Abstract
Objectives
Retrospective analysis of evolution of HIV tropism and association with disease progression in perinatal HIV-1 infection (PaHIV).
Methodology
Eligible patients with PaHIV were grouped as slow, rapid or long-term non-progressors (LTNP). The V3 region of gp120 was sequenced from stored plasma samples and tropism determined by geno2pheno algorithm (FPR 5.75%). Logistic regression with generalised estimating equations assessed factors associated with R5 virus. Time to tropism change was assessed using standard survival methods.
Results
At baseline (n=48) median age was 12 years (IQR 9.3–14.8), 52% were female, 79% were Black African, 96% were non-B subtypes and 81% (39/48) had R5-using virus. Median follow-up was 7.7 years (308.6 person-years), with a median of five (range 1–14) samples per subject (total 252). Analysing all samples, R5 virus was associated with higher current CD4 cell count (median 520 cells/mm3 R5 vs 202 for X4, P=0.0005), LTNP (35% vs 11%, P=0.05), non-Black ethnicity (74% vs 89%, P=0.05) and female gender (55% vs 28%, P=0.005). Twelve of 38 (31%) with R5 virus at baseline switched to X4/dual-using virus, with an estimated 5-year risk of switch of 24.4% (95% CI 9.7–39.2%) predicted by lower current CD4 cell count (unadjusted HR 0.62/50 cells higher, 95% CI 0.47–0.81, P=0.0006). Eleven of 19 (58%) with X4/dual-using virus subsequently had R5 virus at one or more time points.
Conclusion
Maraviroc was a treatment option for 81% at 12 years, falling to 56% at 18 years, with lower CD4 cell count predictive of co-receptor switching. Paediatric studies of CCR5 antagonists should be expedited to ensure they are an early treatment option before tropism switching occurs.
Keywords: co-receptor tropism, perinatal HIV, CCR5 antagonist, children, adolescents
Introduction
HIV cell entry requires binding of the viral gp120 envelope protein to a CD4 receptor and to a chemokine co-receptor, usually either CCR5 or CXCR4. The majority of infants with perinatally acquired HIV-1 infection are infected with viral strains that use the CCR5 chemokine co-receptor (termed R5 virus) to gain cell entry, although occasional infants have been described with viruses that use the CXCR4 co-receptor (termed X4 virus) [1,2]. Similarly, in horizontally infected adults R5 virus is typically the transmitted strain, with the emergence of X4 virus and/or dual tropic R5/X4 virus frequently associated with higher plasma HIV viral loads, lower CD4 cell counts and faster disease progression [3–5]. Whether the emergence of X4 virus is the cause of, or secondary to, immune destruction and the impact of antiretroviral therapy (ART) remains unclear. Much of the data available in adults is for B clade viral strains; however, the majority of perinatally infected children worldwide harbour non-B clade virus and there is some evidence in adult populations suggesting viral tropism differs between clades. With disease progression X4 virus is reported to occur in approximately 50% of adults with subtype B, with lower rates reported for Clade C (0–30%), the commonest HIV subtype globally, accounting for almost half of all infections [6–12].
Paediatric data on the evolution of viral tropism and disease progression are sparse. Cross-sectional analysis of a South African paediatric cohort with predominantly clade C subtypes found 54% of those failing ART had X4 or dual tropic X4/R5-using virus compared to 10% of ART-naïve children; however, the median ages were 7.9 years compared to 0.9 years, respectively [13]. In a cross-sectional analysis of a treatment-experienced, Spanish, perinatally infected adolescent cohort, more than 80% had X4/dual tropic variants [14]. However, longitudinal data assessing the natural history of evolution of X4 virus in paediatric cohorts are lacking and this is of increasing importance with the development of CCR5 antagonists that inhibit HIV-1 replication as a component of ART. Maraviroc is the only CCR5 antagonist currently licensed for use in adults, and paediatric pharmacokinetic safety and efficacy studies in treatment-experienced populations are currently enrolling (NCT00791700). CCR5 antagonists reduce plasma HIV viral load when CCR5 viral strains are present but are not active against X4 and dual tropic R5/X4 populations. Further, the impact of ART on co-receptor switching is unclear, with successful ART in a small number of children (n=6) with X4 virus at baseline associated with the isolation of CCR5 viral strains from persistent CD4 Tcell reservoirs, suggesting theoretically that ART may influence co-receptor usage in the predominant viral strain [12]. Conversely the use of CCR5 antagonists has been associated with the emergence of X4 strains in patients with mixed populations, with the theoretical potential for more rapid disease progression [15].
