Abstract
Background and aims:
Vedolizumab inhibits leucocyte vascular adhesion and migration into the gastrointestinal tract through α4β7 integrin blockade. This agent became available in mid-2014 for the treatment of moderate to severe Crohn’s disease (CD) and UC (UC). The aim of this study was to assess the patterns of use, effectiveness and safety of vedolizumab in an inflammatory bowel disease (IBD) clinical practice.
Methods:
Patients beginning vedolizumab were enrolled with informed consent. A prospective cohort was followed with laboratory, disease activity and quality-of-life assessments made during infusion visits up to week 14. Duration of vedolizumab use, mucosal healing and safety were analysed retrospectively for all patients not captured in the prospective component of this study.
Results:
One hundred and two patients started vedolizumab, with 51 patients (30 CD, 21 UC) followed prospectively. The CD patients exhibited a significant decrease in Crohn’s Disease Activity Index (p = 0.04) and Harvey–Bradshaw index (p < 0.01) by week 14. The UC patients demonstrated improved partial Mayo scores at weeks 6 (p < 0.01) and 14 (p < 0.001). Ninety percent of all CD and UC patients remained on vedolizumab up to week 14. IBD-related quality of life was improved by week 6 in CD and UC cohorts (p = 0.02 and p < 0.01 respectively). Colectomy for lack of response and systemic histoplamosis were notable reasons for early discontinuation of vedolizumab, which was otherwise well tolerated.
Conclusions:
Vedolizumab was efficacious and a high percentage of patients continued this therapy beyond induction dosing. Observed safety signals may be attributed to the refractory IBD disease state of this early-adopting clinical cohort.
Key Words: MAdCAM, vedolizumab, Entyvio, clinical practice
1. Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are the 2 major classifications of human chronic inflammatory bowel disease (IBD). The aetiology of IBD is multifactorial, involving the interaction of host genetic susceptibility and environmental factors that include intestinal microbiota. In IBD, these variables trigger an inappropriate inflammatory response in the gastrointestinal tract that involves lymphocyte infiltration, loss of tolerance to luminal microbiota and a persistent elevation of inflammatory cytokines, including tumour necrosis factor α (TNFα).
Antibody-based drugs targeting TNFα and immune cell adhesion molecules (integrins) are effective for both CD and UC. TNFα inhibitors were first approved in 1998 and are widely used across the world.1 However, a proportion of IBD patients do not respond to these agents initially and many of those who do respond experience loss of effectiveness over time. This therapeutic challenge fuels the interest in and excitement about the clinical application of drugs like natalizumab and vedolizumab, which have the novel mechanisms of antagonizing lymphocyte adhesion and migration into the intestinal mucosa.
Natalizumab targets the α4 integrin subunit on circulating lymphocytes and was the first integrin antagonist to demonstrate efficacy in CD.2 The efficacy and application of natalizumab have also been documented in the clinical practice setting; however, the safety profile of this drug has limited its use.3–5 Vedolizumab (Entyvio, Takeda Pharmaceuticals USA, Inc.) targets the gut-homing α4β7 integrin complex and is now approved for induction and maintenance of remission in both moderate to severe CD and UC, based on randomized, placebo-controlled trials.6–8 The improved safety profile, novel mechanism of action and clinical need for new therapies has led to the rapid adoption of vedolizumab use in clinical practice. The aim of this study was to assess the patterns of clinical use, effectiveness and safety of vedolizumab in an IBD referral practice and to assess the overall durability of vedolizumab as maintenance therapy over the first year in clinical use.
2. Methods
2.1. Study design
All adult IBD patients (age ≥18) initiated on vedolizumab through the Washington University Inflammatory Bowel Disease Center from August 2014 until May 2015 were eligible for enrolment into this prospective observational study. Patients were enrolled both at the academic teaching hospital (Barnes-Jewish) and at the community hospital (Barnes-Jewish West County). A requisite of enrolment was providing written informed consent for this study. Additionally, the duration of clinical use was captured for all patients starting on vedolizumab up to the end of September. For those patients not captured in the prospective study, this was assessed by retrospective chart review. Both components of this study were approved and monitored for compliance by the Washington University Human Research Protection Office (institutional review board).
In all patients, vedolizumab was administered intravenously at 300 mg over 30 min at weeks 0, 2, 6 and 14, consistent with labelling and in accordance with clinical practice protocols. Formal assessments of clinical response were made at these time points during infusion visits. Clinical follow-up visits and changes in treatment were at the treating physician’s discretion. Patient outcomes beyond week 14 were assessed by chart review.
