Abstract
Crohn’s disease [CD] is a chronic progressive and destructive condition. Half of all CD patients will develop bowel damage at 10 years. As in rheumatic diseases, preventing the organ damage consequent to CD complications [fistula, abscess, and/or stricture] is emerging as a new therapeutic goal for these patients in clinical practice. This might be the only way to alter disease course, as surgery is often required for disease complications. Similar to the joint damage in rheumatoid arthritis, bowel damage has also emerged as a new endpoint in disease-modification trials such as the REACT trial. Recently, the Lemann Index [LI] has been developed to measure CD-related bowel damage, and to assess damage progression over time, in order to evaluate the impact of therapeutic strategies in terms of preventing bowel damage. While validation is pending, recent reports suggested that bowel damage is reversible by anti-tumour necrosis factor [TNF] therapy. The Lémann index may play a key role in CD management, and should be implemented in all upcoming disease-modification trials in CD.
Keywords: Crohn’s disease, Lémann index, bowel damage, inflammatory bowel disease, imaging
1. Introduction
Crohn’s disease [CD] is a chronic progressive and destructive inflammatory bowel disease [IBD]. Gut inflammation is generally transmural, involving the small intestine and/or the colon. The chronic inflammatory pattern of the disease, which activates tissue-repairing mechanisms, can lead to irreversible and destructive bowel complications such as strictures, fistulas, or consequent abscesses,1 often requiring surgery.2 Moreover, perianal involvement of the disease can also cause structural and functional damage at that level. The organ damage consequent to CD complications and surgery is likely to progress during the course of the disease3 and, in some cases, can lead to disability.4,5 Data from population-based cohorts show that up to 30% of patients have evidence of bowel damage at baseline, and half of the patients require surgery during 20 years since diagnosis, because of the presence of bowel damage. The risk of postoperative recurrence and consequent bowel damage after the first surgery is around 50% in the following 10 years, eventually leading to further surgical bowel resection6,7
There is increasing evidence that the ultimate goal for the management of CD should go beyond remission of symptoms, to include mucosal healing.8,9,10,11 Notably, the need for surgery has not changed in the past 30 years.12 As surgery is not a cure for CD,13 current therapeutic strategies aim at preventing disease complications.14 These disease-modification-trials represent the next step in the field of CD clinical trials.
Thus, defining and assessing bowel damage using the Lémann Index represent key goals for therapeutic interventions in CD.
2. Lessons from Rheumatoid Arthritis
The concept of damage in immune-mediated disease has been clearly developed in rheumatology, particularly in rheumatoid arthritis [RA].15 Progressive joint damage [from erosions and space-narrowing to complete joint destruction] derived from chronic inflammation is a definite and easy-to-assess outcome that is independent of clinical activity.16,17,18,19,20,21,22,23,24,25 In particular, joint erosions and space narrowing [JSN] are the clearest signs of joint damage in RA.25 These variables have been included in a simple damage score, the modified Sharp’s score. By combining and scoring the presence of erosions and JSN in 16 and 15 areas in the hands, respectively, and from 12 and 6 areas in the feet, respectively, rheumatologists can assess joint damage at each time point and its progression.15 The range of this score goes from 0 [no damage] to 448 [complete destruction].15,26 Similar data are available in ankylosing spondylitis [AS], where findings like subchondral inflammation, and chronic changes, such as erosions and subchondral fatty marrow deposition, are prognostic for disease progression and spinal damage in the long term.19 Several trials show that treating early RA not only to achieve clinical remission, but also to block joint damage progression, is associated with reduced need for joint replacement.27,28,29,30,31,32,33 However, the impact of medications in reducing inflammation and preventing damage is less clear in AS than in RA.24
3. The Need to Define Bowel Damage in Crohn’s Disease
The concept of bowel damage is not new in CD. Disease complications were first described by Crohn et al. in 1932, reporting 14 cases of regional ileitis requiring surgery for complicated disease.34
Usually, a change in disease behaviour according to the Montreal35 or Vienna classification36 from non-stricturing, non-penetrating to stricturing or penetrating behaviour is considered disease progression.37 Based on this outcome measure, longitudinal cohort studies show that from 30% up to 60% of subjects develop bowel damage in the long term.38,39,40,41
