Abstract
Background:
Currently, there is no drug known that is able to eradicate either HIV or HIV-infected host cells. The effectiveness of all available treatments is based on the prevention of viral replication. We investigated whether the monoclonal, CD52 receptor-targeting antibody, alemtuzumab, which is currently approved for the treatment of multiple sclerosis, is able to eliminate HIV-infected immune cells.
Method:
In blood samples from healthy donors and from HIV-1-infected subjects who were either treatment-naïve or resistant to HAART, we studied whether the CD52 expression on T cells and their subsets (CD3, CD4, CD8), B cells (CD19), dendritic cells (CD123) and monocytes (CD11c) is retained in HIV-1 infection and whether alemtuzumab is able to eradicate infected cells, using four-colour flow cytometry.
Results:
We found that CD52 expression on immune cells is retained in HIV-1 infection regardless of CD4 cell count, viral load and treatment status, and is amenable to alemtuzumab-induced depletion.
Conclusions:
For the first time it could be shown in vitro that HIV-1-infected immune cells can be eliminated by using the monoclonal antibody alemtuzumab.
Keywords: alemtuzumab, CD52, T cell elimination, dendritic cells, B cells, monocytes, HIV cure
Background
Treatment of HIV infection has made outstanding progress in the last decades. From a deadly disease, HIV infection has changed – due to the success of highly active antiretroviral therapy (HAART) – to a chronic disease, responsive to suppressive therapy, with viral loads falling below the standard limits of detection and patients being able to live normal lives [1]. However, with more sophisticated methods, virus can still be detected in most patients at levels in the range of 1–50 copies/mL [2]. Although the long-term implications of this persistence have not been established, neurological, cardiovascular and/or malignant consequences cannot be excluded. Another factor to be considered is the toxicity of HAART itself. More importantly, in virologically well-suppressed individuals, increased rates of several long-term, non-AIDS-related diseases such as heart and liver malfunctions, diabetes and cancer have been reported [3]. This concern led to the START study enrolling patients with CD4 cell counts above 500 cells/mm3 and randomisation to immediate treatment or deferred treatment until after the CD4 cell count dropped to 350 cells/mm3. Reported results showed that starting antiretroviral therapy with a CD4 cell count of more than 500 cells/mm3 provided net benefits over starting therapy after the CD4 cell count had declined to 350 cells/mm3 [4]. HAART is now recommended for everyone living with HIV at any CD4 cell count. However, the cost of treating HIV infection may be acceptable in developed countries but it certainly constitutes a major problem in resource-limited countries. These issues have repeatedly raised the question of whether cure of HIV infection is possible.
Current HIV treatments belong to four different mechanistic classes, reverse transcriptase inhibitors (RTI; nucleoside RTIs or ‘NRTIs’ and non-nucleoside RTIs or ‘NNRTIs’), protease inhibitors, entry inhibitors (fusion inhibitors and CCR5-inhibitors) and integrase inhibitors. All of them prevent viral replication, i.e. they have virostatic activity. They eliminate neither the virus nor the infected host cell. As a consequence, a cure of the disease has not been achieved. As soon as HAART is stopped, viral load bounces back within approximately 2 weeks [5]. The reason for this rebound has not been fully elucidated. It has been speculated that latently infected resting memory CD4+ T cells constitute the major reservoir for residual virions [6]. Other suspects include lymphocytes, which are able to produce viral RNA without showing markers of activation [7] as well as monocytes and macrophage lineages. What makes the situation even worse is cell-to-cell spread of HIV even in the presence of antiviral therapy [8]. The ultimate objective of HIV therapy has been and still is the total eradication of virions throughout the body so that HAART can be stopped and the patient is cured [9].
