Abstract
The RCC‐SELECT study showed the correlation between single nucleotide polymorphisms (SNP) in STAT3 gene and survival in metastatic renal cell carcinoma (mRCC) patients with first‐line interferon‐α (IFN‐α). In that study, even patients with STAT3 SNP linked to shorter overall survival (OS) exhibited remarkably improved prognosis. All 180 patients evaluated in the above study were further analyzed for correlation between OS and demographics/clinicopathological parameters. OS was estimated using the Kaplan–Meier method. Associations between OS and potential prognostic factors were assessed using the log‐rank test and the Cox proportional hazards model. The median OS was 42.8 months. Univariate analysis showed that worse Eastern Cooperative Oncology Group‐performance status (ECOG‐PS), high T stage, regional lymph node metastasis, distant metastasis, higher grade, infiltrative growth pattern, the presence of microscopic vascular invasion (MVI), hypercalcemia, anemia, thrombocytopenia and elevated C‐reactive protein were significantly associated with OS. Multivariate analysis revealed that ECOG‐PS (hazard ratio [HR] = 3.665, P = 0.0004), hypercalcemia (HR = 6.428, P = 0.0005) and the presence of MVI (HR = 2.668, P = 0.0109) were jointly significant poor prognostic factors. This is the first study analysing prognostic factors of mRCC patients with first‐line IFN‐α using large cohort of the prospective study. The present study suggests that first‐line IFN‐α is still a useful therapy for mRCC even in the era of molecular targeted therapy.
Keywords: The Era of molecular targeted therapy, interferon‐α, overall survival, prognostic factors, renal cell carcinoma
Renal cell carcinoma (RCC) is one of the most common cancers. The prevalence of RCC and the mortality rate have both increased over the past decade.1 Within the European Union, more than 80 000 new cases were diagnosed in 2012, and there were approximately 35 000 related deaths.2 Despite advances in cross‐sectional imaging techniques and increased opportunity for the incidental detection of RCC during examination for unrelated diseases, approximately 30% of RCC patients already harbor metastatic disease.1 Furthermore, another 20% of patients who were undertaken nephrectomy resulted in development of mRCC during follow‐up.3 Therefore, establishing an optimized treatment strategy for metastatic disease is a major issue that needs to be addressed in order to improve survival of RCC patients.
IFN‐α was among the most commonly used agents for mRCC before molecular targeted agents became available. Although its objective response rate is around 10%4, 5, 6, 7 and the latest guidelines recommend its usage for clear cell carcinoma only by simultaneous administration with bevacizumab,8 for several reasons it is still used as a single agent for Japanese mRCC patients. First, it provides a fairly favorable prognosis in these patients in the cytokine era.9 Second, patients are more likely to achieve complete response than if treated with molecular targeted agents.10 Third, molecular targeted agents induce more frequent adverse events in Japanese mRCC patients than in Caucasian patients.11
In our previous case‐control association study conducted in 2007, a particular SNP in STAT3, frequently observed in Japanese people compared to Caucasian people, predicts a better response to IFN‐α,12 supporting the idea that IFN‐α is a useful therapeutic option in Japanese mRCC patients. Hence, it is reasonable to expect that a substantial fraction of Japanese mRCC patients can still be given IFN‐α as a first‐line therapy even in the post‐cytokine era. In fact, our recent multi‐centre prospective study (the RCC‐SELECT study) demonstrated that STAT3‐2 (rs1905341) is significantly associated with better clinical response (complete response, partial response and stable disease longer than 24 weeks) and better overall survival (OS) in Japanese mRCC patients with first‐line IFN‐α therapy in the post‐cytokine era.10 Notably, in that study, even the one genotypic cohort (with C/T or T/T in STAT3‐2) with shorter survival exhibited prolonged median OS (41.8 months) compared to the previous studies (median OS of the C/C genotype cohort had not been reached at the time of the previous analysis).7, 9 This suggests that the recent prognosis of mRCC patients who received first‐line IFN‐α therapy has been significantly improved compared to the cytokine era regardless of genetic background. However, factors affecting the prognosis of such patients have not yet been fully studied. The aim of the present study is to estimate OS and to identify prognostic factors of mRCC patients whose first‐line therapy was IFN‐α in the era of molecular targeted therapy by analyzing the cohort of the RCC‐SELECT study.
