Abstract
Background and Aims:
Faecal microbiota transplantation is a successful therapy for patients with refractory Clostridium difficile infections. It has also been suggested as a treatment option for inflammatory bowel disease, given the role of the intestinal microbiota in this disease. We assessed the impact of faecal microbiota transplantation in patients with inflammatory bowel disease and studied predictors of clinical (non-)response in microbial profiles of donors and patients.
Methods:
Fourteen refractory patients (8 with ulcerative colitis and 6 with Crohn’s disease) underwent ileocolonoscopy with faecal microbiota transplantation through a nasojejunal (n = 9) or rectal (n = 5) tube. Efficacy was assessed by endoscopic healing at week 8, clinical activity scores and C-reactive protein measurement. Faecal microbiota was analysed by 16S rDNA pyrosequencing.
Results:
There was no significant improvement among the 6 patients with Crohn’s disease at week 8 following faecal microbiota transplantation. One patient experienced temporary clinical remission for 6 weeks. In contrast, 2/8 patients with ulcerative colitis had endoscopic remission at week 8, and of the 6 remaining patients with ulcerative colitis, 1 reported temporary remission for 6 weeks. The donor microbiota richness and the number of transferred phylotypes were associated with treatment success. Persistent increased C-reactive protein 2 weeks after transplantation was predictive of failure of response.
Conclusion:
Faecal microbiota transplantation led to endoscopic and long-term (>2 years) remission in 2 out of 8 ulcerative colitis patients. Higher donor richness was associated with successful transplant. Therefore, faecal microbiota transplantation with donor prescreening could be a treatment option for selected refractory ulcerative colitis patients.
Key Words: Faecal microbiota transplantation (FMT), Roseburia, Oscillibacter
1. Introduction
The microbiota plays an important role in the onset and perpetuation of inflammatory bowel disease (IBD), a chronic disease with onset during childhood or adolescence and leading to symptoms of bloody diarrhoea with abdominal cramps and anorexia. The inflammation has a predilection for the terminal ileum in the case of Crohn’s disease (CD) and the colon starting from the anal margin in the case of ulcerative colitis (UC). Unresolved inflammation may lead to complications such as intestinal strictures or fistulas and abscesses. The contribution of bacteria in the disease pathogenesis has been shown by diverse studies.1,2 Intestinal microbiota are essential for the development of inflammation in murine models of colitis and intensive genetic collaborative studies have identified susceptibility genes involved in the recognition of bacterial peptides and elimination of intracellular bacteria.1,3,4 Furthermore, the intestinal mucosa in postoperative CD remains intact after diversion of the faecal stream, while recurrence of inflammation is observed after exposure of the gut to luminal contents.5
Gut commensal bacteria live in normobiosis with the host and have important metabolic, protective and trophic functions.6 The overall composition of the gut microbiota and the presence or absence of specific species is important for homeostasis and tolerance of the immune system.6,7 The development of high-throughput sequencing technologies has facilitated metagenomic research in determining the complexity and immense diversity of microbial life in various ecological niches.8–10 Metagenomic analysis demonstrated significant interindividual variation in gut microbiota composition,11–13 described as continuous gradients14 or distinct microbiota clusters (‘enterotypes’11 or ‘co-abundance groups’14).
At present there is no clear evidence for a single pathogen causing IBD. On the other hand, marked alterations in microbial communities are observed in IBD patients. Patients with IBD have fewer anti-inflammatory bacteria and/or more proinflammatory bacteria. Such dysbiosis has been well described in CD and more recently also in UC.15–22 A reduction in Faecalibacterium prausnitzii is the most replicated species-specific finding so far and has been confirmed in faecal and mucosal samples.18,20,23–26 This species has anti-inflammatory and immunomodulatory effects in vivo and in vitro.27 In addition to F. prausnitzii, the adherent invasive Escherichia coli (AIEC) is increased in ileal mucosa of CD patients and may sustain inflammation.28–30
Faecal microbiota transplantation (FMT) is defined as the transfer of intestinal microbiota from a healthy donor and aims at restoring a stable microbial community in the gut of the acceptor. Faecal microbiota transplantation has already been shown to be very effective for refractory and recurrent Clostridium difficile infection.31 Given the importance attributed to the intestinal microbiota in IBD, manipulation of its composition by FMT might be a promising treatment if it could restore essential components of the microbiota, thereby reversing the inflammatory processes observed in this disease. Two randomized controlled trials and several case reports using FMT in IBD have been reported recently; most studies were performed in UC patients.32–42 We investigated the safety and efficacy of FMT in CD and UC patients refractory to conventional treatments in an open-label pilot study. We specifically assessed the influence of FMT on the diversity and composition of the microbiota in these patients and searched for predictors of (non-)response to FMT in the microbial profiles of the donors and patients.