Cosnes et al. showed that CD usually starts with a non-stricturing, non-penetrating pattern that evolves to stricturing and/or penetrating during the disease course. However in the same study, up to 20% of patients had stricturing or penetrating complications at onset.38 Similar data were found by Thia et al. 6 in a prospective cohort of 306 patients with newly diagnosed CD, where 18.6% had stricturing or penetrating disease at diagnosis, but a further 66 subjects [26%] developed complications in the first 90 days following diagnosis. More recent data show that up to 50% of patients with newly diagnosed CD already have bowel damage at the time of diagnosis.42
Interestingly, Cosnes et al. evaluated the post-surgical handicap index that was based on location and extent of resection at surgery, thus evaluating the impact of structural damage on the consequent functional damage,43 finding a positive correlation between intestinal resection and functional impairment, defined as diarrhoea and malnutrition. More recently, Peyrin-Biroulet et al. 37 defined bowel damage as the presence of any fistula [including perianal fistulas], abscess, or stricture, defined as wall thickening and luminal narrowing with prestenotic dilatation with a diameter greater than the normal diameter of the small bowel or colon, assessed with imaging techniques such as computerised tomography [CT] or magnetic resonance imaging [MRI].
However, there has not been an international, validated index capable of measuring bowel damage in CD. Based on these results, the International Program to develop New Indexes in Crohn’s disease [IPNIC] group has recently included bowel surgery, disease extent, and presence of stricturing or penetrating disease to develop a quantitative index of bowel damage, the Lémann Index.44,45
4. Quantifying Bowel Damage: the Lémann Index
The assessment of bowel damage needs a complex and integrated evaluation of the entire gastrointestinal tract. Endoscopy is able to assess inflammatory lesions and complications, such as strictures,46,47 but it does not allow for evaluation of CD transmural involvement. Imaging techniques, such as CT and MRI, allow for evaluation of the bowel wall and extra-intestinal involvement [including fistulas and abscesses], as well as perianal disease.3,15,48,49,50,51 In particular, MRI shows 85% accuracy for disease complications and bowel damage when compared with surgical findings, and is highly reproducible [κ value 80%].52 More recently, there is increasing evidence that MRI can help discriminate inflammatory from fibrotic strictures, adding more information on CD-related irreversible structural damage.53,54 However, studies evaluating the role of MRI in assessing long-term damage progression are lacking.
Since 2007, the IPNIC group, including 28 gastroenterologists from 15 countries, one surgeon, two radiologists, and one biostatistician, has been working on the development of the Crohn’s Disease Digestive Damage Score [CD3S], called the Lémann Index.55 They aim to build up a score able to measure cumulative digestive tissue damage, based on a comprehensive assessment of structural bowel damage, including stricturing lesions, penetrating lesions [fistulas and abscesses], and surgical resection or previous surgical procedures, such as stricturoplasty or intestinal diversion, and applicable to different CD settings, such as early or advanced disease, patients with or without history of surgery, or with different CD locations and extension. To build up the index, the entire gastrointestinal tract was divided into four organs [upper tract, small bowel, colon/rectum, and anus] and each organ was divided into different segments, scoring structuring/penetrating on a 4-degree scale [0–3], according to the severity of lesions [Table 1]. Subjects were stratified in each centre according to their present CD location and disease duration [< 2 years, 2–10 years, and 10 years] The study was conducted from August 2008 to December 2010, including 138 patients stratified for disease location [24, 115, 92, and 59 with upper tract, small bowel, colon/rectum, and anus CD location, respectively], and for disease duration [< 2 years since diagnosis, from 2 to 10 years, and > 10 years].45 For each patient, bowel damage was scored globally on a linear visual analogue scale [VAS] and by single organ, by recording and scoring the presence of any stricturing or penetrating lesions and previous surgical procedures, according to their severity. The multivariate analysis defined coefficients both for severity [from grade 0 to grade 3] and location [upper tract, small bowel, colon, and anus]. Good correlation between the Lémann Index and the investigator-based damage assessment was found [0.85, 0.98, 0.90, 0.82 for upper tract, small bowel, colon/rectum, and anus, respectively], with 0.84 overall agreement.45 Further validation and sensitivity-to-change assessment on the IPNIC cohort are planned in the near future.
