Abstract
Aims
To evaluate oral doses of the non-steroidal mineralocorticoid receptor antagonist finerenone given for 90 days in patients with worsening heart failure and reduced ejection fraction and chronic kidney disease and/or diabetes mellitus.
Methods and results
Miner Alocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF) was a randomized, double-blind, phase 2b multicentre study (ClinicalTrials.gov: NCT01807221). Of 1286 screened patients, 1066 were randomized. Patients received oral, once-daily finerenone (2.5, 5, 7.5, 10, or 15 mg, uptitrated to 5, 10, 15, 20, or 20 mg, respectively, on Day 30) or eplerenone (25 mg every other day, increased to 25 mg once daily on Day 30, and to 50 mg once daily on Day 60) for 90 days. The primary endpoint was the percentage of individuals with a decrease of >30% in plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) from baseline to Day 90. A key exploratory endpoint was a composite clinical endpoint of death from any cause, cardiovascular hospitalizations, or emergency presentation for worsening HF until Day 90. Mean age ranged from 69.2 to 72.5 years in different treatment groups (standard deviation 9.7–10.6 years). Decreases in NT-proBNP of >30% from baseline occurred in 37.2% of patients in the eplerenone group and 30.9, 32.5, 37.3, 38.8, and 34.2% in the 2.5→5, 5→10, 7.5→15, 10→20, and 15→20 mg finerenone groups, respectively (P = 0.42–0.88). Except for the 2.5→5 mg finerenone group, the composite clinical endpoint occurred numerically less frequently in finerenone-treated patients compared with eplerenone; this difference reached nominal statistical significance in the 10→20 mg group (hazard ratio 0.56, 95% confidence interval, CI, 0.35; 0.90; nominal P = 0.02), despite the fact that this phase 2 study was not designed to detect statistical significant differences. A potassium level increase to ≥5.6 mmol/L at any time point occurred in 4.3% of patients, with a balanced distribution among all treatment groups.
Conclusion
Finerenone was well tolerated and induced a 30% or greater decrease in NT-proBNP levels in a similar proportion of patients to eplerenone. The finding of reduced clinical events in the finerenone 10→20 mg group should be further explored in a large outcomes trial.
Keywords: Finerenone, Mineralocorticoid receptor antagonists, Worsening heart failure
See page 2115 for the editorial comment on this article (doi:10.1093/eurheartj/ehw155)
Introduction
The steroidal mineralocorticoid receptor antagonists (MRAs) spironolactone1 and eplerenone2 reduce mortality and hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), and are recommended in European and US guidelines for symptomatic patients with HFrEF.3,4 While both spironolactone and eplerenone have been shown to be effective in patients with HFrEF, they may be underused or inconsistently prescribed in hospitalized patients with worsening heart failure (HF) because of a high risk of adverse events.5,6 Furthermore, patients with common co-morbidities of HF such as diabetes mellitus (DM) or chronic kidney disease (CKD) are at particular risk of developing hyperkalemia,7 so may be less likely to receive currently available MRA therapy due to safety concerns. Thus, there is an unmet medical need in patients with worsening HFrEF, and impaired kidney function, which is not being met using currently available MRAs.
Finerenone (BAY 94-8862) is a novel non-steroidal MRA, with higher selectivity towards the mineralocorticoid receptor (MR) compared with spironolactone and stronger MR-binding affinity than eplerenone.8 This combination of potency and selectivity towards the MR9 and a balanced tissue distribution into heart and kidney compared with spironolactone or eplerenone10 could result in more pronounced cardiorenal protection, particularly in high-risk patients with impaired kidney function. The phase 2a minerAlocorticoid Receptor antagonist Tolerability Study (ARTS) trial showed that in patients with HFrEF and mild CKD, finerenone (5.0–10.0 mg/d) had compared efficacy with that of spironolactone (25 or 50 mg/d), with smaller increases in serum potassium level and smaller decreases in estimated glomerular filtration rate (eGFR).11 Further, the phase 2b ARTS-Diabetic Nephropathy trial confirmed the safety of finerenone in patients with diabetic kidney disease.12
The ARTS-HF (Clinicaltrials.gov Identifier: NCT01807221) study was designed to compare the efficacy and safety of five different treatment regimens of once-daily oral doses of finerenone with eplerenone in patients with concomitant type 2 diabetes mellitus (T2DM) and/or CKD, who presented in emergency departments with worsening chronic HFrEF.
Methods
Study design
ARTS-HF has been described in detail elsewhere.13 Briefly, ARTS-HF was a randomized, double-blind, active-comparator-controlled, parallel-group, phase 2b, dose-finding study conducted at 173 centres in 25 countries.7 The study protocol was developed by the steering committee together with the sponsor (Bayer Pharma AG), and was approved by independent ethics committees and/or institutional review boards for all participating centres/countries before the study began. The study was carried out in accordance with Good Clinical Practice guidelines, the guiding principles of the Declaration of Helsinki, and applicable local laws and regulations. All participating patients gave written informed consent. Safety and tolerability were monitored by an independent Data Safety Monitoring Board and a central committee performed blinded adjudication of all hospitalizations and deaths.