2.2. Assessment of clinical response
Demographic and clinical characteristics were collected at first infusion visit and included age, sex, race, smoking status, the Montreal classification of disease location and behaviour, concomitant medications, disease duration, history of surgery and past medication use. Reasons for starting vedolizumab and most recent colonoscopies findings were acquired from chart review.9 At each infusion, up to week 14, assessment of disease activity for CD patients was performed using both the Harvey–Bradshaw index (HBI) and the Crohn’s Disease Activity Index (CDAI).10,11 The HBI was based on patient recall of average CD activity for the last 7 days, which has been shown to be a reliable surrogate for daily diary entries.12
For UC patients, the partial Mayo score, a simplified 9-point version of the Mayo score (remission defined as ≤2 and no subscore >1), was calculated using the patient’s ratings of stool frequency and bleeding components at each infusion.13 The physician’s global assessment of disease activity was taken from the clinical visit closest to the first infusion (±13 days).
Standard laboratory assessments and disease biomarkers for UC and CD patients,14 including complete blood count, liver function tests, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were collected at weeks 0, 6 and 14. The Short Inflammatory Bowel Disease Questionnaire (SIBDQ) was completed at all time points to assess IBD-specific quality of life. Participants rated 10 items on a 7-point Likert scale, ranging from 1 (worst of health) to 7 (best of health). A higher score indicated better quality, with scores ranging from 10 to 70.15
2.3. Mucosal healing
Rates of mucosal healing and endoscopic improvement were assessed in all patients who had colonoscopic evaluations both at baseline (within 2 months of initiation) and after vedolizumab induction (week 6 or later). Patients from both the prospective and the retrospective cohort were eligible. For CD, mucosal ulcerations must have been present at the baseline examination. For UC, patients were included if their baseline endoscopic disease activity was moderate (Mayo score of 2) or greater.
In CD patients, mucosal healing was defined as absence of mucosal ulceration in all segments on the second endoscopic examination (findings consistent with a Simple Endoscopic Score for Crohn’s disease of 0).16 The endoscopist’s final impression of visible CD activity as compared with the baseline examination was used as evidence to determine endoscopic improvement.
Mucosal disease activity in UC was determined by the endoscopist at the time of colonoscopy using the Mayo endoscopic scoring system17 on a drop-down menu available through the ProVation (Minneapolis, MN) electronic report generation system. As in the GEMINI 1 study, a Mayo endoscopic score of 0 or 1 was defined as mucosal healing.7 Patients with an absolute reduction of 1 point in Mayo score from baseline examination as well as those achieving mucosal healing were regarded as showing endoscopic improvement.
2.4. Statistical considerations
Statistical analysis were performed using GraphPad Prism version 5.03 for Windows (San Diego, CA). Categorical variables are shown as percentages and statistical comparisons were performed using Fisher’s exact test. Student’s t-test (paired or unpaired as appropriate) was employed to compare continuous variables between time points (CDAI, CRP, ESR, HBI and partial Mayo score). Changes across more than 2 time points were assessed by one-way ANOVA. A p-value of <0.05 was considered significant. Kaplan–Meier survival analysis was utilized to compare the number of UC and CD patients remaining on vedolizumab starting at week 6 and subsequent infusion weeks.
3. Results
3.1. Characteristics of patients starting vedolizumab
A total of 102 IBD patients started vedolizumab therapy over the study period. Fifty-one of these patients were included in the prospective portion of the study. Reasons for not participating in the prospective study included vedolizumab initiation after closing of the prospective data collection period and missed opportunity to consent prior to the first infusion. All consenting CD (n = 30) and UC (n = 21) patients were included regardless of disease behaviour and severity scores. Table 1 illustrates baseline characteristics of the patients. At the first infusion, CDAI scores were consistent with active disease despite current therapy in over half of the CD patients. Reasons for starting vedolizumab in the remaining CD patients included ongoing disease activity evident on endoscopy (36.7%) and switching from natalizumab (6.7%) for perceived safety reasons associated with JC virus positivity. Patients with UC who were starting on vedolizumab were considered to have ongoing clinical disease activity despite current therapy (90%) or had disease activity on colonoscopy (10%). Patients with CD were more likely than UC patients to have had exposure to more than one TNFα inhibitor therapy before starting vedolizumab (73 vs 38%, p = 0.02). Current smoking was uncommon in both cohorts, a trend observed in our current IBD practice, which includes active efforts towards smoking cessation. Immunomodulator therapy during vedolizumab induction dosing was common in both UC and CD patients.
Table 1.