Materials and Methods
Patients and study design
The RCC‐SELECT study was a multicenter, prospective study evaluating the correlation between the antitumor effects of IFN‐α and 11 SNP in eight genes in 180 patients who were treated with three doses per week of IFN‐α 5 million IU for 12 weeks or longer. Details of this study design, including eligibility criteria and endpoints, have been published previously,10 and the present study includes all patients who were eligible in the previous study. Histology of patients includes 168 clear cell RCC (93.3%), 9 papillary RCC (5.0%) and 3 RCC with unknown histology (1.7%). The protocol of the present study was approved by the ethics committees of all the clinical sites, and all patients provided written informed consent.
Statistical analysis
Overall survival was calculated from the date when the first IFN‐α administration was given to the date of death as a result of any cause or the date of the last follow‐up. OS was analyzed using the Kaplan–Meier method, and median OS and hazard ratio along with 95% confidence limits are described. Associations between OS and potential prognostic factors were assessed using the log‐rank test in univariate analysis. Two‐sided P < 0.05 was considered significant. A stepwise Cox proportional hazards model was performed to determine a combination of factors that were jointly significantly associated with OS. Statistical analyses were performed with SAS v.8.2 (SAS Institute, Cary, NC, USA).
Results
A total of 211 patients with mRCC were enrolled in the previous study, and 203 patients were judged to be eligible, whose characteristics are shown in Table 1.10 Among those patients, 180 patients who could continue the IFN‐α therapy for longer than 12 weeks were evaluated in the RCC‐SELECT study. Data regarding these 180 patients were further analyzed in the present study. The median OS of the patients was 42.8 months (Fig. 1). Then, relationships between patient demographics/clinicopathological parameters and OS were investigated (Table 2). Regarding patient characteristics, better ECOG‐performance status (ECOG‐PS) (0 vs 1) was significantly associated with longer OS (hazard ratio [HR] = 2.728, P = 0.0006). Regarding TNM classification, high T stage (T2, T3 and T4 vs T1) (R = 2.132, P = 0.0298), the presence of regional lymph node metastasis (N1 and N2 vs N0) (HR = 2.092, P = 0.0033) and distant metastasis (M1 vs M0) (HR = 1.961, P = 0.0365) were significantly associated with shorter OS. Regarding pathological features, while expansive growth pattern was related to longer OS than infiltrative growth pattern (HR = 0.571, P = 0.0382), higher grade (G3 vs G1 and G2) (HR = 2.587, P = 0.0002) and the presence of MVI (HR = 3.322, P = 0.0004) were significantly associated with shorter OS. In contrast, neither the presence of sarcomatoid component (HR = 1.657, P = 0.2721) nor sites of metastasis (lung only metastasis vs metastasis involving other sites) (HR = 0.688, P = 0.1131) were related to OS. Regarding laboratory measurements, hypercalcemia (HR = 2.616, P = 0.0010), anemia (HR = 2.214, P = 0.0008), thrombocytosis (HR = 2.704, P = 0.0009) and elevated C‐reactive protein (CRP) (HR = 2.158, P = 0.0013) were significantly associated with shorter OS. Elevated lactate dehydrogenase was not associated with OS (HR = 3.305, P = 0.0782) probably because only two patients (1%) exhibited this abnormality. Finally, the significant variables associated with OS on univariate analysis were entered into a stepwise Cox regression model, resulting in the following three factors: ECOG‐PS (HR = 3.665, P = 0.0004), the presence of MVI (HR = 6.428, P = 0.0005) and hypercalcemia (HR = 2.668, P = 0.0109) (Table 3). Survival curves for patients with or without those factors are shown in Figure 2(a–c).