Patients
Patients were recruited from June 2013 to August 2014 and were eligible for inclusion in the study if they were at least 18 years old and had worsening chronic HFrEF requiring hospitalization and treatment with intravenous diuretics. They also had to have T2DM and/or CKD (i.e. an eGFR of >30 mL/min/1.73 m2 in patients with T2DM and 30–60 mL/min/1.73 m2 in patients without T2DM), have been receiving treatment with evidence-based therapy for HF for at least the previous 3 months, and have a medical history of a left ventricular ejection fraction of 40% or less within the previous 12 months. Patients receiving treatment with spironolactone, eplerenone, renin inhibitors, or potassium-sparing diuretics at presentation had to be able to discontinue those treatments for 24 h before randomization (48 h for spironolactone) and for the duration of the study treatment period. Further details of the inclusion and exclusion criteria have been published previously.13
Dosing, randomization, and masking
Randomization was carried out centrally by an interactive voice/web response system and participants, investigators, and the sponsor's clinical team was blinded to treatment allocation. There were five preplanned finerenone treatment arms and one eplerenone treatment arm. The daily doses in the finerenone treatment arms (presented as ‘initial dose → uptitrated dose’; see ‘Procedures’ section below) were 2.5→5, 5→10, 7.5→15, 10→20, and 15→20 mg. The initial dose in the eplerenone treatment arm was 25 mg every second day; this could be increased to 25 mg once daily and then to 50 mg once daily if both uptitration steps were performed. Uptitration of both study drugs and in all dose groups was performed if serum potassium levels remained ≤5.0 mmol/L. Patients were initially randomized 1:1:1 during the 7 days following hospital presentation to 1 of the 2 lowest preplanned finerenone doses (2.5→5 or 5→10 mg once daily) or eplerenone. The Data Safety Monitoring Board assessed safety and tolerability after randomization of ∼300 patients in January 2014 and decided that the three remaining preplanned finerenone treatment arms could be introduced into the study. The randomization scheme was adapted to achieve a final randomization ratio of ∼1:1.5 between each finerenone dose group and the eplerenone group.
Procedures
Finerenone or eplerenone was administered on top of standard therapy for HF. The planned treatment duration was 90 days, with an additional follow-up period after the cessation of study drug for 30 days. The titration protocol was the same in all treatment groups: if blood potassium concentration was 5.0 mmol/L or less, the initial dose of finerenone or eplerenone was uptitrated at the end of the acute/vulnerable phase (Day 30), and an additional uptitration (eplerenone) or sham uptitration (finerenone) was performed at Day 60. Treatment with the study drug was stopped if the patient had a confirmed blood potassium concentration of 5.6 mmol/L or more.
Outcomes
The primary study objective was to investigate the efficacy (percentage of responders; i.e. the number of patients with a decrease in plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) level of >30% from baseline to Day 90) and safety of different oral doses of finerenone given once daily.
Further exploratory efficacy endpoints included: (i) a composite endpoint of death from any cause, cardiovascular hospitalization, or emergency presentation for worsening chronic HF until Day 90; (ii) change in efficacy biomarkers (B-type natriuretic peptide, NT-proBNP, galectin 3, and N-terminal procollagen III propeptide); (iii) change in scores on health-related quality-of-life (QoL) questionnaires [the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the five-dimension European Quality of Life Questionnaire (EuroQoL)]. Clinical endpoints have been adjudicated by the event committee.
Effects on the following safety parameters were assessed: (i) biomarkers of organ injury (troponin T and cystatin C); (ii) vital signs; (iii) laboratory parameters (including potassium and serum creatinine concentrations, and eGFR; (iv) incidence of adverse events including those of special interest (i.e. an increase in serum potassium concentration to at least 5.6 mmol/L leading to discontinuation or emergency presentation for worsening chronic HF after starting treatment with the study drug).
Statistical analysis
Analyses were performed in three data sets: the safety-analysis set, full-analysis set, and per-protocol set (see Supplementary material online, Appendix). The primary endpoint was assessed in the full-analysis set (with missing data imputed using the last observation carried forward (LOCF): the highest NT-proBNP value from the premature discontinuation and follow-up measurements were used in such cases); a supportive analysis was performed in the per-protocol set and several sensitivity analyses on the imputation method (including on-treatment LOCF, observed case analysis, and random imputation) were performed in the full-analysis set. For the primary endpoint, each finerenone group was compared with the eplerenone group using separate Chi-squared tests with continuity correction. In both cases, a one-sided significance level of 5% was applied. For each contingency table, estimated and two-sided 90% CIs were provided for each treatment groups and treatment differences.
Patients who died prior to Day 90, or who permanently discontinued study drug (≥5 consecutive days) after cardiovascular hospitalization or after emergency hospital presentation for worsening chronic HF, were considered nonresponders to avoid biasing the primary efficacy analysis towards treatment responders.
The composite mortality/morbidity endpoint, its individual components, and cardiovascular mortality were assessed using the life-table and Kaplan–Meier methods. Differences between the finerenone and eplerenone groups were assessed using the log-rank test. This is an exploratory study, i.e. no confirmatory testing on the primary efficacy variable or other variables was performed. Further details of the statistical methods have been reported elsewhere.10
Results
Patients
The disposition of the patients throughout the study is shown in Figure 1. Baseline characteristics were broadly similar across the treatment groups (Table 1). Of the 1066 randomized patients, 72.8% completed the study and 9.4% withdrew consent (Figure 1; see Supplementary material online, Table S1); the majority was receiving recommended pharmacologic therapy for chronic HF. Mean age ranged from 69.2 to 72.5 years in different treatment groups (standard deviation 9.7–10.6 years). The median NT-proBNP concentration at baseline was highest in the eplerenone group (5331 [range 148–47 774] pg/mL) and lowest in the finerenone 15→20 mg dose group (3750 [range 144–45 375] pg/mL). The median BNP concentration at baseline was highest in the finerenone 2.5→5 (715 pg/mL) and lowest in the finerenone 5→10 mg dose group (559 pg/mL).
Figure 1.
