Abstract
Extravascular lung water (EVLW) is a key variable in heart failure management and prognosis, but its objective assessment remains elusive. Lung imaging has been traditionally considered off-limits for ultrasound techniques due to the acoustic barrier of high-impedance air wall. In pulmonary congestion however, the presence of both air and water creates a peculiar echo fingerprint. Lung ultrasound shows B-lines, comet-like signals arising from a hyper-echoic pleural line with a to-and-fro movement synchronized with respiration. Increasing EVLW accumulation changes the normal, no-echo signal (black lung, no EVLW) into a black-and-white pattern (interstitial sub-pleural oedema with multiple B-lines) or a white lung pattern (alveolar pulmonary oedema) with coalescing B-lines. The number and spatial extent of B-lines on the antero-lateral chest allows a semi-quantitative estimation of EVLW (from absent, ≤5, to severe pulmonary oedema, >30 B-lines). Wet B-lines are made by water and decreased by diuretics, which cannot modify dry B-lines made by connective tissue. B-lines can be evaluated anywhere (including extreme environmental conditions with pocket size instruments to detect high-altitude pulmonary oedema), anytime (during dialysis to titrate intervention), by anyone (even a novice sonographer after 1 h training), and on anybody (since the chest acoustic window usually remains patent when echocardiography is not feasible). Cardiologists can achieve much diagnostic gain with little investment of technology, training, and time. B-lines represent ‘the shape of lung water’. They allow non-invasive detection, in real time, of even sub-clinical forms of pulmonary oedema with a low cost, radiation-free approach.
Keywords: Oedema, Lung, Ultrasound, Water
B-lines for imaging pulmonary congestion
Pulmonary congestion is an almost universal finding in patients with acute heart failure. It may be related to heterogeneous mechanisms such as fluid retention with weight gain in patients with reduced ejection fraction and/or fluid redistribution to the lungs, usually without weight variation, in patients with preserved ejection fraction and a noncompliant cardiovascular system.1 Clinical symptoms and signs of pulmonary congestion resulting in interstitial and alveolar oedema are a late event in acute heart failure syndrome. The identification of pulmonary congestion with a reliable and objective non-invasive imaging biomarker would confer a pathophysiological, diagnostic, therapeutic, and prognostic advantage to the clinical cardiologist.1 In patients with impending acute heart failure syndrome, there is often a relatively long incubation period, of days and weeks, during which there is a gradual accumulation of extravascular lung water (EVLW), a term used to describe water within the lungs but outside pulmonary vasculature. Detection and treatment of pulmonary congestion before it is clinically evident can prevent hospitalization and progression of heart failure. Post-discharge freedom from pulmonary congestion is associated with a better prognosis. Therefore, the possibility to image pulmonary oedema at a sub-clinical stage remains an attractive and elusive goal. Several clinical, radiological, and non-imaging methods are currently used for evaluating pulmonary fluid retention, but they are late and inaccurate (physical examination), insensitive and imprecise (chest X-ray), too complex for real time, repeated measurements in sick patients (computerized tomography), or inadequately validated and not widely available (conductance measurements with cardiac devices).1 Lung ultrasound (LUS) assessment of EVLW by B-lines provides an excellent alternative,2,3 expanding the already established role of transthoracic echocardiography in heart failure.4 B-lines can be visualized using the cardiac transducer and appear on the antero-lateral chest scan as multiple laser-like signals arising from the hyper-echoic pleural line, changing throughout the respiratory cycle, with a to-and-fro movement synchronized with respiration (Figure 1). The normal lung is black (no signal); the abnormal wet lung with interstitial pulmonary oedema is black and white (with some B-lines departing from the pleural line); and the lung with alveolar pulmonary oedema is white (confluent B-lines in a fully echogenic lung).
Figure 1.
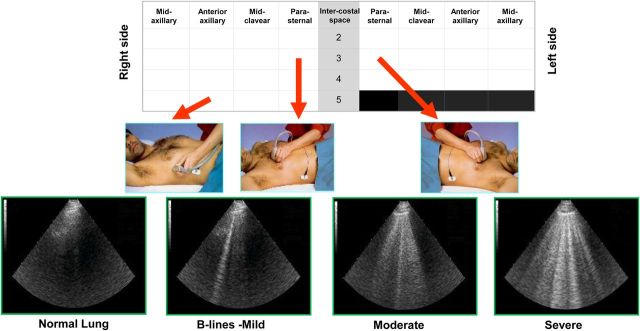
The recommended protocol for evaluating B-lines is performed by scanning 28-region protocol on the anterior chest with the patient in the supine position.3,4,23
The original term of comet-tail artefacts2 was subsequently used interchangeably with ultrasound lung comets.4 Following the consensus on terminology and standards, B-lines is now preferred.5
The term comets intuitively refers to the eye-catching pictorial image, spreading like a rocket or the tail of a comet from the ultrasound transducer to the edge of the screen. B-lines may sound more familiar for radiologists, as the ultrasound counterpart of the well-known radiological Kerley B-lines, a sign of EVLW on chest X-ray. The analogy is however imperfect, because A-lines on chest X-ray (departing from the hila out to the periphery of the lung) are also a sign of abnormal accumulation of EVLW. In contrast, with LUS, ‘A-lines’ refer to the normal appearance of horizontal, equidistant, parallel artefacts originating at regular intervals from the pleural line (visceral and parietal pleura) (Table 1).
