Abstract
Mitochondrial dysfunction underlies numerous age-related pathologies. In an effort to uncover how the detrimental effects of mitochondrial dysfunction might be alleviated, we examined how the nematode C. elegans not only adapts to disruption of the mitochondrial electron transport chain, but in many instances responds with extended lifespan. Studies have shown various retrograde responses are activated in these animals, including the well-studied ATFS-1-dependent mitochondrial unfolded protein response (UPRmt). Such processes fall under the greater rubric of cellular surveillance mechanisms. Here we identify a novel p38 signaling cascade that is required to extend life when the mitochondrial electron transport chain is disrupted in worms, and which is blocked by disruption of the Mitochondrial-associated Degradation (MAD) pathway. This novel cascade is defined by DLK-1 (MAP3K), SEK-3 (MAP2K), PMK-3 (MAPK) and the reporter gene Ptbb-6::GFP. Inhibition of known mitochondrial retrograde responses does not alter induction of Ptbb-6::GFP, instead induction of this reporter often occurs in counterpoint to activation of SKN-1, which we show is under the control of ATFS-1. In those mitochondrial bioenergetic mutants which activate Ptbb-6::GFP, we find that dlk-1, sek-3 and pmk-3 are all required for their life extension.
Author Summary
In humans, mitochondrial dysfunction contributes to numerous age-related diseases, and indeed even aging itself. Yet organisms also have an amazing capacity to compensate for mitochondrial impairment, paradoxically sometimes even living longer for it. This is exemplified in the roundworm Caenorhabditis elegans. In this study we examine how C. elegans with disrupted mitochondrial electron transport chains respond to such dysfunction and delineate a novel signaling cascade that is required for their life extension. Significantly, the components of this pathway are well-conserved in humans.
Introduction
Once considered relatively rare, mitochondrial disorders are now recognized as one of the most common inherited human diseases [1]. Mitochondrial dysfunction is a causative factor in many of the major diseases that limit life-expectancy in humans [2] and is associated with chronic diseases such as type 2 diabetes [3], metabolic syndrome [4], Alzheimer’s disease [5, 6], Parkinson’s disease [7], depression [8], blindness [9] and even aging itself [10–13].
There is hope, however, for coping with, or even overcoming, some forms of mitochondrial dysfunction. In humans, diseases that affect the mitochondrial electron transport chain are pleiotropic and may take years to manifest. Some people remain asymptomatic [14], and there are even examples of spontaneous recovery [15]. This reflects complex interactions with other genes [16] and the environment [17], and suggests that cells are able to adapt to some level of mitochondrial impairment. Even more striking are those organisms that adapt to mitochondrial electron transport chain (ETC) disruption and actually have a longer lifespan as a result of it. This has been reported across phyla–including mice [18]ȁbut has been most extensively studied in the nematode Caenorhabditis elegans [19].
C. elegans’ response to mitochondrial ETC dysfunction is threshold dependent; low levels produce no phenotype, moderate levels can result in increased lifespan, while severe disruption, as in humans, leads to overt pathology and shortened lifespan [20]. Intriguingly, research suggests that pathology resulting from severe mitochondrial dysfunction develops not as a direct consequence, but from the cell’s maladaptive response to the compromised mitochondria. For example, when the p53 homolog, cep-1, is knocked out, worms become long-lived when subjected to levels of mitochondrial disruption that would otherwise shorten lifespan [21]. This gives greater hope that we may be able to target and modulate such responses in humans.
The central role of mitochondria in the pathogenesis of multiple diseases is in part a consequence of their essential role in various cellular processes, including apoptotic signaling [22], ATP production [23], calcium sequestration [24], Fe-S cluster formation [25], immunity [26], nucleotide biosynthesis [27], oxidative stress signaling [28], stem cell maturation [29], steroid biosynthesis and xenobiotic detoxification [30]. The essential nature of mitochondria necessitates their functional status be closely monitored and it is now well established that signaling between the nucleus and mitochondria is bi-directional [31]. So-called retrograde response signaling originates from mitochondria and functions to orchestrate adaptive changes in nuclear gene expression to resolve or reduce mitochondrial stress. A variety of retrograde responses are known and are activated by an assortment of mitochondrial stressors, including depletion or mutation of mtDNA [32], reduced ETC activity [33], reduced mtDNA translation [34], oxidative stress [35], misfolded protein aggregation [36], altered mitochondrial turnover dynamics [37] and exposure to bacterial toxins [38].
Part of how organisms recognize a pathogen attack and activate an immune response is by monitoring their own core cellular functions, including cytosolic protein translation and mitochondrial function [38, 39]. Disruption of such processes is preemptively interpreted as evidence of a pathogen attack. This is a key adaptation for host organisms because the mechanisms of survival and reproduction of pathogens often remain critically dependent upon disrupting core cellular processes, even though pathogens may evolve to evade recognition by other forms of immune surveillance. As one example, many pathogens remain obliged to meet their requirement for iron by stealing it from their host’s mitochondria through use of siderophores [40]. Mitochondrial retrograde responses can be viewed therefore in a much broader sense as signaling elements of the cell surveillance system. The well-studied mitochondrial unfolded protein response (UPRmt) is one type of retrograde response and, in C. elegans, it is activated by a number of bacteria native to its habitat. Interestingly, the UPRmt can also be suppressed by alternate branches of the cell surveillance system when other responses are deemed more urgent [41].
The UPRmt in worms has been well characterized by the Ron and Haynes labs [42] and this process is critical for both development in the face of mitochondrial disruption [43] and for resistance to infection [39]. Two studies utilizing RNAi knockdown of ubl-5 –an important factor mediating the UPRmt response [44]–suggested that the UPRmt may be specifically required for life extension in response to mitochondrial dysfunction [18, 45]. However, ubl-5 may have a constitutive role in mitochondrial homeostasis beyond UPRmt induction, making the UPRmt-specific transcription factor, atfs-1 [43], a better candidate to test the involvement of UPRmt in longevity [46]. Contrary to expectation, not only does constitutively active ATFS-1 fail to extend lifespan [47], removal of atfs-1 by RNAi or mutation does not prevent life extension following mitochondrial disruption by isp-1(qm150) or cco-1 RNAi [46]. These results suggest that activation of the UPRmt may not produce the life extension observed upon mitochondrial dysfunction. Similarly, a recent study on the proteomes of several long-lived mouse models found that longevity correlated with decreased expression of multiple subunits of complexes I, III, IV and V and that this was not accompanied by any activation of the UPRmt [48]. Thus we set out to find other signaling pathways that are triggered independently of atfs-1 in response to mitochondrial dysfunction, and which might instead be required for life extension.
Results
tbb-6 Marks a Novel Signaling Response to Mitochondrial Dysfunction
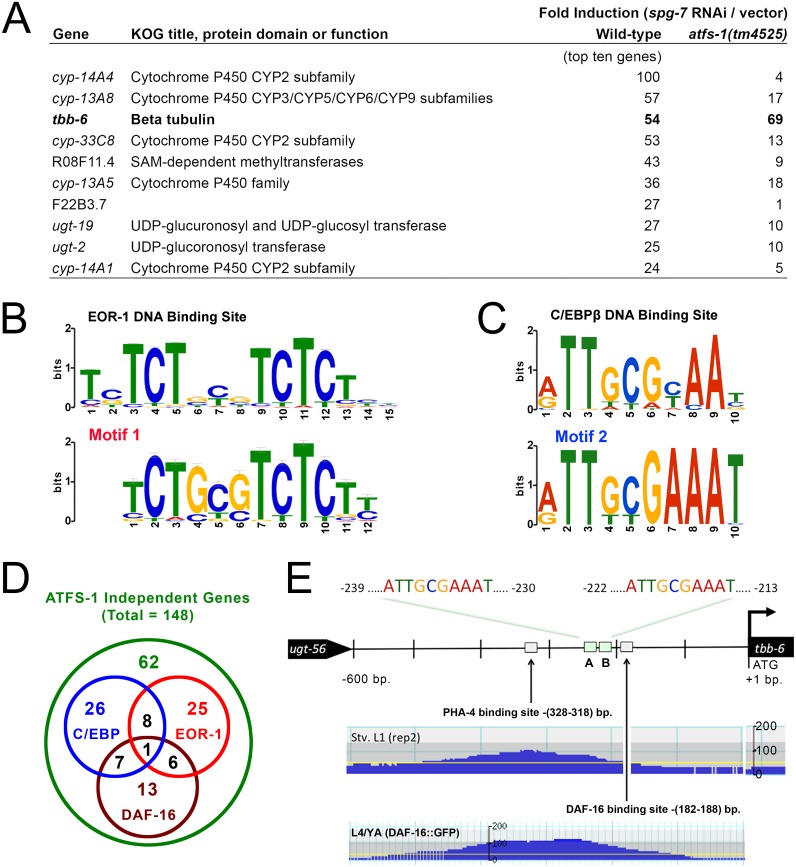
To identify genes in C. elegans that are upregulated independently of ATFS-1 following mitochondrial ETC disruption, we utilized previously published microarray data [43]. We identified 148 genes upregulated more than two-fold in wild-type worms (N2 Bristol) treated with RNAi targeting the mitochondrial metalloprotease spg-7 and which remained elevated in mutant atfs-1(tm4525) worms following the same RNAi treatment (S1 Table). Of these genes, the one showing greatest induction upon mitochondrial disruption was the uncharacterized β-tubulin, tbb-6. It was upregulated more than fifty-fold in wild-type animals, and nearly seventy-fold in atfs-1(tm4525) worms. Indeed, tbb-6 was among the ten most highly upregulated of all genes following spg-7 RNAi treatment and, of these ten, the only one that did not require atfs-1 for its induction (Fig 1A). Promoter analysis of the 148 atfs-1 independent genes identified five motifs that were significantly over-represented: Three motifs were restricted to six small heat shock proteins and all were related to the well-characterized heat shock regulatory element [49]. Forty genes (27%) contained one or more EOR-1 binding motifs (significant at a p-value of 2.1e-43) (Fig 1B and S1 Table), while forty-two genes (28%) contained one or more CCAAT/enhancer binding protein (C/EBP)-like motifs (significant at a p-value of 3.2e-31) (Fig 1C and S1 Table). Interestingly, the two groups of genes containing the latter two motifs were largely independent of each other (Fig 1D and S1 Table). DAF-16/FOXO is a transcription factor best known for its role in life extension following inhibition of the insulin/IGF-1-like signaling pathway in worms [50] but has repeatedly been shown to be uninvolved in life extension following mitochondrial disruption (reviewed in [19]). Recent studies have shown that half of all promoters bound by DAF-16 also contain one or more EOR-1 binding motifs [51], yet of the forty genes that we identified with EOR-1 binding motifs, only seven also contained a DAF-16 binding motif (Fig 1D and S1 Table). DAF-16 binding sites were not significantly over represented in our sample set beyond expectation, even though there were 27 genes containing one or more matches to the DAF-16 binding site consensus (Fig 1D). The promoter of tbb-6 contains two C/EBP motifs, as well as PHA-4 and DAF-16 binding sites (Fig 1E and S1 Table). Collectively, our data hint at the presence of one or more unexplored signaling pathways that are activated independently of the ATFS-1 dependent UPRmt pathway, and which function downstream of mitochondrial disruption to coordinately modulate the expression of multiple genes. Given the extent to which tbb-6 is upregulated upon mitochondrial disruption (~70 fold), we reasoned that tbb-6 expression would serve as a useful marker for a potentially unexplored mitochondrial retrograde response that controls lifespan.
Fig 1. Evidence for a novel signaling pathway activated subsequent to mitochondrial disruption.
(A) Among the ten most highly upregulated genes activated following mitochondrial disruption by spg-7 RNAi, tbb-6 alone does not require atfs-1 for its induction (microarray data from GEO dataset GSE38196). See also S1 Table. (B) 40 of the 148 atfs-1 independent genes activated following spg-7 disruption contain a predicted EOR-1 binding motif (shown in LOGO form aligned against the consensus EOR-1 site which was identified through the C. elegans ModENCODE project (top panel)). (C) 42 of the 148 atfs-1 independent genes activated following spg-7 disruption contain a C/EBP-like promoter motif (shown in LOGO form aligned against the promoter motif bound by human C/EBPβ (top panel)). (D) Venn diagram illustrating the degree of overlap between groups of atfs-1 independent genes that contain C/EBPβ -like, EOR-1 or DAF-16 promoter elements. (E) Promoter region of tbb-6: Sites A and B match the human C/EBPβ consensus motif shown in panel (C). ChiP-Seq data from the C. elegans ModENCODE project [52], reveals a functional DAF-16 binding site, as well as a functional PHA-4 binding site [Stv. L1(rep 2)–starved L1 larvae, 2nd replicate sample set, L4/YA—larval stage 4/young adult].
A tbb-6 Transcriptional Reporter Is Induced following Mitochondrial ETC Disruption
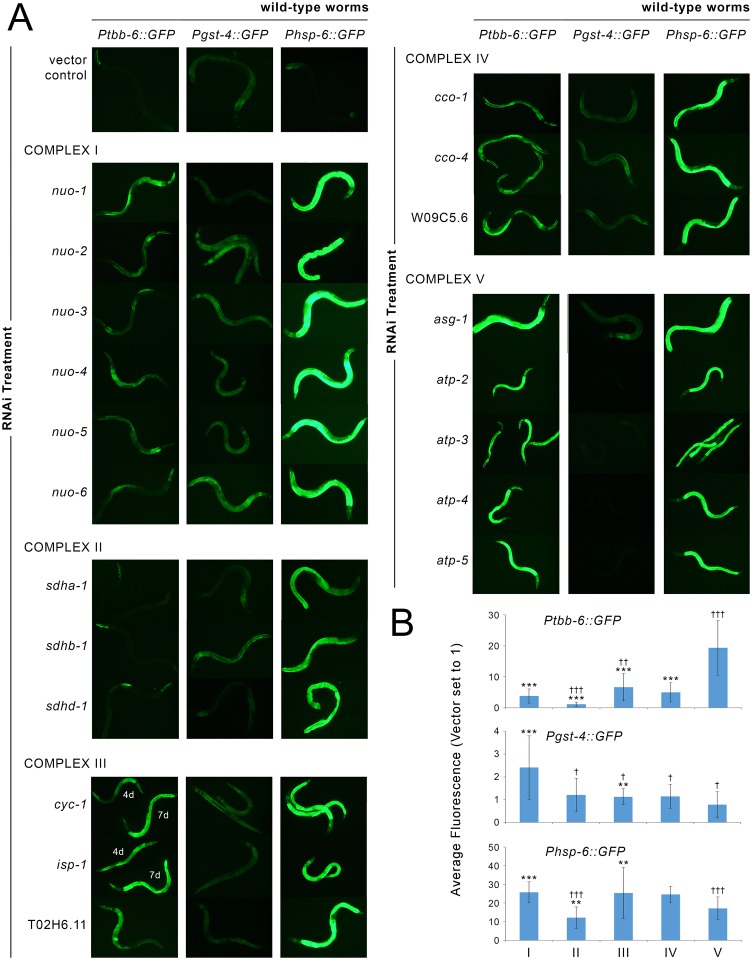
We constructed a Ptbb-6::GFP transcriptional reporter strain and observed background expression in the pharynx, which is consistent with the presence of a PHA-4 binding site in the tbb-6 promoter region (Fig 1E). There was no expression of GFP anywhere else in these worms (see vector control in Fig 2A). We tested whether the Ptbb-6::GFP reporter, like the UPRmt, could be induced upon various RNAi-mediated disruptions to the mitochondrial ETC and related proteins. In all instances where the reporter was activated in adult worms, we observed strongest expression in the intestine (Fig 2A). We also detected faint neuronal expression on some occasions, and during the L4 larval stage Ptbb-6::GFP was often transiently but strongly expressed in the hypodermis. In adult worms, depending upon which respiratory complex was affected, the level of Ptbb-6::GFP expression varied greatly. On average, RNAi knockdown of subunits of complex V led to the highest Ptbb-6::GFP induction. Expression was lower, but still well above background, following knockdown of complex I, III or IV subunits (Figs 2, S1 and S4 and S2 Table). In contrast, using these same RNAi treatments, Pgst-4::GFP expression was most strongly induced upon disruption of complex I (Figs 2 and S2). This reporter is controlled by the oxidative stress sensitive SKN-1/NRF2 transcription factor. With few exceptions, the UPRmt-specific reporter Phsp-6::GFP was robustly induced when any subunit of the electron transport chain was disrupted (Figs 2 and S3). Removing paralogous subunits from our analysis did not change our overall conclusions (S4 Fig). Finally, we also observed induction of Ptbb-6::GFP expression using four additional RNAi clones that disrupt mitochondrial function and can increase lifespan–hsp-6, mrpl-47, mrps-5 and F13G3.7 (orthologous to human SLC25A44) (S5 Fig).
