Abstract
Background and Aims:
Helicobacter pylori infection may protect against some chronic inflammatory diseases. This study examined H. pylori infection and its association with the prevalence of the gastrointestinal diseases Crohn’s disease [CD], ulcerative colitis [UC], and coeliac disease [CeD]. Incident cases in a follow-up period after H. pylori testing were recorded to investigate if protective effects of H. pylori persisted after probable eradication.
Methods:
This was a historical cohort study performed in the Central Denmark Region. Patients were enrolled consecutively from primary health care centres after a urea breath test [UBT] for H. pylori and were then followed for a median of 6 years. The patient’s diseases, country of birth, and gender were acquired from nationwide administrative registries. We used logistic regression to compare the prevalences of CD, UC, and CeD and Cox regression to compare the incidences of CD, UC, and CeD between H. pylori-positive and H. pylori-negative patients, adjusting for confounding variables.
Results:
We found a lower prevalence of CD in H. pylori-positive than in H. pylori-negative patients (odds ratio = 0.36 [0.17–0.75]). There were fewer incident cases of CD in H. pylori-positive than H. pylori-negative patients in the follow-up period (hazard ratio = 0.59 [0.36–0.96]). Similar trends were found for CeD but not for UC.
Conclusions:
H. pylori infection may be a protective factor against the development of CD. However, the incidence of CD is still reduced after UBT and probable H. pylori eradication; thus, H. pylori status may be a marker for other factors that protect against CD.
Keywords: Helicobacter pylori, Crohn’s disease, colitis ulcerative
1. Introduction
Infection with Helicobacter pylori increases the risk of developing ulcers and some upper gastrointestinal cancers.1 Chronic H. pylori infection is common, and approximately 20% of Danish patients with dyspepsia are infected with H. pylori.2 H. pylori infection occurs during childhood.3 Poor sanitation and low socioeconomic standards are associated with increased risk of H. pylori infection, with prevalences in, for example, India, China, and Brazil, of well above 50%.4,5
It has been suggested that H. pylori infection protects against the chronic inflammatory bowel diseases [IBD] Crohn’s disease [CD] and ulcerative colitis [UC]. Several case-control studies have shown a lower prevalence of H. pylori in patients with CD and UC compared with a healthy control group.6,7,8,9 However, these studies either were smaller case-control studies or were performed in a selected cohort who underwent endoscopy and lacked a follow-up period to observe the incidence of CD and UC after H. pylori eradication. A meta-analysis concluded that patients with IBD have a lower prevalence of infection with H. pylori, suggesting a causative relationship between H. pylori infection and IBD. However, that meta-analysis used pooled data to examine IBD and did not distinguish between CD and UC, so it is unclear whether these results apply to both diseases.10
Furthermore, a protective role of H. pylori in the development of coeliac disease [CeD], a chronic inflammatory gastrointestinal disease, is controversial. One study showed that infection with H. pylori reduced the prevalence of CeD, and this association remained strong after adjusting for confounders, such as socioeconomic standards.11 However, another study found an increased CeD prevalence in the presence of H. pylori infection.12
In Denmark, when a patient is found to be H. pylori-positive, the physician is instructed to initiate eradication therapy according to the guidelines of the Danish Society of Gastroenterology and Hepatology.13 The eradication rate after therapy is 87%.14
As results regarding the association between H. pylori infection and IBD have been conflicting, we studied the association between H. pylori infection and the prevalence of CD, UC, and CeD in a cohort of > 50 000 Danish citizens who consecutively performed a first-time urea breath test [UBT]. In a follow-up period, we examined CD, UC, and CeD incidences after UBT, to investigate if potential protective effects of H. pylori persisted after probable eradication.