Table 1.
Summary of patient characteristics
| Patients, number | 180 |
| Age, year, median (range) | 68 (40–85) |
| Sex, number (%) | |
| Male | 147 (81.7) |
| Female | 33 (18.3) |
| ECOG performance status, number (%) | |
| 0 | 161 (89.4) |
| 1 | 19 (10.6) |
| TNM classification, number (%) | |
| T | |
| T1 | 39 (21.7) |
| T2 or above | 138 (76.7) |
| Tx | 3 (1.7) |
| N | |
| N0 | 137 (76.1) |
| N1 or above | 38 (21.1) |
| Nx | 5 (2.8) |
| M | |
| M0 | 39 (21.7) |
| M1 | 138 (76.7) |
| Mx | 3 (1.7) |
| Histologic grade, number (%) | |
| G1 | 15 (8.3) |
| G2 | 109 (60.6) |
| G3 | 48 (26.7) |
| GX | 8 (4.4) |
| Site of metastases, number (%) | |
| Lung only | 120 (66.7) |
| Lung and other sites | 24 (13.3) |
| Other sites | 36 (20.0) |
Figure 1.
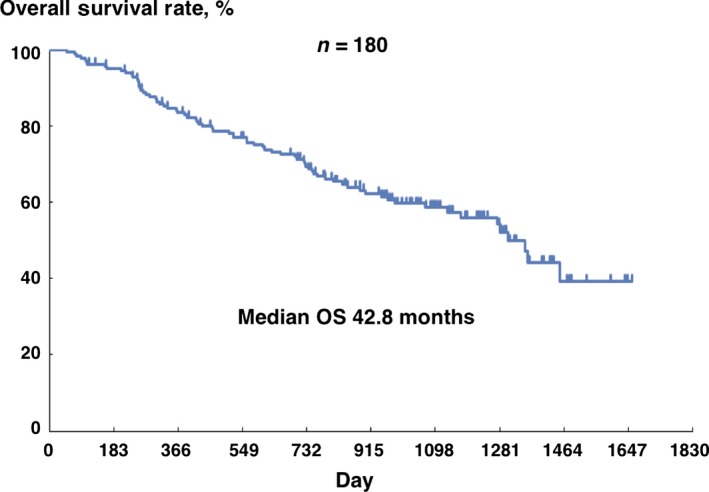
Overall survival of 180 patients with metastatic renal cell carcinoma treated with IFN‐α. Median overall survival (OS) is 42.8 month (95% confidence interval: 37.1‐NA). NA, not available.
Table 2.
Univariate analysis of association between patient demographics/clinicopathological parameters and overall survival
| Factors | N | Median overall survival (days) | Hazard ratio (95% confidence interval) | P‐value |
|---|---|---|---|---|
| Age | ||||
| ≥65 | 108 | 1306 | 0.981 (0.617–1.562) | 0.9363 |
| <65 | 72 | 1352 | ||
| ECOG‐PS | ||||
| 0 | 161 | 1352 | 2.728 (1.496–4.976) | 0.0006 |
| 1 | 19 | 466 | ||
| TNM classification | ||||
| T | ||||
| T1 | 39 | Not reached | 2.132 (1.060–4.291) | 0.0298 |
| T2 or above | 138 | 1274 | ||
| N | ||||
| N0 | 137 | 1453 | 2.092 (1.264–3.464) | 0.0033 |
| N1 or above | 38 | 807 | ||
| M | ||||
| M0 | 39 | 1453 | 1.961 (1.031–3.729) | 0.0365 |
| M1 | 138 | 1282 | ||
| Growth pattern | ||||
| Expansive | 107 | 1352 | 0.571 (0.334–0.977) | 0.0382 |
| Infiltrative | 34 | 736 | ||
| Grade | ||||
| 1 or 2 | 124 | 1453 | 2.587 (1.573–4.254) | 0.0002 |
| 3 | 48 | 728 | ||
| Sarcomatous component | ||||
| No | 169 | 1352 | 1.657 (0.666–4.124) | 0.2721 |
| Yes | 11 | 848 | ||
| Microscopic vascular Invasion | ||||
| No | 50 | Not reached | 3.322 (1.644–6.713) | 0.0004 |
| Yes | 112 | 1070 | ||