Children infected perinatally with HIV-1, with access to ART, are now surviving through adolescence into adulthood. The majority require ART by early childhood, with current WHO guidelines recommending ART in all under fives irrespective of CD4 cell count [16]. With current clinical practice, these individuals are expected to remain on therapy for the foreseeable future, potentially for life [17]. Determining the optimal sequential use of antiretrovirals has become a priority and the most appropriate use of currently available antiretroviral classes, including CCR5 antagonists, requires a clearer understanding of the natural history of the dynamics of co-receptor tropism usage in this population.
This study investigates the evolution of co-receptor usage over time in a perinatally infected paediatric population and includes both ART-experienced and naïve children by retrospective analysis of viral tropism in stored plasma samples over a 10-year period.
Methods
Subjects
Fifty adolescents with perinatally acquired HIV-1 infection were recruited from the ‘Family’ and ‘900’ clinics at St Mary's Hospital, Imperial College Healthcare NHS Trust, London, UK between May 2012 and May 2013. Subjects were selected on criteria that included: aged 10 years or older; confirmed perinatally acquired HIV-1 infection by positive HIV DNA PCR prior to their 10th birthday; positive maternal HIV status; and to have been in follow-up at the study centre for at least 5 years with stored plasma samples available.
Baseline data collection
Baseline data collected were retrieved from patient records and included: age, ethnic group, weight and height, presenting history, nadir CD4 cell count and viral load, CDC disease status, ART history, HIV-1 protease and reverse transcriptase resistance-associated mutations, hepatitis B/C status, CD4 cell count and viral load in response to therapy. Subjects were assigned to a clinical group dependent on the history and age of ART exposure:
-
♦
Group 1: ART initiated after 10th birthday
-
♦
Group 2: ART initiated prior to 5th birthday
-
♦
Group 3: ART naïve at study recruitment
Three clinical groups were selected using age at starting ART as a surrogate marker of disease progression in an era when commencement of ART was driven by CD4 cell count thresholds/clinical disease rather than current universal treatment of all diagnosed infants. Group 2 included children with rapidly progressive disease, presenting and requiring ART in the pre-school years; Group 1 included those with slower disease progression who had survived until at least 10 years of age without ART; and Group 3 those who had yet to require ART on current UK guidance of treatment at CD4 count thresholds of 350 cells/mm3.
Sampling
Stored samples, previously submitted for routine clinical monitoring plasma viral load assay, were identified that had a detectable viral load >500 copies/mL prior to ART or at time of virological failure. Samples were analysed as follows:
-
♦
Groups 1 and 2: yearly prior to treatment initiation and at subsequent time points of virological failure
-
♦
Group 3: at 2-yearly intervals
Additionally all participants had a ‘current’ tropism assay performed at recruitment/latest clinic appointment, using HIV-1 proviral DNA for those suppressed or with low level viraemia (<500 copies/mL) and using plasma RNA if the viral load was greater than 500 copies/mL.
Genotypic analysis of HIV-1 tropism
HIV-1 tropism was assessed according to guidance from BHIVA [19]. Viral RNA was extracted from plasma, reverse-transcribed and the V3 region of the HIV-1 envelope gene PCR amplified and sequenced using in-house primer sequences (unpublished). Each RNA extract was amplified and sequenced in triplicate. Sequences were edited with Sequencher v4.7 (Gene Codes, Ann Arbor, MI) with a cut-off of 20% for minority peaks. Derived sequences were submitted to the ‘geno2pheno’ algorithm ( www.geno2pheno.org) to give a prediction of viral tropism. Sequences were analysed by the clonal analysis algorithm and assigned as R5 or X4 virus using a cut-off false-positive rate of 5.75%, without reference to additional clinical data or the presence of a resistance-associated mutation at codons 11 or 25. For subjects who had low (<500 RNA copies/mL) or undetectable viral loads (<50 copies/mL) at recruitment, sequencing was performed on HIV-1 proviral DNA extracted from peripheral blood mononuclear cells and assigned R5 or X4 tropism based on the same FPR cut-off of 5.75%.