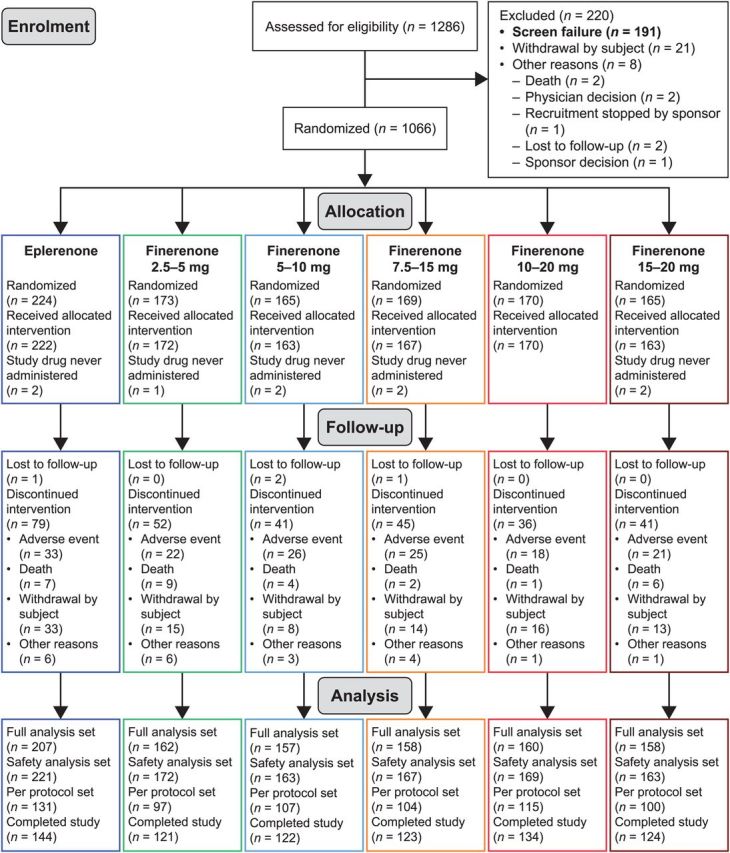
Patient disposition. All patients from the safety-analysis set were considered for the full-analysis set if they had baseline and at least one post baseline plasma N-terminal pro-B-type natriuretic peptide level value or who died, experienced permanent (≥5 consecutive days) withdrawal of study drug after cardiovascular hospitalization, or after emergency presentation for worsening chronic heart failure. The per-protocol set comprised all patients from the full-analysis set with valid plasma NT-proBNP data at visit 7 (Day 60 ± 2) or later and no major protocol deviations.
Table 1.
Patient demographics and clinical characteristics (safety-analysis set)
| Parameter | Eplerenone n = 221 |
Finerenone 2.5→5 mg n = 172 |
Finerenone 5→10 mg n = 163 |
Finerenone 7.5→15 mg n = 167 |
Finerenone 10→20 mg n = 169 |
Finerenone 15→20 mg n = 163 |
Total N = 1055 |
|---|---|---|---|---|---|---|---|
| Mean age (SD) (years) | 72.4 (9.9) | 72.5 (9.7) | 71.8 (10.6) | 69.3 (9.8) | 71.3 (10.23) | 69.2 (10.2) | 71.2 (10.1) |
| Male sex, n (%) | 170 (76.9) | 135 (78.5) | 126 (77.3) | 124 (74.3) | 128 (75.7) | 132 (81.0) | 816 (77.3) |
| Region, n (%) | |||||||
| Europe | 169 (76.5) | 130 (75.6) | 128 (78.5) | 131 (78.4) | 133 (78.7) | 132 (81.0) | 823 (78.0) |
| North America | 14 (6.3) | 9 (5.2) | 8 (4.9) | 15 (9.0) | 13 (7.7) | 10 (6.1) | 69 (6.5) |
| Asia | 7 (3.2) | 7 (4.1) | 4 (2.5) | 5 (3.0) | 7 (4.1) | 7 (4.3) | 37 (3.5) |
| Other | 31 (14.0) | 26 (15.1) | 23 (14.1) | 16 (9.6) | 16 (9.5) | 14 (8.6) | 126 (11.9) |
| New York Heart Association functional class before worsening, n (%) | |||||||
| II | 84 (38.0) | 65 (37.8) | 49 (30.1) | 71 (42.5) | 79 (46.7) | 62 (38.0) | 410 (38.9) |
| III | 121 (54.8) | 92 (53.5) | 98 (60.1) | 89 (53.3) | 80 (47.3) | 89 (54.6) | 569 (53.9) |
| IV | 16 (7.2) | 15 (8.7) | 16 (9.8) | 7 (4.2) | 10 (5.9) | 12 (7.4) | 76 (7.2) |
| Risk factors, (%) | |||||||
| Type 2 diabetes mellitus (without CKD) | 55 (24.9) | 39 (22.7) | 36 (22.1) | 49 (29.3) | 48 (28.4) | 53 (32.5) | 280 (26.5) |
| Type 2 diabetes mellitus with CKD | 84 (38.0) | 68 (39.5) | 71 (43.6) | 59 (35.3) | 60 (35.5) | 56 (34.4) | 398 (37.7) |
| Chronic kidney disease (without T2DM) | 82 (37.1) | 63 (36.6) | 55 (33.7) | 57 (34.1) | 61 (36.1) | 52 (31.9) | 370 (35.1) |
| Ischaemic heart disease | 147 (66.5) | 114 (66.3) | 109 (66.9) | 111 (66.5) | 104 (61.5) | 94 (57.7) | 679 (64.4) |
| Arterial hypertension | 158 (71.5) | 127 (73.8) | 121 (74.2) | 121 (72.5) | 127 (75.1) | 121 (74.2) | 775 (73.5) |
| Atrial fibrillation, ECG at baseline | 107 (48.4) | 64 (37.2) | 70 (42.9) | 61 (36.5) | 68 (40.2) | 62 (38.0) | 432 (40.9) |
| Heart failure medications, n (%) | |||||||
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker at baseline | 173 (78.3) | 131 (76.2) | 129 (79.1) | 130 (77.8) | 127 (75.1) | 134 (82.2) | 824 (78.1) |
| β-Blocker at baseline | 189 (85.5) | 137 (79.7) | 141 (86.5) | 146 (87.4) | 148 (87.6) | 146 (89.6) | 907 (86.0) |
| Mineralocorticoid receptor antagonist at index emergency presentation | 92 (41.6) | 72 (41.9) | 73 (44.8) | 74 (44.3) | 75 (44.4) | 69 (42.3) | 455 (43.1) |
| Median N-terminal pro-B-type natriuretic peptide concentration (pg/mL) | 5331 | 5000 | 4386 | 4085 | 4543 | 3750 | 4517 |
| Median B-type natriuretic peptide concentration, (pg/mL) | 645 | 715 | 559 | 572 | 646 | 570 | 625 |
| Mean potassium concentration (SD), (mmol/L) | 4.1 (0.5) | 4.1 (0.5) | 4.2 (0.5) | 4.2 (0.4) | 4.1 (0.5) | 4.2 (0.5) | 4.1 (0.5) |
| Mean eGFRa (SD), (mL/min 1.73 m2) | 52 (18) | 52 (16) | 52 (16) | 55 (20) | 53 (17) | 55 (19) | 53 (18) |
| eGFRa, ≤60 mL/min 1.73 m2, n (%) | 159 (72) | 120 (70) | 127 (78) | 116 (69) | 120 (71) | 110 (67) | 752 (71) |
