Abstract
Objectives:
Novel treatments for hepatitis C demonstrate high cure rates, but current high prices can be a barrier to rapid global treatment scale-up. Generic competition can rapidly lower drug prices. Using data on exports of raw materials in 2015, we calculated currently feasible generic prices of sofosbuvir and daclatasvir.
Methods:
Data on per-kilogram prices of sofosbuvir and daclatasvir active pharmaceutical ingredients (API) exported from India were extracted from an online database. To the cost of the amount of API needed for a 12-week treatment course, we added cost estimates for formulation (40%), packaging (US$0.35/month), and a mark-up (50%).
Results:
Between 1 January and 15 October 2015, over 5 tons of sofosbuvir were exported, with prices decreasing by US$702/kg/month, and observed prices of US$2501/kg in early September. Over the same period, 84 kg of daclatasvir were exported, with prices decreasing by US$1664/kg/month to US$1897/kg. Using the price estimation algorithm, we estimated the price of a generic sofosbuvir–daclatasvir combination regimen at US$200 per patient for a 12-week treatment course.
Conclusion:
The costs of generic production of sofosbuvir and daclatasvir are rapidly decreasing. Sofosbuvir–daclatasvir combination treatment could be produced for US$200 per patient per 12-week course.
Keywords: sofosbuvir, daclatasvir, hepatitis C, generics
Introduction
Novel direct-acting antivirals (DAAs) have revolutionised treatment options and prognosis for patients with chronic hepatitis C infection. DAAs with superior clinical efficacy include sofosbuvir (Sovaldi, Gilead Sciences) and daclatasvir (Daklinza, Bristol-Myers Squibb). Sofosbuvir–daclatasvir is the only combination treatment that has so far demonstrated consistently high viral suppression rates (93–97%) across genotypes 1–4 [1]. The pricing of these medicines has been heavily criticised by healthcare providers and governments, and despite a high burden of disease globally, meaningful treatment scale-up may not be possible at current prices [2].
Gilead Sciences’ pricing of sofosbuvir differs significantly between countries. The cost of a 12-week course of sofosbuvir is as high as US$84,000 in the US [3]. Branded sofosbuvir is available at a suggested price of US$900/12-week course in 101 named low-income countries [4]. Beyond this, Gilead has issued voluntary licences to 11 Indian generic producers allowing them to produce generic sofosbuvir with a 7% royalty payment to Gilead. These licences allow the export of generic sofosbuvir to the same 101 predefined countries [4,5]. Gilead's licensing and pricing strategy excludes many large middle-income countries that are home to a significant proportion of the world's hepatitis C-infected population: China, Brazil, the Philippines, Turkey, Thailand, Mexico – totalling 38.5 million infected [6].
A 12-week course of daclatasvir is priced at US$63,000 in the US [3]. Bristol-Myers Squibb's (BMS) plans for daclatasvir pricing in low- and middle-income countries have not been announced, but BMS has recently signed an agreement with the Medicines Patent Pool that will allow generic manufacture of daclatasvir. The terms of this agreement are yet to be analysed in detail, but appear to be more permissive in terms of generic production and sale than Gilead's agreements with Indian manufacturers [7].
In 2014, we estimated potential generic prices of DAAs by examination of their synthesis and comparison of their molecular structures to antiretrovirals already generically manufactured. This analysis predicted 12-week course target prices of US$68–136 for sofosbuvir, and US$10–30 for daclatasvir [8]. We have now undertaken an updated analysis for sofosbuvir and daclatasvir, this time using publicly available data on actual exports of DAA active pharmaceutical ingredients (API) to estimate regimen costs. The API is the substance that exerts the therapeutic effect in pharmaceutical products and its price is a key determinant of the cost of production of medicines, for example accounting for 65–90% of the price of antiretroviral medicines in competitive generic markets [1]. Many generic or originator pharmaceutical companies do not produce the API internally and rely on alternative suppliers.
Materials and methods
We gathered current prices of sofosbuvir and daclatasvir from various sources to provide a global price overview, including national pricing databases and online price comparison tools (see Appendix).