2. Materials and Methods
2.1. Study design, participants and setting
This was both a cross-sectional and a historical cohort study. The cohort consisted of 56 001 patients who underwent a urea breath test [UBT] from November 2002 through April 2013, using a system established in 2002 in the eastern part of the Central Denmark Region [population size approximately 700 000] to diagnose and treat H. pylori infections in general practice.2
2.2. Variables
We extracted data from the time of the UBT and forward, including the patient’s Danish Personal Identification Number [CPR; also coding for gender and age] and the result of the test [negative or positive] from local laboratory information systems. Using the CPR, we linked individual records with data from the National Patient Register to identify patients at the time of UBT with CD, UC, and CeD [ICD-10 and ICD-8 codes: CD, K50.x and 563.01; UC, K51.x, 563.19 and 569.04; CeD, K90.0 and 269.00] and patients later diagnosed with one of those diseases. In the follow-up period we only registered incident cases, that is patients who received their first diagnosis of CD, UC, or CeD after UBT. We also linked the individual records to the Civil Registration System to determine each patient’s country of birth. Referrals within 6 months after UBT were studied using department codes for gastroenterology departments and gastric surgery departments, as in Denmark these perform a large proportion of both lower and upper endoscopies.
2.3. Statistical methods
We used logistic regression to compare the prevalences of CD, UC, and CeD between patients with and without H. pylori infection, adjusting for the confounding variables of gender, age, and country of birth [Denmark or any other country]. In the historical cohort study, the cumulative risk of being diagnosed with CD, UC, or CeD was estimated after UBT, using the cumulative incidence function, with death as a competing risk, and those who survived until 1 August 2014 without having developed CD were censored. We used Cox regression to compare CD, UC, and CeD hazard rates between those who tested positive and those who tested negative for H. pylori, adjusting for the confounding variables of gender, age, and country of birth. Descriptive statistics are given as the median and interquartile range [Table 1]. For the statistical comparisons between groups with regard to gender and age distribution, thechi-square test and non-parametric Mann-Whitney test were used, respectively. Prevalence and incidence results are expressed as odds ratios [ORs] or hazard ratios [HRs]. Ratios are given with 95% confidence intervals [CIs].
Table 1.
Descriptive statistics of patients who took a first-time urea breath test [UBT] in general practice in the Central Denmark Region from 2002 to 2013.
| Total | H. pylori-negative | H. pylori-positive | |
|---|---|---|---|
| Total UBT [n] | 60 709 | 48 879 | 11 830 |
| Patients [n] | 56 001 | 45 023 | 10 978 |
| Retest, in 180 days [n] | 1 937 | 1 451 | 486 |
| Retests, ever [n] | 4 708 | 3 856 | 852 |
| Age [years]a | 42.4 [28.7–56.7] | 41.7 [27.8–56.0] | 45.2 [32.5–59.5] |
| Gender [male] | 40.0% | 39.5% | 41.7% |
| Follow-up [years]a | 5.9 [3.3–8.4] | 5.9 [3.3–8.4] | 6.0 [3.3–8.6] |
| Born in DK [n] | 47 044 | 40 923 | 6 121 |
| Born outside DK [n] | 8 957 | 4 100 | 4 857 |
Entries in brackets denote interquartile range.
DK, Denmark.
aMedian [25th to 75th percentile].
2.4. Ethics
The study was approved by the National Board of Health and by the Danish Data Protection Agency [journal number 2007-58-0010]. According to Danish law, approval from the Danish Committee on Health Research Ethics was unnecessary.
3. Results
3.1. Participants and descriptive data
In total, 56 001 individuals underwent a first-time UBT, 47 044 of whom were born in Denmark and 8957 of whom were born outside Denmark. The prevalence of H. pylori in the entire cohort was 20%. Stratification for time of birth showed that people born in 1910–1920 had a prevalence of H. pylori of 34%, whereas people born in 1980–1990 had a prevalence of H. pylori of 11%. The H. pylori prevalence in people born in Denmark was 13% compared with 54% for people born outside Denmark, so we performed sub-analyses according to birthplace. The median follow-up time after UBT was similar for patients who tested positive vs negative for H. pylori [6.0 vs 5.9 years]. H. pylori status did not affect mortality [HR of 0.99, CI: 0.90–1.09]. Of the 10 978 patients who tested positive for H. pylori, 17.6% underwent a follow-up test within 180 days, and 75% of the tests performed in this period were negative, indicating successful eradication of H. pylori. When tested beyond 180 days, 82% of patients were negative. Referrals within 6 months after UBT were comparable for patients who tested positive vs negative for H. pylori [9.59% vs 9.83%, chi-square p = 0.46].
