Abstract
Chemopreventive and potential therapeutic effects of soy isoflavones have been shown to be effective in numerous preclinical studies as well as clinical studies in prostate cancer. Although the inhibition of androgen receptor signaling has been supposed as one mechanism underlying their effects, the precise mechanism of androgen receptor inhibition remains unclear. Thus, this study aimed to clarify their mechanism. Among soy isoflavones, equol suppressed androgen receptor as well as prostate‐specific antigen expression most potently in androgen‐dependent LNCaP cells. However, the inhibitory effect on androgen receptor expression and activity was less prominent in castration‐resistant CxR and 22Rv1 cells. Consistently, cell proliferation was suppressed and cellular apoptosis was induced by equol in LNCaP cells, but less so in CxR and 22Rv1 cells. We revealed that the proteasome pathway through S‐phase kinase‐associated protein 2 (Skp2) was responsible for androgen receptor suppression. Taken together, soy isoflavones, especially equol, appear to be promising as chemopreventive and therapeutic agents for prostate cancer based on the fact that equol augments Skp2‐mediated androgen receptor degradation. Moreover, because Skp2 expression was indicated to be crucial for the effect of soy isoflavones, soy isoflavones may be applicable for precancerous and cancerous prostates.
Keywords: Androgen receptor, isoflavone, prostate cancer, prostate‐specific antigen, Skp2
With the introduction of prostate‐specific antigen (PSA) screening, the prevalence rate of prostate cancer dramatically increased in Europe and North America in the 1980s, followed by Japan in the 1990s. Accordingly, prostate cancer is the most common non‐cutaneous cancer and the second leading cause of cancer‐related mortality among the male population in these countries. Owing to widespread PSA screening, the stage at diagnosis has migrated to earlier stages, accompanied by improved cancer‐specific survival among PSA‐screened populations, as shown in the European Randomized Study of Screening for Prostate Cancer trial undertaken in European countries,1 although a controversial result has been reported from the Prostate, Lung, Colorectal and Ovarian Cancer Screening trial carried out in the USA. However, the latter report suffered from a high contamination rate in the control group, among whom more than 50% underwent PSA screening.2
Thus, because of such a high prevalence rate of prostate cancer and its long latency period, a preventative strategy for prostate cancer appears to attractive, and various agents, including natural and chemical compounds such as vitamin E, selenium, and 5α‐reductase, were studied in large‐scale clinical trials, but failed to show significant results. A large amount of soy isoflavones are consumed in Asian countries, where the prevalence rate of prostate cancer is comparatively low, which suggests that soy isoflavones may be a possible agent for prostate cancer prevention.3, 4 In fact, two randomized clinical trials have examined whether soy proteins and isoflavones can prevent the development of prostate cancer, with both showing a marginal reduction in prostate cancer detection.5, 6, 7, 8 A recent publication has clearly revealed the significant preventative value of soy by meta‐analysis.9 In addition, therapeutic effects of soy isoflavones have also been expected and examined in clinical trials for prostate cancer, especially localized and low‐aggressive cancer.10, 11, 12, 13
As for proof of concept, numerous basic research studies have been undertaken to reveal the significance and the mechanism of soy isoflavones on anticarcinogenesis and their anticancer effects. As a result, chemopreventive and therapeutic effects of soy isoflavones have been shown and several mechanisms of their effects, including the disruption of growth signaling, induction of apoptosis, and inhibition of angiogenesis by phytoestrogens and their antioxidant properties have been proposed.3, 4 Among them, the inhibitory effect on androgen receptor (AR) signaling in prostate cancer has been considered as one of the major effects of soy isoflavones. Davis et al.14 reported that soy isoflavones suppressed AR as well as PSA expression at the transcription level in prostate cancer cells. Later, conversely, Basak et al.15 showed that soy isoflavones similarly suppressed AR at the protein level owing to its loss of stability, but not AR at the mRNA level in prostate cancer cells. Thus, there is controversy regarding the mechanisms regulating AR expression by soy isoflavones.
Therefore, in this study, we aimed to further reveal the mechanism regulating AR expression in prostate cancer cells, as well as the differential effect of isoflavones on cell proliferation in prostate cancer cells. Then, we identified the key molecule regulating AR expression by the most potent equol among soy isoflavones, and revealed its expression in prostate cancer.
Materials and Methods
Cell culture
Human prostate cancer LNCaP and 22Rv1 cells were obtained from ATCC (Manassas, VA, USA) and authenticated by short tandem repeat analysis. Cells were cultured in RPMI‐1640 media (Life Technologies, Carlsbad, CA, USA) supplemented with 10% FBS. LNCaP cells were passaged between 10 and 40 times before use. CxR cells were established and maintained as described previously.16 The cell lines were maintained in a 5% CO2 atmosphere at 37°C.
Antibodies and reagents
Antibodies against AR (sc‐816), p27 Kip1 (sc‐528), and ubiquitin (sc‐271289) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti‐cleaved poly(ADP‐ribose) polymerase (#9541), S‐phase kinase‐associated protein 2 (Skp2) (#4358), and p27 Kip1 (SX53G8.5) antibodies were obtained from Cell Signaling Technology (Cambridge, MA, USA). Anti‐β‐actin (A3854) and anti‐PSA (#1984) antibodies were obtained from Sigma‐Aldrich (St. Louis, MO, USA) and Epitomics (Burlingame, CA, USA), respectively. Equol, genistein, and daidzein were obtained from Sigma‐Aldrich (45405, G6776, and D7802). Dihydrotestosterone (DHT), MG132, and cycloheximide were purchased from Sigma‐Aldrich, CalbioChem (San Diego, CA, USA), and R&D Systems (Minneapolis, MN, USA), respectively.