Table 1.
Primer of lung ultrasound for cardiologists
| Sign | Description | Meaning |
|---|---|---|
| A-lines | Horizontal, parallel lines beyond the pleura | Normal artefacts |
| B-lines | Vertical, comet-tail-like lines fanning out from pleural line | EVLW accumulation |
| Pleural line | Echo dense line | Parietal and visceral pleura |
| Pleural effusion | Echo-free pleura-lung space | Pleural effusion |
The number of B-lines in the antero-lateral chest scan is usually summed to generate a quantitative or semi-quantitative B-line score (Table 2). Up to 2 B-lines per single intercostal space, or up to 5 in the comprehensive antero-lateral chest scan can be a normal finding,2,4 more frequent in the latero-basal areas.
Table 2.
Scoring of B-lines
| Score | Number of B-lines | EVLW |
|---|---|---|
| 0 | ≤5 | Absent |
| 1 | 6–15 | Mild degree |
| 2 | 16–30 | Moderate degree |
| 3 | >30 | Severe degree |
Genesis and determinants of B-lines
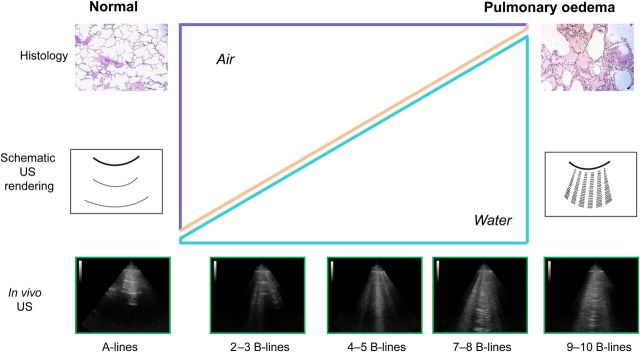
In analogy with the counterpart of radiological B-lines, it was initially proposed that the acoustic interface generating B-lines is the sub-pleural interlobular septa thickened by oedema.2,3,4 This anatomical model is conceptually helpful and appealingly simple, but probably an oversimplification. According to the biophysical model developed from in vitro and ex vivo models, the origin of B-lines is not from a precise anatomic structure (interlobular septa) but rather from reflections of discrete air/fluid interfaces between collapsed, fluid-filled, and well-aerated alveoli.6,7 The appearance of B-lines corresponds to a progressive loss of air per volume of lung tissue with a corresponding increase in relative and absolute content of EVLW (from normal, <5% or <500 mL, to overt pulmonary oedema, >90% or >2000 mL) (Figure 2).
Figure 2.
From left to right panels, the normal lung, then increasing severity of pulmonary oedema. From top to bottom, the corresponding pathology (first row), schematic ultrasound pattern (second row), and in vivo ultrasound texture (bottom). The increase in relative content of water, and decrease of content of air in the lung (middle panel), reshapes the texture of clinical image (last row) from normal, A-lines to abnormal B-lines (water and air mix).2,4
The B-lines are detectable with cardiac, convex, or linear probes, from 2.5 (cardiac) up 7.5 MHz (vascular) transducers.5 With a linear probe, B-lines run in parallel over the screen, whereas with the curvilinear probe, B-lines spread from the same proximal point of convergence.