Fig 2. Ptbb-6::GFP reporter activation following mitochondrial ETC disruption.
(A) RNAi-mediated knockdown of mitochondrial respiratory chain subunits differentially induces Ptbb-6::GFP reporter expression relative to Pgst-4::GFP and Phsp-6::GFP. Shown are representative fluorescence images from a selection of subunits targeted in each complex. Quantified data of multiple replicates for all tested subunits is provided in S1–S3 Figs. (B) Mean change in GFP reporter fluorescence (+/-SD) when the effect of RNAi treatments targeting subunits from each ETC complex are averaged. Two statistical comparisons are shown (Student’s t-test with Bonferroni correction applied for multiple comparisons): Asterisks indicate ETC complex disruptions which, on average, differ significantly in GFP fluorescence relative to knockdown of complex V subunits. Double daggers indicate ETC complex disruptions which, on average, differ significantly in GFP fluorescence relative to knockdown of complex I subunits. (*/†, p<0.01; **/††, p<0.001; ***/ǂǂǂ, p<0.00001) For complex III subunits cyc-1 and isp-1, 4d and 7d refer to 4 day—and 7-day old worms. For comparisons relative to empty vector see S4 Fig.
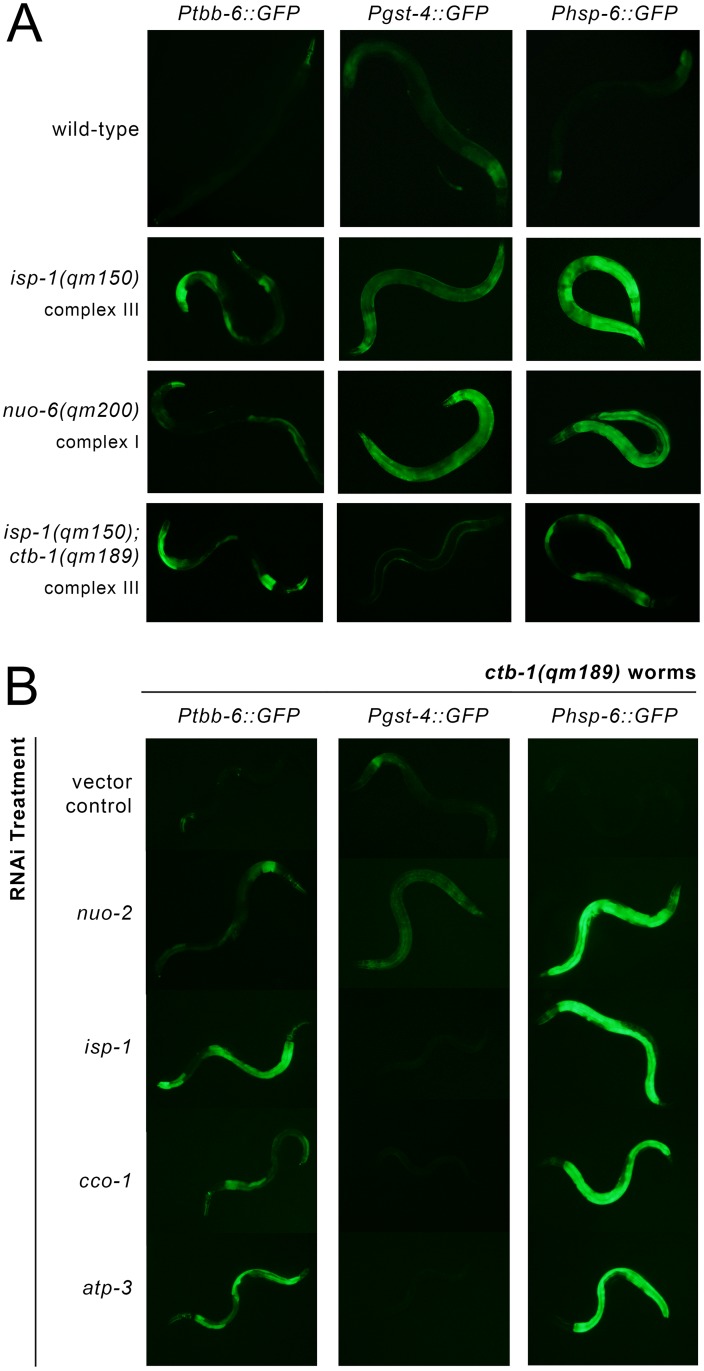
Previous work has suggested that mitochondrial dysfunction resulting from genetic mutations that disrupt subunits of the ETC may invoke retrograde responses that are fundamentally different from those activated following RNAi-knockdown of the same ETC subunits [53]. We crossed our three transcriptional reporters into isp-1(qm150) [54] and nuo-6(qm200) [53] worms to see if their mutations (disrupting complexes III and I, respectively), would also lead to tbb-6 induction. In line with our RNAi data, Ptbb-6::GFP was induced in isp-1(qm150) worms, but markedly less so in nuo-6(qm200) animals. Interestingly, Pgst-4::GFP exhibited the reciprocal expression phenotype, while both isp-1(qm150) and nuo-6(qm200) worms strongly induced the Phsp-6::GFP reporter (Fig 3A). ctb-1(qm189) is a mitochondrial DNA mutation which alters cytochrome b of complex III. This mutation attenuates the slow development of isp-1(qm150) worms but not their extended lifespan [54]. By itself, the ctb-1(qm189) mutation reduces complex III activity by up to 50% compared to wild-type animals [55]. Crossing our three transcriptional reporters into both ctb-1(qm189) and isp-1(qm150); ctb-1(qm189) genetic backgrounds, we observed that Ptbb-6::GFP and Phsp-6::GFP were each induced in isp-1(qm150); ctb-1(qm189) double mutants (but less so than in isp-1(qm150) animals), while there was no expression at all of the Pgst-4::GFP reporter (Fig 3A). ctb-1(qm189) mutants, instead, showed the reciprocal pattern of reporter protein induction (Figs 3B and S6A). These findings are intriguing because within isp-1(qm150); ctb-1(qm189) mutants the ctb-1(qm189) mutation increases the activity of complex I specifically within supercomplex assemblies [55]. A reciprocal relationship between Ptbb-6::GFP and Pgst-4::GFP reporter expression was further underscored when ctb-1(qm189) worms were exposed to RNAi targeting different subunits of the ETC (Figs 3B and S6B). Taken together, these data show that Ptbb-6::GFP is broadly induced by mitochondrial disruption and that its expression appears independent of UPRmt activation. Intriguingly, Ptbb-6::GFP induction exhibits a strong complementarity to SKN-1 activation (Figs 2 and 3). These findings suggest that tbb-6 could indeed reflect activation of a novel retrograde response.
Fig 3. Ptbb-6::GFP reporter expression defines a UPRmt independent pathway.
(A) Ptbb-6::GFP is less strongly induced by mutation of ETC subunits than by RNAi knockdown, whereas Pgst-4::GFP expression displays an opposite pattern. (B) Treatment of ctb-1(qm189) mutants with RNAi targeting complexes I, III, IV or V [nuo-2, isp-1, cco-1 and one-tenth strength atp-3, respectively], underscores the reciprocal relationship between Ptbb-6::GFP and Pgst-4::GFP reporter expression. Quantification data for both (A) and (B) is provided in S6 Fig.
tbb-6 Activation Does Not Depend on DAF-16, SKN-1 nor ATFS-1
It has been shown repeatedly that DAF-16 is unnecessary for the life extension that follows mitochondrial ETC disruption in C. elegans [12, 13, 21, 45, 54, 56, 57]. Since the tbb-6 promoter contains a DAF-16 binding element, we nonetheless tested the role of this transcription factor in tbb-6 promoter activation. Knock-down of daf-16 by RNAi in isp-1(qm150); Ptbb-6::GFP worms failed to block reporter gene induction (Table 1).
Table 1. Targeted screen for factors regulating Ptbb-6::GFP expression and larval development in isp-1(qm150) worms (quantified relative to vector-treated animals).
| RNAi | Gene Name | Gene Function | Ptbb-6::GFP | Development | Reference |
|---|---|---|---|---|---|
| MAPKs | |||||
| B0478.1 | jnk-1 | Jun-N-terminal MAPK (stress response) | no effect | ||
| T07A9.3 | kgb-1 | Jun-N-terminal MAPK (stress response) | no effect | no effect | |
| ZC416.4 | kgb-2 | Jun-N-terminal MAPK (stress response) | no effect | no effect | |
| C49C3.10 | Jun-N-terminal MAPK (stress response) | no effect | no effect | ||
| Y51B9A.9 | Jun-N-terminal MAPK (stress response) | no effect | no effect | ||
| B0218.3 | pmk-1 | p38 MAPK (stress response) | no effect | no effect | |
| F42G8.3 | pmk-2 | p38 MAPK (stress response) | no effect | no effect | |
| F42G8.4 | pmk-3 | p38 MAPK (stress response) | no effect | ||
| F43C1.2 | mpk-1 | ERK MAPK (growth response factor) | no effect | no effect | |
| W06B3.2 | sma-5 | ERK MAPK (development) | delay/arrest | ||
| W06F12.1 | lit-1 | nmo MAPK (development and inflammation) | no effect | no effect | |
| C04G6.1 | mpk-2 | MAPK | no effect | no effect | |
| C05D10.2 | MAPK | no effect | |||
| F09C12.2 | related to MAPK | no effect | |||
| MAP2Ks | |||||
| F35C8.3 | jkk-1 | MAPKK | no effect | ||
| K08A8.1 | mek-1 | MAPKK | no effect | no effect | |
| Y54E10BL.6 | mek-2 | MAPKK | variable | no effect | |
| F42G10.2 | mkk-4 | MAPKK | no effect | ||
| R03G5.2 | sek-1 | MAPKK | no effect | ||
| ZC449.3 | sek-3 | MAPKK | no effect | ||
| F35C8.2 | sek-4 | MAPKK | no effect | no effect | |
| F35C8.1 | sek-5 | MAPKK | no effect | no effect | |
| VZC374L.1 | sek-6 | MAPKK | no effect | no effect | |
| E02D9.1 | MAPKK | no effect | no effect | ||
| MAP3Ks | |||||
| F29C4.1 | daf-1 | MAPKKK | no effect | no effect | |
| C05D2.1 | daf-4 | MAPKKK | no effect | no effect | |
| F33E2.2 | dlk-1 | MAPKKK | no effect | ||
| F13B9.5 | ksr-1 | MAPKKK | no effect | no effect | |
| F58D5.4 | ksr-2 | MAPKKK | no effect | no effect | |
| K11D12.10 | mlk-1 | MAPKKK | no effect | no effect | |
| F52F12.3 | mom-4 | MAPKKK | no effect | no effect | |
| B0414.7 | mtk-1 | MAPKKK | no effect | no effect | |
| F59A6.1 | nsy-1 | MAPKKK | no effect | no effect | |
| K09B11.1 | pik-1 | MAPKKK | no effect | no effect | |
| C24A1.3 | MAPKKK | no effect | no effect | ||
| Y105C5A.x | MAPKKK | no effect | no effect | ||
| Dual-Specificity Phosphatases | |||||
| F08B1.1 | vhp-1 | dual-specificity MAPK phosphatase | arrested | [61] | |
| C04F12.8 | potential dual-specificity MAPK phosphatase | no effect | no effect | ||
| C24F3.2 | potential dual-specificity MAPK phosphatase | no effect | no effect | ||
| F13D11.3 | potential dual-specificity MAPK phosphatase | no effect | no effect | ||
| F28C6.8 | potential dual-specificity MAPK phosphatase | no effect | no effect | ||
| Y54F10BM.13 | potential dual-specificity MAPK phosphatase | delayed | |||
| ZK757.2 | potential dual-specificity MAPK phosphatase | no effect | no effect | ||
| PMK-3 Signaling Pathway Interactors | |||||
| D1005.3 | cebp-1 | bZIP TF; CCAAT-enhancer binding protein | no effect | no effect | [62] |
| C44C8.6 | mak-2 | MAP kinase activated protein kinase | delayed | [63] | |
| F26H9.6* | rab-5 | RAB5 GTPase ortholog | arrested | [64] | |
| C01B7.6* | rpm-1 | E3 ubiquitin ligase | no effect | [65] | |
| F26H9.7 | uev-3 | ubiquitin-conjugating enzyme (E2) variant | no effect | [63] | |
| Transcription Factors modulating Mit Mutant Lifespan | |||||
| C25A1.11 | aha-1 | AHA-1 interacts with AHR-1 and HIF-1 in vitro | no effect | delayed | [33] |
| ZC64.3 | ceh-18 | POU-class homeodomain transcription factor | asynchronous | [33] | |
| ZK652.5* | ceh-23 | homeodomain transcription factor | no effect | no effect | [66] |
| F52B5.5 | cep-1 | ortholog of human tumor suppressor p53 | no effect | no effect | [21] |
| F38A6.3 | hif-1 | hypoxia-induced transcription factor | no effect | no effect | [67] |
| W02C12.3* | hlh-30 | bHLH TF; lipid metabolism | no effect | delayed | [68] |
| T24H10.7 | jun-1 | bZIP TF; development | no effect | [33] | |
| F16H9.2 | nhr-27 | nuclear hormone receptor transcription factor | no effect | no effect | [33] |
| K10C3.6 | nhr-49 | NHR transcription factor; lipid metabolism | no effect | asynchronous | [33] |
| R119.6 | taf-4 | TFIID transcription factor | delayed | [33] | |
| Immune Response | |||||
| C33D3.1* | elt-2 | GATA-type TF; intestinal immunity | no effect | delay/arrest | [69] |
| C50H2.1 | fshr-1 | neuropeptide receptor | no effect | no effect | [70] |
| Y53C10A.12 | hsf-1 | heat-shock TF; stress and immune response | [71] | ||
| K02F3.4 | zip-2 | immune response | no effect | no effect | [72] |
| spg-7 RNAi Induced | |||||
| F45E4.1* | arf-1.1 | ADP-ribosylation factor | no effect | no effect | [43] |
| ZC376.7 | atfs-1 | mitochondrial unfolded protein | delay/arrest | [43] | |
| K01D12.11* | cdr-4 | cadmium responsive | no effect | [43] | |
| F52E1.13* | lmd-3 | oxidative resistance | no effect | [43] | |
| F40F8.7 | pqm-1 | paraquat responsive | no effect | [43] | |
| T19E7.2 | skn-1 | development; oxidative stress response | no effect | [43] | |
| F47H4.10* | skr-5 | homolog of Skp1 in S. cerevisiae | no effect | no effect | [43] |
| T16G1.4* | uncharacterized | no effect | [43] | ||
| Cytoprotection | |||||
| F57H12.1 | arf-3 | ADP-ribosylation factor | no effect | [60] | |
| F56A8.6* | cpf-2 | mRNA cleavage | delayed | [60] | |
| F09G2.4* | cpsf-2 | cleavage and polyadenylation specificity factor | delayed | [60] | |
| D2045.6 | cul-1 | cullin; development | delayed | [60] | |
| R13H8.1 | daf-16 | forkhead box O (FOXO) transcription factor | no effect | no effect | [60] |
| C26C6.5* | dcp-66 | ortholog of NuRD component p66 | no effect | no effect | [60] |
| F47A4.2 | dpy-22 | mediator protein subunit | no effect | [60] | |
| C33D3.1* | elt-2 | GATA-type TF; intestinal immunity | no effect | delay/arrest | [60] |
| H13N06.3* | gob-1 | trehalose-6-phosphatase | variable | delayed | [60] |
| C53A5.3* | hda-1 | histone deacetylase | no effect | [60] | |
| F25B4.6 | hmgs-1 | HMG-CoA synthase | delay/arrest | [60] | |
| F32E10.4* | ima-3 | importin alpha nuclear transport factor | delayed | [60] | |
| C41C4.4* | ire-1 | ER unfolded protein response (UPR) | delayed | [60] | |
| M7.1 | let-70 | E2 ubiquitin conjugating enzyme | delay/arrest | [60] | |