2. Methods
2.1. Patients and donor
Between August 2011 and November 2012, 14 patients consented to undergo FMT (6 CD and 8 UC patients). The study protocol was approved by the ethics committee of University Hospitals Leuven. Only patients with intractable IBD could be included in this pilot study. Patients needed to have failed therapy with immunomodulators and with anti-TNFs. Patients with UC were eligible when they had left-sided colitis or pancolitis and CD patients were eligible if they had extensive involvement of the ileum and/or colon. All but one CD patient had ileocolonic disease and all but one UC patient had extensive colitis (Table 1). Patients were not eligible to participate if they had severe comorbidities (including cardiac, pulmonary, renal and/or hepatic comorbidities), short bowel, a permanent ileostomy or an ileoanal pouch, or if they were unable to provide written informed consent.
Table 1.
Baseline characteristics of the patients.
| Patient | Sex | Age (years) | Diagnosis | Disease duration (years) | Smoking | Concomitant therapy | Sex/age donor (years) | Relation to donor |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 50 | CD | 27 | Current | None | F/47 | Unrelated |
| 2 | F | 29 | CD | 10 | Current | None | F/53 | Daughter |
| 3 | M | 28 | CD | 11 | Ex | Steroids | M/30 | Unrelated |
| 4 | F | 47 | CD | 22 | Never | Steroids | F/55 | Unrelated |
| 5 | M | 38 | UC | 6 | Never | 5-ASA | M/41 | Brother |
| 6 | M | 41 | UC | 13 | Current | 5-ASA, steroids | F/57 | Brother |
| 7 | F | 33 | CD | 16 | Never | None | M/26 | Unrelated |
| 8 | F | 35 | CD (UC-like) | 2 | Never | 5-ASA | F/57 | Unrelated |
| 9 | M | 38 | UC | 14 | Ex | 5-ASA, azathioprine | M/43 | Unrelated |
| 10 | M | 53 | UC | 6 | Never | None | F/34 | Unrelated |
| 11 | M | 39 | UC | 3 | Ex | Infliximab | M/37 | Unrelated |
| 12 | F | 48 | UC | 4 | Ex | None | F/44 | Sister |
| 13 | F | 32 | UC | 6 | Ex | None | F/46 | Unrelated |
| 14 | M | 30 | UC | 3 | Never | 5-ASA | F/27 | Partner |
F, female; M, male; CD, Crohn’s disease; UC, ulcerative colitis; 5-ASA, 5-aminosalicylic acid.
Patients were instructed to select their own donor, either a healthy family member (n = 4; 3 siblings, 1 parent) or a friend (n = 10; 1 partner, no other partners or household members). Exclusion criteria for donors were body mass index (BMI) >30, active smoking, known chronic diseases, antibiotic usage in the past 6 months and detection of inflammation and/or infection in blood and/or faecal assessments. Donor blood was assessed for full blood count and serological testing for hepatitis A, B and C, HIV-1 and 2 and Treponema pallidum. Donor stools were specifically screened for enteropathogens. Bacterial culture was performed to detect the following enteropathogens: Salmonella spp., Shigella spp., Yersinia enterocolitica, Yersinia pseudotuberculosis, Campylobacter spp. and Aeromonas spp. Microscopic examination was performed to search for eggs, cysts and/or larvae of parasites and membrane enzyme-linked immunosorbent assay (ELISA) was done to detect C. difficile toxins A and B, and glutamate dehydrogenase. For the latter, the polymerase chain reaction (PCR) was performed in case of discordance between results of toxin and glutamate assays.
After bowel preparation using a polyethylene glycol solution, patients underwent full ileocolonoscopy with calculation of the Crohn’s Disease Endoscopic Index of Severity (CDEIS), the Simplified Endoscopic Activity Score (SES-CD) and the Mayo endoscopy subscore (for UC) at baseline and week 8 after FMT. Data on C-reactive protein (CRP) and clinical disease activity were collected using the Crohn’s Disease Activity Index (CDAI) for CD and the Mayo score for UC. Patients were followed up for at least 6 months.
2.2. Efficacy endpoints
The primary endpoint for efficacy was the proportion of patients with endoscopic healing at week 8 defined as a Mayo endoscopic subscore of 0 or 1 for UC and SES-CD <3 for CD. Secondary efficacy endpoints included changes in CDAI and Mayo score and changes in CRP. Tolerability of the procedure and adverse events were recorded. In addition, changes in microbial composition were monitored before the start and during follow-up until week 8. All serious adverse events (SAEs), defined as adverse events resulting in hospitalization, were recorded.