The depth should be adjusted according to the body habitus of the patient, with thin patients requiring less depth and obese patients needing greater depth to visualize the pleural line. Neither the width of the sector angle nor the use of harmonic (instead of fundamental) imaging are known to have an impact on the B-lines count, although no systematic data are available.4,5 Higher frequencies and macro probes are useful for the evaluation of pleural line and sub-pleural space and may allow a better image of B-lines, but the overall number does not change significantly by changing the transducer.4,5
The experimental and clinical validation of B-lines for extravascular lung water
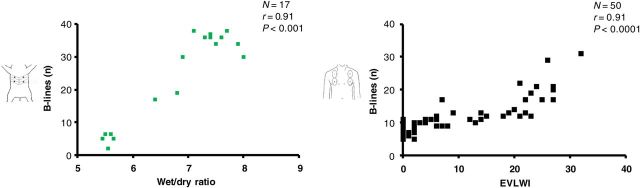
Lung ultrasound has been applied to dogs with spontaneous left-sided heart failure8 and to a pig model of oleic acid-induced lung injury, which mimics human acute respiratory distress syndrome.9,10 In dogs and pigs, the same instrument, transducer, and chest scan technique as used in humans can be adopted, making the translational implications more straightforward. In the pig model, there was a tight, linear correlation between the number of B-lines and wet/dry ratio by gravimetric method post-mortem, which is the gold standard for EVLW9 (Figure 3, left panel). In man, the number of B-lines, even assessed with a limited four-region scan of the anterior chest, correlate closely with invasive evaluation of EVLW,11,12 matching the experimental data (Figure 3, right panel). In intensive care patients with invasive gold standard transpulmonary thermodilution measurements of EVLW, LUS performed markedly better than chest radiography (r = 0.91 vs. r = 0.33), with a 92.3% sensitivity and 91.7% specificity for detecting abnormal values of EVLW.12
Figure 3.
A very good correlation (R = 0.91 in both cases) is shown between B-lines and extravascular lung water evaluated experimentally by gravimetry in pigs with acute lung injury induced by oleic acid (left panel) and clinically by thermodilution in patients with acute lung injury-acute respiratory distress syndrome (right panel).9,12
The same pig model was used to address the equally clinically relevant question as to whether B-lines could detect increased EVLW before the appearance of functional impairment. B-lines showed accumulation of EVLW very early in the course of lung injury in pigs, at a stage when no changes in hemo-gas analytic parameters or chest X-ray findings could be observed10 (Figure 4).
Figure 4.
Sequence of events in a pig model of acute respiratory distress syndrome. B-lines occur early (already significantly increased at 15 min); only at a later stage (at 90 min) were changes in haemogasanalytic parameters significant.10 ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
In the clinical setting, several groups have shown how B-lines correlate reasonably well with the imperfect gold standards of chest X-ray4,13 and CT14 radiological score. The correlation is stronger when intra-patient variations are considered.4
B-lines weakly correlate with clinical congestion score, cardiac peptide levels,15–18 and pulmonary wedge pressures.11,19 This is not unexpected, as in everyday practice, a patient can show variable degrees of B-lines (from absent to severe) for any given level of pulmonary artery wedge pressure, depending on the duration of history of heart failure, speed of changes in pulmonary pressure, characteristics of the alveolar-capillary membrane, oncotic pressure, and lymphatic drainage capacity. B-lines without haemodynamic congestion are found in acute respiratory distress syndrome and environmental pulmonary oedema. B-lines are EVLW, not pulmonary wedge pressure.20
Diagnostic and prognostic value in cardiology patients
B-lines are useful for the identification of cardiogenic origin of dyspnoea in the emergency room, with high sensitivity and excellent specificity, yielding a 94% sensitivity and 92% specificity in differentiating acute heart failure syndrome from non-cardiac causes of acute dyspnoea as shown by a meta-analysis encompassing 1075 patients from seven different studies.21 These results were recently corroborated by a multicentre study which enrolled 1005 patients from seven Italian centres and showed that the LUS-based approach was more accurate than initial clinical work-up, chest X-ray, and natriuretic peptides.22