Table 1.
Reversible and non-reversible parameters included in the Lémann Index.
| Parameters | Reversible? |
|---|---|
| Stricturing lesions | |
| No stricturing lesions | NA |
| Bowel wall thickening < 3 cm | Yes/No |
| Bowel wall thickening >3cm without upper dilatation | Yes/No |
| Frank stricture with upper dilatation | Yes/No |
| Penetrating lesions | |
| No penetrating lesions | NA |
| Superficial ulcerations | Yes |
| Deep transmural ulceration | Yes |
| Phlegmon or any type of fistula | Yes/No |
| Surgical history | |
| None | NA |
| By-pass diversion or stricturoplasty | No |
| Resection | No |
| Perianal lesions | |
| None | NA |
| Simple fistula | Yes |
| Multiple fistulas or any type of abscess | Yes |
| Extensive anal and perianal suppuration, horseshoe abscess, or fistula[s] involving or extending above the elevator plate | Yes |
NA, not applicable.
5. Responsiveness to Change of the Lémann Index
There have been a number of studies conducted recently assessing whether the Lémann Index is sensitive to changes over time, and to determine the utility of changes in the Lémann Index in CD patients56,57,58,59,60 [Table 2]. Gilletta et al. evaluated a retrospective cohort of 221 CD subjects, finding that > 50% of patients had substantial bowel damage [defined as a Lémann Index > 2.0] after 2–10 years since diagnosis. Elevated Lémann Index at first evaluation, as well as duration of clinical activity and intestinal resection, were associated with bowel damage over time56 although intestinal resection is a part of the Lémann Index, so the validity of resection as a predictor of bowel damage, rather than part of it, needs to be clarified by further studies. Duveau et al. retrospectively assessed the variation in the Lémann score in 30 CD subjects,57 finding that the Lémann Index increased in more than one-third of patients during a median follow-up period of 23 months. Moreover, the increase in the Lémann Index was significantly related to disease duration, suggesting that re-evaluations should be appropriately spaced, but no clear recommendation on the right time interval to assess bowel damage by the Lémann Index was indicated.
Table 2.
Summary of clinical studies based on the Lémann Index calculation.
| Study reference | Study design | Study population | Primary outcome | Main results |
|---|---|---|---|---|
| Gilletta et al. 56 | Retrospective cohort analysis | 221 CD subjects up to 10 years FU | LI cut-off for damage | 1.LI > 2.0 is associated with bowel damage 2.High LI at baseline, chronically active disease, intestinal resection were associated with BD progression over time |
| Duveau et al. 57 | Retrospective cohort analysis | 30 CD subjects | LI evolution over time [median time 23 months] | 1/3 of subjects have BD progression over time |
| Fiorino et al. 58 | Prospective cohort | 30 CD subjects starting anti- TNF [median FU 32.5 months] | Efficacy of anti-TNF on BD progression | 1.Anti-TNFs block BD in 83% of subjects. 2.BD progression is associated with higher risk for surgery 3.Cut-off for BD: 4.8 4.Cut-off for BD progression: > 0.3 |
| Bodini et al. 59 | Retrospective cohort analysis | 88 CD subjects treated with anti-TNF, IMM, or 5-ASA Median FU 26 months |
Efficacy of different therapies on LI reduction | Anti-TNFs were more effective than IMM and 5-ASA to reduce the LI overtime |
| Fiorino et al. 60 | Retrospective cohort analysis | 39 CD subjects followed up after surgical resection Median FU [29 months] |
1.Correlation between Rutgeerts’ score and LI 2.Prognostic value of the LI in the postoperative setting |
The LI does not correlate with the Rutgeerts’ score LI progression is associated to POR |
CD, Crohn’s disease; FU, follow-up; TNF: tumour necrosis factor; BD, bowel damage; LI, Lémann Index; IMM, immunomodulators; 5-ASA, 5-aminosalycilic acid; POR: postoperative recurrence.