We extracted data on costs per kilogram of API from a database of Indian export–import logs (www.infodriveindia.com), which allows searching of daily export logs published by Indian customs under a general legal requirement in India (Customs Act 1962 and Notification No. 128/2004-Customs [N.T.]). Information on the breakdown of costs in drug production is sparse, as manufacturers do not normally publish it. We thus make a number of conservative assumptions in order to estimate the costs of various components of production.
To estimate feasible generic prices based on the per-kilogram price of API, we used a previously validated algorithm, which takes into account the additional costs of drug production. Based on standard dosing of sofosbuvir and daclatasvir, we calculated the cost of API for a 12-week course of treatment. The approved dose of sofosbuvir is 400 mg daily, equivalent to 34 g of API for a 12-week course. The approved dose of daclatasvir is 60 mg daily, equivalent to 5 g of API for a 12-week course. We added a 40% margin for formulation – including the cost of excipients, tableting and coating – US$0.35 per month for packaging, and a 50% profit margin on top of all production costs, to arrive at an estimate of a currently feasible price for generic production [1].
We plotted the trend of sofosbuvir and daclatasvir per-kilogram API price changes in 2015, to forecast generic prices possible in the near future. Trend lines were drawn by linear regression weighted by the size of export (bubble size).
Results
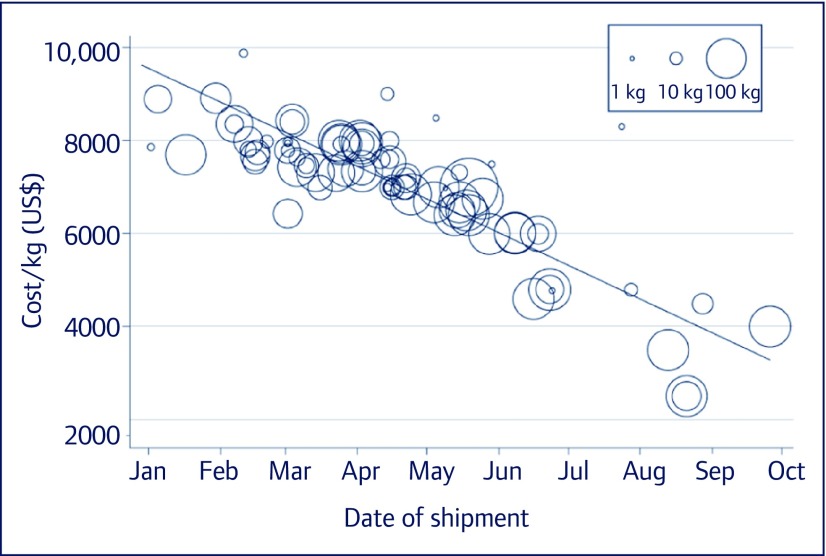
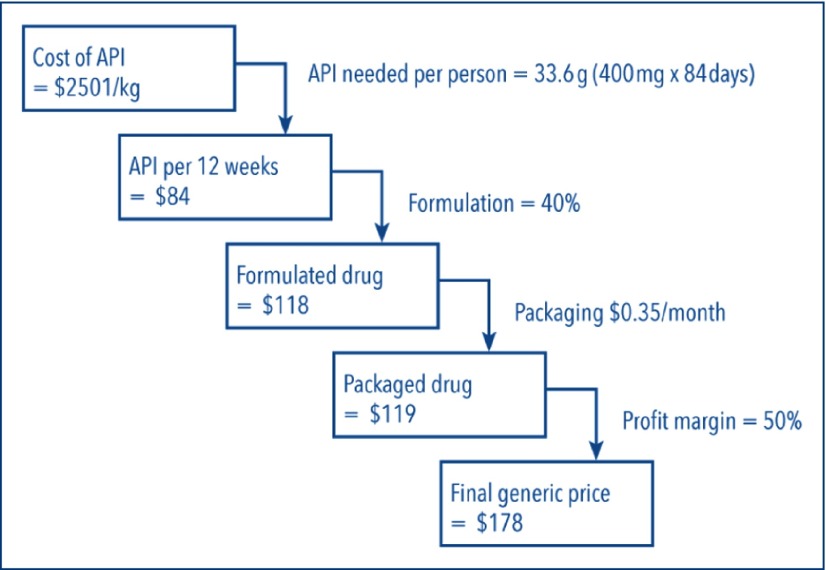
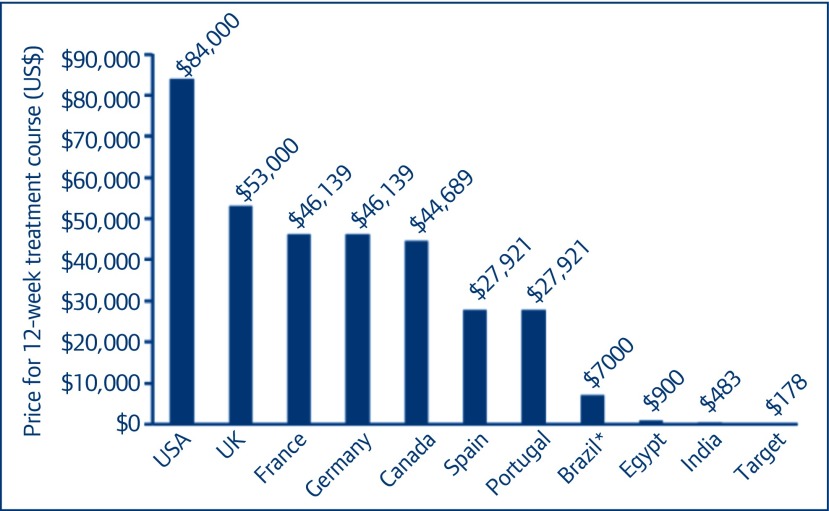
Between 1 January and 15 October 2015 more than 5000 kg of sofosbuvir API were exported from India (Figure 1). This amount would be enough to manufacture a 12-week treatment course for 150,000 people with hepatitis C. Between 1 January and 15 October 2015, the price per kilogram of sofosbuvir fell by a mean US$702/kg/month (95% confidence interval [CI] US$544–860/kg/month), from an average US$8754/kg in January 2015 to multiple large exports in September with a price of US$2501/kg. If sofosbuvir is produced with API procured at the lowest observed high-volume price (US$2501/kg), an amount sufficient to produce one 12-week treatment course (33.6 g) would cost US$84. We calculate that formulating the API into tablets, packaging the product, and adding a 50% profit margin would result in a price of US$178 for a 12-week treatment course of sofosbuvir (Figure 2).
Figure 1.
