Abstract
Increasing evidence has revealed that miR‐199a‐5p is actively involved in tumor invasion and metastasis as well as in the decline of breast cancer tissues. In this research, overexpression of miR‐199a‐5p weakened motility and invasion of breast cancer cells MCF‐7 and MDA‐MB‐231. Upregulation of Ets‐1 increased breast cancer cell invasion, but the mechanism by which miR‐199a‐5p modulates activation of Ets‐1 in breast cancer was not clarified. We investigated the relationship between miR‐199a‐5p and Ets‐1 on the basis of 158 primary breast cancer case specimens, and the results showed that Ets‐1 expression was inversely correlated with endogenous miR‐199a‐5p. Overexpression of miR‐199a‐5p reduced the mRNA and protein levels of Ets‐1 in MCF‐7 and MDA‐MB‐231 cells, whereas anti‐miR‐199a‐5p elevated Ets‐1. siRNA‐mediated Ets‐1 knockdown phenocopied the inhibition invasion of miR‐199a‐5p in vitro. Moreover, luciferase reporter assay revealed that miR‐199a‐5p directly targeted 3′‐UTR of Ets‐1 mRNA. This research revealed that miR‐199a‐5p could descend the levels of β1 integrin by targeting 3′‐UTR of Ets‐1 to alleviate the invasion of breast cancer via FAK/Src/Akt/mTOR signaling pathway. Our results provide insight into the regulation of β1 integrin through miR‐199a‐5p‐mediated Ets‐1 silence and will help in designing new therapeutic strategies to inhibit signal pathways induced by miR‐199a‐5p in breast cancer invasion.
Keywords: Breast cancer, Ets‐1, integrin, invasion, miRNA‐199a
Breast cancer, the most commonly diagnosed type of cancer, comprises 10.4% of all cancer incidence among women.1 Aggressive breast cancers have high potential to become metastatic, a transition that makes clinical intervention more difficult, and may involve chemo‐resistance and damage to the normal immune system.2 Therefore, better elucidation of invasion and metastasis mechanisms is extremely important to improve the therapeutic efficiency of treatments for breast cancer. V‐ets erythroblastosis virus E26 oncogene homolog 1 (Ets‐1) participates in the migration, invasion and angiogenesis of various cancers, including prostate cancer, colon cancer, liver cancer and neuroblastoma.3, 4 However, the mechanisms underlying Ets‐1 expression in breast cancer are still unclear.
MicroRNA (miRNA) are a novel class of short and small non‐coding RNA that take part in post‐transcriptional regulation of gene expression through partial complementary binding with the 3′‐UTR of target mRNA.5, 6 miR‐199a‐5p has been identified in all tumor types, including testicular cancer, hepatocellular cancer and ovarian cancer, and has been associated with cancer migration, invasion and cell growth on the basis of emerging evidence.7, 8 A recent report showed that miR‐199b‐5p declined in breast cancer tissue.9, 10 Moreover, miR‐199a/b are frequently methylated in breast cancer cell lines, and their expression levels inversely correlate with invasive capacity.11
miRNA facilitate the cross‐talk between transcriptional modules and signal transduction pathways. Ectopic expression of miR‐199a attenuates adhesion, migration and invasiveness in vitro partially through IKK/NF‐κB pathway suppression and reduced IL‐8 expression.12 However, the precise function and downstream target of miR‐199a‐5p are still ambiguous in breast cancer. Through experiments, we proved that Ets‐1 is a straightforward target of miR‐199a‐5p, and miR‐199a‐5p‐mediated Ets‐1 silencing leads to a significant decline of β1 integrin, inhibiting the invasion of breast cancer cells.
Materials and Methods
Patient tissue samples
Breast cancer and adjacent control tissue specimens were obtained from 158 patients at the Weifang Medical University Affiliated Hospital after surgical resection. The tumor tissues and adjacent normal tissues were frozen in liquid nitrogen after resection. No patient in this study received chemotherapy or radiation therapy before the surgery. Patients' pathological diagnosis was confirmed by at least two pathologists. Approval to conduct this study was obtained from the Institutional Review Board of Weifang Medical University.
Immunohistochemistry
Paraffin‐embedded tissues were analyzed using immunohistochemical staining, with antibodies specific for Ets‐1 (Abcam, Cambridge, MA, USA). The reactivity degree was assessed by at least two pathologists without knowledge of the clinicopathological features of tumors. The intensity of staining, determined by combining the proportion of positively stained tumor cells, was measured using integrated optical density (IOD) as previously described.13 A staining index (SI) score >mIOD indicated tumors with high expression, and an SI score ≤mIOD indicated low expression.
Cell culture and cell transfection
Human breast cancer cell lines MCF‐7 and MDA‐MB‐231 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cell lines were authenticated based on recovery, growth, viability, morphology and short tandem repeat by the provider. Cell lines were used within 6 months after resuscitation of frozen aliquots and grown in DMEM (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% FBS (Hyclone, Logan, UT, USA). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. Anti‐miR‐199a‐5p or negative control inhibitors and miR‐199a‐5p mimic or the scramble vector (Sangon, Shanghai, China) were transfected into confluent cells with Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions. The 21‐nucleotide siRNA targeting the encoding region of Ets‐1 was chemically synthesized (Sangon) and transfected with Genesilencer Transfection Reagent (Genlantis, San Diego, CA, USA). The scramble siRNA (anti‐NC) was applied as a control.