Statistical analyses
Baseline was defined as the date of the first sample measured on each child. Baseline characteristics of the study population – age, sex, ethnicity, nadir CD4 cell count and viral load, and ART history – were summarised by clinical group, recording absolute numbers, percentages, median and interquartile range (IQR). Amongst those with more than one sample measured, the number and percentage that experienced a change in tropism on at least one occasion was calculated, stratified by clinical group and tropism at the time of the first sample. To further investigate changes in tropism over time, the subset of individuals with initial R5 virus and at least one subsequent sample was considered. Individuals were followed until the date of a change from R5 to X4/dual tropic virus or the last available sample, whichever occurred first. Kaplan–Meier methods were used to investigate the time to tropism switch, and Cox proportional-hazards models were used to investigate factors associated with this. Only univariable results were calculated, due to a small number of events.
Finally, to ensure that information on all samples taken was summarised, logistic regression with generalised estimating equations was used to assess factors associated with the presence of R5 virus. Statistical significance was assumed for P values below 0.05 and analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
Ethical approval
The study was approved by the National Research Ethics Service (NHS Trust Ref 11/LO/1314) and sponsored by Imperial College Healthcare Trust (JROSM0223). Consent was obtained from adolescents aged 16 years and older, with adolescent assent and parental/carer consent obtained for those aged 12–15 years. Each participant received a unique study number, with data pseudo-anonymised under the study number.
Results
Of 50 participants recruited, two were excluded from analysis as all samples (n=3 per patient) from these individuals failed to amplify the V3 region from plasma RNA or proviral DNA and therefore tropism could not be determined.
Baseline data
Of the remaining 48 patients recruited, at baseline, defined as date of earliest retrievable stored sample, the median age was 12 years (IQR 9.3–14.8 years), 52% were female, 79% Black African ethnicity with 46 (96%) patients having non-B clade HIV viral subtypes; most frequently: clade C in 13 (27%), clade A in 10 (21%) and clade A recombinants (A/E, A/D, AE/D, A/G) in 8 (17%). The 48 patients were assigned clinical groups on the basis of their antiretroviral therapy treatment history. Group 1 (n=20): ART initiated after their 10th birthday; Group 2 (n=16) ART pre 5th birthday; and Group 3 (n=11) ART naïve – long-term non progressors (LTNP). One patient had insufficient clinical data to assign to a study group and was excluded from comparator analysis between groups. At baseline, 81% (39/48) had R5 and 19% (9/48) had dual R5/X4 or X4 viruses. The baseline characteristics and viral tropism by clinical group at study entry are described in Table 1. Of the samples, 222/252 (88%) underwent RNA genotyping.
Table 1.
Baseline characteristics of children at time of study entry
| Total* | Group 1: Late ART | Group 2: Early ART | Group 3: LTNP | |
|---|---|---|---|---|
