Abstract
Background:
Inflammatory pouch complications refractory to first-line therapies remain problematic following ileal pouch–anal anastomosis (IPAA) for ulcerative colitis (UC). We evaluated infliximab efficacy and associations with therapeutic response.
Methods:
Data from individuals who underwent colectomy and IPAA for UC (2000–2014) were reviewed. Patients with chronic refractory pouchitis (CP) and Crohn’s disease (CD)-like outcomes treated with infliximab were included. Pre-treatment parameters and response at median 8 (initial) and 48 weeks (sustained) were measured. Complete response was defined as symptomatic and endoscopic resolution with modified Pouchitis Disease Activity Index (mPDAI) <5. Partial response included mPDAI improvement >2. Serum was analysed for Anti-Saccharomyces cerevisiae antibodies (ASCA), anti-OmpC, anti-CBir1 and perinuclear Anti-Neutrophil Cytoplasmic Antibodies (pANCA).
Results:
One hundred and fifty-two patients with CP or a CD-like phenotype were identified. Forty-two were treated with infliximab (33% male; age 32.6±2.6 years, 28.5% CD-like). Post-induction response was achieved in 74% (48% complete) and sustained response in 62.6% (29.6% complete). Mean mPDAI and C-reactive protein declined from 8.5±0.3 to 2±3.4 (p < 0.002) and from 29.48±6.2 to 5.76±1.6mg/L (p < 0.001), respectively. Female gender, smoking and presence of anti-CBir1 were associated with infliximab use (p < 0.01) but not response. Pre-treatment mPDAI <10 (p < 0.01), resolution of rectal bleeding (p < 0.001 ) and week 8 endoscopic activity were associated with sustained response (p = 0.04; odds ratio [OR] 2.2; 95% confidence interval [CI] 1.1–16.5]). More than 2 positive antimicrobial antibody titres were associated with non-response (p < 0.05), but did not retain significance in multivariate analysis (p = 0.197; OR 0.632; 95% CI 0.31–1.2).
Conclusions:
Infliximab can effectively treat inflammatory pouch complications. Pre-treatment mPDAI <10 and early endoscopy may identify responders.
Key Words: Ileal pouch–anal anastomosis, infliximab, inflammatory bowel disease, pouchitis
1. Introduction
Over 20% of patients with ulcerative colitis (UC) may require surgery,1,2 with colectomy followed by ileal-pouch–anal anastomosis (IPAA) the preferred choice for many patients3,4 for medication-refractory, fulminant disease. Pouch complications are relatively common, with de novo inflammation of the ileal reservoir, termed pouchitis, occurring in 12–50% of patients.3,5,6 Current pouchitis medical therapy includes use of antibiotics, mesalamine, corticosteroids, immunomodulators and probiotics, either alone or in combination.7,8
Up to 20% of pouch patients develop chronic refractory pouchitis (CP), defined as no response to 4 weeks of conventional antibiotic treatment.9 Up to 20% can also develop a Crohn’s disease (CD)-like (CDL) phenotype of the pouch, characterized by inflammation in the afferent limb (pre-pouch ileitis), presence of proximal small bowel strictures or perianal/abdominal fistulae unrelated to surgery.10–12 A smaller group (3.8–8%) also develop complications such as pouch fistulae.13 In the setting of standard treatment failure and debilitating symptoms, surgical options, such as defunctioning loop ileostomy with or without pouch excision, are often considered.
As levels of tumour necrosis factor α (TNF-α) are elevated in biopsies of the inflamed pouch, several case series have looked at anti-TNF agents for management of CP and pouch fistulae in an effort to avoid further surgery.14–18 Infliximab has shown some promise, with different degrees of broadly defined clinical response reported over varying follow-ups.15,19 There are no studies specifically investigating infliximab use for CDL outcome.
Prior studies have identified factors associated with inflammatory pouch complications, which include smoking, colonic disease extent preoperatively, primary sclerosing cholangitis (PSC) and other extra-intestinal manifestations.20–22 Family history of CD may also increase the risk of a CD-like phenotype of the pouch.23 In addition, the presence of serological markers, such as pANCA, ASCA and anti-CBir1, in the setting of pouchitis and CDL complications have been evaluated with variable results.24–26,27 There are few studies, however, addressing factors associated with the infliximab response in this setting.
The rationale for this study was to evaluate the efficacy of infliximab in inflammatory pouch complications for both induction and maintenance of response and to assess clinical and serological factors that were associated with starting infliximab and patient response to treatment.
2. Methods
This was a retrospective cohort study of patients with a confirmed pre-colectomy diagnosis of UC and a minimum of 2 years of follow-up after ileostomy closure between January 1, 2000 and June 1, 2014, identified from the IBD and Pelvic Pouch Databases at Mount Sinai Hospital in Toronto, Canada, a large tertiary referral centre. Patients who received infliximab treatment for CP and/or CDL outcome as previously defined by Tyler et al.27 were included.
Chronic refractory pouchitis was defined as mPDAI >5 despite 4 weeks of treatment with appropriate antibiotics or >4 episodes of pouchitis requiring antibiotics per year.
Crohn’s disease-like outcome was defined as abdominal or perianal fistula not related to surgical complication (>1 year post-ileostomy closure) and/or inflammation in the afferent limb (pre-pouch ileum) or more proximal small bowel and/or proximal bowel stricture not related to surgical complication.