| Mean creatinine concentration (SD) (mg/dL) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) |
| Mean urinary albumin:creatinine ratio (SD) (g/kg) | 52 (5) | 50 (5) | 43 (5) | 41 (5) | 41 (5) | 39 (5) | 45 (5) |
| High/very high albuminuria, n (%) | 124 (56.1) | 97 (56.3) | 80 (49.1) | 85 (50.9) | 95 (56.2) | 77 (47.3) | 558 (52.9) |
| Mean systolic blood pressure (SD) (mmHg) | 121 (19) | 119 (16) | 118 (14) | 119 (17) | 116 (17) | 117 (17) | 119 (17) |
| Mean heart rate (SD) (beats/min) | 75 (14) | 73 (13) | 73 (13) | 74 (12) | 74 (12) | 74 (13) | 74 (13) |
| Mean ejection fraction (SD) (%) | 29.8 (7.5) | 29.3 (7.8) | 28.7 (7.4) | 28.5 (7.4) | 29.0 (8.0) | 29.0 (7.5) | 29.1 (7.6) |
SD, standard deviation.
aEstimated glomerular filtration rate was calculated using the modification of diet in renal disease equation.
Dosing
Maximum doses were reached in 77.3% of patients in the finerenone 2.5→5 mg group, 79.1% of the 5→10 mg group, 81.4% of the 7.5→15 mg group, 77.5% of the 10–20 mg group, and 73.6% of the 15→20 mg group. In the eplerenone group, 64.3% of patients reached the 50 mg daily dose. The average daily dose of eplerenone was 38.6 mg.
Efficacy
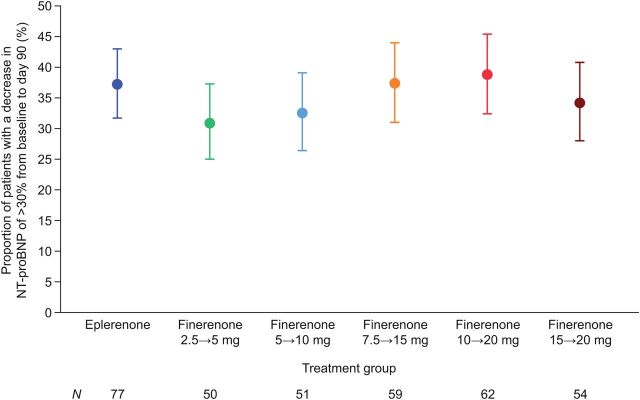
Primary efficacy endpoint
The proportion of patients who had an NT-proBNP level decrease of >30% at Day 90 compared with baseline was similar in the finerenone groups and the eplerenone group in the full-analysis set (Figure 2) and the per-protocol set (see Supplementary material online, Figure S1). The proportions of patients who had an NT-proBNP level decrease of >30% at Day 30 and at Day 60 compared with baseline were also not statistically different across the groups (see Supplementary material online, Figure S2). Sensitivity analyses on the imputation method indicated no relevant effect on the results by the imputation method (see Supplementary material online, Table S2).
Figure 2.
Proportion of patients with a decrease of >30% in plasma N-terminal pro-B-type natriuretic peptide concentration from baseline at Day 90 (full-analysis set). Patients who died prior to Day 90 or who experienced permanent (≥5 consecutive days) withdrawal of study drug after a cardiovascular hospitalization or emergency presentation for worsening chronic heart failure were counted as nonresponders for the primary efficacy analysis.
Summary statistics for the NT-proBNP concentration ratio Day 90: baseline was similar between the groups (see Supplementary material online, Table S3). A mixed-effect model with factors treatment group, comorbidities, MRA use at emergency presentation, region, atrial fibrillation, time, treatment*time, and baseline NT-proBNP value as covariates also showed no differences between the eplerenone group and the finerenone groups (see Supplementary material online, Table S4).
Further exploratory efficacy endpoints
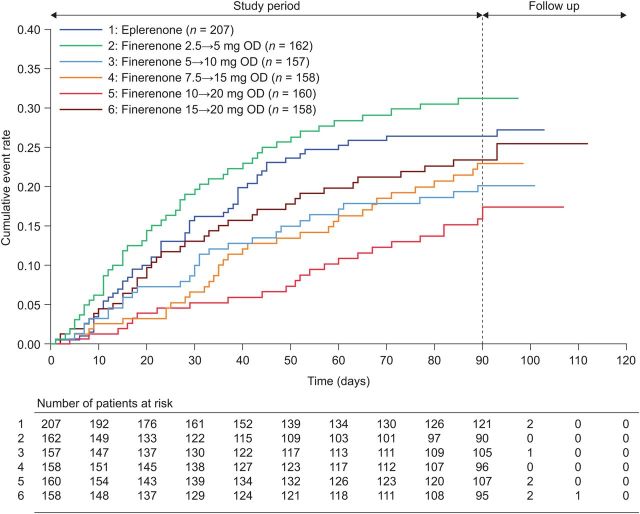
The incidence of the composite endpoint at Day 90 was lower in all finerenone groups compared with the eplerenone group, except for the finerenone 2.5→5 mg group (Figure 3 and see Supplementary material online, Table S5). The incidence of the composite endpoint in the 10→20 mg dose group was nominally improved vs. that in the eplerenone group (hazard ratio, HR: 0.56; 95% CI: 0.35, 0.90; P = 0.02). Similar findings for the 10→20 mg dose group vs. eplerenone were observed for the individual components of death from any cause [HR: 0.13 (95% CI: 0.02, 1.07)], cardiovascular hospitalization [HR: 0.56 (95% CI: 0.34, 0.93)] and emergency presentation to hospital for worsening chronic HF [HR: 0.58 (95% CI: 0.33, 1.02)]; see Supplementary material online, Table S5. The HRs were adjusted for log-transformed baseline NT-proBNP in a post hoc analysis, leading to only slightly higher values (see Supplementary material online, Table S6).