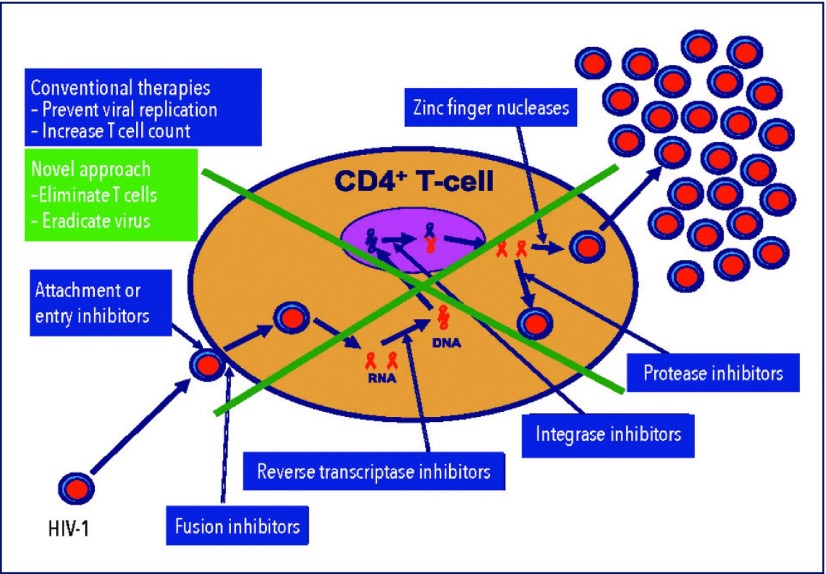
The current basic principles of therapy comprise prevention of viral replication and support for T cell preservation and reconstitution. The objective is to bring down the viral load and to increase the number of T cells. We are following a completely different route. We are looking for means to eradicate virally infected host cells and – since the virus would immediately attack so-far uninfected immune cells – to deplete all potential host cells for a certain period of time. Our hypothesis is that host cells are not only needed for viral replication but also for survival of the virus. Depleting host cells therefore would not only mean that replication is no longer possible but also that the virus would not be able to survive. Accordingly, complete elimination of potential host cells, that is of T cells, lymphocytes, monocytes, macrophages and dendritic cells, for a certain period of time would eradicate not only any virus hiding in these cells but also any freely circulating virus. The basic principle of this proposal compared to conventional HAART is illustrated in Figures 1 and 2.
Figure 1.
Mechanisms of action of HAART and of the proposed new approach. HAART follows the principle ‘prevent viral replication and increase T cell count.’ The new proposal is based on the paradigm ‘eliminate host cells and thereby eradicate the virus.’
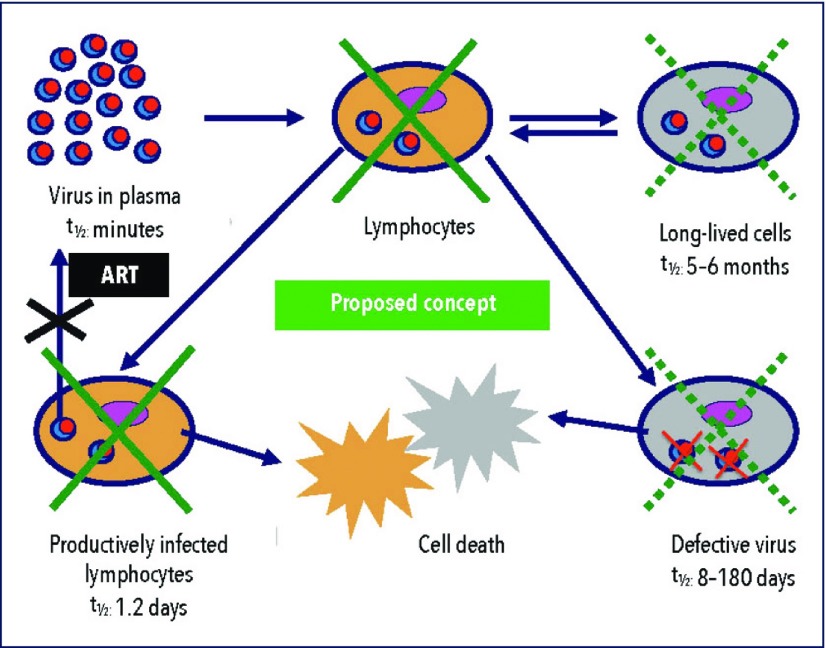
Figure 2.
HIV-1 needs lymphocytes for replication and survival. HIV-1 in plasma (without host cells) is only short-lived with a half-life of minutes: it needs T cells for survival.