Fiorino et al. 58 prospectively evaluated 30 subjects who achieved clinical remission with anti-TNF and were followed up prospectively for a median time of 32.5 months. They found that a Lémann Index of 4.8 was the cut-off value for bowel damage, and that an increase > 0.3 in the Lémann Index was associated with bowel damage progression. Anti-TNF therapy was effective in stopping bowel damage progression in 83% of subjects. Bowel damage progression assessed by the Lémann Index was found to be predictive for major surgery in the follow-up period (hazard ratio [HR] 0.19, p = 0.005).58 These findings are similar to those from a retrospective observational cohort study by Bodini et al. that compared the reduction in the Lémann Index in subjects treated with anti-TNF, azathioprine, and 5-aminosalicylic acid [5-ASA] compounds. They found that anti-TNF therapy was associated with a significantly higher reduction in the Lémann Index than 5-ASA compounds or immunosuppressants.59 In another retrospective study on CD subjects undergoing bowel resection,60 the assessment of endoscopic recurrence by the Rutgeerts’ score and bowel damage by the Lémann Index within 12 months of surgery was found to predict clinical recurrence [HR 0.03, p < 0.0001]. Bowel damage as assessed by the Lémann Index seemed to be independent from endoscopic disease activity as assessed by the Rutgeerts’ score [ρ = 0.26, p = 0.09]. The relationship between the Lémann Index and disease activity over time is summarised in Figure 1.
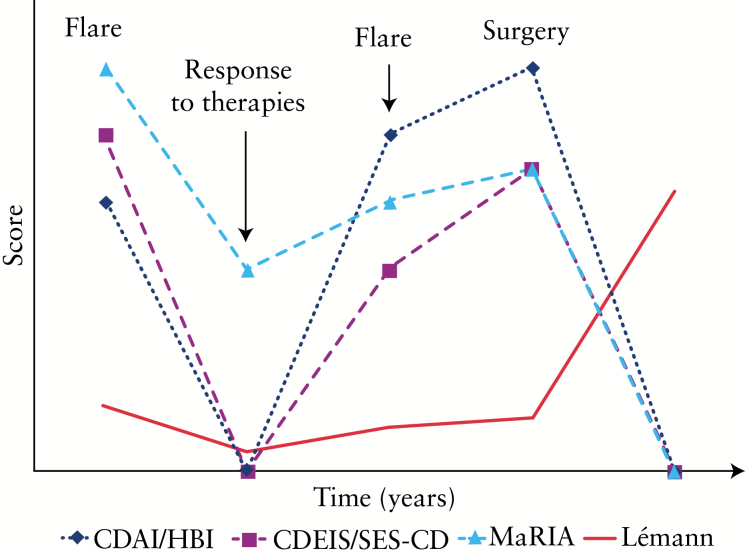
Figure 1.
Changes in the Lémann Index over time compared with activity indexes for Crohn’s disease [CD] (clinical, endoscopic, and magnetic resonance imaging [MRI]-based scores). Independently from disease activity, the ‘residual’ damage after a disease flare remains stable over time and increases after surgery.