Figure 3.
Mortality/morbidity outcomes in patients with worsening chronic heart failure with reduced ejection fraction receiving eplerenone or different doses of finerenone. Cumulative event rates of the composite endpoint of death from any cause, cardiovascular hospitalization, or emergency presentation for worsening chronic heart failure in the full-analysis set.
The changes in concentrations of galectin 3 and N-terminal procollagen III peptide from baseline to Day 90 were small. Mean scores on the KCCQ and the EuroQoL Questionnaire improved at Day 90 compared with baseline in all treatment groups (see Supplementary material online, Table S7).
Safety
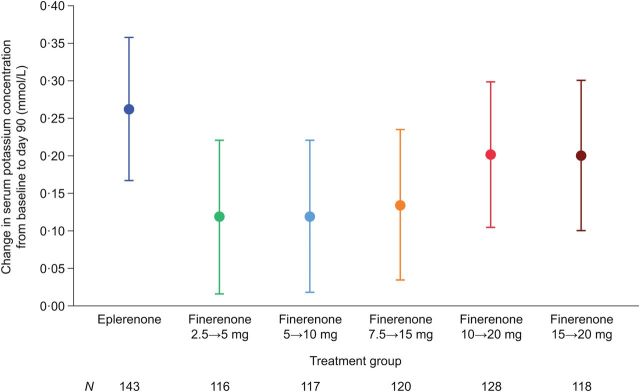
All doses of finerenone had a similar safety profile to that of eplerenone. Incidences of treatment-emergent adverse events were similar between the eplerenone group and all finerenone dose groups (Table 2). Hyperkalaemia (serum potassium concentration ≥5.6 mmol/L) at any time post baseline was observed in 44 patients (4.3%), with a balanced distribution among the finerenone dose groups and the eplerenone group. Five patients (1/212 (0.5%) in the eplerenone group, and 1/164 (0.6%) and 3/160 (1.9%) in the finerenone 7.5→15 and 15→20 mg dose groups, respectively) had a serum potassium concentration >6.0 mmol/L at any time post baseline. Mean change from baseline to Day 90 in serum potassium concentration was greater in the eplerenone group (+0.262 mmol/L) than in each of the finerenone dose groups (+0.119–0.202 mmol/L; Figure 4).
Table 2.
Incidence of treatment-emergent adverse events and hyperkalaemia in patients with worsening chronic heart failure with reduced ejection fraction receiving eplerenone or finerenone (safety-analysis set)
| Adverse event parameter | Incidence, n (%) |
||||||
|---|---|---|---|---|---|---|---|
| Eplerenone n = 221 |
Finerenone 2.5→5 mg n = 172 |
Finerenone 5→10 mg n = 163 |
Finerenone 7.5→15 mg n = 167 |
Finerenone 10→20 mg n = 169 |
Finerenone 15→20 mg n = 163 |
Total N = 1055 |
|
| Any treatment-emergent adverse event | 170 (76.9) | 132 (76.7) | 124 (76.1) | 105 (62.9) | 120 (71.0) | 128 (78.5) | 779 (73.8) |
| Any treatment-emergent serious adverse event | 77 (34.8) | 72 (41.9) | 47 (28.8) | 52 (31.1) | 46 (27.2) | 57 (35.0) | 351 (33.3) |
| Discontinuation due to treatment-emergent adverse event | 32 (14.5) | 21 (12.2) | 25 (15.3) | 25 (15.0) | 17 (10.1) | 21 (12.9) | 141 (13.4) |
| Study drug-related treatment-emergent adverse event | 39 (17.6) | 34 (19.8) | 28 (17.2) | 32 (19.2) | 27 (16.0) | 29 (17.8) | 189 (17.9) |
| Study drug-related treatment-emergent serious adverse event | 9 (4.1) | 10 (5.8) | 7 (4.3) | 11 (6.6) | 6 (3.6) | 11 (6.7) | 54 (5.1) |
| Treatment-emergent adverse events of special interest | 44 (19.9) | 38 (22.1) | 28 (17.2) | 34 (20.4) | 25 (14.8) | 35 (21.5) | 204 (19.3) |
| Hyperkalaemia from baseline to Day 30a | |||||||
| Potassium concentration ≥5.6 mmol/L | 1/178 (0.6) | 1/136 (0.7) | 2/138 (1.4) | 0/142 (0.0) | 2/144 (1.4) | 1/137 (0.7) | 7/875 (0.8) |
| Potassium concentration >6.0 mmol/L | 0/178 (0.0) | 0/136 (0.0) | 0/138 (0.0) | 0/142 (0.0) | 0/144 (0.0) | 1/137 (0.7) | 1/875 (0.1) |
| Hyperkalaemia at any time post baselinea | |||||||
| Potassium concentration ≥5.6 mmol/L | 10/212 (4.7) | 6/165 (3.6) | 6/157 (3.8) | 6/164 (3.7) | 6/165 (3.6) | 10/160 (6.3) | 44/1023 (4.3) |
| Potassium concentration >6.0 mmol/L | 1/212 (0.5) | 0/165 (0.0) | 0/157 (0.0) | 1/164 (0.6) | 0/165 (0.0) | 3/160 (1.9) | 5/1023 (0.5) |
| Emergency presentation for worsening chronic heart failure | 40 (18.1) | 33 (19.2) | 23 (14.1) | 26 (15.6) | 19 (11.2) | 30 (18.4) | 171 (16.2) |
aIncidence of hyperkalaemia is presented as n/n with available data (%).