Patient characteristics.
| Crohn’s disease | Ulcerative colitis | |||
|---|---|---|---|---|
| Age | 49 (n = 30) | 46.2 (n = 21) | ||
| Sex | Female | n = 16 (53.3%) | Female | n = 13 (61.9%) |
| Male | n = 14 (46.7%) | Male | n = 8 (38%) | |
| Race | White | n = 27 (90%) | White | n = 17 (80.9 %) |
| Black | n = 2 (6.7%) | Black | n = 1 (4.8%) | |
| Other | n = 1 (3.3%) | Native American | n = 1 (4.8%) | |
| Other | n = 2 (9.5%) | |||
| Smoking status | Current | n = 1 (3.3%) | Current | n = 0 (0%) |
| Never | n = 23 (76.7%) | Never | n = 18 (85.7%) | |
| Past | n = 6 (20%) | Past | n = 3 (14.3%) | |
| Location (Montreal classification) | L1 | n = 3 (10%) | E1 | n = 6 (28.6%) |
| L2 | n = 4 (13.3%) | E2 | n = 7 (33.3%) | |
| L3 | n = 21 (70%) | E3 | n = 8 (38%) | |
| L4 | n = 2 (6.7%) | |||
| Behaviour (Montreal classification) |
B1 | n = 11 (36.7%) | ||
| B2 | n = 10 (33.3%) | |||
| B3 | n = 4 (13.3%) | |||
| P | n = 5 (16.7%) | |||
| Disease activity at baseline | CDAI | Partial Mayo score | ||
| <150 (remission) | n = 13 (43.3%) | ≤2 (remission) | n = 2 (9.5%) | |
| 150–220 (mild) | n = 8 (26.7%) | 3–4 (mild) | n = 6 (28.6%) | |
| 220–450 (moderate) | n = 7 (23.3%) | 5–6 (moderate) | n = 6 (28.6%) | |
| >450 (severe) | n = 2 (6.7%) | 7– 9 (severe) | n = 7 (33.3%) | |
| HBI | ||||
| <5 (remission) | n = 4 (13.3%) | |||
| 5–7 (mild disease) | n = 4 (13.3%) | |||
| 8–16 (moderate disease) | n = 18 (60%) | |||
| >16 (severe disease) | n = 4 (13.3%) | |||
| Number of prior TNFα inhibitor used | 0 | n = 1 (3.3%) | 0 | n = 5 (23.8%) |
| 1 | n = 7 (23.3%) | 1 | n = 8 (38%) | |
| 2 | n = 11 (36.7%) | 2 | n = 7 (33.3%) | |
| 3 | n = 11 (36.7%) | 3 | n = 1 (4.8%) | |
| Number of prior immunomodulators used (excluding current users) | AZA and/or 6MP | n = 16 (53.3%) | AZA and/or 6MP | n = 6 (28.7%) |
| MTX | n = 6 (20%) | MTX | n = 2 (9.5%) | |
| Number of prior surgeries | 0 | n = 16 (53.3%) | 0 | n = 20 (95.2%) |
| 1 | n = 8 (26.7%) | 1 | n = 1 (4.8%)a | |
| 2 | n = 5 (16.7%) | |||
| 3 | n = 1 (3.3%) | |||
| Steroid use at baseline | Oral | n = 8 (26.7%) | Oral | n = 3 (14.3%) |
| Topical | n = 12 (40%) | Topical | n = 7 (33.3%) | |
| Immunomodulator use at baseline |
Total | n = 21 (70%) | Total | n = 10 (47.6%) |
| AZA and/or 6MP | n = 12 (40%) | AZA and/or 6MP | n = 10 (47.6%) | |
| MTX | n = 9 (30%) | MTX | n = 0 (0%) | |
AZA, azathioprine; 6MP, mercaptopurine; MTX, methotrexate.
a Patient had right hemi-colectomy for adenocarcinoma prior to UC diagnosis.