6. Bowel Damage as a New Endpoint in Disease-modification Trials
Accumulating evidence indicates that we need to look beyond symptoms in Crohn’s disease.10 In this regard, deep remission [clinical and endoscopic remission] has emerged as a new therapeutic goal in both clinical trials9 and clinical practice11 in Crohn’s disease. Disease-modification trials comparing different therapeutic strategies in early CD patients failed to show significant advantages in using early immunosuppression compared with step-up conventional strategy.14,61,62,63 In particular, the REACT trial did not find any significant difference in terms of clinical remission, but CD patients treated with early combined immunosuppression [ECI] had significant lower risks for hospitalisation and surgery at Month 24 compared with the conventional therapeutic approach.14 In this study, the Lémann Index was not used to assess bowel damage progression in the study groups, as it was developed after the start of the enrolment. Similar data were found in the Step-Up Top-Down trial [SUTD] which showed no significant differences in clinical remission at Week 104 in early CD patients although disease complications, such as perianal fistulas, and the need for surgery, were higher in the step-up group compared with the top-down approach.63 Similarly, Baert et al. found that mucosal healing at Year 2, achieved by a top-down approach [infliximab + azathioprine] was associated with a significantly lower rate of new or active perianal fistulas compared with subjects achieving mucosal healing by the conventional step-up approach with steroids [p = 0.0089].61 Moreover, the results from the RAPID trial did not show significant differences in terms of clinical remission and intestinal surgery at Year 3, but there was a significantly lower rate of perianal surgery in subjects treated early with azathioprine than those treated with conventional therapy.62
It is possible that the use of the Lémann Index in those trials would have led to different results, with a different impact of the early therapeutic approach on bowel damage progression in this setting of patients, and might have provided more clear evidence on bowel damage progression as a primary outcome for disease-modification trials.
7. The Lémann Index for Clinicians: Practical Aspects and Perspectives
The possibility of measuring bowel damage opens up new perspectives for clinicians in the management of CD. The Lémann Index may be accurate for assessing bowel damage at a definite time-point [Figure 2]. This may allow the clinician to identify CD patients that are still within the therapeutic window of opportunity8 and thus most likely to benefit from effective therapeutic strategies to avoid progression to complications and surgery. Moreover, integration of the Lémann Index in the evaluation of efficacy of medications may be crucial for the accurate assessment of the impact of therapeutic strategies in the management of CD. Recent data suggest that if the Lémann Index does not increase during follow-up, the risk of progression and of negative outcomes is significantly reduced.42
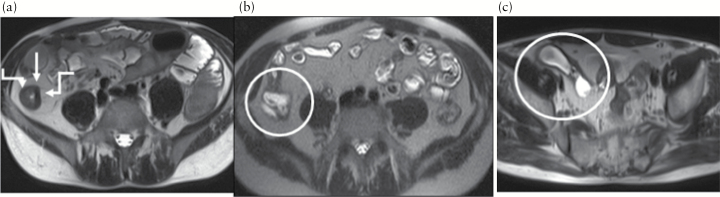
Figure 2.
Evolution of bowel damage over time assessed by magnetic resonance imaging [MRI] in a male patient with 20-cm long stricturing ileal Crohn’s disease at diagnosis. a, between arrows, Lémann Index 0.6, then developed a fistula between ileum and rectum. b, within the circle, Lémann Index 1.6, underwent surgical resection [Lémann Index 8.3 after surgery], and had further anastomotic recurrence. c, within surgery, Lémann Index 9.0. The Lémann Index was able to assess the severity of damage and its progression over time.
Assessment using the Lémann index may guide the clinician in optimising therapeutic strategies in case of an increase in the score. However, it is important to remember that the validity of Lémann Index for non-progression as a target in CD still needs to be confirmed by large long-term controlled trials. Moreover, there have been some issues raised from the preliminary data on the use of the Lémann Index in clinical practice.