| Site of metastasis | ||||
| Lung only | 120 | Not reached | 0.688 (0.432–1.096) | 0.1131 |
| Others | 60 | 1274 | ||
| Lactate dehydrogenase | ||||
| <1.5×ULN | 168 | 1352 | 3.305 (0.807–13.542) | 0.0782 |
| ≥1.5×ULN | 2 | 619 | ||
| Corrected Ca (mg/dL) | ||||
| <10 | 136 | 1352 | 2.616 (1.442–4.744) | 0.0010 |
| ≥10 | 21 | 583 | ||
| Hemoglobin (mg/dL) | ||||
| ≤LLN | 89 | 1070 | 2.214 (1.372–3.573) | 0.0008 |
| >LLN | 87 | Not reached | ||
| C‐reactive protein (mg/dL) | ||||
| <0.3 | 81 | 1361 | 2.158 (1.334–3.491) | 0.0013 |
| ≥0.3 | 94 | 848 | ||
| Platelet count (/μL) | ||||
| <400 000 | 154 | 1361 | 2.704 (1.468–4.980) | 0.0009 |
| ≥400 000 | 22 | 524 | ||
Table 3.
Multivariate analysis of association between patient factorsand overall survival
| Factors | P‐value | Hazard ratio | 95% CI |
|---|---|---|---|
| ECOG‐PS (0 vs 1) | 0.0004 | 3.665 | 1.785–7.527 |
| Microscopic vascular invasion (No vs Yes) | 0.0005 | 6.428 | 2.254–18.332 |
| Corrected Ca (mg/dL) (<10 vs ≥10) | 0.0109 | 2.668 | 1.253–5.682 |
Figure 2.
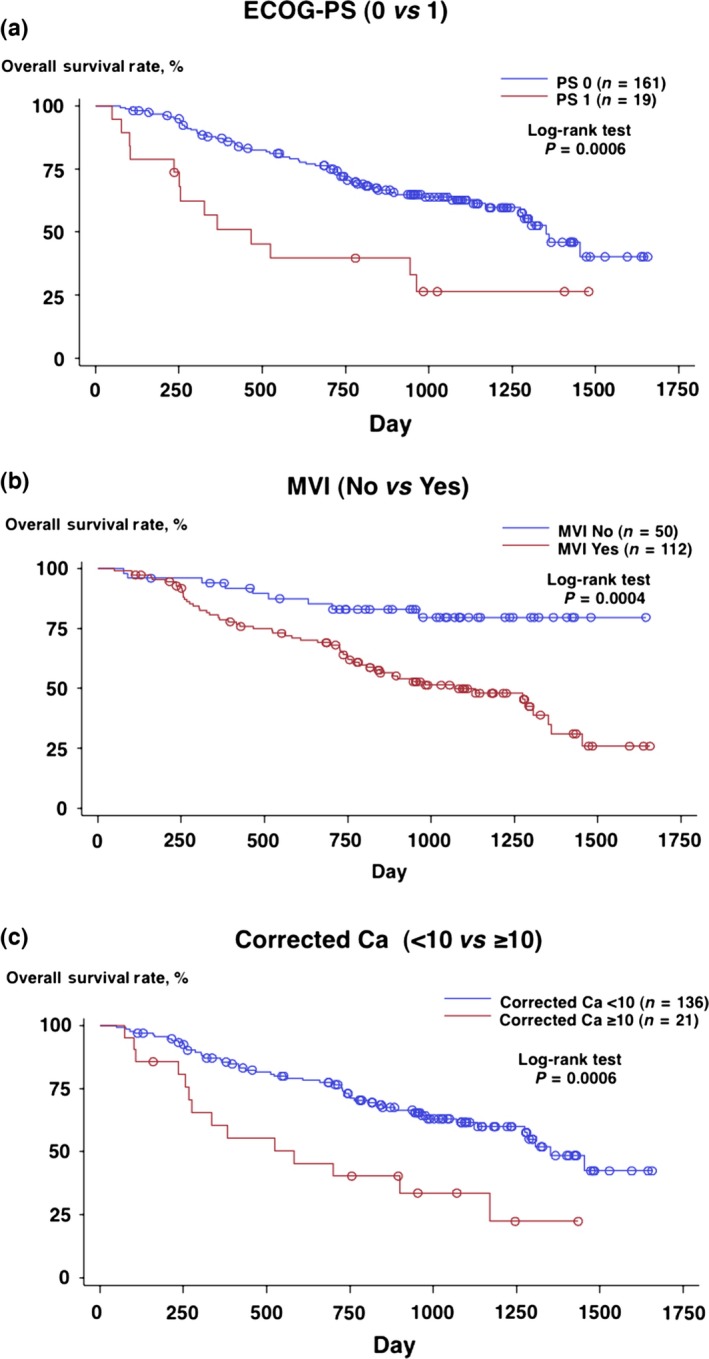
Overall survival of patients treated with IFN‐α: (a) ECOG‐PS 0 versus 1, (b) with versus without microscopic vascular invasion (MVI) and (c) corrected Ca++ <10 mg/dL versus 10 mg/dL or above.
Discussion
This is an extended analysis of the RCC‐SELECT study10 that confirmed the correlation between the clinical response of IFN‐α therapy for mRCC and a STAT3 SNP (rs1905341). We observed a marked improvement in patients' OS (42.8 months) when compared to patients' in studies in the cytokine era with either the same or different racial backgrounds.7, 9, 13 It is of note that the present study is based on the cohort of the first and hitherto largest prospective study on mRCC patients whose first‐line therapy was IFN‐α in the post‐cytokine era.12 This endorses the accuracy of the results for the following reasons: (i) the collection of precise data regarding the patient demographics and clinical parameters; and (ii) the careful follow‐up until events took place or the observation was finished. Although there are some differences in patient demographics (e.g. PS, metastasis