Abstract
Background
Limited data are available on factors associated with HIV-RNA viral load (VL) among antiretroviral treatment (ART)-naïve key populations in concentrated epidemics.
Methods
We conducted a cross-sectional survey of 1211 adult ART-naïve patients at 19 HIV clinics in Ho Chi Minh City (HCMC), Vietnam. Data collection included a standardised questionnaire, routine laboratory testing, hepatitis serology and HIV VL. Correlation between CD4 cell count and VL was assessed across all participants. In 904 participants not meeting Vietnam criteria for ART (CD4 cell count >350 cells/mm3, WHO clinical stage 1 or 2 and not pregnant), multivariate analyses were conducted to assess factors associated with HIV VL.
Results
Pre-ART patients had a median age of 31 years and 54% were male. Median CD4 cell count was 533 cells/mm3. Median HIV VL was 17,378 copies/mL; 60% had VL greater than 10,000 copies/mL and 16% had VL above 100,000 copies/mL. Although declining CD4 cell count was correlated with rising VL across all CD4 cell counts, correlation of VL with CD4 cell counts between 351 and 500 cell/mm3 was not significant. On multivariate linear regression, higher HIV VL was independently associated with male sex, men who have sex with men (MSM), CD4 cell count 351–500, HIV diagnosis within the previous 6 months, and hepatitis B (HBV). Lower HIV VL was independently associated with hepatitis C (HCV).
Conclusions
The majority of HIV patients who were not eligible for ART in HCMC in 2014 had HIV VL greater than 10,000 copies/mL. These data support expanded eligibility of ART to all HIV patients with the goal of treatment as prevention. This study is also among the first to demonstrate that MSM had a higher VL than women and heterosexual men and highlights the need for improved outreach and linkages to HIV care for this high-risk group.
Keywords: HIV, ART naïve, treatment as prevention, HIV viral load, Vietnam
Introduction
Numerous case–control, cohort and modelling studies have concluded that the strongest predictor of per exposure HIV transmission risk is HIV-RNA viral load (VL) of the HIV-infected person [1–7]. The Rakai Project Study Group in Uganda found that transmission to heterosexual partners increased with VL strata in the infected partner and that more than 75% of infections were transmitted from individuals with VL>10,000 copies/mL [4]. Another African study concluded that 90% of new HIV infections could be eliminated by treating only people living with HIV (PLHIV) with HIV VL>10,000 copies/mL [3].
Various factors have been associated with HIV VL. A number of studies have found 35–50% lower HIV VL among females relative to males [7–12]. Acute HIV infection is characterised by very high VL and increased transmission risk [7,13–17].
Studies examining HIV viral load among persons who inject drugs (PWID) are conflicting. One study in France demonstrated a small but statistically significant 0.35–0.60 log10 increase in HIV VL among active PWID relative to former PWID not on ART [18]. However, another study among female PWID in New York City did not find a correlation between HIV VL and use of cocaine, methadone or injecting heroin [19].
Genital ulcer disease increases HIV VL and rates of HIV transmission [2,4,6,7,20]. A meta-analysis on treatment of co-infections found a small but significant inverse relationship between syphilis treatment and HIV viral load [21]. In addition, one retrospective analysis found untreated primary or secondary syphilis increased HIV VL on average by 66% and decreased CD4 cell count on average by 62 cells/mm3 [22].
In Vietnam, the HIV epidemic is concentrated among men who have sex with men (MSM), PWID and female sex workers (FSW). The majority of PLHIV in Vietnam are not yet on ART and many continue to engage in risky behaviour, presenting a significant risk for HIV transmission. Targeted combined prevention strategies, including expanded treatment to members of key populations not currently eligible for ART, could potentially decrease HIV transmission in the community. The purpose of this study was to determine HIV VL and to identify clinical, biological, behavioural and demographic factors associated with HIV VL among pre-ART patients in Vietnam.
Methods
Brief overview of study setting
Ho Chi Minh City (HCMC) is the largest city in Vietnam, with a population of 8 million inhabitants [23]. The province has a large population of PLHIV, largely concentrated among PWID, MSM and FSW. The reported 2013 prevalence of HIV infection among these three key populations was 18%, 5% and 15%, respectively [24]. As of September 2013, there were 26,249 PLHIV enrolled in HIV care, with 24,115 on ART and 2134 designated as pre-ART [25].
Study population, recruitment and entry criteria
This was a cross-sectional assessment of all pre-ART patients in all 19 district-based HIV outpatient clinics (OPCs) in HCMC operating at the time of the study. No sampling or randomisation was employed; all pre-ART patients at each OPC who met the inclusion criteria and gave informed consent were enrolled in the evaluation.
Enrolment was conducted between November 2013 and July 2014 during routine visits when routine blood work was scheduled. The inclusion criteria were that individuals be 18 years of age or older, with documented HIV infection, enrolled in pre-ART care, and not known to be eligible for ART based on Vietnam Ministry of Health guidelines. Exclusion criteria were for individuals to be unable or unwilling to give informed consent, or any previous or current use of ART. Criteria for ART initiation in the Vietnam national treatment program at that time was a CD4 cell count ≤350 cells/mm3 or WHO clinical stage 3 or 4. Pregnant and breast-feeding women in Vietnam were eligible for antiretroviral-based prevention of mother-to-child transmission.