3.2. Crohn’s disease
The prevalence of CD at the time of UBT among H. pylori-positive patients was 0.07% compared with 0.24% among H. pylori-negative patients, with an OR of 0.36 [CI: 0.17–0.75] after adjustment for age and gender [Table 2], indicating a protective role of H. pylori against CD, even after stratification for place of birth. For the group born in Denmark, the CD prevalence was 0.10% among H. pylori-positive patients compared with 0.24% among H. pylori-negative patients, with an OR of 0.38 [CI: 0.17–0.87] after adjustment for age and gender, whereas the group born outside Denmark had a CD prevalence at 0.04% among H. pylori-positive patients compared with 0.14% for H. pylori-negative patients (OR = 0.28 [CI: 0.06–1.38]).
Table 2.
Patients diagnosed with Crohn’s disease [CD], ulcerative colitis [UC], and coeliac disease [CeD] after urea breath test [UBT] in the Helicobacter pylori-negative and -positive groups. Events [n], odds ratios [ORs], hazard ratios [HRs], and 95% confidence intervals [CIs] are shown.
| N | Patient at time of UBT [n] | [%] | OR | [95% CI] | Patients after UBT [n] | HR | [95% CI] | ||
|---|---|---|---|---|---|---|---|---|---|
| H. pylori-negative | 45 023 | CD | 116 | 0.24 | 173 | REF | |||
| UC | 308 | 0.68 | 250 | REF | |||||
| CeD | 41 | 0.09 | 74 | REF | |||||
| H. pylori-positive | 10 978 | CD | 8 | 0.07 | 0.36a | [0.17–0.75] | 21 | 0.59b | [0.36–0.96] |
| Unadjusted | 0.30 a | [0.15–0.62] | 0.49 b | [0.31–0.78] | |||||
| UC | 63 | 0.57 | 0.89a | [0.67–1.18] | 45 | 0.90b | [0.64–1.27] | ||
| Unadjusted | 0.89 a | [0.64–1.10] | 0.73 b | [0.53–1.00] | |||||
| CeD | 5 | 0.05 | 0.65a | [0.24–1.73] | 10 | 0.59b | [0.29–1.22] | ||
| Unadjusted | 0.50 a | [0.20–1.27] | 0.55 b | [0.28–1.06] | |||||
REF, reference value; unadjusted values shown by italics.
aOdds ratio and confidence interval compared with H. pylori-negative.
bHazard ratio and confidence interval compared with patients who were H. pylori-negative before urea breath test.
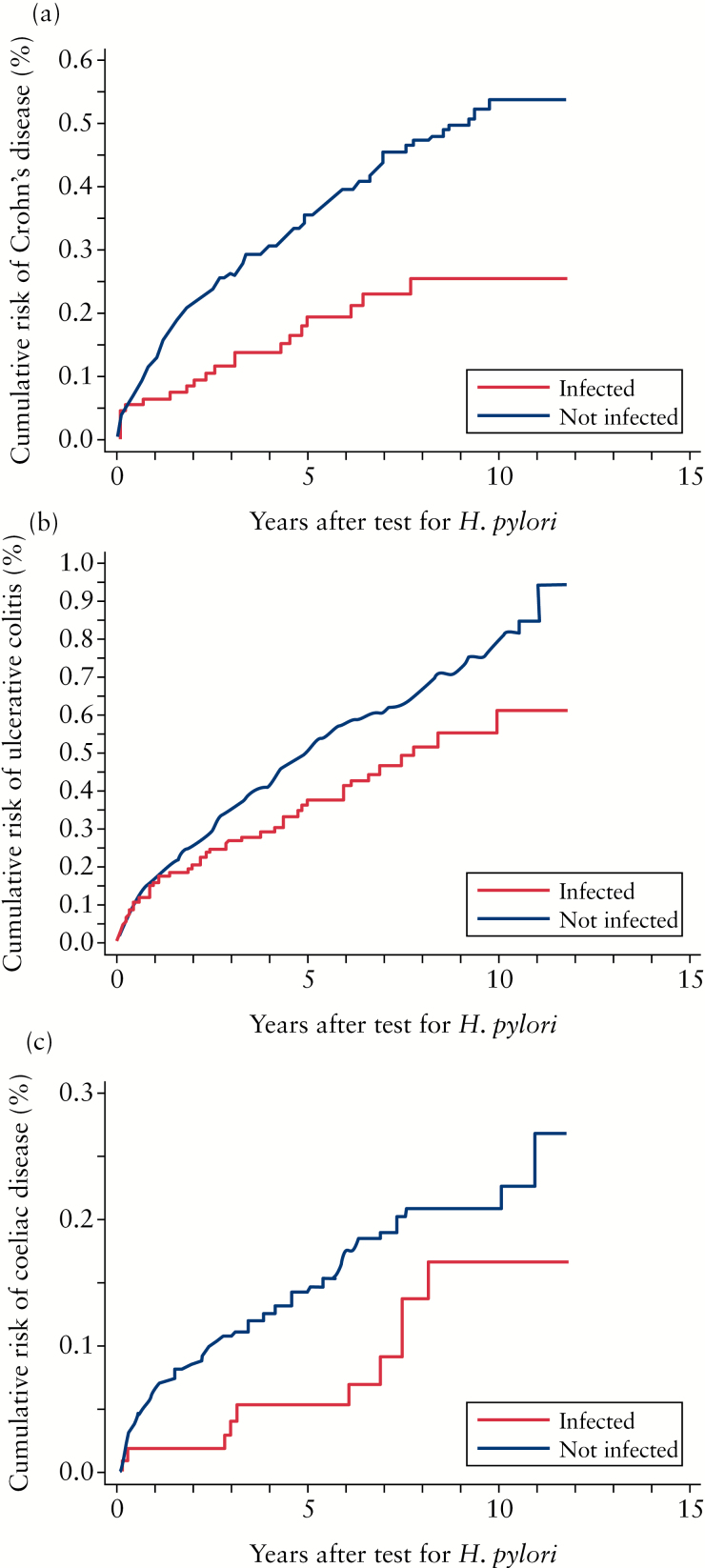