Figure 4.
Mean change in serum potassium concentration from baseline to Day 90 in patients with worsening chronic heart failure with reduced ejection fraction receiving eplerenone or different doses of finerenone. Changes were assessed by analysis of covariance with the factors treatment group, comorbidities, mineralocorticoid receptor antagonist use at emergency presentation to hospital, region, and the baseline value as covariates.
Mean systolic blood pressure decreased by <3 mmHg from baseline to Day 90 in all treatment groups. The size of the decrease was similar in the eplerenone group and the finerenone 5→10 mg, 7.5→15 and 10→20 mg dose groups. No clear dose relationship was observed in the finerenone groups (see Supplementary material online, Table S8).
Mean eGFR (as measured using the modification of diet in renal disease equation) increased slightly from baseline to Day 90 in the two lowest finerenone groups and decreased in the other groups (see Supplementary material online, Table S9). The proportion of patients experiencing a decrease in eGFR of ≥25, ≥30, ≥40, and ≥57% was low across all groups (see Supplementary material online, Table S10) and there was no statistically significant change in heart rate between groups (see Supplementary material online, Table S11).
There were five renal events leading to hospitalization: two in the eplerenone group and one each in the finerenone 2.5→5, 7.5→15, and 15→20 mg dose groups.
Discussion
Mineralocorticoid receptor antagonists have not been systematically studied in patients with worsening chronic HFrEF who require emergency admission to hospital. However, evidence from the use of MRAs in other patient groups1,2,14 and the intense neurohormonal activation in patients with worsening HF3,15 suggests that the MR may be an important therapeutic target in this population with very high morbidity and mortality despite current therapy.
ARTS-HF is the first clinical trial to compare the novel nonsteroidal MRA finerenone with eplerenone in this population with a high unmet medical need i.e. patients with worsening HFrEF requiring emergency treatment and who also have DM and/or CKD. This period of acute worsening is a crucial time for treatment of patients with HFrEF because mortality and morbidity are known to increase following emergency presentation. In this high-risk population, finerenone was well tolerated with a safety profile comparable with that of eplerenone. The proportion of patients who had an NT-proBNP level decrease of >30% from baseline to Day 90 was similar in the finerenone and eplerenone groups. Although the absolute decrease in NT-proBNP was highest in the eplerenone group, this may be due to the fact that this group had the highest NT-proBNP levels at baseline. However, the incidences of the exploratory composite endpoint of death from any cause, cardiovascular hospitalization, or emergency presentation for worsening HF at Day 90 were lower in most finerenone groups compared with eplerenone. Changes in other efficacy biomarkers and QoL questionnaires were similar between finerenone and eplerenone groups.
Eplerenone was chosen as the active comparator for ARTS-HF because it was considered to be more suitable than spironolactone for patients with DM (owing to the metabolic effects of spironolactone)16,17 or CKD (owing to the safety profile of spironolactone in patients with moderate CKD)13,18 Given that >70% of the study population had an eGFR ≤60 mL/min/1.73 m2 at baseline, the starting dose of eplerenone chosen (25 mg every second day) is in line with the recommended starting dose of eplerenone in stable patients with HFrEF and CKD.2,19
Overall, patients in the finerenone 10→20 mg dose group had the greatest reduction in the composite outcome including death from any cause, cardiovascular hospitalization, or emergency presentation to hospital, compared with patients in the eplerenone group (HR: 0.56; 95% CI: 0.35, 0.90).
Together, these observations suggesting a better outcome in the finerenone 10→20 mg group compared with the other finerenone groups and the eplerenone group, combined with a safety profile that is comparable with that in the other groups, indicate that the 10 mg once-daily dose of finerenone, uptitrated to 20 mg after 30 days, would provide the best balance of safety and efficacy for further investigation in larger clinical trials.
There are mechanisms that may explain the decrease in the clinical composite endpoint in the finerenone (10→20 mg) group. Finerenone has a unique pharmacodynamic profile, which is considered to be the consequence of different physicochemical properties in comparison with steroidal MRAs. Physicochemical drug properties have a strong impact on plasma protein binding, vascular transport, and tissue penetration and distribution. Moreover, finerenone has a different mode of mineralocorticoid receptor inactivation compared with steroidal MRAs,9 which may lead to a pronounced suppression of downstream gene expression leading to hypertrophy and, thus, improved organ protection.10 However, the reason why outcomes seem to be improved, particularly in the finerenone 10→20 mg compared with the 15→20 mg group, is unclear at this stage.
Both finerenone and eplerenone were well tolerated overall, with a similar rate of adverse events across all study groups and a low incidence of renal events leading to hospitalization. Overall, 4.3% of patients experienced serum potassium concentration ≥ 5.6 mmol/L. A potassium concentration >6.0 mmol/L was observed in only five patients (0.5%), with no such elevations observed in the finerenone 2.5→5 mg, 5→10 and 10→20 mg dose groups.
