Abstract
Background and Aims:
Ulcerative colitis [UC] is associated with colonic mucosa barrier defects and bacterial dysbiosis, but these features may simply be the result of inflammation. Therefore, we sought to assess whether these features are inherently abrogated in the terminal ileum [TI] of UC patients, where inflammation is absent.
Methods:
TI biopsies from paediatric inflammatory bowel disease [IBD] subsets [Crohn’s disease [CD; n = 13] and UC [n = 10]], and non-IBD disease controls [n = 12] were histologically graded, and alcian blue/periodic acid-Schiff stained biopsies were quantified. The mucosal barrier was assessed for mucin [MUC2], immunoglobulin [Ig]A, IgG, and total bacteria (fluorescence in-situ hybridisation [FISH probe EUB338]) by immunofluorescence. The regulation of mucin secretion was investigated by NLRP6 gene expression and immunofluorescence. The composition of the active mucosa-associated microbiota was explored by sequencing the 16S rRNA amplicon generated from total RNA.
Results:
Despite the absence of ileitis, UC patients displayed ileal barrier depletion illustrated by reductions in mucin-containing goblet cells and mucin production and altered epithelial NLRP6 expression. In both CD patients with ileitis and UC patients with normal histology, bacteria coated with IgA and IgG penetrated the TI mucin layer. Biopsy 16S rRNA sequencing revealed a reduction in α-diversity by three methods [Shannon, Simpson, and Equitability indices] between UC and non-IBD paediatric patients.
Conclusions:
These findings suggest an underlying defect in the UC-afflicted intestinal tract even in the absence of inflammation, implicating barrier and microbial changes as primary abnormalities in UC that may play a causative role in disease development.
Keywords: Ulcerative colitis, mucosal barrier, mucin
1. Introduction
The gastrointestinal tract includes a vulnerable single-cell epithelial layer adjacent to the lumen, where bacteria reside and contribute to the regulation of a healthy intestinal barrier.1,2 The bacterial-epithelial contact is minimised by a mucous film, mainly consisting of neutral and acidic mucin glycoproteins produced by goblet cells.3 The colonic mucous film is uniform, with an attached and rather sterile thick inner layer protecting the mucosa, and a loose outer layer that provides a nutrient source for resident bacteria.4 In contrast, the intestinal barrier of the small bowel consists of a patchy mucin layer originating from the crypts and released apically to the protruding villi, that along with adherent bacteria is flushed to the colon.5 In addition to mucin secretions, the intestinal barrier is reinforced with secretory immunoglobulin [Ig]A and IgG and constitutively-secreted antimicrobial peptides, produced mainly by Paneth cells in the small intestine.6,7,8,9
Inflammatory bowel diseases (IBD; including Crohn’s disease [CD] and ulcerative colitis [UC]) are chronic conditions of the digestive tract. CD is characterized by patchy, potentially panenteric, transmural inflammation frequently involving the terminal ileum [TI], whereas UC by definition is restricted to the colonic mucosa, with the exception of limited ‘backwash ileitis’ and non-specific gastritis.10,11 The cause of IBD is unknown, but a combination of genetic predisposition, environmental factors, and a dysregulated inflammatory response to the resident microbiota are considered important for the pathogenesis of IBD.12,13 IBDs are associated with alterations of the gut microbiota, such as a loss in bacterial diversity and shifts in the microbiota that are often correlated with active disease.14,15 Interestingly, siblings of patients with CD who have an elevated risk of developing the disease also display a loss in bacterial diversity, suggesting that alterations of the gut microbiota, and especially an increase in taxa associated with pro-inflammatory responses, might contribute to the observed pathologies.16
In CD the colonic mucous layer is thicker, and gene polymorphisms [eg NOD2 and ATG16L1] have been linked to autophagy defects in ROS-mediated mucin secretion and abnormal secretion of antimicrobial peptides from Paneth cells in the ileum.17,18,19 Recently, a causal role for dysbiotic bacteria in the development of CD-like ileitis and consequential reduction in antimicrobial peptides was shown in a murine model.20 In UC, the colonic mucous layer is thinner and Paneth cell function is not affected in the ileum.21 Depletion of the mucosal barrier in inflamed UC has also been linked to alterations in MUC2 [the most abundant mucin in the TI and colon], bacterial penetration of the barrier, and increased mucolytic bacteria.22,23,24 Evidence suggests that the mucosal barrier depletion could be due to abrogation of core mucin biosynthesis by endoplasmic reticulum stress.25 In addition, the Nod-like receptor pyrin domain-containing protein 6 [NLRP6] has a functional role in mucin exocytosis,26 and NLRP6 -/- mice are more susceptible to chemically-induced colitis.27 Although no link between NLRP6 expression and UC has been firmly established in humans, the thinning of the mucosal barrier could be partly regulated by NLRP6 expression.
There is compelling evidence that supports the link between alterations in the mucosa-associated microbiota and defects in the mucosal barrier in IBD. However, whether the microbial dysbiosis and barrier defects are prerequisites to IBD and contribute to these pathologies or are just consequences of local inflammation, remains unknown. In this study, we investigated whether these alterations are inherent features of UC by characterizing the microbiome and mucosal barrier in the TI in UC patients, which is devoid of inflammation. We compared findings with those obtained in CD patients and non-IBD disease controls and specifically chose to focus on children, as they are less likely to show other confounding effects.
2. Materials and Methods
2.1. Consent and ethics approval
Consent from patients was obtained and the study was approved by the Health Research Ethics Board at the University of Alberta [Study ID Pro00023820], Edmonton, AB, Canada.
2.2. Patient criteria
For all patients, bowel cleansing was standardized using Picosalax®: sodium picosulfate and magnesium oxide before endoscopy. Diagnosis of CD and UC was based on endoscopic, clinical, and histopathological findings using the Paris classifications.28 UC subjects endoscopically or histologically diagnosed with backwash ileitis were excluded from the study. Inclusion criteria included: 3–18 years old; parent/child understood and gave consent/assent; patient undergoing endoscopy; IBD: diagnosed clinically, confirmed by endoscopy28; non-IBD disease controls: children requiring endoscopy but without mucosal pathology [ie coeliac disease and enteropathy were excluded]. Controls may not have been completely healthy (eg may have had abdominal pain, chronic diarrhoea, benign polyps, or irritable bowel syndrome [IBS, excluding post-infectious IBS]) but were excluded if they had gut inflammation on histology or endoscopy. Exclusion criteria included: exposure to antibiotics or probiotics within a month preceding endoscopy; exposure to motility drugs [with the exception of bowel preparation]; presence of gut luminal disorder [other than IBD] or inflammation on histology [in non-IBD controls]; other significant comorbidities [eg diabetes, arthritis, neurological disease].