Migration assay
Cancer cells were cultured in 6‐well plates until a monolayer formed before being scraped with a straight line across the dish with the fine end of 200‐μL pipette tips. Plates were washed twice with PBS to remove detached cells and incubated with the complete growth medium. Cell migration was photographed using 10 high‐power fields at 0 and 24 h post‐induction of injury. The migrated cells in the wounded region were photographed under the microscope.
Invasion assay
Transwell analysis was performed using a culture medium‐treated 6.5‐mm Transwell chamber with 8.0‐μm polycarbonate membranes. Briefly, the 8‐μm pore size filters were coated with 100 μL of 1 mg/mL matrigel (BD Biosciences, San Jose, CA, USA). Homogeneous single cell suspensions were added to the upper chambers and allowed to invade for 24 h. Non‐migratory cells were scraped off from the top of the Transwell with a cotton swab. The cells attached to the bottom side of the membrane were fixed by methanol, stained with 5% crystal violet and examined by light microscopy. Quantification of invaded cells was performed according to published criteria.
Western blot analysis
Cell lysates were prepared and subjected to 12% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio‐Rad, Hercules, CA, USA) for Ets‐1 (Abcam), FAK, Src, AKT, mTOR (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β‐actin (Maxim, Fuzhou, China) detection. An enhanced chemiluminescence substrate kit (Beyotime Biotechnology, Haimen, China) was used for the chemiluminescent detection of signals with autoradiography film.
RNA reversed transcription and quantitative real‐time PCR assays
The total RNA, including small RNA, was extracted from the clinical specimens or from the breast cancer cells with a miRVana MicroRNA Isolation Kit (Invitrogen, Carlsbad, CA, USA) and subjected to reverse transcription. Stem‐loop qRT‐PCR was performed using the Premix Ex Taq mix (TaKaRa, Dalian, China) according to the manufacturer's instructions, and the samples were run on an iQ5 Multicolor Real‐time PCR Detection System (Bio‐Rad). Thermal reaction cycles of 95°C for 30 s and 40 repetitions of 95°C for 5 s and 60°C for 30 s were carried out. GAPDH and 18S RNA were used as internal controls to determine the relative expressions of mRNA and miRNA, respectively (the primers are shown in Table 1). The relative expression of RNA was calculated using the comparative Ct method. Low and high categories of miR‐199a‐5p expression are designated according to a median cut‐off value.
Table 1.
The primer sequences of qRT‐PCR
| Gene | Primer | Sequence |
|---|---|---|
| mir‐199a‐5p | Stem‐loop primer | 5′‐CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGacaggtag‐3′ |
| Forward primer | 5′‐ACACTCCAGCTGGGcccagt‐3′ | |
| Reverse primer | 5′‐tggtgtcgtggagtcg‐3′ | |
| Ets‐1 | Forward primer | 5′‐tggagtcaacccagcctatc‐3′ |
| Reverse primer | 5′‐tctgcaaggtgtctgtctgg‐3′ | |
| 18S | Forward primer | 5′‐ ggccctgtaattggaatgagtccac‐3′ |
| Reverse primer | 5′‐gctcccaagatccaactacgagctt‐3′ | |
| GAPDH | Forward primer | 5′‐aaggtgaaggtcggagtcaa‐3′ |
| Reverse primer | 5′‐aatgaaggggtcattgatgg‐3′ |
Luciferase reporter assay
Human Ets‐1 3′‐UTR containing the putative binding site of miR‐199a‐5p, as well as its identical sequence with a deletion of the miR‐199a‐5p seed sequence (mutant), was amplified by PCR, inserted into the luciferase reporter vector pGL3 (Promega, Madison, WI, USA), and validated by sequencing. Cancer cells were plated at 1 × 105 cells/well on 24‐well plates and transfected with pGL3‐Ets‐1 3′‐UTR or its mutant construct. Consequently, 24‐h post‐transfection, the firefly and Renilla luciferase activities were measured according to the dual‐luciferase assay manual (Promega). The Renilla luciferase signal was normalized to firefly luciferase signal for each individual analysis. All experiments were repeated at least three times independently.
Chromatin immunoprecipitation
ChIP assays were performed according to the instructions of the ChIP Assay Kit from (Upstate Biotechnology, Lake Placid, NY, USA). MDA‐MB‐231 cells were transfected with a vector that expressed Ets‐1 for 48 h, and the equivalent of 2 × 106 cells was used per ChIP reaction by using mouse anti‐Ets‐1. Normal mouse IgG or normal rabbit IgG was used as a control antibody. Immunoprecipitated and input DNA were analyzed using PCR. The primers used for Ets‐1 were as follows: forward primer: 5′‐TTGTCTGTAGATATATTTAAAT‐3′; reverse primer: 5′‐TATTTATGAGACAATTACACAA‐3′.