First, structural bowel damage should be irreversible by definition, whereas the Lémann Index has been shown to be reduced by effective therapies. The presence of parameters of inflammation in the score calculation is the main reason for such discrepancy. As examples, ulcers are definitively related to active inflammation and are clearly reversible damage, bowel wall thickening is also a parameter of disease activity,64 and strictures can be a result of inflammation or fibrosis and can be reversible under effective therapy65 [Table 1]. Fiorino et al. found that the Lémann Index can significantly change after treatments for acute inflammation, but it remains stable in the maintenance phase, suggesting that ‘residual bowel damage’ as a baseline measurement for disease progression over time should actually be the starting point of disease progression. There is no clear indication on what the right time to assess ‘residual damage’ is, but previous studies based on resolution of inflammation assessed by MRI would suggest at minimum 26 weeks from baseline.66 Moreover, this may suggest the need for refinement by including only non-reversible parameters,67 although the concomitance between inflammation and structural damage due to repair processes can be a major limitation. This is the main reason why the Lémann Index would differ from the modified Sharp Score, since the parameters for RA are more clearly independent from active inflammation and are definitively irreversible.
Second, there are no clear cut-offs to discriminate the presence of bowel damage and clinically meaningful changes over time. Gilletta et al. found that a Lémann Index > 2.0 was related to the presence of bowel damage, although this value was based on patients undergoing surgery for complications.56 Fiorino et al. propose a cut-off of 4.8, based on a blinded independent clinical evaluation by a gastroenterologist.58 Because there is no objective definition for bowel damage, the proposed cut-offs could be considered valid, but not validated, and need confirmation.
Finally, the Lémann Index remains complex to calculate, since it requires the combination of disease history, endoscopy, imaging, and clinical evaluation, and it is difficult to use in clinical practice, especially for non-trained physicians.
In conclusion, the Lémann Index has the potential to be a powerful tool for measuring the real impact of therapeutic strategies on long-term outcomes in CD, in particular in terms of prevention of bowel damage progression. Further studies are needed to clarify the cut-offs for bowel damage and to confirm the sensitivity-to-changes and the validity of changes assessed by the Lémann Index as a therapeutic target.
Achieving deep remission in CD in order to prevent the development of bowel damage, with the final aim of reducing the need for surgery and changing patients’ lives, may be the ultimate therapeutic goals in CD.
Funding
None.
Conflict of Interest
The authors declare no relevant conflict of interest
Acknowledgments
Medical writing assistance was provided by Marie Cheeseman.
References
- 1. Freeman HJ. Natural history and clinical behavior of Crohn’s disease extending beyond two decades. J Clin Gastroenterol 2003;37:216–9. [DOI] [PubMed] [Google Scholar]
- 2. Szabò H, Fiorino G, Spinelli A, et al. Review article: anti-fibrotic agents for the treatment of Crohn’s disease-lessons learnt from other diseases. Aliment Pharmacol Ther 2010;31:189–201. [DOI] [PubMed] [Google Scholar]
- 3. Pariente B, Peyrin-Biroulet L, Cohen L, Zagdanski AM, Colombel JF. Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol 2011;197:42–9. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 5. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. ; the International Programme to Develop New Indexes for Crohn’s Disease [IPNIC] group. . Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012;:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EVJ. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota [1970–2004]. Am J Gastroenterol 2012;107:1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr Drug Targets 2014;15:1056–63. [DOI] [PubMed] [Google Scholar]
- 9. Levesque BG, Sandborn WJ, Ruel J, Feagan BG, Sands BE, Colombel JF. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51 e1. [DOI] [PubMed] [Google Scholar]
- 10. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 11. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 12. Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut 2011;60:1178–81. [DOI] [PubMed] [Google Scholar]