Knockdown analysis using siRNAs
The following double‐stranded RNA 25‐bp oligonucleotides were commercially generated (Life Technologies): Skp2 #1, 5′‐CUUCCUCGCUGUUGCUCAGGCUGUC‐3′ (sense) and 5′‐GACAGCCUGAGCAACAGCGAGGAAG‐3′ (antisense); and Skp2 #2, 5′‐UAGAGAGCAAGGCUGCAAAGGAGUC‐3′ (sense) and 5′‐GACUCCUUUGCAGCCUUGCUCUCUA‐3′ (antisense). Prostate cancer cells were transfected with siRNA using Lipofectamine 2000 (Life Technologies) according to the manufacturer's protocol.
RNA isolation, reverse transcription, and quantitative real‐time PCR
Total RNA was extracted using the NucleoSpin RNA II kit (Clontech, Palo Alto, CA) and reverse transcribed using iScript (Bio‐Rad, Hercules, CA, USA). The resulting cDNAs were diluted to 1:5–10 and served as templates for real‐time PCR using TaqMan Gene Expression Assays for full‐length AR (Hs00907244_m1), AR V7 (custom‐made), PSA (Hs00426859_g1), Skp2 (Hs00185584_m1), β‐actin (Hs00185584_m1), and GAPDH (Hs02758991_g1) (all from Life Technologies) and TaqMan Gene Expression Master Mix (Life Technologies) on a CFX Connect Real‐Time System (Bio‐Rad). The transcript levels of target genes were normalized to the corresponding GAPDH transcript levels. All values represent the results of at least three independent experiments.
Western blot analysis
Whole‐cell extracts and Western blot analyses were prepared as described previously.17, 18, 19, 20 Briefly, the concentration of the prepared protein extracts was quantified using a Protein Assay (Bio‐Rad) based on the Bradford method. Aliquots (20 μg protein) were separated by 4–20% SDS‐PAGE and transferred to PVDF microporous membranes (GE Healthcare Bio‐Sciences, Piscataway, NJ, USA) using a semi‐dry blotter. The membranes were then incubated with the primary antibodies for 1 h at room temperature, followed by incubation with peroxidase‐conjugated secondary antibodies for 40 min at room temperature. The bound antibodies were visualized using an ECL kit (GE Healthcare Bio‐Sciences), and images were obtained using an image analyzer (Ez‐Capture MG; ATTO, Tokyo, Japan).
Co‐immunoprecipitation assay
Co‐immunoprecipitation assays were carried out as previously described.17 Briefly, whole‐cell extracts (500 μg) were incubated for 2 h at 4°C with 1.0 μg anti‐AR antibody and p27 antibody with 20 μL protein A/G agarose (Santa Cruz Biotechnology). The immunoprecipitated samples were washed three times, and the immunoprecipitated samples and pre‐immunoprecipitated samples (50 μg) as inputs were subjected to Western blot analysis with the indicated antibodies.
Cytotoxicity analysis
Cytotoxicity analysis was carried out as described previously.18, 19, 20 Briefly, prostate cancer cells (2 × 103) were seeded in 96‐well plates. On the following day, cells were cultured with FBS or charcoal‐stripped serum (CSS)‐supplemented media with or without 1 nM DHT for 24 h and various concentrations of equol were applied. After 48 h, the surviving cells were stained using the alamarBlue assay (TREK Diagnostic Systems, Cleveland, OH, USA) at 37°C for 180 min. The absorbance of each well was measured using an ARVO MX plate reader (Perkin Elmer, Waltham, MA, USA). The results are representative of at least three independent experiments.
Statistical analysis
The statistical significance of most differences between groups was calculated using Prism 19 software (GraphPad, San Diego, CA, USA). All data were assessed using Student's t‐test. Levels of statistical significance were set at P < 0.05.
Results
Equol potently inhibits AR and PSA expression
We examined which soy isoflavones, including equol, genistein, and daidzein, were most potent for suppressing AR signaling. As shown in Figure 1(a), LNCaP cells showed impairment of AR expression after treatment with soy isoflavones. Consistently, expression of the major AR‐target gene, PSA, was also reduced by soy isoflavones. However, AR mRNA expression was little affected by soy isoflavones (Fig. 1b). In contrast, PSA mRNA expression was significantly inhibited by soy isoflavones (Fig. 1b), suggesting non‐transcriptional regulation of AR expression by equol. Interestingly, equol, which is a major metabolite of daidzein, most potently inhibited AR and PSA expression compared with genistein and daidzein. Therefore, we used equol in subsequent experiments.
Figure 1.