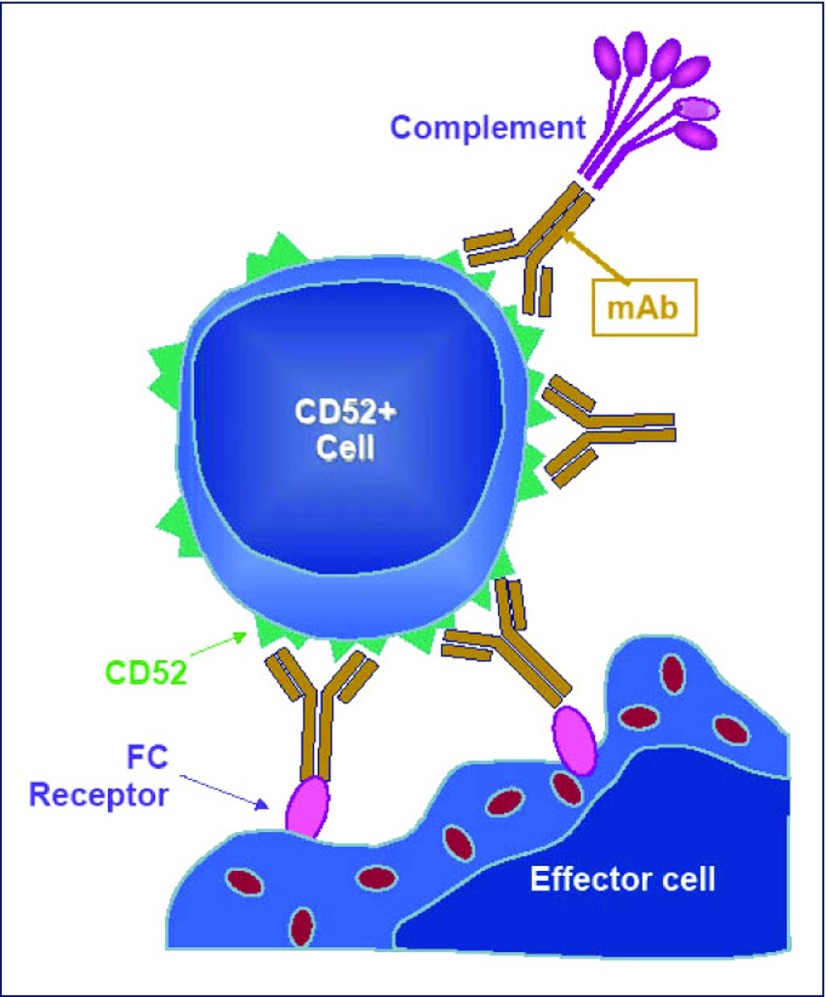
There are currently several different ways available to lyse immune cells. We selected the CD52-targeting monoclonal antibody, alemtuzumab (Campath, Lemtrada), for this purpose. This drug is a humanised, IgG1 kappa monoclonal antibody directed against the cell surface antigen CD52 [10] and is the most potent monoclonal antibody for the elimination of lymphocytes, monocytes and macrophages currently available. The mechanism of action of alemtuzumab is summarised in Figure 3. It has been shown that a single intravenous dose of 3 mg is able to completely eliminate all circulating lymphocytes in humans for several months [11] although the half-life of the drug in blood varies between 15 and 20 days, as reported by Morris et al. [12] or only 6 hours as published by Hale et al. [13]. Biodistribution of alemtuzumab as evaluated in mice is characterised by high uptake in bone marrow, the lymphoid tissues and in the mononuclear phagocyte system. Staining was also observed in the male reproductive system and in the skin [14].
Figure 3.
Mechanism of action of alemtuzumab. Alemtuzumab lyses lymphocytes via complement fixation, antibody-dependent cell-mediated cytotoxicity (ADCC), and by induction of apoptosis. Secondary pathways include cytokine release and T cell and complement activation. Since in vitro, not all of these mechanisms are active, T cell depletion is not complete.
Alemtuzumab had been approved and was widely used under the trade name Campath or Mabcampath for the treatment of chronic lymphocytic leukaemia (CLL) [15] and is under investigation in other haematological malignancies [16]. The drug is now approved by the FDA and the EMA for the treatment of multiple sclerosis using the trade name Lemtrada [17] and is widely used off-label in stem cell [18] and solid organ transplantation [19]. It is well known and has been repeatedly published that alemtuzumab only incompletely eliminates immune cells in vitro in contrast to the situation in vivo [11] where immune cells are immediately and completely eliminated by a single dose of alemtuzumab. Nevertheless, we decided to test this drug in vitro before testing in HIV-infected individuals to see first, whether the CD52 receptor is retained in HIV infection and, second, whether alemtuzumab can still bind to this receptor and lyse HIV-infected cells.
In our study we investigated the expression of the CD52 antigen on various immune cells in peripheral whole blood samples obtained from HIV-infected individuals who included responders and non-responders to HAART, with different CD4 cell counts and viral loads. We also investigated the in vitro depletion kinetics of these immune cells by alemtuzumab.
Methods
CD52 expression on immune cells
The percentage and density of CD52 receptors on T cells and T cell subsets (CD3, CD4, CD8), B cells (CD19), dendritic cells (CD123) and monocyte-derived dendritic cells (CD11c) was determined in samples from healthy volunteers (n=3) and from HIV-1 patients (n=21, detailed demographic data were available from 15 subjects) with different CD4 cell counts and viral loads, and who included responders and non-responders to HAART, by incubating alemtuzumab at room temperature (RT) for 30 minutes in 100 μL heparinised whole blood at a concentration of 10 μg/mL together with monoclonal antibodies against CD3/CD4/CD8 in one test tube, against CD19 in a second test tube, and against CD11c and CD123 in a third test tube. Whole blood was used to perform the assay instead of peripheral blood mononuclear cells (PBMCs) because it would be more representative of the situation in vivo, there would be less manipulation of the sample and it was technically simple to perform. Red blood cells (RBCs) were then lysed with FACS lysing solution and washed once with phosphate-buffered solution (PBS). Alemtuzumab was counterstained with 5 μL of anti-human IgG/Fc antibody (dilution 1:10) and incubated at room temperature for 30 minutes. The cells were washed once with PBS and re-suspended in 100 μL of 0.5% paraformaldehyde. The percentage and intensity of CD52 expression were measured by four-colour flow cytometry.
