Abstract
Asbestos‐induced mesothelial carcinogenesis is currently a profound social issue due to its extremely long incubation period and high mortality rate. Therefore, procedures to prevent malignant mesothelioma in people already exposed to asbestos are important. In previous experiments, we established an asbestos‐induced rat peritoneal mesothelioma model, which revealed that local iron overload is a major cause of pathogenesis and that the induced genetic alterations are similar to human counterparts. Furthermore, we showed that oral administration of deferasirox modified the histology from sarcomatoid to the more favorable epithelioid subtype. Here, we used i.p. administration of desferal to evaluate its effects on asbestos‐induced peritoneal inflammation and iron deposition, as well as oxidative stress. Nitrilotriacetate was used to promote an iron‐catalyzed Fenton reaction as a positive control. Desferal significantly decreased peritoneal fibrosis, iron deposition, and nuclear 8‐hydroxy‐2′‐deoxyguanosine levels in mesothelial cells, whereas nitrilotriacetate significantly increased all of them. Desferal was more effective in rat peritoneal mesothelial cells to counteract asbestos‐induced cytotoxicity than in murine macrophages (RAW264.7). Furthermore, rat sarcomatoid mesothelioma cells were more dependent on iron for proliferation than rat peritoneal mesothelial cells. Because inflammogenicity of a fiber is proportionally associated with subsequent mesothelial carcinogenesis, iron elimination from the mesothelial environment can confer dual merits for preventing asbestos‐induced mesothelial carcinogenesis by suppressing inflammation and mesothelial proliferation simultaneously.
Keywords: Asbestos, chelator, desferal, iron, mesothelioma
Asbestos is a natural silicate mineral. During the last century, huge amounts have been used in industry due to its excellent characteristics of resistance to heat, acid, and friction with flexibility and economic merits as a material.1, 2 However, fine asbestos fibers are present in the air of mines, factories, or construction workplaces and can pass through the respiratory system to the lung parenchyma and, finally, after decades, to the parietal pleura of affected personnel, in the event that proper preventive strategies were not taken. In the 1960s, epidemiologists already recognized the association between asbestos exposure and malignant mesothelioma (MM).2 Despite a variety of efforts, MM remains among those tumors with the poorest prognosis when diagnosed. Its incidence is increasing worldwide, but it is resistant to currently available therapeutic methods.3, 4, 5 Therefore, it is necessary to find efficient strategies to prevent, slow, and cure MM.
We were therefore prompted to elucidate the carcinogenic mechanism of asbestos‐induced MM. Our previous preclinical studies revealed that the pathogenesis of asbestos‐induced mesothelial carcinogenesis is closely associated with local iron overload,6 which promotes the Fenton reaction and leads to oxidative DNA damage and, finally, to a variety of mutations. Iron overload has generally been associated with carcinogenesis.7, 8, 9, 10 Simple i.p. injection of each one of the three kinds of commercially used asbestos (chrysotile, crocidolite, and amosite) efficiently causes peritoneal MM in rats, with extremely similar genomic alterations and prominent iron deposition.6 Irrespective of the types of asbestos, postadministration of nitrilotriacetate (NTA; iron chelating agent to promote the Fenton reaction)11, 12 significantly shortened the periods of carcinogenesis, confirming the major role of iron in this process.13, 14 In the case of chrysotile, which does not include iron in itself, its hemolytic activity and the subsequent adsorption of hemoglobin were important,15 in addition to the general affinity of asbestos to histones.16 We also reported that i.p. injection of iron saccharate with NTA in rats induced MM and homozygous deletion of the Cdkn2a/2b tumor suppressor gene, supporting the hypothesis that iron overload is responsible for asbestos‐induced mesothelial carcinogenesis.17
Based on these findings, we recently used an oral iron chelating agent, deferasirox, to prevent MM in a rat peritoneal MM model, and we found that this preventive treatment leads to a favorable histological transition of MM from the sarcomatoid subtype to the epithelioid subtype.18 Here we used a classic iron‐chelating agent, desferal (desferrioxamine), to evaluate whether this treatment is useful for the prevention of MM after exposure to asbestos using rat and cell culture models.
Materials and Methods
Chemicals
Desferal was purchased from Novartis Pharma (Basel, Switzerland), and NTA disodium salt was purchased from Nacalai Tesque (Kyoto, Japan). Holotransferrin (iron‐saturated transferrin, human) was from Sigma (St. Louis, MO, USA).