| F38H4.9* | let-92 | catalytic subunit of protein phosphatase 2A | delay/arrest | [60] | |
| T27C4.4* | lin-40 | component of NuRD complex | delayed | [60] | |
| C25H3.6* | mdt-26 | mediator; development | no effect | [60] | |
| ZC581.1* | nekl-2 | serine threonine protein kinase | arrested | [60] | |
| T23H2.5* | rab-10 | RAB-like GTPase | no effect | [60] | |
| C35C5.1* | sdc-2 | regulates X transcription | no effect | delayed | [60] |
| F46A9.5* | skr-1 | ubiquitin ligase complex component | no effect | no effect | [60] |
| C06A8.2* | snpc-1.1 | small nuclear RNA activating complex | delayed | [60] | |
| C23H3.4 | sptl-1 | serine palmitoyltransferase; development | arrested | [60] | |
| F19B6.2 | ufd-1 | ubiquitin selection chaperone | delay/arrest | [60] | |
| C46C2.1* | wnk-1 | WNK-type protein kinase homolog | no effect | no effect | [60] |
| F53F4.11* | an ortholog of human RSL1D1 | no effect | delayed | [60] | |
| Surveillance | |||||
| F40F12.7 | cbp-3 | CREB binding protein | arrested | [26] | |
| F31E3.1 | ceh-20 | homeodomain transcription factor | no effect | [26] | |
| Y47G6A.23 | lpd-3 | lipid metabolism | variable | no effect | [26] |
| R05D11.3 | ran-4 | nuclear transport factor; development | variable | delayed | [26] |
| Y54E10BR.5 | signal peptidase complex subunit | no effect | no effect | [26] | |
| Mitochondria-associated degradation (MAD) | |||||
| C06A1.1 | cdc-48.1 | AAA-ATPase | arrested | ||
| C41C4.8 | cdc-48.2 | AAA-ATPase | arrested | ||
| F59E12.5 | npl-4.2 | ubiquitin selection chaperone | arrested | ||
| F19B6.2 | ufd-1 | ubiquitin selection chaperone | delay/arrest | [60] | |
| K06H7.3 | vms-1 | VCP/Cdc48-associated (controversial role) | no effect | no effect | [73–75] |
| Other | |||||
| Y116A8C.12* | arf-6 | ADP-ribosylation factor | no effect | ||
| C06A1.1 | cdc-48.1 | ubiquitin selection chaperone; ERAD | arrested | ||
| C41C4.8 | cdc-48.2 | ubiquitin selection chaperone; ERAD | arrested | ||
| C35D10.9 | ced-4 | programmed cell death | no effect | no effect | [76] |
| F56D2.7 | ced-6 | cell-corpse engulfment during apoptosis | no effect | no effect | |
| R13H8.1 | daf-16 | forkhead box O (FOXO) transcription factor | no effect | no effect | |
| C26D10.5 | eff-1 | involved in cell fusions | no effect | no effect | |
| F52E1.7 | hsp-17 | heat-shock protein chaperone | no effect | no effect | |
| C09H6.2* | lin-10 | required for polarized protein localization | no effect | ||
| F59E12.5 | npl-4.2 | ER-associated protein degradation (ERAD) | arrested | ||
| F55B12.5 | nrf-5 | lipid-binding transportation protein | no effect | no effect | |
| F29B9.4 | psr-1 | apoptotic pathway | no effect | no effect | |
| C03C10.4 | rei-1 | RAB-11 GEF activity | no effect | [77] | |
| F10D11.1 | sod-2 | mitochondrial superoxide dismutase | no effect | [78] | |
| C44H4.5* | tap-1 | TGF-beta activated kinase | no effect | no effect | |
| F42D1.2 | tatn-1 | tyrosine amino transferase | no effect | no effect | |
| T04H1.9 | tbb-6 | beta-tubulin | no effect | no effect | [43] |
| R13F6.4 | ten-1 | teneurin | no effect | ||
| ZK524.2 | unc-13 | regulator of neurotransmitter release | no effect | [79] | |
| K06H7.3 | vms-1 | VCP/Cdc48-associated mito stress responsive | no effect | no effect | [73] |
| D2030.9 | wdr-23 | negative regulator of SKN-1 | delayed | [80] | |
| T20F10.1 | wts-1 | integrity of apical intestinal membrane | no effect | no effect | |
| C03C10.4 | mitochondrial ribosome interacting protein | no effect |
* RNAi clone was sequence not verified
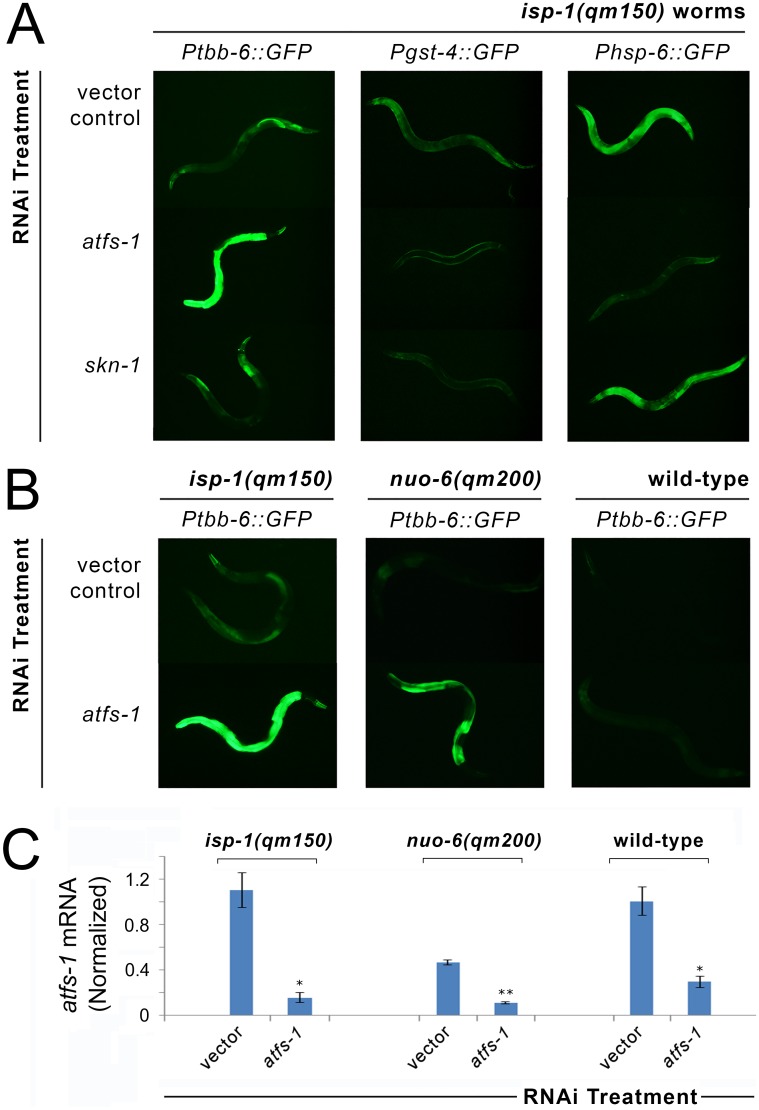
In worms, ATFS-1 is the master transcriptional regulator of the UPRmt, while SKN-1 is the key NRF-2 like transcription factor that responds to oxidative- and xenobiotic stresses [58]. The activation of both proteins has been reported in Mit mutants [20, 43]. As expected from our analysis of the spg-7 microarray data, ATFS-1 is not required for Ptbb-6::GFP activation. In Fig 4A, we show that removal of atfs-1 by RNAi completely blocked both Phsp-6::GFP [43] and Pgst-4::GFP induction in isp-1(qm150) Mit mutants, while Ptbb-6::GFP was not reduced. The dependency of SKN-1 activation on ATFS-1 following mitochondrial dysfunction has not been previously reported. Intriguingly, our data show that not only does SKN-1 sit downstream of ATFS-1, but it may also have a role in activation of downstream UPRmt components; treating isp-1(qm150) and nuo-6(qm200) worms with skn-1 RNAi completely blocked Pgst-4::GFP expression but also mildly, but significantly, attenuated Phsp-6::GFP induction (Figs 4A and S7A and S7B). In contrast to the other two reporters, Ptbb-6::GFP induction by isp-1(qm150) was not only undiminished by atfs-1 RNAi, it was markedly further activated (see Fig A in S7 Fig for quantitation). Surprisingly, in nuo-6(qm200) worms, which minimally induce Ptbb-6::GFP, we also observed hyperactivation of Ptbb-6::GFP following atfs-1 removal (Fig B in S7 Fig, quantification in Fig C in S7 Fig). Furthermore, our data reveal that atfs-1 RNAi specifically induces Ptbb-6::GFP expression in the context of mitochondrial dysfunction, because the reporter remained unchanged when wild type worms were treated with the same atfs-1 RNAi (Fig 4B). This difference in phenotype was unlikely to be due to differences in the efficacy of RNAi knockdown between the strains (Fig 4C). Thus tbb-6 is not only daf-16, atfs-1 and skn-1 independent, but it is activated complementary to UPRmt and oxidative stress signaling.
Fig 4. Ptbb-6::GFP reporter expression defines a UPRmt independent pathway.
(A) RNAi knockdown of atfs-1 blocks Phsp-6::GFP expression, as reported [43], but dramatically further upregulates Ptbb-6::GFP in both isp-1(qm150) and nuo-6(qm200) worms. Surprisingly, atfs-1 RNAi also turned off Pgst-4::GFP. RNAi knockdown of skn-1 in both isp-1(qm150) and nuo-6(qm200) worms (data for latter worms is also provided in S7 Fig), turns off Pgst-4::GFP, as reported [59], but has no effect on Ptbb-6::GFP (and Phsp-6::GFP) expression. (B) Upregulation of Ptbb-6::GFP following atfs-1 removal is only observed in the context of ETC dysfunction. (C) Quantitative of atfs-1 mRNA in worms of panel (B). Bars represent mean (+/- SD); n = 3 biological replicates/condition. Asterisks indicate significant knockdown of atfs-1 mRNA on atfs-1 RNAi relative to vector (Student’s t-test, *p<0.001, **p<0.0001).
tbb-6 Marks a New Branch of the Cell Surveillance System
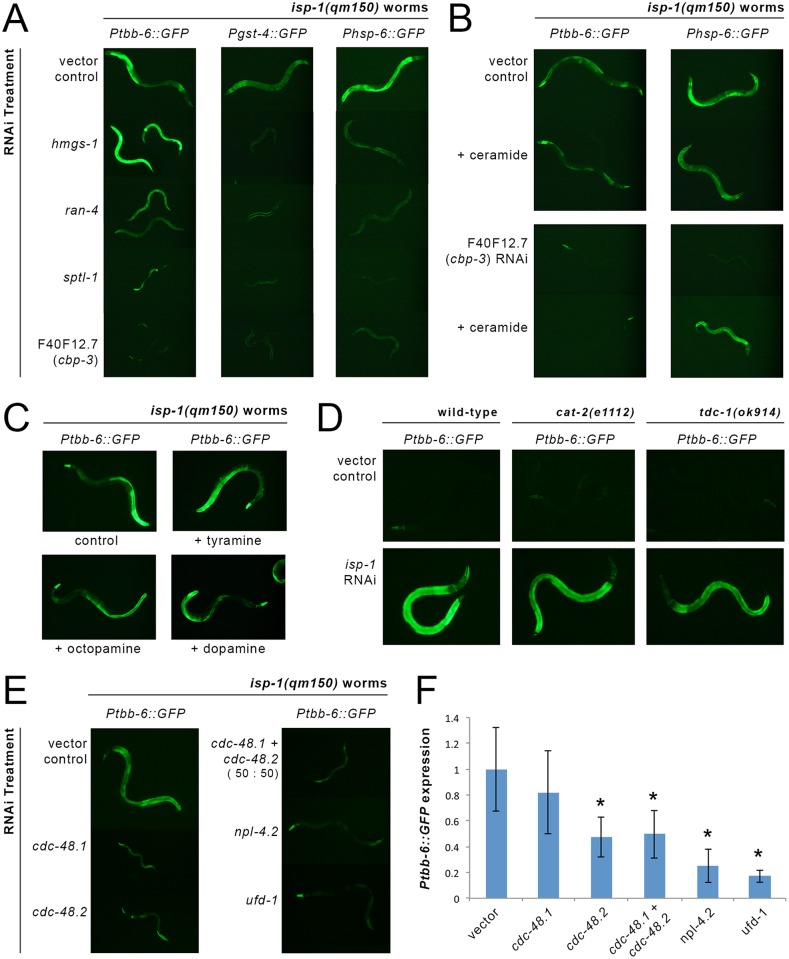
A number of genes function epistatically to ATFS-1 in response to various forms of mitochondrial disruption and this has been linked to their role in synthesizing mevalonate (hmgs-1) and ceramide (ran-4, sptl-1 and F40F12.7) [26]. Both hmgs-1 and sptl-1 have also been previously reported to be required for other cellular surveillance responses, including induction of Pgst-4::GFP upon treatment with azide [60]. To test whether any of these genes are also required for induction of tbb-6, we assayed the effect of RNAi knockdown of each on our isp-1(qm150) transcriptional reporter lines (Table 1 and S3 Table). As reported, RNAi against hmgs-1, ran-4, sptl-1 and F40F12.7 largely blocked Phsp-6::GFP induction by isp-1(qm150). The effect of these same RNAi on Ptbb-6::GFP expression was strikingly different. Like loss of atfs-1, neither hmgs-1, ran-4 nor sptl-1 were required for Ptbb-6::GFP induction (Fig 5A) and knockdown of hmgs-1 or sptl-1 further upregulated Ptbb-6::GFP (quantified in S8 Fig). Only F40F12.7 RNAi completely blocked Ptbb-6::GFP induction in isp-1(qm150) worms (Figs 5A and S8). The protein encoded by F40F12.7 is predicted to act as a transcriptional coactivator and bears significant orthology with CREB-binding proteins and thus from hereon we will refer to it as CBP-3.
Fig 5. Ptbb-6::GFP marks a new cell surveillance pathway.
(A, B) Among genes known to function epistatically to atfs-1 in its role in activating the UPRmt [26, 60], only F40F12.7/ cbp-3 is also required for Ptbb-6::GFP expression (A). The role of cbp-3 in the Ptbb-6::GFP pathway is distinct from its role in the UPRmt, since ceramide addition only replaces the requirement for cbp-3 in UPRmt activation (B). (C, D) Monoamine neurotransmission and neuromodulation are dispensable for Ptbb-6::GFP activation. Neither dietary supplementation of L-tyramine, octopamine or dopamine (C), nor genetic inactivation of catecholamine synthesis (D), alters Ptbb-6::GFP activation following mitochondrial ETC disruption. (E, F) RNAi-mediated inhibition of core MAD pathway genes strongly inhibit Ptbb-6::GFP induction by isp-1(qm150) worms. Quantitative fluorescence imaging data is provided in panel F. (n = 4–7 worms per condition; asterisks indicate significantly (p<0.025) different relative to vector-treated animals).
Liu and colleagues reported that loss of Phsp-6::GFP expression in animals treated with cbp-3 (F40F12.7), ran-4 or sptl-1 RNAi could be rescued by exogenous application of C24 ceramide [26]. Using the cbp-3 RNAi, we replicated this effect on Phsp-6::GFP expression in isp-1(qm150) worms. In contrast, ceramide had no effect on the recovery of Ptbb-6::GFP expression (Fig 5B). Thus, of all the genes reported to be epistatic to atfs-1 and the UPRmt, only cbp-3 is also required for tbb-6 activation, but in a manner independent of ceramide.
Innate Immune Response Regulators Are Not Required for tbb-6 Pathway Induction
Many pathogens secrete toxins that interfere with mitochondrial function [26]. Consequently, C. elegans respond to mitochondrial dysfunction as a pathogen attack and indeed the UPRmt activates genes involved in innate immunity [38, 39]. Numerous other signaling pathways have reported roles in pathogen response. To test whether tbb-6 might be part of an immune response separate from the UPRmt, we assayed Ptbb-6::GFP expression in isp-1(qm150) worms upon RNAi knockdown of four genes reported to mount cellular defenses against infection: elt-2 [69], fshr-1 [70], hsf-1 [71] and zip-2 [72]. None of these treatments diminished Ptbb-6::GFP expression (Table 1 and S3 Table), further suggesting that tbb-6 marks a novel branch of the cell surveillance system.
Transcription Factors Known to Be Required for Mit Mutant Life-Extension Are Dispensable for tbb-6 Pathway Induction
Since several transcription factors have already been implicated in the life extension of isp-1(qm150) worms [21, 33, 67, 81, 82], we tested whether any are required for tbb-6 expression. The genes we tested included: aha-1, ceh-18, ceh-23, cep-1, hif-1, hlh-30, jun-1, nhr-27, nhr-49, and taf-4. RNAi knock-down of each showed no attenuation of Ptbb-6::GFP expression in isp-1(qm150) worms (Table 1, see also S3 Table). Indeed, some of these RNAi treatments resulted in further upregulation of Ptbb-6::GFP; most notably, taf-4 RNAi dramatically upregulated intestinal Ptbb-6::GFP in isp-1(qm150) but not in otherwise wild-type worms.