Immune cell depletion by alemtuzumab
A concentration of 10 μg/mL of alemtuzumab was incubated with 100 μL heparinised blood for 4 hours. Rabbit complement was added as exogenous complement to ensure efficient cell lysis. After the 4-hour incubation, the cells were again processed for CD52 expression under the same procedure as described above for the CD52 expression. The percentage of surviving cells was determined by flow cytometry. Experiments were performed in triplicate.
HIV-1 proviral DNA detection from the immune cell pellets
DNA from PBMCs after RBC lysis from three patients who were HAART responders (VL<50 copies/mL) and from three patients who had experienced HAART treatment-failure and harboured the M184V drug-resistance mutation, were analysed before and after incubation with alemtuzumab by in-house multiplex HIV-1 gag and pol polymerase chain reactions (PCR) to confirm whether the HIV-infected cells were still present. Although total eradication of lymphocytes is not possible in this in vitro model, the same DNA samples were subjected to HIV-1 drug-resistance genotypic testing in order to assess whether the alemtuzumab-mediated immune cell killings may lead to eradication of M184V-infected cells. The method for the PCR in brief is as follows:
-
•
HIV-1 gag – outer primers targeted HIV-1 gag: gag-728 (5′ GCA GCA AAG TCA GCC AAA ATT ACC 3′), and gag-1146 (5′ CCT TTT ATA GAT GTC TCC CAC TGG 3′). Inner primers: gag-844 (5′ TTT TAA CCC AGA AGT AAT ACC CAT G 3′), and gag-1070 (5′ GTT CCT GCT ATG TCA CTT CCC CTT 3′).
-
•
HIV-1 pol – outer primers: JA-17 (5′ TAC AGG AGC AGA TGA TAC AG 3′), and JA-20 (5′ CCT GGC TTT AAT GTT ACT GG 3′). Inner primers: JA-18 (5′ GGA AAC CAA AAA TGA TAG GG 3′), and JA-19 (5′ ATT ATG TTG ACA GGT GTA GG 3′).
The first PCR reactions were performed at 94 °C for 5 minutes, 94 °C for 30 seconds, 52 °C for 30 seconds, 72 °C for 30 seconds for a total of 40 cycles, then at 72 °C for 7 minutes. The second PCR reactions were performed at 94 °C for 5 minutes, 94 °C for 30 seconds, 50 °C for 30 seconds, 72 °C for 30 seconds for a total of 35 cycles, then at 72 °C for 7 minutes and kept at 4 °C.
Results
CD52 expression on various immune cells
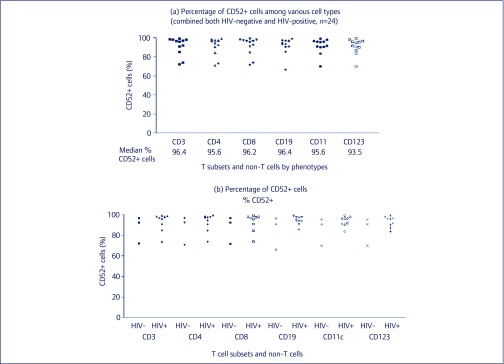
We found that almost all T cells (CD3+), T helper cells (CD4+), CD8+ T cells, B cells, CD11c+ cells and plasmacytoid dendritic cells (pDCs, CD123+) expressed CD52 receptors. The median CD52 positivity of these immune cells was more than 90% regardless of HIV infection (Figure 4). The difference of mean fluorescent intensity (MFI) between HIV-negative and HIV-positive groups could not be calculated because of the small sample size (n=2 for HIV-negative and n=9 for HIV-positive individuals) but a trend of higher MFI was observed in HIV-negative (mean [SD], range of HIV-negative versus HIV-positive individuals 82.70 [16.74], 70.86–94.53 versus 63.49 [30.79], 33.66–117.42, respectively.
Figure 4.
CD52 expression on immune cells. (a) CD52 expression on T cells (CD3+ cells), CD4+, CD8+ T cell subsets. (b) B cells, monocytes, plasmacytoid dendritic cells, and other CD11c+ cells in HIV-seronegative individuals (HIV-) and in HIV-seropositive individuals (HIV+).