Asbestos and animals
Chrysotile and crocidolite fibers from the Union for International Cancer Control (Geneva, Switzerland) were suspended in physiological saline (5 mg/mL) and autoclaved prior to injection into rats. The suspension of asbestos was sonicated for 30 min immediately before use to prevent the aggregation of the fibers. We used another iron chelator, NTA,19 to promote the Fenton reaction12, 20 with the dose previously described6, 21 for a comparison with the effects of desferal. Thirty‐five 6‐week‐old specific pathogen‐free male Wistar rats (Shizuoka Laboratory Animal Center, Shizuoka, Japan) were divided into the following seven groups of five animals each: saline treatment of the same volume as asbestos suspension, as controls; chrysotile treatment; chrysotile and NTA treatment; chrysotile and desferal treatment; crocidolite treatment; crocidolite and NTA treatment; and crocidolite and desferal treatment. The asbestos‐treated groups received two i.p. injections of 5 mg chrysotile or crocidolite (total 10 mg) during the first week. Nitrilotriacetate (80 mg/kg) or desferal (100 mg/kg) were injected i.p. once a week for a total of 5 weeks, starting from the second week after the asbestos injection (Fig. 1a). The animals were euthanized 24 h after the last injection of NTA/desferal or at the corresponding time, and autopsies were carried out. The animal experiment committee of Nagoya University Graduate School of Medicine (Nagoya, Japan) approved this experiment.
Figure 1.

Body weights and macroscopic view of peritoneal cavity after asbestos injection in rats in the presence or absence of iron chelators. (a) Body weight changes. (b) Macroscopic peritoneal appearance 5 weeks after injection of asbestos. Intraperitoneal injection of asbestos caused chronic inflammation and fibrosis in the peritoneal cavity, which was aggravated by nitrilotriacetate (NTA) (arrows show deformation of edge of hepatic lobes, indicating severe serosal fibrosis) and ameliorated by desferal. n.s., statistically not significant (means ± SEM) *P < 0.05.
Histological and immunohistochemical analyses
The tissues were fixed with 10% buffered neutral formalin solution. After dehydration, tissue specimens were subjected to paraffin embedding, cut to a 3‐μm thickness and stained with HE, Masson trichrome for fibrosis, or Perl's iron staining. For immunohistochemical analyses, the avidin–biotin complex method with peroxidase was used, and quantitative analyses were undertaken both for areas and integrated density as described22 except for ImageJ was used (https://imagej.nih.gov/ij/) as a software (previously NIH image was used).
Antibodies
An anti‐8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) mouse mAb N45.1 (Nikken Seil, Fukuroi, Japan) was used to evaluate oxidative stress at the cellular level with immunohistochemistry.22
Fibrosis index
The fibrosis index was determined as previously described.23
Serum ferritin assay
Commercial kits were used to determine the levels of rat serum ferritin (PRA011; Mitsubishi Chemical Safety Institute Ltd, Uto, Japan).
Cell culture
Rat peritoneal mesothelial cells (RPMC) and murine macrophages (RAW264.7) were cultured in RPMI‐1640 medium containing 10% FBS as previously described.23 Malignant mesothelioma cell lines from rats were established 24 and cultured as described.25
Viable cell evaluation
Viable cells were either counted by Trypan blue dye exclusion or quantified with an MTT assay (Sigma). The cells were counted 48 h after exposure to desferal with simultaneous pretreatment with different concentrations of holotransferrin using Trypan blue and expressed as percentages of control cell counts.
Statistics
Data are shown as the means ± SEM. We used an unpaired t‐test, which was modified for unequal variances when necessary. Statistical analyses were carried out with Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
Results
Protocol for animal experiments and body weight
To optimize the experimental protocol for rats, we carried out a preliminary 5‐week experiment to determine the maximal non‐toxic dose of desferal in order to avoid false negative results. Based on the results of the preliminary experiment, we injected rats with 100 mg/kg desferal i.p. once a week for a total of 5 weeks. We weighed the rats weekly and observed that repeated NTA treatment after asbestos injection caused body weight reduction in both the chrysotile and crocidolite groups (Fig. 1a), which could be attributed to the promotion of the Fenton reaction. In contrast, desferal treatment did not result in significant difference in body weight from the saline control group (Fig. 1a).