2.3. Biopsy collection
Biopsies were collected during endoscopy from normal-appearing TI and either immersed in formalin for histology [n ≥ 2 per patient], fixed in methanol Carnoy’s solution for preservation of the mucous layer and the mucosal barrier [n ≥ 2 per patient], or individually snap-frozen for quantitative reverse-transcription polymerase chain reaction [qRT-PCR] [n = 1 per patient] and 16S ribosomal-RNA next-generation sequencing [n = 1 per patient].
2.4. Histology assessment and mucous layer analyses
Biopsies fixed in neutral-buffered formalin were paraffin-embedded using standard laboratory techniques. The biopsies were serially sectioned using a microtome [2 µm] and haematoxylin and eosin-stained with an autostainer [Leica Microsystems, Concord, ON, Canada]. Coded slides were evaluated by a single pathologist, blinded to patient details, based on a modified histology score [see Supplementary Table 1, available as Supplementary data at ECCO-JCC online].29 Biopsies fixed in methanol Carnoy’s were stained for mucin-containing goblet cells and mucous production using alcian blue/periodic acid-Schiff [AB/PAS], as described in detail in the Supplementary Data and shown in Supplementary Figure 1 [available as Supplementary data at ECCO-JCC online]. Briefly, sections from the coded slides consisting of complete villus crypt length were imaged for each patient at 200X magnification. Quantitative analyses for mucin-containing goblet cells and mucous production around the epithelial lining and lumen were performed using ImageJ software 1.48v.30
2.5. Fluorescence in-situ hybridisation [FISH] and immunofluorescence
Biopsies fixed in methanol Carnoy’s were used to identify bacteria, assess the mucosal barrier, and examine regulation of mucous secretion by NLRP6. Methanol Carnoy’s fixation preserved the mucous and bacterial layer and reduced non-specific binding and autofluorescence compared with formalin fixation [data not shown]. FISH probe EUB338 was used to identify all members of the bacteria kingdom [Integrated DNA Technologies, Toronto, ON, Canada] as detailed in the Supplementary Data. To control for non-specific binding, a bacteria probe NON338 was applied, and showed no non-specific binding [data not shown]. The mucosal barrier was assessed in biopsies [detailed in the Supplementary Data] by immunofluorescence with staining for MUC2 [rabbit anti-human MUC2, Santa Cruz Biotechnology, Santa Cruz, CA, USA], IgA, and IgG [rabbit anti-human IgA or IgG antibodies, Abcam, Toronto, ON, Canada]. To exclude non-specific binding, an IgG isotype [rabbit anti-human, Abcam] was applied, and showed no non-specific binding [data not shown]. The regulation of mucous secretion was assessed by staining for NLRP6 [goat anti-human NLRP6, Santa Cruz Biotechnology] with secondary antibody in absence of primary antibody serving as control.
2.6. Quantitative reverse-transcription PCR
Total RNA extraction and qRT-PCR for gene expression of NLRP6 [Table 1] are described in the Supplementary Data. Glyceraldehyde 3-phosphate dehydrogenase [GAPDH, Integrated DNA Technologies, Toronto, ON, Canada] primer was used as a housekeeping gene for relative quantification of transcript amount.
Table 1.
Primers used for quantitative reverse-transcription polymerase chain reaction [qRT-PCR].
| Target | Sense and antisense primers [5′→3′] | Annealing temperature [°C] | Amplicon size [bp] |
|---|---|---|---|
| NLRP6 | GCTGCAGATTGGTTGCTG TGGTGCCTTGAGAACTGCT |
60 | 96 |
| GAPDH | CCCACTCCTCCACCTTTGAC ATGAGGTCCACCACCCTGTT |
60 | 115 |
NLRP6, Nod-like receptor pyrin domain-containing protein 6; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; bp, base pairs.
2.7. 16S rRNA high-throughput sequencing of metabolically active ileal bacteria
To determine the composition of metabolically-active bacteria, next-generation sequencing of 16S rRNA amplicons generated by polymerase chain reaction [PCR] from cDNA generated from RNA isolated from ileal biopsies was performed using an Illumina MiSeq platform.31 16S rRNA gene libraries were prepared following the 16S Sample Preparation Guide 15044223 A [Illumina, San Diego, CA, USA]. Briefly, cDNA from biopsies was used as template in nested PCR using primers suitable for amplification of the V3 and V4 variable regions of the 16S rRNA gene with overhang MiSeq sequencing adapters [Forward 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGC CTACGGGNGGCWGCAG-3′; Reverse 5′- GTCTCGTGGGCTCG GAGATGTGTATAAGAGACAGGACTACHVGGGTAT CTAATCC-3′], producing amplicons of approximately ~550bp. PCR cycle programme consisted of 25 cycles with denaturing at 95°C [30 s], annealing at 55°C [30 s], and elongation at 72°C [30 s]. PCR amplicons were purified using AMPure XP paramagnetic beads [Beckman Coulter, Brea, CA] and subjected to indexing PCR using Nextera XT indices [Illumina] according manufacturer’s protocols.