sites and frequency of prior nephrectomy) between the previous and present studies in addition to study design, the greatest contributing factor is the emergence of molecular targeted agents. Because the patient enrollment of the original RCC‐SELECT study commenced in December 2006, only 18 months before the introduction of sorafenib and sunitinib for mRCC in Japan, it is highly likely that the vast majority of patients enrolled in this study benefited from those molecular targeted agents following first‐line IFN‐α therapy. Notably, Shinohara et al.14 report in their retrospective study that the median OS was 38.2 months in the mRCC patients with the same racial background whose first‐line therapy was cytokine in the era of molecular‐targeted therapy. This strongly supports the result of the present study that the prognosis of mRCC patients whose first‐line therapy was IFN‐α has been remarkably improved.
Intriguingly, in an extended analysis of the AXIS trial comparing axitinib versus sorafenib as a second‐line treatment for mRCC, patients previously treated with cytokines including IFN‐α exhibited significantly longer progression free survival and OS from the start of first‐line therapy than those previously treated with sunitinib in both axitinib and sorafenib treated groups.15 This suggests that first‐line IFN‐α therapy followed by VEGF‐TKI may provide longer survival than sequencing two different VEGF‐TKI in a substantial fraction of mRCC patients. Considering the result of the landmark trial comparing IFN‐α versus sunitinib by Motzer et al.16 such a result appears paradoxical. However, there are some possible explanations for this outcome: (i) tumors may have acquired resistance to VEGF‐TKI during first‐line sunitinib treatment, resulting in diminished efficacy with second‐line VEGF‐TKI;17 and (ii) administration of cytokines including IFN‐α provokes generation and persistence of memory T cells that can recognize tumor epitopes,18 and such memory T cells might be ignited by peptide released from dying tumor cells during VEGF‐TKI treatment,19 resulting in enhancement of anti‐cancer immunity.