Ethics
This research study was reviewed by FHI 360 Office of International Research Ethics, Protection of Human Subjects Committee and the Ho Chi Minh City Provincial AIDS Committee Institutional Review Board.
Data collection
After providing informed consent, participants were guided through a structured questionnaire by trained clinic staff. The questionnaire included demographics, HIV status of primary sex partner, symptoms of upper respiratory (URI) or viral infections, symptoms of STIs, injection drug use history, and sexual behaviour. Other information extracted from the medical record included HIV transmission risk-group category and the date of the first HIV-positive test.
Routine laboratory investigations included complete blood count, liver function and CD4 cell count. If not performed within the previous 1 year, testing was carried out for HBsAg, anti-HCV antibody and syphilis serology (VDRL, RPR and/or TPHA). An additional 6 mL of blood was taken for an HIV viral load test.
Routine blood tests were performed at the local laboratory in each district using standard commercial assays. All viral load testing was performed at the Pasteur Institute in HCMC using a validated and external quality-controlled real-time reverse transcriptase PCR assay (generic HIV viral load assay, Biocentric, Bandol, France), which had a level of detection of 250 copies/mL [26,27].
Data analysis
Viral load was analysed both as a continuous variable and as a dichotomous categorical variable. The two categories for dichotomous HIV viral load were less than and greater than or equal to 10,000 (4.0 log10) copies/mL. This cut-off value was based on previous studies showing a higher risk for HIV transmission above 10,000 copies/mL in patients not taking ART.
‘Recent HIV diagnosis’ was defined as first positive HIV test within the previous 6 months. ‘Sexually active’ was defined as having any sex during the previous 30 days. ‘Multiple sex partners’ was defined as having two or more sex partners within the previous 30 days. STI symptoms included any one or more of genital ulcer, dysuria, urethral discharge (men) or vaginal discharge (women). ‘URI or viral symptoms’ was defined as report of any three or more of the following symptoms: headache, fever, chills, cough, sputum, nasal congestion, throat pain, muscle aches, swollen lymph nodes, fatigue and rash. ‘Syphilis serology’ was defined as the result of TPHA, VDRL and/or RPR testing. Access to VDRL and RPR testing was limited in some clinics.
The analysis population was pre-ART subjects, defined as those who did not meet Vietnam MOH criteria for ART at the time of the study. Because current CD4 cell count could not be determined prior to enrolment, several subjects initially included in the evaluation were subsequently found to meet criteria for ART after the results of the CD4 cell count were known and were excluded from the bivariate and multivariate analyses of associations with HIV VL.
Associations between categorical variables and dichotomous HIV VL were evaluated using the Chi-squared test or Fisher's exact test. Comparisons of continuous variables between HIV VL groups were assessed using t-test and ANOVA tests. The non-parametric versions of these tests (i.e. Mann–Whitney or Kruskal–Wallis tests) were used if normality assumptions were not met.
For testing the association between categorical and continuous log10 transformed VL, we used t-tests or ANOVA tests. The non-parametric versions of these tests (i.e. Mann–Whitney, Kruskal–Wallis tests) were used if normality assumptions were not met. For assessing the association between log10 HIV VL and continuous variables, we used Spearman correlation coefficients with 95% confidence intervals.
Multivariate logistic regression was used to evaluate predictors for the dichotomous variable of HIV VL>10,000 copies/mL. A second log–linear regression model was used to evaluate predictors for log10 transformed HIV VL as a continuous variable. Age, WHO clinical stage, injection-drug user (IDU) status, gender, sexual orientation, HBsAg and anti-HCV were included in both models. Additional variables significant at P<0.10 in respective bivariate analyses were included using a backward selection process. Final tests were assessed for significance at the 5% level with two-sided comparisons.
Results
Participant characteristics
A total of 1231 patients gave informed consent and enrolled in the study representing 58% of pre-ART patients registered in public OPCs at the start of the study. Data on number of refusals to participate was not systematically reported, but refusals were less than 5% at those sites that reported these data. Of those patients enrolled, 20 patients were missing either VL or CD4 data, or had previous CD4 cell count ≤350 cells/mm3, and were excluded from analysis. Another 307 patients had a CD4 count ≤350 cells/mm3 at the time of enrolment and therefore met criteria for ART. These patients were excluded from the outcome analysis but were included in the CD4 cell count versus VL correlation testing. The final analysis population size was 904 for the bivariate and multivariate analyses and 1211 for the CD4 cell count versus VL correlation analysis.
Characteristics of the study population are shown in Table 1. The median age was 31 (range 18–64). The majority were male (54%), aged 26–35 (55%), married (57%), and WHO clinical stage 1 (89%).
Table 1.