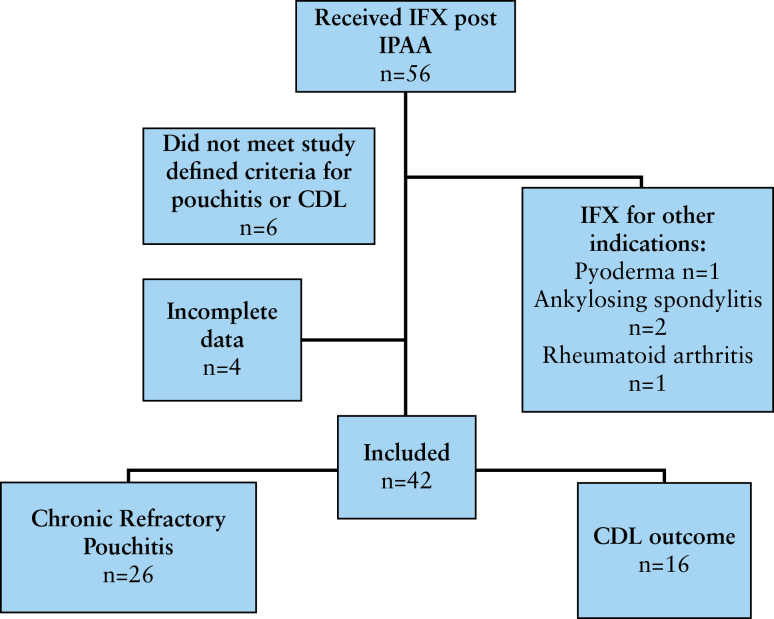
Exclusion criteria included <3 doses of infliximab, patients reclassified with CD after pathology review of original surgical specimens, and patients receiving infliximab for other indications (for a flow diagram of patient inclusion see Figure 1).
Figure 1.
Flow diagram of patient inclusion.
Study data were collected by thorough chart review and patient interview. Pre-colectomy disease extent was characterized using the Montreal classification.28 Clinical data collected included gender, dates of diagnosis, colectomy and pouch surgeries and infliximab commencement, age at IBD diagnosis, times from diagnosis to colectomy and pouch formation, family history, smoking status, extra-intestinal manifestations (including arthralgias, osteoporosis, erythema nodosum, pyoderma gangrenosum, primary sclerosing cholangitis [PSC] and ocular inflammation) and post-surgical outcomes. Adverse post-surgical outcomes (outlet obstruction, anal strictures or rectal cuff inflammation) were recorded but did not lead to exclusion. The clinical course of the pouch, including time to first episode of pouchitis, treatment of pouchitis, time from pouchitis to infliximab, presence of pre-pouch ileitis and presence of fistula within or beyond 1 year of surgery, was documented. Ethics approval for this study was provided by the Research Ethics Board of Mount Sinai Hospital.
Blood samples were obtained by venipuncture and collected using standard serum or serum separation tubes at the time of enrolment for a larger cohort study.27 Samples were spun and serum was aliquoted and stored at –80°C. Serological assays for anti-microbial antibodies (AMAs) were carried out at Prometheus Laboratories (San Diego, CA) using enzyme-linked immunosorbent assays (ELISAs), as previously described.27 Antibody tests were considered positive if titres were greater than cut-off limits provided by Prometheus: ASCA immunoglobulin (Ig) A >20; ASCA IgG >40; pANCA >12.1, (pANCA indirect immunofluorescence microscopy [IFA] DNAse-sensitive); anti-OmpC >16.4; anti-CBir1 >21 (units: EU/mL).
Induction therapy with infliximab consisted of infusions of 5mg/kg at 0, 2 and 6 weeks. Maintenance started with 5mg/kg of infliximab every 8 weeks and was adjusted according to response. Concomitant use of immunomodulators, antibiotics, steroids, 5-ASA, probiotics and anti-diarrhoeals was recorded. Adverse events attributed to infliximab were noted, and differentiated if adverse events led to discontinuation.
2.1. Outcome measures
Pre-treatment parameters were measured within 4 weeks of initiating treatment. Initial response was assessed at 8 (6–11) weeks (median and interquartile range [IQR]) and sustained response at 48 (36–61) weeks. Clinical response evaluation was based on endoscopic, clinical and radiographic data and a modified Pouch Disease Activity Index (mPDAI) (Supplementary material Table 1).Previous work has established an mPDAI cut point of ≥5 for active pouchitis (sensitivity 97%, specificity 100%).29
2.2. Outcome definitions
Complete response was defined as return to baseline pre-pouchitis stool frequency with mucosal healing at endoscopy, mPDAI score <5 and improvement ≥2; fistulae drainage ceased; and improvement closure on imaging/examination.
Partial response was improvement without return to baseline stool frequency, or return to baseline with persistent inflammation at endoscopy and mPDAI improvement of ≥2; and fistulae showing marked reduction in drainage with or without improvement on imaging.
No response was defined as minimal or no clinical improvement, persistent inflammation on endoscopy, mPDAI score >5 and change of < 2; and fistulae showing minimal or no reduction in drainage.
Mucosal healing was defined as description at endoscopy of normal mucosa with histological verification of the same, where available.
Sustained response was defined as response noted at a median of week 48, either partial or complete.
2.3. Statistical analysis
Descriptive statistics are reported as mean/SE or median/IQR for continuous variables, and frequency and percentage for categorical variables. Fisher’s exact and Kruskal–Wallis tests, using post hoc Bonferroni correction for multiple testing, detected differences in clinical and serological variables between sub-groups. A logistic regression model was applied to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of clinical and serological factors with p-values <0.1 in univariate analyses, with sustained clinical response as the dependent variable. For all analyses, 2-sided tests were used; p-values ≤0.05 were deemed significant. Analyses were performed using SPSS version 22 (IBM, NY).
