of global HCV treatment [27].
Entecavir (Baraclude) was developed by Bristol-Myers Squibb (BMS), with total sales of more than US$6 billion in the decade since its launch [28–30]. At an approximate price of $15,000/patient-year in the US, however, this represents only 400,000 patient-years of treatment. This statement is not to dispute the logic of originator drug pricing, but it does serve to highlight the differences in approach between providing patented drug treatments for high-income populations versus providing mass treatment of generic drugs for low- and middle-income countries.
This paper analyses the global prices of entecavir, estimates its potential cost of manufacture for several million patient-years of treatment, and proposes mechanisms to expand the treatment of CHB globally.
Methods
Efficacy of entecavir
Clinical trials were identified in PubMed using the search term ‘entecavir OR baraclude’ and filters ‘Clinical Trial’ and ‘Humans’. This returned 171 results on 11 January 2015. Studies were assessed for design type and interventions used. The summary of product characteristics (SmPC) [31] and package insert for entecavir [32] were also reviewed.
Calculation of treatment cost
We estimated a reasonable market demand for generic entecavir to provide incentive for effective competition to reach low production costs: a mass of 2 tonnes of the active pharmaceutical ingredient (API) per year was assumed. This figure was established in iterative discussions with API suppliers as a ‘reasonable figure’ to capture some economies of scale in API production. For finished pharmaceutical production (FPP), this represents roughly 4 billion tablets, which would allow for optimised production pricing. Except in patients with suspected resistance, the once-daily dose of entecavir is 0.5 mg, which would require 0.18 g of drug per person per year. Therefore, 2 tonnes of entecavir would be sufficient to treat 11 million people per year, which is less than 5% of those living with CHB globally [1]. Current entecavir API producers were identified online and surveyed by email for pricing quotes at a volume of 2,000 kg. Twenty companies were surveyed, representing a mix of generic producers with or without Stringent Regulatory Authority (SRA) approvals for entecavir or other drugs. There was no strong correlation between quoted pricing and SRA approvals. Companies did offer that SRA-approved material would cost between 20% and 45% more than non-SRA API.
To calculate a target price for entecavir, we estimated the cost of the API, excipients, tableting, shipping and packaging. We calculate the cost of production based on these estimates, and add a 50% mark-up to give a realistic target price. We use upper estimates throughout and assume relatively inefficient manufacture and logistics to provide a conservative estimate of the cost of entecavir production.
Patent coverage
To analyse entecavir patent coverage, relevant patents were identified, and relevant legal cases were reviewed. The lowest prices of entecavir were identified in nine countries, using published figures for government procurement where possible, as well as online price comparison tools and personal communications with global pharmaceutical pricing experts. Lowest prices for these drugs in the US were gathered from online price comparison tools. References for entecavir prices in different countries are provided in Appendix 1.
Prices for generic TDF, lamivudine, adefovir and emtricitabine were collected from prices published in the World Health Organization's Global Price Reporting Mechanism (GPRM) [33] for SRA-approved products. We also reviewed prices reported by the Clinton Health Access Initiative (CHAI) [34] and Médecins Sans Frontières (MSF) [35]. Figures reported by CHAI represent maximum ‘ceiling’ prices that manufacturers may charge governments of countries in the CHAI Procurement Consortium [34]. Prices in the Médecins Sans Frontières (MSF) Untangling The Web report originate from surveys sent to the manufacturers by MSF. GPRM lists prices of medicines derived from completed purchases reported by agencies such as the Global Fund, PEPFAR, UNITAID and others. Prices listed as lowest global prices in Table 1 are prices reported by GPRM, as these represent ‘real-world’ values, that is minimum prices of completed transactions in 2014
Results
Efficacy in randomised trials
Entecavir showed significantly higher rates of viral suppression than lamivudine (3TC) in both HBeAg-positive and HBeAg-negative patients, in two randomised trials (Table 2). Only one trial directly comparing entecavir to TDF was found, the results of which were not statistically significant [36]. Other studies were identified, but were excluded due to having a retrospective, non-randomised or open-label study design, or for not being direct comparator studies. The ClinicalTrials.gov database lists one ongoing trial in which TDF and entecavir are directly compared; however, this study is focused on renal function and is of an open-label, case–control design [37].
Table 2.
Double-blind randomised controlled trials for entecavir: 48-week results
| Trial | HBeAg | Endpoint | Entecavir 0.5 mg OD (%) | Lamivudine 100 mg OD (%) |
|---|---|---|---|---|
| ETV-022 [57] | Positive | HBV DNA
<300 copies/mL |
236/354 (67%) | 129/355 (36%) |
| ETV-027 [58] | Negative | HBV DNA
<300 copies/mL |
293/325 (90%) | 225/313 (72%) |
P values for all results in table are <0.001. More than 80% of patients in both studies were treatment naïve, with similar proportions in both arms
The European SmPC and the package insert for TDF cite studies comparing TDF with adefovir, but do not include direct comparisons of TDF with either entecavir or lamivudine monotherapies [38,39].