2.3. FMT procedure
A total of 200 g of spontaneous stools from healthy donors collected during the 48 h before FMT (and kept at 4°C) were homogenized with 400 mL sterile saline and administered in two times through a nasojejunal tube immediately following ileocolonoscopy and via the same route on the next day in the first 9 patients. As patient 9 developed an aspiration pneumonia following vomiting on the nasojejunal tube, the delivery method was changed thereafter for safety reasons to administration via a rectal tube in all remaining patients. The preparation of the stool mixture was not altered, however.
2.4. Microbiome analysis
For microbiome analyses, stool samples of patients were collected just before bowel cleansing and FMT (= baseline) and at several time-points after the treatment (Supplementary Table 1). Stool samples were aliquoted and stored at –80°C within 6 h after collection. The sample that was used to make the stool mixture was analysed for each donor. DNA of fresh frozen stools was isolated according to Godon et al.43 and subsequently sent to BGI Tech Solutions (Hong Kong) Co.
The V3–V5 region of the bacterial 16S rDNA was amplified, introducing the 454 Life Sciences primer B (forward) and A (reverse) adapter sequences as well as a unique 10-base multiplex identifier (specific for each sample) and 2-base linker sequence (both reverse) and processed on a 454 GS-FLX sequencer with titanium sequencing chemistry. Sequencing outputs were filtered according to quality (average >25) and length (minimum 400, maximum 600) using the software package mothur. Chimera detection was carried out using UCHIME against the ‘Gold’ database (http://drive5.com/uchime/uchime_download.html). A subset of 5000 sequences per sample was used for the analyses. Clustering of sequences was performed using UCLUST and a 97% level operational taxonomic unit (OTU) table was created with Perl scripts. Classification of sequences was performed using RDP Classifier software.
Bacterial community comparisons were performed using the Vegan R package. Bray–Curtis dissimilarity was analysed using constrained principle component analysis (capscale) and analysis of dissimilarity using adonis in R to test differences in various groups. All results were corrected for multiple testing.
3. Results
3.1. Efficacy of FMT in CD and UC
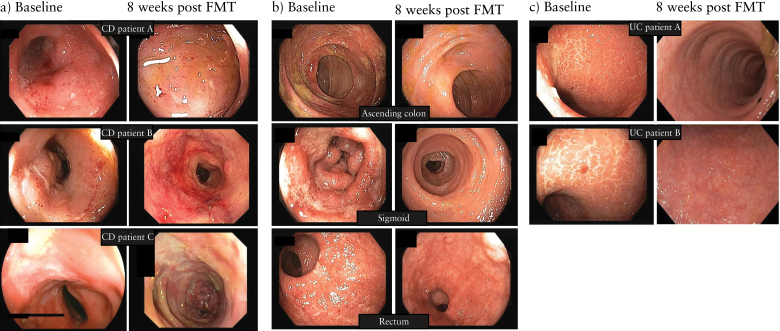
Table 2 shows the overall results for clinical and endoscopic evolution following FMT. None of the CD patients had endoscopic healing at week 8 or clinical (CDAI) or biological (CRP) response (Table 2, Figure 1a). One patient with UC-like CD reported temporal improvement in clinical symptoms for 6 weeks (Figure 1b). This patient had a normal CRP at all time-points, including baseline. In contrast, 2 out of the 8 UC patients went into complete remission and were still in remission in October 2014 (3 and 2.5 years) (Figure 1c). One additional UC patient improved transiently until week 6.
Table 2.
IBD activity scores at baseline and at week 8.
| Week 0 | Week 8 | p-value | |
|---|---|---|---|
| Crohn’s disease | n = 6 | n = 6 | |
| CDAI median (IQR) | 290 (243–359) | 235 (167–330) | 0.24 |
| CRP, mg/L, median (IQR) | 10.9 (4.9–24.7) | 6.7 (4.3–16.5) | 0.49 |
| CDEIS, median (IQR) | 11.8 (9.5–17.2) | 14.7 (6.7–17.4) | 0.89 |
| SES-CD, median (IQR) | 17.5 (17–19.5) | 12 (8–20) | 0.94 |
| Ulcerative colitis | n = 8 | n = 7 | |
| Mayo endoscopic subscore (IQR) | 3 (2.8–3) | 3 (2.5–3) | 0.47 |
| Total Mayo score (IQR) | 8.5 (7–9.5) | 7 (3.5–8.5) | 0.37 |
| CRP, mg/L, median (IQR) | 3.4 (2.2–5.7) | 10.8 (3.3–12.5) | 0.06 |
CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; CDEIS, Crohn’s Disease Endoscopic Index of Severity; SES-CD, Simplified Endoscopic Activity Score; IQR, interquartile range.