In the years following UBT, those who tested positive for H. pylori were less likely to be diagnosed with CD than those who tested negative, with a hazard ratio of 0.59 [CI: 0.36–0.96] [Table 2 and Figure 1a].
Figure 1.
Incidence of Crohn′s disease [CD], ulcerative colitis [UC], and coeliac disease [CeD] after urea breath test [UBT] with presumed Helicobacter pylori eradication after positive test. The dotted line represents patients who were H. pylori-negative by UBT, and the solid line represents patients who were H. pylori-positive by UBT. The x-axis shows time [years] whereas the y-axis shows cumulative risk [%, please note that the scale differs for the three diagnoses]. A: Cumulative risk of CD after UBT. A significantly increased incidence of CD was found in patients who were H. pylori-negative at UBT: hazard ratio 0.59 (95% confidence interval [CI]: 0.36–0.96). B: Cumulative risk of UC after UBT. A trend of an increased incidence of UC was found in patients who were H. pylori-negative by UBT: hazard ratio, 0.90 [95% CI: 0.64–1.27]. C: Cumulative risk of CeD after UBT. A trend of increased incidence of CeD was found in patients who were H. pylori-negative by UBT: hazard ratio 0.59 [95% CI: 0.29–1.22].
3.3. Ulcerative colitis
In the same population, the prevalence of UC among H. pylori-positive patients was 0.57% compared with 0.68% among H. pylori-negative patients, with an OR of 0.89 [CI: 0.67–1.18] after adjustment for age and gender [Table 2]. The group born in Denmark had a prevalence of UC among H. pylori-positive patients of 0.74% compared with 0.71% among H. pylori-negative patients, with an OR of 0.87 [CI: 0.63–1.20] after adjustment for age and gender. The group born outside Denmark had a lower prevalence of UC among both H. pylori-positive patients [0.37%] and H. pylori-negative patients [0.39%], with an OR of 0.93 [CI: 0.47–1.82] after adjustment for age and gender.
The risk of developing UC after UBT was lower for those who tested positive for H. pylori, but the difference was not statistically significant after adjustment for confounders (HR of 0.90 [CI: 0.64–1.27]) [Table 2 and Figure 1b].