Chemical structure and cost of manufacture
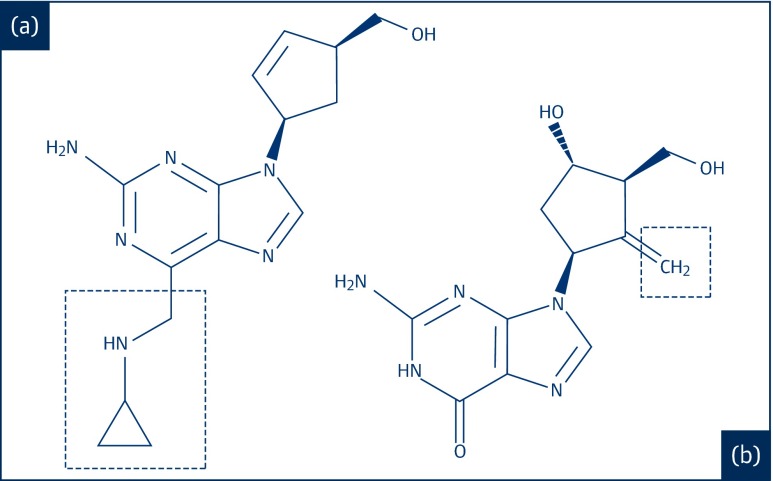
Entecavir is a guanosine nucleoside analogue. Its full chemical name is 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3- (hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate, and its molecular weight is 295.3. The structure of entecavir is shown in Figure 1. There are three chiral centres in the structure and entecavir is synthesised as the 1S, 3R, 4S enantiomer [32]. The chemical structure of entecavir is related to the nucleoside analogue abacavir, which is sold as an FPP for under $200/person-year in low-income countries [33–35]. Both structures contain 5-membered carbocycles substituted with a hydroxymethyl group in position 3 and a purine analogue heterocycle. Entecavir is made by a substantially different, and more expensive, chemical synthesis than traditional nucleosides and abacavir. However, the dose used is very low (0.5 mg), providing an opportunity for large-scale treatment at low cost.
Figure 1.
The chemical structures of (a) abacavir and (b) entecavir
(from PubChem)
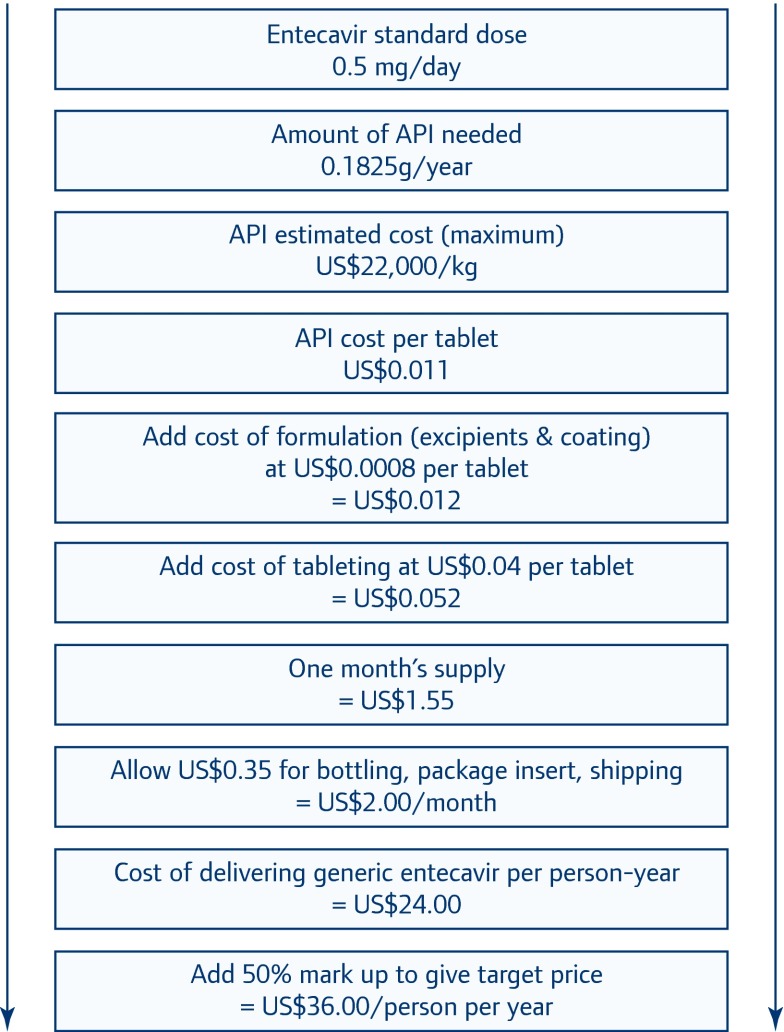
A flowchart displaying the calculations and assumptions is given in Figure 2, and described below. The standard daily dose for entecavir is 0.5 mg. Thus, the volume of API required is 0.1825 grams per patient-year. At a volume demand of 2 tonnes/year, generic manufacturers of the API estimated charges of $11,000–22,000/kg. The API manufacturers interviewed were a mix of those with and without SRA-approved production; there was no strong correlation between prices quoted and SRA approvals. Companies who were specifically questioned offered that SRA-approved pricing would be about 20–45% higher than for non-SRA approved API. Pricing at $11,000–22,000/kg results in the cost of API being $0.0055–0.011 per tablet at the standard 0.5 mg dose. We assumed the cost of the API to be the higher extreme of this range ($22,000/kg).
Figure 2.