3.2. Clinical effectiveness and outcomes for CD
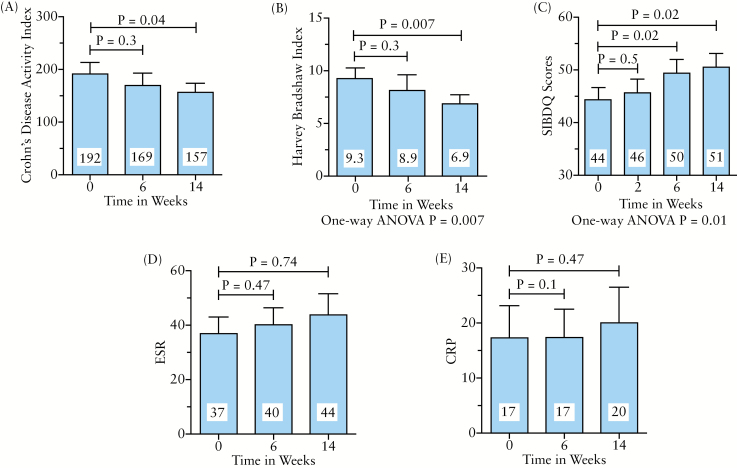
The CD patients available for analysis are presented in Figure 1 and the outcomes data are summarized in Figure 2. Of the seven patients who did not complete the study to week 14, none failed to complete for reasons consistent with a lack of efficacy. Of CD patients who started vedolizumab, 90.5% continued up to and including week 14. These patients exhibited a significant decrease in CDAI (averaging 35 points) and HBI (averaging 2.4 points) by week 14. While disease activity scores were not significantly improved by week 6, health-related quality of life scores were improved as early as week 6 of vedolizumab treatment and continued to be so at week 14. Biomarkers of disease activity did not improve in this vedolizumab-treated CD cohort (Figure 2D, E). After 14 weeks on vedolizumab, nearly half of CD patients were able to stop corticosteroids (43%, p = 0.17).
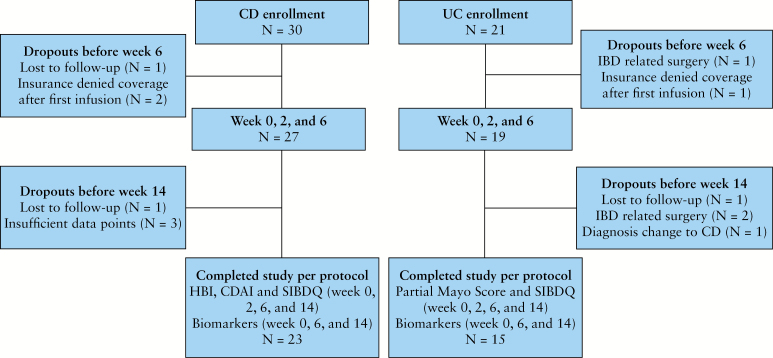
Figure 1.
Flow diagram of eligible patients at inclusion and during study phases.
Figure 2.
Clinical effectiveness data for Crohn’s disease patients. Twenty-three patients completed the 14-week study per protocol. Their data are presented as mean ± SEM for (A) Crohn's Disease Activity Index, (B) Harvey–Bradshaw index, (C) Short Inflammatory Bowel Disease questionnaire (SIBDQ), with higher scores indicating a better disease related quality of life, (D) erythrocyte sedimentation rate and (E) C-reactive protein. The p-values were calculated using paired Student’s t-test or one-way ANOVA as indicated.
3.3. Clinical effectiveness and outcomes in UC
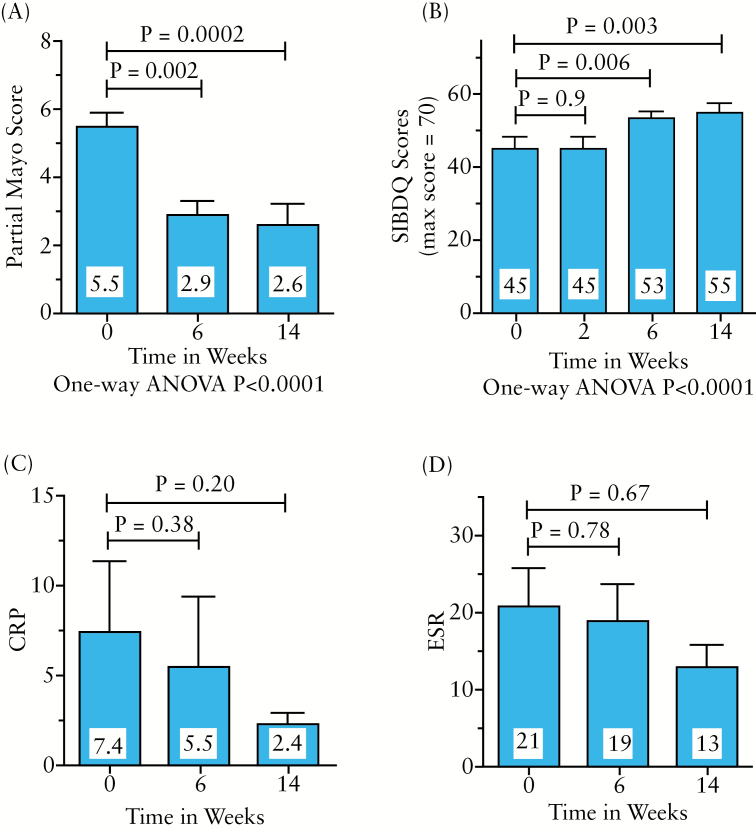
Figure 1 illustrates the patients available for analysis at the various time points and the outcomes data are summarized in Figure 3. Three patients did not reach week 14 of vedolizumab due to reasons consistent with a lack of response (colectomy). Seventy-one percent of UC patients who started vedolizumab completed week 14 and had complete data available for analysis. These patients demonstrated highly significant improvements in partial Mayo scores at weeks 6 (–2.6, p = 0.002) and 14 (–2.9, p = 0.0 002). Of patients with clinically active disease at vedolizumab initiation, 55% reached clinical remission by 14 weeks. Improvement in health-related quality of life was also noted in these vedolizumab-treated UC patients at these same time points. A non-significant decrease in mean CRP and ESR levels was also present (Figure 3C, D). Notably, 73% of UC patients taking oral or topical corticosteroids were able to wean from these medications by week 14 (p = 0.02).