2.8. Bioinformatic analysis
16S ribosomal rRNA gene libraries were sequenced using a paired-end workflow that includes demultiplexing of libraries and trimming of adapters. Since the average quality of read2 was low, only the read1 was used for analyses as follows. Sequence quality was inspected starting from the 5’ end and the 3’ moiety was trimmed off whenever a stretch of 3 nt with quality scores smaller than 30 [Q < 30] was found, using the split_libraries_fastq.py script from QIIME32 with default parameters. Sequences were clustered into operational taxonomic units [OTUs] and depurated from chimeric sequences using the UPARSE pipeline.33 Taxonomy was assigned using the RDP classifier.34 OTUs with a minimal counts fraction less than 0.00005 were filtered out for subsequent analyses. Bray-Curtis dissimilarities were calculated from the contingency table [OTU table], followed by principal coordinates analysis [PCoA].35 To determine whether relative abundance at the family level differed in IBD subsets compared with non-IBD, the change in abundance for each family was calculated with the following formula: [mean abundance in CD or UC] / [mean abundance of the non-IBD controls] normalized to the mean abundance of the family level in non-IBD controls. Families with abundance < 1% were grouped into ‘Other bacteria’ for clarity of representation. Shannon, Simpson, and Equitability α-diversity indices were calculated using the alpha_diversity.py script from QIIME.
Characterization of the microbial community at lower taxonomic levels was done by minimum entropy decomposition [MED]36 using the quality-controlled sequences described above. This method allows resolution of the 16S rRNA gene tags with single nucleotide differences. The taxonomic identification of the closest relative to each MED node was achieved by blasting each node sequence against the 16S ribosomal rRNA and nr/nt databases from NCBI. The top hit was extracted by parsing BLAST reports with in-house Perl scripts. In those instances when MED nodes were identical to more than one reference sequence in the databases, the top hit was kept. The raw data of all libraries generated during this study are publicly available at the Sequence Read Archive [SRA] portal of the National Center for Biotechnology Information [NCBI] under accession number SRP064817.
2.9. Statistical analyses
Continuous variables were expressed as median with range. The statistical analyses were performed using GraphPad Prism [GraphPad Software version 5, San Diego, CA]. Comparisons between the histology scores, mucous-goblet cells, mucous secretion, and NLRP6 gene expression were made by an unpaired non-parametric two-tailed Mann-Whitney U test. Bacterial α-diversity comparisons were made by one-way analysis of variance with Bonferroni’s multiple comparison post-test. Comparisons between bacterial taxa were made by Benjamini-Hochberg false discovery rate corrections.
3. Results
3.1. Patient characteristics
Clinical characteristics of non-IBD controls and patients with CD and UC are shown in Table 2. Compared with the majority of CD patients who had moderate disease activity, UC patients consisted mostly of females, many of whom were in remission or with mild disease activity. All UC patients had a normal TI confirmed by endoscopy and biopsies. Out of the 10 UC patients, 8 had a history of pancolitis, 1 had left-sided colitis, and 1 did not have active UC at time of endoscopy and previous disease extent was not clearly documented. More UC patients were receiving treatment, likely reflecting a larger number of follow-up studies in this group. Backwash ileitis was absent in all UC patients—to eliminate the possibility that outcomes may be affected by inflammation.
Table 2.
Patient characteristics.
| Patient characteristic | Non-IBD | CD | UC |
|---|---|---|---|
| N | 12 | 13 | 10 |
| Mean age, years [range] | 11.7 [7–16] | 12.9 [4–17] | 13.2 [6–17] |
| Gender [F/M] | 5/7 | 5/8 | 8/2 |
| Ileal biopsies [N; mean/patient] | 6.4 | 6.0 | 6.2 |
| Number of crypts-villi analysed [patient mean ± S.D.] | 11±4 | 9±3 | 13±4 |
| Disease activitya | NA | 3/2/7/1 | 4/4/2/0 |
| Diagnosis [new/follow-up] | NA | 7/6 | 1/9 |
| Treatments [%] | |||
| Proton pump inhibitor | - | 8 | 0 |
| Azathioprine | - | 15 | 50 |
| Infliximab | - | 15 | 30 |
| Prednisone | - | 0 | 40 |
| 5-aminosalicylic acid | - | 0 | 50 |
| Sulphasalazine | - | 8 | 10 |
| Methotrexate | - | 15 | 10 |
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; SD, standard deviation; NA, not applicable; F, female; M, male.
aDisease activity [remission/mild/moderate/severe] was based on Paediatric Crohn’s Disease Activity Index [PCDAI] and Paediatric Ulcerative Colitis Activity Index [PUCAI].
3.2. Mucin depletion and bacteria-mucosa interaction in the non-inflamed TI of UC
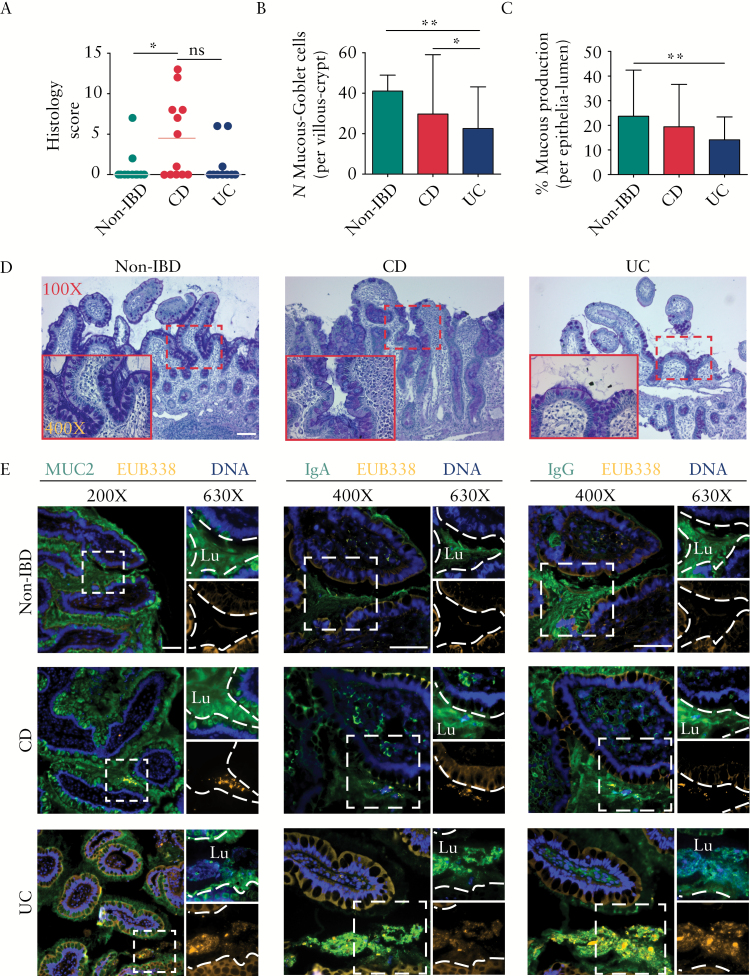
To examine ileal inflammation, biopsies were scored blindly [Figure 1A]. Histology [see Supplementary Figure 2, available as Supplementary data at ECCO-JCC online)] showed marked epithelial damage, architectural changes, infiltration of mononuclear cells in the lamina propria, and infiltration of polymorphonuclear cells in the lamina propria and epithelium in CD patients. These features were not found in non-IBD and UC patients, confirming absence of inflammation in the TI of UC patients. To determine the condition of the mucous layer, we quantified mucin-containing goblet cells [Figure 1B] and mucin production [Figure 1C] of AB/PAS stained ileal biopsies [representative images shown in Figure 1D]. We found a significant reduction of mucin-containing goblet cells per villous crypt units [UC vs non-IBD, p < 0.01; UC vs CD, p < 0.05], and depletion of mucin production [UC vs. non-IBD, p < 0.01]. Surprisingly, there was no significant difference in these parameters between non-IBD and CD patients.