Neither Octopamine nor Dopamine Modulates tbb-6 Expression
Durieux and colleagues demonstrated that mitochondrial disruption confined to neurons was sufficient to both increase lifespan and induce a UPRmt response cell non-autonomously in the intestine [45]. Recently, Burkewitz and colleagues showed that mitochondrial morphology in worms is modulated by neurotransmitters; specifically, when neurons perceive a low energy state via AMPK signaling, neuronal octopamine release is switched off, causing mitochondria in distal tissues to assume a more fused and elongated morphology [83]. Similar mitochondrial morphology has been previously reported in Mit mutants [12]. Taken together, these observations suggest that neuronal mitochondrial dysfunction may alter mitochondrial morphology and lifespan of the whole worm through neurotransmitter or neurohormonal signaling. Furthermore, it is possible that tbb-6 upregulation in the gut may not be the result of local mitochondrial disruption but of signaling from neurons with compromised mitochondria.
We tested the involvement of octopamine and related neurotransmitters in tbb-6 regulation via complementary approaches. First, we simply increased neurotransmitter availability in isp-1(qm150) worms through exogenous application of octopamine, dopamine, and L-tyramine. Second, we removed these neurotransmitters/neurohormones by crossing our Ptbb-6::GFP reporter into cat-2(e1112) and tdc-1(ok914) mutant backgrounds—genes required for synthesis of dopamine and octopamine, respectively—and asked if there was constitutive reporter activation. In short, neither treatment affected Ptbb-6::GFP expression. Specifically, when isp-1(qm150); Ptbb-6::GFP reporter worms of various larval stages were transferred to plates supplemented with octopamine, dopamine, or L-tyramine, and GFP expression subsequently monitored over several days, under no condition was Ptbb-6::GFP expression altered relative to untreated control animals (Fig 5C). Likewise, absence of tdc-1 or cat-2 did not constitutively induce Ptbb-6::GFP, nor did it enhance Ptbb-6::GFP expression in animals fed isp-1 RNAi relative to control-treated worms (Fig 5D). Finally, unc-13 is required for neurotransmitter release [79]. When we treated isp-1(qm150); Ptbb-6::GFP worms with unc-13 RNAi, we again observed no diminution of Ptbb-6::GFP reporter expression (Table 1). We conclude that neither octopamine, dopamine nor L-tyramine modulates tbb-6 expression.
Inhibition of the Mitochondrial-Associated Degradation (MAD) Pathway Blocks TBB-6::GFP Induction
Segref and colleagues [84] have presented evidence for a novel cell surveillance mechanism that is active in both human cells and worms following mitochondrial respiratory dysfunction. They showed that activity of the ubiquitin/proteosome system (UPS) is specifically repressed in the cytosol following insult to various mitochondrial respiratory chain and matrix bioenergetic targets, and that this response is strongly exacerbated by removal of SKN-1. This reduction in cytosolic UPS activity was not due simply to exhaustion of ATP levels, instead UPS activity could be recovered by increasing the assembly and activity of the 26S proteosome, or by addition of N-acetyl cysteine. These findings indicate that the 26S proteosome becomes limiting under conditions of mitochondrial bioenergetic stress, and the authors speculated that the 26S proteosome was re-directed to the outer mitochondrial membrane (OMM) as part of the Mitochondrial-associated Degradation (MAD) pathway. The MAD pathway functions analogously to the endoplasmic reticulum-associated degradation (ERAD) pathway [85] to retrieve and degrade dysfunctional OMM proteins [86, 87]. Both pathways utilize overlapping machinery, in particular the conserved AAA-ATPase Ccd48/VCP/p97, as well as the ubiquitin-binding and Ccd48-binding heterodimeric cofactor UFD1/NPL4, to dislodge ubiquitinated proteins from each respective organelle and chauffeur them to the 26S proteosome for degradation. Specificity is obtained through additional co-factors that recognize ubiquitinated proteins in each compartment and also bind to the core complex. Wu and colleagues [74], recently confirmed using yeast that disruption to the mitochondrial respiratory chain, the matrix protein folding environment, or mitochondrial oxidative stress, are all sufficient to strongly activate the MAD pathway.
We tested if MAD pathway activity plays a role in controlling the expression of TBB-6::GFP in isp-1(qm150) worms. ufd-1 encodes the sole UFD1 ortholog in C. elegans. Yeast two-hybrid analyses have shown this protein interacts with both CDC-48.1 and CDC-48.2, as well as NPL-4.2 [88]. RNAi-mediated inhibition of all four genes reduced Ptbb-6::GFP expression (Fig 5E and 5F and Table 1). These findings imply that the signal for Ptbb-6::GFP reporter activation is downstream of MAD pathway activation and they raise the intriguing possibility that reduced UPS activity in the cytosol might result in stabilization and activation of a cytosolic signaling pathway that ultimately leads to upregulation of tbb-6 expression.
tbb-6 Is Under MAPK Control
Mitogen activated protein kinase (MAPK) cascades are conserved across eukaryotes as cytosolic signaling pathways that respond both to mitogens and to stressful stimuli. The p38 family of MAPKs respond to a variety of stressors and play an integral role in activating the immune response [89]. C. elegans is no exception; the p38 MAPK, PMK-1, is crucial to immunity [90] and activates both SKN-1 (Nrf2) [91] and DAF-16 (FOXO) [92] in response to oxidative stress. The other family of stress-activated protein kinases are the c-Jun N-terminal kinases (JNKs), which perform a vast repertoire of functions [93] and may mediate the mammalian UPRmt [42]. One of the C. elegans JNKs, KGB-1, is involved in cellular surveillance and pathogen aversion [38], and acts in a competitive manner with the UPRmt [41].
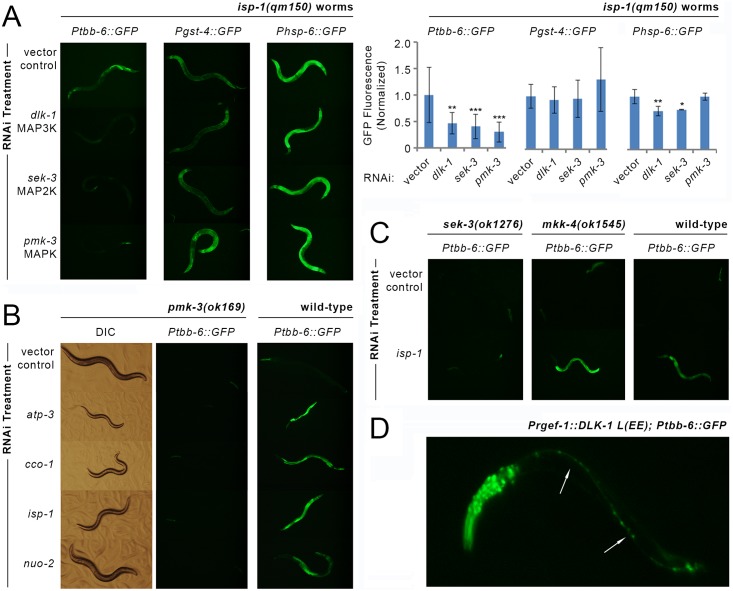
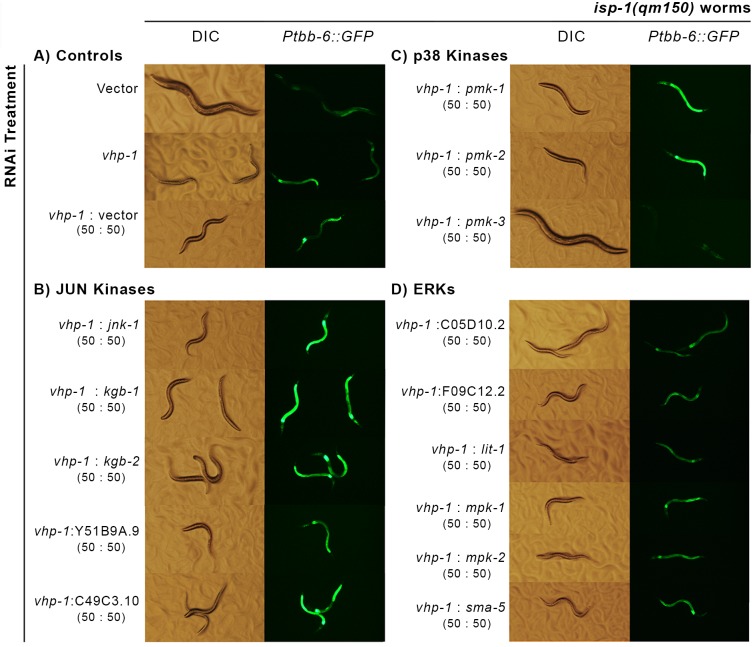
We tested for a role of MAPK signaling in tbb-6 expression by assaying whether RNAi-mediated knockdown of each of the 14 known C. elegans MAPKs blocked reporter induction in isp-1(qm150); Ptbb-6::GFP worms (Table 1). Significantly, pmk-3 RNAi alone completely blocked Ptbb-6::GFP expression (Fig 6A). Knockdown of two uncharacterized MAPKs had a weak inhibitory effect (C05D10.2, F09C12.2), while jnk-1 and sma-5 further upregulated Ptbb-6::GFP expression. All other MAPKs were without effect. The requirement for pmk-3 in Ptbb-6::GFP activation was not unique to isp-1(qm150) mutants; it was also readily apparent in isp-1(qm150); ctb-1(qm189) worms (Fig A in S9 Fig), and even in nuo-6(qm200) worms which only show weak Ptbb-6::GFP induction (Fig B in S9 Fig). Moreover, using a reciprocal approach, pmk-3(ok169) mutants containing the Ptbb-6::GFP reporter failed to induce GFP when cultured on various RNAi targeting subunits of the mitochondrial ETC, including isp-1 (Fig 6B and Fig C in S9 Fig). Notably, while inactivation of pmk-3 completely blocked Ptbb-6::GFP induction outside the pharynx, it had no effect on Phsp-6::GFP expression in either isp-1(qm150) (Fig 6A) or nuo-6(qm200) mutant animals (Fig B in S9 Fig), and it further upregulated Pgst-4::GFP in isp-1(qm150) worms that previously only showed moderate induction of this reporter (Fig 6A). We next used RNAi to map additional upstream elements of the pmk-3 MAPK signaling cascade and tested all 10 known MAPK kinases (MAP2K), and 12 MAPK kinase kinases (MAP3K) (Table 1). We found the uncharacterized MAP2K sek-3, and the well characterized MAP3K dlk-1, both to be unequivocally required for Ptbb-6::GFP upregulation in isp-1(qm150), isp-1(qm150); ctb-1(qm189) and nuo-6(qm200) animals (Figs 6A, S9A and S9B). Knockdown of four other MAP2Ks had milder effects on Ptbb-6::GFP induction: knockdown of mkk-4 consistently increased reporter expression while the expression phenotype produced by mek-2 knockdown was highly variable, with some worms very dark and others very bright. Knockdown of either jkk-1 or sek-1 both reduced Ptbb-6::GFP expression, but not to the extent produced by sek-3 knockdown. (Table 1). Thus, we conclude that a novel MAPK cascade consisting of DLK-1, SEK-3 and PMK-3 is required in worms for mitochondrial bioenergetic disruption to induce Ptbb-6::GFP.
Fig 6. Ptbb-6::GFP expression requires a MAPK signal cascade.
(A) RNAi-mediated disruption of the MAP3K/MAP2K/MAPK pathway defined by DLK-1 → SEK-3 → PMK-3 blocks induction of Ptbb-6::GFP in isp-1(qm150) worms but not Pgst-4::GFP nor Phsp-6::GFP reporter expression. Graph provides quantification of reporter expression level, normalized to vector-control RNAi (Mean+/-SD, n = 12–18 worms/ RNAi treatment). Asterisks indicate significant difference relative to vector (one-way ANOVA and ad hoc using Dunnett’s Multiple Comparisons Test, *p<0.05, **p<0.01, ***p<0.001). See also S9 Fig and S3 Table. (B) pmk-3(ok169) null mutants show the expected reduction in size upon RNAi knockdown of mitochondrial respiratory subunits, but are incapable of inducing Ptbb-6::GFP. (C) MKK-4 is not required for Ptbb-6::GFP induction following mitochondrial ETC disruption by isp-1 RNAi, unlike SEK-3. (D) Neuron-specific expression of a constitutively active form of DLK-1 acts cell autonomously to activate Ptbb-6::GFP expression. There is no induction of Ptbb-6::GFP in intestinal or other cells. (Arrows mark ventral nerve cord).
Both DLK-1 and PMK-3 play important roles in axon and synapse development [65, 94, 95] as well as efficient axon regeneration [96]. In these capacities, DLK-1 and PMK-3 function with the MAP2K, MKK-4 [65]. While our RNAi-based approach for identifying MAP2Ks essential for Ptbb-6::GFP expression in isp-1(qm150) mutants did not detect a role for mkk-4, but instead sek-3 (Table 1), we independently verified this result using mkk-4(ok1545) and sek-3(ok1276) loss-of-function mutants. We crossed our Ptbb-6::GFP reporter into both mutant backgrounds and monitored GFP induction when worms were treated with isp-1 RNAi. Consistent with our earlier observation, only loss of sek-3, and not mkk-4, abrogated GFP expression. The mkk-4(ok1545) mutation, in fact, enhanced Ptbb-6::GFP reporter expression over and above that of control worms (Fig 6C).
It has been reported previously that a DLK-1::GFP translational fusion reporter, expressed under the control of the endogenous DLK-1 promoter, localizes specifically to neurons, accumulates in axonal boutons, and is tightly controlled by the E3 ubiquitin ligase, rpm-1 [62]. We have shown that expression of our Ptbb-6::GFP transcriptional reporter in isp-1(qm150) mutants is strongly activated in intestinal cells, and less so in neurons (Fig 2). This expression occurs in a dlk-1, sek-3 and pmk-3 dependent manner (Fig 6A–6C). To determine whether neuronal DLK-1 signaling functions non-cell autonomously to mediate the intestinal expression of Ptbb-6::GFP, we expressed a constitutively-active form of DLK-1 [62] exclusively in the neurons of Ptbb-6::GFP reporter worms. Under these conditions, Ptbb-6::GFP fluorescence was detected only in neuronal cells and not in the intestine (Fig 6D), opening the intriguing possibility that DLK-1 is expressed in cells outside of neurons or is activated differently under conditions of mitochondrial dysfunction (see Discussion).
Hyperactivation of PMK-3 Arrests Mit Mutant Development
While Mit mutants typically exhibit a collection of co-segregating phenotypes—including delayed development, smaller size, and extended lifespan, these phenotypes can, in fact, be separated [20]. This was first demonstrated by the discovery of the isp-1(qm150); ctb-1(qm189) double mutant which, as previously mentioned, exhibits an attenuated delay in development but the same extended lifespan as isp-1(qm150) [54]. We proceeded to test for a role of PMK-3 in development and lifespan.
RNAi knockdown of pmk-3 neither accelerated nor delayed the development of either isp-1(qm150) or nuo-6(qm200) Mit mutants. To test the effect of further upregulation of PMK-3, we reasoned that knocking down a negative regulator of MAPKs should result in hyperactivation of PMK-3. Dual-specificity phosphatases (DUSPs) act as negative regulators of MAPKs [97]. We used BLAST to identify potential DUSPs in C. elegans and assayed isp-1(qm150) development and Ptbb-6::GFP induction upon RNAi knockdown of each (Table 1). Most treatments had no effect on either phenotype. Of the two that did, the most dramatic was knockdown of VHP-1, a DUSP known to act preferentially on the stress-activated protein kinases—the JNKs and p38s. Significantly, vhp-1 RNAi dramatically further upregulated Ptbb-6::GFP and arrested both isp-1(qm150) and nuo-6(qm200) worms at the L3 larval stage (Figs 7 and S10A). Upregulation of Ptbb-6::GFP by vhp-1 RNAi was also observed in isp-1(qm150); ctb-1(qm189) mutants (Fig A in S9 Fig). This response was specific to the context of mitochondrial disruption, as wild-type worms cultured on vhp-1 RNAi displayed only minimal hypodermal induction of Ptbb-6::GFP (S11 Fig) and, as has been previously reported, did not arrest but matured into smaller adults [98]. To confirm that the arrest of isp-1(qm150) worms upon vhp-1 knockdown was due to hyperactivation of PMK-3, we assayed both isp-1(qm150) and wild-type worm development following simultaneous knockdown of vhp-1 in combination with either of the 14 annotated worm MAPKs (Figs 7 and S11). No MAPK RNAi had any effect on vhp-1 arrest with the notable exception of pmk-3, which resulted in a near total rescue of the phenotype; that is, isp-1(qm150) worms grown on a 1:1 combination of vhp-1 and pmk-3 RNAi by-passed L3 larval arrest and matured into fertile adults (Fig 7). It is possible that use of a combination RNAi approach differentially reduced the efficacy of vhp-1 RNAi specifically in combination with pmk-3 RNAi; this too would permit worms to continue development. To exclude this possibility, we constructed a nuo-6(qm200); pmk-3(ok169) double mutant and then examined its ability to proceed through development when cultured on full strength vhp-1 RNAi. Like isp-1(qm150) mutants, nuo-6(qm200) single mutants normally arrest under these conditions. Genetic removal of pmk-3, however, allowed these worms to by-pass larval arrest and produce offspring (Fig A in S10 Fig).