Characteristics of pre-ART study participants (n=904)
| Total n (%) |
|
|---|---|
| HIV WHO clinical stage | |
| Stage 1 | 804(88.9) |
| Stage 2 | 100(11.1) |
| Province of residence | |
| HCMC | 732(81.0) |
| Other | 172(19.0) |
| Sex | |
| Male | 487(53.9) |
| Female | 417(46.1) |
| Age | |
| 18–25 | 152(16.8) |
| 26–35 | 497(55.0) |
| 36–64 | 255(28.2) |
| Highest education level | |
| No schooling | 27(3.0) |
| Primary(1–5) | 157(17.4) |
| Secondary(6–9) | 346(38.3) |
| High school(10–12) | 238(26.3) |
| College/University | 136(15.0) |
| Marital status | |
| Married | 514(56.9) |
| Divorced/widowed | 125(13.8) |
| Single | 265(29.3) |
| Currently lives with other people | |
| Alone | 87(9.6) |
| With other people | 817(90.4) |
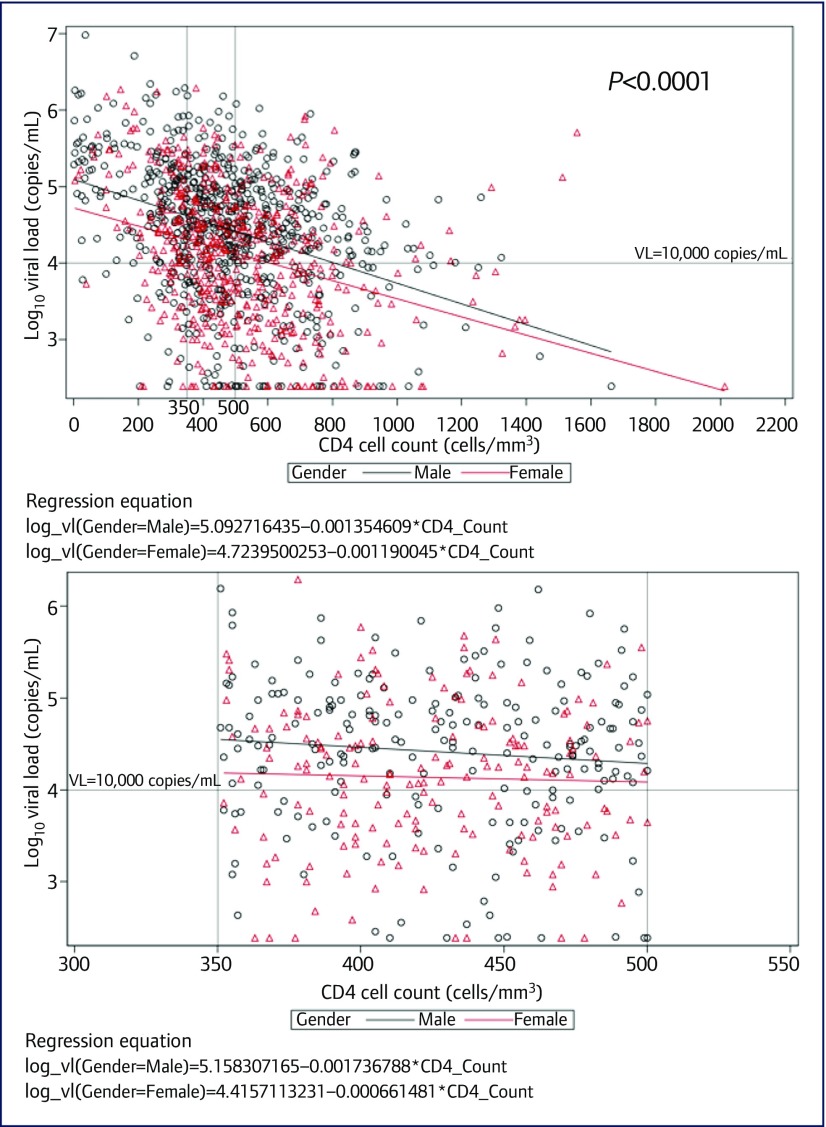
The ranges of CD4 cell count and HIV VL are shown in Figure 1. The median CD4 was 533 cells/mm3 (interquartile range [IQR] 439–681). The majority (58%) had CD4>500 cells/mm3. A small proportion (12%) had VL below 1000 copies/mL, 27% had VL 1000–9999 copies/mL, and 61% had VL>10,000 copies/mL, a level associated with higher risk for onward HIV transmission [3,4]. Of note, 16% had VL above 100,000 copies/mL. Lower CD4 cell count was associated with higher VL (P<0.0001). Overall, CD4 cell count and HIV VL were significantly and negatively correlated, such that as CD4 cell count declined, HIV VL increased (Figure 1a). In CD4 cell count ranges below 350 and above 500 cells/mm3 the negative correlation with HIV VL was statistically significant (P<0.0001 and P<0.0001, respectively). However, within the 351–500 cells/mm3 range the correlation between CD4 cell count and viral load was not significant (Figure 1b). Differences in linear trends by gender can also be seen in Figure 1.
Figure 1.
Scatterplot of CD4 vs HIV VL in (a) total population (n=1211), and (b) patients with CD4 cell counts 351–500 cells/mm3
Risk behaviour
Risk behaviour disaggregated by sexual orientation and gender is presented in Table 2. MSM represented 17% of the total sample and approximately one-third (31%) of all males in the study population. Among those diagnosed with HIV in the previous 6 months, 40% were MSM, 30% were non-MSM males, and 30% were female (P<0.001). MSM had significantly higher HIV VL: 80% of MSM had HIV VL ≥10,000 copies/mL while only 64% of non-MSM males and 51% of females had HIV VL in that range (P<0.0001). Mean HIV VL was 0.51 log10 copies/mL higher in MSM when compared to females, or an average of 30,199 copies/mL in MSM versus 9332 copies/mL in females. Non-MSM males had a mean HIV viral load of 15,849 copies/mL.