| 20 | 16 | 11 | ||
| CD4 (cells/mm3) | Median (IQR) | 460 (330–760) | 810 (520–1000) | 670 (510–810) |
| CD4 group | ≤200 | 1 (5%) | 0 (0%) | 0 (0%) |
| 201–350 | 5 (25%) | 2/15 (13.3%) | 2 (18.2%) | |
| 351–500 | 5 (25%) | 1/15 (6.7%) | 0 (0%) | |
| 501+ | 9 (45%) | 12/15 (80.0%) | 9 (81.8%) | |
| VL (copies/mL) | Median (IQR) | 11435 | 1223 | 3991 |
| (1691–44431) | (157–10,359) | (2067–7514) | ||
| VL group (n) | <500 | 4 (20%) | 5/15 (33.3%) | 1 (9.1%) |
| 500–10,000 | 5 (25%) | 6/15 (40.0%) | 8 (72.7%) | |
| 10,001–50,000 | 6 (30%) | 3/15 (20.0%) | 2 (18.2%) | |
| 50,001+ | 5 (25%) | 1/15 (6.7%) | 0 (0%) | |
| Clade | C | 7/17 (41.2%) | 3/13 (23.1%) | 3/8 (37.5%) |
| Non-C | 10/17 (58.8%) | 10/13 (76.9%) | 5/8 (62.5%) | |
| Ethnicity | Black African | 17 (85%) | 11 (68.8%) | 10 (90.9%) |
| Other | 3 (15%) | 5 (31.3%) | 1 (9.1%) | |
| Gender | Female | 9 (45%) | 9 (56.3%) | 7 (63.7%) |
| Male | 11 (55%) | 7 (43.8%) | 4 (36.4%) | |
| Age | Median (IQR) | 12 (9–15) | 11 (8–13) | 15 (13–17) |
| Tropism | R5 | 15 (75%) | 12 (75.0%) | 11 (100%) |
| X4 | 2 (10%) | 2 (12.5%) | 0 (0%) | |
| Dual/mixed | 3 (15%) | 2 (12.5%) | 0 (0%) |
One child (with R5 virus at study entry) had insufficient clinical data to assign to a study group
LTNP: long-term non-progressors; IQR: interquartile range
Follow-up: association with R5 virus
Median duration of follow-up was 7.7 years with a total of 308.6 person-years recorded. Of the 37 who had ever received ART, 15 (42%) were dual, and 22 (58%) ≥ triple class exposed at latest follow-up. HIV-1-associated resistance mutations at any time point were documented in 19/37 (51%) of ART-exposed patients: 7/37 (19%) single-, 9/37 (24%) dual- and 3/37 (8%) triple-class mutations.
A median of five (range 1–14) samples per subject, totalling 252 samples, were successfully sequenced and viral tropism determined. Analysing all 252 samples, the presence of R5 virus was associated with: a higher current CD4 cell count, median 520 cells/mm3 for subjects with R5 virus vs 202 cells/mm3 for X4 virus (P=0.0005), being a long term non-progressor (Group 3) versus ever requiring ART (35% vs 11% P=0.05), non-Black African ethnicity (74% vs 89%; P=0.05) and female gender (55% vs 28%; P=0.005) (Table 2). No difference was recorded between groups starting ART early and late.
Table 2.
Factors associaed with having a CCR5-using virus, considering all samples over follow-up
| Total samples | CCR5 | CXCR4 | Dual / mixed | P | |
|---|---|---|---|---|---|
| 252 | 203 | 36 | 13 | ||
| Method used | DNA | 21 (10.3%) | 6 (16.7%) | 3 (23.1%) | 0.12 |
| RNA | 182 (89.7%) | 30 (83.3%) | 10 (76.9%) | ||
| CD4 (cells/mm3) | Median (IQR) | 520 (346–730) | 202 (68–327) | 300 (145–775) | 0.0005 |
| CD4 group | ≤200 | 21/191 (11.0%) | 21/35 (60%) | 4/12 (33.3%) | |
| 201–350 | 30/191 (15.7%) | 9/35 (25.7%) | 3/12 (25.0%) | ||
| 351–500 | 39/191 (20.4%) | 4/35 (11.4%) | 1/12 (8.3%) | ||
| 501+ | 101/191 (52.9%) | 1/35 (2.9%) | 4/12 (33.3%) | ||
| VL (copies/mL) | Median (IQR) | 5,874 | 22,024 | 12,938 | 0.3403 |
| (886–17,568) | (591–24,995) | (158–21,289) | |||
| VL group | <500 | 44/192 (22.9%) | 9 (25%) | 5/12 (41.7) | |
| 500–10,000 | 68/192 (35.4%) | 10 (27.8%) | 2/12 (16.7%) | ||