Fig 7. A single MAPK is required for the induction of Ptbb-6::GFP following mitochondrial bioenergetic disruption.
(A) RNAi knockdown of the dual-specificity phosphatase vhp-1 in isp-1(qm150) worms leads to hyperactivation of Ptbb-6::GFP and L3 larval arrest. (B-D) Inhibition of pmk-3 uniquely rescues both larval arrest and blocks reporter expression among all fourteen JNK (B), p38 (C) and ERK-type (D) MAPKs.
PMK-3 Is Required for Mit Mutant Life Extension
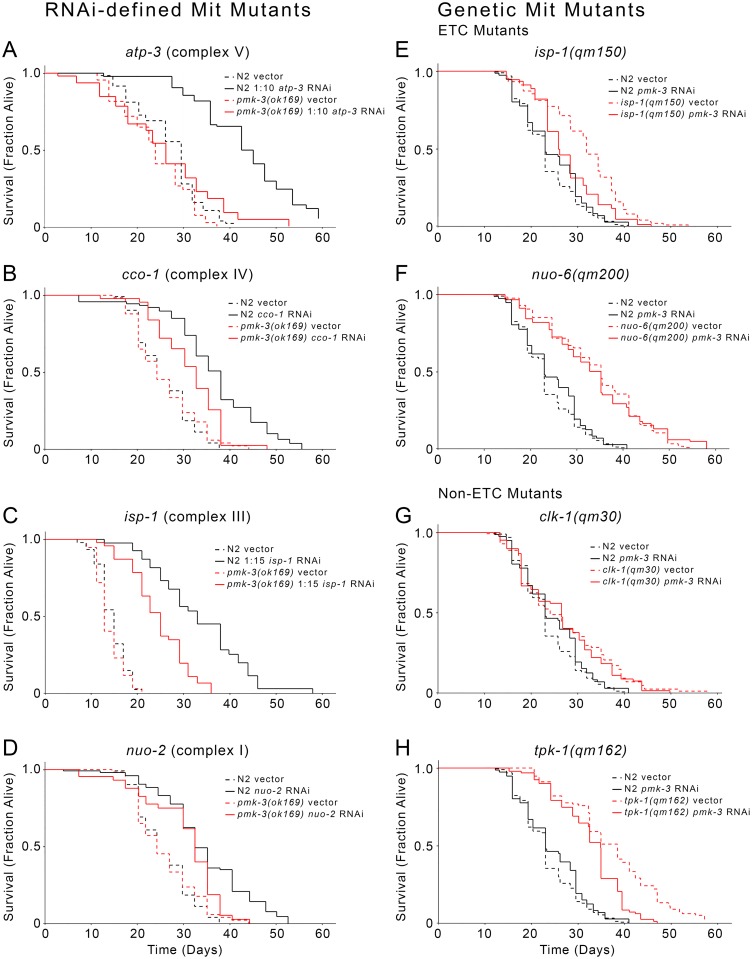
We next tested whether PMK-3 is required for Mit mutant longevity, again using independent approaches through use of both genetic and reciprocal RNAi-mediated mitochondrial disruption. We first treated wild-type and pmk-3(ok169) null worms with RNAi targeting nuo-2 (complex I), isp-1 (complex III), cco-1 (complex IV), or atp-3 (complex V). Life extension of wild type worms on these particular RNAi constructs has been well-characterized by us and others [13, 20, 99]. By itself, the pmk-3(ok169) null mutation had no effect on lifespan, yet on each of the four RNAi treatments, pmk-3 null worms had significantly attenuated life extension relative to wild-type animals (Fig 8A–8D). The effect of the pmk-3 mutation on atp-3 RNAi was especially pronounced, with one replicate showing a complete absence of life extension (Fig 8A and S4 Table).
Fig 8. Role of pmk-3 in Mit mutant life extension.
(A-D) pmk-3(ok169) worms showed significantly attenuated life extension upon RNAi knockdown of specific mitochondrial ETC subunits. (E-H) isp-1(qm150) and tpk-1(qm162), but not nuo-6(qm200) or clk-1(qm30) mitochondrial ETC mutants display significantly attenuated life extension following RNAi-mediated removal of pmk-3. For these four panels, lifespan curves represent averages of two or more independent experiments. Significance values, N, and lifespan statistics are provided in S4 Table. Raw lifespan data is provided in S5 Table.
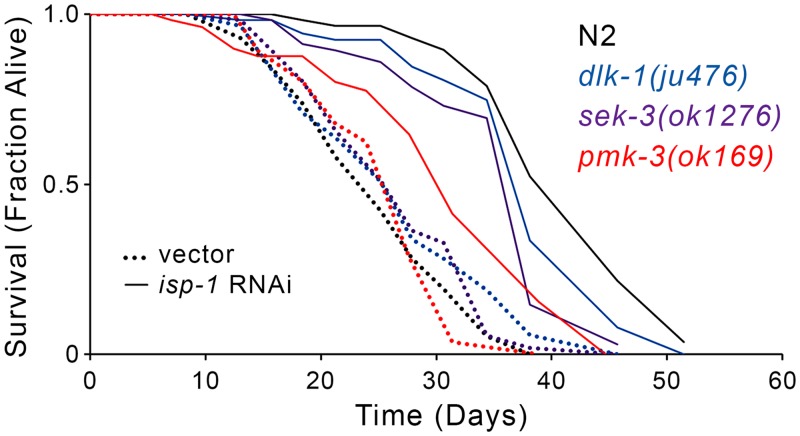
We next used a reciprocal approach to test the effect of pmk-3 RNAi on the lifespan of four genetically-defined Mit mutants: isp-1(qm150), nuo-6(qm200), clk-1(qm30) and tpk-1(qm162). The latter two mutants indirectly affect the mitochondrial electron transport chain [100]: clk-1 encodes demethoxyubiquinone mono-oxygenase, an enzyme required for ubiquinone biosynthesis, while tpk-1 disrupts the TCA cycle by limiting thiamine, which is essential for α-ketoacid dehydrogenase activity. We found that pmk-3 knockdown significantly (p-value < 0.001) attenuated the life extension of isp-1(qm150) and tpk-1(qm162) mutants, but had no effect on either clk-1(qm30) or nuo-6(qm200) animals (Fig 8E–8H). Intriguingly, the selective requirement for pmk-3 in the life extension of only some Mit mutants correlated both with the specific respiratory complex that was targeted, and induction of Ptbb-6::GFP. For example, pmk-3 knockdown had moderate to no effect on the lifespan of animals with disrupted complex I activity, namely nuo-6(qm200) mutants and worms with RNAi-induced nuo-2 knockdown, which only moderately or weakly induce Ptbb-6::GFP, respectively, in line with the effect on lifespan following pmk-3 removal. In contrast, pmk-3 knockdown significantly attenuated life extension in the context of complex III, IV or V disruption, as in isp-1(qm150) mutants and worms with RNAi knockdown of isp-1, cco-1 or atp-3, all conditions which strongly induce Ptbb-6::GFP. Finally, we tested the effect of knocking out the upstream components of the MAPK cascade on lifespan following complex III disruption. Similar to pmk-3(ok169), both dlk-1(ju476) and sek-3(ok1276) mutants have lifespans close to wild-type animals cultured on vector alone, but have dramatically attenuated life extension when exposed to isp-1 RNAi (Fig 9), emphasizing the specific requirement of this pathway for longevity in the face of mitochondrial disruption. Significantly, while several genes have been found to be required for Mit mutant longevity (Table 1), this is the first demonstration of a mitochondrial stress response required for life extension in relation to specific forms of mitochondrial dysfunction.
Fig 9. dlk-1, sek-3 and pmk-3 are required for life extension following RNAi-mediated disruption of isp-1.
Mutations in dlk-1(ju476), sek-3(ok1276) and pmk-3(ok169) attenuate the life extending effects of isp-1 RNAi relative to wild type (N2) worms (N = 60 worms/condition). Full lifespan statistics in S4 Table.
TBB-6 Plays a Minor Role in Life Extension following PMK-3 Activation
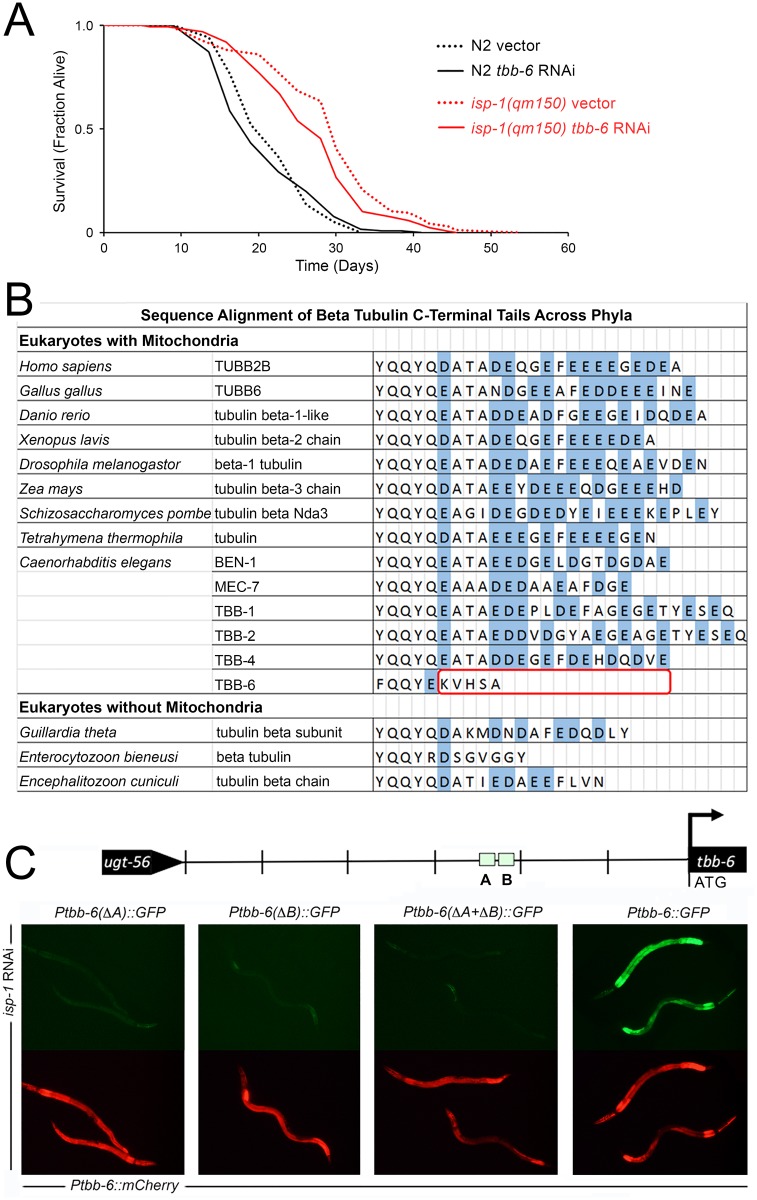
We have used Ptbb-6::GFP throughout this study as a marker of activation of a potential mitochondrial retrograde response. Expression of this reporter positively correlates with life extension across multiple ETC disruptants (compare S1 Fig and Fig 8), and tbb-6 was one of the most highly upregulated of all genes following spg-7 RNAi treatment (Fig 1A). We tested whether tbb-6 itself plays a role in life extension following ETC disruption. When isp-1(qm150) worms were cultured on tbb-6 RNAi, a mild (~7%) but significant (p < 0.01) reduction in lifespan was observed (Fig 10A). We speculate that TBB-6 may have a function in regulating ADP entry into mitochondria (Fig 10B, and Discussion).
Fig 10. RNAi-mediated inhibition of tbb-6 mildly inhibits the life extension of isp-1(qm150) Mit mutants—potentially by mediating voltage-dependent anion channel (VDAC) activity.
(A) Survival analysis of isp-1(qm150) and wild type (N2) worms cultured on RNAi to tbb-6 or vector control (pL4440). The lifespan of isp-1(qm150) mutants is reduced by ~7% following knockdown of tbb-6. (Combined data from replicate experiments, Log rank test p < 0.003, N = 126–206 worms/condition). Full lifespan statistics in S4 Table. (B) C-termini of β-tubulins from various species. Non-C. elegans data derived from Fig 6A of Rostovtseva and colleagues [101]). (C) Removal of either of the two C/EBP-like motifs in the tbb-6 promoter of Ptbb-6::GFP abrogates GFP reporter expression. Transgenic lines contain mCherry under the control of the wild-type tbb-6 promoter as an internal control.
A Role for CCAAT/Enhancer Binding Proteins in PMK-3 Signaling
We identified two C/EBP-like motifs present in the promoter of tbb-6 (Fig 1E). As a first step toward identifying transcription factors that function downstream of PMK-3, we tested whether either of these motifs was required for Ptbb-6::GFP activation following mitochondrial ETC disruption. We used site-directed mutagenesis to selectively remove each site, as well as both sites together. These mutated promoters were then coupled to GFP, and finally co-injected into worms along with mCherry expressed under control of the wild-type tbb-6 promoter. As expected, removal of both promoter elements completely abolished the ability of isp-1 RNAi to induce GFP (Fig 10C).
Discussion
In this study we have identified a novel MAPK cascade which is required in worms for life extension following mitochondrial bioenergetic dysfunction. We do not know whether this signaling cascade simply acts during development and is essentially a permissive factor that allows mitochondrial retrograde response signaling to occur, whether the cascade functions as a bona fide retrograde response that controls longevity directly, or whether it forms part of a signaling pathway that is activated in distal cells as a consequence of mitochondrial dysfunction in unrelated tissues (that is, cell non-autonomous signaling). At present we favor the notion that DLK-1, SEK-3 and PMK-3 function as a true retrograde response based on the following supporting evidence: (i) tbb-6 is the most highly upregulated gene among the 148 atfs-1 independent gene set that we initially described. When we coupled GFP to a copy of the tbb-6 promoter and treated worms with various ETC insults, this reporter was most strongly expressed in the gut, the same tissue that the bona fide UPRmt reporter Phsp-6::GFP was activated, suggesting tbb-6 is activated in cells directly experiencing mitochondrial stress (Fig 2). (ii) The novel DLK-1, SEK-3 and PMK-3 stress cascade, which we show is essential for tbb-6 induction, functions cell autonomously; that is, when we constitutively activated DLK-1 in neurons we observed expression of Ptbb-6::GFP only in neurons (Fig 6D). (iii) The Mitochondrial-associated degradation (MAD) pathway functions within cells experiencing mitochondrial dysfunction to extract and remove damaged outer mitochondrial membrane proteins. Inhibiting core elements of this pathway should exacerbate mitochondrial dysfunction and enhance any stress signaling to distal tissues. Despite this, RNAi-mediated inhibition of MAD components in isp-1(qm150) mutants did not enhance Ptbb-6::GFP expression, rather, it suppressed it (Table 1). This finding argues that tbb-6 is induced cell autonomously following MAD pathway activation in cells directly experiencing mitochondrial damage.