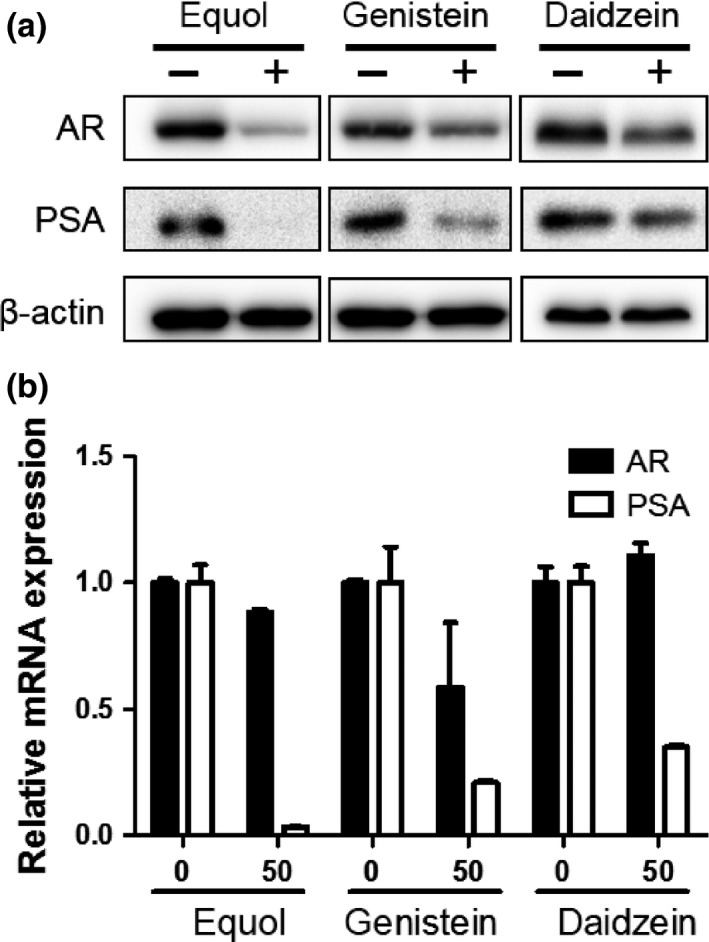
Soy isoflavones inhibit androgen receptor (AR) and prostate‐specific antigen (PSA) expression. (a, b) LNCaP cells were treated with 50 μM equol, genistein, or daidzein for 48 h. (a) Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies. (b) After extraction of total RNA and synthesis of cDNA, quantitative real‐time PCR was carried out with primers and probes specific for AR,PSA, and GAPDH. The transcript levels of AR and PSA from non‐treated cells were defined as 1. Boxes, mean; bars ± SD.
Androgen receptor protein and cell proliferation suppressed by equol with or without androgens
To examine the effect of androgens on AR degradation by equol, LNCaP cells were incubated in CSS‐supplemented media with or without DHT and treated with equol. When LNCaP cells were treated with equol, the AR protein level decreased with or without DHT (Fig. 2a). Consistently, PSA mRNA levels were also decreased by equol with or without DHT, although equol repeatedly failed to affect AR mRNA levels (Fig. 2b). Cell proliferation was also retarded by equol with or without DHT (Fig. 2c). The inhibitory effects of equol on AR activity and cell proliferation were intriguingly prominent in DHT treated cells compared with DHT untreated cells (Fig. 2), suggesting that the effect of equol is partially androgen‐dependent.
Figure 2.
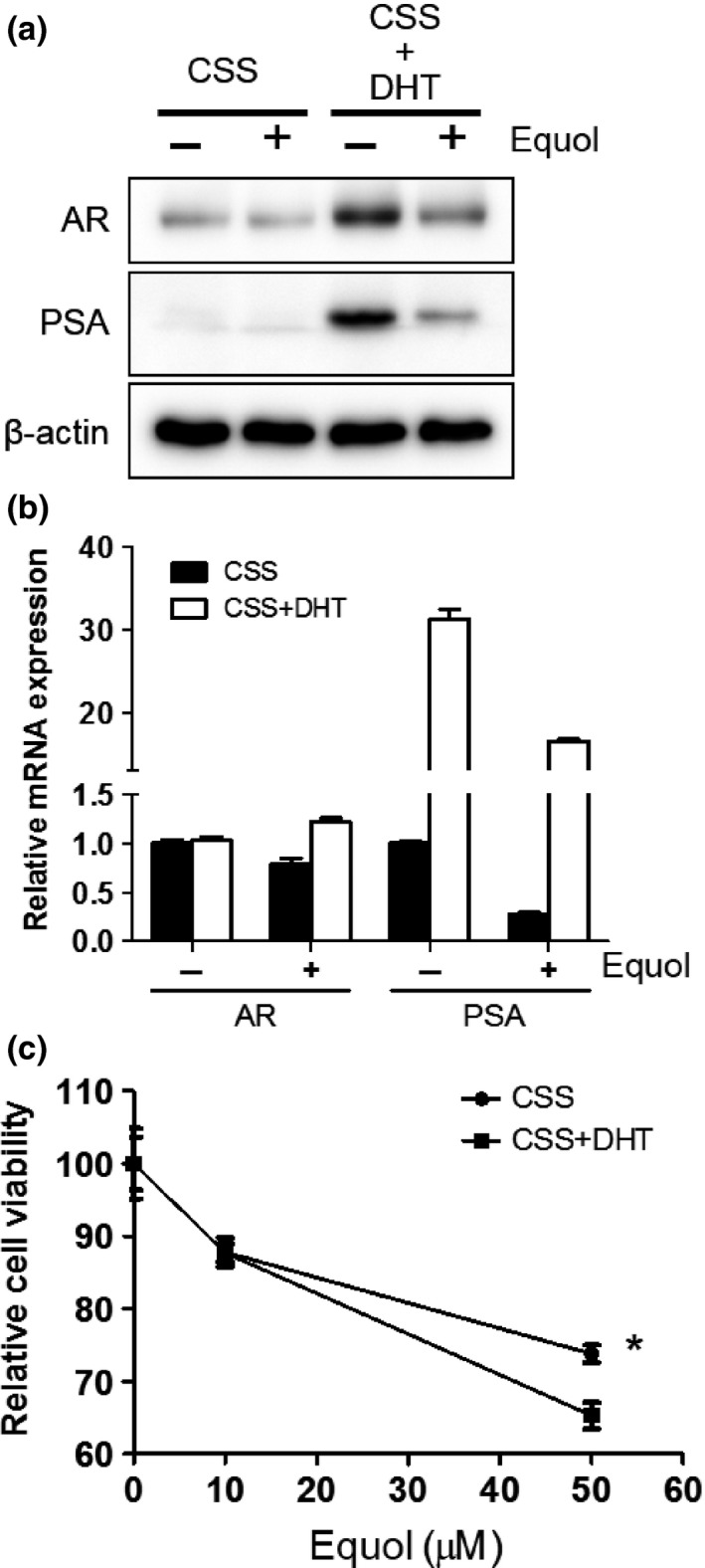
Androgen receptor (AR) protein and cell proliferation are suppressed by equol with or without androgens. (a, b) LNCaP cells incubated in charcoal‐stripped serum (CSS)‐supplemented media with or without 10 nM dihydrotestosterone (DHT). Equol was supplemented in media after 24 h. Cells were harvested at 48 h after equol treatment. (a) Whole‐cell extracts from these cells were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies. (b) After extraction of total RNA and synthesis of cDNA, quantitative real‐time PCR was undertaken using primers and probes specific for AR,PSA, and GAPDH. AR and PSA transcript levels from non‐treated cells were defined as 1. Boxes, mean; bars ± SD. (c) LNCaP cells were seeded into 96‐well plates and incubated in CSS‐supplemented media with or without 1 nM DHT. On the following day, various concentrations of equol were applied. After incubation for 72 h, cell survival was analyzed by cytotoxicity assay. Cell survival in the absence of equol corresponds to 100. Boxes, mean; bars ± SD. *P < 0.05. PSA, prostate‐specific antigen.