In the present study, multivariate analysis revealed that PS, hypercalcemia and MVI are the independent prognostic factors. Hypercalcemia is the most frequent paraneoplastic complication of mRCC, being manifested in up to 20% of RCC patients during the course of their disease20, 21 and known to imply a poor prognosis because of its frequent association with disseminated disease. Previous reports suggested that humoral factors such as PTHrP,22 IL‐623 and prostaglandins24 may play roles in hypercalcemia either directly or indirectly by increasing bone resorption. Since Motzer et al.7 identified hypercalcemia as an independent prognostic factor in mRCC patients treated with IFN‐α, it has been included in other prognosis prediction models for mRCC from the cytokine era to the target therapy era.7, 25, 26, 27, 28 Interestingly, the largest retrospective analysis so far regarding prognostic factors for survival in mRCC patients treated with sunitinib revealed that hypercalcemia is the only factor among the MSKCC's criteria7 for OS in multivariate analysis.28 Therefore, hypercalcemia may be a prognostic factor for metastatic renal cell carcinoma regardless of the type of systemic therapy.
The importance of MVI as a prognostic factor in RCC is also well documented in the published literature.29, 30, 31 It is histologically obvious that MVI requires a local destruction of the venous wall by the tumor cells. However,, it is also significantly associated with other prognostic factors such as tumor size, Fuhrman grade, pathological stage and the presence of sarcomatoid elements.29, 30, 31 This suggests that MVI is not merely a manifestation of tumor cell entry into the circulation, but also an indicator of malignant potential. However, the previous studies indicating MVI as a prognostic factor were based on the analysis of patients with clinically localized RCC who underwent radical or partial nephrectomy, but not those with metastasis at the time of diagnosis. Conversely, in previous studies investigating survival of mRCC patients subjected to systemic therapy, MVI was not shown as a prognostic factor. This is probably because not all patients that participated in such studies underwent prior nephrectomy; therefore, analyses regarding the relationship between survival and histological details of tumors may not have been feasible. In this sense, this is the first study showing that MVI is a prognostic factor also in mRCC patients who received systemic therapy because it includes prior nephrectomy as a study criterion that enables analysis for correlation between histological factors such as MVI and overall survival in mRCC patients.
A limitation of the present study is that it only included patients with limited racial background. Hence, external validation with other racial backgrounds is needed. The present study suggests that IFN‐α is still a valid agent for mRCC for first‐line treatment even in the era of molecular targeted therapy. For such a treatment strategy, PS, hypercalcemia and MVI are independent predictors of survival, which will be particularly useful information for selecting appropriate patients. Although IFN‐α appeared inferior to molecular targeted agents in the previous clinical trial settings,16, 32, 33 we have to reconsider its actual efficacy and contemporary role in mRCC treatment in the context of sequential therapy with molecular targeted agents. Further studies are warranted to identify optimal combination of IFN‐α and following molecular targeted agents for attaining better outcomes.
Disclosure Statement
Yoshiaki Kawano has received honorarium from Pfizer. Masatoshi Eto has received honoraria from Bayer, Pfizer, Novartis, GlaxoSmithKline and research funding from Pfizer and Novartis. Tomomi Kamba has received honoraria from Pfizer, GlaxoSmithKline and Dainippon Sumitomo. Hideaki Miyake has received honoraria from Pfizer and Novartis. Takao Kamai has received honorarium from Pfizer. Hirotsugu Uemura has received honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline, research funding from Pfizer and Novartis, and fees for promotional materials from Pfizer, Bayer and Novartis. Taiji Tsukamoto has received honorarium from Pfizer. Kazuo Nishimura has received honoraria from Bayer, Pfizer and Novartis. Osamu Ogawa has received honoraria from Bayer, Pfizer, Novartis, GlaxoSmithKline and research funding from Pfizer and Novartis. Seiji Naito has served as a consultant for Ono and received honoraria from Pfizer, Novartis and GlaxoSmithKline. All remaining authors have no conflicts of interest to declare.