Although MRA use has been limited by safety concerns in patients with HF and concomitant diabetes or renal impairment, previous studies have shown that their clinical benefits are maintained in these patient groups. In RALES,20 spironolactone efficacy was maintained in patients with an eGFR <60 mL/min/1.73 m2. In EMPHASIS-HF, the cumulative rate of the primary endpoint (HF hospitalization or cardiovascular mortality) remained significantly reduced in the eplerenone group compared with the placebo group in the subset of patients with DM or renal dysfunction.6 In EPHESUS, a history of DM and an eGFR ≤60 mL/min/1.73 m2 were each identified as risk factors for developing a serum potassium concentration ≥6.0 mEq/L, but did not alter the cardiovascular benefit of eplerenone.21
Introduction of a novel neurohormonal blocking agent into clinical practice in patients with HFrEF already on standard therapy may be challenging, particularly when the new agent should replace a cornerstone drug of this regimen. In the present study, 43% of patients were already on MRA therapy with spironolactone or eplerenone at baseline. In those patients, spironolactone and eplerenone were discontinued 48 or 24 h before randomization, respectively. This strategy was well tolerated and, thus, ARTS-HF provides some practical insights into how these agents should be introduced in the clinical practice. Pending the results of further clinical studies, it is possible that this non-steroidal MRA will provide an alternative therapy option for patients with HF in particular in those who are at high risk of developing hyperkalaemia.
Study limitations
The primary efficacy endpoint in ARTS-HF was based on a surrogate marker of efficacy: the proportion of patients who experienced a >30% reduction in NT-proBNP levels. Prospective studies showed that decreases in plasma NT-proBNP concentrations of 30% or more correlate with improved prognosis22–32 but also changes in natriuretic peptides by several drugs were not related to improved outcome in clinical trials.33–35 N-terminal pro-B-type natriuretic peptide levels may be increased in patients with atrial fibrillation and HF compared with those without atrial fibrillation. A factor to account for atrial fibrillation was included when performing an analysis of covariance for the absolute change in NT-proBNP levels. There were differences in NT-proBNP at baseline between the groups, which were also considered within the ANCOVA for absolute change in NT-proBNP levels by adjusting for logarithmized baseline NT-proBNP.10
In addition, the composite of death from any cause, cardiovascular hospitalizations, or emergency presentations for worsening HF was only a secondary endpoint and the observed event rate was low. Therefore, these findings need to be confirmed by subsequent studies with higher event rates, and longer treatment duration and follow-up, in order to draw firm conclusions.
Conclusions
ARTS-HF is the first clinical trial to compare the novel nonsteroidal MRA finerenone with eplerenone in a unique and vulnerable patient population. In patients with worsening HFrEF requiring hospitalization and who also had DM and/or CKD, finerenone reduced levels of NT-proBNP to a similar extent to that of eplerenone with a good safety profile. The present study provides the grounds for the further evaluation of this novel non-steroidal MRA in the setting of a phase 3 study and also indicates that 10→20 mg is the most suitable dosage scheme to be tested.
Authors' contributions
A.P.: performed statistical analysis; S.-Y.K., C.N.: handled funding and supervision; G.F., B.P.: handled funding and supervision; Steering Committee: acquired the data, conceived and designed the research; G.F., S.-Y.K., C.N., B.P.: drafted the manuscript; all other authors: made critical revision of the manuscript for key intellectual content.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The study was designed by the Steering Committee in collaboration with the sponsor (Bayer Pharma AG). The sponsor also had a role in data collection and performed the statistical analysis. The corresponding author had responsibility for the manuscript and decision to submit for publication. All authors participated in manuscript revision and vouch for the accuracy and completeness of the data reported. Funding to pay the Open Access publication charges for this article was provided by the Bayer Pharma AG.
Conflict of interest: S.D.A., M.B., M.G., L.K., H.K., A.P.M., B.P., P.P., A.A.V., F.Z., and G.F. were members of the Steering Committee for ARTS-HF. S.D.A. has received consulting fees from Bayer Pharma AG. M.B. has received research support and speaker honoraria from Bayer Pharma AG, Boehringer Ingelheim GmbH, Medtronic, Inc., Pfizer Inc., Servier, and St Jude Medical, Inc. G.F. is a member of the Steering Committee of trials sponsored by Bayer Pharma AG, Cardiorentis, the European Commission, Medtronic, Novartis, and Vifor Pharma. M.G. has received consulting fees for Bayer Pharma AG, Cardiocell, J&J, Novartis, Otsuka Pharmaceutical, and Takeda. H.K. has received research grants and consulting fees from Bayer Pharma AG. L.K. has been a speaker for Bayer Pharma AG, Novartis, and Servier. N.K.K., S.-Y.K., G.P., and C.N. are employees of Bayer Pharma AG. A.P.M. is a member of the Steering Committee of trials sponsored by Bayer Pharma AG, Novartis, Abbott Vascular, and Cardiorentis. A.P. provided clinical trial support funded by Bayer Pharma AG. B.P. has received consulting fees for Bayer Pharma AG, Eli Lilly and Pfizer Inc.; has stock options in Relypsa Inc.; and has a patent pending on site-specific delivery of eplerenone to the myocardium. P.P. received speaker honoraria and advisory-board-member honoraria from Bayer Pharma AG. A.A.V. has received consulting and speaker fees and/or research grants from Amgen, Bayer Pharma AG, Cardio3Biosciences, Celladon, Novartis, Merck/MSD, Servier, Takeda, Trevena, and Vifor Pharma and is supported by research grants from the European Commission (FP7-242209-BIOSTAT-CHF) and the Dutch Heart Foundation. F.Z. is a consultant or has received honoraria from Air Liquide, Bayer Pharma AG, Biomérieux, Biotronik, Boston Scientific, Janssen, Novartis, Pfizer, Resmed, Servier, St Jude, and Takeda; has received speaker fees from Mitsubishi; has stocks in Cardiorenal Diagnostics; and has received grants or research support from BG Medicine and Roche Diagnostics. B.P. has received consulting fees from Bayer Pharma AG, Pfizer, Astra Zeneca, Relypsa, Stealth peptides, Forest Laboratories, scPharmaceuticals, Sarfez; has stock options in Relypsa, scPharmaceuticals;and has a patent pending on the site-specific delivery of eplerenone to the myocardium.