Figure 3.
Clinical effectiveness data for ulcerative colitis patients. Fifteen patients completed the 14-week study per protocol. Their data are presented as mean ± SEM for (A) partial Mayo score, (B) Short Inflammatory Bowel Disease questionnaire (SIBDQ), with higher scores indicating a better disease-related quality of life, (C) erythrocyte sedimentation rate and (D) C-reactive protein. The p-values were calculated using paired Student’s t-test or one-way ANOVA as indicated.
3.4. Endoscopic mucosal healing and response
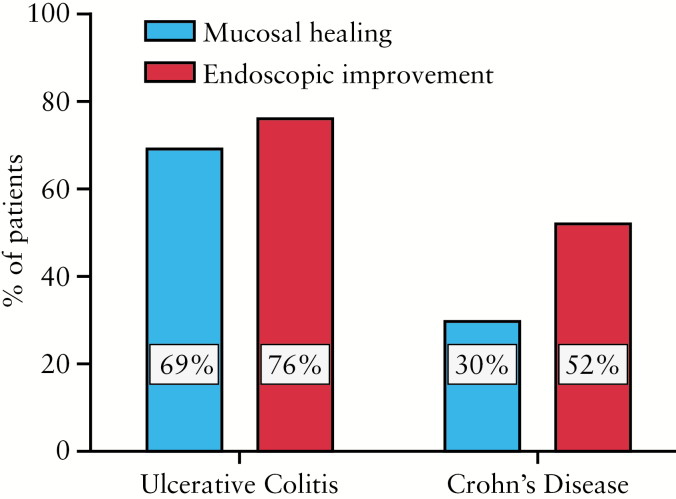
A portion of all patients met criteria for assessment of mucosal healing (29 UC and 27 CD). The median time to post-vedolizumab induction colonoscopy was 22 weeks for both UC and CD. The range was 9–47 weeks for UC and 12–52 weeks for CD. Rates of mucosal healing and endoscopic improvement were higher in UC than CD and are presented in Figure 4.
Figure 4.
Mucosal healing on vedolizumab. Twenty-nine UC and 27 CD patients had data available to determine endoscopic evidence of mucosal healing after initiation of vedolizumab as described in the text (section 2).
3.5. Tolerability of vedolizumab
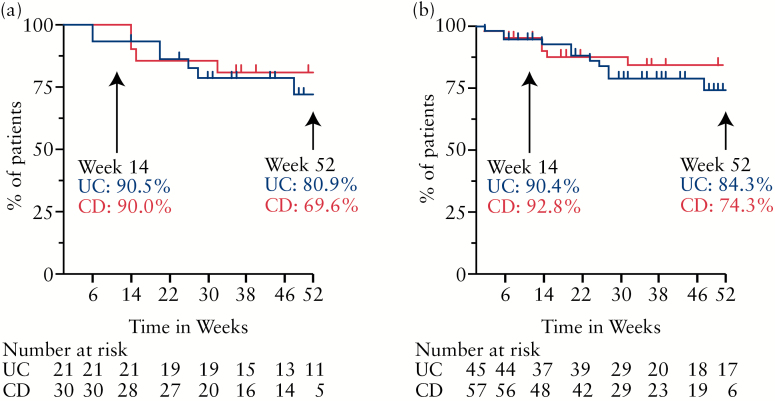
Vedolizumab was generally well tolerated in this population. Altogether, over 90% of the prospectively followed patients and the total vedolizumab cohort received their week-14 dose of vedolizumab (Figure 5). The majority of patients remained on vedolizumab well beyond this time point, with most discontinuations occurring prior to the fourth dose. In this study, the median length of vedolizumab use in the CD cohort was 29 weeks (range 2–63 weeks, interquartile range [IQR] 28 weeks). The median length of vedolizumab use in the UC cohort was 34 weeks (range 2–64 weeks, IQR 38 weeks). One patient experienced an anaphylactic reaction on the third dose, requiring corticosteroids, antihistamines, epinephrine and hospitalization. This patient had exhausted other medical options and was able to continue vedolizumab after participating in an allergist-supervised desensitization protocol.