Acknowledgments
This study was supported in part by a grant‐in‐aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant No 16390466).
Cancer Sci 107 (2016) 1013–1017
Funding Information
Ministry of Education, Culture, Sports, Science and Technology, Japan (16390466).
Clinical Trial Registration Number: UMIN000000731.
References
- 1. Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008; 34: 193–205. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374–403. [DOI] [PubMed] [Google Scholar]
- 3. Athar U, Gentile TC. Treatment options for metastatic renal cell carcinoma: a review. Can J Urol 2008; 15: 3954–66. [PubMed] [Google Scholar]
- 4. Negrier S, Escudier B, Lasset C et al Recombinant human interleukin‐2, recombinant human interferon alfa‐2a, or both in metastatic renal‐cell carcinoma. Groupe Français d'Immunothérapie. N Engl J Med 1998; 338: 1272–8. [DOI] [PubMed] [Google Scholar]
- 5. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R, Group EOfRaToCEG . Radical nephrectomy plus interferon‐alfa‐based immunotherapy compared with interferon alfa alone in metastatic renal‐cell carcinoma: a randomised trial. Lancet 2001; 358: 966–70. [DOI] [PubMed] [Google Scholar]
- 6. Flanigan RC, Salmon SE, Blumenstein BA et al Nephrectomy followed by interferon alfa‐2b compared with interferon alfa‐2b alone for metastatic renal‐cell cancer. N Engl J Med 2001; 345: 1655–9. [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon‐alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002; 20: 289–96. [DOI] [PubMed] [Google Scholar]
- 8. Escudier B, Porta C, Schmidinger M et al Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014; 25(Suppl 3): iii49–56. [DOI] [PubMed] [Google Scholar]
- 9. Naito S, Yamamoto N, Takayama T et al Prognosis of Japanese metastatic renal cell carcinoma patients in the cytokine era: a cooperative group report of 1463 patients. Eur Urol 2010; 57: 317–25. [DOI] [PubMed] [Google Scholar]
- 10. Eto M, Kamba T, Miyake H, et al STAT3 polymorphism can predict the response to interferon‐α therapy in patients with metastatic renal cell carcinoma. Eur Urol 2013; 63: 745–52. [DOI] [PubMed] [Google Scholar]
- 11. Uemura H, Shinohara N, Yuasa T et al A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 2010; 40: 194–202. [DOI] [PubMed] [Google Scholar]
- 12. Ito N, Eto M, Nakamura E, et al STAT3 polymorphism predicts interferon‐alfa response in patients with metastatic renal cell carcinoma. J Clin Oncol 2007; 25: 2785–91. [DOI] [PubMed] [Google Scholar]
- 13. Shinohara N, Nonomura K, Abe T et al A new prognostic classification for overall survival in Asian patients with previously untreated metastatic renal cell carcinoma. Cancer Sci 2012; 103: 1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shinohara N, Obara W, Tatsugami K et al Prognosis of Japanese patients with previously untreated metastatic renal cell carcinoma in the era of molecular‐targeted therapy. Cancer Sci 2015; 106: 618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escudier B, Michaelson MD, Motzer RJ et al Axitinib versus sorafenib in advanced renal cell carcinoma: subanalyses by prior therapy from a randomised phase III trial. Br J Cancer 2014; 110: 2821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Motzer RJ, Hutson TE, Tomczak P et al Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med 2007; 356: 115–24. [DOI] [PubMed] [Google Scholar]
- 17. Motzer RJ, Escudier B, Tomczak P et al Axitinib versus sorafenib as second‐line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013; 14: 552–62. [DOI] [PubMed] [Google Scholar]