- 13. Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther 2012;35:625–33. [DOI] [PubMed] [Google Scholar]
- 14. Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease [REACT]: a cluster randomised controlled trial. Lancet 2015. doi: 10.1016/s0140-6736[15]00068-9. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 15. Fiorino G, Peyrin-Biroulet L, Danese S. Bowel damage assessment in Crohn’s disease by magnetic resonance imaging. Curr Drug Targets 2012;13:1300–7. [DOI] [PubMed] [Google Scholar]
- 16. Agarwal SK. Core management principles in rheumatoid arthritis to help guide managed care professionals. J Manag Care Pharm 2011;17:S03–8. [PubMed] [Google Scholar]
- 17. Boers M, Felson DT. Clinical measures in rheumatoid arthritis: which are most useful in assessing patients? J Rheumatol 1994;21:1773–4. [PubMed] [Google Scholar]
- 18. Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis 2008;67:794–800. [DOI] [PubMed] [Google Scholar]
- 19. Madsen KB, Schiottz-Christensen B, Jurik AG. Prognostic significance of magnetic resonance imaging changes of the sacroiliac joints in spondyloarthritis-a follow-up study. J Rheumatol 2010;37:1718–27. [DOI] [PubMed] [Google Scholar]
- 20. Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004;50:36–42. [DOI] [PubMed] [Google Scholar]
- 21. Ostergaard M, Hansen M, Stoltenberg M, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum 1999;42:918–29. [DOI] [PubMed] [Google Scholar]
- 22. Palosaari K, Vuotila J, Takalo R, et al. Bone oedema predicts erosive progression on wrist MRI in early RA-a 2-yr observational MRI and NC scintigraphy study. Rheumatology [Oxford] 2006;45:1542–8. [DOI] [PubMed] [Google Scholar]
- 23. Rudwaleit M, Jurik AG, Hermann KG, et al. Defining active sacroiliitis on magnetic resonance imaging [MRI] for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- 24. Schett G, Coates LC, Ash ZR, Finzel S, Conaghan PG. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther 2011;13:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landewe RB, Strand V, Conaghan PG, van der Heijde D. Damage and progression on radiographs in individual joints: data from pivotal randomized controlled trials. J Rheumatol 2011;38:2018–22. [DOI] [PubMed] [Google Scholar]
- 26. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 27. Moura CS, Abrahamowicz M, Beauchamp ME, et al. Early medication use in new-onset rheumatoid arthritis may delay joint replacement: results of a large population-based study. Arthritis Res Ther 2015;17:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ciubotariu E, Gabay C, Finckh A. Joint damage progression in patients with rheumatoid arthritis in clinical remission: do biologics perform better than synthetic antirheumatic drugs? J Rheumatol 2014;41:1576–82. [DOI] [PubMed] [Google Scholar]
- 29. Graudal N, Hubeck-Graudal T, Tarp S, Christensen R, Jurgens G. Effect of combination therapy on joint destruction in rheumatoid arthritis: a network meta-analysis of randomized controlled trials. PLoS One 2014;9:e106408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heimans L, Wevers-deBoer KV, Ronday HK, et al. Can we prevent rapid radiological progression in patients with early rheumatoid arthritis? Clin Rheumatol 2015;34:163–6. [DOI] [PubMed] [Google Scholar]
- 31. Scott DL, Ibrahim F, Farewell V, et al. Randomised controlled trial of tumour necrosis factor inhibitors against combination intensive therapy with conventional disease-modifying antirheumatic drugs in established rheumatoid arthritis: the TACIT trial and associated systematic reviews. Health Technol Assess 2014;18:1–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott DL, Ibrahim F, Farewell V, et al. Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial BMJ 2015;350:h1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sugihara T, Ishizaki T, Hosoya T, et al. Structural and functional outcomes of a therapeutic strategy targeting low disease activity in patients with elderly-onset rheumatoid arthritis: a prospective cohort study [CRANE]. Rheumatology [Oxford] 2015;54:798–807. [DOI] [PubMed] [Google Scholar]
- 34. Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA 1984;251:73–9. [DOI] [PubMed] [Google Scholar]
- 35. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology Can J Gastroenterol 2005;19[Suppl A]: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 36. Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000;6:8–15. [DOI] [PubMed] [Google Scholar]