Statistical analysis
All data are expressed as mean ± standard error. The software SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Student's t‐test and one‐way ANOVA were used to determine the significance of two groups and multiple groups. Spearman's correlation coefficient was used to assess the correlation between miR‐199a‐5p expression and Ets‐1 expression. Differences with P < 0.05 were considered significant in all statistical analyses.
Results
miR‐199a‐5p inhibited cell migration and invasion in breast cancer cells
Northern blot analysis demonstrated the overexpression of miR199a‐5p (data not shown). Upregulation of miR‐199a‐5p blocked the ability of cells to migrate 24 h after plating (Fig. 1a,b), causing a reduction in the ability of MDA‐MB‐231 cells to invade compared with the scramble vector group by Transwells (Fig. 1c,d). Transfecting anti‐miR‐199a‐5p inhibitor into MCF‐7 cells downregulated the miR‐199a‐5p expression (data not shown) and increased cell migration and invasion capabilities (Fig. 1e,f).
Figure 1.
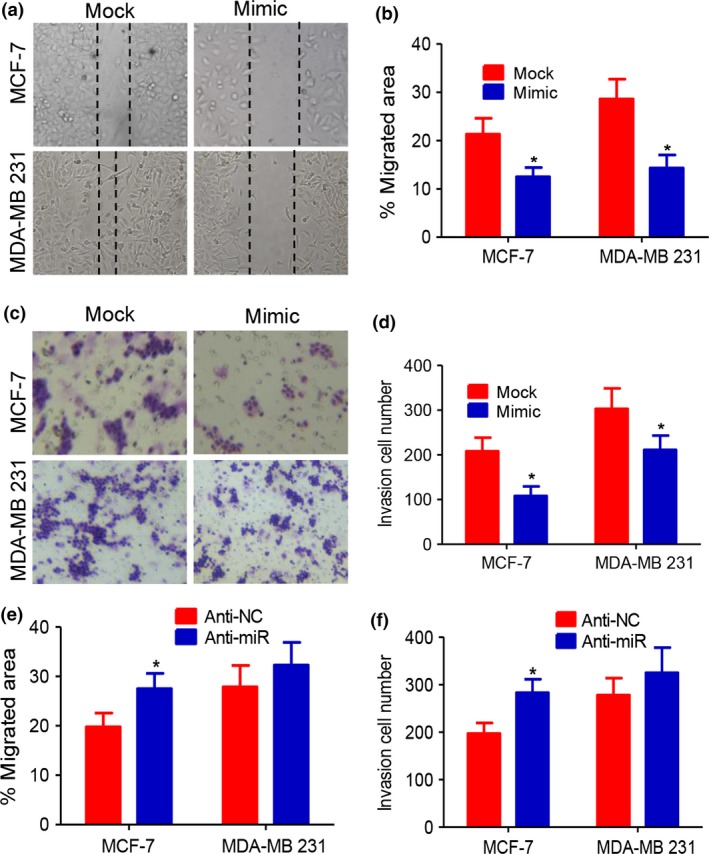
miR‐199a‐5p suppresses migration and invasion of breast cancer cells. (a, b) Migration of MDA‐MB‐231 and MCF‐7 cells in migration assays was impaired with miR‐199a‐5p overexpression. (c, d) Invasion assay indicated the decreased invasion capabilities of miR‐199a‐5p overexpression in MDA‐MB‐231 and MCF‐7 cells compared with scramble vector group. (e, f) Anti‐miR‐199a‐5p inhibitor increased cell migration and invasion capabilities (*P < 0.05).
Ets‐1 was highly expressed and negatively related with endogenous miR‐199a‐5p levels in breast cancer tissues
Ets‐1 protein, expressed in both tumor cell nuclei and cytoplasm, was highly expressed in 66 of 158 (42.0%) human breast cancer samples (Fig. 2a). Breast cancer tissues have higher Ets‐1 transcript level than para‐carcinoma tissues (Fig. 2b), whereas miR‐199a‐5p was downregulated in breast cancer tissues compared with para‐carcinoma tissue (Fig. 2c). Lower miR‐199a‐5p expression was also observed in breast cancer tissues with higher Ets‐1 immunostaining (Fig. 2d). An inverse correlation between Ets‐1 transcript levels and miR‐199a‐5p expression in breast cancer tissues was also discovered (Fig. 2e). These results revealed that Ets‐1 expression in primary breast cancer tissues was inversely correlated with endogenous miR‐199a‐5p levels.
Figure 2.