Effect of equol on AR expression in CxR and 22Rv1 cells
We analyzed the effect of equol in CxR cells, which are castration‐resistant derivatives of LNCaP cells. Interestingly, equol induced downregulated AR expression at the protein level (Fig. 3a), but not at the mRNA level (Fig. 3b). Accordingly, PSA expression was reduced by equol at both mRNA and protein levels (Fig. 3a,b), although the inhibitory effects on AR and PSA suppression by equol was less prominent compared with that in LNCaP cells (Fig. 1b). However, in another castration‐resistant cell line, 22Rv1, full‐length AR as well as AR variant (AR V7) expression was little affected by equol at both mRNA and protein levels (Fig. 3c,d).
Figure 3.
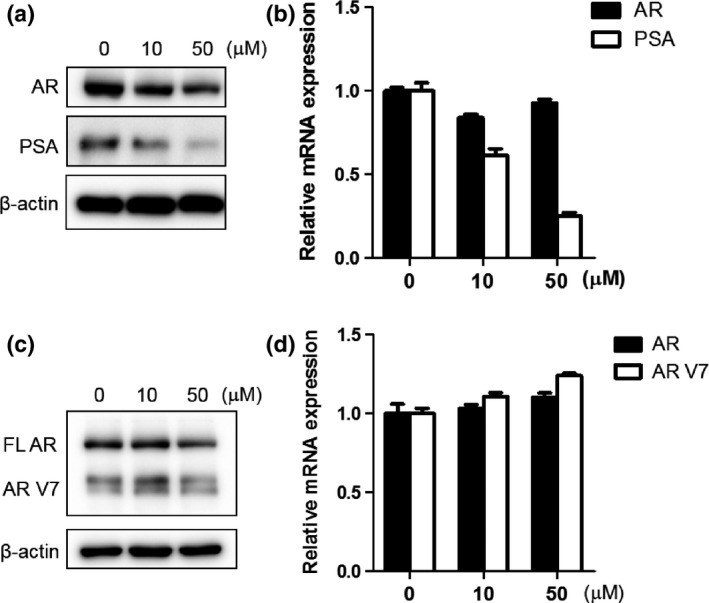
Effect of equol on androgen receptor (AR) and prostate‐specific antigen (PSA) expression in CxR and 22Rv1 cells. (a, c) CxR (a) and 22Rv1 (c) cells were treated with indicated concentrations of equol for 48 h. Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies. (b, d) CxR (b) and 22Rv1 (d) cells were treated with indicated concentrations of equol for 48 h. After extraction of total RNA and cDNA synthesis, quantitative real‐time PCR was undertaken using primers and probes specific for AR,AR V7,PSA, and GAPDH. Transcript levels of AR and PSA from non‐treated CxR and 22Rv1 cells were defined as 1. Boxes, mean; bars ± SD.
Equol reduces cell viability most prominently in LNCaP cells, compared with CxR and 22Rv1 cells
Subsequently, we examined cytotoxicity by equol in LNCaP, CxR, and 22Rv1 cells. As shown in Figure 4(a), LNCaP cells showed prominent susceptibility to equol. However, equol had little or no effect in CxR and 22Rv1 cells, respectively. The cleaved form of poly(ADP‐ribose) polymerase, indicating an induction of cellular apoptosis, increased in LNCaP cells but not in CxR or 22Rv1 cells in the presence of equol (Fig. 4b).
Figure 4.