| 10,001–50,000 | 65/192 (33.9%) | 12 (33.3%) | 4/12 (33.3%) | ||
| 50,001+ | 15/192 (0.08%) | 5 (13.9%) | 1/12 (8.3) | ||
| Clade | C | 58/174 (33.3%) | 14/34 (41.2%) | 4 (30.8%) | 0.35 |
| Non-C | 116/174 (66.7%) | 20/34 (58.8%) | 9 (69.2%) | ||
| Group | 1 Late ART | 74/201 (36.8%) | 21 (58.3%) | 9 (69.2%) | 0.12 |
| 2 Early ART | 76/201 (37.8%) | 11 (30.6%) | 4 (30.8%) | 0.06 | |
| 3 LTNP | 51/201 (25.3%) | 4 (11.1%) | 0 (0%) | ||
| Ethnicity | Black African | 150 (73.9%) | 32 (88.9%) | 9 (69.2%) | 0.05 |
| Other | 53 (26.1%) | 4 (11.1%) | 4 (30.8%) | ||
| Sex | Female | 112 (55%) | 10 (27.8%) | 7 (53.8%) | 0.005 |
| Male | 91 (44.8%) | 26 (72.2%) | 6 (46.2%) | ||
| Age (years) | Median (IQR) | 16 (13–18) | 17 (15–19) | 17 (5–11) | 0.0339 |
Comparing CCR5 vs CXCR4: calculated from logistic regression with GEEs to account for repeated samples taken on children
IQR: interquartile range; GEE: generalised estimating equation
Tropism switch over time
Three children had only one evaluable sample and were excluded from analysis of tropism switching over the duration of the study period. During a median of 7.7 years of follow-up 19/45 (42.2%) had at least one change in their tropism (Table 3). Lower current CD4 cell count predicted co-receptor switch [unadjusted hazard ratio (HR)=0.62 per 50 cells higher; 95% confidence interval (CI) 0.47–0.81; P=0.0006] but not viral load (P=0.81), age (P=0.85) or clinical group (P=0.56) (Table 4).
Table 3.
Proportion of participants who experienced tropism switch, by tropism at first sample and clinical group
| n | Tropism changed at least once over follow-up | |||
|---|---|---|---|---|
| Total* | 45 | 19 (42.2%) | ||
| Tropism at first sample | R5 | 38 | 12 (30.8%) | |
| X4 | 2 | 2 (100.0%) | ||
| Dual | 5 | 5 (100.0%) | ||
| Group | 1 Late ART | 19 | 10 (52.6%) | |
| 2 Early ART | 14 | 7 (50.0%) | ||
| 3 LTNP | 11 | 2 (18.2%) | ||
| Tropism at first sample and group | 1 Late ART | R5 | 14 | 5 (35.7%) |
| X4 | 2 | 2 (100.0%) | ||
| Mixed | 3 | 3 (100.0%) | ||
| 2 Early ART | R5 | 12 | 5 (41.7%) | |
| X4 | 0 | – | ||
| Mixed | 2 | 2 (100.0%) | ||
| 3 LTNP | R5 | 11 | 2 (18.2%) | |
| X4 | 0 | – | ||
| Mixed | 0 | – |
45 children with ≥2 samples recorded were eligible for inclusion (one child with initial R5 and two with X4 virus were excluded), of whom 44 had sufficient clinical data to be assigned to a clinical group (one further child with initial X4 virus excluded)
Table 4.
Factors associated with a switch in viral tropism
| Hazard ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Current
CD4 cell count |
Per 50 cells higher | 0.62 | 0.47–0.81 | 0.0006 |
| Group | 1 Late ART
2 Early ART 3 LTNP |
2.45
1.54 1.00 (reference) |
0.46–12.99
0.28–8.48 |
0.56 |
| Current viral load | Per 1 log10 copies/mL higher | 0.92 | 0.46–1.84 | 0.81 |
| Age | Per year older | 1.02 | 0.85–1.21 | 0.85 |
Results from Cox proportional hazards regression model (univariate due to limited number of events)
CI: confidence interval
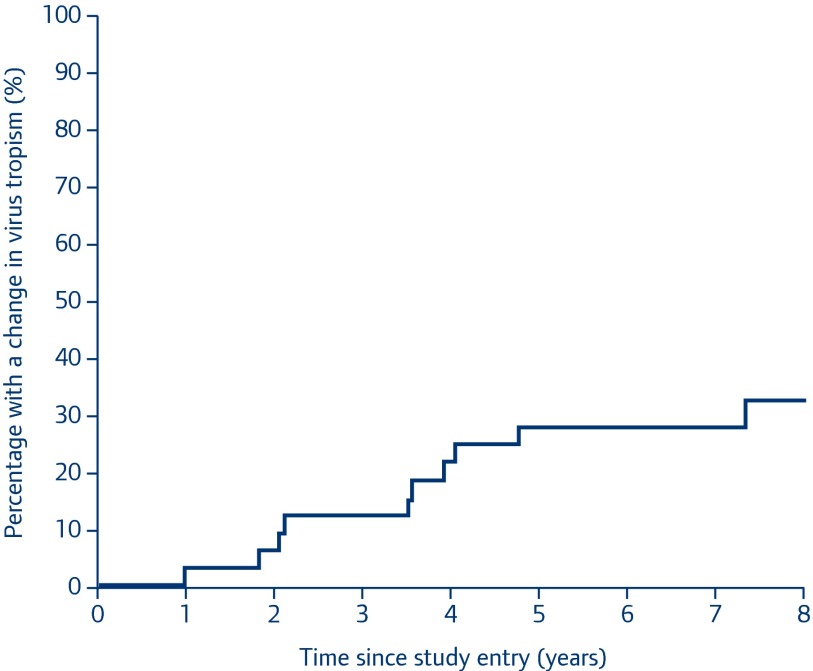
Of the 39 patients with R5 virus at baseline, 38 (97%) had at least one follow-up sample successfully sequenced. A switch from R5 to dual or X4 occurred in 12/38 (30.8%) during the median 7.7 years of follow-up (Table 3). The estimated 5-year risk of tropism switch from R5 to dual tropic/X4 virus was 24.4% (95% CI 9.7–39.2%), with estimated percentages of a change in tropism at 1 year of 2.8% (95% CI 0.0–8.2%) and 2 years of 5.7% (95% CI 0.0–13.4%) (Figure 1).