Cost per kg of sofosbuvir API exports from January to mid-October 2015, weighted by volume of purchase (bubble size)
Figure 2.
Calculation of target generic price for sofosbuvir
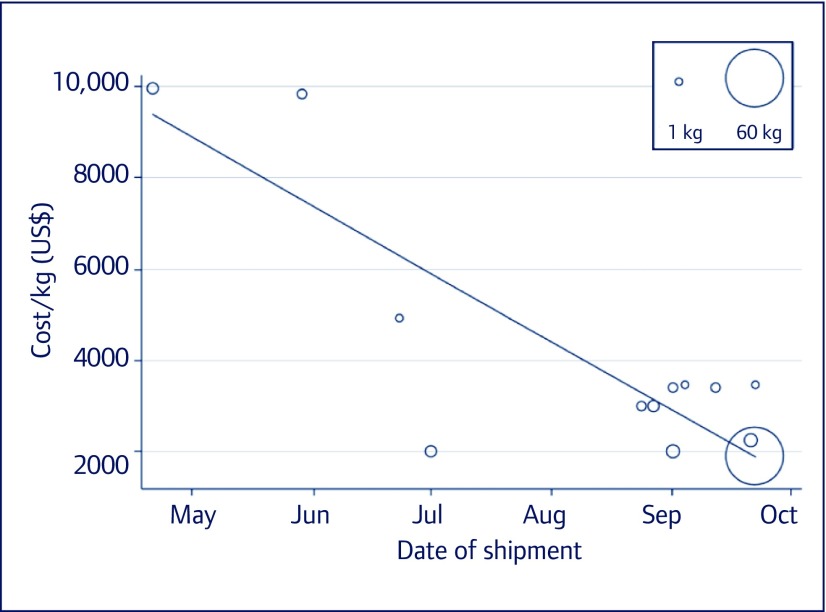
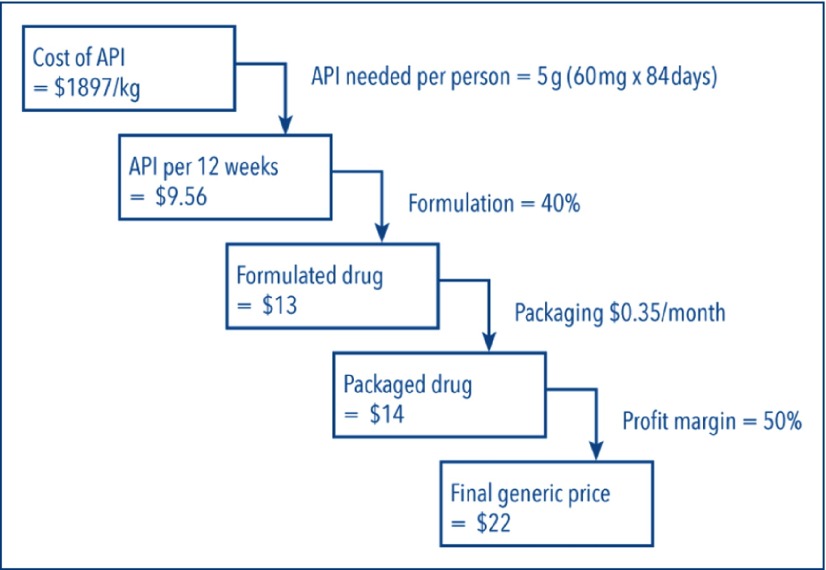
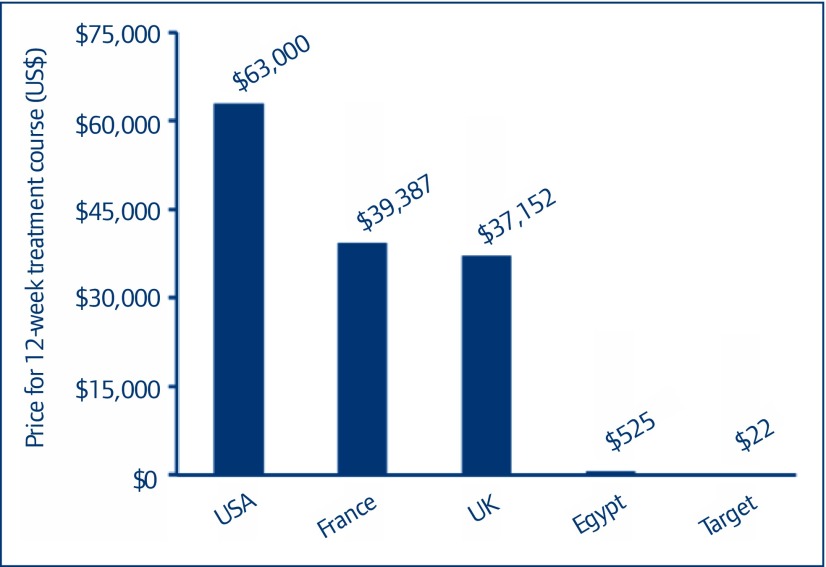
The available data show the first exports of daclatasvir API from India being shipped in early May 2015; between 1 May and 15 October we identified 14 shipments totalling 84 kg, enough to manufacture 17,000 12-week treatment courses (Figure 3). The price of daclatasvir API fell over 5 months, at a mean rate of US$1664/kg/month (95% CI US$1064–2265/kg/month), from US$9982/kg in May 2015 to US$1897/kg by mid-October 2015. Using the same algorithm for generic price estimation as for sofosbuvir, the most recently identified per-kilogram price of daclatasvir API would yield a generic price of US$22 per 12-week treatment course (Figure 4). The price of a 12-week course of sofosbuvir–daclatasvir combination treatment could therefore be US$200 in the near future.
Figure 3.
Cost per kg of daclatasvir API exports from May to mid-October 2015, weighted by volume of purchase (bubble size)
Figure 4.
Calculation of target generic price for daclatasvir
Some 99% of exported sofosbuvir, and 95% of exported daclatasvir, by volume, were shipped to Cairo, Egypt.