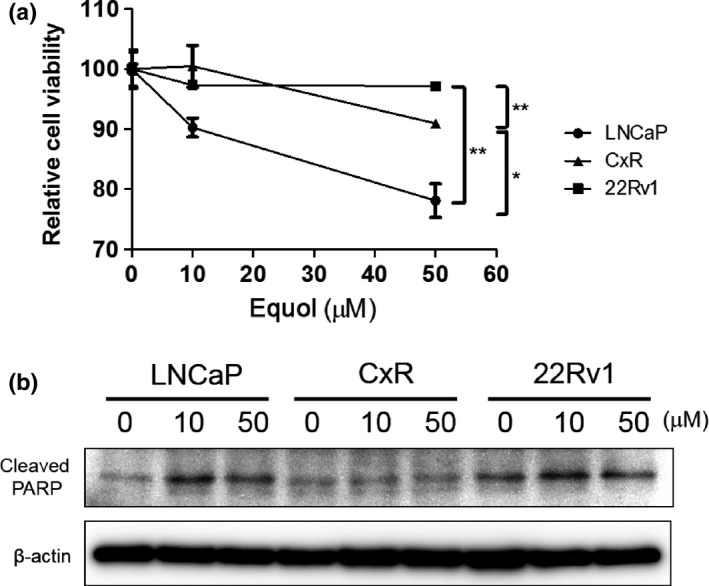
Equol suppresses cell proliferation most prominently in LNCaP. (a) LNCaP, CxR, and 22Rv1 cells were seeded into 96‐well plates. The following day, various concentrations of equol were applied. After incubation for 48 h, cell survival was analyzed by a cytotoxicity assay. Cell survival in the absence of equol corresponds to 100. Boxes, mean; bars ± SD. *P < 0.05; **P < 0.01. (b) LNCaP, CxR, and 22Rv1 cells were treated with indicated concentrations of equol for 48 h. Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies. PARP, poly(ADP‐ribose) polymerase.
Equol suppresses AR expression through AR degradation
To investigate the mechanism of AR repression by equol, LNCaP cells were treated with cycloheximide, inhibiting de novo protein synthesis, or MG132, inhibiting proteasome degradation. As shown in Figure 5, although equol alone induced AR protein degradation, MG132 blocked AR degradation by equol. However, cycloheximide reversed the AR level changes by equol, indicating that equol suppresses AR expression through proteasome degradation. Intriguingly, MG132 treatment induced a truncated form of AR, especially with equol (Fig. 5), as previously reported.21
Figure 5.
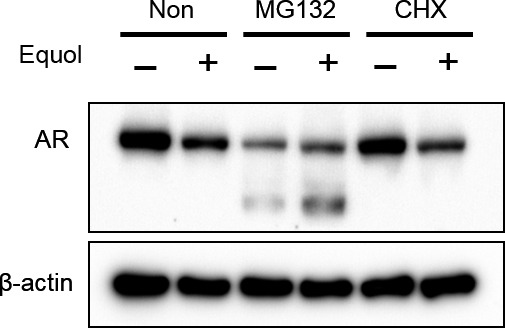
Equol suppresses androgen receptor (AR) expression through AR degradation. LNCaP cells were cultured with or without (Non) 5 mM MG132 or 1 mg/mL cycloheximide (CHX) during equol treatment for 48 h. Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies.
Androgen receptor degraded by equol through interaction between AR and Skp2
It has been reported that Skp2 regulates AR expression through ubiquitin‐mediated degradation.22 To assess the role of Skp2 on AR expression after treatment of equol, we transfected Skp2‐specific siRNA into LNCaP cells and then examined AR expression and cell viability. In the presence of equol, knockdown of Skp2 resulted in restored AR expression, which was accompanied by PSA induction, and overcame cell growth inhibition compared with control siRNA (Fig. 6a,b). These results suggest that equol induced AR degradation through Skp2. Next, we examined whether equol treatment affects the interaction between AR and Skp2. Interestingly, the interaction between AR and Skp2 was increased in the presence of equol, even though the number of non‐immunoprecipitated Skp2 and AR proteins was decreased by equol (Fig. 6c).
Figure 6.
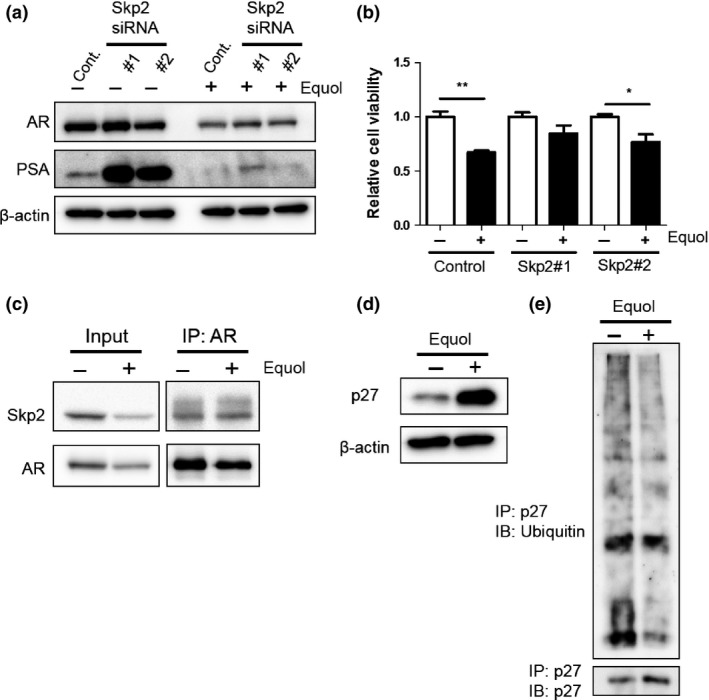
Equol predominantly induces androgen receptor (AR) degradation by S‐phase kinase‐associated protein 2 (Skp2). (a) LNCaP cells were transfected with 50 nM control siRNA, Skp2 siRNA #1, or Skp2 siRNA #2, and treated with 50 μM equol at 24 h after incubation. Cells were harvested 48 h later. Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies. (b) LNCaP cells transfected with 50 nM control siRNA, Skp2 siRNA #1, or Skp2 siRNA #2 were seeded into 96‐well plates. On the following day, 50 μM equol was applied. After incubation for 48 h, cell survival was analyzed by a cytotoxicity assay. Cell survival in the absence of equol corresponds to 100. Boxes, mean; bars ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. (c) LNCaP cells were treated with equol for 48 h. Whole‐cell extracts were prepared and immunoprecipitated (IP) with an anti‐AR antibody. Presence of Skp2 and AR in whole cell extracts and IPs was determined by anti‐AR and anti‐Skp2 antibody, respectively. Western blot analysis was carried out with the indicated antibodies. (d) LNCaP cells were treated with equol for 48 h. Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies. (e) Whole‐cell lysates were prepared from equol‐treated LNCaP cells and IP with an anti‐p27 antibody and immunoblotted (IB) with antibodies for ubiquitin and p27.