Figure 1.
Kaplan–Meier estimate of probability of tropism switch from R5 to X4/dual tropic virus over study follow-up (n=38)
Tropism reversion
Of 19 patients who ever had X4/dual tropic virus and at least one further sample sequenced, 11/19 (58%) were predicted to have harboured R5 virus solely at one or more subsequent time points. Over time, 5/11 had three or more switches between R5 and X4/dual tropic virus, three of whom were R5 at latest follow-up.
Discussion
In this cohort of adolescents with perinatally acquired HIV-1 infection, more than 80% had R5 virus at 12 years of age, indicating that CCR5 antagonists may be a treatment option as a component of ART. The estimated 5-year risk of tropism switch from R5 to X4/dual tropic virus was 24% with, as in adult studies, lower CD4 cell count predictive of co-receptor switching [4]. As the cohort entered adult care and approach their third decade living with HIV, the proportion with R5 virus at all time points sequenced had fallen to just over 50%. This is comparable to a cross-sectional genotypic co-receptor tropism analysis of 55 Spanish adolescents, median age 18.2 years, 90% clade B, of whom 40% harboured X4/dual tropic strains [18]. As such, almost half of perinatally infected adolescents currently enter adult care with reduced treatment options for HIV. In this small cohort no difference was seen in tropism switching between those who started ART early in life, at less than 5 years of age, and those who started later, at over 10 years of age, and may reflect the small numbers and heterogeneity of CD4 cell count thresholds at diagnosis and subsequently starting ART within the cohort.
Additionally, due to the small sample size (36 samples with CXCR4 virus on 18 children), we were unfortunately unable to perform multivariate analysis. Therefore the observed univariate differences in tropism switching between genders and ethnicity may be due to confounding factors.
In routine clinical practice a genotypic co-receptor tropism sequence, performed within 3 months prior to starting/switching ART, determines whether a CCR5 antagonist is a potentially active agent within an ART regimen [19]. Surprisingly, more than a half of those ever sequenced shown to harbour X4 virus reverted to R5 virus at a subsequent time point. The relatively frequent ‘switching’ between R5 and X4 virus, and vice versa, demonstrated in this cohort, highlights a need for vigilant clinical monitoring of virological response in children and adolescents receiving maraviroc. On current evidence, detection of X4 virus at any time should be considered long-lasting and preclude the use of CCR5 antagonists. Clinical outcome data for the use of maraviroc in paediatric patients following tropism determination by genotypic testing methods are limited. However, recent preliminary data suggest that CCR5 antagonists may have a therapeutic role in patients with a mixture of R5 and X4 viruses and warrant further investigation, particularly in those with multidrug resistance and limited treatment options [20,21].