3.4. Coeliac disease
The prevalence of CeD among H. pylori-positive patients was 0.05% compared with 0.09% among H. pylori-negative patients, with an OR of 0.65 [CI: 0.24–1.73] after adjustment for age and gender [Table 2]. For the group born in Denmark, the prevalence of CeD among H. pylori-positive patients was 0.05% compared with 0.10% among H. pylori-negative patients (OR = 0.58 [CI: 0.18–1.88]), and for the group born outside Denmark, the prevalence of CeD among H. pylori-positive patients was 0.04% compared with 0.05% among H. pylori-negative patients (OR = 0.85 [CI: 0.12–6.07]).
After UBT, the risk of developing CeD was lower in patients who tested positive for H. pylori than in those who tested negative, but the difference was not statistically significant, with a hazard ratio of 0.59 [CI: 0.29–1.22] [Table 2 and Figure 1c].
4. Discussion
We found that H. pylori-negative patients had a higher prevalence of CD than did H. pylori-positive patients. Even after UBT and probable eradication, CD was more frequently diagnosed in the H. pylori-negative group during the follow-up period compared with the group of patients who were H. pylori-positive before follow-up. We also found a similar inverse relationship between H. pylori and CeD, but it was not statistically significant.
This study had several strengths. The cohort was large, with 56 000 patients tested in general practice; therefore, these findings are likely generalisable. The cohort was followed for several years with healthcare information available both before and after UBT. The validity of the diagnoses is high in the Danish National Patient Registry for CD and UC.15 In the Danish National Patient Registry, CeD is known to be underdiagnosed 10-fold, but we have no reasons to expect this not to divide equally between the two groups.16 When performed correctly, the sensitivity and specificity of the UBT used in this study to detect H. pylori are both approximately 95%, and this method is one of two recommended non-invasive methods to detect H. pylori according to Maastricht IV.17,18 Hence, this study likely provides precise information about H. pylori status and valid information about the prevalence and incidence of CD and UC in a Danish population.
However, the study also had limitations. Adjustment for patient socioeconomic status would have strengthened our analysis because H. pylori infection is linked to low socioeconomic standards, and high socioeconomic standards have been linked to CD 19 and CeD.20 Likewise, information about drug prescriptions could have substantiated probable eradication after a positive H. pylori test, but precise information was not obtainable.
Several biases could have led to overestimation of CD, UC, or CeD incidence in the H. pylori-negative group. First, if patients with dyspepsia were H. pylori-negative, they would be more likely to be tested for other diseases that cause abdominal discomfort, eg CD, UC, and CeD, and then misclassified. Second, people with abdominal symptoms due to undiagnosed CD, UC, or CeD are more likely to be tested for H. pylori, found to be negative, and later diagnosed with CD, UC, or CeD. In fact, we did see an increased incidence just after the UBT in all groups, probably due to established contact with doctors. However, we found no differences in referrals between H. pylori-negative and -positive, so the association between H. pylori and lower incidence of CD cannot be explained by a lower probability of being referred for work-up in hospital. Both of these biases are only relevant in the incidence analysis and should be eliminated by the long observation period, as active CD leads to severe symptoms that are not likely to be endured for a longer period without further contact with health care professionals. A bias towards underestimation of CD in the H. pylori-positive group could have been present because metronidazole, which is often used to eradicate H. pylori, reduces the activity of CD for years after metronidazole treatment.21 Again, the long follow-up period should have eliminated this bias. Concomitantly, our results could be biased towards lower H. pylori prevalence in patients with CD, UC, or CeD, as people with frequent health care contact are more likely to receive antibiotics due to other causes, which could have led to H. pylori eradication. However, a previous study whose control group consisted of patients with chronic obstructive lung disease found that a history of antibiotic use did not play a role in the negative association between H. pylori and CD.22 Overall, the above-mentioned biases should not affect the conclusions of this study.
The H. pylori prevalences observed in this study matched those previously described for the Danish population; and the lower prevalence in more recent birth cohorts has also been described before, and is attributed to the improving socioeconomic standards and sanitary conditions in Denmark.2 After testing positive for H. pylori, 82% of patients showed eradication of the infection when they were retested, which accords with a previous Danish study that reported an eradication rate of 87%.14 Even though UBT retest was not performed routinely, we expect similar eradication rates to be present or even higher in the remainder of H. pylori-positive patients, as they are less likely to be retested if symptoms are relieved after treatment. We found a stable follow-up period in which mortality was unaffected by H. pylori status, so we were able to compare disease incidence between the two groups with nearly identical follow-up times.