Because Skp2 is one of ubiquitin ligase to regulate tumor suppressor genes, including CDK inhibitor p27,23 we tested the hypothesis that p27 degradation is suppressed during AR degradation. In LNCaP cells, equol treatment resulted in p27 accumulation (Fig. 6d). Then, we tested the ubiquitination of p27 in LNCaP cells after treatment with equol and found that the ubiquitination of p27 was suppressed.
Expression of Skp2 in prostate cancer cell lines
Skp2 expression was important to suppress AR expression in the presence of equol. We found the effect of equol was different between cell lines (Fig. 4). Then, we examined Skp2 expression levels in prostate cancer cell lines. As shown in Figure 7, Skp2 expression levels were decreased in LNCaP, CxR, and 22Rv1 cells, in descending order, at both mRNA and protein levels.
Figure 7.
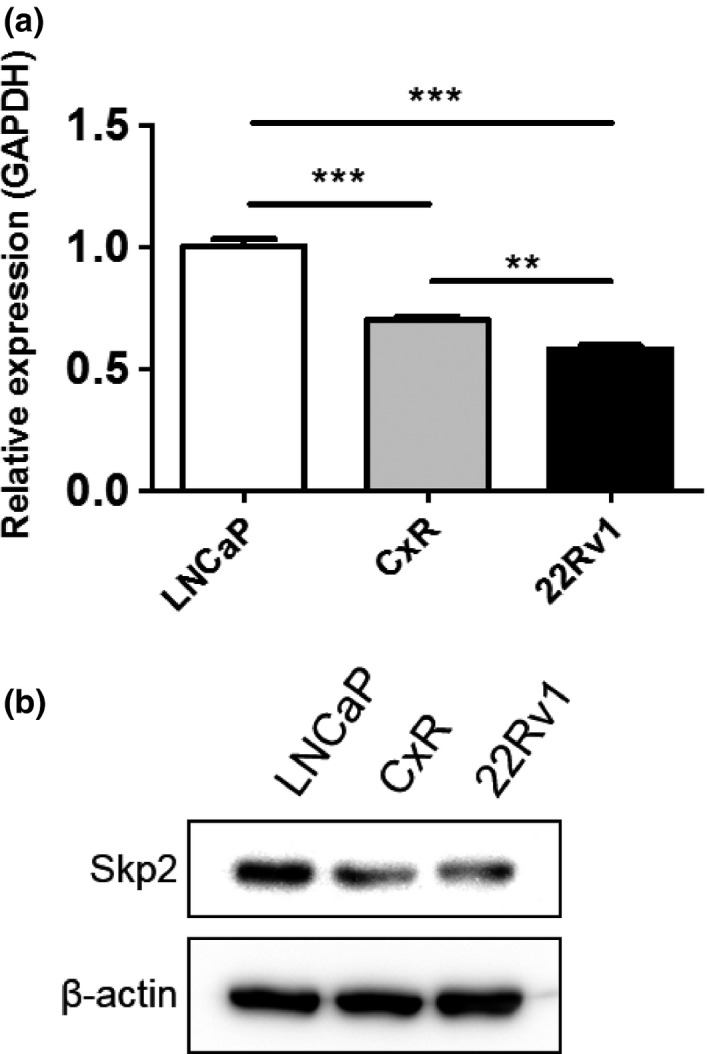
S‐phase kinase‐associated protein 2 (Skp2) expression in prostate cancer cells. (a, b) LNCaP, CxR, and 22Rv1 cells were treated with 50 μM equol for 48 h. (a) After extraction of total RNA and cDNA synthesis, quantitative real‐time PCR was undertaken with primers and probes specific for Skp2 and GAPDH. Skp2 transcript levels from LNCaP cells were defined as 1. Boxes, mean; bars ± SD. **P < 0.01; ***P < 0.001. (b) Whole‐cell extracts were subjected to SDS‐PAGE, and Western blot analysis was carried out with the indicated antibodies.