Alemtuzumab-mediated immune cell lysis
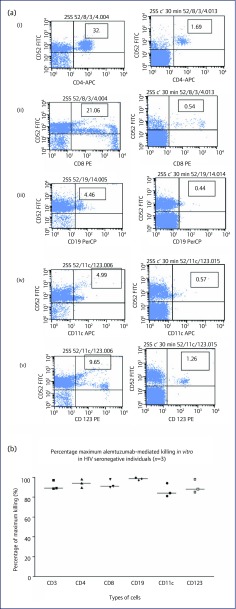
Samples from HIV-1-seronegative subjects were investigated. In Figure 5a, the results of an HIV-1-seronegative individual show alemtuzumab-mediated depletion of 87% of plasmacytoid dendritic cells (CD52+/CD123+) to 95% depletion of CD4+ T cells. The maximum alemtuzumab-mediated depletion ranged from 86% for CD11c+ cells (monocytes) (Figure 5b) to 98% in B cells (CD19+). The kinetic patterns of all three individuals were similar.
Figure 5.
Alemtuzumab-induced immune cell depletion: results from HIV-seronegative individuals. (a) Results from an HIV-seronegative individual: in vitro alemtuzumab-induced lysis of various immune cells at 30 minutes without (left panels), and with (right panels) complement added. Type of immune cells: (i) CD4 cells; (ii) CD8 cells; (iii) B cells; (iv) monocytes; and (v) plasmacytoid dendritic cells. (b) Maximum cell depletion (%) by alemtuzumab in blood samples from HIV-seronegative individuals at 1-hour incubation with complement added.
The kinetics and the maximum percentages of cell lysis in samples from HIV-1-infected individuals seemed to be different compared to those of seronegative subjects (Figure 6). Figure 6a shows the depletion results of an HIV-seropositive patient. Figures 6b and 6c show the results of all nine patients at 1 and 4 hours incubation with alemtuzumab together with the added complement, respectively. The median percentage of CD3+, CD4+, CD8+, CD19+, CD11c+ and CD123+ depletion in samples from seropositive (versus seronegative) subjects was 58% versus 89%, 52% versus 93%, 60% versus 91%, 68% versus 99%, 49% versus 85%, and 59% versus 88%, respectively.
Figure 6.
Immune cell depletion. (a) Results from an HIV-seropositive individual. In vitro alemtuzumab-induced lysis of various immune cells at 4 hours without (left panels), and with (right panels) complement added. Type of immune cells: (i) CD4 cells (76% depletion); (ii) CD8 cells (67% depletion); (iii) B cells (81% depletion); (iv) monocytes (52% depletion); and (v) plasmacytoid dendritic cells (74% depletion). Maximum cell depletion (%) by alemtuzumab in blood samples from HIV-seropositive individuals (n=9) after incubation for (b) 1 hour and (c) 4 hours.
A comparison of samples from patients with virological success (viral load <50 copies/mL) and virological failure (viral loads >1000 copies/mL) who carried at least one lamivudine-associated resistance mutation showed that alemtuzumab-mediated immune cell lysis was similar between the groups (data not shown).
HIV-1 proviral DNA and detection of M184V drug resistance mutation
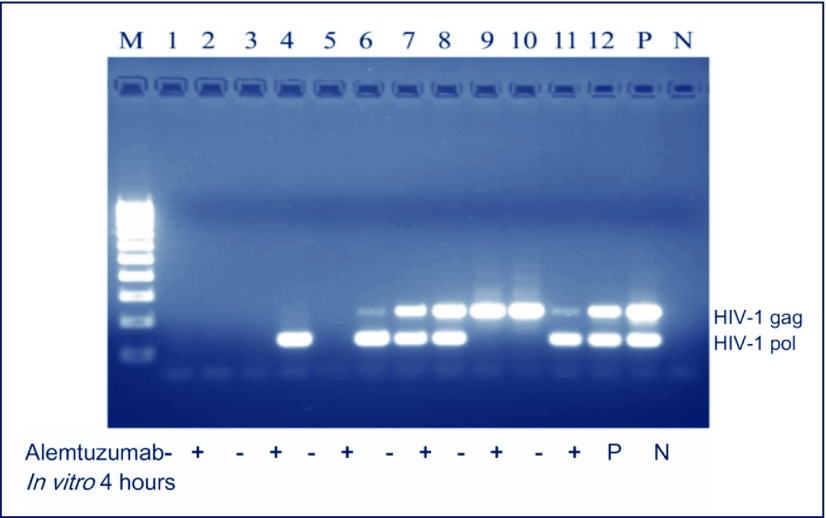
Expectedly, since the lysis of lymphocytes was not complete in vitro (Figure 7), HIV-1 proviral DNA could still be detected in the samples from patients whose HAART had failed and had viral loads of >1000 copies/mL, regardless of alemtuzumab treatment (Figure 4). In two of three virologically well-suppressed patients, HIV-1 proviral DNA could be detected in only the alemtuzumab-treated PBMCs but not in untreated ones. In the two patients with HAART failure and whose HIV-1 reverse transcriptase gene was amplifiable, M184V (lamivudine resistance-associated mutation) proviral DNA was detected before and after incubation with alemtuzumab.