Assumptions and calculation of generic entecavir target price
Typical excipients used in entecavir tablets include povidone, crospovidone, lactose monohydrate, magnesium stearate and microcrystalline cellulose [32]. Of these, the most expensive by far is povidone, which costs $11.70–15.27 per kilogram. Commercial Baraclude tablets weigh 205 mg and contain approximately 60% (120.5 mg) lactose monohydrate and 32% (65 mg) microcrystalline cellulose [40]. The typical tablet is likely to contain a maximum of 15% povidone as a solubility enhancing agent [41]. We have used cost figures for the prices of excipients to estimate a reasonable maximum excipient cost of $0.0008 per tablet. The combined cost of API and excipient comes to $0.012 per tablet.
We assume the cost of tableting (combining ingredients, pressing pills and quality control) to be about $0.04 per tablet. Thus, our conservative estimate of the price of API, excipient, coating and tableting is $0.052 per tablet. For comparison, generic placebo tablets, which contain all the excipients of an active tablet, are generally listed at values between $0.01 and $0.05. A personal interview with the Director of generic product development at a large pharmaceutical company suggests that typical tablet production costs (including excipients) are between $0.01 and $0.03/tablet; informal feedback from Supply Chain Management Systems (Arlington, VA) is that tablets typically cost about $0.03 to manufacture for the PEPFAR programme. These costs include the price of excipients but not of API.
The larger component of the cost of treatment with entecavir is primary and secondary packaging and distribution. We assume a full (primary and secondary) packaging cost of $1.00/bottle, with 30 dosage units (1 month's supply) in each bottle. Allowing $0.33 for packaging and distribution per bottle, our final estimate for the cost of delivering generic entecavir treatment is $2/month, or $24/person-year. Adding a 50% profit margin gives a target price of $36/person-year.
The cost of the active pharmaceutical ingredient is about $2–4/year. We used the higher estimate for API cost, and at $4 this represents 11% of our target price. Packaging, shipping and mark-up represent 48% of our target price.
The current lowest price for TDF API is $220 per kilogram [42]. The amount of API needed ($0.066 for a 300 mg dose) therefore costs six times more than the API needed for entecavir ($0.011 for a 0.5 mg dose).
Patent expiry
Table 3 shows patent numbers and expiry dates in selected countries. The basic patents on entecavir were filed in 1990 and 1991. The basic patent for entecavir in the US was invalidated in 2014 (Appendix 2). In China and Brazil, the basic patents expired in 2011 (Appendix 2). In Europe, having received a Supplementary Protection Certificate of 5 years, and a 6-month extension for paediatric indication, patents on entecavir will expire in 2017. In some cases, confirmation of these extensions is pending (Appendix 2). In all these countries, there are a number of formulation patents in force.
Table 3.
Expiry dates of basic patents on entecavir in selected countries
| Country | Number | Expiry date | Status |
|---|---|---|---|
| US | US5206244 | 21 February 2015* | Invalidated |
| UK | EP0481754 | 15 April 2017*† | Extension in force |
| France | EP0481754 | 1 April 2017* | Extension in force |
| Germany | EP0481754 | 16 April 2017*† | Extension in force |
| India | 213457 | 2 July 2022‡ | Unclear |
| China | CN1030916 | 20 November 2011 | Expired |
| Brazil | PP 1100846-6 B1 | 18 October 2010 | Expired |
| South Africa | 1991/07894 | 2011‡ | Expired |
References for patents and term extensions can be found in Appendix 2
Expiry date of extended patent term
Date shown assumes granting of 6-month paediatric extensions, requests for which have been filed with the European Patent Office
Expiry date estimated as 20 years after filing
A number of generics companies produce entecavir. TEVA sells generic entecavir in the US [43]. Cipla, Zydus and Ranbaxy manufacture versions in India [44]. TEVA is SRA-approved while Cipla, Zydus, and Ranbaxy have applied for SRA approvals. Several manufacturers produce generic entecavir in China [45,46]. While the basic patent for entecavir has lapsed in South Africa, Doctors Without Borders has reported that generic entecavir is not being produced or imported due to fears that the originator company will rapidly take out ‘evergreening’ patents, benefiting from South Africa's system of granting patents [47]. It is unclear whether generic competition exists in Brazil. All entecavir bought by the Ministry of Health in Brazil has been supplied by a subsidiary of Bristol-Myers Squibb (Appendix 1).
Patent protection for TDF expires in 2018 in the US and the EU [48] (Table 1). Many key patent applications for tenofovir (in its various forms) have been rejected by the Indian patent office, and generic production began there in 2005 [49]. While some patent applications are still pending in India, Gilead Sciences signed a voluntary license (VL) agreement with the Medicines Patent Pool in 2006, allowing generic production of TDF by Indian companies irrespective of patent applications for the treatment of HIV and HBV [49,50]. However, this licence is limited to Indian generic manufacturers, and disallows export to many middle-income countries with large HIV and HBV epidemics, such as China, Brazil, Russia, Egypt and Ukraine [50]. Despite a disputed patent application on TDF in Brazil, the government has announced that generic TDF will be produced locally [49]. Following negotiations for discounts, one of the patents for TDF was revoked in China in 2013 [51].