Figure 5.
Patients remaining on vedolizumab. Kaplan–Meier analysis illustrating the rate of vedolizumab continuance for both ulcerative colitis and Crohn’s disease patients carried out to week 52 as available. (A) Prospective study cohort (n = 51; UC = 21, CD = 30). (B) All patients starting vedolizumab (n = 102; UC = 45, CD = 57). The numbers of subjects at risk are listed by time point in weeks.
3.6. Safety of vedolizumab
Several adverse events occurred in this IBD population starting vedolizumab in the first year of its approval, as summarized in Table 2. In total, 29% (6 of 21) of UC patients and 37% (11 of 30) of CD patients in the prospective cohort experienced adverse events during this time period. The rate of adverse events was much lower in the retrospective cohort. Some patients experienced more than one adverse event. Many of these were considered serious, but few were considered directly related to vedolizumab.
Table 2.
Adverse events during vedolizumab therapy.
| Prospective cohort (n = 51) | CD patients (11 of 30) |
UC patients (6 of 21) |
|---|---|---|
| Before week 14 | IBD-related surgeries (n = 2) | Total proctocolectomy (n = 2) |
| Histoplasmosis (n = 1) | Fever within 24h of first dose (n = 1) | |
| Anaphylaxis (n = 1) | Conjunctivitis (n = 1) | |
| Rash (n = 1) | ||
| After week 14 | IBD-related surgeries (n = 4) | Total proctocolectomy (n = 1) |
| Basal cell carcinoma (n = 1) | Diverting loop ileostomy (n = 1) | |
| CMV colitis followed by new Hodgkin's lymphoma diagnosis and subsequent death (n = 1) | ||
| Retrospective cohort (n = 115) | CD patients (2 of 34) |
UC patients (2 of 30) |
| Before week 14 | Total proctocolectomy (n = 2) | |
| After week 14 | IBD-related surgeries (n = 2) |
In UC, 3 patients were found to have an inadequate response to vedolizumab and underwent total proctocolectomy. Two of these patients went to colectomy prior to week 14, while 1 went to surgery at week 30 for increased disease activity. One additional patient in the UC cohort had a diverting loop ileostomy for a rectal stricture at week 20 with a subsequent change in diagnosis to CD. Two other individuals developed adverse events potentially related to vedolizumab within the induction period. The first individual developed a fever 24 hours after the second dose, which resolved spontaneously within a day. The other patient developed conjunctivitis, which required topical antibiotics 1 week after the first dose. Both continued on therapy.
In CD, several serious adverse events occurred. Six patients required CD-related surgery. The surgery was related to ongoing disease activity or stricture in 5 of these patients, while in 1 patient the operation was a takedown of loop ileostomy after a demonstration of colonic healing. All patients but 1, who underwent a total proctocolectomy with end ileostomy, remained on vedolizumab after surgery. There was one new basal cell carcinoma diagnosis in a patient who had previously been on azathioprine.
Two patients with CD treated with vedolizumab had particularly notable serious adverse events. The first was a 72-year-old female with CD who was changed to vedolizumab 8 weeks after her last dose of infliximab based on ongoing clinical and endoscopic disease activity. Biopsies from a colonoscopy after 14 weeks of therapy demonstrated cytomegalovirus (CMV). The patient was started on oral ganciclovir and continued on vedolizumab. Eight weeks later the patient was diagnosed with Hodgkin’s lymphoma. Prior to starting cancer treatment, the patient developed sepsis and acute kidney injury, and died.