Figure 7.
Detection of HIV-1 proviral DNA. Results show the gel electrophoresis of HIV-1 gag- and pol-specific polymerase chain reactions from the paired samples of three patients successfully treated with HAART (plasma HIV-1 viral loads <50 copies/mL) and from three patients experiencing treatment failure (viral loads >1000 copies/mL). Numbers 1–6 represent successful HAART and numbers 7–12 treatment failure. Odd numbers are samples without alemtuzumab and even numbers with alemtuzumab added to the culture for 4 hours.
M: molecular markers; P: positive control; N: negative control.
Discussion
The results of our experiments indicate that the CD52 receptor is retained in T cells of HIV-1-infected subjects and is able to bind alemtuzumab to a similar extent as in healthy volunteers. This finding is in agreement with a paper by Wu et al. [20] who determined a whole set of cell surface antigens in HIV-positive individuals and who reported that CD52 is expressed and can be used as a marker in these patients. Furthermore, our data show that T cell lysis by alemtuzumab can also be induced in blood samples obtained from HIV-1-infected subjects. The extent of alemtuzumab-induced cell lysis in samples from HIV-infected subjects was statistically not different from that of samples obtained from HIV-uninfected subjects. However, whereas all samples from HIV-negative subjects responded to alemtuzumab-induced cell depletion at rates of 80–100%, samples from HIV-infected patients showed a higher variability. Additionally, the rate of cell lysis seemed to be slower in samples from HIV patients compared to those from healthy subjects.
In general, in vitro depletion of different immune cells by alemtuzumab is not complete. This is in contrast to the situation in vivo, where circulating immune cells are completely eliminated [11] due to additional mechanisms such as antibody-dependent cellular cytotoxicity (ADCC), which is the major driver of alemtuzumab-induced cell lysis.
Adding fresh complement after 30 minutes of in vitro incubation with alemtuzumab increased the extent of cell depletion in some of the partial responders, but had little or no effect in others (data not shown). HIV and HIV-infected cells have been reported to be intrinsically resistant to complement-mediated depletion [21] although the complement system is highly activated in HIV infection and AIDS. However, due to deposition of C3, mannose-binding lectin and complement regulatory proteins such as decay-accelerating factor, membrane co-factor protein, CD59, and soluble factor H on the cell surface, virions and virus-infected cells may be partially protected from complement-mediated lysis. Our experiments indicate that this protective shielding system can be circumvented by the use of alemtuzumab, rendering infected cells sensitive to complement-mediated lysis. The situation may improve further in vivo, where the upregulated complement system might constitute a large-enough resource for increased complement-induced cell depletion following alemtuzumab binding to the CD52 receptor.
More importantly, in vivo the major contributor of alemtuzumab-induced cell lysis, ADCC, will come into effect. Natural killer (NK) cells play a major role in ADCC of virions and HIV-infected cells [22]. Their number and phenotype are subject to dramatic changes at different stages of HIV infection. Early on, NK cells are highly activated in HIV-infected subjects compared to normal subjects. Later on, their number decreases and NK cell receptor expression becomes significantly different, leading to a shift from activating to inhibitory phenotype. Accordingly, alemtuzumab-induced depletion of HIV-infected cells should be particularly effective in the early stages of HIV infection when both complement and NK cells are upregulated.
Another interesting question relates to dosing of alemtuzumab in HIV patients. Weinblatt et al. [11] have shown that a single intravenous dose of 3 mg alemtuzumab is able to completely eliminate all peripheral lymphocytes in rheumatoid arthritis patients. Assuming distribution of the antibody in the intravascular space of a 70-kg subject with 70% of body water, the concentration of alemtuzumab would be 0.06 μg/mL. In our experiments we found that in vitro, 2 μg/mL is less effective in cell depletion than 10 μg/mL, stressing again the importance of ADCC in comparison to complement-dependent cytotoxicity alone.
Ginaldi et al. [23] estimated that 125 mg of alemtuzumab is required to saturate all of the CD52 binding sites in a healthy subject assuming that the number of lymphocytes is 1012 and the number of CD52 binding sites per cell is 5×105. According to the results published by Weinblatt [11], saturation of all available binding sites is not necessary for complete lymphocyte depletion. CD52 is expressed on peripheral blood lymphocytes, tonsillar cells, thymocytes, monocytes and macrophages, but not on granulocytes, platelets, erythrocytes and haematopoietic stem cells [24]. Using radioisotopes, the CD52 cell density on peripheral blood lymphocytes has been estimated at 500,000 antigens per cell [20]. This means that approximately 5% of the cell surface is covered with CD52 [25].