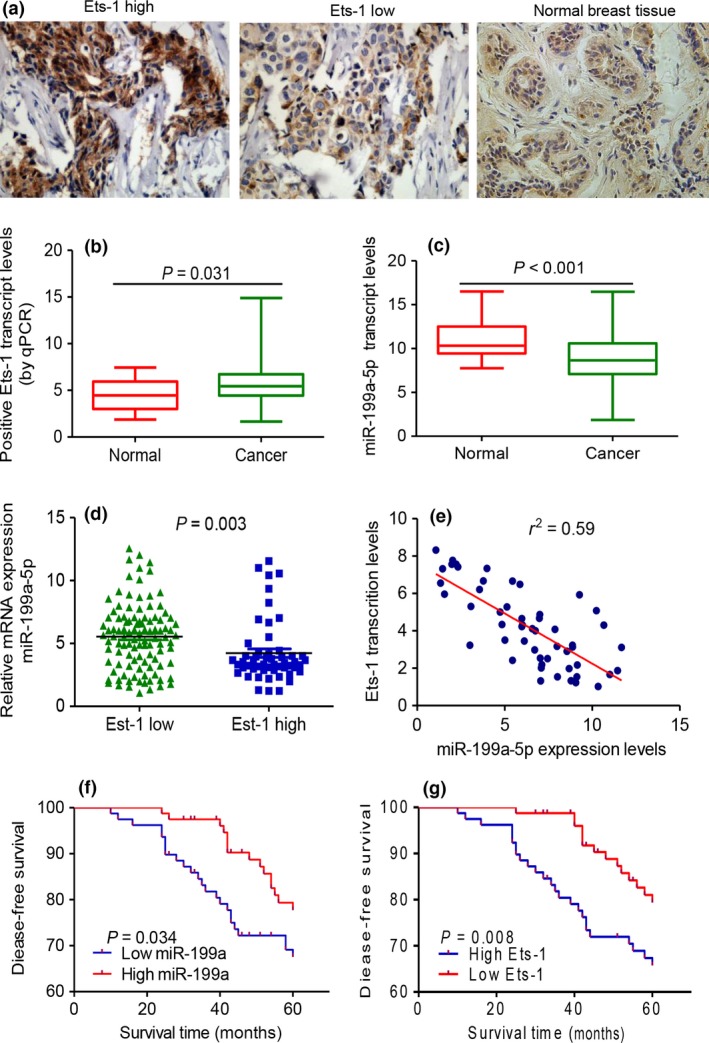
Inverse correlation between miR‐199a‐5p and Ets‐1 expression in breast specimens. (a) Ets‐1 immunostaining was located at the nuclei or cytoplasm of cancer cells in breast cancer specimens. (b) A significant difference between Ets‐1 high group and Ets‐1 low group was observed. (c) miR‐199a‐5p was downregulated in breast cancer tissues than in normal tissues by RT‐PCR. (d, e) In breast cancer tissues, lower miR‐199a‐5p expression was accompanied with higher immunostaining of Ets‐1. An inverse correlation between miR‐199a‐5p expression and Ets‐1 transcription levels in breast cancer tissues was observed. (f, g) Kaplan–Meier analysis of disease‐free survival was analyzed according to miR‐199a‐5p and Ets‐1 expression levels, respectively.
We next analyzed the correlation among the expression of miR‐199a‐5p and Ets‐1 and the clinicopathologic characteristics of breast cancer patients. Correlation regression analysis showed that low expression of miR‐199a‐5p was significantly correlated with tumor size, lymph nodes metastasis and HER‐2 status. High expression levels of Ets‐1 were correlated with tumor size, lymph node metastasis, and status of ER, PR and HER‐2 (Table 2). Furthermore, Kaplan–Meier analysis was performed to assess the correlation between miR‐199a‐5p and disease free survival (DSF) of breast cancer patients. The results showed that low miR‐199a‐5p expression and high Ets‐1 expression in breast cancer tissues was significantly associated with poor DSF (Fig. 2f,g).
Table 2.
Clinical relevance of miR‐199a‐5p andEts‐1in breast cancer
| Feather | miR‐199a‐5p | Ets‐1 | ||||
|---|---|---|---|---|---|---|
| Low | High | P‐value | Low | High | P‐value | |
| Age | 0.617 | 0.200 | ||||
| ≤45 | 33 | 37 | 39 | 31 | ||
| >45 | 44 | 42 | 40 | 48 | ||
| Tumor size (cm) | 0.010a | 0.001a | ||||
| ≤2 | 42 | 26 | 24 | 44 | ||
| >2 | 37 | 53 | 55 | 35 | ||
| Histological grade | 0.053 | 0.022a | ||||
| I | 28 | 17 | 16 | 29 | ||
| II, III | 51 | 62 | 63 | 50 | ||
| Clinical stage | 0.061 | 0.044a | ||||
| I, II | 57 | 47 | 46 | 58 | ||
| III, IV | 22 | 34 | 33 | 21 | ||
| Lymph nodes metastasis | 0.017a | 0.001a | ||||
| Positive | 45 | 30 | 28 | 47 | ||
| Negative | 34 | 49 | 51 | 30 | ||
| ER | 0.143 | 0.03a | ||||
| Positive | 63 | 55 | 68 | 50 | ||
| Negative | 16 | 24 | 15 | 25 | ||
| PR | 0.365 | 0.005a | ||||
| Positive | 53 | 51 | 64 | 40 | ||
| Negative | 26 | 18 | 25 | 39 | ||
| HER‐2 | 0.003a | 0.022a | ||||
| Positive | 14 | 31 | 29 | 16 | ||
| Negative | 65 | 48 | 50 | 63 | ||
These P‐values are significant.