Figure 1.
Ulcerative colitis [UC] patients demonstrate mucous barrier reduction and an elevated mucosa-bacterial interaction even in the absence of ileitis. [A] Scatterplot representation of histology showed ileitis in Crohn’s disease [CD] and its absence in UC and non-inflammatory bowel disease [IBD] groups. [B] Compared with the non-IBD and CD groups, the mean numbers of mucin-containing goblet cells per villous crypt were significantly reduced in the terminal ileum [TI] of the UC group. [C] Compared with the non-IBD group, mucous production expressed as % of epithelial surface covered with mucin [see Supplementary Figure S1, available as Supplementary data at ECCO-JCC online] in the epithelia-lumen was significantly reduced in the UC group. [D] Representative AB/PAS-stained TI biopsies showing reduced mucin in UC. Bar is 100 µm; inset image is 400X. [E] Immunostaining for mucin [MUC2], IgA, IgG, and bacteria, showing bacteria virtually absent from the mucosal layer in non-IBD patients. In contrast, in CD and UC bacteria were in close proximity to the epithelial lining with an increase in association with IgA and IgG. Lu, lumen; images showing mucin or Ig [top right] and bacteria [bottom right] were cropped digitally using the ZEN software, and the dashed lines were digitally added to mark the epithelial surface; bar is 50 µm. Statistically significant differences between two groups are indicated by an asterisk [p < 0.05] or double asterisk [p < 0.01]; ns, not significant. Note: the epithelial tissue structures visualised [shown in orange] are caused by autofluorescence resulting from fluorescence in-situ hybridisation [FISH]; in contrast, bacteria appear as clear orange clumps.
To determine if the mucosal barrier depletion increased bacterial penetration into the mucous layer and Ig coating, ileal biopsies were stained for bacteria, mucin, IgA, and IgG and imaged by florescence microscopy [Figure 1E]. In non-IBD patients, few to no bacteria were found in the mucous layer. The mucosal barrier consisted of secreted mucin and mucin-containing goblet cells, and IgA and IgG secretions were mostly limited to the lumen. In both CD and UC patients, bacteria penetrated into the mucin layer and were associated with IgA and IgG attachment, as seen in serial sections. An increase of IgA and IgG in CD, and increase in IgG in UC, were observed in the lamina propria, in addition to the lumen, suggesting immune activation within the mucous layer at the site of bacterial penetration.
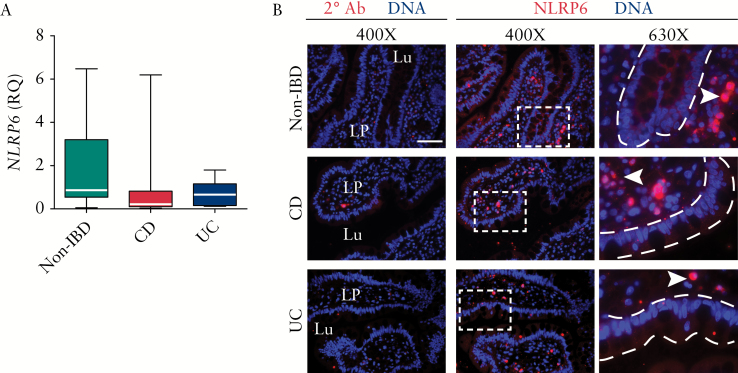
3.3. Ileal NLRP6 expression is altered in IBD
NLRP6 has been shown to regulate colonic mucous secretion in mice,26 but its role in humans or in the ileum is not known. We hypothesised that NLRP6 may have a similar role of regulating mucin in the TI. In order to determine whether the reduction in mucous secretion could be due in part to dysregulation of ileal NLRP6, its expression was analysed by quantitative reverse transcription polymerase chain reaction [qRT-PCR]. Median and interquartile range [IQR] for NLRP6 expression in CD and UC were 0.21 [0.10–0.40] and 0.65 [IQR 0.16–0.93], respectively, vs 0.86 [IQR 0.76–2.34] for non-IBD controls. We found a trend for reduced NLRP6 expression in IBD subsets but this did not reach statistical significance [Figure 2A]. As qRT-PCR will not distinguish between different cellular sources, we used immunofluorescence of the NLRP6 protein to determine the cellular localisation of the protein, and found NLRP6 to be expressed in the epithelial layer as well as the lamina propria in non-IBD patients [Figure 2B]. In contrast, the expression was well distributed in the lamina propria but reduced in the epithelial layer of CD and UC patients, suggesting a redistribution of NLRP6 in IBD, which could, in theory, provide a mechanism for reduced mucin production in IBD. This further suggests that qRT-PCR does not provide a complete picture of the reduction in NLRP6 in UC, as the gene appears to be differentially regulated in particular cells.
Figure 2.