Almost half of the adolescents were triple-class ART experienced and, of those adolescents who had ever received ART, one-third had accumulated mutations associated with resistance to two or more drug classes, further limiting future ART treatment options. Globally, 30% of children with PaHIV are triple-class experienced, with up to 80% of multidrug-resistant paediatric patients harbouring X4/dual tropic viruses [14]. Paediatric studies of CCR5 antagonists should be expedited, particularly as early use of such agents may ensure they are a viable treatment option for those currently facing a lifetime on antiretroviral therapy.
Consideration should, however, be given to existing data from adult studies. In a post hoc analysis of the MERIT study in patients with R5 virus confirmed by enhanced sensitivity Trofile assay, maraviroc was non-inferior to efavirenz in treatment-naïve adults infected with R5 virus out to 5 years of follow-up [22]. More recently a treatment-naïve nucleoside-sparing strategy of boosted darunavir with maraviroc was stopped due to increased virological failure compared to the standard of care of boosted darunavir with tenofovir/emtricitabine [23].
Initial paediatric data suggest that maraviroc is safe, well tolerated and effective with both an oral solution and paediatric tablets in development [24,25]. A4001031, a Phase IV study in treatment-experienced children age 2 to <18 years harbouring R5 virus is ongoing and due to report in 2019. Given the central nervous system penetration of maraviroc, the potential role in younger children with advanced disease and HIV encephalopathy is of particular interest.
Limitations to this study include a relatively small retrospective cohort born in the pre-ART era that may not be representative of the current cohort of children growing up with HIV. Having survived in the pre-HAART era, when half of children born with HIV died before their second birthday, this cohort will not include children with rapidly progressive disease who potentially have higher rates of tropism switching from R5 to X4 virus. Conversely, in the current era of universal ART for all children diagnosed in the first 5 years of life under World Health Organization (WHO) guidelines and at higher CD4 cell count thresholds for older children, current paediatric populations may have lower rates of tropism switching, providing viral suppression and CD4 cell counts are maintained, which can be a challenge, particularly through adolescence. As failure on maraviroc is rarely associated with resistance, it has the potential to preserve future treatment options and provides a well-tolerated option for a population with limited treatment options.
In conclusion half of the children born with HIV will enter adult life with X4/dual tropic virus, for whom the existing CCR5 antagonist may not be an effective component of ART. Further investigation into the optimal sequencing of antiretroviral classes is required for this unique population who currently face a lifetime on antiretroviral therapy.
References
- 1. Cavarelli M, Scarlatti G. Phenotypic variation in human immunodeficiency virus type 1 transmission and disease progression. Dis Markers 2009; 27: 121– 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Church JD, Huang W, Mwatha A et al. HIV-1 tropism and survival in vertically infected Ugandan infants. J Infect Dis 2008; 197: 1382– 1388. [DOI] [PubMed] [Google Scholar]
- 3. Shepherd J, Jacobson L, Qiao W et al. Emergence and persistence of CXCR4-tropic HIV-1 in a population of men from the Multicenter AIDS Cohort Study. J Infect Dis 2008; 198: 1104– 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bidwell Goetz M, Leduc R, Kostman J et al. Relationship between HIV co-receptor tropism and disease progression in persons with untreated chronic HIV Infection. J Acquir Immune Defic Syndr 2009; 50: 259– 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daar ES, Kesler KL, Petropoulos CJ et al. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin Infect Dis 2007; 45: 643– 649. [DOI] [PubMed] [Google Scholar]
- 6. Briz V, Poveda E, Mar Gonzalez M et al. Impact of antiretroviral therapy on viral tropism in HIV-infected patients followed longitudinally for over 5 years. J Antimicrob Chemother 2008; 61: 405– 410. [DOI] [PubMed] [Google Scholar]