Overexpression of miR‐199a‐5p downregulated Ets‐1 expression through post‐transcriptional repression
Upregulated expression of miR‐199a‐5p in MDA‐MB‐231 cells generated a decrease in the endogenous Ets‐1 protein level compared with that of the mock cells, with consistent results observed in MCF‐7 cells (Fig. 3a,b). qRT‐PCR demonstrated that transfection of miR‐199a‐5p mimic resulted in decreased Ets‐1 levels by approximately 62% in MDA‐MB‐231 cells and by approximately 58% in MCF‐7 cells (Fig. 3c). We further performed miR‐199a‐5p knockdown experiments by transfecting anti‐miR‐199a‐5p or scramble miRNA (anti‐NC) into cancer cells. Inhibition of miR‐199a‐5p significantly elevated the protein and mRNA level of Ets‐1 compared with those transfected with anti‐NC (Fig. 3d–f).
Figure 3.
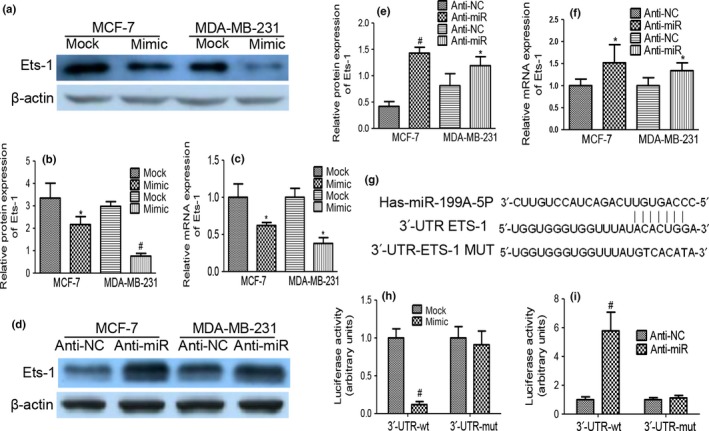
Overexpression of miR‐199a‐5p downregulated Ets‐1 expression directly. (a, b) Western blot indicated that stable transfection of miR‐199a‐5p precursor resulted in decreased protein levels of Ets‐1 in MDA‐MB‐231 and MCF‐7 cells. (c) qRT‐PCR revealed the decreased mRNA levels of Ets‐1 in miR‐199a‐5p precursor‐transfected MDA‐MB‐231 and MCF‐7 cells when compared with those transfected with mock vector. (d–f) Elevated protein and mRNA levels of Ets‐1 with the use of anti‐miR‐199a‐5p inhibitor compared with those transfected with anti‐NC. (g) Sequence alignment of human miR‐199a‐5p and the 3′‐UTR of Ets‐1. (h) Luciferase assay shows that miR‐199a‐5p inhibited wild type but not mutated Ets‐1 3′‐UTR reporter activity. (i) Knockdown of miR‐199a‐5p increased the luciferase activity in MCF‐7 and MDA‐MB‐231 cells compared with scramble group. (*P < 0.05, #P < 0.01).
Identification of miR‐199a‐5p binding sites in the Ets‐1 3′‐UTR
We searched putative‐predicted miR‐199a‐5p targets by using publicly available and commonly used programs (TargetScan, miRBase and PicTar). A sequence was located at bases 2708–2715 of the Ets‐1 3′‐UTR (NM_001143820), which was highly complementary with the seed sequence of miR‐199a‐5p (Fig. 3g). Ectopic expression of miR‐199a‐5p inhibited the expression of the reporter vector containing the wild‐type sequence of Ets‐1 3′‐UTR, but not the reporter vector containing the mutation of the seed‐miR‐199a‐5p binding site in MCF‐7 cells (Fig. 3h). Moreover, knockdown of miR‐199a‐5p increased the luciferase activity in MCF‐7 cells (Fig. 3i). These data showed that Ets‐1 was a direct and specific target of miR‐199a‐5p.
Knockdown of Ets‐1 directly impeded β1 integrin expression
Treatment with miR‐199a‐5p mimic in MDA‐MB‐231 and MCF‐7 cells could give rise to a sharp decline in protein and mRNA levels of β1 integrin subunit (Fig. 4a). In contrast, miR‐199a‐5p inhibitor upregulated overexpression of β1 integrin subunit (Fig. 4b). As delineated in Figure 4(c), β1 integrin dramatically enhanced invasion ability in the miR‐199a‐5p mimic group. Given that no miR‐199a‐5p binding site was noted in the 3′‐UTR of β1 integrin, we ruled out the possibility that miR‐199a‐5p may indirectly target β1 integrin. Here, we confirmed that knockdown of Ets‐1 could lessen the level of β1 integrin and decrease the migration and invasion capabilities of miR‐199a‐5p overexpression breast cancer cells in vitro. Figure 4(d) indicates that, at least in MCF‐7, β1 integrin overexpression augmented invasion in the absence of Ets‐1.
Figure 4.