Table 2.
Sexual behaviour, STI, hepatitis serology and IDU by sexual orientation and gender.
| MSM (N=153) n (%) |
Non-MSM male (N=334) n (%) |
Female (N=417) n (%) |
Total (N=904) 2 n (%) |
P-value | |||
|---|---|---|---|---|---|---|---|
| HIV status of regular sex partner | |||||||
| Positive | 32(26.4) | 106(37.7) | 266(68.7) | 404(51.2) | <0.001 | ||
| Negative/unknown | 89(73.6) | 175(62.3) | 121(31.3) | 385(48.8) | |||
| Multiple sex partners in last 30 days 1 | |||||||
| Yes | 20(29.9) | 5(2.8) | 7(2.6) | 32(6.2) | <0.001 | ||
| No | 47(70.1) | 175(97.2) | 260(97.4) | 482(93.8) | |||
| Received money for sex in last 30 days 1 | |||||||
| Yes | 3(4.5) | 0(0.0) | 2(0.7) | 5(1.0) | 0.0146 | ||
| No | 64(95.5) | 182(100) | 267(99.3) | 513(99.0) | |||
| Any STI symptoms | |||||||
| Yes | 9(5.9) | 22(6.6) | 94(22.5) | 125(13.8) | <0.001 | ||
| No | 144(94.1) | 312(93.4) | 323(77.5) | 779(86.2) | |||
| History of IDU | |||||||
| Yes | 15(9.8) | 182(54.5) | 25(6.0) | 222(24.6) | <0.001 | ||
| No | 138(90.2) | 152(45.5) | 392(94.0) | 682(75.4) | |||
| Syphilis serology | |||||||
| Positive | 31(20.3) | 22(6.7) | 6(1.5) | 59(6.6) | <0.001 | ||
| Negative | 122(79.7) | 306(93.3) | 407(98.5) | 835(93.4) | |||
| HBsAg | |||||||
| Positive | 20(13.2) | 50(15.1) | 31(7.5) | 101(11.3) | 0.004 | ||
| Negative | 131(86.8) | 282(84.9) | 383(92.5) | 796(88.7) | |||
| Anti-HCV | |||||||
| Positive | 18(11.9) | 183(55.3) | 53(12.8) | 254(28.3) | <0.001 | ||
| Negative | 133(88.1) | 148(44.7) | 361(87.2) | 642(71.7) | |||
| HIV viral load(copies/mL) | |||||||
| 0–999 | 7(4.6) | 39(11.7) | 60(14.4) | 106(11.7) | <0.001 | ||
| 1000–9999 | 23(15.0) | 81(24.3) | 144(34.5) | 248(27.4) | |||
| 10,000–99,999 | 92(60.1) | 154(46.1) | 163(39.1) | 409(45.2) | |||
| ≥100,000 | 31(20.3) | 60(18.0) | 50(12.0) | 141(15.6) | |||
| Recently diagnosed HIV infection | |||||||
| Yes | 64(41.8) | 49(14.7) | 48(11.5) | 161(17.8) | <0.001 | ||
| No | 89(58.2) | 285(85.3) | 369(88.5) | 743(82.2) | |||
STI: sexually transmitted infections; IDU: injection drug use; MSM: men who have sex with men.
Among those who reported sex in last 30 days. Some participants did not answer all risk-behaviour questions.
Some factors have lower totals due to missing data.
One-quarter (n=222) of the sample had a history of injection-drug use. Non-MSM males (55%) were much more likely to have IDU history than MSM (10%) and females (6%). Only 32 (14%) of PWID reported injecting in the previous 7 days and only three of these reported sharing needles. Only 28 (13%) were on methadone maintenance treatment (MMT). PWID were more likely than others to be diagnosed for more than 3 years (54% vs 36%, P<0.0001) and to have hepatitis C infection (85% vs 10%, P<0.0001).
Predictors of HIV viral load
Bivariate analysis between categorical variables and HIV VL greater than 10,000 copies/mL is presented in Table 3. Results of the multivariate models are shown in Table 4. Factors independently associated with log10 transformed HIV VL and HIV VL>10,000 copies/mL were MSM, non-MSM male, CD4 351–500 cells/mm3, recent HIV diagnosis and HBsAg. Positive anti-HCV was associated with lower HIV VL. When hepatitis C was replaced with PWID in the multivariate analyses, we found that injection-drug use was also negatively associated with viral load ≥10,000 copies/mL (odds ratio [OR] 0.65, 95% confidence interval [CI] 0.44–0.97) and did not significantly change the level of association of the other independent variables with HIV VL (data not shown).
Table 3.