3. Results
One hundred and fifty-two patients classified as having a CP or CDL outcome were identified from the IPAA database (1983 patients for the time period studied). Fifty-six patients received infliximab after IPAA. Four patients did not meet criteria outlined for a CP or CDL outcome or had mPDAI <5 pre-induction. Six patients had incomplete data or were lost to follow-up. Four received infliximab for non-IBD indications. Forty-two patients receiving infliximab thus met criteria for inclusion. All included patients retained a diagnosis of UC following examination of biopsies and colectomy specimens. Sixty-five percent had CRP and 28.5% had a CDL phenotype (Figure 1).
Patient demographics and disease characteristics for these 42 patients divided into infliximab responders and non-responders are listed in Table1. Mean disease duration from diagnosis to pouch creation was 63 months (range 5–300 months). Mean time from restoration of faecal stream following IPAA to the first episode of pouchitis was 35.2 months (range 1–159 months). Mean time from IPAA to infliximab treatment was 87.1 months (range 1–136 months).
Table 1.
Demographic characteristics of patients treated with infliximab for pouch complications.
| Characteristic | Infliximab cohort (n = 42) |
Infliximab responder (n = 26) |
Infliximab non-responder (n = 16) |
p-value |
|---|---|---|---|---|
| Gender (% male) | 25 | 25 | 25 | 0.6 |
| Age at diagnosis (y; median) | 24 | 21 | 25 | 0.3 |
| Age at IPAA (y; median) | 32 | 25 | 33 | 0.1 |
| Pre-treatment CRP (mg/L; mean) | 29 | 20 | 45 | 0.32 |
| Pre-colectomy colitis extent (% with pancolitis) | 85 | 91 | 82 | 0.4 |
| Presence of extra-intestinal manifestations (%) | 29.6 | 60 | 20 | 0.17 |
| Presence of PSC (%) | 0 | 0 | 0 | – |
| Smoking status (% current smokers) | 26 | 16 | 30 | 0.3 |
| Family history of IBD (%) | 14.8 | 16 | 14 | 0.6 |
| Time to surgery from UC diagnosis (y; mean, SD) | 5.2 (4.2) | 3.6 (4.3) | 5.6 (5.0) | 0.2 |
| Time to pouchitis from surgery (y; mean, SD) | 2.1 (3.0) | 2.6 (5.1) | 1.7(3.8) | 0.18 |
| Time from pouchitis to infliximab (y; mean, SD) | 3.1 (4.0) | 2.3(4.1) | 3.9 (5.0) | 0.04* |
| Pre-treatment mPDAI (median; IQR) | 8 (7.5–10.5) | 8 (6–9) | 10 (8–11) | 0.02* |
| CD-like phenotype (%) | 29 | 31 | 25 | 0.5 |
| Pre-colectomy anti-TNF (%) | 74 | 76 | 68 | 0.8 |
| Concomitant immunomodulator (%) | 26 | 31 | 25 | 0.7 |
CD, Crohn’s disease; CRP, C-reactive protein; IBD, inflammatory bowel disease; IPAA, ileal pouch–anal anastomosis; IQR, interquartile range; PSC, primary sclerosing cholangitis; TNF, tumour necrosis factor; UC ulcerative colitis.
Pre-colectomy infliximab exposure occurred in 74.1%. Patients were treated with antibiotics and other therapies for pouchitis/pouch inflammation for 56.5 months (mean; range 1–169) prior to infliximab. Twenty-six were on combination therapy with immunomodulators (thiopurine or methotrexate). Additional therapies (antibiotics, steroids, mesalamine, probiotics or anti-diarrhoeals) were used in 89%.
Mean CRP pre-infliximab was 29.5±6.1mg/L. Mean mPDAI score pre- treatment was 8.58±0.7. Pouchoscopy within 4 weeks of induction confirmed active inflammation in all included patients prior to infliximab, with pre-pouch ileitis in 13/42 (31%). Fistulae were identified clinically, endoscopically or radiographically in 10/42 (23.8%), of which 5 developed within 1 year post-IPAA. A CDL phenotype was identified in 12/42 (28.5%) patients.
3.1. Response to infliximab post-induction
At a median of 8 weeks, 48% had achieved complete response (mPDAI <5 and mucosal healing). A further 26% had a partial response (reduction in mPDAI of ≥2 without return to baseline and without complete mucosal healing). Twenty-six percent had no initial response but were retained on treatment (Table 2).
Table 2.
Outcomes with infliximab treatment (total cohort; n = 42).
| Outcome | Post-induction | Maintenance |
|---|---|---|
| Clinical response (%) | 74 (48 complete) | 62.6 (29.6) |
| Mucosal healing (%) | 44.4 | 29.6 |
| mPDAI | 5(2.5–6) | 22.5 (2–7.5) |
| CRP (mg/L) | 15±2 | 5.7 ± 1.1 |
CRP, C-reactive protein; mPDAI, modified Pouchitis Disease Activity Index.
3.2. Response to infliximab during maintenance
At a median of 48 weeks 29.6% sustained complete response and a further 33% sustained partial response. Thirty-eight percent did not have a sustained response (Table 2). There was a significantly reduced CRP across the group from 29.48±6.2mg/L (mean ± SE) prior to induction to 5.76±1.6mg/L at median week 48; p < 0.001; Table 2).
Ten patients had fistulizing pouch disease. After induction, 50% of these had complete response, 50% had partial response and 0/10 had no response. Long-term follow-up was available for 9/10, with 1/9 sustaining complete response, 7/9 with partial response and 1/9 with no response.
During follow-up 21.6% patients had a pouch excision or diversion ileostomy. Median time from original operation to pouch excision and/or diversion ileostomy was 7 years (IQR 2–11.5). Interestingly, median time from initial infliximab to surgery in this sub-group was only 9 months (IQR 3–13.5) and 75% of those who underwent a pouch excision had not responded to infliximab treatment.