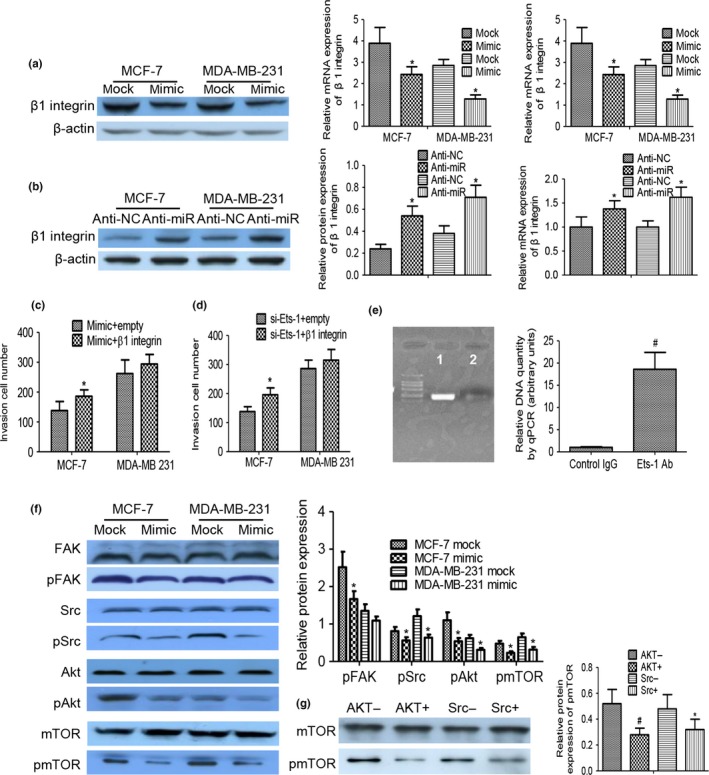
Knockdown of Ets‐1 directly impeded β1 integrin expression and the downstream FAK/Src/AKT/mTOR signaling pathway. (a, b) Western blot analysis and qRT‐PCR findings indicated that miR‐199a‐5p mimic resulted in decreased protein and RNA levels of β1 integrin, and inhibition of miR‐199a‐5p downregulated expression of β1 integrin. (c) Overexpression of β1 integrin recovered the cell invasion inhibition mediated by miR‐199a‐5p. (d) The inhibition of invasion by overexpressing miR‐199a‐5p by using knockdown of Ets‐1 was abrogated with treatment with β1 integrin overexpression. (e) ChIP analysis of the interaction of endogenous Ets‐1 with the β1 integrin promoter. Chromatin fragments from MDA‐MB‐231cells were immunoprecipitated with anti‐Ets‐1 (lane 1) and control normal rabbit IgG (lane 2) were displayed by qPCR and agarose gel electrophoresis. (f) Western blot analyses of pFAK/pSrc/pAKT/pmTOR expression in MDA‐MB‐231 and MCF‐7 cells transfected with miR‐199a‐5p overexpressing or scramble vector. (g) pmTOR could be inactivated by inhibition of Akt or Src (*P < 0.05, #P < 0.01).
A ChIP‐seq experiment was performed with breast cancer cells to confirm that endogenous Ets‐1 interacted with chromatin fragments containing the β1 integrin promoter. PCR results showed a specific band when DNA sequences were precipitated with Ets‐1 antibodies, whereas control IgG did not show any band in the analysis (Fig. 4e). A similar result was detected using MCF‐7 breast cancer cells, which display a high level of endogenous Ets‐1 (data not shown).
miR‐199a‐5p overexpression inhibited FAK/Src/AKT/mTOR signaling pathway
FAK/Src signaling regulates tumor metastasis through various mechanisms, including PI3K/Akt cascade in cancer cells.14 Activated Akt affects multiple downstream targets, one of which is mTOR pathway. We investigated whether FAK/Src/AKT/mTOR axis in breast cancer MDA‐MB‐231 and MCF‐7 cells was inhibited with miR‐199a‐5p overexpressing or scramble vector, respectively. As shown in Figure 4(f), treatment of cells with miR‐199a‐5p overexpressing prevented FAK/Src/AKT/mTOR phosphorylation, and the inhibitory effect was not displayed in the scramble vector group. When the miR‐199a‐5p mimic group was treated with AKT or Src inhibitor (AKT inhibitor GSK690693, Src inhibitor AZD0530), the mTOR phosphorylation was relieved (Fig. 4g).
Discussion
miR‐199a‐5p is significantly downregulated in many human tumor types and is related to tumor malignancy,15 but its role in breast cancer is not well understood. In this study, we investigated the miRNA expression in breast cancer and adjacent normal tissue samples by using qRT‐PCR, and we found that the expression of miR‐199a‐5p declined, and that downregulation of miR‐199a‐5p was correlated with tumor size, lymph nodes metastasis and HER‐2 expression status. Low miR‐199a‐5p expression in breast cancer tissues indicated poor survival of the patients. To test the potential roles of the decreased expression of miR‐199a‐5p in breast cancer, we performed functional assays using breast cancer cell line with stable overexpression of miR‐199a‐5p. Our data showed that miR‐199a‐5p exerted a significant inhibitory effect on the motility of breast cancer cells, whereas miR‐199a‐5p inhibitor recovered breast cancer cell formation. In addition, endogenous mature miR‐199a attenuated multi‐drug resistance in ovarian cancer stem‐like cells by regulating the expression of its target gene CD44.16 These data showed that the miR‐199a was involved in the behavior of tumor cells and may be a potential target of tumor immunotherapy.