Basic patent protection for lamivudine expired in 2010 in the US, Europe [48] and China [35], and WHO-prequalified or SRA-approved lamivudine is available at very low cost from Indian generic producers (Table 1) [35]. GlaxoSmithKline held an additional patent for use in CHB treatment, which expired in 2014 in the US [52] and 2012 in the EU [53].
Current prices
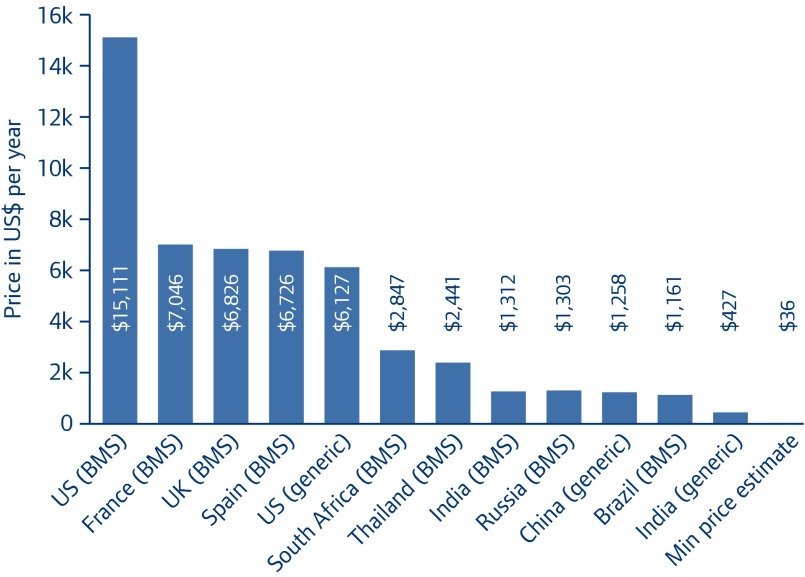
As shown in Figure 3, entecavir is bought at a wide range of prices worldwide, with the generic version in India sold for less than 3% of the price of the originator drug in the US, and less than 7% of the price of the generic sold in US pharmacies (NB the generic versions of entecavir sold in India are not approved by SRA, nor are they WHO-prequalified, although their manufacturers have been approved nationally according to WHO Good Manufacturing Practice guidelines). In the UK, France and Spain, where the drug is under patent until early 2017, prices are similar at around US$7,000/patient per year – slightly less than half the price in the US. The prices of the originator drug offered to India, Russia and Brazil are similar. The price of generic entecavir in China is similar to the prices of the originator drug in India, Russia and Brazil.
Figure 3.
The lowest price for entecavir 0.5 mg in selected countries
(per patient per year)
(References for individual prices can be found in Appendix 2.)
The lowest prices for originator and generic TDF and lamivudine, in the US and globally, are far lower than the cheapest prices for entecavir. Originator TDF costs $10,718 in the United States – roughly two-thirds of the price of originator entecavir (Table 1). The lowest global price for a completed sale of generic, SRA-approved TDF is $38 per person-year. In the US, originator lamivudine costs $2,627 while generic lamivudine is $1,047; however, the global cheapest is $10/person-year (WHO prequalified). The global lowest prices of TDF and lamivudine are also significantly lower than previous independent estimates for lowest price based on analysis of production costs [27]. These prices, however, have in many cases been negotiated for the procurement of the drug for only HIV treatment.
Discussion
Hepatitis B infection leads to approximately 686,000 deaths per year. This life-threatening viral infection could be controlled using entecavir treatment costing $36/person-year. This conservatively estimated target price of $36 is far below the $15,111 that is charged for the originator version in the US and the more than $6,000 charged for originator versions in Europe and Teva's generic version in the US.
At this price, entecavir would be the cheapest recommended first-line monotherapy for treating chronic hepatitis B, achieving prices below those of TDF. Entecavir priced at $36/patient-year could allow expansion of chronic hepatitis B treatment in low- and middle-income countries, avoiding significant numbers of cases of cirrhosis and/or primary liver cancer, and yielding massive savings in high-income countries.
We have estimated the target price conservatively, including a generous 50% profit margin on top of total production and shipping costs. Generic production at this price would thus be highly sustainable, and attract competitors. While 2 tonnes/year of entecavir does not sound like a large volume, it is clear from our discussions with API suppliers that there will be fierce competition in such a market. The reason for this is simple: 2 tonnes of entecavir at $22,000/kg represents US$44 million in annual API sales. By comparison, at an API price of approximately $220/kg, API producers would have to sell 200 tonnes of TDF API in order to achieve the same revenue. At $22,000/kg the margin per kg on entecavir API sales will also be vastly larger than for TDF. There is the potential for entecavir to be priced below $36/person-year, if lower profit margins were accepted by generic companies.
If sold at or near the target price, entecavir would be cheaper than the cheapest versions of TDF, its therapeutic alternative. The global minimum price for TDF is reported variously in different resources. The WHO Global Price Reporting Mechanism (GPRM) reports a minimum price of TDF of $38 in 2014, and a median (of transactions with medical aid agencies) of $46 [33]. The CHAI Antiretroviral ceiling prices list sets a ceiling of $54 for TDF [34].