Desferal quenches asbestos‐induced inflammation of somatic cavity
Asbestos injection with or without repeated NTA post‐treatment constantly caused severe fibrous peritonitis, inducing dull edge and spherization of the lobes of liver (Fig. 1b). In contrast, post‐treatment with desferal after asbestos injection counteracted these effects (Fig. 1b). Notably, rats treated with desferal after asbestos injection showed markedly reduced fibrosis on the serosal surface, whereas NTA enhanced it (Fig. 2a). For quantitation, we calculated the fibrosis index, which confirmed that the findings are statistically significant (Fig. 2b).
Figure 2.
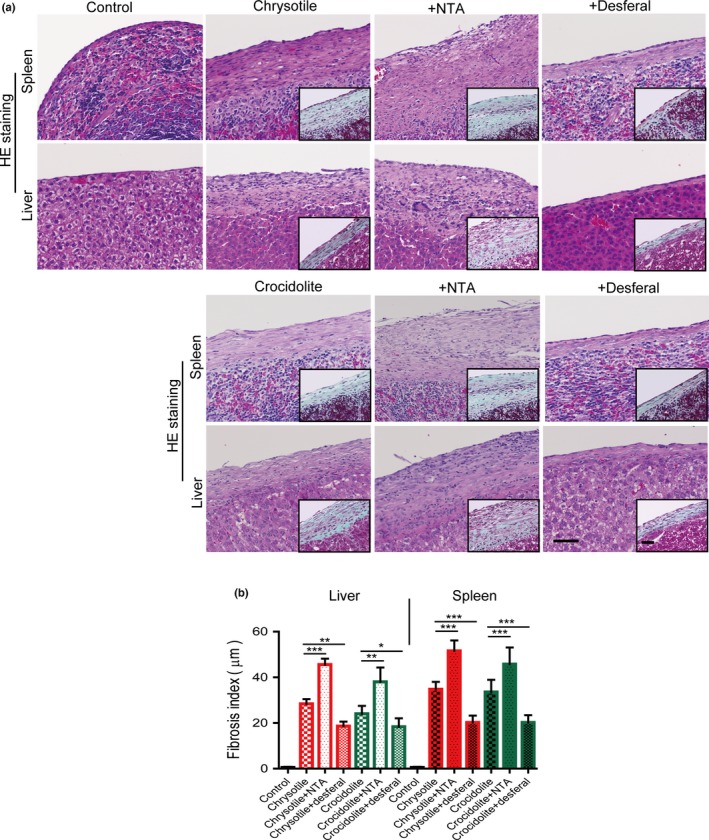
Microscopic findings of serosal surface of the spleen and liver in rats 5 weeks after asbestos injection in the presence or absence of iron chelators and fibrosis index. (a) Typical observation with HE and Masson trichrome (insets) staining (bar = 50 μm). (b) Fibrosis index was calculated by measuring the distance from the peritoneal surface to the parenchyma of spleen or liver (means ± SEM). *P < 0.05; **P < 0.01; ***P < 0.001. NTA, nitrilotriacetate.
Desferal reduces asbestos‐induced local iron deposition
Perl's iron staining on tissue specimens showed that desferal reduced stainable iron on the serosal surface of the liver and spleen, whereas NTA increased iron deposits (Fig. 3a). Asbestos injection caused an increase in the serum ferritin level compared to the control, which was further increased by NTA treatment, though desferal post‐treatment reduced it (Fig. 3b).
Figure 3.

Quantitation of iron deposition on the surface of the spleen and liver and serum ferritin levels in rats 5 weeks after asbestos injection. (a) Iron deposition (hemosiderin) by Perl's iron staining was observed on the surface of the spleen and liver 5 weeks after asbestos injection, which was aggravated by nitrilotriacetate (NTA) and ameliorated by desferal (bar = 50 μm). (b) Serum ferritin levels were in proportional association with iron deposition (means ± SEM). *P < 0.05; **P < 0.01; ***P < 0.001.
Desferal inhibits asbestos‐induced 8‐OHdG generation in mesothelial cells
After i.p. injection of chrysotile or crocidolite, nuclear immunostaining of 8‐OHdG was observed in the mesothelia covering the spleen and liver (Fig. 4a). Desferal post‐treatment decreased immunostaining, whereas NTA increased it (Fig. 4a). Quantitation of 8‐OHdG immunohistochemistry on mesothelial cells confirmed statistical significance among each group with the analysis of both area and integrated density (Fig. 4b,c).