Ileal NLRP6 location is altered in inflammatory bowel disease [IBD]. [A] NLRP6 gene expression in ileal biopsies as measured by quantitative reverse-transcription polymerase chain reaction [qRT-PCR] [relative quantification, RQ]. Data are expressed as median with interquartile range, minimum and maximum. [B] Representative immunofluorescence of biopsies stained for NLRP6 protein showed expression in the lamina propria and proportionately distributed throughout epithelial cells of non-IBD group, with lower and intermittent epithelial distribution in both IBD subsets. Lu, lumen; LP, lamina propria; arrowhead, NLRP6-expressing cell in the LP; bar is 50 µm. The dashed lines were digitally added to mark the epithelial surface.
3.4. Bacterial diversity at the ileal mucosal layer is reduced in IBD
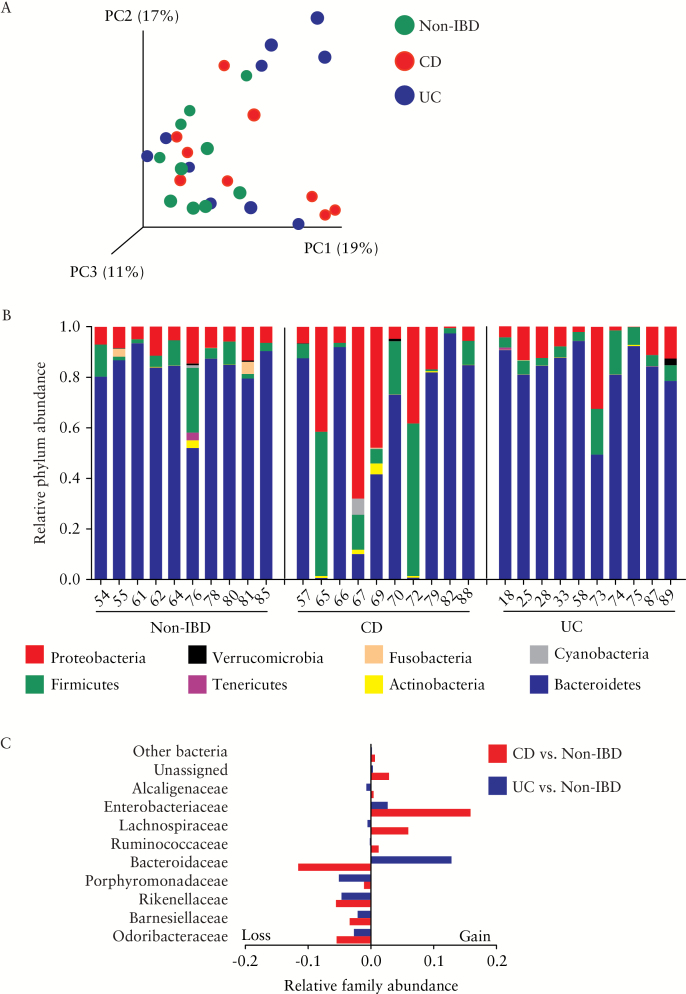
To assess whether the mucosal microbiota was altered in the ileum of UC patients, the bacterial community in biopsies was characterised by 16S rRNA. Being based on RNA, this procedure gives a more accurate representation of the metabolically active bacteria as compared with sequencing based on DNA. We calculated Bray-Curtis dissimilarities and displayed them by principal coordinates analyses [PCoA; Figure 3A], but did not observe a complete separation of samples by disease type on any of the principal coordinates. Taxonomic bins at the phylum [Figure 3B] and family levels [Figure 3C] showed no statistically significant differences between the groups after correction for multiple comparisons. However, within the CD group, patients showed a trend to harbour higher proportions of Proteobacteria and Firmicutes and underrepresentation of Bacteroidetes. When the abundance of bacterial taxa in IBD subsets were compared with non-IBD controls at the family level, the compositional differences of CD patients included higher proportions of Enterobacteriaceae and Lachnospiraceae, and loss in Bacteroidaceae, whereas a relative higher abundance of Bacteroidaceae was observed in UC.
Figure 3.
Perturbations in ileal microbial composition in inflammatory bowel disease [IBD]. [A] Principal coordinates analysis [PCoA] of BrayCurtis dissimilarity showed approximately half of Crohn’s disease [CD] and ulcerative colitis [UC] samples had a microbiota composition that was different from most non-IBD patients. [B] Relative abundance at the phylum level for each individual patient [identified by the patient study code number] showing extensive changes in several CD patients. [C] Relative abundance at family level [calculated as change in total fraction compared with non-IBD] showed gains in Enterobacteriaceae in IBD subsets, with Bacteroidaceae loss in CD and gain in UC. No statistically significant difference between the groups was found using BenjaminiHochberg false discovery rate correction.