It is also important to note the differences in logistical requirements between TDF and entecavir. Because of the large difference in dose (300 mg for TDF; 0.5 mg for entecavir), for every tonne of entecavir API produced by a manufacturer, 600 tonnes of TDF API would need to be produced for the same number of patient-years of treatment. Besides logistical differences, the active pharmaceutical ingredients used in entecavir and TDF, at standard doses, differ by a factor of six – $0.011 per pill for entecavir, and $0.066 for TDF [42]. The differences in API cost and dosage underline the potential for competitive production of entecavir achieving lower prices per patient than TDF.
Current prices for generic entecavir (Figure 3), while far lower than originator prices, are still significantly higher than our estimate. The global lowest price of entecavir is $427/patient-year from a producer who is not SRA-approved at this time. In the US, the difference between lowest generic price and the target price is even more dramatic. This price disparity between target generic price and observed current generic prices may be explained by a self-perpetuating cycle between the high price and usage: high prices lead to entecavir being used at relatively low volumes, and these low volumes make demand appear to be low, leading to insufficient generic competition. At the same time, this may simply reflect a lack in efforts by buyers (such as national healthcare systems, bulk purchasers) to negotiate lower prices. These processes are clearly counterproductive to the recommendations of guidelines for use of entecavir as a first-line monotherapy for chronic hepatitis B [6,10].
Increasing the number of patients treated with entecavir rather than other drugs is both recommended by guidelines, and will allow further reduction in price by increasing purchase volumes.
Besides making widespread treatment possible with the recommended therapies, production of affordable entecavir would allow a two-pronged approach to eradication of hepatitis B globally. While vaccine use has been expanded dramatically [54] with considerable effects in curbing HBV, treatment for the over 350 million who are chronically infected could prevent the onward transmission of the virus.
There are significant rates of co-infection with HCV and HIV, and there is the potential for resistance of one virus to drugs being used to treat another. Therefore combined testing for HBV, HCV and HIV should be strongly encouraged to optimise selection of treatment.
Lastly, affordable entecavir would provide a second-line treatment for those patients experiencing the toxicity-associated side effects of TDF such as nephrotoxicity and effects on bone density [6,9,13].
The cost of manufacturing entecavir is clearly not linked to current prices. The cost of patented medicines in high-income countries is hypothetically reflective of three considerations: (1) the inherent value of treatment; (2) consideration granted for the investment in bringing a new product(s) to the market; and (3) incentive to reward innovation. With this paper, we are not entering into a discussion of what entecavir or other treatments under patent should cost in high-income countries. However, many people, even in high-income countries, do not receive the current standard of treatment, or in fact, any treatment at all for hepatitis B. The Global Fund, UNICEF, PEPFAR and other NGOs target making medicines accessible to patients in low- to middle-income countries. As NGOs consider the opportunity to scale up treatment of hepatitis B in low- to middle-income countries, it is of value to the broader community to understand the potential minimum cost of large-scale treatment for CHB using standard-of-care treatments.
We are estimating the potential cost for accessing treatment for several million patients per year from generic suppliers in a competitive market. This is a much larger scale of treatment than currently exists worldwide. It is highly unlikely that the originator's cost of manufacturing entecavir even approaches our estimated potential cost of treatment, given that the number of patients on treatment and volume demand is much lower than for our estimates. We do, however, believe that our analysis suggests that large-scale treatment can potentially be accessed for considerably less than the lowest current pricing for generic access.
In conclusion, encouraging widespread competitive generic production of entecavir would allow dramatic price reductions and rapid scale-up of HBV treatment globally, with a well-tolerated regimen that has a high barrier to resistance.
Acknowledgements
Appendix 1
Appendix 1.
References for prices of entecavir in selected countries
| Country | Price for 360 pills of 0.5 mg entecavir (US$) | Ref number |
|---|---|---|
| US (originator) | 15110.64 | [1] |
| US (generic in store) | 6126.72 | [1] |
| France (originator) | 7045.57 | AG Hasenknopf, personal communication November 2014 |
| UK (originator) | 6826.03 | [2] |
| Spain | 6762.42 | [3] |
| South Africa | 2846.99 | [4] |
| Thailand | 2440.77 | [5] |
| India (originator) | 1312.49 | [6] |
| India (generic) | 426.69 | [6] |
| Russia (government ceiling price; originator) | 1302.88 | [7] |
| China (generic) | ||
| Hunan | 1421.54 | [8] |
| Shandong | 1013.34 | [9] |
| Beijing | 1258.56 | [10] |
| Henan | 1337.82 | [11] |
| Mean for four provinces: | 1257.82 | |
| Brazil | 1161.13 | [12] |
Prices in other currencies were converted to US$ using exchange rates at 19th December 2014
- 1. GoodRx [Internet]. 2014. [ cited 2014 Dec 30]. Available from: www.goodrx.com/
- 2. Baraclude® [Internet]. British National Formulary 2014. [ cited 2015 Jan 6]. Available from: www.medicinescomplete.com/mc/bnf/current/PHP3873-baraclude.htm
- 3. Colegio de Farmaceuticos de Ponteverda Consulta de Precios de Medicamentos [Internet]. 2014. [ cited 2014 Dec 19]. Available from: www.cofpo.org/index.php/medices.html?file=tl_files/Listados/PreciosMedicamentos.pdf
- 4. South African Medicine Price Registry Database of Medicine Prices [Internet]. 2014. [ cited 2015 Jan 1]. Available from: www.mpr.gov.za/Publish/ViewDocument.aspx?DocumentPublicationId=1761
- 5. Drug And Medical Supply Information Center Ministry of Public Health [Internet]. 2014. [ cited 2014 Dec 19]. Available from: http://dmsic.moph.go.th/
- 6. DrugsUpdate.com [Internet]. [ cited 2014 Dec 30]. Available from: www.drugsupdate.com/
- 7. [Internet]. 2014. [ cited 2014 Dec 19]. Available from: http://grls.rosminzdrav.ru/PriceLims.aspx?Torg=&Mnn=&Mnf=&Barcode=&Order=&All=0&PageSize=8&orderby=pklimprice&orderType=desc&pagenum=1
- 8. The Medical Concentrative Purchase of Hunan Province [Internet]. Available from: www.hnyycg.gov.cn/HomePage/Index.aspx
- 9. Shangdong Yaopin Jizhong Caigou Wang [Internet]. Available from: www.sdyypt.net/Website/Index.aspx
- 10. BJMB [Internet]. Available from: www.bjmbc.org.cn/index/index.aspx
- 11. Henan Medicine Purchase Service Center (HMPC) [Internet]. Available from: http://www.hnyyzb.com.cn/HomePage/Default_new.aspx
- 12. Transparência Pública. Licitações – Advanced search for ‘entecavir’. [Internet]. [ cited 20 15 Jan 6]. Available from: www3.transparencia.gov.br/TransparenciaPublica/jsp/licitacoes/licitacaoBuscaAvancada.jsf?consulta2=5&camposDefault=true&CodigoOrgao=null
Appendix 2
Appendix 2.