Figure 4.
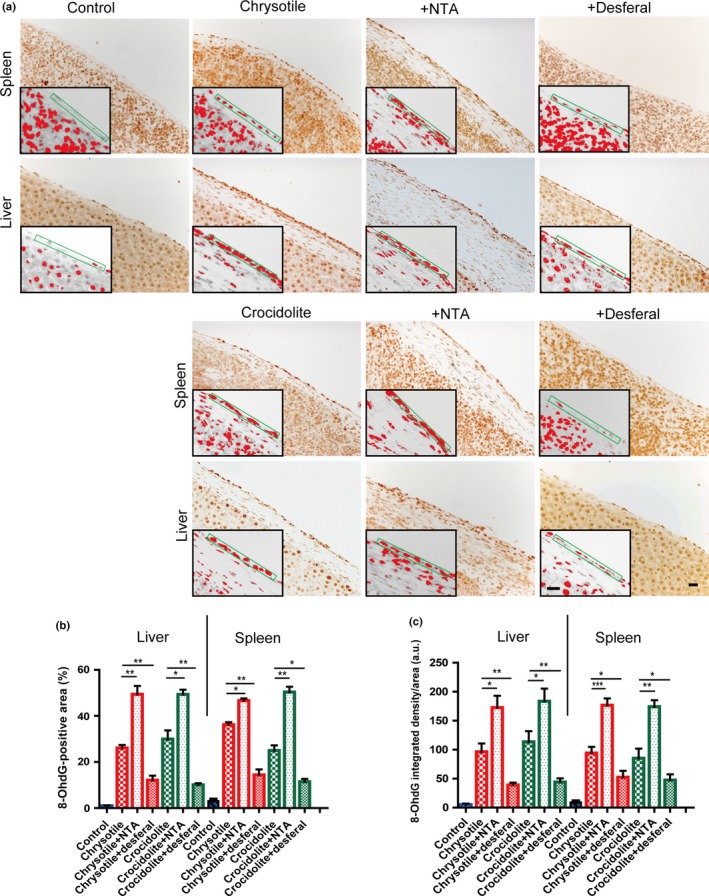
Immunohistochemical analysis of 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in rat peritoneal mesothelial cells after asbestos injection in the presence or absence of iron chelators. (a) Nuclear immunostaining of surface mesothelial cells were observed 5 weeks after asbestos injection, which was aggravated by nitrilotriacetate (NTA) and ameliorated by desferal (bar = 50 μm). (b,c) Quantitative analysis of immunostained area (b) and integrated density per area (c) (means ± SEM). *P < 0.05; **P < 0.01; ***P < 0.001. a.u., arbitrary unit.
Desferal decreases asbestos‐induced cytotoxicity in mesothelial cells but not in macrophages
We evaluated cell viability after asbestos exposure by MTT assay in RPMC and RAW264.7. Desferal protected mesothelial cells from asbestos‐induced cell injury, but this effect was not observed in macrophages (Fig. 5).
Figure 5.
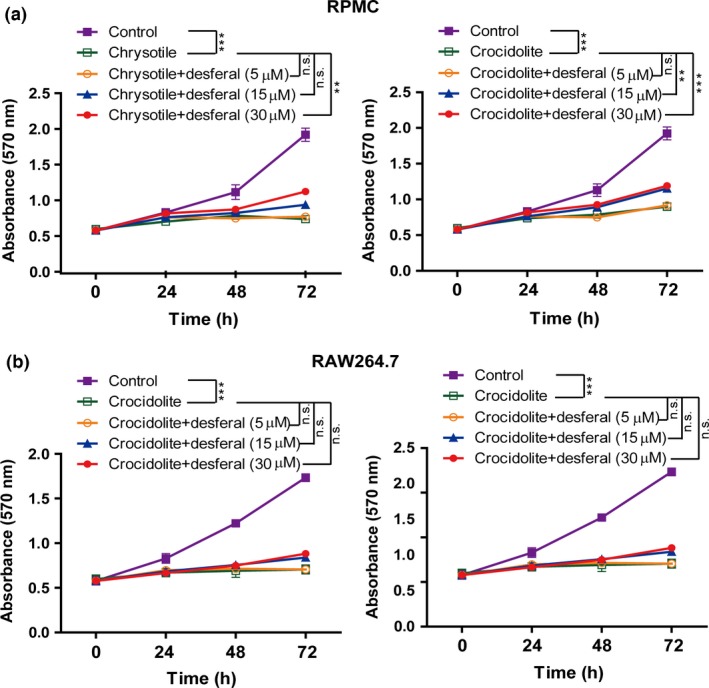
Differential effect of desferal on asbestos‐induced cytotoxicity between rat peritoneal mesothelial cells (RPMC) and murine macrophages (RAW264.7) by MTT assay. (a) Dose‐dependent effects on mesothelial cells. (b) Effects on macrophage cells. Means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., statistically not significant.