Promoter analysis of the 148 atfs-1 independent genes identified in this study revealed significant enrichment of two transcription factor binding sites in essentially non-overlapping gene sets (Fig 1). One group of 42 genes shared a DNA motif closely related to the DNA binding site of mammalian CCAAT/enhancer binding proteins (C/EBP transcription factors). We showed that this motif is used in signaling mitochondrial ETC stress as its removal from the promoter of the Ptbb-6::GFP reporter blocked induction by isp-1 RNAi. A second group of 40 genes contained an EOR-1 binding element. Future studies will address the role of EOR-1 in Mit mutant longevity, which is a likely proposition given that EOR-1 is an essential component of a recently-described longevity response mediated by EGF in adult worms [102, 103]. Suffice to say, we have found that RNAi to EOR-1 does not block Ptbb-6::GFP expression, raising the possibility that more than one signaling pathways may function in the longevity control of Mit mutants. In this regard, the genes under EOR-1 control that are essential for the longevity response mediated by EGF in adults worms [103], are over-represented in our 148 gene set (10 out of a total of 503 up- or down-regulated genes, hypergeometric probability <0.003). Recently, EOR-1 was also implicated in the genetic response to dietary restriction and the set of genes under its control were enriched in mitochondrial targets [104]. Moreover, while DAF-16 binding elements were not significantly enriched in our 148 gene set, we nonetheless found 27 genes that contained DAF-16 binding sites (Fig 1D). Kumar and colleagues [51] recently described a signature set of 37 genes that directly bound DAF-16 in all DAF-16 chromatin-binding studies to date. It has been repeatedly shown that DAF-16 is not required for the Mit phenotype yet, surprisingly, four of these 37 core DAF-16 binding genes are present in our set of 148 atfs-1 independent genes. Based on our sample size, this is unlikely to have occurred by chance (hypergeometric probability <0.0002). We predict that EOR-1 and the transcription factor(s) that binds the CCAAT/enhancer binding protein site, will work in concert to turn on a novel kind of hybrid stress response in Mit mutants. If true, this idea would be in line with the remarkable study of Stroustrup and colleagues [105] that showed Mit mutants in particular (and to a lesser extent, dietary restriction), did not simply temporally scale lifespan, as various other genetic and environmental interventions that also extend life did, but instead fundamentally changed the way worms age. In further support of such a possibility, a search for enriched functional GO terms among the 148 atfs-1 independent genes using DAVID [106], revealed a significant (q-value < 0.05) enrichment of genes encoding FBOX-containing proteins (8 genes), small heat shock proteins (sHSPs, 6 genes), and gene clusters involved in aging (12 genes), ER-nuclear signaling and cytochrome P450 activity. FBOX proteins are components of SCF ubiquitin E3 ligase complexes that play important roles in protein turnover [107], along with sHSPs. The FBOX cluster was enriched in genes containing the CCAAT motif, while the sHSPs and other clusters were enriched in genes containing EOR-1 motifs.
Based on the well-established roles of other MAPKs, we speculate that PMK-3 controls the activity of one or more transcription factors. Again, we do not know if DLK-1, SEK-3 and PMK-3 function prior to, or after, mitochondrial bioenergetic stress, but if it is after then we predict likely targets could be members of the CCAAT/enhancer binding proteins (C/EBP transcription factors), since removal of either of the two C/EBP-like binding motifs in the promoter of tbb-6 blocked Ptbb-6::GFP reporter induction, which we also showed is dependent upon PMK-3. In C. elegans, there are three transcription factors orthologous to mammalian C/EBPs, namely, CEBP-1, CEBP-2, and ZIP-4. These belong to a broader category of transcription factors known as bZIPs for the basic leucine zipper domain which binds the DNA. Intriguingly, both ATFS-1 and SKN-1 are themselves bZIP transcription factors, suggesting possible mechanisms for the complementary nature with our novel retrograde pathway: ATFS-1 may bind and compete with C/EBP-like proteins for the same promoter elements, or they might share a common protein binding partner. We have already tested the bZIP jun-1 for its known requirement in Mit mutant longevity [33] and cebp-1 for its known role with pmk-3 in neuron morphology [108]. Since neither of these diminished Ptbb-6::GFP activation by isp-1(qm150), our efforts will now be focused on the remaining bZIP transcription factors in C. elegans.
In mammals, C/EBPδ is known to act in a calcium-activated, mitochondrial retrograde response [109], raising the possibility that increases in cytoplasmic calcium following mitochondrial depolarization could also be involved in the novel MAPK pathway that we have identified in this study. Interestingly, C/EBP proteins are known to recruit CREB-binding protein (CBP) [110]. One possible function for CBP-3, the CBP ortholog which we found to be essential for Ptbb-6::GFP induction in this study, might be that it is needed to directly interact with transcription factors that bind to C/EBP motifs. If CBP-3 functions downstream of PMK-3, one prediction is its removal by RNAi should permit isp-1 animals cultured on vhp-1 RNAi to resume larval development, analogous to co-treatment with pmk-3 RNAi. We have found this not to be the case, although it did block Ptbb-6::GFP hyperactivation (Fig B in S10 Fig). It is difficult to interpret the significance of this result, however, since cbp-3 RNAi itself causes isp-1(qm150) worms to arrest [26] and we found it to result in sterility and early mortality even in wild-type worms. Clearly, cbp-3 has essential roles, including in ceramide biosynthesis [26], and whether it plays a specific or general role in signaling mitochondrial dysfunction shall require further investigation.
The previously described roles for PMK-3 relate to a MAPK cascade required for both neuronal development [65] and axon regeneration [111]. Interestingly, while this MAPK cascade is also initiated by DLK-1 (MAP3K), it utilizes the MAP2K MKK-4, instead of SEK-3 which we found to be required for Ptbb-6::GFP induction. Whether these differences in MAP2K usage are mediated by different DLK-1 isoforms or reflect different tissues of activation remains a question for future study. Intriguingly, it was recently shown that sensory neurons of Mit mutants have reduced functionality relative to wild-type animals [112], suggesting there could be competition for DLK-1 by the two MAP2Ks in the same tissue and that increased neuronal response time may be the payoff for long term survival under stress. In our studies, both genetic and RNAi-mediated removal of mkk-4 failed to reduce Ptbb-6::GFP expression. The same RNAi construct was employed previously and shown to block SKN-1 activation induced by oxidative stress [113]. These findings further highlight the complementarity between the PMK-3 and SKN-1 signaling pathways that we have discovered in this work. DLK-1 also been implicated in Wallerian degeneration in mammals and flies [114], the active process by which severed axons self-destruct. This is especially interesting because DLK-1 is coupled to JNK activation in this pathway, via MKK4/7 and the NAD+ sensor and adaptor protein SARM1 [115]. Presumably other adaptor proteins could act to modulate DLK-1 target proteins in a different setting, and this may be what is behind the novel DLK-1, SEK-3, PMK-3 signaling pathway that we have identified in this study [116].
MAPK signaling is highly conserved across phyla, and p38 signaling has been implicated in numerous pathologies. However, most studies have looked at the role of the p38α isoform, to the extent that it is referred to simply as p38 in much of the literature [117]. However, the four mammalian p38 isoforms differ in expression across tissues as well as in their substrate specificity, and inhibition of different isoforms can produce opposite effects [118], limiting the potential for broad spectrum p38 inhibitors in ameliorating disease. The complexity of p38 MAPK signaling is similar in worms: The three isoforms exhibit differential tissue specificity and methods of activation [119]. In worms, the p38 that behaves most like the well-studied mammalian p38α is PMK-1, which, as stated previously, is activated by oxidative stress and plays an essential role in immunity. Further study of the other two p38 isoforms in C. elegans is likely to shed light on the roles of the less studied mammalian isoforms as well.
Finally, while we used Ptbb-6::GFP as a marker of PMK-3 activity that somehow permitted a functional Mit mutant longevity response following complex III, IV and V disruption, we also showed that tbb-6 itself is required for life extension following mitochondrial disruption. TBB-6 is unusual among β-tubulins in that its C-terminus is notably truncated relative to other β-tubulins (Fig 9B). Rostovtseva and colleagues [101] have reported that the C-termini of β-tubulins which are enriched in glutamate can plug the voltage-dependent anion channel (VDAC) and reduce ADP entry into mitochondria [101]. This finding raises the intriguing possibility that TBB-6 may function to enhance ADP entry into mitochondria under stressed conditions. The identification of the precise mechanism by which TBB-6 functions to extend life, along with the mode of action of DLK-1, SEK-3 and PMK-3 in mitochondrial stress-induced longevity, stands to be an exciting area for future investigation.
Methods
Identification of atfs-1 Independent Gene Set and Promoter Analysis
Gene Expression Omnibus dataset GSE38196, first described in [43], was used to identify genes upregulated independently of atfs-1 following mitochondrial dysfunction (spg-7 RNAi). Full details of our procedure to isolate atfs-1 independent genes from this dataset is provided in S1 Text. The MEME Suite of tools (v4.10.1) [120] was used to identify enriched DNA elements (ungapped) among the promoter regions of the identified gene subset. We limited our search to 400 bp of the most proximal 5’ sequence of each gene. MAST [121], was used to locate DAF-16 binding sites using a weighted matrix based on the consensus identified by Kumar and colleagues [51].
Nematode Strains and Maintenance
A complete list of C. elegans strains used in this study is provided in S6 Table. All strains were maintained at 20°C on standard NGM-agar plates [20].
Transgene Construction and Transgenic Strain Generation
Recombinant array construction, microinjection procedures and choice of strain background are detailed in S1 Text and S6 Table.
Feeding RNAi
Feeding RNAi and RNAi dilution studies were performed as previously described [20]. Details regarding either the source or construction of feeding RNAi constructs is provided in Supplemental Experimental Procedures (S1 Text).
Fluorescence Imaging and Quantification
Images of first day adult worms were captured using an Olympus DP71 CCD camera connected to an Olympus SZX16 fluorescence dissecting microscope. Where relevant, images were quantified using ImageJ software (NIH). A one-way ANOVA, or Student’s t-test with correction applied for multiple testing was employed, as indicated in figure legends.
atfs-1 mRNA Quantification
qRT-PCR was used to measure the efficacy of atfs-1 RNAi knockdown in Fig 4C. Details of strain culturing, mRNA extraction, cDNA synthesis, primer design for qRT-PCR analysis, data normalization and statistical testing are provided in S1 Text.
Lifespan Analyses
Lifespan studies were performed as described previously [20]. Use of FudR was avoided. The first day of adulthood was designated as day one. Data was analyzed using the log rank test and Cox’s proportional hazard model. A full description of all lifespan experiments is provided in S4 Table. Raw lifespan data is provided in S5 Table.
Supporting Information
All ETC subunits targeted by feeding RNAi in the current analysis—subunits are organized by complex (see S2 Table for a list of all known ETC subunits in C. elegans). Graphed data shows change in GFP fluorescence (mean +/-SD) following treatment of Ptbb-6::GFP reporter worms with each feeding RNAi. Data is normalized relative to vector-control treated worms. Each condition is the average fluorescence of between 3–12 worms. Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing). Red daggers indicate subunits with paralogs.
(TIF)
All ETC subunits targeted by feeding RNAi in the current analysis—subunits are organized by complex (see S2 Table for a list of all known ETC subunits in C. elegans). Graphed data shows change in GFP fluorescence (mean +/-SD) following treatment of Pgst-4::GFP reporter worms with each feeding RNAi. Data is normalized relative to vector-control treated worms. Each condition is the average fluorescence of between 3–12 worms. Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing). Red daggers indicate subunits with paralogs.
(TIF)
All ETC subunits targeted by feeding RNAi in the current analysis—subunits are organized by complex (see S2 Table for a list of all known ETC subunits in C. elegans). Graphed data shows change in GFP fluorescence (mean +/-SD) following treatment of Phsp-6::GFP reporter worms with each feeding RNAi. Data is normalized relative to vector-control treated worms. Each condition is the average fluorescence of between 3–12 worms. Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing). Red daggers indicate subunits with paralogs.
(TIF)
Data presented in Fig 2B of main text is reproduced on left. All ETC subunits with paralogs (marked with red daggers in S1–S3 Figs) were removed from the initial analysis and then GFP fluorescence re-averaged across each complex (+/-SD). Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing,* p<0.01, **p<0.001, ***p<0.0001).
(TIF)
RNAi clones targeting non-ETC mitochondrial targets and which have previously have been reported to increase lifespan also induce Ptbb-6::GFP expression. Targets include the mitochondrial ribosome machinery (B0261.4/mrpl-47 [12] and mrps-5 [122]); the solute carrier protein F13G3.7 [12] and the UPRmt response protein hsp-6 [123].
(TIF)
(A) Constitutive GFP fluorescence in wild-type, isp-1(qm150), nuo-6(qm200), ctb-1(qm189) and isp-1(qm150); ctb-1(qm189) mutants carrying the listed reporter construct was averaged over 8–50 adult worms. Data is presented as relative GFP fluorescence (mean +/-SD). Two sets of statistical comparisons were undertaken: Asterisks indicate significant difference relative to wild-type control while hash indicates significant difference between ctb-1(qm189)) and ctb-1(qm189); isp-1(qm150) (Student’s t-test, p<0.05 before Bonferroni correction for multiple testing,* p<0.017, **p<0.001, ***p<0.0001, # p<0.05, ##p<0.002). (B) Wild-type worms and ctb-1(qm189) worms containing the listed GFP reporter construct were cultured on bacterial feeding RNAi targeting atp-3 (1/10th strength), cco-1, isp-1 or nuo-2 and GFP fluorescence quantified as described. Asterisks indicate significantly different relative to vector control of the same genetic background (Student’s t-test, p<0.05 before Bonferroni correction for multiple testing, *p<0.01, **p<0.001, ***p<0.0001).
(TIF)
(A) isp-1(qm150) worms carrying Ptbb-6::GFP, Pgst-4::GFP or Phsp-6::GFP reporter genes were exposed to atfs-1 or skn-1 feeding RNAi then GFP fluorescence was quantified in day one adults. Data is presented as mean (+/- SD) normalized to vector-control treated animals. Asterisks indicate significant difference relative to vector control-treated worms (Student’s t-test, p<0.01; n = 12 worms/condition, from four biological replicates). (B) RNAi knockdown of skn-1 in nuo-6(qm200) worms turns off Pgst-4::GFP, as reported [59], but has no effect on Ptbb-6::GFP nor Phsp-6::GFP expression. RNAi knockdown of atfs-1 blocks Phsp-6::GFP expression, as reported [43], but dramatically further upregulates Ptbb-6::GFP. Surprisingly, atfs-1 RNAi also turned off Pgst-4::GFP. (C) isp-1(qm150), nuo-6(qm200) and wild type worms containing the Ptbb-6::GFP reporter were cultured on RNAi to atfs-1 and GFP fluorescence quantified as described in (A). Asterisks indicate significantly different relative to vector control-treated worms (Student’s t-test, unequal variance, ns = not significant,*p<0.001, **p<0.0001).
(TIF)
isp-1(qm150) worms containing Ptbb-6::GFP, Pgst-4::GFP or Phsp-6::GFP were cultured on feeding RNAi targeting components of the cellular surveillance pathway known to function upstream of atfs-1 [26, 60]. GFP fluorescence was quantified when vector-control worms reached adulthood. Size-corrected fluorescence data is presented as mean fluorescence (+/- SD) normalized to vector-control treated animals. Asterisks indicate significant difference relative to vector control-treated worms (Student’s t-test, p<0.001; n = 6 worms/condition, from two biological replicates).
(TIF)
(A) Induction of Ptbb-6::GFP expression in isp-1(qm150);ctb-1(qm189) worms is blocked by pmk-3, sek-3 and dlk-1 RNAi. Both atfs-1 and vhp-1 RNAi result in increased reporter fluorescence. Data is presented as mean (+/- SD) normalized to vector-control treated animals. Asterisks indicate significant difference relative to vector control-treated worms (Student’s t-test, Bonferroni corrected for multiple testing p<0.01; n = 5 worms/condition, from a single biological replicate). (B) The weak induction of Ptbb-6::GFP in nuo-6(qm200) worms is blocked when animals are exposed to dlk-1, sek-3 or pmk-3 RNAi, but none of these treatments have any effect on Pgst-4::GFP or Phsp-6::GFP reporter expression. (C) Wild type worms co-treated with RNAi targeting pmk-3 and either atp-3 or isp-1 (both at 1/10th strength) are unable to induce Ptbb-6::GFP expression.
(TIF)
(A) nuo-6(qm200); Ptbb-6GFP worms arrest growth when cultured on vhp-1 RNAi (top row). Larval arrest is by-passed following the genetic removal of pmk-3 in pmk-3(ok169); nuo-6(qm200);Ptbb-6::GFP worms (bottom row). (B) Knockdown of pmk-3 by bacterial feeding RNAi uniquely rescued both the larval arrest and blocked Ptbb-6::GFP reporter expression of isp-1(qm150); Ptbb-6::GFP worms co-cultured on vhp-1 RNAi (top panel, data copied from Fig 7, main text). Unlike pmk-3 knockdown, cbp-3 knockdown does not overcome the growth arrest induced by vhp-1 knockdown.
(TIF)
(A) RNAi-mediated knockdown of vhp-1 in Ptbb-6::GFP worms results in weak hypodermal GFP fluorescence and smaller adult worms. The decrease in adult size is proportional to vhp-1 RNAi dose. (B-D) RNAi-mediated knockdown of none of the 14 known MAPKs in C. elegans inadvertently increases adult size.