Figure 1.
Endoscopic images of inflammatory bowel disease patients at baseline and 8 weeks after faecal microbiota transplantation. (a) Three representative Crohn’s disease patients showing lack of endoscopic improvement. (b) Representative endoscopic images of the patient with ulcerative colitis-like Crohn’s disease showing partial endoscopic healing. (c) Representative endoscopic images of ulcerative colitis patients showing complete endoscopic remission.
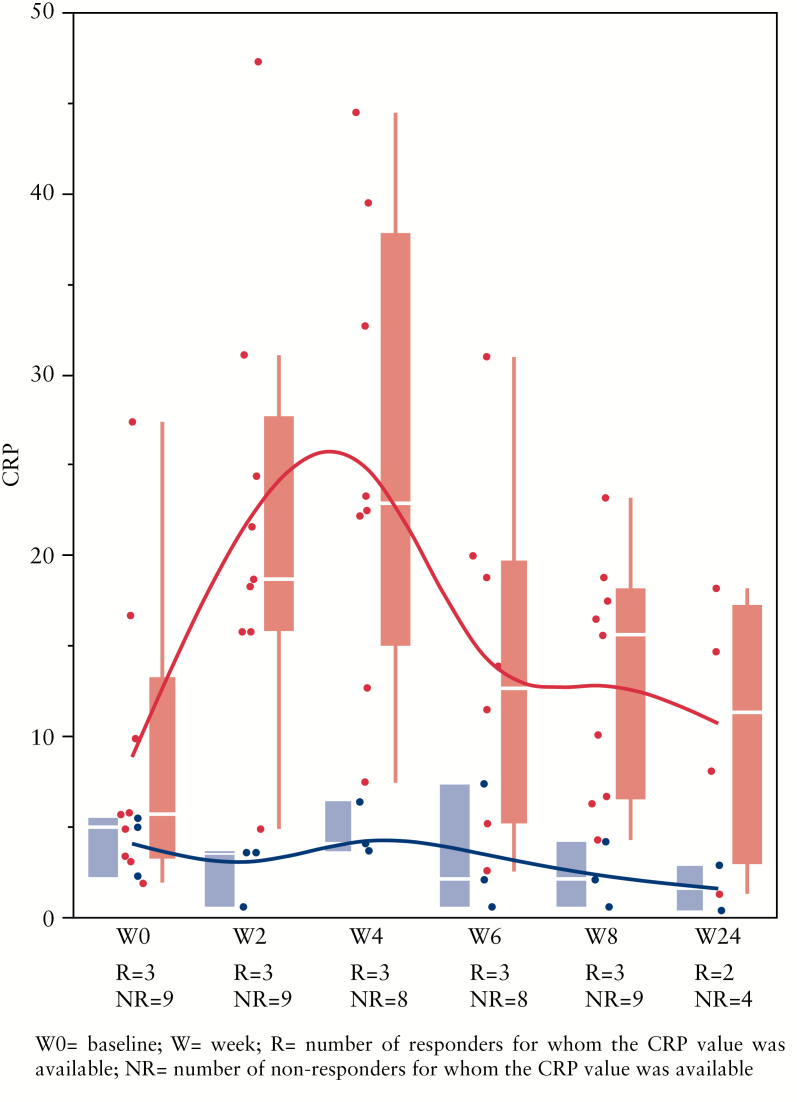
There were no differences at baseline in age, gender, BMI, CRP, haematocrit, disease duration, disease activity and/or smoking between patients who responded to FMT and those who did not respond (all differences not significant). In patients that did not respond to FMT, an increase in CRP within the first 2 weeks after FMT was observed (p = 0.0159) (Figure 2), in both non-responding CD and non-responding UC patients.
Figure 2.
C-reactive protein (CRP) values over time for responders (blue) and non-responders (red) to faecal microbiota transplantation (FMT). A significant difference between successful and non-successful FMT was already observed by week 2 (p = 0.0159). R, number of responders for whom the CRP value was available; NR, number of non-responders for whom the CRP value was available.