Global price overviews shown in Figures 5 and 6 put our target price estimates in context. Our calculated target price for sofosbuvir is 99.8% below the current US price, about 99.6% below current prices in the EU, 80% below the price offered by Gilead in Egypt, and 63% below the current lowest price globally (Figure 5). Our calculated target price for daclatasvir is 99.97% below the current US price, about 99.9% below current prices in the EU, and 96% below the current lowest price globally (Figure 6).
Figure 5.
Prices of sofosbuvir in different countries. *Price in Brazil based on expert opinion
Figure 6.
Prices of daclatasvir in different countries
Discussion
Continued competition in the API market, optimisation of the manufacturing process, and reductions in profit margin could bring the generic price down further. These medicines could follow the precedent set by antiretrovirals, in which rapid price reductions followed generic competition, allowing global treatment scale-up. First-line antiretroviral regimens now cost as little as US$100 per patient per year [9].
Monitoring data on exported API offers an insight into the costs of drug production, details of which are not usually made public by (originator or generic) pharmaceutical manufacturers. The observed prices are only made ‘visible’ in these databases because the API has passed through Indian customs. These API costs are thus likely to include profit margins of unknown size as they are sold by one company to another, and may include an additional margin for international tariffs. API sales within India are likely to be at lower prices per kilogram, and companies synthesising API internally are likely to have lower costs than those assumed in our calculations. Both of these factors have the effect of increasing our calculated generic prices – that is, making our price reduction predictions conservative.
Our study is limited to considering the Indian API market. Indian generic production is well established as sufficient to meet global demands, supplying, for example, around 80% of global antiretrovirals [10].
Nearly all of the observed exports were shipped to Egypt, the country with the world's highest prevalence of hepatitis C, and where key sofosbuvir patents were rejected [11]. Given that there are at least 11 generics manufacturers in India – some or all of whom are likely to be manufacturing API in-house, benefitting from technology transfer of Gilead's manufacturing process – and that there are multiple generics companies in Egypt that have expressed plans to manufacture sofosbuvir, we can reasonably infer that the demonstrated API price decrease is due to competition between multiple Egyptian buyers and/or multiple Indian API producers [4].
As part of drug procurement, countries and international agencies need to be assured of the quality of the product. Our estimates are limited with regard to calculating prices for generic sofosbuvir and daclatasvir versions that would attain Western market approval, because it is not possible to ascertain the quality of the API being exported from India from the data sources used in this analysis. There are, however, numerous reasons to believe that the API is of good quality: many companies currently producing generic sofosbuvir are doing so under licence from Gilead, which includes a full technology transfer of the manufacturing process. Many are well-known generic manufacturers, and have had their manufacturing practices certified by national regulators. Many also supply Western markets with other medications. The World Health Organization is currently working with several companies to arrange quality assurance of generic versions of sofosbuvir through its ‘prequalification programme’ [12]. In previous communications with generic manufacturers, the added cost of producing API that satisfies stringent regulatory authority (SRA) requirements has been reported at 20–45% of the cost of API alone [13]. Thus, even assuming that the API exports captured in this study are of inferior quality, producing SRA-approved generics would not dramatically affect the conclusions of this study, and these added costs could be rapidly offset by the continuing downward trends in API price.
For significant global treatment scale-up, a simplified and cost-efficient protocol of diagnostic and monitoring tests could be used, in which genotyping is not used before starting sofosbuvir–daclatasvir treatment [1,14]. Global treatment expansion would benefit from proof-of-principle studies assessing the ability to achieve high viral suppression rates with generic sofosbuvir–daclatasvir, minimal diagnostic tests, and without genotyping. As generic sofosbuvir is in the early stages of entering markets, bioequivalence data should immediately be made publicly available to encourage confidence in the quality of new generics.
Sofosbuvir with daclatasvir, currently the most effective pan-genotypic combination treatment, could be sustainably produced at a price of US$200. The price for the requisite laboratory tests for diagnosis and treatment monitoring, if using a pan-genotypically effective regimen like sofosbuvir–daclatasvir, has been reported as US$56 per patient [1,14]. By combining this estimate of testing costs with our present cost-based price estimates [1], We propose that testing, treatment and monitoring is currently possible at US$256 per patient, for a 12-week sofosbuvir–daclatasvir regimen, with no genotyping. At current trends, these per-patient costs could show progressive reductions below this price in the next 2–3 years.