B-lines have a limited specificity and can be found in the area surrounding isolated alveolar consolidations, from infectious, infiltrative, or traumatic lung disease. Oedematous (wet) B-lines cannot be readily distinguished from fibrotic (dry) B-lines associated with thickening of sub-pleural intralobular or interlobular septa, as found for instance in systemic sclerosis.2,4,5 The consideration of the limited specificity is also important in assessing the diagnostic value and high sensitivity of LUS for detection of pulmonary congestion, which is strongly dependent on patient selection and comorbidities. In addition, it is not always possible with B-lines alone to separate EVLW accumulation due to heart failure or acute respiratory distress syndrome, although the latter may show a more inhomogeneous and irregular pattern, sub-pleural consolidation, highly fragmented pleural line, and multiple B-lines alternating with spared areas.5,20 Differentiation by LUS should include consideration of the clinical context and may be supported by other modalities such as echocardiography, which readily detects abnormal cardiac or valvular function and increased pulmonary artery pressure during acute heart failure syndrome.3,4
B-lines also have a striking prognostic value, shown in patients with heart failure,23–25 acute coronary syndrome,26 and dialysis.27 Persistent B-lines at pre-discharge assessment are associated with a more malignant prognosis than B-lines on admission only.28,29 End-stage kidney disease patients with concomitant heart failure with very severe congestion (>60 B-lines) had a 4.2-fold increased risk of death, compared with patients having no or mild congestion (<15 B-lines).27 The prognostic value is independent and additive over established clinical, imaging, and laboratory markers, such as NYHA class of dyspnoea, pulmonary congestion signs such as crackles, left ventricular ejection fraction, pulmonary artery systolic pressure, or cardiac natriuretic peptide levels.23–27
Lung ultrasound also provides a useful biomarker to assess the time course of lung water changes following interventions, since within minutes or a few hours, B-lines in heart failure patients are substantially reduced after diuretics,3,30 or following dialysis.31
B-lines stress echocardiography
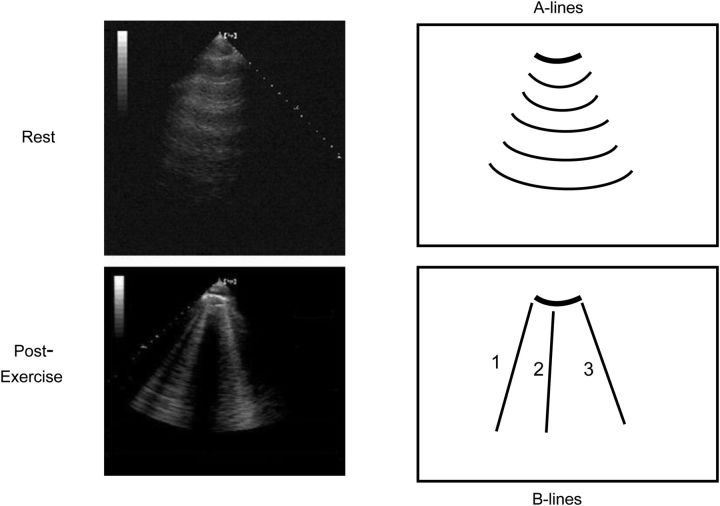
Exercise may result in the sudden appearance or increase of B-lines on the chest in heart failure patients (Figure 5), in whom an increase in left ventricular filling pressures may occur with or without inducible ischaemia.32,33 When B-lines develop or worsen during exercise, resting levels of cardiac peptides and pulmonary pressures are higher, as are the chances of an unfavourable outcome during short-term follow-up.33
Figure 5.
Lung ultrasound (third right intercostal space) at rest (upper panel) and immediately after exercise (lower panel). The exercise-induced appearance of B-lines eclipses the normal pattern of A-lines present at rest.32
In addition, exercise-induced B-lines are detectable in patients with chronic mountain sickness during exercise performed at 3.600 m, but not in healthy high-altitude dwellers.34 The rapid lung interstitial fluid accumulation can be prevented by oxygen inhalation.
Stress echo with B-lines can also be performed outdoors, with pocket size instruments, in an entirely different setting of ecological stress. The diagnostic target is the diagnosis, or early subclinical identification, of life-threatening pulmonary oedema. In this challenging but fascinating context, LUS detects B-lines in 20–50% of super-fit subjects in extreme physiology settings, such as high altitude35,36 or deep underwater apnoea diving37,38 (Figure 6).
Figure 6.
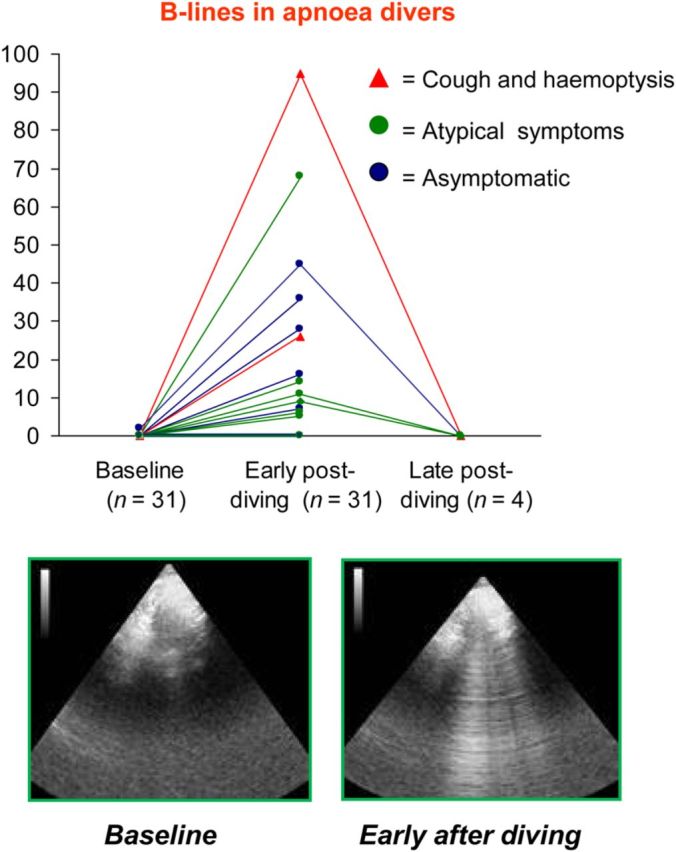
Outdoor stress echo with lung ultrasound for the diagnosis of subclinical pulmonary oedema. A typical image of lung ultrasound before (left panel, normal) and soon after (right panel, B-lines) apnoea diving. Scan is made by a sonographer on the boat supporting divers in Red Sea.37
Therefore, stress lung ultrasound may be useful to detect exercise-induced heart failure and also to appreciate lung interstitial fluid lung accumulation in the setting of extreme physiology (Table 3).