Infliximab dose optimization was undertaken in 61% (n = 26). Sixty-eight percent (21 patients) with pre-colectomy infliximab exposure (total = 31) underwent dose optimization compared with 45% (5/11) of those who had not been previously exposed, though this was not statistically significant (p = 0.23). Infliximab antibodies and trough levels (Prometheus assay) were measured in 68% of those who had been dose-optimized. Of those who underwent dose optimization, 70% achieved response. The primary indication for checking levels was secondary loss of response. Thirty-three percent (n = 4) had detectable antibody to infliximab; 3 were switched to adalimumab and 1 underwent dose optimization to 10mg/kg every 6 weeks. Twenty-five percent (n = 3) had low trough levels and underwent dose optimization (two had interval reduction to 6 weeks, one had a simultaneous dose increase [to 10mg/kg] and interval reduction [to 6 weeks]). Three patients (25%) had acceptable trough levels (>3 μg/mL), 1 of these was switched to another biologic and 2 had pouch excision. One patient had low-titre antibody to infliximab and a detectable trough, and had a dose escalation. Five patients were dose-optimized based on clinical assessment alone and all 5 continued treatment.
Of subjects switched from infliximab, 8 (19.5%) switched to adalimumab, 1 (3.2%) to ustekinumab and 1 (3.2%) to golimumab. Three subjects who were switched to adalimumab subsequently underwent pouch excision. Indications for switching included 5 adverse reactions (3 infusion reactions with 1 anaphylaxis, 1 rash and 1 pneumonitis) and 5 with loss of response (primary or secondary). None of the patients with infusion reactions were on immunomodulators at the time of reaction. All infusion reactions occurred more than 1 year after initiation of infliximab. Overall, infliximab was used for a median of 49 months (IQR 11.5–71.5). Thirty-nine percent of patients remained on infliximab as of June 2014.
3.3. Associations with starting infliximab
In assessing associations with treatment, we first assessed clinical factors that may have predisposed patients to receiving infliximab for their pouch complications. We compared clinical characteristics across patients who had received infliximab with those identified with CP and CDL who had not received infliximab (Table 3). Female gender (p < 0.01) and current smoking (p = 0.03) were associated with infliximab treatment.
Table 3.
Clinical demographics of refractory pouch inflammation cohort according to whether the patients had infliximab treatment or no biologic treatment.
| Feature | Infliximab cohort (n = 42) | Responders (n = 26) | Non-responders (n = 16) | No infliximab cohort (n = 110) | CP (n = 60) | CDL (n = 50) | p-value |
|---|---|---|---|---|---|---|---|
| Gender, n (% male) | 11 (25) | 7 (25) | 4 (25) | 54 (51) | 33 (55) | 22 (44) | 0.01 |
| Duration from UC diagnosis to surgery (y, SD) | 5.2 (4.2) | 3.6 (4.3) | 5.6 (5.0) | 6.8 (6.9) | 7.2 (6.7) | 6.2 (7.5) | 0.13 |
| Age at diagnosis (y, median) | 24 | 21 | 25 | 29 | 29 | 29 | 0.67 |
| Age at surgery (y, median) | 32 | 25 | 33 | 37 | 37 | 36 | 0.45 |
| UC extent pre-operation (%) | 0.29 | ||||||
| E1 | 0 | 0 | 0 | 1.9 | 0 | 2 | |
| E2 | 17 | 15.3 | 18.75 | 6.3 | 10 | 4 | |
| E3 | 83 | 84.6 | 81.25 | 91.8 | 90 | 94 | |
| Family history IBD (%) | 6 (14.8) | 3 | 3 | 22 (19) | 13 | 9 | 0.3 |
| Smoking status | 0.03 | ||||||
| Never smoked (%) | 18 | 12 | 6 | 41.8 | 46.6 | 36 | |
| Ex-smoker (%) | 13 | 7 | 6 | 46.3 | 46.6 | 46 | |
| Current smoker (%) | 11 (26) | 4 | 7 | 11.9 | 6.7 | 18 | |
| Extra-intestinal manifestations (%) | 19 | 11.5 | 31 | 21 | 16.6 | 26 | 0.9 |
| PSC (%) | 0 | 0 | 0 | 5.4 | 6.6 | 4 | 0.17 |
CDL, Crohn’s disease-like phenotype; CP, chronic refractory pouchitis; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; UC, ulcerative colitis.
3.4. Associations with response to infliximab
Univariate analyses were then carried out to examine associations with infliximab response. Only covariates with p-value <0.1 were entered into the multivariate analysis. The binary for the multivariate regression was the presence of sustained response (partial and complete) at a median of 48 weeks. The covariates that had p-values <0.1 were initial mPDAI <10 (p = 0.01), presence of endoscopic response at week 8 (p = 0.015), presence of >2 positive antimitochondrial antibody titres (p = 0.04) and need for dose intensification (p = 0.02). Age, gender, pre-pouch disease extent, smoking and concomitant immune modulation were not significantly associated with sustained infliximab response. The CDL phenotype did not negatively affect response to infliximab (p = 0.143). There was no association between pre-treatment CRP and infliximab response (p = 0.874). Eleven patients (26%) were on steroid therapy at the time of induction with infliximab. There was no significant association noted between steroid use and response (p = 0.46). Exposure to infliximab prior to colectomy and IPAA also did not appear to affect subsequent response significantly (p = 0.3).