- 7. Moyle G, Wildfire A, Mandalia S et al. Epidemiology and predictive factor for chemokine receptor use in HIV-1 infection. J Infect Dis 2005; 191: 866– 872. [DOI] [PubMed] [Google Scholar]
- 8. Huang W, Elhleman S, Toma J et al. Coreceptor tropism in human immunodeficiency virus type 1 subtype D: high prevalence of CXCR4 tropism and heterogeneous composition of viral populations. J Virol 2007; 81: 7885– 7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esbjornsson J, Mansson F, Martinez-Arias W et al. Frequent CXCR4 tropism of HIV-1 subtype A and CRF02_AG during late stage disease: indication of an evolving epidemic in West Africa. Retrovirology 2010; 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connell BJ, Michler K, Capovilla A et al. Emergence of X4 usage among HIV-1 subtype C: evidence for an evolving epidemic in South Africa. AIDS 2008; 22: 896– 899. [DOI] [PubMed] [Google Scholar]
- 11. Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenges of HIV-1 subtype diversity. N Engl J Med 2008; 358: 1590– 1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Equils O, Garratty E, Wei LS. Recovery of replication-competent virus from CD4 T cell reservoirs and change in coreceptor use in human immunodeficiency virus type 1-infected children responding to highly active antiretroviral therapy. J Infect Dis 2000; 182: 751– 757. [DOI] [PubMed] [Google Scholar]
- 13. Green TN, Archary M, Gordon ML et al. Drug resistance and coreceptor usage in HIV type 1 subtype C-infected children initiating or failing highly active antiretroviral therapy in South Africa. AIDS Hum Retroviruses 2012; 28: 324– 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Briz V, García D, Méndez-Lagares G et al. High prevalence of X4/DM-tropic variants in children and adolescents infected with HIV-1 by vertical transmission. Pediatr Infect Dis J 2012; 31: 1048– 1052. [DOI] [PubMed] [Google Scholar]
- 15. Fatenheuer G, Nelson M, Lazzarin A et al. Subroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med 2008; 359: 1442– 1455. [DOI] [PubMed] [Google Scholar]
- 16. WHO March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Available at: www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_march2014/en/ ( accessed June 2015). [PubMed]
- 17. Bamford A, Turkova A, Lyall EGH et al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med 2015; Feb 3. doi: 10.1111/hiv.12217. [ Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Biagio A, Parisini A, Bruzzone B et al. Genotypic determination of HIV tropism in a cohort of patients perinatally infected with HIV-1 and exposed to antiretroviral therapy. HIV Clin Trials 2014; 15: 45– 50. [DOI] [PubMed] [Google Scholar]
- 19. Geretti A-M, Mackie N. Determining HIV tropism in clinical practice (2009). Available at: www.bhiva.org/tropism-guidelines.aspx ( accessed June 2015).
- 20. Surdo M, Balestra E, Saccomandi P. Inhibition of dual/mixed tropic HIV-1 isolates by CCR5-inhibitors in primary lymphocytes and macrophages. PLoS One 2013; 8: e68076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavarelli M, Mainetti L, Pignataro AR et al. Complexity and dynamics of HIV-1 chemokine receptor usage in a multidrug-resistant adolescent. AIDS Res Hum Retroviruses 2014; 30: 1243– 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper DA, Heera J, Ive P et al. Efficacy and safety of maraviroc vs. efavirenz in treatment-naive patients with HIV-1: 5-year findings. AIDS 2014; 28: 717– 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stellbrink HJ, Pulik P, Szlavik J et al. Maraviroc (MVC) dosed once daily with darunavir/ritonavir (DRV/r) in a 2 drug-regimen compared to emtricitabine/-tenofovir (TDF/FTC) with DRV/r; 48-week results from MODERN (Study A4001095). International AIDS Conference. Melbourne, Australia. July 2014. Abstract TUAB0101.
- 24. Giaquinto C, Keet L, Fortuny C et al. Safety and efficacy of maraviroc in CCR5-tropic HIV-1 infected children. International AIDS Conference. Kuala Lumpur, Malaysia July 2014. Abstract MOAB0103.
- 25. Nyrienda M, Mackie NE, Kaye S, Foster C. Recommendation for the off licence use of maraviroc in children with PaHIV-I infection by a regional paediatric virtual clinic. BHIVA/BASHH Annual Conference. Liverpool, UK April 2014. Abstract O7.