References for patents listed in Table 3
- 1. United States Court of Appeals for the Federal Circuit. Bristol-Myers Squibb Company v. Teva Pharmaceuticals USA, Inc. [Internet]. 2014. Available from: http://e-foia.uspto.gov/Foia/RetrievePdf?system=FCA&flNm=13-1306_1
- 2. FDA Orange Book Appl No 021798 [Internet] [ cited 2014 Dec 22]. Available from: www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021798&Product_No=001&table1=OB_Rx
- 3. Zahler R, Slusarchyk WA. Hydroxymethyl (methylenecyclopentyl) purines and pyrimidines [Internet]. South Africa; 1991/07894, 1991. [ cited 2014 Dec 20]. Available from: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=30&f=G&l=50&co1=AND&d=PTXT&s1=5,206,244&OS=5,206,244&RS=5,206,244
- 4. European Patent Office Global patent index – EP 0481754 A3 [Internet]. 2015. [ cited 2015 Jan 2]. Available from: https://data.epo.org/gpi/EP0481754A3-hydroxymethyl-methylenecyclopentyl-purines-and-pyrimidines
- 5. DPMA Registerauskunft des Deutschen Patent- und Markenamtes (DPMA) [Internet]. [ cited 2015 Jan 3]. Available from: https://register.dpma.de/DPMAregister/pat/register?AKZ=1220060000690
- 6. Sprockel OL, Harianawala A, Desai D, Fakes MG, Colonno RJ.. A pharmaceutical composition for treating hepatitis b infection [Internet]. India; 213457, 2008. [ cited 2015 Jan 3]. Available from: www.allindianpatents.com/patents/213457
- 7. Singh K. HC rejects BMS’ plea on sale of hepatitis B generic by Ranbaxy. Economic Times [Internet]. New Delhi; 2010. Sep 23 [ cited 2015 Jan 2]. Available from: http://articles.economictimes.indiatimes.com/2010-09-23/news/27574785_1_generic-version-ranbaxy-mg-tablets
- 8. Espacenet INPADOC legal status: CN1030916 (C) – 1996-02-07 [Internet] 2014. [ cited 2015 Jan 2]. Available from: http://worldwide.espacenet.com/publicationDetails/inpadoc?CC=CN&NR=1030916C&KC=C&FT=D&ND=&date=19960207&DB=&locale=en_EP
- 9. Instituto Nacional da Propriedade Industrial Consulta à Base de Dados do INPI [Internet] 2015. [ cited 2015 Jan 3]. Available from: https://gru.inpi.gov.br/pPI/servlet/LoginController?action=Login&BasePesquisa=Patentes
- 10. Slusarchyk WA, Zahler R.. Composte, e, composição antiviral útil e processo para tratar o vírus do herpes simples 1 e 2, vírus varicella zoster e citomegalovírus humano [Internet]. Brazil; PP 1100846-6 B1, 1990. Available from: http://gru.inpi.gov.br/
- 11. Companies and Intellectual Property Commission Public Design & Patent Search [Internet]. 2015. [ cited 2015 Jan 3]. Available from: http://patentsearch.cipc.co.za/patents/patentsearch.aspx?search=basic
Source of funding
This project was not funded.
Conflicts of interest
DG and JF and report no conflicts of interest. AH has received consultancy payments from Janssen, not connected with this project. GC has received consultancy payments and funding for clinical trials from pharmaceutical companies not connected with this project. SB has received consultancy payment and research funding from BMS and Gilead not connected with this project.
Authors’ contributions
AH designed the project. AH and GC supervised the study team. DG conducted the systematic review of treatments and additional searches. JF analysed the costs of production of the treatments. All authors critically reviewed the manuscript.