- 18. Gallagher KM, Lauder S, Rees IW, Gallimore AM, Godkin AJ. Type I interferon (IFN alpha) acts directly on human memory CD4+ T cells altering their response to antigen. J Immunol 2009; 183: 2915–20. [DOI] [PubMed] [Google Scholar]
- 19. Wiita AP, Hsu GW, Lu CM, Esensten JH, Wells JA. Circulating proteolytic signatures of chemotherapy‐induced cell death in humans discovered by N‐terminal labeling. Proc Natl Acad Sci USA 2014; 111: 7594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papworth K, Grankvist K, Ljungberg B, Rasmuson T. Parathyroid hormone‐related protein and serum calcium in patients with renal cell carcinoma. Tumour Biol 2005; 26: 201–6. [DOI] [PubMed] [Google Scholar]
- 21. Pepper K, Jaowattana U, Starsiak MD et al Renal cell carcinoma presenting with paraneoplastic hypercalcemic coma: a case report and review of the literature. J Gen Intern Med 2007; 22: 1042–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kao PC, Klee GG, Taylor RL, Heath H. Parathyroid hormone‐related peptide in plasma of patients with hypercalcemia and malignant lesions. Mayo Clin Proc 1990; 65: 1399–407. [DOI] [PubMed] [Google Scholar]
- 23. Paule B, Clerc D, Rudant C et al Enhanced expression of interleukin‐6 in bone and serum of metastatic renal cell carcinoma. Hum Pathol 1998; 29: 421–4. [DOI] [PubMed] [Google Scholar]
- 24. Robertson RP, Baylink DJ, Marini BJ, Adkison HW. Elevated prostaglandins and suppressed parathyroid hormone associated with hypercalcemia and renal cell carcinoma. J Clin Endocrinol Metab 1975; 41: 164–7. [DOI] [PubMed] [Google Scholar]
- 25. Escudier B, Choueiri TK, Oudard S et al Prognostic factors of metastatic renal cell carcinoma after failure of immunotherapy: new paradigm from a large phase III trial with shark cartilage extract AE 941. J Urol 2007; 178: 1901–5. [DOI] [PubMed] [Google Scholar]
- 26. Choueiri TK, Garcia JA, Elson P et al Clinical factors associated with outcome in patients with metastatic clear‐cell renal cell carcinoma treated with vascular endothelial growth factor‐targeted therapy. Cancer 2007; 110: 543–50. [DOI] [PubMed] [Google Scholar]
- 27. Heng DY, Xie W, Regan MM et al External validation and comparison with other models of the International Metastatic Renal‐Cell Carcinoma Database Consortium prognostic model: a population‐based study. Lancet Oncol 2013; 14: 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Motzer RJ, Escudier B, Bukowski R et al Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer 2013; 108: 2470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Poppel H, Vandendriessche H, Boel K et al Microscopic vascular invasion is the most relevant prognosticator after radical nephrectomy for clinically nonmetastatic renal cell carcinoma. J Urol 1997; 158: 45–9. [DOI] [PubMed] [Google Scholar]
- 30. Lang H, Lindner V, Letourneux H, Martin M, Saussine C, Jacqmin D. Prognostic value of microscopic venous invasion in renal cell carcinoma: long‐term follow‐up. Eur Urol 2004; 46: 331–5. [DOI] [PubMed] [Google Scholar]
- 31. Dall'Oglio MF, Antunes AA, Sarkis AS et al Microvascular tumour invasion in renal cell carcinoma: the most important prognostic factor. BJU Int 2007; 100: 552–5. [DOI] [PubMed] [Google Scholar]
- 32. Escudier B, Szczylik C, Hutson TE et al Randomized phase II trial of first‐line treatment with sorafenib versus interferon Alfa‐2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27: 1280–9. [DOI] [PubMed] [Google Scholar]
- 33. Hudes GR, Berkenblit A, Feingold J, Atkins MB, Rini BI, Dutcher J. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol 2009; 36(Suppl 3): S26–36. [DOI] [PubMed] [Google Scholar]