Table 3.
B-lines stress echocardiography: from indoor to outdoor
| Stress echo application | Indoors | Outdoors |
|---|---|---|
| Instrument | High cost, high weight | Low cost, light weight |
| Subjects | Patients with exertional dyspnoea or heart failure | Very fit normals |
| Stress | Semi-supine exercise | Extreme physical activitya |
| Environment | Echo laboratory | Ecologicalb |
| Scan anterior chest | 28 regions (full information) | Four regions (to save time) |
| Stress physiology | Artificial | Real life |
| Management changes | Titrating diuretic therapy | Stopping exposurec |
aApnoea diving, marathon, triathlon, and trekking.
bDesert, high-altitude, and deep sea.
cAt a subclinical stage, preventing life-threatening pulmonary oedema.
Lung ultrasound in extreme physiology setting is perhaps the best example of a new paradigm of ‘ecologic’ stress echocardiography, performed outside the controlled hospital setting, where some conditions—for instance, mental stress, psychological discomfort, environmental aggression, or extreme physiology states—cannot be reproduced.39
Advantages
Lung ultrasound is radiation-free, which will reduce the need for chest-X rays in cardiology patients; such patients have commonly been subjected to increased levels of diagnostic radiation exposure.40 Lung ultrasound requires an imaging time of a few minutes. Cardiac transducers work well for LUS, since they are designed with a small footprint, allowing easy scanning between rib interspaces.
The methodology and findings of B-lines are similar in pigs, dogs, and man.8–10
In consecutive patients studied in a cardiology environment, such as echocardiography laboratory or intensive care unit, the feasibility of LUS approaches 100%, with intra- and inter-observer variability consistently <5% and <10%, respectively,3,11,18 and a mean difference of 0.3 B-lines between readers.25 The reproducibility is excellent; thus, B-lines are ideally suited as a biomarker to assess intra-patient changes over time.
B-lines are appealingly simple to use, to learn, and to teach. A 1-h training session and a pocket size instrument are sufficient for a novice sonographer to reach the same accuracy in counting B-lines of a highly experienced cardiologist with top-level equipment.41,42 B-lines are readily obtainable; their assessment should be considered in the primary care of the dyspnoic patient.
Lung ultrasound study provides considerable information beyond assessing the presence of simple B-lines, from pleural effusion to consolidations and pneumothorax. As B-lines originate from the visceral pleura, their simple presence proves that the visceral pleura is opposing the parietal pleura, thus excluding pneumothorax at that point. The absence of any movement of the pleural line, either horizontal (sliding) or vertical (pulse), coupled with the absence of B-lines strongly supports a clinical suspicion of pneumothorax.5,20
Limitations
For LUS, large wound dressings and subcutaneous emphysema may limit access in <1% of subjects. Soft tissue oedema or morbid obesity can degrade the quality of images. In general, LUS complements poor acoustic windows, and its feasibility is nearly 100%.20
Lung ultrasound only evaluates the sub-pleural cortical area, extending for a depth of 2–4 cm.
The separation of wet, watery B-lines from fibrotic, dry, B-lines as can be found in systemic sclerosis can be difficult with an isolated LUS assessment: clinical context, integration with echocardiography and dynamic evaluation are recommended.43 An accurate way to identify wet B-lines is to follow their acute variation within minutes, since they increase during exercise32,33 or volume loading20 and decrease following diuretics3,30 or dialysis31 (Table 4). There are also posture-dependent changes in lung water, with 25% more B-lines in the supine compared with the sitting position in patients with acute heart failure.44
Table 4.