Discussion
Soy isoflavones consist of several types of components, such as more abundant daidzein and genistein and less abundant glycitein, as well as equol as a metabolizer of daidzein. Previously, equol has been shown to be more active compared with other soy isoflavones.24, 25 In line with previous studies, this study showed that equol exerted the most powerful effect to suppress AR expression in LNCaP cells compared with non‐metabolized soy isoflavones, such as daidzein and genistein, suggesting a crucial role metabolizing daidzein into equol by intestinal flora, as suggested by Akaza.26
Previously, there has been some controversy regarding AR regulation by soy isoflavones. The present study revealed that AR protein expression by equol was regulated by the proteasomal pathway, but not through transcriptional or translational mechanisms, which supports the results of Basak et al.,15 but not those of Davis et al.14 However, it should be noted that differences in experimental conditions, such as exposure duration to the agent, might affect results. Previously, Basak et al. showed that histone deacetylase inhibition by genistein leads to the hyperacetylation of the AR‐stabilizing chaperone heat shock protein 90, which undermines the chaperone function and accelerates ubiquitination and degradation of client proteins including AR.27 Similarly, Li et al.28 reported that AR activity was regulated by isoflavones through Akt/Foxo3a/glycogen synthase kinase‐3β signaling, resulting in inhibition of AR translocation into the nucleus, thereby promoting AR degradation. For AR ubiquitination, the cascade of ubiquitin ligases from E1 to E3 plays a crucial role. Among these proteins, several E3 ubiquitin ligases have been reported to modulate AR function. Previous studies have shown that the MDM229 and Skp222 E3 ligases promote AR depletion and inhibit AR function, whereas RNF630 and Siah231 E3 ligases play opposite roles. Because MDM2 has been shown to be downregulated by the soy isoflavone genistein,32 suggesting inconsistent effects of soy isoflavone on AR and MDM2, we focused on Skp2 as a possible mediator of AR degradation by the soy isoflavone equol. Next, we successfully revealed the functional role of Skp2 that promoted the interaction with AR and augmented AR degradation in the presence of equol. Previously, soy isoflavones have been shown to bind to the AR ligand‐binding domain and act as an anti‐androgen,33 as well as to affect the expression of not only AR but also estrogen receptor α/β and progesterone receptor in prostate cancer cells.34, 35, 36 In addition, AR mutation (T877A) in LNCaP cells might influence the effect of soy isoflavones, as it has been known that AR mutation can affect ligand specificity.37 These reports suggest the possibility that these functions also influence the growth inhibition in equol‐treated LNCaP cells. Consistently, our study has shown that equol inhibited AR function as well as cell proliferation in androgen‐dependent LNCaP cells in a partially androgen‐dependent manner.
As a member of the F‐box protein family, Skp2 is responsible for the degradation of several tumor suppressors, such as p2738 and Foxo1.39 We showed that equol induced accumulation of p27. These results suggest that expression of tumor suppressor genes including p27 was maintained through equol promoting AR degradation by Skp2.
In addition, this study has shown that Skp2 expression was decreased in castration‐resistant CxR and 22Rv1 cells, compared with androgen‐dependent LNCaP cells. This decrease in Skp2 would bring about decreased AR degradation and thereby increased AR expression. The increased AR degradation40 as well as augmented AR expression by various mechanisms41 are well known to be major causes for obtaining castration resistance in prostate cancer. Therefore, decreased expression of Skp2 may be one cause of castration resistance in castration‐resistant CxR and 22Rv1 cells.
In this study, we showed that 50 μM equol effectively suppressed AR expression and cell growth. This concentration is higher than ordinary serum and prostate tissue concentrations of equol and isoflavones.42, 43 Therefore, to obtain high concentration levels of equol in prostate tissue, the development of methods to increase equol bioavailability by increasing the absorption rate using bacterial conversion from daidzein into equol would be required, as suggested by Akaza.26
Taken together, this study has provided proof of concept to use soy isoflavones, especially equol, for chemoprevention and therapeutics of prostate cancer based on the mechanism that equol augmented Skp2‐mediated AR degradation. Moreover, Skp2 expression is crucial for the effect of soy isoflavones on AR expression as well as prostate cancer proliferation, suggesting that soy isoflavones may be applicable for precancerous and cancerous prostates expressing abundant Skp2.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by Kakenhi grants (15K20095, 25462483, 25462484, and 26861273) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), a Grant‐in‐Aid for Scientific Research on Innovative Areas (221S0001) from the Scientific Support Programs for Cancer Research from MEXT, Japan, and the Suzuki Foundation for Urological Medicine, Japan. The authors are grateful to Edanz Group Japan for editorial assistance, and Ms. Noriko Hakoda and Ms. Eriko Gunshima for their technical assistance.
Cancer Sci 107 (2016) 1022–1028
Funding Information
Ministry of Education, Culture, Sports, Science, and Technology of Japan (15K20095, 25462483, 25462484, 26861273, 221S0001); Suzuki Foundation for Urological Medicine, Japan.
References
- 1. Schroder FH, Hugosson J, Roobol MJ et al Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow‐up. Lancet 2014; 384: 2027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andriole GL, Crawford ED, Grubb RL 3rd et al Mortality results from a randomized prostate‐cancer screening trial. N Engl J Med 2009; 360: 1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horie S. Chemoprevention of prostate cancer: soy isoflavones and curcumin. Korean J Urol 2012; 53: 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol 2014; 140: 116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamilton‐Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low‐grade prostate cancer. Nutr Cancer 2008; 60: 7–13. [DOI] [PubMed] [Google Scholar]
- 6. Hamilton‐Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Isoflavone‐rich soy protein isolate suppresses androgen receptor expression without altering estrogen receptor‐beta expression or serum hormonal profiles in men at high risk of prostate cancer. J Nutr 2007; 137: 1769–75. [DOI] [PubMed] [Google Scholar]
- 7. Hamilton‐Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Soy protein isolate increases urinary estrogens and the ratio of 2:16alpha‐hydroxyestrone in men at high risk of prostate cancer. J Nutr 2007; 137: 2258–63. [DOI] [PubMed] [Google Scholar]