There are several limitations to this analysis. The prices of API exported from India do not include trade tariffs when imported into other countries – these tariffs could vary by country of import, but a typical figure would be 12% of the cost of the exported API. In addition, costs of shipping would need to be accounted for. These are normally in the range of 8% of the price of the API. Even if these combined costs of tariffs and shipping were included, sofosbuvir could still cost in the region of $215 per 12-week course of treatment, and daclatasvir $27, both of which are still well below current sales prices. There is no generic supplier of HCV DAAs that has so far received WHO pre-qualification – this is used as a mark of high-quality drug production, and is used to assess the quality of supplies of antiretrovirals. There could be additional investment involved when generic companies apply for WHO pre-qualification. Current prices of DAAs are highly variable between countries, even within income brackets, and most hepatitis C infections occur in countries that are not covered by Gilead's voluntary licensing for sofosbuvir. Compulsory licences could be considered by countries as a mechanism to provide access to generic DAAs [15].
The feasibility and cost-efficiency of large-scale hepatitis C treatment, in terms of drug costs, is demonstrated theoretically in our analyses. Expansion of global treatment will rely on programmes that pioneer low-cost diagnosis and simplified monitoring, and such programmes could drive further price reductions in all components of treatment and care. When drug costs reach the affordable levels that we believe can be feasibly attained within the next year, other operational challenges will remain in scaling up global hepatitis C treatment. Lowering drug costs will be a first step.
Acknowledgements
Funding
This research was sponsored by a grant from UNITAID. The publication of study results was not contingent on the sponsor's approval or censorship of the manuscript.
Conflicts of interest
All authors declare no conflicts of interest.
Contributions
AH designed the study. BS, DG, and JF conducted the cost analyses. AH and DG drafted the manuscript. All authors critically reviewed and approved the manuscript.
Appendix
Sofosbuvir prices
-
1.
Hepatitis C Online. HCV medications: sofosbuvir. Available at: www.hepatitisc.uw.edu/page/treatment/drugs/sofosbuvir-drug (accessed December 2015).
-
2.
Service des relations avec la clientele, Quebec. List of Medications. Available at: https://www.prod.ramq.gouv.qc.ca/DPI/PO/Commun/PDF/Liste_Med/Liste_Med/liste_med_2015_07_24_en.pdf (accessed December 2015).
-
3.
France: Taylor P. PMLiVE. France agrees lowest Sovaldi pricing in EU. Government brings cost of hepatitis C drug to €5,000 below list price. 21 November 2014. Available at: www.pmlive.com/pharma_news/france_agrees_lowest_sovaldi_pricing_in_eu_618661 (accessed December 2015).
-
4.
Germany: Taylor P. PMLiVE. Gilead agrees price cut for Sovaldi in Germany. Same sum as agreed with French government last November. 13 February 2015. Available at: www.pmlive.com/pharma_news/gilead_agrees_price_cut_for_sovaldi_in_germany_649304 (accessed December 2015).
-
5.
Spain: Lamata F, Gálvez R, Pita Barros P, Sánchez Caro J. Accesso a los nuevos medicamentos: el ejemplo de la hepatitis C. Available at: www.actasanitaria.com/documentos/acceso-los-nuevos-medicamentos-el-ejemplo-de-la-hepatitis-c-costes-precios-y-patentes/ (accessed December 2015).
-
6.
UK: British National Formulary. Available at: https://www.medicinescomplete.com/mc/bnf/current/ (accessed December 2015.
-
7.
Medicines Patent Pool. Licences in the MPP. Available at: www.medicinespatentpool.org/current-licences/ (accessed December 2015).
-
8.
Fick M, Hirschler B. Reuters. Gilead offers Egypt new hepatitis C drug at 99 percent discount. 21 March 2014. Available at: www.reuters.com/article/2014/03/21/us-hepatitis-egypt-gilead-sciences-idUSBREA2K1VF20140321 (accessed December 2015).
-
9.