A second individual, a 40-year-old female with severe ileal and colonic CD, started vedolizumab 4 weeks after stopping adalimumab, to which she had inadequate clinical and endoscopic responses. She remained on weekly subcutaneous methotrexate (25mg per week) during vedolizumab induction. One week after the second dose (7 weeks after the last adalimumab dose), she developed worsening diarrhoea and a sepsis-like syndrome and was admitted to the intensive care unit requiring mechanical ventilation and blood pressure support. A colonoscopy demonstrated moderate to severe disease activity, with biopsies subsequently demonstrating heavy infiltration with the fungal organism Histoplasma capsulatum. A diagnosis of disseminated histoplasmosis was confirmed as the organism was also recovered from the lung and blood cultures. Her urine histoplasma antigen was quantitatively above the maximum range for test sensitivity. Given the timing of symptom development and the high burden of fungal organisms in the colon biopsies, it was suspected that vedolizumab had a role in precipitating the decline in her clinical condition even if the predisposition to acquiring the histoplasma had occurred with prior IBD therapies. Therapy for CD was stopped and amphotericin was initiated with an eventual transition to voriconazole. Over an 8-week hospitalization, the patient recovered and was discharged to remain on histoplasma-suppressive therapy indefinitely. She subsequently received repeat induction therapy with vedolizumab while remaining on methotrexate, which led to steroid-free clinical remission, which she has maintained for more than 6 months.
Concomitant medical therapy and the timing between the last dose of TNFα inhibitor and initiation of vedolizumab is likely to influence the observed safety signal. In the GEMINI trials, a TNFα inhibitor washout period of at least 8 weeks was required. In this study, a third of UC patients had taken a TNFα inhibitor within 1 year prior to starting vedolizumab. Half of these patients received their first dose of vedolizumab less than 8 weeks after the last dose of TNFα inhibitor. Seventy percent of CD patients switched from a TNFα inhibitor to vedolizumab, with two-thirds of these patients switching in less than 8 weeks. Among UC and CD patients starting vedolizumab less than 8 weeks after the last dose of TNFα inhibitor, the average timeframe was 2 weeks, with a range of 0–7 weeks.
4. Discussion
In this prospective study in a clinical practice setting, we provide detailed data on the patient characteristics as well as clinical effectiveness, tolerability and safety of vedolizumab for treatment of chronic IBD. This therapy led to improvements in clinical disease activity as well as quality of life for both UC and CD patients. Adverse events were common, but rarely attributable specifically to vedolizumab.
Vedolizumab is the first widely prescribed drug in this new class of biologic therapies for IBD that target leucocyte trafficking. The strong need for new therapeutic mechanisms was evidenced in our patient population, of which the majority had previously received 2 or more TNFα inhibitors and more than half were taking concurrent immunomodulators and/or corticosteroids at the time of starting vedolizumab.
Currently, there is only information from one other clinical practice cohort of patients receiving vedolizumab. This report also evaluated a refractory IBD population who had started vedolizumab in its first year after approval by the US Food and Drug Administration (FDA).18 The current study differs from this published report in that all effectiveness data were collected prospectively and conclusions about effectiveness were based on defined disease activity indices. Additionally, we included all patients starting on vedolizumab without exclusion criteria other than refusal to provide informed consent. The inclusion of all patients starting on vedolizumab was intentional as the study’s aim was not only to capture the effectiveness and safety of vedolizumab in a clinical practice cohort, but also to understand the patient characteristics of those starting vedolizumab. This sampling strategy is relevant as it has been found that patient populations that meet strict inclusion criteria mandated by clinical trials are not necessarily representative of those encountered in the clinical practice setting.19
Clinical effectiveness of vedolizumab was evident in both the CD and UC populations. Reductions in clinical disease activity were evident at 6 weeks for UC patients and by week 14 for CD patients. These findings are consistent with observations from clinical trials, which indicated earlier response to vedolizumab in UC than CD.6–8 We did observe an overall improvement in IBD-related quality of life by week 6 and persisting to week 14 in both the CD and UC cohorts. Perhaps the most important measurable sign of perceived benefit to vedolizumab was the finding that 90% of both CD and UC patients remained on this therapy after week 14. Stool, urine and serum samples were collected along with standard laboratory test results and should provide an important repository for assessment of novel predictive biomarkers.
Mucosal healing was also evident in both CD and UC after vedolizumab induction. Observed rates of mucosal healing in our UC cohort were higher than that observed in the GEMINI 1 study, where 40.9 and 51.6% of patients achieved mucosal healing at weeks 6 and 52 respectively using an every 8 weeks. While we used the same criteria for mucosal healing, our clinical practice-based study differed, as not all patients had 2 endoscopic evaluations, early colectomy patients (at <14 weeks, n = 3) were excluded, and there was variance in the timeframe for second colonoscopic evaluation after vedolizumab initiation. Mucosal healing in CD was not reported in either the GEMINI 2 study or in the previous clinical practice report on vedolizumab. Thus, this report represents the first available data assessing CD mucosal healing in response to vedolizumab.