Summary of bivariate analyses
| HIV viral load ≥10,000 copies/mL (N=550) n (%) |
HIV viral load <10,000 copies/mL (N=354) n (%) |
Total (N=904) 2 n (%) |
P-value | |
|---|---|---|---|---|
| HIV WHO clinical stage | ||||
| Stage 1 | 479(87.1) | 325(91.8) | 804(88.9) | 0.0273 |
| Stage 2 | 71(12.9) | 29(8.2) | 100(11.1) | |
| Sex | ||||
| Male | 337(61.3) | 150(42.4) | 487(53.9) | <0.0001 |
| Female | 213(38.7) | 204(57.6) | 417(46.1) | |
| Age | ||||
| 18–25 | 107(19.5) | 45(12.7) | 152(16.8) | 0.0199 |
| 26–35 | 287(52.2) | 210(59.3) | 497(55.0) | |
| 36–64 | 156(28.4) | 99(28.0) | 255(28.2) | |
| Highest education level | ||||
| No school | 13(2.4) | 14(4.0) | 27(3.0) | 0.0175 |
| Primary(1–5) | 90(16.4) | 67(18.9) | 157(17.4) | |
| Secondary(6–9) | 201(36.5) | 145(41.0) | 346(38.3) | |
| High(10–12) | 147(26.7) | 91(25.7) | 238(26.3) | |
| College/university | 99(18.0) | 37(10.5) | 136(15.0) | |
| Marital status | ||||
| Married | 287(52.2) | 227(64.1) | 514(56.9) | <0.0001 |
| Divorced/Widowed | 72(13.1) | 53(15.0) | 125(13.8) | |
| Single | 191(34.7) | 74(20.9) | 265(29.3) | |
| Recently diagnosed HIV infection | ||||
| Yes | 124(22.5) | 37(10.5) | 161(17.8) | <0.0001 |
| No | 426(77.5) | 317(89.5) | 743(82.2) | |
| URI/viral symptoms | ||||
| Yes | 179(32.5) | 87(24.7) | 266(29.5) | 0.0119 |
| No | 371(67.5) | 265(75.3) | 636(70.5) | |
| Any STI symptoms | ||||
| Yes | 73(13.3) | 52(14.7) | 125(13.8) | 0.5470 |
| No | 477(86.7) | 302(85.3) | 779(86.2) | |
| MSM(males only) | ||||
| Yes | 123(36.5) | 30(20.0) | 153(31.4) | 0.0003 |
| No | 214(63.5) | 120(80.0) | 334(68.6) | |
| Multiple sex partners in last 30 days 1 | ||||
| Yes | 24(8.0) | 8(3.7) | 32(6.2) | 0.0463 |
| No | 275(92.0) | 207(96.3) | 482(93.8) | |
| Received money for sex in last 30 days 1 | ||||
| Yes | 3(1.0) | 2(0.9) | 5(1.0) | 1.0000 |
| No | 300(99.0) | 213(99.1) | 513(99.0) | |
| History of IDU | ||||
| Yes | 127(23.1) | 95(26.8) | 222(24.6) | 0.2016 |
| No | 423(76.9) | 259(73.2) | 682(75.4) | |
| Current CD4 count(cells/mm3) | ||||
| 351–500 | 256(46.5) | 128(36.2) | 384(42.5) | 0.0020 |
| >500 | 294(53.5) | 226(63.8) | 520(57.5) | |
| Syphilis test result | ||||
| Positive | 44(8.1) | 15(4.3) | 59(6.6) | 0.0254 |
| Negative | 500(91.9) | 335(95.7) | 835(93.4) | |
| HBsAg | ||||
| Positive | 72(13.2) | 29(8.3) | 101(11.3) | 0.0228 |
| Negative | 474(86.8) | 322(91.7) | 796(88.7) | |
| Anti-HCV | ||||
| Positive | 142(26.1) | 112(31.9) | 254(28.3) | 0.0577 |
| Negative | 403(73.9) | 239(68.1) | 642(71.7) | |
Among those who reported sex in last 30 days. Some participants did not answer all risk-behaviour questions.
Some factors have lower totals due to missing data.
Table 4.
Results of multivariate analysis
| Logistic regression for HIV VL ≥10,000 copies/mL | Linear regression for log10 HIV VL | |||
|---|---|---|---|---|
| Adjusted OR (95% CI) |
P-value | Parameter estimates (95% CI) |
P-value | |
| WHO clinical stage | ||||
| Stage 1 | Reference | Reference | ||
| Stage 2 | 1.5 (0.93–2.42) | 0.0962 | 0.06 (−0.11–0.24) | 0.4913 |
| Gender/MSM | ||||
| MSM | 3.08 (1.91–4.95) | <0.0001 | 0.38 (0.22–0.55) | <0.0001 |
| Male (Non-MSM) | 1.98 (1.4–2.8) | 0.0001 | 0.26 (0.12–0.39) | 0.0002 |
| Female | Reference | Reference | ||
| Age | ||||
| 18–25 | 1.1 (0.69–1.77) | 0.688 | 0.06 (-0.12–0.24) | 0.5163 |
| 26–35 | 1 (0.72–1.38) | 0.9861 | -0.04 (-0.17–0.09) | 0.5386 |
| 36–64 | Reference | Reference | ||
| Current CD4 cell count (cells/mm3) | ||||
| 351–500 | 1.46 (1.09–1.94) | 0.0106 | 0.24 (0.13–0.35) | <0.0001 |
| >500 | Reference | Reference | ||
| Recently diagnosed HIV infection | ||||
| Yes | 1.82 (1.2–2.78) | 0.0053 | 0.21 (0.06–0.37) | 0.0051 |
| No | Reference | Reference | ||
| URI/viral symptoms | ||||
| Yes | 1.4 (1.02–1.92) | 0.0375 | 0.11 (-0.02–0.23) | 0.0863 |
| No | Reference | Reference | ||
| HBsAg | ||||
| Positive | 1.64 (1.02–2.65) | 0.0414 | 0.19 (0.01–0.36) | 0.0348 |
| Negative | Reference | Reference | ||
| Anti-HCV | ||||
| Positive | 0.64 (0.45–0.91) | 0.0132 | -0.15 (-0.28–-0.01) | 0.0362 |
| Negative | Reference | Reference | ||
Discussion
These results reveal a large proportion of participants with elevated HIV viral load among pre-ART patients in HCMC. More than 60% of the pre-ART population had VL above 10,000 copies/mL and 16% had viral loads above 100,000 copies/mL. This is significantly higher than the 34% of patients with CD4 cell counts >350 cells/mm3 found to have HIV viral load greater than 10,000 copies/mL in a recent study in South Africa [28].