References
- 1. Trépo C, Chan HLY, Lok A.. Hepatitis B virus infection. Lancet 2014; 384: 2053– 2063. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization Hepatitis. Available at: www.who.int/csr/disease/hepatitis/en/ ( accessed March 2015).
- 3. World Health Organization Hepatitis B: fact sheet number 204. Available at www.who.int/mediacentre/factsheets/fs204/en/ ( accessed March 2015).
- 4. GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 385: 117– 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Funk ML, Rosenberg DM, Lok ASF.. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat 2002; 9: 52– 61. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012; 57: 167– 185. [DOI] [PubMed] [Google Scholar]
- 7. Chu C-J, Lee S-D.. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol 2008; 23: 512– 520. [DOI] [PubMed] [Google Scholar]
- 8. Kourtis A, Bulterys M.. HIV-HBV coinfection: a global challenge. N Engl J Med 2012; 366: 1749– 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lok ASF, McMahon BJ.. Chronic hepatitis B: update 2009. Hepatology 2009; 50: 661– 662. [DOI] [PubMed] [Google Scholar]
- 10. Liaw Y-F, Kao J-H, Piratvisuth T et al. . Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatology Int 2012; 6: 531– 561. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. WHO, Geneva; 2015. Available at: http://who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ ( accessed March 2015). [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence Antiviral treatment in adults with chronic hepatitis B. NICE; 2014. Available at: http://pathways.nice.org.uk/pathways/hepatitis-b-chronic/antiviral-treatment-in-adults-with-chronic-hepatitis-b#path=view%3A/pathways/hepatitis-b-chronic/antiviral-treatment-for-chronic-hepatitis-b.xml&content=view-index ( accessed March 2015).
- 13. Gill US, Zissimopoulos A, Al-shamma S et al. . Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J Infect Dis 2015; 211: 374– 382. [DOI] [PubMed] [Google Scholar]
- 14. Tenney DJ, Rose RE, Baldick CJ et al. . Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years-of therapy. Hepatology 2009; 49: 1503– 1514. [DOI] [PubMed] [Google Scholar]
- 15. NIH press release NIH-sponsored study identifies superior drug regimen for preventing mother-to-child HIV transmission. November 2014. Available at: www.nih.gov/news/health/nov2014/niaid-17.htm ( accessed March 2015).
- 16. Ha NB, Ha NB, Garcia RT et al. . Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology 2009; 50: 727– 734. [DOI] [PubMed] [Google Scholar]
- 17. Günthard HF, Aberg JA, Eron JJ et al. . Antiretroviral treatment of adult HIV Infection: 2014 recommendations of the International Antiviral Society-USA panel. JAMA 2014; 312: 410– 425. [DOI] [PubMed] [Google Scholar]
- 18. McMahon MA, Jilek BL, Brennan TP et al. . The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007; 356: 2614– 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papatheodoridis G V, Chan HL-Y, Hansen BE et al. . Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 2015; 62: 956– 967. [DOI] [PubMed] [Google Scholar]
- 20. Abbas Z, Siddiqui AR.. Management of hepatitis B in developing countries. World J Hepatol 2011; 3: 292– 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liaw Y-F. Antiviral therapy of chronic hepatitis B: opportunities and challenges in Asia. J Hepatol 2009; 51: 403– 410. [DOI] [PubMed] [Google Scholar]
- 22. Papatheodoridis GV, Tsochatzis E, Hardtke S, Wedemeyer H.. Barriers to care and treatment for patients with chronic viral hepatitis in Europe: a systematic review. Liver Int 2014; 34: 1452– 1456. [DOI] [PubMed] [Google Scholar]
- 23. Papatheodoridis G, Sypsa V, Kantzanou M et al. . Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey. J Viral Hepat 2014; 22: 409– 415. [DOI] [PubMed] [Google Scholar]
- 24. Allard N, Maclachlan J, Cowie BC.. The cascade of care for Australians living with chronic hepatitis B: measuring access to diagnosis, management and treatment. Aust N Z J Public Health 2015: doi: 10.1111/1753-6405.12345. [DOI] [PubMed] [Google Scholar]
- 25. Cohen C, Holmberg SD, McMahon BJ et al. . Is chronic hepatitis B being undertreated in the United States? J Viral Hepat 2011; 18: 377– 383. [DOI] [PubMed] [Google Scholar]
- 26. Tedder RS, Rodger AJ, Fries L et al. . The diversity and management of chronic hepatitis B virus infections in the United Kingdom: a wake-up call. Clin Infect Dis 2013; 56: 951– 960. [DOI] [PubMed] [Google Scholar]
- 27. Hill A, Khoo S, Fortunak J et al. . Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; 58: 928– 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bristol-Myers Squibb Company Form 10-K: Annual report pursuant to section 13 or 15 (d) of the securities exchange act of 1934. 2007.
- 29. Bristol-Myers Squibb Company Form 10-K: Annual report pursuant to section 13 or 15 (d) of the securities exchange act of 1934. 2010.
- 30. Bristol-Myers Squibb Company Form 10-K: Annual report pursuant to section 13 or 15 (d) of the securities exchange act of 1934. 2013.
- 31. European Medicines Agency Baraclude (entecavir). Summary of product characteristics. Available at: www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000623/human_med_000670.jsp&mid=WC0b01ac058001d124 ( accessed March 2015).