After binding to CD52, alemtuzumab causes a release of inflammatory cytokines and induction of cell death through any of the host-effector mechanisms, i.e. complement-dependent cytotoxicity [26], ADCC via its IgG Fc region [27] and by direct apoptosis [28]. The release of various cytokines and inflammatory mediators may result in an enhancement of HIV replication in this setting if HIV-transmissible cells cannot be totally eliminated. This may explain our observation why in HAART responders with undetectable plasma HIV viral loads, incomplete depletion of T cells by alemtuzumab led to detectable HIV-1 DNA. Another possible explanation is that due to lysis of HIV-infected immune cells, viral DNA was released from the cells and became detectable. Multiplex PCR findings showed that only one of the two HIV-1 target genes was positive in the samples of two patients. The most likely explanation for this observation is viral diversity.
Alemtuzumab has already been used in HIV-infected patients. Tan et al. [29] performed renal transplantation in three HIV recipients using alemtuzumab pre-conditioning (30 mg) and low-dose tacrolimus for post-transplant immunosuppression. All patients had good graft function, and none has experienced acute rejection. No infectious or surgical complications occurred and CD4 cell counts were slowly recovering. HIV viral loads remained undetectable. However, since HAART was resumed after transplantation (Tan, personal communication), clear conclusions on alemtuzumab-induced T cell lysis cannot be drawn from this study.
Vivanco et al. [30] reported on four cases of hepatitis C in HIV-positive patients who underwent kidney transplantation and received alemtuzumab pre-conditioning for induction. Hütter et al. [31] reported on an HIV-1-infected patient with acute myeloid leukaemia who received stem-cell transplantation from a donor who was homozygous for CCR5 delta32, which confers high resistance against HIV-1 infection. Following stem-cell transplantation, the patient has remained HIV-1 virus-free without resuming HAART and may be considered cured. Hütter explained this result by a complete shift from a heterozygous genotype to a homozygous delta32/delta32 genotype, which was achieved 61 days after transplantation. However, this result may also be explained by T cell depletion during and after transplantation due to rabbit antithymocyte globulin and gentuzumab, two potent lymphocyte-depleting agents [32].
Conclusions
We were able to demonstrate for the first time that HIV-infected immune cells retain functioning CD52 receptors and can effectively be depleted by alemtuzumab.
In order to investigate whether this approach could potentially open up new horizons for the treatment of HIV infection and AIDS, a study in HIV-infected patients is warranted. Early use in the disease would be most appropriate although individuals whose HAART has failed may also be amenable to treatment with alemtuzumab. In the latter case, a clinical trial would allow study of whether alemtuzumab is able to deplete resistance-associated mutant-infected cells and thereby re-initiate susceptibility to HAART. Proof-of-concept studies of HIV reservoirs and potential functional cure among HIV-infected patients clinically indicated for alemtuzumab treatment is also warranted.
Acknowledgements
The study was performed with funding from Bayer Healthcare, Berlin, Germany, where WK was employed at that time. KR is supported as the Senior Research Scholar, the Thailand Research Fund (TRF); and the Research Professor Program, Chulalongkorn University, Bangkok, Thailand.
Authors’ contributions
WK devised the underlying concept of HIV eradication by T cell depletion using a CD52-targeting antibody, developed the design of the studies together with KR and SS, and drafted the manuscript. SS and SB carried out the experiments. KR supervised the studies. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1. The Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fischer M, Günthard HF, Opravil M et al. Residual HIV-RNA levels persist for up to 2.5 years in peripheral blood mononuclear cells of patients on potent antiretroviral therapy. AIDS Res Hum Retroviruses 2000; 16: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 3. Bedimo R. Non-AIDS-defining malignancies among HIV-infected patients in the highly active antiretroviral therapy era. Curr HIV/AIDS Rep 2008; 5: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 27; 373: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davey RT, Davey RT Jr, Bhat N et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA 1999; 96: 15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chomont N, El-Far M, Ancuta P et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Z, Schuler T, Zupancic M et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 1999; 286: 1353–1357. [DOI] [PubMed] [Google Scholar]
- 8. Sigal A, Kim JC, Balazs AB et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 2011, 477: 95–98. [DOI] [PubMed] [Google Scholar]
- 9. Lafeuillade A, Stevenson M.. The search for a cure for persistent HIV reservoirs. AIDS Rev 2011; 13: 63–66. [PubMed] [Google Scholar]
- 10. Waldmann H, Hale G.. CAMPATH: from concept to clinic. Phil Trans R Soc B 2005; 360: 1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinblatt ME, Maddision PJ, Bulpitt KJ et al. Campath-1H, a humanized monoclonal antibody, in refractory rheumatoid arthritis. An intravenous dose-escalation study. Arthritis Rheum 1995; 38:1589–1594. [DOI] [PubMed] [Google Scholar]
- 12. Morris E, Rebello P, Thomson K et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood 2003; 102: 404–406. [DOI] [PubMed] [Google Scholar]
- 13. Hale G, Rebello P, Brettman LR et al. Blood concentrations of alemtuzumab and antiglobulin responses in patientswith chronic lymphocytic leukemia following intravenous or subcutaneousroutes of administration. Blood 2004; 104: 948–955. [DOI] [PubMed] [Google Scholar]
- 14. Genzyme Canada Product Monograph MabCampath. 2010.