We found that a majority of pre-ART patients had WHO clinical stage 1 and CD4>500 cells/mm3. The low number of patients with clinical stage 2 may be due to underreporting of clinical symptoms perceived by patients and doctors to be minor and not meeting criteria for ART. It is also possible that some patients with CD4 cell counts in the range 350–500 cells/mm3 were diagnosed with clinical stage 3 or 4 conditions, such as oral thrush, weight loss or pulmonary TB, which are commonly reported among PLHIV in Vietnam and would make them eligible for ART.
Almost half (46%) of our study participants were female. This proportion may not be representative of the HIV population in the community as women in Vietnam are more likely to enrol in care earlier and have greater retention in care [29,30]. However, our data also suggest that women may be a low-risk group for HIV transmission. Women had significantly lower VL than men and among the 417 women enrolled in our study, 98% reported having no or one regular sex partner, 63% reported that their regular sex partner was already HIV-infected, 6% reported ever injection drug use, and only 0.5% reported sex work. This suggests that most of the women enrolled acquired HIV from their husbands or male partners, had few other sex partners, and had low potential for transmitting the virus to others.
Similar to previous studies, we found higher HIV VL in men versus women [8–10,31]. It is not clear why MSM had higher VL than non-MSM males. The fact that MSM represented 40% of the participants with recent HIV diagnosis but only 17% of the entire sample is an indication of the changing epidemiology of HIV infection in Vietnam; and recent HIV diagnosis was independently associated with higher VL. However, in the multivariate analyses MSM was significantly associated with elevated HIV VL even after controlling for recent HIV diagnosis.
This study is the first known to us to identify higher VL among HBV co-infected patients. Based on our study results, earlier expanded treatment for those PLHIV co-infected with HBV would be likely to have significant long-term benefits to individual patients as well as the potential for reduced transmission of both HIV and HBV in the community. The preferred first-line ART regimen in Vietnam includes the drugs tenofovir and lamivudine, which are also effective against hepatitis B. Indeed, the most recent Vietnam HIV treatment guidelines revised in 2015 recommend early treatment of patients with HBV or HCV co-infection [32].
This study is also one of the first to show lower VL among persons co-infected with hepatitis C, a finding also reported in the RESINA Cohort in Germany [33]. However, the biological mechanism for this phenomenon has not been elucidated. The majority (86%) of PWID were positive for anti-HCV, suggesting that anti-HCV may be a surrogate marker for injection drug use among PLHIV. PWID were more likely to be diagnosed for longer periods of time, suggesting that as a risk group they were infected and identified in the more distant past.
Our study has a number of limitations not previously mentioned. The cross-sectional nature of the study could not assess when patients acquired HIV infection or how VL evolves over time. We cannot exclude the possibility that VL varies over time within individuals. Very few participants reported high-risk behaviour including active drug use, sex work and other high-risk sexual behaviour. These behaviours may have been underreported because they are stigmatised and we relied on patient self-report in a survey conducted by clinic staff. Similar to HIV epidemics in other countries, MSM in HCMC constitute both an increasing proportion of new HIV transmissions and a long-underserved population [34–40]. Compared to PWID, MSM in HCMC were significantly more likely to have been recently diagnosed with HIV, suggesting a shifting trend in HIV transmission in HCMC from injection drug use to homosexual sex. Increasing HIV transmission among MSM in Vietnam would also support the implementation of other biomedical HIV prevention strategies such as pre-exposure prophylaxis (PrEP).
The Vietnam Ministry of Health revised the national HIV treatment guidelines in 2015 to expand ART eligibility to include CD4 cell counts less than 500 cells/mm3 as well as groups at high-risk for HIV transmission, such as PWID, FSW, MSM and those with HIV-uninfected sex partners [32]. Our findings support implementation of the 2015 revised ART guidelines within the goal of treatment as prevention. However, in order to reduce HIV transmission in the community, expanded eligibility for ART needs to be combined with outreach and improved services for those most likely to transmit HIV [14]. Our study underscores the need for updated service delivery models that target groups with higher VL and higher levels of risk behaviour, especially MSM. Effectively addressing the HIV epidemic among MSM in HCMC will require improved provision and access to MSM-friendly HIV prevention and care services [41]. PWID will need greater access to harm-reduction methods including opioid substitution therapy and needle exchange programs. Only then will the goals of treatment as prevention be truly achievable.
Acknowledgements
The authors thank the US Agency for International Development (USAID) for funding this study under its cooperative agreement #AID-486-A-11-00011 ‘Sustainable Management of HIV/AIDS Response and Transition to Technical Assistance’ (SMART TA) Project. The contents, opinions, and statements in this article are the sole responsibility of the authors and do not necessarily represent the views or opinions of the funding agency.