The dual nature of B-lines: wet or dry
| B-lines nature | Wet | Dry |
|---|---|---|
| Dominant component | Water | Fibrosis |
| Underlying pathology | Heart Failure | Interstitial lung disease |
| Pleural line | Regular, smooth | Irregular, thickened |
| Effects of diuretics/dialysis | Acute decrease | No change |
| From sitting to supine | Acute increase | No change |
| Effects of volume loading | Acute increase | No change |
| Effects of exercise | Acute increase | No change |
Future perspectives
The diagnosis will become more objective through simple soft computing algorithm.46 Major scientific societies might clear the field from persisting terminology confusion and the lack of standardization and better tailor the LUS to the needs of the cardiological imaging community. Large-scale prognostic validation in heart failure patients undergoing exercise stress echo is under way as a specific subproject of the ‘Stress Echo 2020’ study endorsed by the Italian Society of Echocardiography. Randomized multicentre studies are testing the hypothesis that B-line-guided therapy may improve clinical outcome in high-risk haemodialysis patients with cardiomyopathy. Therefore, the dissemination of the technique due to its obvious merits should go in parallel with an adequate assessment in outcome studies. The clinical use of the technique still is ancillary. Large-scale, randomized trials are needed to document to what extent its use would benefit the cardiac patient with a failing heart and to appreciate its limitations which could result in inappropriate therapy.
Current recommendations
Bedside LUS was recognized in a scientific statement of ESC since 2010 as a ‘potentially useful way to assess pulmonary congestion’,1 and recommended in 2015 as a first-line test in the evaluation of suspected acute heart failure to assess pulmonary congestion,47,48 since ‘in reasonably expert hands LUS maybe equally or more informative than chest X-ray allowing also an important time saving’.48 The 2012 recommendations of an international committee on point-of-care LUS recognized LUS as ideally suited for ‘monitoring pulmonary congestion changes in heart failure patients as they disappear or clear upon adequate medical treatment’.5
According to the 2016 EACVI recommendations, during exercise stress echo the acute increase in B-lines detected by LUS is a feasible way for demonstrating that the symptom ‘dyspnoea when exercising’ is related to pulmonary congestion due to backward heart failure.49
EACVI recommendations for use of pocket size devices explicitly list ‘semi-quantification of EVLW’ among the top 8 indications for pocket size devices.50
Conclusions
B-lines are a sign of interstitial syndrome of mixed origin (fibrosis, inflammation, and congestion), and in acute or chronic heart failure patients they mostly represent the direct, positive image of lung water, for a long time a forbidden fruit for the clinical cardiologist. Through the lung water teaser, the cardiologist will become familiar with the new diagnostic world of LUS, a friendly neighbour of transthoracic echocardiography, of critical help in many different and frequent clinical situations, from diagnosis and semi-quantification of pleural effusion to fluid management in cardiogenic shock to pneumothorax identification in differential diagnosis of dyspnoea.5,20,45 The rapidly dynamic nature of B-lines separates wet B-lines made by water (decreased by diuretics) from dry B-lines made of connective tissue. B-lines can be used anywhere (even in extreme environmental conditions with pocket size devices), by anyone (including novice sonographer) on anybody (since the superficial acoustic window that is required usually is feasible even when echocardiography is not feasible). Rarely, in cardiac imaging, we have so much diagnostic gain with so little technological, training, and time pain. Lung ultrasound can quantify lung oedema noninvasively in real time, even at an early subclinical stage, with user-friendly, low cost, radiation-free, and direct imaging of EVLW.
Authors’ contributions
E.P., P.A.P. performed statistical analysis, handled funding and supervision, acquired the data, conceived and designed the research, drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Funding
The study was partially funded by CNR-MIUR (National Research Council, Italian Ministry of University and Research) Ageing subproject (Progetto PI.P02 Progetto di Interesse-Invecchiamento). Funding to pay the Open Access publication charges for this article was provided by Italian National Research Council.
Conflict of interest: none declared.