Desferal inhibits proliferation of MM, but not mesothelial cells
We carried out MTT assay in vitro using RPMC and two asbestos‐induced rat MM cell lines, SM1 and SM2 derived from the sarcomatoid subtype,25 for comparison. Desferal inhibited the growth of MM cells in a time‐ and dose‐dependent manner (Fig. 6a). Two MM cell lines revealed similar results after 48‐h treatment with 15–30 μM desferal. However, no similar effects were observed in RPMC after treatment with desferal.
Figure 6.
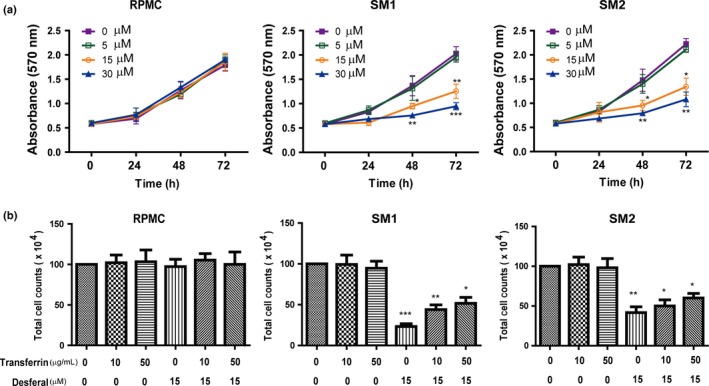
Antiproliferative effects of desferal on malignant mesothelioma (MM) cells and its modulation by holotransferrin. (a) Desferal dose‐dependently inhibited the proliferation of asbestos‐induced rat MM cell lines (SM1 and SM2) but not rat peritoneal mesothelial cells (RPMC). (b) Pretreatment with holotransferrin partially reduced the inhibitory effects of desferal in MM cells. SM, sarcomatoid mesothelioma.
In order to determine whether the inhibition of MM proliferation by desferal is iron‐dependent, we treated the cells with 10 or 50 μg/mL holotransferrin with or without desferal (Fig. 6b). Cytostatic effects of desferal were partially ameliorated by the pretreatment with holotransferrin in SM cell lines. Pretreatment with holotransferrin alone showed no effects on cell growth of mesothelial or MM cells (Fig. 6b).
Discussion
In our previous chemoprevention experiment against asbestos‐induced mesothelial carcinogenesis in rats, deferasirox (given orally) significantly induced mesenchymal epithelial transition in MM to a favorable histological subtype.18 Thus, iron removal therapy was shown to be effective in asbestos‐induced mesothelial carcinogenesis. However, due to potential side‐effects of renal toxicity after repeated treatment, it is not ethically easy to apply this to humans who have been already exposed to asbestos.26, 27 In the present study, we used another iron chelating agent, desferal, for this purpose.
Desferal has been clinically used since the 1970s by the parenteral route for patients with iron overload, resulting from a variety of pathologies such as genetic hemochromatosis28 and repeated transfusion.29 We used peritoneal injections of asbestos to induce mesothelial carcinogenesis in the peritoneal cavity of rats.6 In combination with in vitro experiments using cultured cells, our results here indicate dual merits of desferal against asbestos‐induced mesothelial carcinogenesis, including inhibition of carcinogenesis and of the proliferation of established MM.