The authors thank and acknowledge the dedication and hard work of the study team leaders, members and clinic providers who implemented this critical research activity:
HCMC Provincial AIDS Committee: Tran Thinh, Van Hung, Luong Quoc Binh, Huynh Tan Tien, Pham Thị Mong Thuong, Dinh Quoc Thong.
FHI 360 Vietnam: Nguyen Nhat Quang, Hoang Nguyen Bao Tram, Nga Doan Vu Tuyet, Tran Khanh Trang.
OPC District 1: Nguyen Viet Trung, Nguyen Sang Duyen, Le Van Khanh, Nguyen Thi Yen, Nguyen Thi Mai Duong, Pham Thi Hang, Tran Thi Phung, Nguyen Ngo Anh Tu, Le Thị Kim Vien, Dinh Thi Bich.
OPC District 2: Vu Duc Khoi, Nguyen Thi Hong Cuc, Nguyen Thi Tiep, Duong Thi Minh Thu.
OPC District 3: Le Thi Hong, Vu Thi Khoan, Phan Chi Tin.
OPC District 4: Cao Kim Van, Le Thanh Tu, Tran Thi Thanh Truc, Phạm Thi Uyen Tram, Luu Thanh Thuy.
OPC District 5: Nguyen Thanh Son, Truong Thi Thu Thuy, Nguyen Thi Hong Van, Nguyen Thi Mai Thi.
OPC District 6: Do Thi Hong Thanh, Nguyen Quoc Trung, Tran Thi Thanh Ngan, Tran Dang Khoa.
OPC District 7: Nguyen Anh Tuyet, Nguyen Trong Minh Tan, Vo Duc Minh, Nguyen Thị Loan Thao.
OPC District 8: Nguyen Ngọc Thoa, Tran Thi Hong, Nguyen Ngoc Hai, Dinh Thị Phuong.
OPC District 9: Tran Anh Hien, Nguyen Thị Nguyet, Nguyen Thi Tu, Tạ Thi Thao.
OPC District 10: Bui Thi Thu Phuong, Le Minh Tri, Phan Minh Duc, Vuonng Tu Cuong, Do Phuong Thao.
OPC District 12: Nguyen Cong Cuong, Vo Duy Binh, Dinh Thi Thu Hang, Truong Thi Kim Nguyen, Tran Minh Luan.
OPC District Thu Duc: Nguyen Thị Thu Hang, Le Thi Thao, Nguyen Thị Thu Ha, Mai Thuy Van.
OPC District Binh Thanh: Quach Kim Ung, Le Thi Thu, Dang Ngọc Phuong, Nguyen Thi Kim Hoang.
OPC District Phu Nhuan: Tran Huu Duc, Tran Van Dang, Hoang Dinh Hoan, Cao Thi Ngoc Hiep.
OPC District Tan Binh: Vuu Tuan Khanh, Tạ Hong Nguyen, Mai Ngoc Anh, Mai Minh Man, Voo Van Vo, Hoang Thi Tham, Nguyen Khanh Chi.
OPC District Binh Tan: Vo Huu Phuoc, Phan Tri Dung, Phạm Van Dang, Nguyen Thanh Tung, Nguyen Phan Thanh Truyen.
OPC District Hoc Mon: H Loan Niekdam, Huynh Ngoc Mai Phuong Thao, Nguyen Thị Xuan Trang, Vu Thi Kim Anh, Tran Thi Le Quyen.
OPC District Binh Chanh: Le Thi Cam Ha, Phạm Ngoc Hue, Pham Mong Tuyen, Lai Phuoc Xuan, Huynh Kim Thuy, Do thi Bich Lieu, Phạm Anh Tuan, Le Phuong Uyen, Le Xuan Moi.
OPC District Go Vap: Thach Thi Ca, Le Thi Hoang Bich, Le Thị Nguyet Thu, Le Thi Phuong Thao, Nguyen Thi Ngoc Dung.
Conflict of interests
All authors report no conflict of interests.