References
- 1. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading heart failure in acute heart failure: a scientific statement from the acute heart failure committee of the Heart failure association of the European Society of cardiology and endorsed by the European Society of Intensive Care medicine. Eur J Heart Failure 2010;12:423–433. [DOI] [PubMed] [Google Scholar]
- 2. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997;156:1640–1646. [DOI] [PubMed] [Google Scholar]
- 3. Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004;93:1265–1270. [DOI] [PubMed] [Google Scholar]
- 4. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr 2006;19:356–363. [DOI] [PubMed] [Google Scholar]
- 5. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577–591. [DOI] [PubMed] [Google Scholar]
- 6. Soldati G, Inchingolo R, Smargiassi A, Sher S, Nenna R. Ex-vivo lung sonography: morphologic – ultrasound relationship. Ultrasound Med Biol 2012;38:1169–1170. [DOI] [PubMed] [Google Scholar]
- 7. Spinelli A, Vinci B, Tirella A, Matteucci M, Gargani L, Ahluwalia A, Domenici C, Picano E, Chiarelli P. Realization of a poro-elastic ultrasound replica of pulmonary tissue. Biomatter 2012;2:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rademacher N, Pariaut R, Pate J, Saelinger C, Kearney MT, Gaschen L. Transthoracic lung ultrasound in normal dogs and dogs with cardiogenic pulmonary edema: a pilot study. Vet Radiol Ultrasound 2014;55:447–452. [DOI] [PubMed] [Google Scholar]
- 9. Jambrik Z, Gargani L, Adamicza A, Kaszaki J, Varga A, Forster T, Boros M, Picano E. B-lines quantify the lung water content: a lung ultrasound versus lung gravimetry study in acute lung injury. Ultrasound Med Biol 2010;36:2004–2010. [DOI] [PubMed] [Google Scholar]
- 10. Gargani L, Lionetti V, Di Cristofano G, Bevilacqua G, Recchia FA, Picano E. Early detection of acute lung injury uncoupled to hypoxemia in pigs using ultrasound lung comets. Crit Care Med 2007;35:2769–2774. [DOI] [PubMed] [Google Scholar]
- 11. Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. Ultrasound comet-tail images. A comparative study with wedge pressure and extravascular lung water. Chest 2005;127:1690–1695. [DOI] [PubMed] [Google Scholar]
- 12. Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, Kruse JM. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care 2015;19:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med 2008;28:585–591. [DOI] [PubMed] [Google Scholar]
- 14. Baldi G, Gargani L, Abramo A, D'Errico L, Caramella D, Picano E, Giunta F, Forfori F. Lung water assessment by lung ultrasonography in intensive care: a pilot study. Intensive Care Med 2013;39:74–84. [DOI] [PubMed] [Google Scholar]
- 15. Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, Picano E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail 2008;10:70–77. [DOI] [PubMed] [Google Scholar]
- 16. Liteplo AS, Marill KA, Villen T, Miller RM, Murray AF, Croft PE, Capp R, Noble VE. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009;16:201–210. [DOI] [PubMed] [Google Scholar]
- 17. Prosen G, Klemen P, Štrnad M, Grmec S. Combination of lung ultrasound (a comet-tail sign) and N-terminal pro-brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Crit Care 2011;15:R114; 10.1186/cc10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miglioranza MH, Gargani L, Sant'Anna RT, Rover MM, Martins VM, Mantovani A, Weber C, Moraes MA, Feldman CJ, Kalil RA, Sicari R, Picano E, Leiria TL. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141–1151. [DOI] [PubMed] [Google Scholar]
- 19. Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, Luberto L, Anile A, Cerutti E, Radeschi G, Frascisco MF. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014;121:320–327. [DOI] [PubMed] [Google Scholar]
- 20. Lichtenstein D. Lung ultrasound in the critically ill. Review. Annals Intensive Care 2014;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med 2014;21:843–852. [DOI] [PubMed] [Google Scholar]
- 22. Pivetta E, Goffi A, Lupia E, Tizzani M, Porrino G, Ferreri E, Volpicelli G, Balzaretti P, Banderali A, Iacobucci A, Locatelli S, Casoli G, Stone MB, Maule MM, Baldi I, Merletti F, Cibinel GA, SIMEU Group for Lung Ultrasound in the Emergency Department in Piedmont. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU Multicenter Study. Chest 2015;148:202–210. [DOI] [PubMed] [Google Scholar]
- 23. Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007;13:830–835. [DOI] [PubMed] [Google Scholar]
- 24. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail 2015;21:548–554. [DOI] [PubMed] [Google Scholar]
- 25. Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016;37:1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bedetti G, Gargani L, Sicari R, Gianfaldoni ML, Molinaro S, Picano E. Comparison of prognostic value of echocardiographic risk score with the Thrombolysis in Myocardial Infarction (TIMI) and Global Registry in Acute Coronary Events (GRACE) risk scores in acute coronary syndrome. Am J Cardiol 2010;106:1709–1716. [DOI] [PubMed] [Google Scholar]