- 37. Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut 2010; 59: 141–147 [DOI] [PubMed] [Google Scholar]
- 38. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 2005;11:3971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut 2001;49:777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vester-Andersen MK, Prosberg MV, Jess T, et al. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol 2014;109:705–14. [DOI] [PubMed] [Google Scholar]
- 42. Fiorino G, Peyrin-Biroulet L, Bonifacio C, et al. MRE and colonoscopy findings in early Crohn’s disease predict the course of the disease: a prospective observational cohort study. Gastroenterology 2013;7:S88. [Google Scholar]
- 43. Cosnes J, de Parades V, Carbonnel F, et al. Classification of the sequelae of bowel resection for Crohn’s disease. Br J Surg 1994;81:1627–31. [DOI] [PubMed] [Google Scholar]
- 44. Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011;17:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pariente B, Mary JY, Danese S, et al. Development of the Lemann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 2015;148:52–63 e3. [DOI] [PubMed] [Google Scholar]
- 46. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 47. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif [GETAID]. Gut 1989;30:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease Inflamm Bowel Dis 2011;17:1073–80. [DOI] [PubMed] [Google Scholar]
- 49. Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64–79. [DOI] [PubMed] [Google Scholar]
- 50. Jauregui-Amezaga A, Rimola J, Ordas I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut 2014;64:1397–402. [DOI] [PubMed] [Google Scholar]
- 51. Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease, Aliment Pharmacol Ther 2011;34:125–45. [DOI] [PubMed] [Google Scholar]
- 52. Spinelli A, Fiorino G, Bazzi P, et al. Preoperative magnetic resonance enterography in predicting findings and optimizing surgical approach in Crohn’s disease. J Gastrointest Surg 2014;18:83–90; discussion 90–1. [DOI] [PubMed] [Google Scholar]
- 53. Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015;110:432–40. [DOI] [PubMed] [Google Scholar]
- 54. Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 2014;24:619–29. [DOI] [PubMed] [Google Scholar]
- 55. Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011;17:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gilletta C, Lewin M, Bourrier A, et al. Changes in the Lemann Index Values During the First Years of Crohn’s Disease. Clin Gastroenterol Hepatol 2015;13:1633–40. [DOI] [PubMed] [Google Scholar]
- 57. Duveau N, Azahaf M, Panchal H, Nachury M, Colombel JF, Pariente B. Evolution of the Lemann index in Crohn’s disease: a retrospective study. J Crohns Colitis 2015;9[Suppl 1]:S57. [Google Scholar]
- 58. Fiorino G, Bonifacio C, Allocca M, et al. Bowel damage as assessed by the Lemann Index is reversible on anti-TNF therapy for Crohn’s disease. J Crohns Colitis 2015;9:633–9. [DOI] [PubMed] [Google Scholar]
- 59. Bodini G, Savarino V, Baldissarro I, De Maria C, Yehia L, Savarino E. Biological therapy is able to modify the disease progression of Crohn’s disease preventing its long-term associated disability- - a study performed by using the Lèmann Score. J Crohns Colitis 2015;9:S317–S8. [Google Scholar]
- 60. Fiorino G, Bonifacio C, Allocca M, et al. Clinical utility of the Lemann index and Rutgeerts score to predict postoperative course of Crohn’s disease: a retrospective single-center cohort study. J Crohns Colitis 2015; 9[ Suppl 1]:S193. [Google Scholar]
- 61. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010;138:463–8; quiz e10-1. [DOI] [PubMed] [Google Scholar]
- 62. Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn’s Disease: a randomized controlled trial. Gastroenterology 2013;145:758–65 e2; quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 63. D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- 64. Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 2011;17:1759–68. [DOI] [PubMed] [Google Scholar]
- 65. Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort study [CREOLE]. J Crohns Colitis 2015;9:S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Assche G, Herrmann KA, Louis E, et al. Effects of infliximab therapy on transmural lesions as assessed by magnetic resonance enteroclysis in patients with ileal Crohn’s disease. J Crohns Colitis 2013; .7: .950–7. [DOI] [PubMed] [Google Scholar]
- 67. Panes J. What is disease progression in Crohn’s disease and how can it be measured? J Crohns Colitis 2015;9:599–600. [DOI] [PubMed] [Google Scholar]