3.5. Initial mPDAI <10 and early mucosal healing are associated with sustained infliximab response
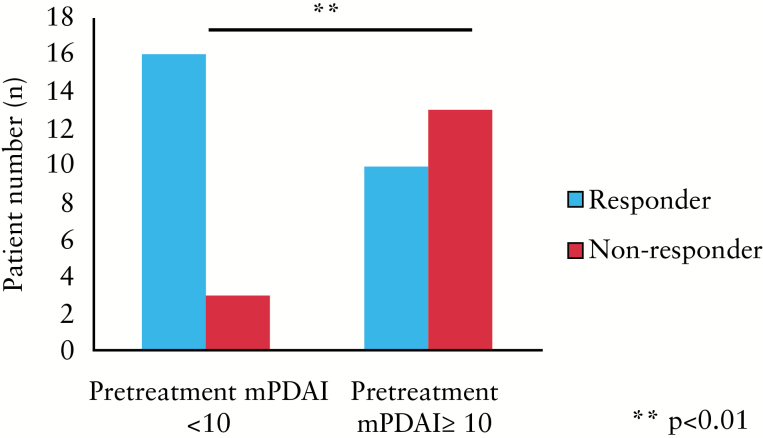
Patients with pre-treatment mPDAI <10 were significantly more likely to have both sustained clinical and endoscopic response to infliximab (p = 0.01, χ2 test; Figure 2). Interestingly, patients with initial mPDAI <10 were more likely to have mucosal healing post-induction (p = 0.03). Looking at the components of the initial mPDAI, rectal bleeding (r = –0.54, p = 0.007) and endoscopic activity (r = –0.49, p = 0.01) sub-scores both significantly correlated inversely with sustained response, while stool frequency, urgency and presence of fever were not correlated with sustained response. A number of parameters measured at week 8 were also associated with sustained response to infliximab. mPDAI (r = 0.56, p = 0.02) and rectal bleeding (r = –0.748, p = 0.001) at week 8 correlated significantly with sustained response. Endoscopic response at week 8 was significantly associated with sustained response (p = 0.015, Fisher’s exact test). This association retained significance in multivariate logistic regression analyses (p = 0.04; OR 2.2; 95% CI 1.1–16.5).
Figure 2.
A modified Pouchitis Disease Activity Index (mPDAI) <10 is associated with sustained clinical response to infliximab at a median follow-up of 48 weeks (p < 0.01). Clinical response was recorded as an improvement without return to baseline frequency, or return to baseline with persistent inflammation at endoscopy and mPDAI improvement of ≥2; complete response was judged as mPDAI <5 and return to baseline, and fistulae showing marked reduction in drainage with or without improvement on imaging.
3.6. Infliximab responders have fewer positive AMA titres
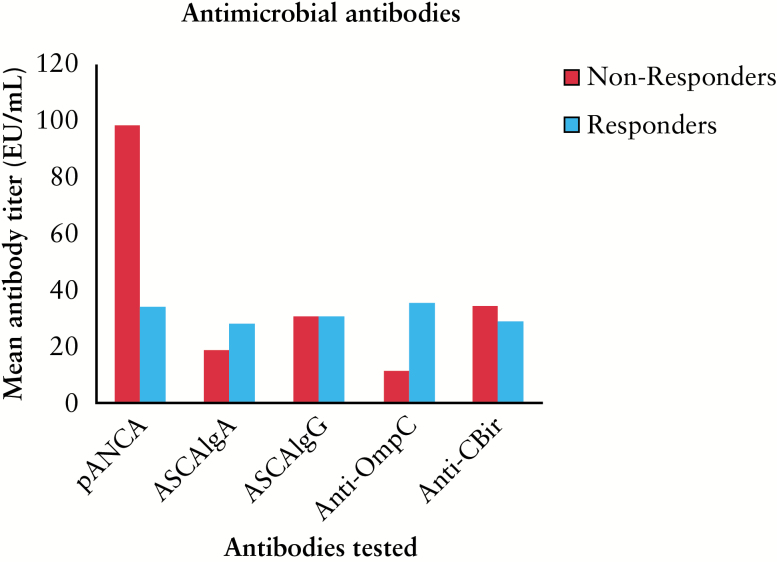
Three hundred and ninety-nine post-IPAA patients had available AMA serology. Of these, 117 had CP or a CDL phenotype. Twenty-eight had been treated with infliximab (69% of the infliximab-treated cohort presented here). Using serological markers confirmed the heterogeneous nature of patients with inflammatory pouch complications. Prevalence of positive antibodies was compared with those in the larger populations from which this cohort was derived. There was a high proportion of subjects positive for anti-CBir1 (52%) compared with the larger chronic pouchitis population (28.3%; p < 0.01), more similar to levels in the group with a CDL outcome (53.5%).27 There was no difference in anti-CBir1 positivity in infliximab-treated CP vs CDL. The CDL phenotype was associated with >2 positive titres (p < 0.01) but did not affect the infliximab response (p = 0.836). Anti-CBir1 did not correlate with fistulae or pouch excision in this cohort (p = 0.645 and p = 0.836, respectively (Table 4). Mean pANCA was higher (98) in non-responders compared with responders (34.2; p = 0.052).
Table 4.
Prevalence of antibody titres in infliximab-treated patients compared with total CP and CDL cohorts.
| Antibody | % Positive in this cohort (total) | Infliximab responders | Infliximab non-responders | % Positive (CDL cohort) | % Positive (CP uncomplicated) | p-value |
|---|---|---|---|---|---|---|
| (n = 27) | (n = 19) | (n = 8) | (n = 67) | (n = 50) | ||
| pANCA | 63 | 63 | 62.5 | 43 | 55 | 0.22 |
| ASCA IgA | 25.9 | 21.1 | 37.5 | 18 | 16 | 0.56 |
| ASCA IgG | 14.8 | 15.8 | 12.5 | 14 | 6 | 0.35 |
| Anti-OmpC | 59 | 57.9 | 62.5 | 44 | 36 | 0.25 |
| Anti-CBir1 | 51.9 | 47.4 | 62.5 | 53.5 | 28.3 | 0.01 |
ASCA, Anti-Saccharomyces cerevisiae antibodies; CDL, Crohn’s disease-like phenotype; CP, chronic refractory pouchitis; pANCA, perinuclear anti neutrophil cytoplasmic antibodies.