3.2. Safety
Five SAEs were reported in a total of 4 patients (Supplementary Table 2). In 4 patients, high fever developed within a few hours after FMT. An urgent CT scan was performed in the first 2 patients on the day following FMT because of an accompanying sharp increase in CRP. Signs of severe colitis were seen without perforation and/or abscess and fever disappeared spontaneously within 2 days following FMT. In the other patients who developed fever, a conservative approach with clinical observation was used and the same pattern was observed, with disappearance of fever after <48 hours.
One patient vomited after FMT with aspiration and bilateral pneumonia on chest X-ray. This patient was treated with broad-spectrum antibiotics for 14 days and received a second FMT 8 weeks later upon the patient’s specific request as the UC symptoms had not improved, by the rectal route. He has been in remission since.
To compare the bacterial composition between patients that developed fever and the patients that did not, we excluded the patient who had pneumonia since we could not determine establish whether the FMT also contributed to his fever. When comparing the most abundant genera (average abundance >1%) at baseline in non-responding patients, a non-significant trend of lower Alistipes (p = 0.052) and higher Escherichia/Shigella (p = 0.051) abundance was found in samples of patients developing fever (n = 3) compared with those who did not (n = 6).
3.3. Bacterial profiling
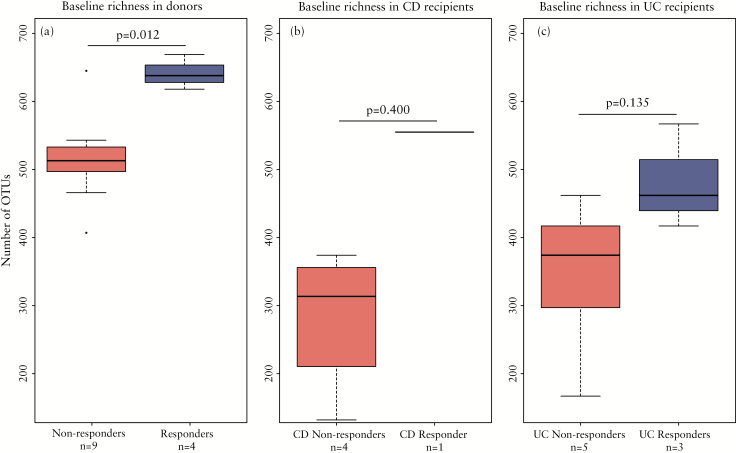
As expected, the baseline richness, characterized as the number of different bacterial OTUs, was overall higher in healthy donors compared with patients. For the microbial analysis, we compared the patients that did not respond (n = 10) with the patients that experienced clinical response for 6 weeks or more (n = 4). Interestingly, significantly higher bacterial richness was found in donors whose stools resulted in successful FMT (Wilcoxon test, p = 0.012; Figure 3a). Although non-significant, the same trend of higher richness at baseline was found in patients who successfully responded to the FMT with UC patients displaying a slightly higher richness compared with CD patients (Figure 3b, c). However, species richness between patients and their corresponding donors at baseline did not statistically differ between responders versus non-responders (Wilcoxon test, p = 0.5697). Combined, these results indicate that the absolute richness of the stool sample used for FMT and not the relative richness compared with the recipient is a marker for treatment success.
Figure 3.
Baseline richness associated with treatment success. Bacterial richness at baseline by comparing the number of operational taxonomic units (OTUs) in (a) samples of donors, (b) Crohn’s disease patients and (c) ulcerative colitis patients. On the x-axis the samples are separated based on (partial) response to the faecal microbiota transplantation.
After FMT, species richness increased in all patients. At week 8, a trend of higher bacterial richness in patients responding to FMT than in those that did not respond to FMT was observed (Wilcoxon test, p = 0.05833).
3.4. Transfer of phylotypes
To identify clinically relevant microbial signals, we focused on effectively transferred phylotypes (defined as phylotypes that are higher in donors than in patient at baseline, that increase after transplantation in the patients and are detected with at least 1% relative abundance post-transplant in patients).33
Based upon this definition, we found in total 11 phylotypes to be transferred in our cohort.
Patients that did not respond to FMT had a median number of 1.5 transferred phylotypes, which was significantly lower than the number in patients that responded at least temporarily to the FMT, who had a median of 5 transferred phylotypes (Wilcoxon test, p = 0.017).