(TIF)
List of C. elegans genes up-regulated independently of atfs-1 following exposure to spg-7 RNAi. Shown are gene descriptions and fold induction in the presence and absence of atfs-1 relative to vector control.
(XLSX)
Ptbb-6::GFP, Pgst-4::GFP and Phsp-6::GFP transcriptional reporter expression in wild-type worms following RNAi-mediated disruption of nuclear-encoded mitochondrial proteins.
(XLSX)
Ptbb-6::GFP, Pgst-4::GFP and Phsp-6::GFP transcriptional reporter expression and development in isp-1(qm150) worms following RNAi-mediated disruption of nuclear-encoded mitochondrial proteins.
(XLSX)
Survival analyses (Excel sheet #1), and Genotype:RNAi interaction terms (Excel sheet #2).
(XLSX)
Raw survival data for the lifespan analyses used to generate S4 Table.
(XLSX)
List of strains employed in current study, along with a description of relevant strain constructions.
(XLSX)
Detailed list of experimental protocols.
(DOCX)
Acknowledgments
Several strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Dr. Cole Haynes (Memorial Sloan Kettering Center, NY) for depositing spg-7 microarray data and providing reagents, Dr. Alexander Soukas (Harvard, MA), Danielle Garsin (UTHSC, Houston, TX) and Dr. Yishi Jin (UCSD, CA) for reagents. We also thank both Jonathan Dorigatti and Oxana Radetskaya (UTHSCSA, San Antonio, TX) for technical help. Finally, we thank the reviewers and editors for helpful criticism.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support was provided by the National Institutes of Health [AG-047561 (SLR), AG044768 (ALF), AG013319 (ALF), ES017761 (ALF), T32 AG021890 (EM)]; the National Institute for General Medical Sciences NIGMS [K12GM111726 (MBB)], the Glenn Foundation for Medical Research (EM) and the South Texas Veteran's Affairs Healthcare System (ALF). Funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders—past, present and future. Biochimica et biophysica acta. 2004;1659(2–3):115–20. 10.1016/j.bbabio.2004.09.005 . [DOI] [PubMed] [Google Scholar]
- 2.Lane RK, Hilsabeck T, Rea SL. The role of mitochondrial dysfunction in age-related diseases. Biochim Biophys Acta. 2015. 10.1016/j.bbabio.2015.05.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieusset J. Contribution of mitochondria and endoplasmic reticulum dysfunction in insulin resistance: Distinct or interrelated roles? Diabetes Metab. 2015. 10.1016/j.diabet.2015.02.006 . [DOI] [PubMed] [Google Scholar]
- 4.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond). 2008;114(3):195–210. . [DOI] [PubMed] [Google Scholar]
- 5.Brown AM, Gordon D, Lee H, Caudy M, Hardy J, Haroutunian V, et al. Association of the dihydrolipoamide dehydrogenase gene with Alzheimer's disease in an Ashkenazi Jewish population. Am J Med Genet B Neuropsychiatr Genet. 2004;131B(1):60–6. Epub 2004/09/25. 10.1002/ajmg.b.30008 . [DOI] [PubMed] [Google Scholar]
- 6.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19(14):2040–1. 10.1096/fj.05-3735fje . [DOI] [PubMed] [Google Scholar]
- 7.Dagda RK, Chu CT. Mitochondrial quality control: insights on how Parkinson's disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J Bioenerg Biomembr. 2009;41(6):473–9. Epub 2009/12/17. 10.1007/s10863-009-9255-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. 2015;112(11):3463–8. Epub 2015/03/04. 10.1073/pnas.1500877112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadun AA, Chicani CF, Ross-Cisneros FN, Barboni P, Thoolen M, Shrader WD, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Archives of neurology. 2012;69(3):331–8. Epub 2012/03/14. 10.1001/archneurol.2011.2972 . [DOI] [PubMed] [Google Scholar]
- 10.Melov S, Lithgow GJ, Fischer DR, Tedesco PM, Johnson TE. Increased frequency of deletions in the mitochondrial genome with age of Caenorhabditis elegans. Nucleic Acids Res. 1995;23(8):1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rea S. CLK-1/Coq7p is a DMQ mono-oxygenase and a new member of the di-iron carboxylate protein family. FEBS Lett. 2001;509(3):389–94. [DOI] [PubMed] [Google Scholar]
- 12.Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33(1):40–8. . [DOI] [PubMed] [Google Scholar]
- 13.Dillin A, Hsu A-L, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, et al. Rates of Behavior and Aging Specified by Mitochondrial Function During Development. Science. 2002;298(5602):2398–401. [DOI] [PubMed] [Google Scholar]
- 14.Limongelli A, Schaefer J, Jackson S, Invernizzi F, Kirino Y, Suzuki T, et al. Variable penetrance of a familial progressive necrotising encephalopathy due to a novel tRNA(Ile) homoplasmic mutation in the mitochondrial genome. Journal of medical genetics. 2004;41(5):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu TK, Wang AG, Yen MY, Liu JH. Leber's hereditary optic neuropathy masquerading as optic neuritis with spontaneous visual recovery. Clinical & experimental optometry. 2014;97(1):84–6. 10.1111/cxo.12100 . [DOI] [PubMed] [Google Scholar]
- 16.Hudson G, Carelli V, Spruijt L, Gerards M, Mowbray C, Achilli A, et al. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am J Hum Genet. 2007;81(2):228–33. Epub 2007/08/02. 10.1086/519394 ; PubMed Central PMCID: PMCPmc1950812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkman MA, Yu-Wai-Man P, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, et al. Gene-environment interactions in Leber hereditary optic neuropathy. Brain: a journal of neurology. 2009;132(Pt 9):2317–26. 10.1093/brain/awp158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497(7450):451–7. 10.1038/nature12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munkácsy E, Rea SL. The paradox of mitochondrial dysfunction and extended longevity. Exp Gerontol. 2014;56(0):221–33. 10.1016/j.exger.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rea SL, Ventura N, Johnson TE. Relationship Between Mitochondrial Electron Transport Chain Dysfunction, Development, and Life Extension in Caenorhabditis elegans. PLoS Biol. 2007;5(10):e259 Epub 2007/10/05. 06-PLBI-RA-2325 [pii] 10.1371/journal.pbio.0050259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura N, Rea SL, Schiavi A, Torgovnick A, Testi R, Johnson TE. p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell. 2009;8(4):380–93. Epub 2009/05/07. ACE482 [pii] 10.1111/j.1474-9726.2009.00482.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li MX, Dewson G. Mitochondria and apoptosis: emerging concepts. F1000Prime Rep. 2015;7:42 10.12703/P7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholls DG, Ferguson SJ. Bioenergetics3. London: Academic Press; 2002. 297 p. [Google Scholar]
- 24.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. 10.1152/physrev.00057.2003 . [DOI] [PubMed] [Google Scholar]
- 25.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. Epub 2013/04/09. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Samuel BS, Breen PC, Ruvkun G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature. 2014;508(7496):406–10. Epub 2014/04/04. 10.1038/nature13204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khutornenko AA, Roudko VV, Chernyak BV, Vartapetian AB, Chumakov PM, Evstafieva AG. Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):12828–33. Epub 2010/06/23. 10.1073/pnas.0910885107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Souza AD, Parish IA, Krause DS, Kaech SM, Shadel GS. Reducing Mitochondrial ROS Improves Disease-related Pathology in a Mouse Model of Ataxia-telangiectasia. Mol Ther. 2013;21(1):42–8. Epub 2012/09/27. 10.1038/mt.2012.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243–56. 10.1038/nrm3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson EF, Stout CD. Structural diversity of eukaryotic membrane cytochrome p450s. J Biol Chem. 2013;288(24):17082–90. 10.1074/jbc.R113.452805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnould T, Michel S, Renard P. Mitochondria Retrograde Signaling and the UPR(mt): Where Are We in Mammals? Int J Mol Sci. 2015;16(8):18224–51. 10.3390/ijms160818224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240(1):98–103. . [DOI] [PubMed] [Google Scholar]
- 33.Khan M, Ligon M, Temmer L, Hufnal B, Farber IR, Munkácsy E, et al. TAF-4 is Required for the Life Extension of isp-1, clk-1 and tpk-1 Mit Mutants. Aging (Albany NY). 2013;10(Oct 5):741–58. Epub 2013/10/11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michel S, Canonne M, Arnould T, Renard P. Inhibition of mitochondrial genome expression triggers the activation of CHOP-10 by a cell signaling dependent on the integrated stress response but not the mitochondrial unfolded protein response. Mitochondrion. 2015;21:58–68. 10.1016/j.mito.2015.01.005 . [DOI] [PubMed] [Google Scholar]
- 35.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40(3):356–61. Epub 2008/02/05. ng.2007.50 [pii] 10.1038/ng.2007.50 . [DOI] [PubMed] [Google Scholar]
- 36.Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-Like Protein 5 Positively Regulates Chaperone Gene Expression in the Mitochondrial Unfolded Protein Response. Genetics. 2006;174(1):229–39. 10.1534/genetics.106.061580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiavi A, Maglioni S, Palikaras K, Shaik A, Strappazzon F, Brinkmann V, et al. Iron-Starvation-Induced Mitophagy Mediates Lifespan Extension upon Mitochondrial Stress in C. elegans. Curr Biol. 2015;25(14):1810–22. 10.1016/j.cub.2015.05.059 . [DOI] [PubMed] [Google Scholar]
- 38.Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149(2):452–66. Epub 2012/04/17. 10.1016/j.cell.2012.02.050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, Haynes CM. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature. 2014;516(7531):414–7. 10.1038/nature13818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirienko NV, Ausubel FM, Ruvkun G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2015;112(6):1821–6. 10.1073/pnas.1424954112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Runkel ED, Liu S, Baumeister R, Schulze E. Surveillance-activated defenses block the ROS-induced mitochondrial unfolded protein response. PLoS genetics. 2013;9(3):e1003346 10.1371/journal.pgen.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochimica et biophysica acta. 2013;1833(2):410–6. 10.1016/j.bbamcr.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337(6094):587–90. Epub 2012/06/16. 10.1126/science.1223560 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295(5554):502–5. [DOI] [PubMed] [Google Scholar]
- 45.Durieux J, Wolff S, Dillin A. The Cell-Non-Autonomous Nature of Electron Transport Chain-Mediated Longevity. Cell. 2011;144(1):79–91. S0092-8674(10)01434-0 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett CF, Vander Wende H, Simko M, Klum S, Barfield S, Choi H, et al. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nature communications. 2014;5:3483 10.1038/ncomms4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauthan M, Ranji P, Aguilera Pradenas N, Pitot C, Pilon M. The mitochondrial unfolded protein response activator ATFS-1 protects cells from inhibition of the mevalonate pathway. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):5981–6. 10.1073/pnas.1218778110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miwa S, Jow H, Baty K, Johnson A, Czapiewski R, Saretzki G, et al. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun. 2014;5:3837 10.1038/ncomms4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin J, Ananthan J, Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988;8(9):3761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–4. [DOI] [PubMed] [Google Scholar]
- 51.Kumar N, Jain V, Singh A, Jagtap U, Verma S, Mukhopadhyay A. Genome-wide endogenous DAF-16/FOXO recruitment dynamics during lowered insulin signalling in C. elegans. Oncotarget. 2015;6(39):41418–33. 10.18632/oncotarget.6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerstein MB, Lu ZJ, Van Nostrand EL, Cheng C, Arshinoff BI, Liu T, et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330(6012):1775–87. Epub 2010/12/24. 10.1126/science.1196914 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W, Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9(3):433–47. Epub 2010/03/30. 10.1111/j.1474-9726.2010.00571.x . [DOI] [PubMed] [Google Scholar]
- 54.Feng J, Bussière F, Hekimi S. Mitochondrial Electron Transport Is a Key Determinant of Life Span in Caenorhabditis elegans. Developmental Cell. 2001;1(November):633–44. [DOI] [PubMed] [Google Scholar]
- 55.Suthammarak W, Morgan PG, Sedensky MM. Mutations in mitochondrial complex III uniquely affect complex I in Caenorhabditis elegans. J Biol Chem. 2010;285(52):40724–31. Epub 2010/10/26. M110.159608 [pii] 10.1074/jbc.M110.159608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen M, Hsu A-L, Dillin A, Kenyon C. New Genes Tied to Endocrine, Metabolic, and Dietary Regulation of Lifespan from a Caenorhabditis elegans Genomic RNAi Screen. PLoS Genetics. 2005;1(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y, Sun H. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell. 2007;6(4):489–503. Epub 2007/07/05. ACE302 [pii] 10.1111/j.1474-9726.2007.00302.x . [DOI] [PubMed] [Google Scholar]
- 58.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17(15):1882–93. 10.1101/gad.1107803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahn NW, Rea SL, Moyle S, Kell A, Johnson TE. Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem J. 2008;409(1):205–13. 10.1042/bj20070521 [DOI] [PubMed] [Google Scholar]
- 60.Shore DE, Carr CE, Ruvkun G. Induction of cytoprotective pathways is central to the extension of lifespan conferred by multiple longevity pathways. PLoS Genetics. 2012;8(7):e1002792 Epub 2012/07/26. 10.1371/journal.pgen.1002792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuno T, Hisamoto N, Terada T, Kondo T, Adachi M, Nishida E, et al. The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J. 2004;23(11):2226–34. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan D, Jin Y. Regulation of DLK-1 kinase activity by calcium-mediated dissociation from an inhibitory isoform. Neuron. 2012;76(3):534–48. Epub 2012/11/13. 10.1016/j.neuron.2012.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trujillo G, Nakata K, Yan D, Maruyama IN, Jin Y. A ubiquitin E2 variant protein acts in axon termination and synaptogenesis in Caenorhabditis elegans. Genetics. 2010;186(1):135–45. 10.1534/genetics.110.117341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park EC, Glodowski DR, Rongo C. The ubiquitin ligase RPM-1 and the p38 MAPK PMK-3 regulate AMPA receptor trafficking. PLoS ONE. 2009;4(1):e4284 Epub 2009/01/28. 10.1371/journal.pone.0004284 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120(3):407–20. . [DOI] [PubMed] [Google Scholar]
- 66.Walter L, Baruah A, Chang HW, Pace HM, Lee SS. The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biology. 2011;9(6):e1001084 Epub 2011/06/30. 10.1371/journal.pbio.1001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SJ, Hwang AB, Kenyon C. Inhibition of Respiration Extends C. elegans Life Span via Reactive Oxygen Species that Increase HIF-1 Activity. Curr Biol. 2010;20(23):2131–6. Epub 2010/11/26. S0960-9822(10)01374-6 [pii] 10.1016/j.cub.2010.10.057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lapierre LR, De Magalhaes Filho CD, McQuary PR, Chu CC, Visvikis O, Chang JT, et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat Commun. 2013;4:2267 Epub 2013/08/09. 10.1038/ncomms3267 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Block DH, Twumasi-Boateng K, Kang HS, Carlisle JA, Hanganu A, Lai TY, et al. The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult C. elegans. PLoS Genet. 2015;11(5):e1005265 10.1371/journal.pgen.1005265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powell JR, Kim DH, Ausubel FM. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc Natl Acad Sci U S A. 2009;106(8):2782–7. 10.1073/pnas.0813048106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh V, Aballay A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci U S A. 2006;103(35):13092–7. 10.1073/pnas.0604050103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estes KA, Dunbar TL, Powell JR, Ausubel FM, Troemel ER. bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107(5):2153–8. 10.1073/pnas.0914643107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, et al. A stress-responsive system for mitochondrial protein degradation. Mol Cell. 2010;40(3):465–80. 10.1016/j.molcel.2010.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu X, Li L, Jiang H. Doa1 targets ubiquitinated substrates for mitochondria-associated degradation. J Cell Biol. 2016;213(1):49–63. 10.1083/jcb.201510098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Esaki M, Ogura T. Cdc48p/p97-mediated regulation of mitochondrial morphology is Vms1p-independent. J Struct Biol. 2012;179(2):112–20. 10.1016/j.jsb.2012.04.017 . [DOI] [PubMed] [Google Scholar]
- 76.Yee C, Yang W, Hekimi S. The Intrinsic Apoptosis Pathway Mediates the Pro-Longevity Response to Mitochondrial ROS in C. elegans. Cell. 2014;157(4):897–909. Epub 2014/05/13. 10.1016/j.cell.2014.02.055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakaguchi A, Sato M, Sato K, Gengyo-Ando K, Yorimitsu T, Nakai J, et al. REI-1 Is a Guanine Nucleotide Exchange Factor Regulating RAB-11 Localization and Function in C. elegans Embryos. Dev Cell. 2015;35(2):211–21. 10.1016/j.devcel.2015.09.013 . [DOI] [PubMed] [Google Scholar]