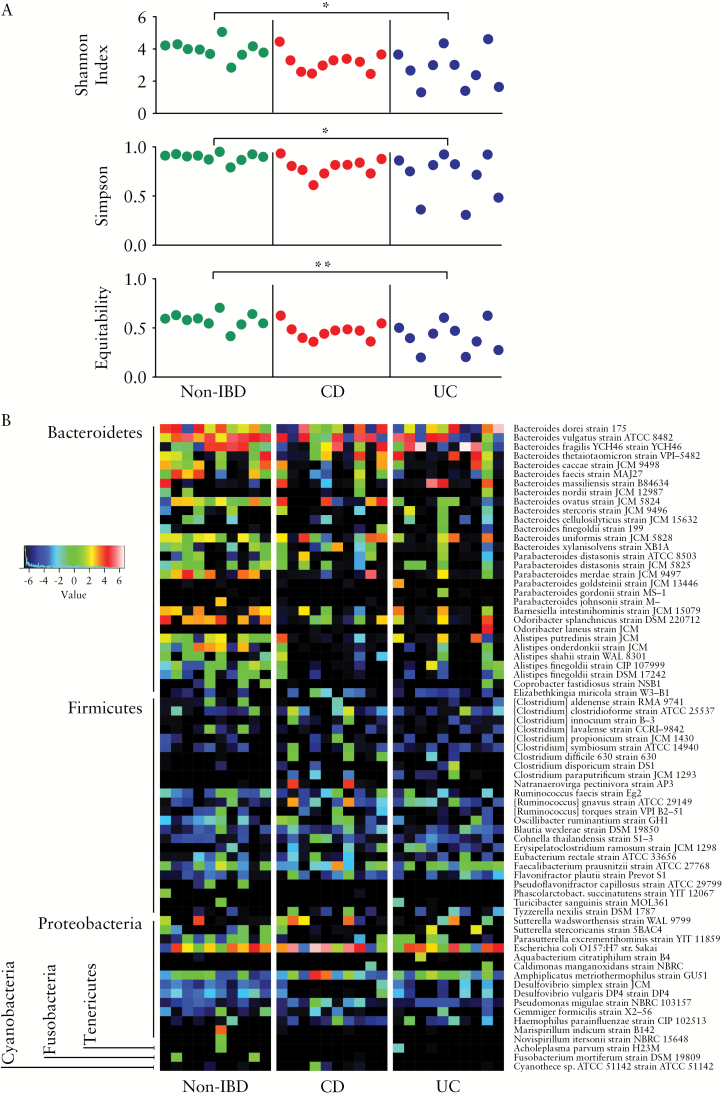
Bacterial diversity within patients [α-diversity], using the Shannon [p < 0.05], Simpson [p < 0.05], and Equitability indices [p < 0.01] were significantly reduced in UC vs non-IBD [Figure 4A]. No differences in bacterial α-diversity were observed between CD vs non-IBD and CD vs UC patients. To increase the resolution of our analysis, we implemented minimum entropy decomposition36 and used BLAST to characterise the ileal microbiome at the species level [Figure 4B]. After applying Benjamini-Hochberg false discovery rate corrections, no taxon was identified as significantly different between the groups. We identified a general trend for reduction in the abundance of bacterial populations within the Bacteroidetes and Proteobacteria phyla in UC and CD patients compared with controls, including decreases in Bacteroides ovatus in UC, Alistipes putredinis, two Alistipes finegoldii phylotypes, Desulfovibrio simplex, and Desulfovibrio vulgaris in CD, and Parabacteroides merdae and Alistipes onderdonkii in both CD and UC.
Figure 4.
Bacterial α-diversity comparison and heatmap of relative bacterial species abundance. [A] Bacterial α-diversity of individual patients. Statistical comparison between groups, made by one-way analysis of variance with Bonferroni’s multiple comparison post-test, indicated reduced diversity in ulcerative colitis [UC] compared with non-inflammatory bowel disease [IBD]. Asterisk, p < 0.05; double asterisk, p < 0.01. Statistical comparison between non-IBD and CD, as well as CD and UC, did not show significance. [B] Minimum entropy decomposition nodes were identical [100% similarity; coverage 100%] to the reported species/strain. However, in many cases, more than one identical sequence was found in the subject database and therefore the reported taxa represent only taxonomical approximations. Data represented as log2. Patterns of alterations at the species level are observed; however, no statistically significant difference was found between the groups when using Benjamini-Hochberg false discovery rate correction.
4. Discussion
Our aim was to investigate the TI of paediatric UC patients in order to assess the mucous barrier and microbial composition in a non-inflamed bowel section, and evaluate whether changes in the barrier and bacterial communities occurred even in the absence of inflammation. If this were the case, it would suggest that these changes are not the result of inflammation alone but rather possible independent and primary contributors to pathogenesis. Our results indicate a loss in mucin-containing goblet cells and depletion of the mucous layer in the non-inflamed TI of paediatric patients with UC compared with CD and non-IBD groups. This suggests that mucous layer depletion, typically associated with active UC in the colon,37,38,39 may be caused by an underlying systemic defect in the gut epithelial lining [even outside the colon] and is not simply the result of inflammation. Recent findings using confocal laser endomicroscopy have shown an increase in epithelial cell loss and barrier dysfunction in the normal duodenum of UC patients40 and support our observations of intestinal epithelial changes in non-inflamed gut sections in UC.
At the mucosal barrier, we show increases in bacterial penetration into the mucosal layer in both IBD subsets, whereas bacteria were virtually absent in biopsies from non-IBD patients, suggesting not only thinning but also a functional deficiency of the protective TI mucosal barrier. The absence of mucin-attached bacteria in non-IBD patients could be due to the rigorous process of fixation, which could displace loosely adherent bacteria, but we would have expected to see a similar effect in IBD patients as well. Colonic reduction of mucin granules and a thinner mucous layer have been previously reported, with increased bacterial penetration in active UC23 and increased mucosa-associated bacteria in children with IBD.41 However, it was not clear whether such features are just the result of local inflammation. In contrast, our study provides direct evidence that barrier defects and bacterial dysbiosis are inflammation-independent hallmarks of UC. In addition, the bacteria at the mucosal surface displayed coating with IgA and IgG in IBD subsets in our study, which could be the result of an exaggerated immune response to the ileal microbiota [or an appropriate response to abnormally penetrating bacteria], indicating another basic defect in the non-inflamed TI. Recently, it was shown that CD patients deployed high IgA and IgG responses against microbes, and those receiving anti-tumour necrosis factor [TNF]α therapy had lower antibody response.42 Other investigators have shown the importance of IgA coating of bacteria in identifying colitogenic bacteria.43 Moreover, bacteria-specific IgG immune responses in intestinal mucosal washings are increased in CD, with more prominence against commensal bacteria in UC.44
We identified a modest, but not statistically significant, reduction in NLRP6 expression, which in humans was believed to be solely expressed in the cytoplasm of endothelial cells in the small intestine.45 The NLRP6 protein has been implicated in maintaining intestinal homeostasis by regulating epithelial repair, proliferation, mucin secretion, and the colonic microbiota in mice26 but has not been rigorously studied in humans. This suggests that underlying epithelial aberrations may be partially involved in impaired mucin secretion to the lumen. In fact, MUC2 misfolding and aberrant expression in colonic crypts of non-inflamed and inflamed UC suggest defects in mucin secretion and precedence to inflammation.25 Other inflammasome-related molecules, as well as autophagy,18 could also affect mucin secretion and are the focus of future research. In addition, basic helix-loo-helix Hath1 and zinc-finger Kuppel-like Factor 4 transcription factors, which are responsible for goblet cell differentiation, have been associated with goblet cell depletion in the upper colonic crypts of inflamed UC and may provide further mechanistic insight into our findings.46