Vedolizumab’s safety profile in clinical practice is anticipated to be good based on clinical trial data and the drug’s specificity for the gut.20 However, adverse events were common in our study and included surgeries, a systemic fungal infection and a death. Most of the serious adverse events were in patients with severe disease at inclusion while waiting for FDA and insurer approval of vedolizumab. This delay and the duration of insufficiently effective immunosuppressive therapies leading up to the initiation of vedolizumab likely amplified the effect size of this observation. Patients included in the retrospective cohort had shorter intervals between the clinical decision to start vedolizumab and initiation after insurance approval. We anticipate that the rate of severe adverse events will diminish with time as insurance coverage and availability expand. Moreover, it may improve further if the positioning of vedolizumab in clinical care paradigms evolves to an earlier time point, as some suggest it should.21 Still, treating clinicians must be aware of the potential for serious adverse events to develop in patients starting and continuing on vedolizumab.
Limitations should be noted when interpreting the current study. The study had an open-label design and the overall number of patients included was significantly smaller than those in published clinical trials. These factors and the cohort size precluded subgroup analyses for predictors of effectiveness. However, these are balanced by the major strength of this study, which is that it provides detailed insight into real-life IBD clinical practice in a prospectively followed cohort. While there are limitations to a single-institution study, this regional referral centre attracts patients from a diverse metropolitan and multi-state catchment area of more than 3 million people.
In summary, vedolizumab was well tolerated and led to clinical improvements in the majority of IBD patients in a clinical practice setting. Adverse events were higher than expected, but may be attributed to the refractory nature of this IBD population. These findings are in strong support of a role for vedolizumab in clinical practice, but should also draw attention to the importance of monitoring this patient population for therapy-related complications.
Funding
This work was supported by National Institutes of Health research grants (DK089016 to MAC and P30 DK052574 to the Washington University School of Medicine Division of Gastroenterology). Additional support was provided by the Givin’ It All For Guts Foundation (www.givinitallforguts.org) to MAC. No commercial entities supported this research.
Conflict of Interest
Dr Ciorba reports grants from NIH-NIDDK, grants from NIH-NIAID, grants from the Longer Life Foundation, personal fees from UCB, personal fees from AbbVie and personal fees from Takeda, all unrelated to the submitted work.
Acknowledgments
The authors greatly appreciate the assistance of the infusion centre staff at both Barnes-Jewish Hospital and Barnes-Jewish West County Hospital and the Washington University IBD Center clinical staff.
References
- 1. Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol 2015;12:537–45. [DOI] [PubMed] [Google Scholar]
- 2. Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology 2007;132:1672–83. [DOI] [PubMed] [Google Scholar]
- 3. Chen CH, Kularatna G, Stone CD, Gutierrez AM, Dassopoulos T. Clinical experience of natalizumab in Crohn's disease patients in a restricted distribution program. Ann Gastroenterol 2013;26:189–90. [PubMed] [Google Scholar]
- 4. Juillerat P, Wasan SK, Fowler SA, et al. Efficacy and safety of natalizumab in Crohn's disease patients treated at 6 Boston academic hospitals. Inflamm Bowel Dis 2013;19:2457–63. [DOI] [PubMed] [Google Scholar]
- 5. Sakuraba A, Keyashian K, Correia C, et al. Natalizumab in Crohn's disease: results from a US tertiary inflammatory bowel disease center. Inflamm Bowel Dis 2013;19:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–27.e3. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 9. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:9A–13A. [DOI] [PubMed] [Google Scholar]
- 10. Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 11. Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 12. Henao MP, Bewtra M, Osterman MT, et al. Measurement of inflammatory bowel disease symptoms: reliability of an abbreviated approach to data collection. Inflamm Bowel Dis 2015;21:2262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res 2012;159:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol 1996;91:1571–8. [PubMed] [Google Scholar]
- 16. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 17. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 18. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis 2015; 21:2879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–7; quiz e78. [DOI] [PubMed] [Google Scholar]
- 20. Hagan M, Cross RK. Safety of vedolizumab in the treatment of Crohn's disease and ulcerative colitis. Expert Opin Drug Saf 2015;14:1473–9. [DOI] [PubMed] [Google Scholar]
- 21. Bryant RV, Sandborn WJ, Travis SP. Introducing vedolizumab to clinical practice: who, when, and how? J Crohns Colitis 2015;9:356–66. [DOI] [PubMed] [Google Scholar]