In our subacute experiments using rats, desferal significantly prevented asbestos‐induced fibrosis of the peritoneal surface on the spleen and liver. Concomitantly, local iron deposition, serum ferritin representing general iron stores, and 8‐OHdG levels of mesothelial cells were significantly reduced with desferal treatment. We also injected another iron chelating agent with an opposing effect, NTA, to enhance the iron‐catalyzed Fenton reaction and compared the observed effects to those obtained with desferal. The results of NTA injection were opposite to those obtained with desferal, suggesting that iron deposition and inflammation are closely associated with fiber‐induced carcinogenesis. Of note, our previous findings using various carbon nanotubes of different diameters indicated that the degree of fibrosis and iron deposits observed in subacute experiments is predictive of subsequent mesothelial carcinogenesis.23, 30
Mechanistically, desferal was also effective against asbestos‐induced toxicity in mesothelial cells in vitro, but not in macrophages. This suggests that the iron uptake and/or inhibition of iron release are key for asbestos‐mediated cytotoxicity in mesothelial cells and that resistance to asbestos‐induced cytotoxicity differs between mesothelial cells and macrophages, presumably due to the difference in iron metabolism.
Additionally, desferal inhibited the proliferation of MM cells. This was not observed in non‐transformed mesothelial cells and was at least partially recovered by iron supplementation with holotransferrin. Accordingly, more iron is required for neoplastic growth than for non‐neoplastic cells.7 Indeed, desferal has been suggested as an anticancer agent in several cancers, including breast cancer.31, 32
In conclusion, desferal presents anticarcinogenic potential against asbestos‐induced carcinogenesis from two distinct aspects, anti‐inflammation and antiproliferation. Unfortunately, there is a limitation in terms of application of this technique to humans due to the invasive nature of intrapleural administration. However, this technique may be applied intrapleurally at the diagnostic biopsy of pleura under thoracoscopy, or i.v. desferal may be used with other conventional chemotherapy or radiation. Further studies are warranted to determine the preventive in vivo activity of desferal against asbestos‐induced mesothelial carcinogenesis.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported, in part, by the National Cancer Center Research and Development Fund (25‐A‐5), Grants‐in‐aid for Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24390094; 221S0001‐04; 24108008), and the Yasuda Medical Foundation. The authors wish to thank Nobuaki Misawa for excellent technical assistance with the pathologic specimens.
Cancer Sci 107 (2016) 908–915
Funding Information: National Cancer Center; Ministry of Education, Culture, Sports, Science and Technology of Japan; Yasuda Medical Foundation
References
- 1. Roggli VL, Oury TD, Sporn TA. (eds.). Pathology of asbestos‐associated diseases. 2 edn New York: Springer Verlag, 2004. [Google Scholar]
- 2. IARC, WHO . Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens; Part C: Arsenic, Metals, Fibres, and Dusts. Lyon, France, 2012; 219–309.
- 3. Robinson B, Lake R. Advances in malignant mesothelioma. N Engl J Med 2005; 353: 1591–603. [DOI] [PubMed] [Google Scholar]
- 4. Robinson B, Musk A, Lake R. Malignant mesothelioma. Lancet 2005; 366: 397–408. [DOI] [PubMed] [Google Scholar]
- 5. Thompson JK, Westbom CM, Shukla A. Malignant mesothelioma: development to therapy. J Cell Biochem 2014; 115: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang L, Akatsuka S, Nagai H et al Iron overload signature in chrysotile‐induced malignant mesothelioma. J Pathol 2012; 228: 366–77. [DOI] [PubMed] [Google Scholar]
- 7. Toyokuni S. Iron‐induced carcinogenesis: the role of redox regulation. Free Radic Biol Med 1996; 20: 553–66. [DOI] [PubMed] [Google Scholar]
- 8. Toyokuni S. Oxidative stress and cancer: the role of redox regulation. Biotherapy 1998; 11: 147–54. [DOI] [PubMed] [Google Scholar]
- 9. Toyokuni S. Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep 2002; 7: 189–97. [DOI] [PubMed] [Google Scholar]