Our study showed that people infected with H. pylori had a lower prevalence of CD. This finding has previously been documented in several case-control studies and a meta-analysis.6,7,8,9,10 This association was observed whether the subjects were born in or outside Denmark. These results suggest that H. pylori protects against CD. Even after H. pylori diagnosis and probable eradication, more patients were diagnosed with CD among those who were H. pylori-negative prior to follow-up than among those who were H. pylori-positive prior to follow-up. This finding has not been previously shown.
The mechanism by which H. pylori protects against CD is not known, but it has been suggested that H. pylori induces the development of FoxP3+ regulatory T cells and impairs dendritic cell maturation, which could contribute to decreased inflammation.23,24 As we observed a difference in CD incidence between the two groups, even after UBT and probable eradication, there may be factors other than H. pylori that contributed to our findings. This is compatible with another hypothesis, the ‘hygiene hypothesis’, which states that improved sanitary conditions reduce infection load in general and, consequently, reduce exposure to bacterial antigens which may trigger autoinflammation. If that hypothesis is true, then H. pylori is merely an indicator of other infectious agents and poor sanitary conditions in infancy and can be considered an associative marker of a lifestyle protective against these gastrointestinal diseases rather than a causal factor. However, as H. pylori infection occurs during childhood, one could argue that the long duration of H. pylori infection is of greater significance to the development of CD than the 6 years [the follow-up period of this study] without H. pylori infection. This is supported by the fact that H. pylori seems to protect against CD even in children, indicating that infection during childhood is particularly important for gaining the protective effects of H. pylori.25
We found UC to be more frequent in H. pylori-negative patients than in H. pylori-positive patients, but this difference was not statistically significant. Other studies have found a negative correlation between H. pylori infection and UC, and our negative finding may represent a type 2 error.6,10 However, after stratification into groups born inside and outside Denmark, the prevalence of UC was identical in H. pylori-positive vs H. pylori-negative patients. Stratification further revealed that the group born outside Denmark had a lower prevalence of UC and a considerably higher prevalence of H. pylori, which may indicate susceptibility bias causing the UC risk to be underestimated in the H. pylori-positive group. In conjunction, we did not find any difference in UC incidence between H. pylori-negative patients and H. pylori-positive patients after UBT. Hence, we were not able to confirm a protective effect of H. pylori against UC. The negative association observed between H. pylori infection and CD compared with the lack of an association between H. pylori infection and UC may represent a biological difference between CD and UC, as H. pylori infects the upper gastrointestinal tract and UC is limited to the colon.
Fewer H. pylori-positive patients than H. pylori-negative patients had CeD, but this difference was not statistically significant. Lebwohl and colleagues showed a strong negative association between CeD and H. pylori. 11 That study had a large cohort of patients who underwent oesophagogastroduodenoscopy and a high CeD prevalence compared with our cohort, which may explain why our association was not statistically significant. The prevalence of recognised CeD in Denmark is estimated to be 0.1–0.2%, which accords with our data.26 After UBT, CeD was more frequently diagnosed in the group that tested negative for H. pylori before follow-up, but this difference was not statistically significant.
4.1. Conclusion
This study, which was cross-sectional and was the first large historical cohort study on this topic, showed that H. pylori infection may protect against CD. However, a negative association between H. pylori infection and CD was observed during the follow-up period after UBT, and possible eradication of factors other than H. pylori may have caused or contributed to this phenomenon.
Funding
The study was performed without external funding.
Conflict of Interest
None.
Author Contributions
All authors contributed to study planning. Data were mainly collected by JD, LC, and HV. PJ and LG performed most data analysis. As lead author, LB wrote the body of the manuscript but all authors contributed and approved the final version of the manuscript.