**p < 0.01, Kruskal–Wallis test.
The number of positive AMA titres was lower in responders (2±0.4) compared with non-responders (3±0.5; p < 0.05). Positive titres (>2) were negatively associated with remission; however, this did not retain significance in multivariate analysis (p = 0.197; OR 0.632; 95% CI 0.31–1.2). AMA prevalence in infliximab responders and non-responders is demonstrated in Figure 3. The mean raw value of ASCA IgG was significantly higher in non-responders, but was still lower than the threshold for a positive test (15.2 vs 7.3, p = 0.015; Kruskal–Wallis test).
Figure 3.
Mean antimicrobial antibody titres in infliximab responders and non-responders. Mean pANCA was higher in non-responders (p = 0.052). Mean total antibody titres (EU/mL) are shown for patients with sustained complete response to infliximab and patients who did not sustain response. An antibody test was considered positive if titres were greater than the cut-off limits as provided by Prometheus: ASCA IgA >20 EU/mL; ASCA IgG >40 EU/mL; pANCA >12.1 EU/mL pANCA IFA DNAse-sensitive; anti-OmpC >16.4 EU/mL; anti-CBir1 >21 EU/mL.
4. Discussion
Patients with CP or CDL outcome in the pouch are a difficult population to treat, and limited data exist regarding advanced medical therapy once traditional treatments fail. The results of the present study support the use of infliximab as an effective treatment for inflammatory pouch complications and indicate that a pre-treatment mPDAI of ≤10 may help predict successful sustained response. These data also suggest that infliximab is an option even in those who have previously failed biologic treatment pre-colectomy.
Response rates in our cohort are comparable with several other smaller case series.18,19 One study showed that 84.6% of 26 patients had a complete or partial response following induction and 58.3% had an ongoing response after 21.5 months.14 Viscidio et al.15 published a study of 7 patients with 100% response to infliximab at week 10 and at 11 months, with mPDAI reduced from 12 to 5. Ferrante et al. noted that 79% had at least a partial response after induction and 56% had an ongoing clinical response at a median of 20 months.18 Barreiro’s group also demonstrated that short-term responses were high (complete response in 21% and partial response in 64%). At week 52, 45.5% had an ongoing response.19 Fistula response was not recorded separately and criteria for response were certainly less rigid than in this study. That said, CRP and mPDAI were reduced. In these studies, immunomodulators were used concomitantly in 82 and 54.5% of patients respectively. Our study had similar favourable findings, with high levels of short-term response (74%) and 48% with complete response. Patients with pouch fistula also showed a significant response, with 56% complete response and 44% partial response. CRP and mPDAI were significantly decreased (p < 0.001). A longer-term response to infliximab is demonstrated in the majority (62.6%) of cases presented here, with half of the patients continuing treatment for over 5 years, a surrogate marker of ongoing response. These results suggest but do not confirm that long-term remission using infliximab is possible in this problematic condition.
It is worth noting that in the available literature where combination therapy has been used for pouch problems, much larger numbers discontinue medication secondary to infectious complications compared with our observations.18 A unique feature of this cohort is that relatively few received combination immunosuppression (26%) compared with other studies (54–100%).15,18,19 This did not adversely affect treatment response, however. Four patients on infliximab alone experienced infusion reactions, resulting in discontinuation for 3 of the 4. It is widely acknowledged that combination therapy reduces infusion reactions.30 The question thus arises as to whether it is worthwhile using combination therapy in this scenario.
One potential confounding factor in our study is other medication usage (antibiotics /steroids), the impact of which is difficult to assess retrospectively. Switching to another anti-TNF is likely to have been addressed, as patients who discontinued infliximab were not included in further analysis. This study was not designed to specifically look at effectiveness of the second anti-TNF, but of those switched to adalimumab 2 ultimately required pouch excision, 4 achieved response, albeit with dose escalation and concomitant immunomodulation in 3 of the 4, and 1 was switched to ustekinumab. Li et al. 17 recently assessed short-term response to adalimumab in 17 patients with chronic pouchitis or pouch fistula and showed 76.5% response after 4 weeks.
Few studies have attempted to assess clinical predictors of therapy in refractory pouch problems. Here we demonstrate clearly that early mucosal healing and pre-treatment mPDAI ≤10 are favourable outcome associations. One previous study suggested that mPDAI may be predictive of response, but this was not presented in the data.19 Interestingly, pre-treatment rectal bleeding was the only symptom that significantly correlated with sustained remission, while urgency and stool frequency did not appear to have a major bearing on the change in mPDAI score. This is consistent wit h previous work showing a poor correlation between pouchitis symptoms and objective disease activity as measured at endoscopy.31
It is also worth noting in this cohort that previous exposure to infliximab, which is now commonly used in patients with refractory UC as a rescue therapy32 and more recently in accelerated higher dosing regimens,33 did not appear to affect infliximab response in the setting of pouchitis. A recent meta-analysis suggests that pre-colectomy infliximab exposure increases early pouch-specific complications,34 but there is little evidence to assess its use as a long-term therapeutic in the scenario of inflammatory pouch complications.