- 27. Zoccali C, Torino C, Tripepi R, Tripepi G, D'Arrigo G, Postorino M, Gargani L, Sicari R, Picano E, Mallamaci F, on behalf of the Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 2013;24:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gargani L, Pang PS, Frassi F, Miglioranza MH, Dini FL, Landi P, Picano E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound 2015;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, Tritto I, Zannad F, Girerd N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015;17:1172–1181. [DOI] [PubMed] [Google Scholar]
- 30. Facchini C, Malfatto G, Giglio A, Facchini M, Parati G, Branzi G. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J Cardiovasc Med (Hagerstown) 2015[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 2009;135:1433–1439. [DOI] [PubMed] [Google Scholar]
- 32. Agricola E, Picano E, Oppizzi M, Marino G, Zangrillo A, Margonato A. Assessment of stress-induced pulmonary interstitial edema by chest ultrasound during exercise echocardiography and its correlation with left ventricular function. J Am Soc Echocardiogr 2006;19:457–463. [DOI] [PubMed] [Google Scholar]
- 33. Scali MC, Simionuc A, Mandoli GE, Dini FL, Marzilli M, Picano E. The added value of exercise-echocardiography in heart failure patients: assessing dynamic changes in extravascular lung water. Eur Heart J Cardiovascul Imaging 2015;16 (suppl 2):S102–S129. [Google Scholar]
- 34. Pratali L, Rimoldi SF, Rexhaj E, Hutter D, Faita F, Salmòn CS, Villena M, Sicari R, Picano E, Allemann Y, Scherrer U, Sartori C. Exercise induces rapid interstitial lung water accumulation in patients with chronic mountain sickness. Chest 2012;141:953–958. [DOI] [PubMed] [Google Scholar]
- 35. Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest 2007;131:1013–1018. [DOI] [PubMed] [Google Scholar]
- 36. Pratali L, Cavana M, Sicari R, Picano E. Frequent subclinical high-altitude pulmonary edema detected by chest sonography as ultrasound lung comets in recreational climbers. Crit Care Med 2010;38:1818–1823. [DOI] [PubMed] [Google Scholar]
- 37. Frassi F, Pingitore A, Cialoni D, Picano E. Chest sonography detects lung water accumulation in healthy elite apnea divers. J Am Soc Echocardiogr 2008;21:1150–1155. [DOI] [PubMed] [Google Scholar]
- 38. Boussuges A, Coulange M, Bessereau J, Gargne O, Ayme K, Gavarry O, Fontanari P, Joulia F. Ultrasound lung comets induced by repeated breath-hold diving, a study in underwater fishermen. Scand J Med Sci Sports 2011;21:e384–e392. [DOI] [PubMed] [Google Scholar]
- 39. Picano E, Pellikka PA. Stress echo applications beyond coronary artery disease. Eur Heart J 2014;35:1033–1040. [DOI] [PubMed] [Google Scholar]
- 40. Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, Bar O, Maccia C, Pierard L, Sicari R, Plein S, Mahrholdt H, Lancellotti P, Knuuti J, Heidbuchel H, Di Mario C, Badano LP. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014;35:665–672. [DOI] [PubMed] [Google Scholar]
- 41. Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Mottola G. Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc Ultrasound 2006;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiem AT, Cham CH, Ander DS, Kobylivker AN, Manson WC. Comparison of expert and novice sonographers’ performance in focused lung ultrasonography in dyspnea (FLUID) to diagnose patients with heart failure syndrome. Acad Emer Med 2015;22:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gargani L, Doveri M, D'Errico L, Frassi F, Bazzichi ML, Delle Sedie A, Scali MC, Monti S, Mondillo S, Bombardieri S, Caramella D, Picano E. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford) 2009;48:1382–1387. [DOI] [PubMed] [Google Scholar]
- 44. Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T, Lewis EF. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care 2015;4:326–332. [DOI] [PubMed] [Google Scholar]
- 45. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brattain LJ, Telfer BA, Liteplo AS, Noble VE. Automated B-line scoring on thoracic sonography. J Ultrasound Med 2013;32:2185–2190. [DOI] [PubMed] [Google Scholar]
- 47. Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, Flachskampf FA, Hassager C, Pasquet A, Gargani L, Galderisi M, Cardim N, Haugaa KH, Ancion A, Zamorano JL, Donal E, Bueno H, Habib G. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular care Association. Eur Heart J Cardiov Imaging 2015;16:119–146. [DOI] [PubMed] [Google Scholar]
- 48. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite-Moreira AF, Bellou A, Anker SD, Filippatos G. (2015) Recommendations management on pre-hospital and hospital of acute heart failure: a consensus paper of the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur Heart J 2015;17:544–558. [DOI] [PubMed] [Google Scholar]
- 49. Lancellotti P, Pellikka PA, Budts W, Chaudry F, Donal E, Dulgheru R, Edvarsen T, Garbi M, Ha JW, Kane G, Kreeger J, Mertens L, Pibarot P, Picano E, Ryan T, Tsutsui J, Varga A. Recommendations for the clinical use of stress echocardiography in non-ischemic heart disease: joint document of the European Association of Cardiovascular imaging and the American Society of Echocardiography. Eur Heart J Cardiov Imaging 2016. [DOI] [PubMed] [Google Scholar]
- 50. Sicari R, Galderisi M, Voigt JU, Habib G, Zamorano JL, Lancellotti P, Badano LP. The use of pocket-size imaging devices: a position statement of the European Association of Echocardiography. Eur J Echocardiogr 2011;12:85–87. [DOI] [PubMed] [Google Scholar]