References
- 1. Franceschi F, Zuccala G, Roccarina D, et al. Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol 2014;234–42. [DOI] [PubMed] [Google Scholar]
- 2. Dahlerup S, Andersen RC, Nielsen BS, et al. First-time urea breath tests performed at home by 36,629 patients: a study of Helicobacter pylori prevalence in primary care. Helicobacter 2011;16:468–74. [DOI] [PubMed] [Google Scholar]
- 3. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. [DOI] [PubMed] [Google Scholar]
- 4. Lehours P, Yilmaz O. Epidemiology of Helicobacter pylori infection. Helicobacter 2007;12[Suppl 1]: 1–3. [DOI] [PubMed] [Google Scholar]
- 5. Calvet X, Ramirez Lazaro MJ, Lehours P, et al. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 2013;18[Suppl 1]:5–11. [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Zhong B, Chao K, et al. Role of Helicobacter species in Chinese patients with inflammatory bowel disease. J Clin Microbiol 2011;49:1987–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonnenberg A, Genta RM. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012;35:469–76. [DOI] [PubMed] [Google Scholar]
- 8. Ando T, Watanabe O, Ishiguro K, et al. Relationships between Helicobacter pylori infection status, endoscopic, histopathological findings, and cytokine production in the duodenum of Crohn’s disease patients. J Gastroenterol Hepatol 2008;23[Suppl 2]: S193–7. [DOI] [PubMed] [Google Scholar]
- 9. Xiang Z, Chen YP, Ye YF, et al. Helicobacter pylori and Crohn’s disease: a retrospective single-center study from China. World J Gastroenterol 2013;19:4576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luther J, Dave M, Higgins PD, et al. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis 2010;16:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebwohl B, Blaser MJ, Ludvigsson JF, et al. Decreased Risk of Celiac Disease in Patients With Helicobacter pylori Colonization. Am J Epidemiol 2013;178:1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konturek PC, Karczewska E, Dieterich W, et al. Increased prevalence of Helicobacter pylori infection in patients with celiac disease. Am J Gastroenterol 2000;95:3682–3. [DOI] [PubMed] [Google Scholar]
- 13. Bytzer P, Dahlerup JF, Eriksen JR, et al. Diagnosis and treatment of Helicobacter pylori infection. Dan Med Bull 2011;58:C4271. [PubMed] [Google Scholar]
- 14. Roug S, Madsen LG. Importance of post-treatment follow-up to secure sufficient eradication therapy for Helicobacter pylori. Dan Med J 2012;59:A4553. [PubMed] [Google Scholar]
- 15. Fonager K, Sorensen HT, Rasmussen SN, et al. Assessment of the diagnoses of Crohn’s disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol 1996;31:154–9. [DOI] [PubMed] [Google Scholar]
- 16. Horwitz A, Skaaby T, Karhus LL, et al. Screening for celiac disease in Danish adults. Scand J Gastroenterol 2015;50:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaira D, Vakil N. Blood, urine, stool, breath, money, and Helicobacter pylori. Gut 2001;48:287–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection - the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–64. [DOI] [PubMed] [Google Scholar]
- 19. Economou M, Pappas G. New global map of Crohn’s disease: Genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis 2008;14:709–20. [DOI] [PubMed] [Google Scholar]
- 20. Whyte LA, Kotecha S, Watkins WJ, et al. Coeliac disease is more common in children with high socio-economic status. Acta Paediatr 2014;103:289–94. [DOI] [PubMed] [Google Scholar]
- 21. Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology 1995;108:1617–21. [DOI] [PubMed] [Google Scholar]
- 22. Pronai L, Schandl L, Orosz Z, et al. Lower prevalence of Helicobacter pylori infection in patients with inflammatory bowel disease but not with chronic obstructive pulmonary disease - antibiotic use in the history does not play a significant role. Helicobacter 2004; 9: 278–83. [DOI] [PubMed] [Google Scholar]
- 23. Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 2011;121:3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rad R, Brenner L, Bauer S, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 2006;131:525–37. [DOI] [PubMed] [Google Scholar]
- 25. Roka K, Roubani A, Stefanaki K, et al. The prevalence of Helicobacter pylori gastritis in newly diagnosed children with inflammatory bowel disease. Helicobacter 2014;19:400–5. [DOI] [PubMed] [Google Scholar]
- 26. Dydensborg S, Toftedal P, Biaggi M, et al. Increasing prevalence of coeliac disease in Denmark: a linkage study combining national registries. Acta Paediatr 2012;101:179–84. [DOI] [PubMed] [Google Scholar]