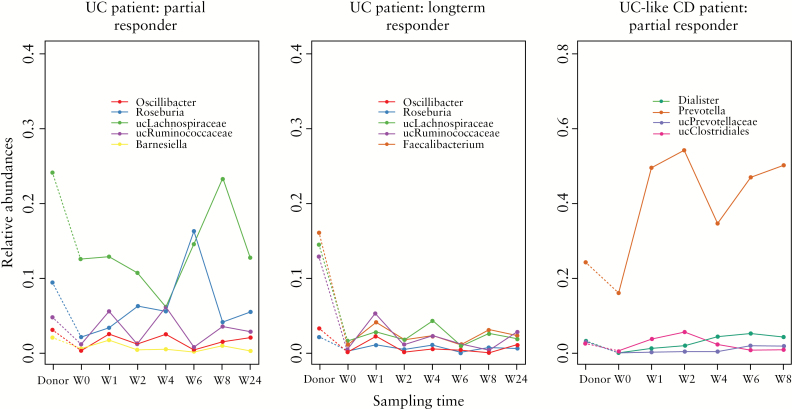
In UC patients, 4 out of 5 of the transferred phylotypes were shared between two patients who had a successful FMT, namely Roseburia, Oscillibacter, unclassified Lachnospiraceae and unclassified Ruminococcaceae (Figure 4) (post-FMT samples of the third patient were lacking). Of them, Roseburia and Oscillibacter were only transferred in the two successful FMT cases but not in any of the other patients in our cohort. Only 1 CD patient reported temporary improvement of symptoms and the transfer of 4 microbial phylotypes – Dialister, Prevotella, unclassified Prevotellaceae and unclassified Clostridiales – was observed in this patient (Figure 4).
Figure 4.
Markers of successful faecal microbiota transplantation (FMT). Evolution over time of phylotypes that were transferred in patients with (partial) response to FMT.
We also assessed transfer efficiency, i.e. the percentage of donor phylotypes that were absent in the patient at baseline and were transferred from the donor to the patient. However, no statistical difference in transfer efficiency was found between responders (median efficiency 74%, range 50–76%) and non-responders (median efficiency 63%, range 14–83%) to FMT.
4. Discussion
Manipulation of the intestinal microbiota has been acknowledged as a treatment for refractory C. difficile diarrhoea following the excellent results of a sham-controlled study.31 Faecal microbiota transplantation has since been proposed for a variety of other inflammatory disorders associated with dysbiosis, such as metabolic syndrome, irritable bowel syndrome and inflammatory bowel disease.35 Several case reports or small case series have reported on the success rates of FMT but not all of these analysed the changes that occurred in the microbiota with FMT.34,36,42 Recently, two large randomized control trials with FMT in UC were published.38,39 Both trials were discontinued due to futility. Of note, the study of Moayyedi and colleagues38 nevertheless demonstrated the efficacy of FMT in inducing remission in active UC patients.
We studied FMT in 14 patients with therapy-refractory CD or UC and used 16S rDNA sequencing to investigate who could benefit from this therapy. We also investigated whether analysis of donor and recipient prior to FMT could improve success rates. A first observation was that the success rate was higher in UC than in CD. In CD, only 1 patient (of note, a patient with UC-like CD) reported a transient symptomatic improvement for 6 weeks but no clinical or endoscopic efficacy was observed at week 8 in the CD patients. In UC, one patient reported transient symptom improvement for 6 weeks and 2 patients were in prolonged remission for 2.5 and 3 years respectively following FMT. The success rate we observed is similar to what was recently reported by Rossen and colleagues39 in their randomized controlled trial.39 Furthermore, we found that increased CRP levels at week 2 were an early marker of failure. This is an important finding as it could allow early rescue therapy in those IBD patients that will not benefit from FMT.
Most reports of successful FMT have been reported in UC patients,33,34 except for a case series from China, where impressive clinical improvement was shown in CD patients.42 However, no endoscopy data were available in that study. Suskind and colleagues40 also reported a highly successful FMT study in CD; however, they studied a cohort of tumour necrosis factor-naive paediatric CD patients, whose phenotype is very different from that of the CD patients we assessed here. Nevertheless, the reason why FMT would preferentially work in UC is intriguing, as dysbiosis has first and mainly been described in CD.15,18 An explanation may lie in the mucosa-adherent bacteria, which are more important in CD patients, as shown by the role of AIEC.28–30 Also, the transmural inflammatory character of CD may necessitate longer treatment cycles, although this has never been proved. Moreover, we previously showed, using laser capture microdissection and 16S rDNA sequencing of selected microscopic CD lesions, that there are significant changes in the composition and location of the gut microbiome in CD.44 Some of the abnormal findings persisted even after macroscopic mucosal healing. Finally, microbiota dysbiosis in UC is generally milder than in CD patients, with higher microbial richness in UC (see also Figure 3b and c) and microbial profiles are closer to those of healthy individuals, thus being potentially easier to shift.15
Faecal microbiota transplantation provoked significant fever in 4 out of 14 patients. This was also described in a case series from Vienna, where all 5 UC patients reported fever following FMT.33 A potential explanation includes a transient translocation of bacteria with release of proinflammatory cytokines into the systemic circulation. Here, we found a trend of lower Alistipes and higher Escherichia/Shigella abundances in baseline samples of patients that developed fever.