- 8. Miyanaga N, Akaza H, Hinotsu S et al Prostate cancer chemoprevention study: an investigative randomized control study using purified isoflavones in men with rising prostate‐specific antigen. Cancer Sci 2012; 103: 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta‐analysis of randomized controlled trials. BJU Int 2014; 113: E119–30. [DOI] [PubMed] [Google Scholar]
- 10. deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone‐rich soy extract on prostate‐specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer 2010; 62: 1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar NB, Kang L, Pow‐Sang J et al Results of a randomized phase I dose‐finding trial of several doses of isoflavones in men with localized prostate cancer: administration prior to radical prostatectomy. J Soc Integr Oncol 2010; 8: 3–13. [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar NB, Krischer JP, Allen K et al A Phase II randomized, placebo‐controlled clinical trial of purified isoflavones in modulating steroid hormones in men diagnosed with localized prostate cancer. Nutr Cancer 2007; 59: 163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lazarevic B, Boezelijn G, Diep LM et al Efficacy and safety of short‐term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo‐controlled, double‐blind Phase 2 clinical trial. Nutr Cancer 2011; 63: 889–98. [DOI] [PubMed] [Google Scholar]
- 14. Davis JN, Kucuk O, Sarkar FH. Expression of prostate‐specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog 2002; 34: 91–101. [DOI] [PubMed] [Google Scholar]
- 15. Basak S, Pookot D, Noonan EJ, Dahiya R. Genistein down‐regulates androgen receptor by modulating HDAC6‐Hsp90 chaperone function. Mol Cancer Ther 2008; 7: 3195–202. [DOI] [PubMed] [Google Scholar]
- 16. Shiota M, Yokomizo A, Tada Y et al Castration resistance of prostate cancer cells caused by castration‐induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene 2010; 29: 237–50. [DOI] [PubMed] [Google Scholar]
- 17. Imada K, Shiota M, Kohashi K et al Mutual regulation between Raf/MEK/ERK signaling and Y‐box‐binding protein‐1 promotes prostate cancer progression. Clin Cancer Res 2013; 19: 4638–50. [DOI] [PubMed] [Google Scholar]
- 18. Itsumi M, Shiota M, Yokomizo A et al PMA induces androgen receptor downregulation and cellular apoptosis in prostate cancer cells. J Mol Endocrinol 2014; 53: 31–41. [DOI] [PubMed] [Google Scholar]
- 19. Shiota M, Yokomizo A, Takeuchi A et al Inhibition of protein kinase C/Twist1 signaling augments anticancer effects of androgen deprivation and enzalutamide in prostate cancer. Clin Cancer Res 2014; 20: 951–61. [DOI] [PubMed] [Google Scholar]
- 20. Yokomizo A, Shiota M, Kashiwagi E et al Statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate 2011; 71: 298–304. [DOI] [PubMed] [Google Scholar]
- 21. Harada N, Inoue K, Yamaji R, Nakano Y, Inui H. Androgen deprivation causes truncation of the C‐terminal region of androgen receptor in human prostate cancer LNCaP cells. Cancer Sci 2012; 103: 1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li B, Lu W, Yang Q, Yu X, Matusik RJ, Chen Z. Skp2 regulates androgen receptor through ubiquitin‐mediated degradation independent of Akt/mTOR pathways in prostate cancer. Prostate 2014; 74: 421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutterluty H, Chatelain E, Marti A et al p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol 1999; 1: 207–14. [DOI] [PubMed] [Google Scholar]
- 24. Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr 2010; 140: 1355S–62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr 2010; 140: 1363S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akaza H. Prostate cancer chemoprevention by soy isoflavones: role of intestinal bacteria as the “second human genome”. Cancer Sci 2012; 103: 969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bali P, Pranpat M, Bradner J et al Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 2005; 280: 26729–34. [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK‐3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem 2008; 283: 27707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation‐dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J 2002; 21: 4037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu K, Shimelis H, Linn DE et al Regulation of androgen receptor transcriptional activity and specificity by RNF6‐induced ubiquitination. Cancer Cell 2009; 15: 270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qi J, Tripathi M, Mishra R et al The E3 ubiquitin ligase Siah2 contributes to castration‐resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell 2013; 23: 332–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li M, Zhang Z, Hill DL, Chen X, Wang H, Zhang R. Genistein, a dietary isoflavone, down‐regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res 2005; 65: 8200–8. [DOI] [PubMed] [Google Scholar]
- 33. Mahmoud AM, Zhu T, Parray A et al Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS ONE 2013; 8: e78479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fritz WA, Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein down‐regulates androgen and estrogen receptor expression in the rat prostate. Mol Cell Endocrinol 2002; 186: 89–99. [DOI] [PubMed] [Google Scholar]
- 35. Mahmoud AM, Al‐Alem U, Ali MM, Bosland MC. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J Steroid Biochem Mol Biol 2015; 152: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog 2007; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veldscholte J, Ris‐Stalpers C, Kuiper GG et al A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti‐androgens. Biochem Biophys Res Commun 1990; 173: 534–40. [DOI] [PubMed] [Google Scholar]
- 38. Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin‐mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1999; 1: 193–9. [DOI] [PubMed] [Google Scholar]
- 39. Frescas D, Pagano M. Deregulated proteolysis by the F‐box proteins SKP2 and beta‐TrCP: tipping the scales of cancer. Nat Rev Cancer 2008; 8: 438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gregory CW, Johnson RT Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res 2001; 61: 2892–8. [PubMed] [Google Scholar]
- 41. Shiota M, Yokomizo A, Naito S. Oxidative stress and androgen receptor signaling in the development and progression of castration‐resistant prostate cancer. Free Radic Biol Med 2011; 51: 1320–8. [DOI] [PubMed] [Google Scholar]
- 42. Akaza H, Miyanaga N, Takashima N et al Is daidzein non‐metabolizer a high risk for prostate cancer? A case‐controlled study of serum soybean isoflavone concentration. Jpn J Clin Oncol 2002; 32: 296–300. [DOI] [PubMed] [Google Scholar]
- 43. Gardner CD, Oelrich B, Liu JP, Feldman D, Franke AA, Brooks JD. Prostatic soy isoflavone concentrations exceed serum levels after dietary supplementation. Prostate 2009; 69: 719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