- 32. Bristol-Myers Squibb Company Full prescribing information: Baraclude (entecavir). 2014. Available at: http://packageinserts.bms.com/pi/pi_baraclude.pdf ( accessed March 2015).
- 33. World Health Organization Global Price Reporting Mechanism for HIV, tuberculosis and malaria. 2014. Available at: www.who.int/hiv/amds/gprm/en/ ( accessed March 2015).
- 34. Clinton Health Access Initiative Antiretroviral (ARV) ceiling price list. 2013. Available at: www.clintonhealthaccess.org/files/CHAI%20ARV%20Ceiling%20Price%20August%202013.pdf ( accessed March 2015).
- 35. MSF Access campaign Untangling the web of antiretroviral price reductions, 17th edition July 2014. Available at: www.msfaccess.org/sites/default/files/MSF_UTW_17th_Edition_4_b.pdf ( accessed March 2015).
- 36. Liaw Y-F, Sheen I-S, Lee C-M et al. . Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology 2011; 53: 62– 72. [DOI] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov Search for ‘entecavir AND tenofovir’.
- 38. eMC Viread 245 mg film-coated tablets. Summary of Product Characteristics. 2014. Available at: https://www.medicines.org.uk/emc/history/9008 ( accessed March 2015).
- 39. Gilead Sciences Inc Full prescribing information: Viread (tenofovir). 2012. Available at: www.gilead.com/~/media/Files/pdfs/medicines/hiv/viread/viread_pi.pdf ( accessed March 2015).
- 40. Yousaf AM, Jee J-P, Hwang SR et al. . Development of direct compression entecavir 0.5 mg-loaded tablet exhibiting enhanced content uniformity. Powder Technol 2014; 267: 302– 308. [Google Scholar]
- 41. Desai D, Li D, Harianawala A et al. . Solubilization of entecavir by povidone to overcome content uniformity challenges for low-dose tablet formulations. Pharm Dev Technol 2013; 18: 1305– 1313. [DOI] [PubMed] [Google Scholar]
- 42. Import-Export data showing FOB pricing of shipments from India to manufacturers in other countries, reviewed by JF. Available at: www.infodriveindia.com ( accessed December 2014)
- 43. Press release Teva announces launch of generic Baraclude tablets, 0.5 mg and 1 mg, in the United States. Available at: www.tevapharm.com/news/?itemid=%7BB77E1E3B-2E97-41EA-9519-72CBF420DBC7%7D ( accessed March 2015)
- 44. DrugsUpdate.com Entavir from Cipla, Entecavir – Baraclude to Entehep. Available at: www.drugsupdate.com/brand/generic/Entecavir/44641 ( accessed March 2015).
- 45. PRNewswire-Asia Kun Run Biotechnology Announces That They Have Obtained the Manufacturing Approval for Entecavir from the China State Food and Drug Administration. PR Newswire. Haikou, China; 17 June 2010. Available at: http://www.prnewswire.com/news-releases/kun-run-biotechnology-announces-that-they-have-obtained-the-manufacturing-approval-for-entecavir-from-the-china-state-food-and-drug-administration-96550799.html ( accessed March 2015).
- 46. Wang J. Clinical utility of entecavir for chronic hepatitis B in Chinese patients. Drug Des Develop 2014; 8: 13– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Press release World Hepatitis Day: TAC joins organisations calling for improved access to HepB vaccines & therapies. Johannesburg, 2014. July Available at: www.tac.org.za/news/world-hepatitis-day-tac-joins-organisations-calling-improved-access-hepb-vaccines-therapies ( accessed January 2015).
- 48. Gilead Sciences Inc Form 10-K: Annual report pursuant to section 13 or 15 (d) of the securities exchange act of 1934. 2012.
- 49. MSF Access campaign Untangling the web of antiretroviral price reductions, 16th edition 2013. Available at: www.msfaccess.org/content/untangling-web-antiretroviral-price-reductions-16th-edition ( accessed March 2015).
- 50. The Medicines Patent Pool Licences in the MPP: Gilead Sciences. 2012. www.medicinespatentpool.org/current-licences/ ( accessed January 2015).
- 51. Palmer E. China revokes patent on Gilead's Viread. FiercePharma. Available at: www.fiercepharma.com/story/china-revokes-patent-gileads-viread/2013-08-07 ( accessed March 2015).
- 52. GlaxoSmithKline Annual report 2013. www.gsk.com/media/325156/annual-report-2013.pdf (p. 229; accessed March 2015).
- 53. GlaxoSmithKline Annual report 2010. www.gsk.com/media/279952/annual-report-2010.pdf (p. 15; accessed March 2015)
- 54. Cui Y, Jia J.. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 2013; 28 Suppl 1: 7– 10. [DOI] [PubMed] [Google Scholar]
- 55. GoodRx [Internet]. 2014. [ cited 2014 Dec 30]. Available from: www.goodrx.com/
- 56. DrugsUpdate.com Medicine INN name searched on 6 Jan 2015.
- 57. Chang T, Gish R, Man R et al. . A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006; 354: 1001– 1010. [DOI] [PubMed] [Google Scholar]
- 58. Lai C-L, Shouval D, Lok AS et al. . Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006; 354: 1011– 1020. ( Erratum in: N Engl J Med 2006; 354: 1863.) [DOI] [PubMed] [Google Scholar]