MSF Briefing Document. Strategies to Secure Access to Generic Hepatitis C Medicines. May 2015. Overcoming patent and regulatory barriers to secure access to generic hepatitis C medicines. Available at: http://www.msfaccess.org/sites/default/files/MSF_assets/HepC/Docs/HepC_brief_OvercomingbarriersToAccess_ENG_2015.pdf (accessed December 2015).
Daclatasvir prices
-
10.
Hepatitis C Online. HCV medications: daclatasvir. Available at: www.hepatitisc.uw.edu/page/treatment/drugs/daclatasvir (accessed December 2015).
-
11.
UK: British National Formulary. Available at: https://www.medicinescomplete.com/mc/bnf/current/ (accessed December 2015).
-
12.
Swan T. 2014 Pipeline Report. Hepatitis C Pipeline. Available at: www.pipelinereport.org/sites/g/files/g575521/f/201407/HCV%20Treatment.pdf (accessed December 2015).
-
13.
Egypt: Andrieux-Meyer I, Cohn J, Affonso de Araujo E, Hamid S. Disparity in market prices for hepatitis C virus direct-acting drugs. Lancet 2015; 3: e676–e7.
References
- 1. Van de Ven N, Fortunak J, Simmons B et al. Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology 2015; 61: 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill A, Cooke G. Hepatitis C can be cured globally, but at what cost? Science 2014; 345: 141–142. [DOI] [PubMed] [Google Scholar]
- 3. Hepatitis C Online HCV medications. Available at: www.hepatitisc.uw.edu/page/treatment/drugs (accessed December 2015).
- 4. Gilead Sciences Inc Chronic hepatitis C treatment expansion: generic manufacturing for developing countries. 2015. Available at: http://www.gilead.com/~/media/files/pdfs/other/hcv%20generic%20agreement%20fast%20facts%20101615.pdf?la=en (accessed December 2015).
- 5. Gilead Sciences Inc 2014 original HCV license agreement. Available from: www.gilead.com/~/media/files/pdfs/other/2014_original_hcv_licensing_agreement.pdf?la=en (accessed December 2015).
- 6. Taro T. A step back for millions of people with hepatitis C. ITPC press release. 19 September 2014. Available at: tpcglobal.org/a-step-back-for-millions-of-people-with-hepatitis-c/ (accessed December 2015).
- 7. Bristol-Myers Squibb HCV Developing World Strategy. Available at: www.bms.com/responsibility/access-to-medicines/Pages/HCV-developing-world-strategy.aspx (accessed December 2015).
- 8. Hill A, Khoo S, Fortunak J et al. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; 58: 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MSF Access campaign Untangling the web of antiretroviral price reductions, 17th edition – July 2014. Available at: msfaccess.org/content/untangling-web-antiretroviral-price-reductions-17th-edition-%E2%80%93-july-2014 (accessed December 2015).
- 10. Waning B, Diedrichsen E, Moon S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc 2010; 13: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MSF Access campaign MSF Access Campaign response to Gilead's deal with generic companies for sofosbuvir and ledipasvir. 15 September 2015. Available at: www.msfaccess.org/content/msf-access-campaign-response-gilead%E2%80%99s-deal-generic-companies-sofosbuvir-and-ledipasvir (accessed December 2015).
- 12. Beyer P. The perspective of the World Health Organization. 2015. Available from: http://www.agcm.it/en/component/joomdoc/events/contributions/Peter_BEYER_WHO.pdf/download.html (accessed December 2015).
- 13. Hill A, Gotham D, Cooke G et al. Analysis of minimum target prices for production of entecavir to treat hepatitis B in high- and low-income countries. J Virus Erad 2015; 1: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohn J, Roberts T, Amorosa V et al. Simplified diagnostic monitoring for hepatitis C, in the new era of direct-acting antiviral treatment. Curr Opin HIV AIDS 2015; 10: 369–373. [DOI] [PubMed] [Google Scholar]
- 15. Andrieux-Meyer I, Cohn J, Affonso de Araujo E, Hamid S. Disparity in market prices for hepatitis C virus direct-acting drugs. Lancet 2015; 3: e676–e677. [DOI] [PubMed] [Google Scholar]