In general, a favourable clinical outcome with infliximab appears to be the consequence of sustained therapeutic drug levels, and the current literature supports a practice of dose adjustment to obtain this and avoid immunogenicity.35 Fifty-one percent of this cohort underwent dose optimization to retain response. Although it is likely that efficacy is reduced with second-line exposure compared with naive patients, the numbers studied and the availability of drug levels for this retrospective cohort limits our ability to demonstrate this, and in clinical practice the number of TNF-naive patients with pouchit is is likely to remain low. Thus, though it is essential to recognize the spectrum of mechanisms affecting response and loss of response, given the relatively good response rates seen, previous exposure should not preclude the use of infliximab in this setting, but the clinician should have a low threshold for considering therapeutic drug monitoring and appropriate dose optimization to retain response.
The different AMA prevalence seen in post-IPAA patients compared with a general IBD cohort is likely due to microbial flora alteration in the ileal pouch compared with the flora in the ileum of a UC patient prior to colectomy. A higher frequency of CD-related AMAs was previously reported in patients with severe UC requiring colectomy.22,27 The presence of AMAs has been associated with adverse pouch outcomes, including pouchitis and CDL phenotype.24,26,27,36,37 pANCA has been associated with chronic pouchitis,25,27,36 ASCA with CDL phenotype23,26 and anti-CBir1 with complex chronic pouchitis27,36 and CDL outcome.27 Their association with infliximab response is thus of interest as a potential prognostic indicator. This infliximab-treated group had a high proportion of anti-CBir1 (53%). This may reflect an inherent selection bias for disease complexity and higher inflammatory burden in this cohort. Anti-CBir1 did not, however, correlate with the need for further surgery or treatment failure, indicating that infliximab is still a viable treatment option in these patients. There was also a higher rate of ASCA IgG (14.8%) compared with previous studies, which could suggest more complex or heterogeneous disease. Previous data have shown that the number of positive antibodies may influence outcome.27 Extending this concept, these data also show that >2 positive antibodies may influence infliximab response, though not definitively. It is worth noting that not all patients in this cohort had undergone AMA testing. AMA testing had been carried out in a larger cohort of pouch patients for an earlier study and interest in the possible effect of AMA status on infliximab response prompted the inclusion of these results in this study. One confounding factor in these data may relate to the timing of AMA testing in relation to infliximab treatment, which was not consistent in this population. Another potential bias is that the AMA testing was carried out in patients during a specific earlier time period (2008–2011) so associations with response may also be reflective of clinical practice changing over time, and these results ought to be interpreted in this context.
Given the retrospective design and small population observed, to fully characterize the infliximab response in refractory pouch inflammation, is difficult without further prospective study to evaluate the clinical, genetic and microbiome risk variants of these patients as the sample size may affect the significance of the results noted. Notwithstanding, in the interim this cohort is among the largest and most well-characterized currently available and these data support the accumulation of literature advocating infliximab use in appropriate patients with refractory inflammatory pouch complications.
Funding
OBK is funded by a CIHR/CAG Fellowship Award for Inflammatory Bowel Disease. MSS is supported by the Gale and Graham Wright Diseases Research Chair.
Conflict of Interest
OBK, MR, ADT, JMS, ZC: none. AHS received research grants from Abbvie, Amgen, Pfizer, Millennium Honorarium for Educational Event Presentations. GRG received research support and consulting fees from Abbvie and Takeda. MSS received research support and consulting fees from Janssen, Abbvie, Takeda and Prometheus.
Author Contributions
OBK: study design and planning; acquisition, analysis and interpretation of data; manuscript drafting. MR: data collection and analysis; manuscript drafting. ADT: data collection and analysis; critical revision of the manuscript for important intellectual content. JMS: accessing data; critical revision of the manuscript for important intellectual content. ZC, AHS, GRG: acquisition of data; critical revision of the manuscript for important intellectual content. MSS: study concept and design; planning of the study; acquisition, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
OBK and MSS accept full responsibility for the conduct of the study. This study conforms to the guidelines set out by the STROBE statement for observational studies (cohort, case–control and cross-sectional studies): www.strobe-statement.org/index.
Supplementary Data
Supplementary data to this article can be found online at ECCO-JCC online
Previous Presentations
This work has been presented as oral presentations at Canadian Digestive Disease Week: Research Topics, Banff, Alberta, Canada, February 2015, and at Advances in IBD, Orlando, Florida, USA, December 2015.
Acknowledgments
We thank Harden Huang and Brenda O’Connor for maintaining and providing access to the Mount Sinai Hospital Pelvic Pouch Database. Prometheus Laboratories provided antimicrobial assays and infliximab level/antibody testing.