Supplementary Material
Acknowledgements
This study was funded by Bayer Pharma AG. Charlotte Cookson DPhil of Oxford PharmaGenesis™ provided medical writing support funded by Bayer Pharma AG.
References
- 1. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 2. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 3. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 5. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 6. Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, Pitt B. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol 2013;62:1585–1593. [DOI] [PubMed] [Google Scholar]
- 7. Loutradis C, Tolika P, Skodra A, Avdelidou A, Sarafidis PA. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: A Nested Case-Control Study. Am J Nephrol 2015;42:351–360. [DOI] [PubMed] [Google Scholar]
- 8. Liu LC, Schutte E, Gansevoort RT, van der Meer P, Voors AA. Finerenone: third-generation mineralocorticoid receptor antagonist for the treatment of heart failure and diabetic kidney disease. Expert Opin Investig Drugs 2015;24:1123–1135. [DOI] [PubMed] [Google Scholar]
- 9. Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Perez S, Heckroth H, Nitsche A, Erguden JK, Gielen-Haertwig H, Schlemmer KH, Mittendorf J, Paulsen H, Platzek J, Kolkhof P. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem 2012;7:1385–1403. [DOI] [PubMed] [Google Scholar]
- 10. Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, Eitner F, Albrecht-Kupper B, Schafer S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 2014;64:69–78. [DOI] [PubMed] [Google Scholar]
- 11. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013;34:2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM, Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) Study Group. Effect of finerenone in patients with diabetic nephropathy. JAMA 2015;314:884–894.26325557 [Google Scholar]
- 13. Pitt B, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Nowack C, Kim SY, Pieper A, Kimmeskamp-Kirschbaum N, Filippatos G. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail 2015;17:224–232. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 15. Girerd N, Pang PS, Swedberg K, Fought A, Kwasny MJ, Subacius H, Konstam MA, Maggioni A, Gheorghiade M, Zannad F. Serum aldosterone is associated with mortality and re-hospitalization in patients with reduced ejection fraction hospitalized for acute heart failure: analysis from the EVEREST trial. Eur J Heart Fail 2013;15:1228–1235. [DOI] [PubMed] [Google Scholar]
- 16. Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail 2009;2:608–615. [DOI] [PubMed] [Google Scholar]
- 17. Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A1(c) levels in patients with chronic heart failure. Am Heart J 2010;160:915–921. [DOI] [PubMed] [Google Scholar]
- 18. Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011;32:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. SPC Eplerenone 25 mg film-coated tablets https://www.medicines.org.uk/emc/medicine/29837 .
- 20. Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012;60:2082–2089. [DOI] [PubMed] [Google Scholar]
- 21. Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008;118:1643–1650. [DOI] [PubMed] [Google Scholar]
- 22. Pascual-Figal DA, Domingo M, Casas T, Gich I, Ordonez-Llanos J, Martinez P, Cinca J, Valdes M, Januzzi JL, Bayes-Genis A. Usefulness of clinical and NT-proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur Heart J 2008;29:1011–1018. [DOI] [PubMed] [Google Scholar]
- 23. Verdiani V, Ognibene A, Rutili MS, Lombardo C, Bacci F, Terreni A, Nozzoli C. NT-ProBNP reduction percentage during hospital stay predicts long-term mortality and readmission in heart failure patients. J Cardiovasc Med 2008;9:694–699. [DOI] [PubMed] [Google Scholar]
- 24. Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004;110:2168–2174. [DOI] [PubMed] [Google Scholar]
- 25. Berry C, Murphy NF, De Vito G, Galloway S, Seed A, Fisher C, Sattar N, Vallance P, Hillis WS, McMurray J. Effects of aldosterone receptor blockade in patients with mild-moderate heart failure taking a beta-blocker. Eur J Heart Fail 2007;9:429–434. [DOI] [PubMed] [Google Scholar]
- 26. Frantz RP, Olson LJ, Grill D, Moualla SK, Nelson SM, Nobrega TP, Hanna RD, Backes RJ, Mookadam F, Heublein D, Bailey KR, Burnett JC. Carvedilol therapy is associated with a sustained decline in brain natriuretic peptide levels in patients with congestive heart failure. Am Heart J 2005;149:541–547. [DOI] [PubMed] [Google Scholar]
- 27. Fruhwald FM, Fahrleitner-Pammer A, Berger R, Leyva F, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, Daubert JC, Cleland JG. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony. Eur Heart J 2007;28:1592–1597. [DOI] [PubMed] [Google Scholar]
- 28. Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann-Zalan I, Hoersch S, Katus HA. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 2004;110:1780–1786. [DOI] [PubMed] [Google Scholar]
- 29. Iraqi W, Rossignol P, Angioi M, Fay R, Nuee J, Ketelslegers JM, Vincent J, Pitt B, Zannad F. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation 2009;119:2471–2479. [DOI] [PubMed] [Google Scholar]
- 30. Maggioni AP, Anand I, Gottlieb SO, Latini R, Tognoni G, Cohn JN. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol 2002;40:1414–1421. [DOI] [PubMed] [Google Scholar]
- 31. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Matsui T, Kinoshita M. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001;37:1228–1233. [DOI] [PubMed] [Google Scholar]
- 32. Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, Konstam MA. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail 2010;3:347–353. [DOI] [PubMed] [Google Scholar]
- 33. Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, Bevilacqua M, Salio M, Cardano P, Dunselman PH, Holwerda NJ, Tognoni G, Cohn JN, Valsartan Heart Failure Trial Investigators. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2002;106:2454–2458. [DOI] [PubMed] [Google Scholar]
- 34. Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M, SURVIVE Investigators. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007;297:1883–1891. [DOI] [PubMed] [Google Scholar]
- 35. Khand AU, Chew PG, Douglas H, Jones J, Jan A, Cleland JG. The effect of carvedilol on B-type natriuretic peptide and cardiac function in patients with heart failure and persistent atrial fibrillation. Cardiology 2015;130:153–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.