We found significant loss in bacterial α-diversity, namely in their abundance and evenness in the UC subset compared with non-IBD. In the non-inflamed TI of UC, this diversity loss suggests that microbial alterations occurred independently of inflammation. However, our study cannot delineate the mechanisms by which dysbiosis occurs nor how this relates to our findings regarding the mucous layer. Most importantly, it is not clear whether the reduced microbial diversity is the cause or result of the depleted barrier, or if the depleted mucous layer causes the dysbiosis. Overall community composition differences between groups of patients did not show a complete separation, possibly due to the heterogeneous mixture of patients including treatments and disease status. At the family and species levels, a general trend for reductions in members of the Bacteroidetes taxa and increased relative abundance of Firmicutes and Proteobacteria members were found in both IBD subsets vs non-IBD controls. However, these findings did not reach statistical significance. In concordance, other studies characterising the mucosa-associated microbiota of treatment-naïve children with CD,47 or adults with IBD,48 have observed depletion of Bacteroidetes, with increase in Proteobacteria. In CD, a trend of higher abundance in unclassified Enterobacteriaceae was notable, a possible reflection of the inflammatory environment with changes in oxygen exposure at the mucosal surface.49 At the species level, in agreement with relative family abundance changes, dominance by some Bacteroides species, and loss of others were noted in UC, a possible reflection of mucin degradation as a carbon source.50 Although others have shown increases in mucolytic Ruminococcus and a reduction in Akkermansia in the colon of UC,24 we did not find such imbalances in the TI. Contrary to what others have reported previously,51 ,52 sulphate-reducing Desulfovibrio spp. was not enriched in the TI of UC patients, and instead decreased in CD. This is not surprising, as Desulfovibrio degrade acidic mucin which is found in the colon,52 and AB/PAS staining showed mostly neutral mucins in the ileum. These findings highlight the unique features of the TI in UC, which is clearly a different environment from the colon.
Although not statistically significant, with inherent variability of microbial composition at the individual level and need for correction for multiple comparisons, these findings do suggest biologically relevant changes in microbial composition in the non-inflamed TI of UC patients. Nevertheless, some of these trends point to relevant alterations that could contribute to disease pathogenesis.
Some limitations of our study should be mentioned. The small number of recruits in this pilot study was insufficient to reach statistical power in some of the measurements, highlighting the potential for type 2 errors. Direct and indirect treatment effects on the microbiota, through reduction of inflammation, are possible and need to be considered as possible confounders in this study; however, including treated patients allows for analysis of various degrees of bowel inflammation. Bowel cleansing before endoscopy may have altered the ileal microbiota51 and the mucous layer,23 but this treatment was standard for all groups and ileoscopy is not possible without bowel preparation. Whereas it is possible that this may have differentially affected some of our outcomes, all patients required this treatment and achieved similar bowel cleansing effects using the same agent. In addition, the disease control group of non-IBD children with some [although minimal] intestinal symptoms do not represent the perfect ‘healthy’ controls. However, it was unethical to involve completely healthy children in a study requiring endoscopy. With regard to the ileal mucous barrier, due to the loose structure and patchy secretion of mucin in the TI, analysis of barrier thickness comparable to the colonic barrier was not feasible.37 Hence, the quantification of mucous production was defined as the percentage of mucin covering the epithelial layer and lumen. Furthermore, goblet cells were digitally quantified only if the goblet cells were filled with mucin, and the data presented here were not a direct measurement of goblet cell numbers. We also recognise that functional microbial analyses are lacking, and that shotgun metagenomics, metatranscriptomics, and/or metabolomics data would assist to better understand a potential role of the microbiota in disease, but full shotgun metagenomic sequencing from biopsies is not feasible due to predominance of human DNA.
Taken together, we show that the mucosal barrier in the non-inflamed ileum of UC paediatric patients is abrogated with reduction in mucous secretion, increase in bacterial penetration and Ig response, and loss of bacterial diversity. We support previous reports of shifts in the IBD microbiota; however, we show that these changes are also present in the absence of inflammation, in the TI. We cannot correlate the loss or gain of a single bacterial species with disease activity, reaffirming that IBD involves the non-specific imbalance of the microbiota. It is possible that the microbial and phenotypic changes in paediatric IBD are unique and distinct from those shown for adult IBD, but studying children is more likely to provide insights into pathogenesis given that children are less likely to have other comorbidities, will have had less exposure to some environmental factors [eg smoking], and diagnosis in childhood is more likely to be closer to the time of exposure to a causative agent. These findings suggest that paediatric UC is likely to be caused by a systemic mucosal barrier defect with abnormal bacterial colonisation, and that these changes precede [and possibly promote] inflammation. If future studies confirm our findings, mucosal barrier integrity and dysbiotic gut microbiota could become targets for prevention and therapeutic avenues.
Funding
This work was supported by grants awarded to EW by Alberta Innovates-Health Solutions [AIHS] and Women & Children’s Health Research Institute [WCHRI]. Infrastructure in EW’s laboratory is funded by the Centre of Excellence for Gastrointestinal Inflammation and Immunity Research [CEGIIR] at the University of Alberta and the Alberta Inflammatory Bowel Disease Consortium, which is supported by an AIHS Interdisciplinary Team Grant. MA is the recipient of Postdoctoral Fellowships from Canadian Institutes of Health Research/Canadian Association of Gastroenterology [CIHR/CAG] and AIHS. DZ is the recipient of a graduate studentship from WCHRI. JJ and GKSW are funded through Alberta Innovates Technology Futures-Innovates Centres of Research Excellence [AITF-iCORE]. The funders had no role in study design, collection, or interpretation of data.
Conflict of Interest
None of the conflicts are directly related to this research. EW has received advisory board honoraria from Janssen and AbbVie.