- 15. Hillmen P, Skotnicki AB, Robak T et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007; 25: 5616–5623. [DOI] [PubMed] [Google Scholar]
- 16. Ravandi F, O’Brien S.. Alemtuzumab in CLL and other lymphoid neoplasms. Cancer Invest 2006; 24: 718–725. [DOI] [PubMed] [Google Scholar]
- 17. Hirst CL, Pace A, Pickersgill TP et al. Campath 1-H treatment in patients with aggressive relapsing remitting multiple sclerosis. J Neurol 2008; 255: 231–238. [DOI] [PubMed] [Google Scholar]
- 18. Delgado J, Thomson K, Russell N et al. Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: a British Society of Blood and Marrow Transplantation Study. Blood 2006; 107: 1724–1730. [DOI] [PubMed] [Google Scholar]
- 19. Ciancio G, Burke III W.. Alemtuzumab (Campath-1H) in kidney transplantation. Am J Transplant 2008; 8: 15–20. [DOI] [PubMed] [Google Scholar]
- 20. Wu JQ, Dyer WB, Chrisp J et al. Longitudinal microarray analysis of cell surface antigens on peripheral blood mononuclear cells from HIV+ individuals on highly active antiretroviral therapy. Retrovirology 2008: 5: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Datta PK, Rappaport J.. HIV and complement: hijacking an immune defence. Biomed Pharmacother 2006; 60: 561–568. [DOI] [PubMed] [Google Scholar]
- 22. Alter G, Altfeld M.. NK cell function in HIV-1 infection. Curr Mol Med 2006; 6: 621–629. [DOI] [PubMed] [Google Scholar]
- 23. Ginaldi L, De Martinis M, Matutes E et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leukemia Res 1998; 22:185–191. [DOI] [PubMed] [Google Scholar]
- 24. Ratzinger G, Reagan JL, Heller G et al. Differential CD52 expression by distinct myeloid dendritic cell subsets: implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood 2003; 101: 1422–1429. [DOI] [PubMed] [Google Scholar]
- 25. Osterborg A, Dyer MJ.. CAMPATH-1H in the treatment of lymphoid malignancies In: Cheson BD, ed. Monoclonal Antibody Therapy of Hematologic Malignancies. Abingdon, UK: Darwin Scientific Publishing, 2001; pp. 135–150. [Google Scholar]
- 26. Golay J, Manganini M, Rambaldi A, Introna M.. Effect of alemtuzumab on neoplastic B cells. Haematologica 2004; 89: 1476–1483. [PubMed] [Google Scholar]
- 27. Dyer MJS, Hale G, Hayhoe FGJ, Waldmann H.. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood 1989; 73: 1431–1439. [PubMed] [Google Scholar]
- 28. Byrd JC, Shinn CA, Jansure J. CAMPATH-1H induces apoptosis in human chronic lymphocytic leukemia cells (CLL) in vitro independent of complement mediated lysis or Fca receptor ligation (abstract 556). Blood 1999; 15 (Suppl 1): 126a. [Google Scholar]
- 29. Tan HP, Kaczorowski DJ, Basu A et al. Living-related donor renal transplantation in HIV-recipients using alemtuzumab preconditioning and steroid-free tacrolimus monotherapy: a single center preliminary experience. Transplantation 2004; 78: 1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vivanco M, Friedmann P, Xia Y et al. Campath induction in HCV and HCV/HIV-seropositive kidney transplant recipients. Transplant Int 2013; 26: 1016–1026. [DOI] [PubMed] [Google Scholar]
- 31. Hütter G, Nowak D, Mossner M et al. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N Engl J Med 2009; 360: 692–698. [DOI] [PubMed] [Google Scholar]
- 32. Stauch D, Dernier A, Sarmiento ME et al. targeting of natural killer cells by rabbit antithymocyte globulin and Campath-1H: similar effects independent of specificity. PLoS ONE 2009; 4: e4709. [DOI] [PMC free article] [PubMed] [Google Scholar]