- 78.Hunter T, Bannister WH, Hunter GJ. Cloning, expression, and characterization of two manganese superoxide dismutases from Caenorhabditis elegans. J Biol Chem. 1997;272(45):28652–9. . [DOI] [PubMed] [Google Scholar]
- 79.Kohn RE, Duerr JS, McManus JR, Duke A, Rakow TL, Maruyama H, et al. Expression of multiple UNC-13 proteins in the Caenorhabditis elegans nervous system. Mol Biol Cell. 2000;11(10):3441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29(10):2704–15. Epub 2009/03/11. MCB.01811-08 [pii] 10.1128/MCB.01811-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walter L, Baruah A, Chang HW, Pace HM, Lee SS. The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS biology. 2011;9(6):e1001084 10.1371/journal.pbio.1001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lapierre LR, De Magalhaes Filho CD, McQuary PR, Chu CC, Visvikis O, Chang JT, et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nature communications. 2013;4:2267 10.1038/ncomms3267 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burkewitz K, Morantte I, Weir HJ, Yeo R, Zhang Y, Huynh FK, et al. Neuronal CRTC-1 Governs Systemic Mitochondrial Metabolism and Lifespan via a Catecholamine Signal. Cell. 2015;160(5):842–55. 10.1016/j.cell.2015.02.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segref A, Kevei E, Pokrzywa W, Schmeisser K, Mansfeld J, Livnat-Levanon N, et al. Pathogenesis of human mitochondrial diseases is modulated by reduced activity of the ubiquitin/proteasome system. Cell Metabolism. 2014;19(4):642–52. Epub 2014/04/08. 10.1016/j.cmet.2014.01.016 . [DOI] [PubMed] [Google Scholar]
- 85.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458(7237):453–60. 10.1038/nature07962 . [DOI] [PubMed] [Google Scholar]
- 86.Karbowski M, Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol. 2011;23(4):476–82. 10.1016/j.ceb.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu S, Peng G, Wang Y, Fang S, Karbowski M. The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell. 2011;22(3):291–300. 10.1091/mbc.E10-09-0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mouysset J, Kahler C, Hoppe T. A conserved role of Caenorhabditis elegans CDC-48 in ER-associated protein degradation. J Struct Biol. 2006;156(1):41–9. 10.1016/j.jsb.2006.02.015 . [DOI] [PubMed] [Google Scholar]
- 89.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annual review of immunology. 2002;20:55–72. 10.1146/annurev.immunol.20.091301.131133 . [DOI] [PubMed] [Google Scholar]
- 90.Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297(5581):623–6. 10.1126/science.1073759 . [DOI] [PubMed] [Google Scholar]
- 91.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19(19):2278–83. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kondo M, Yanase S, Ishii T, Hartman PS, Matsumoto K, Ishii N. The p38 signal transduction pathway participates in the oxidative stress-mediated translocation of DAF-16 to Caenorhabditis elegans nuclei. Mechanisms of ageing and development. 2005;126(6–7):642–7. 10.1016/j.mad.2004.11.012 . [DOI] [PubMed] [Google Scholar]
- 93.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiology and molecular biology reviews: MMBR. 2006;70(4):1061–95. 10.1128/MMBR.00025-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nix P, Hisamoto N, Matsumoto K, Bastiani M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc Natl Acad Sci U S A. 2011;108(26):10738–43. Epub 2011/06/15. 10.1073/pnas.1104830108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Vaart A, Rademakers S, Jansen G. DLK-1/p38 MAP Kinase Signaling Controls Cilium Length by Regulating RAB-5 Mediated Endocytosis in Caenorhabditis elegans. PLoS Genet. 2015;11(12):e1005733 10.1371/journal.pgen.1005733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nix P, Hammarlund M, Hauth L, Lachnit M, Jorgensen EM, Bastiani M. Axon regeneration genes identified by RNAi screening in C. elegans. J Neurosci. 2014;34(2):629–45. 10.1523/JNEUROSCI.3859-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14(1):6–16. . [PubMed] [Google Scholar]
- 98.Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Current biology: CB. 2001;11(3):171–6. . [DOI] [PubMed] [Google Scholar]
- 99.Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS genetics. 2005;1(1):119–28. 10.1371/journal.pgen.0010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butler JA, Mishur RJ, Bhaskaran S, Rea SL. A metabolic signature for long life in the Caenorhabditis elegans Mit mutants. Aging Cell. 2013;12(1):130–8. Epub 2012/11/24. 10.1111/acel.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A. 2008;105(48):18746–51. 10.1073/pnas.0806303105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iwasa H, Yu S, Xue J, Driscoll M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging Cell. 2010;9(4):490–505. 10.1111/j.1474-9726.2010.00575.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu G, Rogers J, Murphy CT, Rongo C. EGF signalling activates the ubiquitin proteasome system to modulate C. elegans lifespan. Embo j. 2011;30(15):2990–3003. Epub 2011/06/16. 10.1038/emboj.2011.195 ; PubMed Central PMCID: PMCPmc3160178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou L, Wang D, Chen D, Liu Y, Zhang Y, Cheng H, et al. A Systems Approach to Reverse Engineer Lifespan Extension by Dietary Restriction. Cell Metab. 2016;23(3):529–40. 10.1016/j.cmet.2016.02.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stroustrup N, Anthony WE, Nash ZM, Gowda V, Gomez A, López-Moyado IF, et al. The temporal scaling of Caenorhabditis elegans ageing. Nature. 2016;advance online publication. 10.1038/nature16550 http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature16550.html-supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. 10.1038/nprot.2008.211 . [DOI] [PubMed] [Google Scholar]
- 107.Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol. 2013;14(6):369–81. 10.1038/nrm3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bounoutas A, Kratz J, Emtage L, Ma C, Nguyen KC, Chalfie M. Microtubule depolymerization in Caenorhabditis elegans touch receptor neurons reduces gene expression through a p38 MAPK pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(10):3982–7. Epub 2011/03/04. 10.1073/pnas.1101360108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guha M, Avadhani NG. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13(6):577–91. Epub 2013/09/06. 10.1016/j.mito.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR. CCAAT/enhancer-binding protein family members recruit the coactivator CREB-binding protein and trigger its phosphorylation. J Biol Chem. 2003;278(38):36959–65. 10.1074/jbc.M303147200 . [DOI] [PubMed] [Google Scholar]
- 111.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323(5915):802–6. Epub 2009/01/24. 10.1126/science.1165527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maglioni S, Schiavi A, Runci A, Shaik A, Ventura N. Mitochondrial stress extends lifespan in C. elegans through neuronal hormesis. Exp Gerontol. 2014;56:89–98. 10.1016/j.exger.2014.03.026 . [DOI] [PubMed] [Google Scholar]
- 113.Kell A, Ventura N, Kahn NW, Johnson TE. Activation of SKN-1 by novel kinases in Caenorhabditis elegans Free Radical Biology & Medicine. 2007;in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghosh-Roy A, Chisholm AD. Caenorhabditis elegans: a new model organism for studies of axon regeneration. Dev Dyn. 2010;239(5):1460–4. 10.1002/dvdy.22253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron. 2016;89(3):449–60. 10.1016/j.neuron.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Witzel F, Maddison L, Bluthgen N. How scaffolds shape MAPK signaling: what we know and opportunities for systems approaches. Front Physiol. 2012;3:475 10.3389/fphys.2012.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Risco A, Cuenda A. New Insights into the p38gamma and p38delta MAPK Pathways. Journal of signal transduction. 2012;2012:520289 10.1155/2012/520289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Javadov S, Jang S, Agostini B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: therapeutic perspectives. Pharmacol Ther. 2014;144(2):202–25. Epub 2014/06/14. 10.1016/j.pharmthera.2014.05.013 ; PubMed Central PMCID: PMCPmc4185221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berman K, McKay J, Avery L, Cobb M. Isolation and characterization of pmk-(1–3): three p38 homologs in Caenorhabditis elegans. Mol Cell Biol Res Commun. 2001;4(6):337–44. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma W, Noble WS, Bailey TL. Motif-based analysis of large nucleotide data sets using MEME-ChIP. Nat Protoc. 2014;9(6):1428–50. 10.1038/nprot.2014.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bailey TL, Gribskov M. Combining evidence using p-values: application to sequence homology searches. Bioinformatics. 1998;14(1):48–54. . [DOI] [PubMed] [Google Scholar]
- 122.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497(7450):451–7. 10.1038/nature12188 http://www.nature.com/nature/journal/v497/n7450/abs/nature12188.html-supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ventura N, Rea SL. Caenorhabditis elegans mitochondrial mutants as an investigative tool to study human neurodegenerative diseases associated with mitochondrial dysfunction. Biotechnology Journal. 2007;2(5):584–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All ETC subunits targeted by feeding RNAi in the current analysis—subunits are organized by complex (see S2 Table for a list of all known ETC subunits in C. elegans). Graphed data shows change in GFP fluorescence (mean +/-SD) following treatment of Ptbb-6::GFP reporter worms with each feeding RNAi. Data is normalized relative to vector-control treated worms. Each condition is the average fluorescence of between 3–12 worms. Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing). Red daggers indicate subunits with paralogs.
(TIF)
All ETC subunits targeted by feeding RNAi in the current analysis—subunits are organized by complex (see S2 Table for a list of all known ETC subunits in C. elegans). Graphed data shows change in GFP fluorescence (mean +/-SD) following treatment of Pgst-4::GFP reporter worms with each feeding RNAi. Data is normalized relative to vector-control treated worms. Each condition is the average fluorescence of between 3–12 worms. Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing). Red daggers indicate subunits with paralogs.
(TIF)
All ETC subunits targeted by feeding RNAi in the current analysis—subunits are organized by complex (see S2 Table for a list of all known ETC subunits in C. elegans). Graphed data shows change in GFP fluorescence (mean +/-SD) following treatment of Phsp-6::GFP reporter worms with each feeding RNAi. Data is normalized relative to vector-control treated worms. Each condition is the average fluorescence of between 3–12 worms. Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing). Red daggers indicate subunits with paralogs.
(TIF)
Data presented in Fig 2B of main text is reproduced on left. All ETC subunits with paralogs (marked with red daggers in S1–S3 Figs) were removed from the initial analysis and then GFP fluorescence re-averaged across each complex (+/-SD). Asterisks indicate significantly different from vector (Student’s t-test, p<0.05 before Sidak-Bonferroni correction for multiple testing,* p<0.01, **p<0.001, ***p<0.0001).
(TIF)
RNAi clones targeting non-ETC mitochondrial targets and which have previously have been reported to increase lifespan also induce Ptbb-6::GFP expression. Targets include the mitochondrial ribosome machinery (B0261.4/mrpl-47 [12] and mrps-5 [122]); the solute carrier protein F13G3.7 [12] and the UPRmt response protein hsp-6 [123].
(TIF)
(A) Constitutive GFP fluorescence in wild-type, isp-1(qm150), nuo-6(qm200), ctb-1(qm189) and isp-1(qm150); ctb-1(qm189) mutants carrying the listed reporter construct was averaged over 8–50 adult worms. Data is presented as relative GFP fluorescence (mean +/-SD). Two sets of statistical comparisons were undertaken: Asterisks indicate significant difference relative to wild-type control while hash indicates significant difference between ctb-1(qm189)) and ctb-1(qm189); isp-1(qm150) (Student’s t-test, p<0.05 before Bonferroni correction for multiple testing,* p<0.017, **p<0.001, ***p<0.0001, # p<0.05, ##p<0.002). (B) Wild-type worms and ctb-1(qm189) worms containing the listed GFP reporter construct were cultured on bacterial feeding RNAi targeting atp-3 (1/10th strength), cco-1, isp-1 or nuo-2 and GFP fluorescence quantified as described. Asterisks indicate significantly different relative to vector control of the same genetic background (Student’s t-test, p<0.05 before Bonferroni correction for multiple testing, *p<0.01, **p<0.001, ***p<0.0001).
(TIF)
(A) isp-1(qm150) worms carrying Ptbb-6::GFP, Pgst-4::GFP or Phsp-6::GFP reporter genes were exposed to atfs-1 or skn-1 feeding RNAi then GFP fluorescence was quantified in day one adults. Data is presented as mean (+/- SD) normalized to vector-control treated animals. Asterisks indicate significant difference relative to vector control-treated worms (Student’s t-test, p<0.01; n = 12 worms/condition, from four biological replicates). (B) RNAi knockdown of skn-1 in nuo-6(qm200) worms turns off Pgst-4::GFP, as reported [59], but has no effect on Ptbb-6::GFP nor Phsp-6::GFP expression. RNAi knockdown of atfs-1 blocks Phsp-6::GFP expression, as reported [43], but dramatically further upregulates Ptbb-6::GFP. Surprisingly, atfs-1 RNAi also turned off Pgst-4::GFP. (C) isp-1(qm150), nuo-6(qm200) and wild type worms containing the Ptbb-6::GFP reporter were cultured on RNAi to atfs-1 and GFP fluorescence quantified as described in (A). Asterisks indicate significantly different relative to vector control-treated worms (Student’s t-test, unequal variance, ns = not significant,*p<0.001, **p<0.0001).
(TIF)
isp-1(qm150) worms containing Ptbb-6::GFP, Pgst-4::GFP or Phsp-6::GFP were cultured on feeding RNAi targeting components of the cellular surveillance pathway known to function upstream of atfs-1 [26, 60]. GFP fluorescence was quantified when vector-control worms reached adulthood. Size-corrected fluorescence data is presented as mean fluorescence (+/- SD) normalized to vector-control treated animals. Asterisks indicate significant difference relative to vector control-treated worms (Student’s t-test, p<0.001; n = 6 worms/condition, from two biological replicates).
(TIF)
(A) Induction of Ptbb-6::GFP expression in isp-1(qm150);ctb-1(qm189) worms is blocked by pmk-3, sek-3 and dlk-1 RNAi. Both atfs-1 and vhp-1 RNAi result in increased reporter fluorescence. Data is presented as mean (+/- SD) normalized to vector-control treated animals. Asterisks indicate significant difference relative to vector control-treated worms (Student’s t-test, Bonferroni corrected for multiple testing p<0.01; n = 5 worms/condition, from a single biological replicate). (B) The weak induction of Ptbb-6::GFP in nuo-6(qm200) worms is blocked when animals are exposed to dlk-1, sek-3 or pmk-3 RNAi, but none of these treatments have any effect on Pgst-4::GFP or Phsp-6::GFP reporter expression. (C) Wild type worms co-treated with RNAi targeting pmk-3 and either atp-3 or isp-1 (both at 1/10th strength) are unable to induce Ptbb-6::GFP expression.
(TIF)
(A) nuo-6(qm200); Ptbb-6GFP worms arrest growth when cultured on vhp-1 RNAi (top row). Larval arrest is by-passed following the genetic removal of pmk-3 in pmk-3(ok169); nuo-6(qm200);Ptbb-6::GFP worms (bottom row). (B) Knockdown of pmk-3 by bacterial feeding RNAi uniquely rescued both the larval arrest and blocked Ptbb-6::GFP reporter expression of isp-1(qm150); Ptbb-6::GFP worms co-cultured on vhp-1 RNAi (top panel, data copied from Fig 7, main text). Unlike pmk-3 knockdown, cbp-3 knockdown does not overcome the growth arrest induced by vhp-1 knockdown.
(TIF)
(A) RNAi-mediated knockdown of vhp-1 in Ptbb-6::GFP worms results in weak hypodermal GFP fluorescence and smaller adult worms. The decrease in adult size is proportional to vhp-1 RNAi dose. (B-D) RNAi-mediated knockdown of none of the 14 known MAPKs in C. elegans inadvertently increases adult size.
(TIF)
List of C. elegans genes up-regulated independently of atfs-1 following exposure to spg-7 RNAi. Shown are gene descriptions and fold induction in the presence and absence of atfs-1 relative to vector control.
(XLSX)
Ptbb-6::GFP, Pgst-4::GFP and Phsp-6::GFP transcriptional reporter expression in wild-type worms following RNAi-mediated disruption of nuclear-encoded mitochondrial proteins.
(XLSX)
Ptbb-6::GFP, Pgst-4::GFP and Phsp-6::GFP transcriptional reporter expression and development in isp-1(qm150) worms following RNAi-mediated disruption of nuclear-encoded mitochondrial proteins.
(XLSX)
Survival analyses (Excel sheet #1), and Genotype:RNAi interaction terms (Excel sheet #2).
(XLSX)
Raw survival data for the lifespan analyses used to generate S4 Table.
(XLSX)
List of strains employed in current study, along with a description of relevant strain constructions.
(XLSX)
Detailed list of experimental protocols.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.