Author Contributions
MA and EW conceived and designed the experiments. MA, DZ, MWC, HQH, and EW collected and processed biopsies. MA, DZ, and RV performed the experiments. CS was responsible for histology scoring. All authors analysed and interpreted the data. MA, JJ, and IM were responsible for figures preparation and statistical analyses. MA, DZ, RV, JJ, IM, and EW drafted the manuscript. All authors approved the final version.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Acknowledgments
We graciously thank the patients and their families for providing samples, and the IBD nurses and endoscopy staff at Stollery Children’s Hospital. We would like to thank Allen Fu for his help in experiment preparations and technical procedures. We would like to acknowledge the Applied Genomic Core, Faculty of Medicine and Dentistry, at the University of Alberta. We also thank Drs Richard N. Fedorak and Karen L. Madsen from CEGIIR for providing material support.
References
- 1. El Aidy S, Merrifield CA, Derrien M, et al. The gut microbiota elicits a profound metabolic reorientation in the mouse jejunal mucosa during conventionalisation. Gut 2013;62:1306–14. [DOI] [PubMed] [Google Scholar]
- 2. Wlodarska M, Willing BP, Bravo DM, Finlay BB. Phytonutrient diet supplementation promotes beneficial clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci Rep 2015;5:9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanchez de Medina F, Romero-Calvo I, Mascaraque C, Martinez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis 2014;20:2394–404. [DOI] [PubMed] [Google Scholar]
- 4. Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 2013;10:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansson ME. Mucus layers in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:2124–31. [DOI] [PubMed] [Google Scholar]
- 6. Kamada N, Sakamoto K, Seo SU, et al. Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe 2015;17:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antoni L, Nuding S, Weller D, et al. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohns Colitis 2013;7:e652–64. [DOI] [PubMed] [Google Scholar]
- 8. Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011;4:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaiko GE, Stappenbeck TS. Host-microbe interactions shaping the gastrointestinal environment. Trends Immunol 2014;35:538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ushiku T, Moran CJ, Lauwers GY. Focally enhanced gastritis in newly diagnosed pediatric inflammatory bowel disease. Am J Surg Pathol 2013;37:1882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeRoche TC, Xiao SY, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep [Oxf] 2014;2:178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe 2015;17:577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Missaghi B, Barkema HW, Madsen KL, Ghosh S. Perturbation of the human microbiome as a contributor to inflammatory bowel disease. Pathogens 2014;3:510–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014;146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michail S, Durbin M, Turner D, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis 2012;18:1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hedin C, van der Gast CJ, Rogers GB, et al. Siblings of patients with Crohn’s disease exhibit a biologically relevant dysbiosis in mucosal microbial metacommunities. Gut 2015, 10.1136/gutjnl-2014–308896. [DOI] [PubMed] [Google Scholar]
- 17. Boltin D, Perets TT, Vilkin A, Niv Y. Mucin function in inflammatory bowel disease: An update. J Clin Gastroenterol 2013;47:106–11. [DOI] [PubMed] [Google Scholar]
- 18. Patel KK, Miyoshi H, Beatty WL, et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J 2013;32:3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sadaghian Sadabad M, Regeling A, de Goffau MC, et al. The ATG16l1-t300a allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn’s disease patients. Gut 2015;64:1546–52. [DOI] [PubMed] [Google Scholar]
- 20. Schaubeck M, Clavel T, Calasan J, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2015, 10.1136/gutjnl-2015-309333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wehkamp J, Harder J, Weichenthal M, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2003;9:215–23. [DOI] [PubMed] [Google Scholar]
- 22. Larsson JM, Karlsson H, Crespo JG, et al. Altered o-glycosylation profile of muc2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis 2011;17:2299–307. [DOI] [PubMed] [Google Scholar]
- 23. Johansson ME, Gustafsson JK, Holmen-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014;63:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Png CW, Linden SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 2010;105:2420–8. [DOI] [PubMed] [Google Scholar]
- 25. Heazlewood CK, Cook MC, Eri R, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008;5:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wlodarska M, Thaiss CA, Nowarski R, et al. Nlrp6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 2014;156:1045–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elinav E, Strowig T, Kau AL, et al. Nlrp6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011;145:745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 29. Villanacci V, Antonelli E, Geboes K, Casella G, Bassotti G. Histological healing in inflammatory bowel disease: A still unfulfilled promise. World J Gastroenterol 2013;19:968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider CA, Rasband WS, Eliceiri KW. Nih image to imagej: 25 years of image analysis. Nat Methods 2012;9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16s ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013;41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caporaso JG, Kuczynski J, Stombaugh J, et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edgar RC. Uparse: Highly accurate otu sequences from microbial amplicon reads. Nat Methods 2013;10:996–8. [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of RRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007;73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lozupone C, Knight R. Unifrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005;71:8228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eren AM, Morrison HG, Lescault PJ, Reveillaud J, Vineis JH, Sogin ML. Minimum entropy decomposition: Unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J 2015;9:968–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pullan RD, Thomas GA, Rhodes M, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994;35:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol Res Pract 2013;2013:431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fyderek K, Strus M, Kowalska-Duplaga K, et al. Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J Gastroenterol 2009;15:5287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim LG, Neumann J, Hansen T, et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2014;20:892–900. [DOI] [PubMed] [Google Scholar]
- 41. Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006;55:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frehn L, Jansen A, Bennek E, et al. Distinct patterns of IgG and IgA against food and microbial antigens in serum and feces of patients with inflammatory bowel diseases. PLoS One 2014;9:e106750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palm NW, de Zoete MR, Cullen TW, et al. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014;158:1000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 1996;38:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gremel G, Wanders A, Cedernaes J, et al. The human gastrointestinal tract-specific transcriptome and proteome as defined by RNA sequencing and antibody-based profiling. J Gastroenterol 2014, 10.1007/s00535-014-0958-7. [DOI] [PubMed] [Google Scholar]
- 46. Gersemann M, Becker S, Kubler I, et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009;77:84–94. [DOI] [PubMed] [Google Scholar]
- 47. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012;13:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lennon G, Balfe A, Earley H, et al. Influences of the colonic microbiome on the mucous gel layer in ulcerative colitis. Gut Microbes 2014;5:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O’Brien CL, Allison GE, Grimpen F, Pavli P. Impact of colonoscopy bowel preparation on intestinal microbiota. PLoS One 2013;8:e62815. [DOI] [PMC free article] [PubMed] [Google Scholar]