A large body of experimental evidence supports an essential role for ETS‐1 during tumor invasion.17 Higher levels of Ets‐1 protein and mRNA were observed in breast cancer tissues than in adjacent control tissue.18 The inhibition of migration and invasion of cancer cells through direct targeting of ETS‐1 is consistent with the role of dysregulation of extracellular matrix proposed for miR‐199.19 The miR family has diverse functions in different cell contexts; thus, we investigated whether miR‐199a‐5p could influence the Ets‐1 expression in breast cancer. In this experiment, lower levels of Ets‐1 mRNA and protein were detected in breast cancer cells with the treatment of miR‐199a‐5p mimic compared with mock cells. In contrast, transfection of anti‐miR‐199a‐5p inhibitors into breast cancer cells elevated the protein levels of Ets‐1. Ectopic expression of miR‐199a‐5p inhibited the expression of the reporter vector containing the wild‐type sequence of Ets‐1 3′‐UTR, but not the reporter vector containing the mutation of the seed‐miR‐199a‐5p binding site, indicating that Ets‐1 is a direct and specific target of miR‐199a‐5p, which could diminish levels of Ets‐1 mRNA and protein in breast cancer.
Ets‐1 regulates many genes, such as MMP9, MMP13 and integrin.20, 21 We identified several ETS‐binding sites in the promoter regions of the human β1 integrin genes. Integrins belong to a cell surface adhesion molecular family and are composed of non‐covalently associated α and β subunits. Integrin heterodimeric connected with extracellular matrix ligands leads to the activation of adhesion‐dependent intracellular signaling pathways.22 In a previous study, we found that αvβ6 integrin binds with FN in an RGD‐dependent manner and is associated with invasion and poor prognosis in breast cancer.23 High levels of β1 integrin correlate with lower survival rates in patients with invasive breast cancer.24 β1 integrin‐deficient MDA‐MB‐231 cells acquired more epithelial‐like morphologies and features in human triple negative breast cancer.25
We also performed knockdown of Ets‐1 by using the siRNA. Our findings demonstrated that knockdown of Ets‐1 resulted in decreased expression of Ets‐1 and β1 integrin in breast cancer cells. Capabilities of migration and invasion were attenuated after knockdown of Ets‐1 in MCF‐7 and MDA‐MB‐231 cells. Binding of Ets‐1 protein to the β1 integrin promoter was further verified by ChIP in breast cancer MCF‐7 cells. The qRT‐PCR results showed a specific band when DNA sequences were precipitated with Ets‐1 antibodies. The data revealed that β1 integrin expression was regulated via Ets‐1 binding to the promoter of β1 integrin.
Integrin‐mediated adhesion can trigger the activation of numerous signaling intermediates, such as FAK, Src and integrin‐linked kinase.26 Integrin binding to extracellular matrix ECM ligands leads to FAK clustering and auto‐phosphorylation, which, in turn, leads to the formation of a FAK/Src complex and the activation of AKt/mTOR.27 Treatment of cells with miR‐199a‐5p mimic was used to determine whether FAK/Src/AKT/mTOR axis was affected. Western blot analysis revealed that pFAK/pSrc/pAKT/pmTOR were reduced by overexpression of miR‐199a‐5p. Furthermore, miR‐199a‐5p‐mediated inactivation of pmTOR could be secured by inhibition of Src or Akt. In short, these data showed that FAK/Src/AKT/mTOR signaling pathway was inhibited by miR‐199a‐5p overexpression.
In conclusion, we are the first to report that miR‐199a‐5p plays a suppressive role in β1 integrin‐induced breast cancer invasion by directly targeting Ets‐1. The downstream signaling pathway of FAK/Src/AKT/mTOR was impeded by miR‐199a‐5p overexpression. This finding advances our understanding of the mechanisms of breast cancer invasion, which may lead to the development of miR‐199a‐5p‐based strategies for treating the disease and set forth effective therapeutic approaches in the progression of breast cancer.
Disclosure Statement
The authors have no conflict of interest to declare.
Cancer Sci 107 (2016) 916–923
Funding Information
National Nature Scientific Foundation of China (81573717); The Natural Science Foundation of Shandong Province (2015ZRB14132, ZR2009CM047).