The route of administration is still a matter of debate. Whereas oral administration through a nasojejunal tube would avoid the need to perform endoscopies, we observed one aspiration pneumonia in a young 38-year-old patient, without comorbidities, due to vomiting after placement of the tube and administration of the donor stools. Altering the route of administration to rectal instillation did not appear to affect the success rate of FMT in our study – although statistical analyses were hampered by the small sample size – but also several authors previously reported that FMT success was not affected by the route of administration.35,45,46 Based on these data, we currently recommend that nasojejunal administration of FMT should not be used, to avoid the risk of aspiration pneumonia as a complication of FMT. Moayyedi and colleagues38 used weekly retention enemas for 6 weeks with a supernatant of faecal solution as the active treatment, without pretreatment with bowel lavage or antibiotics, and demonstrated this to be an effective treatment for active UC. Using this gentler approach, they also induced a significant increase in microbiota diversity in the treatment group compared with the placebo group.
The most important limitation of our study remains its small sample size, which hampered thorough statistical analyses of several of our observations. Moreover, selection bias cannot be excluded. Nevertheless, where previous studies mainly focused on the patients, we were able to show here that the microbiota of the donor is a major factor in treatment success. This finding is supported by the observation of Moayyedi and colleagues38 that successful FMT was highly donor-dependent. We demonstrate for the first time that higher absolute baseline richness in donors is associated with successful FMT. Besides high richness, a successful donor also needs to have a transferable dose of biologically important phylotypes. The latter is in line with the finding of Rossen and colleagues,39 who observed a shift in the microbiota of patients receiving the active treatment towards their respective donor.
Among the phylotypes that were transferred, we revealed Roseburia and Oscillibacter to be specific for a successful bacterial imprint to induce remission in UC. Although these observations did not reach significance, it is noteworthy that Oscillibacter abundance has been negatively correlated to CD in earlier studies.47,48 Roseburia is a butyrate-producing genus and Angelberger and colleagues33 also reported colonization of donor-derived Roseburia faecis in their patient that had a successful FMT. Moreover, in previous work an inverse relation between disease activity and the abundance of Roseburia hominis in UC patients was demonstrated.20
Although we observed overlap in phylotypes that were transferred in both responders and non-responders, a higher number of transferred phylotypes was a key point for a transplant to work. A successful donor should thus enable sufficient enrichment of the microbiota of a patient to induce remission. At week 8, we indeed observed a trend of higher bacterial richness in samples of responders, in line with the accumulating evidence of a correlation between microbial richness and overall health.13,49
In conclusion, we observed a difference in efficacy following FMT between patients with CD and UC. CRP was an early marker of failure of FMT and higher baseline richness in the donor sample was crucial for successful bacterial enrichment and to induce remission. As UC patients tend to have higher baseline microbial richness compared with CD patients, this probably further contributes to the difference in success between the two IBD phenotypes. Based on our data, FMT with donor prescreening could therefore be a potential treatment for UC patients refractory to standard medical therapy. Gradual enrichment of the faecal microbiota by repeated FMT with high-richness donors should be considered to further improve the success rate of FMT in IBD.
Funding
This work was supported by the Geconcerteerde Onderzoekacties (GOA) of KU Leuven (grant number GOA/11/015). Marie Joossens and Kathleen Machiels are postdoctoral fellows and Séverine Vermeire, Marc Ferrante and Gert Van Assche are senior clinical investigators of the Research Foundation – Flanders (FWO).
Conflict of Interest
None.
Author Contributions
Study concept and design: Vermeire, Raes. Acquisition, analysis or interpretation of data: Vermeire, Joossens, Verbeke, Wang, Machiels, Sabino, Ferrante, Van Assche, Rutgeerts, Raes. Drafting of the manuscript: Vermeire, Joossens. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Joossens, Wang. Obtained funding: Vermeire, Joossens, Machiels, Ferrante, Van Assche. Administrative, technical or material support: Machiels, Sabino, Verbeke, Ferrante, Van Assche, Rutgeerts. Study supervision: Vermeire, Raes. Final approval of manuscript as submitted: all authors.
Conference Presentation
Initial results of this study, on Crohn’s disease patients only, were given in a presentation titled ‘Pilot Study on the Safety and Efficacy of Faecal Microbiota Transplantation in Refractory Crohn’s Disease’ at Digestive Disease Week (DDW) 2012 in San Diego, USA.