References
- 1. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- 2. Allison J, Herrinton LJ, Liu L, et al. Natural history of severe ulcerative colitis in a community-based health plan. Clin Gastroenterol Hepatol 2008;6:999–1003. [DOI] [PubMed] [Google Scholar]
- 3. Marcello PW, Roberts PL, Schoetz DJ, Jr, et al. Long-term results of the ileoanal pouch procedure. Arch Surg 1993;128:500–3. [DOI] [PubMed] [Google Scholar]
- 4. Cohen Z, McLeod RS, Stern H, et al. The pelvic pouch and ileoanal anastomosis procedure. Surgical technique and initial results. Am J Surg 1985;150:601–7. [DOI] [PubMed] [Google Scholar]
- 5. Dayton MT, Larsen KR, Christiansen DD. Similar functional results and complications after ileal pouch-anal anastomosis in patients with indeterminate vs ulcerative colitis. Arch Surg 2002;137:690–4. [DOI] [PubMed] [Google Scholar]
- 6. Simchuk EJ, Thirlby RC. Risk factors and true incidence of pouchitis in patients after ileal pouch-anal anastomoses. World J Surg 2000;24:851–6. [DOI] [PubMed] [Google Scholar]
- 7. Holubar SD, Cima RR, Sandborn WJ, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev 2010;16:CD001176. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Pardi DS. Clinical management of pouchitis. Gastroenterology 2004;127:1809–14. [DOI] [PubMed] [Google Scholar]
- 9. Ferrante M, Declerck S, De Hertogh G, et al. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis 2008;14:20–8. [DOI] [PubMed] [Google Scholar]
- 10. Shen B, Remzi FH, Brzezinski A, et al. Risk factors for pouch failure in patients with different phenotypes of Crohn’s disease of the pouch. Inflamm Bowel Dis 2008;14:942–8. [DOI] [PubMed] [Google Scholar]
- 11. Goldstein NS, Sanford WW, Bodzin JH. Crohn’s-like complications in patients with ulcerative colitis after total proctocolectomy and ileal pouch-anal anastomosis. Am J Surg Pathol 1997;21:1343–53. [DOI] [PubMed] [Google Scholar]
- 12. Shen B, Remzi FH, Lavery IC, et al. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol 2008;6:145–58. [DOI] [PubMed] [Google Scholar]
- 13. Loftus EV, Jr, Friedman HS, Delgado DJ, et al. Colectomy subtypes, follow-up surgical procedures, postsurgical complications, and medical charges among ulcerative colitis patients with private health insurance in the United States. Inflamm Bowel Dis 2009;15:566–75. [DOI] [PubMed] [Google Scholar]
- 14. Colombel JF, Ricart E, Loftus EV, Jr, et al. Management of Crohn’s disease of the ileoanal pouch with infliximab. Am J Gastroenterol 2003;98:2239–44. [DOI] [PubMed] [Google Scholar]
- 15. Viscido A, Habib FI, Kohn A, et al. Infliximab in refractory pouchitis complicated by fistulae following ileo-anal pouch for ulcerative colitis. Aliment Pharmacol Ther 2003;17:1263–71. [DOI] [PubMed] [Google Scholar]
- 16. Calabrese C, Gionchetti P, Rizzello F, et al. Short-term treatment with infliximab in chronic refractory pouchitis and ileitis. Aliment Pharmacol Ther 2008;27:759–64. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Lopez R, Queener E, et al. Adalimumab therapy in Crohn’s disease of the ileal pouch. Inflamm Bowel Dis 2012;18:2232–9. [DOI] [PubMed] [Google Scholar]
- 18. Ferrante M, D’Haens G, Dewit O, et al. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis 2010;16:243–9. [DOI] [PubMed] [Google Scholar]
- 19. Barreiro-de Acosta M, Garcia-Bosch O, Souto R, et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis 2012;18:812–7. [DOI] [PubMed] [Google Scholar]
- 20. Abdelrazeq AS, Kandiyil N, Botterill ID, et al. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis 2008;10:805–13. [DOI] [PubMed] [Google Scholar]
- 21. Kartheuser AH, Dozois RR, Wiesner RH, et al. Complications and risk factors after ileal pouch-anal anastomosis for ulcerative colitis associated with primary sclerosing cholangitis. Ann Surg 1993;217:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleshner P, Ippoliti A, Dubinsky M, et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2007;5:952–8. [DOI] [PubMed] [Google Scholar]
- 23. Melmed GY, Fleshner PR, Bardakcioglu O, et al. Family history and serology predict Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum 2008;51:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aisenberg J, Legnani PE, Nilubol N, et al. Are pANCA, ASCA, or cytokine gene polymorphisms associated with pouchitis? Long-term follow-up in 102 ulcerative colitis patients. Am J Gastroenterol 2004;99:432–41. [DOI] [PubMed] [Google Scholar]
- 25. Sandborn WJ, Landers CJ, Tremaine WJ, et al. Antineutrophil cytoplasmic antibody correlates with chronic pouchitis after ileal pouch-anal anastomosis. Am J Gastroenterol 1995;90:740–7. [PubMed] [Google Scholar]
- 26. Dendrinos KG, Becker JM, Stucchi AF, et al. Anti-Saccharomyces cerevisiae antibodies are associated with the development of postoperative fistulas following ileal pouch-anal anastomosis. J Gastrointest Surg 2006;10:1060–4. [DOI] [PubMed] [Google Scholar]
- 27. Tyler AD, Milgrom R, Xu W, et al. Antimicrobial antibodies are associated with a Crohn’s disease-like phenotype after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2012;10:507–12. [DOI] [PubMed] [Google Scholar]
- 28. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003;46:748–53. [DOI] [PubMed] [Google Scholar]
- 30. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 31. Ben-Bassat O, Tyler AD, Xu W, et al. Ileal pouch symptoms do not correlate with inflammation of the pouch. Clin Gastroenterol Hepatol 2014;12:831–7. [DOI] [PubMed] [Google Scholar]
- 32. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805–11. [DOI] [PubMed] [Google Scholar]
- 33. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:330–5. [DOI] [PubMed] [Google Scholar]
- 34. Selvaggi F, Pellino G, Canonico S, et al. Effect of preoperative biologic drugs on complications and function after restorative proctocolectomy with primary ileal pouch formation: systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:79–92. [DOI] [PubMed] [Google Scholar]
- 35. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9. [DOI] [PubMed] [Google Scholar]
- 36. Fleshner P, Ippoliti A, Dubinsky M, et al. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2008;6:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White E, Melmed GY, Vasiliauskas EA, et al. A prospective analysis of clinical variables, serologic factors, and outcome of ileal pouch-anal anastomosis in patients with backwash ileitis. Dis Colon Rectum 2010;53:987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]