References
- 1. Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 2004; 6: 229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobson JR, Taipaleenmaki H, Hu YJ et al hsa‐mir‐30c promotes the invasive phenotype of metastatic breast cancer cells by targeting NOV/CCN3. Cancer Cell Int 2014; 14: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deves C, Renck D, Garicochea B et al Analysis of select members of the E26 (ETS) transcription factors family in colorectal cancer. Virchows Arch 2011; 458: 421–30. [DOI] [PubMed] [Google Scholar]
- 4. Cao P, Feng F, Dong G et al Estrogen receptor alpha enhances the transcriptional activity of ETS‐1 and promotes the proliferation, migration and invasion of neuroblastoma cell in a ligand dependent manner. BMC Cancer 2015; 15: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li W, Zhai L, Zhao C, Lv S. MiR‐153 inhibits epithelial–mesenchymal transition by targeting metadherin in human breast cancer. Breast Cancer Res Treat 2015; 150: 501–9. [DOI] [PubMed] [Google Scholar]
- 6. Liu QS, Zhang J, Liu M, Dong WG. Lentiviral‐mediated miRNA against liver‐intestine cadherin suppresses tumor growth and invasiveness of human gastric cancer. Cancer Sci 2010; 101: 1807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang SH, Zhou JD, He QY, Yin ZQ, Cao K, Luo CQ. MiR‐199a inhibits the ability of proliferation and migration by regulating CD44‐Ezrin signaling in cutaneous squamous cell carcinoma cells. Int J Clin Exp Pathol 2014; 7: 7131–41. [PMC free article] [PubMed] [Google Scholar]
- 8. Raimondi L, Amodio N, Di Martino MT et al Targeting of multiple myeloma‐related angiogenesis by miR‐199a‐5p mimics: in vitro and in vivo anti‐tumor activity. Oncotarget 2014; 5: 3039–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Persson H, Kvist A, Rego N et al Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res 2011; 71: 78–86. [DOI] [PubMed] [Google Scholar]
- 10. Yi H, Liang B, Jia J et al Differential roles of miR‐199a‐5p in radiation‐induced autophagy in breast cancer cells. FEBS Lett 2013; 587: 436–43. [DOI] [PubMed] [Google Scholar]
- 11. Mudduluru G, Ceppi P, Kumarswamy R, Scagliotti GV, Papotti M, Allgayer H. Regulation of Axl receptor tyrosine kinase expression by miR‐34a and miR‐199a/b in solid cancer. Oncogene 2011; 30: 2888–99. [DOI] [PubMed] [Google Scholar]
- 12. Dai L, Gu L, Di W. MiR‐199a attenuates endometrial stromal cell invasiveness through suppression of the IKKbeta/NF‐kappaB pathway and reduced interleukin‐8 expression. Mol Hum Reprod 2012; 18: 136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang JH, Chen XT, Wen ZS et al High expression of GOLPH3 in esophageal squamous cell carcinoma correlates with poor prognosis. PLoS ONE 2012; 7: e45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu QF, Liu Y, Fan Y et al Alpha‐enolase promotes cell glycolysis, growth, migration, and invasion in non‐small cell lung cancer through FAK‐mediated PI3K/AKT pathway. J Hematol Oncol 2015; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung HH, Davis AJ, Lee TL et al Methylation of an intronic region regulates miR‐199a in testicular tumor malignancy. Oncogene 2011; 30: 3404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng W, Liu T, Wan X, Gao Y, Wang H. MicroRNA‐199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer‐initiating cells. FEBS J 2012; 279: 2047–59. [DOI] [PubMed] [Google Scholar]
- 17. Li C, Wang Z, Chen Y et al Transcriptional silencing of ETS‐1 abrogates epithelial‐mesenchymal transition resulting in reduced motility of pancreatic cancer cells. Oncol Rep 2015; 33: 559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furlan A, Vercamer C, Bouali F et al Ets‐1 controls breast cancer cell balance between invasion and growth. Int J Cancer 2014; 135: 2317–28. [DOI] [PubMed] [Google Scholar]
- 19. Chan YC, Roy S, Huang Y, Khanna S, Sen CK. The microRNA miR‐199a‐5p down‐regulation switches on wound angiogenesis by derepressing the v‐ets erythroblastosis virus E26 oncogene homolog 1‐matrix metalloproteinase‐1 pathway. J Biol Chem 2012; 287: 41032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh S, Basu M, Roy SS. ETS‐1 protein regulates vascular endothelial growth factor‐induced matrix metalloproteinase‐9 and matrix metalloproteinase‐13 expression in human ovarian carcinoma cell line SKOV‐3. The Journal of Biological Chemistry 2012; 287: 15001–15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Kamoshida G, Matsuda A, Katabami K et al Involvement of transcription factor Ets‐1 in the expression of the alpha3 integrin subunit gene. FEBS J 2012; 279: 4535–46. [DOI] [PubMed] [Google Scholar]
- 22. Russo MA, Paolillo M, Sanchez‐Hernandez Y et al A small‐molecule RGD‐integrin antagonist inhibits cell adhesion, cell migration and induces anoikis in glioblastoma cells. Int J Oncol 2013; 42: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W, Liu Z, Zhao C, Zhai L. Binding of MMP‐9‐degraded fibronectin to beta6 integrin promotes invasion via the FAK‐Src‐related Erk1/2 and PI3K/Akt/Smad‐1/5/8 pathways in breast cancer. Oncol Rep 2015; 34: 1345–52. [DOI] [PubMed] [Google Scholar]
- 24. Yao ES, Zhang H, Chen YY et al Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res 2007; 67: 659–64. [DOI] [PubMed] [Google Scholar]
- 25. Scalici JM, Harrer C, Allen A et al Inhibition of alpha4beta1 integrin increases ovarian cancer response to carboplatin. Gynecol Oncol 2014; 132: 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raymond K, Faraldo MM, Deugnier MA, Glukhova MA. Integrins in mammary development. Semin Cell Dev Biol 2012; 23: 599–605. [DOI] [PubMed] [Google Scholar]
- 27. Abraham J. PI3K/AKT/mTOR pathway inhibitors: the ideal combination partners for breast cancer therapies? Expert Rev Anticancer Ther 2014; 15: 1–18. [DOI] [PubMed] [Google Scholar]