Acknowledgments
The authors would like to thank Karolien Van den Broeck and colleagues of the IBD clinical trial team for their help in the follow-up of the patients and donors, Leen Rymenans for excellent technical assistance with the DNA extractions and Falk Hildebrand for his assistance with the first bioinformatics analysis.
References
- 1. Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 3. Onderdonk AB, Hermos JA, Bartlett JG. The role of the intestinal microflora in experimental colitis. Am J Clin Nutr 1977;30:1819–25. [DOI] [PubMed] [Google Scholar]
- 4. Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutgeerts P, Geboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- 6. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003;361:512–9. [DOI] [PubMed] [Google Scholar]
- 7. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009;9:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterson J, Garges S, Giovanni M, et al. The NIH Human Microbiome Project. Genome Res 2009;19:2317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 13. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 14. Jeffery IB, Claesson MJ, O’Toole PW, et al. Categorization of the gut microbiota: enterotypes or gradients? Nat Rev Microbiol 2012;10:591–2. [DOI] [PubMed] [Google Scholar]
- 15. Erickson AR, Cantarel BL, Lamendella R, et al. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS One 2012;7:e49138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011;60:631–7. [DOI] [PubMed] [Google Scholar]
- 19. Lepage P, Hasler R, Spehlmann ME, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011;141:227–36. [DOI] [PubMed] [Google Scholar]
- 20. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014;63:1275–83. [DOI] [PubMed] [Google Scholar]
- 21. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006;55:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010;139:1844–54. [DOI] [PubMed] [Google Scholar]
- 23. Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, et al. Abnormal microbiota composition in the ileocolonic mucosa of Crohn’s disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 2006;12:1136–45. [DOI] [PubMed] [Google Scholar]
- 24. Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 2009;15:1183–9. [DOI] [PubMed] [Google Scholar]
- 25. Swidsinski A, Loening-Baucke V, Vaneechoutte M, et al. Active Crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis 2008;14:147–61. [DOI] [PubMed] [Google Scholar]
- 26. Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis 2009;15:653–60. [DOI] [PubMed] [Google Scholar]
- 27. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 2008;105:16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 1998;115:1405–13. [DOI] [PubMed] [Google Scholar]
- 29. Glasser AL, Boudeau J, Barnich N, et al. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect Immun 2001;69:5529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kleessen B, Kroesen AJ, Buhr HJ, et al. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol 2002;37:1034–41. [DOI] [PubMed] [Google Scholar]
- 31. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile . N Engl J Med 2013;368:407–15. [DOI] [PubMed] [Google Scholar]
- 32. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther 2012;36:503–16. [DOI] [PubMed] [Google Scholar]
- 33. Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 2013;108:1620–30. [DOI] [PubMed] [Google Scholar]
- 34. Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003;37:42–47. [DOI] [PubMed] [Google Scholar]
- 35. Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2012;9:88–96. [DOI] [PubMed] [Google Scholar]
- 36. Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility and efficacy trial results. J Gastroenterol Hepatol 2015;20:51–8. [DOI] [PubMed] [Google Scholar]
- 37. Kump PK, Grochenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis 2013;19:2155–65. [DOI] [PubMed] [Google Scholar]
- 38. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102–9. [DOI] [PubMed] [Google Scholar]
- 39. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110–8. [DOI] [PubMed] [Google Scholar]
- 40. Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis 2015;21:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verbeke KA, Boesmans L, Boets E. Modulating the microbiota in inflammatory bowel diseases: prebiotics, probiotics or faecal transplantation? Proc Nutr Soc 2014;73:490–7. [DOI] [PubMed] [Google Scholar]
- 42. Zhang FM, Wang HG, Wang M, et al. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World J Gastroenterol 2013;19:7213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Godon JJ, Zumstein E, Dabert P, et al. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol 1997;63:2802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Hertogh G, Lemmens B, Verhasselt P, et al. Assessment of the microbiota in microdissected tissues of Crohn’s disease patients. Int J Inflam 2012;2012:505674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009;15:285–9. [DOI] [PubMed] [Google Scholar]
- 46. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 2014;58:1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mondot S, Kang S, Furet JP, et al. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm Bowel Dis 2011;17:185–92. [DOI] [PubMed] [Google Scholar]
- 48. Papa E, Docktor M, Smillie C, et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One 2012;7:e39242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brussow H. Microbiota and healthy ageing: observational and nutritional intervention studies. Microb Biotechnol 2013;6:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]