References
- 1. Garcia PM, Kalish LA, Pitt J et al. . Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med 1999; 341: 394–402. [DOI] [PubMed] [Google Scholar]
- 2. Gray RH, Wawer MJ, Brookmeyer R et al. . Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001; 357: 1149–1153. [DOI] [PubMed] [Google Scholar]
- 3. Lingappa JR, Hughes JP, Wang RS et al. . Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One 2010; 5: e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quinn TC, Wawer MJ, Sewankambo N et al. . Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342: 921–929. [DOI] [PubMed] [Google Scholar]
- 5. Butler DM, Smith DM, Cachay ER et al. . Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS 2008; 22: 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hughes JP, Baeten JM, Lingappa JR et al. . Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wawer MJ, Gray RH, Sewankambo NK et al. . Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005; 191: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 8. Ballesteros-Zebadua P, Villarreal C, Cocho G et al. . Differences in HIV-1 viral loads between male and female antiretroviral-untreated Mexican patients. Arch Med Res 2013; 44: 296–301. [DOI] [PubMed] [Google Scholar]
- 9. Farzadegan H, Hoover DR, Astemborski J et al. . Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352: 1510–1514. [DOI] [PubMed] [Google Scholar]
- 10. Grinsztejn B, Smeaton L, Barnett R et al. . Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4(+) T-cell count. Antivir Ther 2011; 16: 1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Visnegarwala F, Raghavan SS, Mullin CM et al. . Sex differences in the associations of HIV disease characteristics and body composition in antiretroviral-naive persons. Am J Clin Nutr 2005; 82: 850–856. [DOI] [PubMed] [Google Scholar]
- 12. Sterling TR, Lyles CM, Vlahov D et al. . Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180: 666–672. [DOI] [PubMed] [Google Scholar]
- 13. Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS 2008; 3: 10–15. [DOI] [PubMed] [Google Scholar]
- 14. West GR, Corneli AL, Best K et al. . Focusing HIV prevention on those most likely to transmit the virus. AIDS Educ Prev 2007; 19: 275–288. [DOI] [PubMed] [Google Scholar]
- 15. Vanhems P, Lecomte C, Fabry J. Primary HIV-1 infection: diagnosis and prognostic impact. AIDS Patient Care STDS 1998; 12: 751–758. [DOI] [PubMed] [Google Scholar]
- 16. Pilcher CD, Tien HC, Eron JJ Jr. et al. . Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis 2004; 189: 1785–1792. [DOI] [PubMed] [Google Scholar]
- 17. Brenner BG, Roger M, Routy JP et al. . High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 2007; 195: 951–959. [DOI] [PubMed] [Google Scholar]
- 18. Carrieri MP, Tamalet C, Vlahov D et al. . Relationship between HIV-1 viral load and continued drug use in untreated infected injection drug users. Addict Biol 1999; 4: 197–202. [DOI] [PubMed] [Google Scholar]
- 19. Thorpe LE, Frederick M, Pitt J et al. . Effect of hard-drug use on CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr 2004; 37: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 20. Gray RH, Li X, Wawer MJ et al. . Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis 2004; 189: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 21. Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis 2010; 10: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchacz K, Patel P, Taylor M et al. . Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS 2004; 18: 2075–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. General Statistics Office Statistical Handbook of Vietnam 2014. Hanoi: Statistical Publishing House; 2014. [Google Scholar]
- 24. 2013 Sentinel Surveillance Report. Ho Chi Minh City Provincial AIDS Committee. 2014.
- 25. Year 2013 Care and treatment program D28 report. Ho Chi Minh City AIDS Committee. 2014.
- 26. Vietnam Ministry of Health Antiretroviral therapy cohort assessment and HIV drug resistance early warning indicators. Hanoi: Medical Publishing House: 2010.
- 27. Cost of providing HIV care and treatment services in Vietnam. Ministry of Health, Vietnam. 2010.
- 28. Govender S, Otwombe K, Essien T et al. . CD4 counts and viral loads of newly diagnosed HIV-infected individuals: implications for treatment as prevention. PLoS One 2014; 9: e90754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rangarajan S, Tram HN, Todd CS et al. . Risk Factors for Delayed Entrance into Care after Diagnosis among Patients with Late-Stage HIV Disease in Southern Vietnam. PLoS One 2014; 9: e108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen DB, Do NT, Shiraishi RW et al. . Outcomes of antiretroviral therapy in Vietnam: results from a national evaluation. PLoS One 2013; 8: e55750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown A, Aghaizu A, Murphy G, Delpech V. Predictors for high viraemia among a treatment-naive national HIV cohort in the United Kingdom. HIV Med 2013; 14 (Suppl. 2): 52. [Google Scholar]
- 32. Vietnam MInistry of Health. Decision No. 3655 Hanoi 2015.
- 33. Reuter S, Oette M, Wilhelm FC et al. . Prevalence and characteristics of hepatitis B and C virus infections in treatment-naive HIV-infected patients. Med Microbiol Immunol 2011; 200: 39–49. [DOI] [PubMed] [Google Scholar]
- 34. Griensven F, Thienkrua W, McNicholl J et al. . Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS 2013; 27: 825–832. [DOI] [PubMed] [Google Scholar]
- 35. Beyrer C, Baral SD, Griensven F et al. . Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Griensven F, Holtz TH, Thienkrua W et al. . Temporal trends in HIV-1 incidence and risk behaviours in men who have sex with men in Bangkok, Thailand, 2006–13: an observational study. Lancet HIV 2015; 2: e64–e70. [DOI] [PubMed] [Google Scholar]
- 37. Vietnam Administration of AIDS Control (VAAC) 2013 HIV Sentinel Surveillance survey (HSS). Hanoi: Ministry of Health; 2014. [Google Scholar]
- 38. Estimates and Projections Technical Working Group Preliminary results of the HIV Estimates and Projection in Viet Nam 2013. Hanoi: Ministry of Health; 2013. [Google Scholar]
- 39. Vietnam Administration for AIDS Control National Strategy on HIV/AIDS Prevention and Control (Issued with Decision 608/QD-TTg dated May 25,2012 of the Prime Minister) 2012.
- 40. Colby D, Cao NH, Doussantousse S. Men who have sex with men and HIV in Vietnam: a review. AIDS Educ Prev 2004; 16: 45–54. [DOI] [PubMed] [Google Scholar]
- 41. Hoang HT, Mai TDA, Nguyen NA et al. . Needs assessment on the use of health services among men who have sex with men in Ho Chi Minh City, Vietnam. LGBT Health 2015; 2: 341–345. [DOI] [PubMed] [Google Scholar]