- 10. Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 2009; 100: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson RL, Bishop WE, Campbell RL. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. Crit Rev Toxicol 1985; 15: 1–102. [DOI] [PubMed] [Google Scholar]
- 12. Toyokuni S, Sagripanti JL. Iron‐mediated DNA damage: sensitive detection of DNA strand breakage catalyzed by iron. J Inorg Biochem 1992; 47: 241–8. [DOI] [PubMed] [Google Scholar]
- 13. Toyokuni S. Mechanisms of asbestos‐induced carcinogenesis. Nagoya J Med Sci 2009; 71: 1–10. [PMC free article] [PubMed] [Google Scholar]
- 14. Toyokuni S. Iron overload as a major targetable pathogenesis of asbestos‐induced mesothelial carcinogenesis. Redox Rep 2013; 19: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagai H, Ishihara T, Lee WH et al Asbestos surface provides a niche for oxidative modification. Cancer Sci 2011; 102: 2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kubo Y, Takenaka H, Nagai H, Toyokuni S. Distinct affinity of nuclear proteins to the surface of chrysotile and crocidolite. J Clin Biochem Nutr 2012; 51: 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Q, Akatsuka S, Yamashita Y et al Homozygous deletion of CDKN2A/2B is a hallmark of iron‐induced high‐grade rat mesothelioma. Lab Invest 2010; 90: 360–73. [DOI] [PubMed] [Google Scholar]
- 18. Nagai H, Okazaki Y, Chew SH, Misawa N, Yasui H, Toyokuni S. Deferasirox induces mesenchymal‐epithelial transition in crocidolite‐induced mesothelial carcinogenesis in rats. Cancer Prev Res 2013; 6: 1222–30. [DOI] [PubMed] [Google Scholar]
- 19. Anderson C, Danylchuk KD. The effect of chronic administration of trisodium nitrilotriacetate (Na3NTA) on the haversian remodelling system in dogs. J Environ Pathol Toxicol 1979; 3: 413–20. [PubMed] [Google Scholar]
- 20. Toyokuni S, Sagripanti J‐L. DNA single‐ and double‐strand breaks produced by ferric nitrilotriacetate in relation to renal tubular carcinogenesis. Carcinogenesis 1993; 14: 223–7. [DOI] [PubMed] [Google Scholar]
- 21. Okada S, Hamazaki S, Toyokuni S, Midorikawa O. Induction of mesothelioma by intraperitoneal injections of ferric saccharate in male Wistar rats. Br J Cancer 1989; 60: 708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toyokuni S, Tanaka T, Hattori Y et al Quantitative immunohistochemical determination of 8‐hydroxy‐2′‐deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate‐induced renal carcinogenesis model. Lab Invest 1997; 76: 365–74. [PubMed] [Google Scholar]
- 23. Nagai H, Okazaki Y, Chew S et al Diameter of multi‐walled carbon nanotubes is a critical factor in mesothelial injury and subsequent carcinogenesis. Proc Natl Acad Sci U S A 2011; 108: E1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Usami N, Fukui T, Kondo M et al Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients. Cancer Sci 2006; 97: 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang L, Yamashita Y, Chew SH et al Connective tissue growth factor and β‐catenin constitute an autocrine loop for activation in rat sarcomatoid mesothelioma. J Pathol 2014; 233: 402–14. [DOI] [PubMed] [Google Scholar]
- 26. Kontoghiorghes GJ. Deferasirox: uncertain future following renal failure fatalities, agranulocytosis and other toxicities. Expert Opin Drug Saf 2007; 6: 235–9. [DOI] [PubMed] [Google Scholar]
- 27. Dubourg L, Laurain C, Ranchin B et al Deferasirox‐induced renal impairment in children: an increasing concern for pediatricians. Pediatr Nephrol 2012; 27: 2115–22. [DOI] [PubMed] [Google Scholar]
- 28. Tavill AS. Diagnosis and management of hemochromatosis. Hepatology 2001; 33: 1321–8. [DOI] [PubMed] [Google Scholar]
- 29. Propper RD, Cooper B, Rufo RR et al Continuous subcutaneous administration of deferoxamine in patients with iron overload. N Engl J Med 1977; 297: 418–23. [DOI] [PubMed] [Google Scholar]
- 30. Nagai H, Okazaki Y, Chew SH et al Intraperitoneal administration of tangled multiwalled carbon nanotubes of 15 nm in diameter does not induce mesothelial carcinogenesis in rats. Pathol Int 2013; 63: 457–62. [DOI] [PubMed] [Google Scholar]
- 31. Hoke EM, Maylock CA, Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radical Biol Med 2005; 39: 403–11. [DOI] [PubMed] [Google Scholar]
- 32. Rao VA. Iron chelators with topoisomerase‐inhibitory activity and their anticancer applications. Antiox Redox Signal 2013; 18: 930–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


